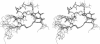Abstract
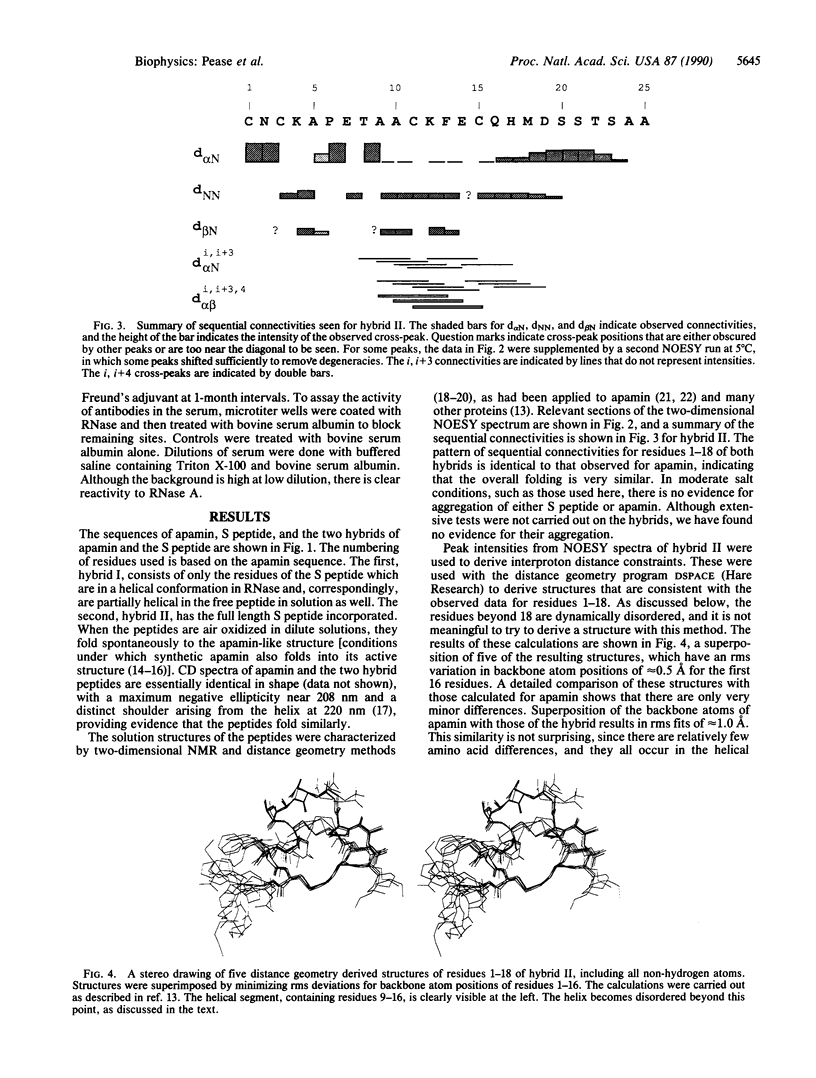
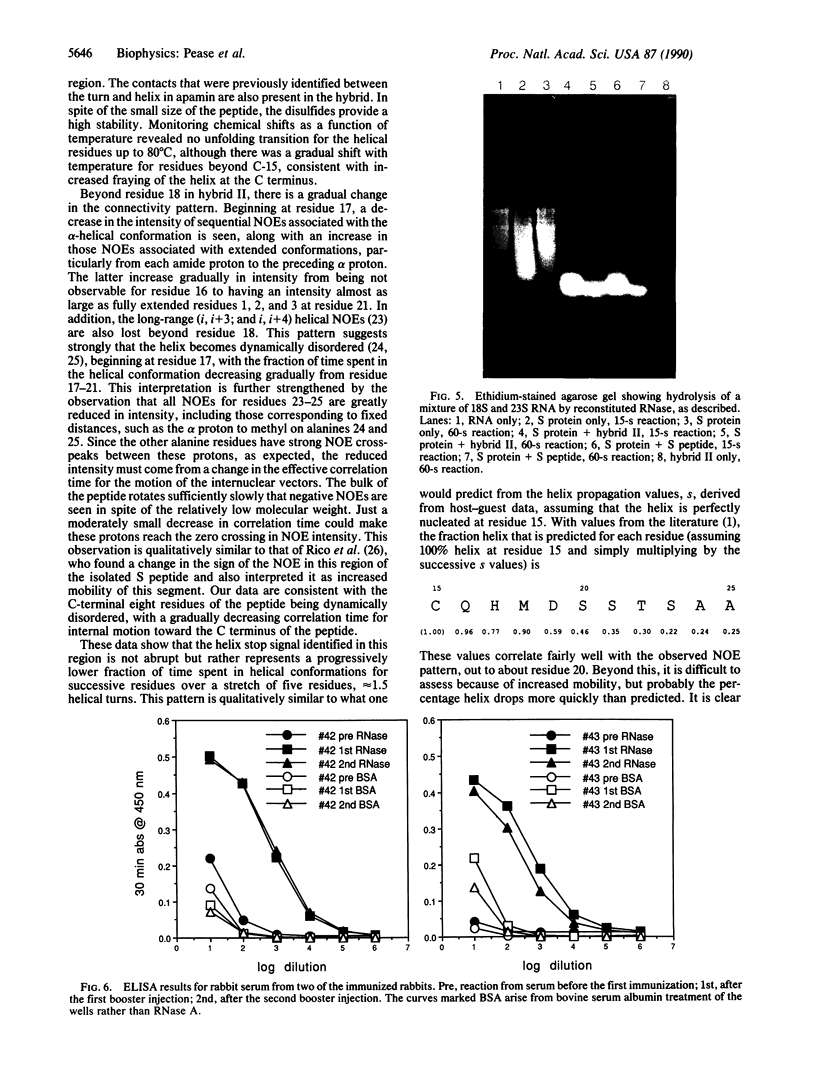
Peptides have been synthesized that have hybrid sequences, partially derived from the bee venom peptide apamin and partially from the S peptide of ribonuclease A. The hybrid peptides were demonstrated by NMR spectroscopy to fold, forming the same disulfides and basic three-dimensional structure as native apamin, containing a beta-turn and an alpha-helix. These hybrids were active in complementing S protein, reactivating nuclease activity. In addition, the hybrid peptide was effective in inducing antibodies that cross-react with the RNase, without conjugation to a carrier protein. The stability of the folded structure of this peptide suggests that it should be possible to elicit antibodies that will react not only with a specific sequence, but also with a specific secondary structure. Hybrid sequence peptides also provide opportunities to study separately nucleation and propagation steps in formation of secondary structure. We show that in S peptide the alpha-helix does not end abruptly but rather terminates gradually over four or five residues. In general, these hybrid sequence peptides, which fold predictably because of disulfide bond formation, can provide opportunities for examining structure-function relationships for many biologically active sequences.
Full text
PDF

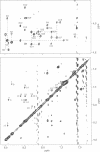
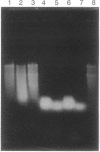
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bierzynski A., Kim P. S., Baldwin R. L. A salt bridge stabilizes the helix formed by isolated C-peptide of RNase A. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2470–2474. doi: 10.1073/pnas.79.8.2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. E., Klee W. A. Helix-coil transition of the isolated amino terminus of ribonuclease. Biochemistry. 1971 Feb 2;10(3):470–476. doi: 10.1021/bi00779a019. [DOI] [PubMed] [Google Scholar]
- Callewaert G. L., Shipolini R., Vernon C. A. The disulphide bridges of apamin. FEBS Lett. 1968 Aug;1(2):111–113. doi: 10.1016/0014-5793(68)80033-x. [DOI] [PubMed] [Google Scholar]
