Abstract
Neuroimaging data indicate that episodic memory (i.e., remembering specific past experiences) and episodic simulation (i.e., imagining specific future experiences) are associated with enhanced activity in a common set of neural regions, often referred to as the core network. This network comprises the hippocampus, parahippocampal cortex, lateral and medial parietal cortex, lateral temporal cortex, and medial prefrontal cortex. Evidence for a core network has been taken as support for the idea that episodic memory and episodic simulation are supported by common processes. Much remains to be learned about how specific core network regions contribute to specific aspects of episodic simulation. Prior neuroimaging studies of episodic memory indicate that certain regions within the core network are differentially sensitive to the amount of information recollected (e.g., the left lateral parietal cortex). In addition, certain core network regions dissociate as a function of their timecourse of engagement during episodic memory (e.g., transient activity in the posterior hippocampus and sustained activity in the left lateral parietal cortex). In the current study, we assessed whether similar dissociations could be observed during episodic simulation. We found that the left lateral parietal cortex modulates as a function of the amount of simulated details. Of particular interest, while the hippocampus was insensitive to the amount of simulated details, we observed a temporal dissociation within the hippocampus: transient activity occurred in relatively posterior portions of the hippocampus and sustained activity occurred in anterior portions. Because the posterior hippocampal and lateral parietal findings parallel those observed previously during episodic memory, the present results add to the evidence that episodic memory and episodic simulation are supported by common processes. Critically, the present study also provides evidence that regions within the core network support dissociable processes.
Keywords: Episodic memory, recollection, hippocampus, parietal, fMRI
1. Introduction
1.1. Episodic Memory and Episodic Simulation
Humans have the ability to re-experience past experiences (i.e., episodic memory) and to pre-experience hypothetical future experiences (i.e., episodic simulation; Schacter, Addis, Buckner, 2008; Suddendorf & Corballis, 2007; Szpunar, Spreng, & Schacter, 2014; Tulving, 2005). For example, one can mentally relive the last time they went to a Metallica concert. In contrast, if one is trying to decide whether to go to a Metallica concert for the first time, one can draw on details of similar concerts one has attended in the past to simulate the future experience and decide whether to go. According to the constructive episodic simulation hypothesis (Schacter & Addis, 2007), the ability to simulate future experiences draws on many of the same mental processes that support episodic memory. Specifically, episodic memory supports the construction of future experiences by providing access to episodic details that can be recombined into novel events.
1.2. The Core Network
A number of studies have provided evidence for the idea that many of the processes that support episodic simulation are shared with episodic memory (for reviews, see Benoit & Schacter, 2015; Schacter & Madore, 2016; Schacter et al., 2012; Szpunar, 2010). For example, one of the most consistent neuroimaging findings is that of the recruitment of a common set of neural regions during episodic memory and episodic simulation (for a recent review and meta-analysis, see Benoit & Schacter, 2015). These regions include the hippocampus, parahippocampal cortex, lateral and medial parietal cortex, lateral temporal cortex, and medial prefrontal cortex. Several researchers have argued that the observed neural overlap constitutes evidence for a core network that supports both the reconstruction of past episodes and the construction of future episodes (e.g., Buckner & Carroll, 2007; Hassabis & Maguire, 2007; Schacter & Addis, 2007; Schacter et al., 2012).
Recent studies have begun to identify the specific contributions of individual regions within the core network to episodic simulation (e.g., Andrews-Hanna, Reidler, Sepulcre, Poulin, & Buckner, 2010; Benoit, Gilbert, & Burgess, 2011; Benoit, Szpunar, & Schacter, 2014; Gaesser, Spreng, McLelland, Addis, & Schacter, 2013; Gerlach, Spreng, Madore, & Schacter, 2014; Hassabis et al., 2014; Martin, Schacter, Corballis, & Addis, 2011; Palombo, Hayes, Peterson, Keane, & Verfaellie, 2016; Szpunar, St. Jacques, Robbins, Wig, & Schacter, 2014; Szpunar, Jing, Benoit, & Schacter, 2015; Xu, Yuan, & Lei, 2016; for discussion, see Roberts, Schacter, & Addis, 2017; Schacter et al., 2012). For example, by manipulating the content of simulated episodes, Szpunar et al. (2014) demonstrated that activation in specific core-network regions are associated with processing different types of simulated content (e.g., simulating social scenarios was associated with neural activity in the posterior cingulate and lateral temporal cortex and simulating locations in those scenarios was associated with retrosplenial and parahippocampal activity). In a related study, Hassabis et al. (2014) demonstrated that core network regions such as the lateral temporal cortex and posterior cingulate are associated with processing personality characteristics of the people comprising an episodic simulation (e.g., agreeableness and extraversion, respectively). In another set of studies (Gaesser et al., 2013; Martin et al., 2011), greater hippocampal activity during episodic simulation was predictive of subsequent memory for the simulation, thus indicating that the hippocampus is involved in encoding a simulated future event into memory.
1.3. Current Aim and Study
Although the foregoing and related studies represent a promising beginning, much remains to be learned about how specific core network regions contribute to specific aspects of episodic simulation. The aim of the present functional magnetic resonance imaging (fMRI) study is to elucidate the contributions of individual regions within the core network to two specific features of episodic simulation: 1) whether simulation-related activities in different regions of the core network differ in their sensitivity to the timecourse of simulation (i.e., whether neural activity is transient or sustained), and 2) whether engagement of the regions depends on the amount of