- Cosand W. L., Merrifield R. B. Concept of internal structural controls for evaluation of inactive synthetic peptide analogs: synthesis of [Orn13,14]apamin and its guanidination to an apamin derivative with full neurotoxic activity. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2771–2775. doi: 10.1073/pnas.74.7.2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyson H. J., Rance M., Houghten R. A., Lerner R. A., Wright P. E. Folding of immunogenic peptide fragments of proteins in water solution. I. Sequence requirements for the formation of a reverse turn. J Mol Biol. 1988 May 5;201(1):161–200. doi: 10.1016/0022-2836(88)90446-9. [DOI] [PubMed] [Google Scholar]
- Dyson H. J., Rance M., Houghten R. A., Wright P. E., Lerner R. A. Folding of immunogenic peptide fragments of proteins in water solution. II. The nascent helix. J Mol Biol. 1988 May 5;201(1):201–217. doi: 10.1016/0022-2836(88)90447-0. [DOI] [PubMed] [Google Scholar]
- Granier C., Pedroso Muller E., Van Rietschoten J. Use of synthetic analogs for a study on the structure-activity relationship of apamin. Eur J Biochem. 1978 Jan 2;82(1):293–299. doi: 10.1111/j.1432-1033.1978.tb12023.x. [DOI] [PubMed] [Google Scholar]
- Habermann E., Reiz K. G. Zur Biochemie der Bienengiftpeptide Melittin und Apamin. Biochem Z. 1965 Nov 15;343(2):192–203. [PubMed] [Google Scholar]
- Harper J. W., Auld D. S., Riordan J. F., Vallee B. L. Enzymatically active angiogenin/ribonuclease A hybrids formed by peptide interchange. Biochemistry. 1988 Jan 12;27(1):219–226. doi: 10.1021/bi00401a033. [DOI] [PubMed] [Google Scholar]
- Hider R. C., Ragnarsson U. A comparative structural study of apamin and related bee venom peptides. Biochim Biophys Acta. 1981 Jan 30;667(1):197–208. doi: 10.1016/0005-2795(81)90080-5. [DOI] [PubMed] [Google Scholar]
- Kim P. S., Baldwin R. L. A helix stop signal in the isolated S-peptide of ribonuclease A. 1984 Jan 26-Feb 1Nature. 307(5949):329–334. doi: 10.1038/307329a0. [DOI] [PubMed] [Google Scholar]
- Kim P. S., Bierzynski A., Baldwin R. L. A competing salt-bridge suppresses helix formation by the isolated C-peptide carboxylate of ribonuclease A. J Mol Biol. 1982 Nov 25;162(1):187–199. doi: 10.1016/0022-2836(82)90168-1. [DOI] [PubMed] [Google Scholar]
- Nelson J. W., Kallenbach N. R. Persistence of the alpha-helix stop signal in the S-peptide in trifluoroethanol solutions. Biochemistry. 1989 Jun 13;28(12):5256–5261. doi: 10.1021/bi00438a050. [DOI] [PubMed] [Google Scholar]
- Nelson J. W., Kallenbach N. R. Stabilization of the ribonuclease S-peptide alpha-helix by trifluoroethanol. Proteins. 1986 Nov;1(3):211–217. doi: 10.1002/prot.340010303. [DOI] [PubMed] [Google Scholar]
- Nieto J. L., Jiménez M. A., Rico M., Santoro J., Herranz J. Nuclear Overhauser effects in aqueous solution as dynamic probes in short linear peptides. FEBS Lett. 1988 Oct 24;239(1):83–87. doi: 10.1016/0014-5793(88)80550-7. [DOI] [PubMed] [Google Scholar]
- Pease J. H., Wemmer D. E. Solution structure of apamin determined by nuclear magnetic resonance and distance geometry. Biochemistry. 1988 Nov 1;27(22):8491–8498. doi: 10.1021/bi00422a029. [DOI] [PubMed] [Google Scholar]
- RICHARDS F. M., VITHAYATHIL P. J. The preparation of subtilisn-modified ribonuclease and the separation of the peptide and protein components. J Biol Chem. 1959 Jun;234(6):1459–1465. [PubMed] [Google Scholar]
- Rico M., Nieto J. L., Santoro J., Bermejo F. J., Herranz J., Gallego E. Low-temperature 1H-NMR evidence of the folding of isolated ribonuclease S-peptide. FEBS Lett. 1983 Oct 17;162(2):314–319. doi: 10.1016/0014-5793(83)80779-0. [DOI] [PubMed] [Google Scholar]
- Wagner G., Braun W., Havel T. F., Schaumann T., Go N., Wüthrich K. Protein structures in solution by nuclear magnetic resonance and distance geometry. The polypeptide fold of the basic pancreatic trypsin inhibitor determined using two different algorithms, DISGEO and DISMAN. J Mol Biol. 1987 Aug 5;196(3):611–639. doi: 10.1016/0022-2836(87)90037-4. [DOI] [PubMed] [Google Scholar]
- Wemmer D., Kallenbach N. R. Structure of apamin in solution: a two-dimensional nuclear magnetic resonance study. Biochemistry. 1983 Apr 12;22(8):1901–1906. doi: 10.1021/bi00277a025. [DOI] [PubMed] [Google Scholar]
- Wüthrich K., Billeter M., Braun W. Polypeptide secondary structure determination by nuclear magnetic resonance observation of short proton-proton distances. J Mol Biol. 1984 Dec 15;180(3):715–740. doi: 10.1016/0022-2836(84)90034-2. [DOI] [PubMed] [Google Scholar]
- van Rietschoten J., Granier C., Rochat H., Lissitzky S., Miranda F. Synthesis of apamin, a neurotoxic peptide from bee venom. Eur J Biochem. 1975 Aug 1;56(1):35–40. doi: 10.1111/j.1432-1033.1975.tb02204.x. [DOI] [PubMed] [Google Scholar]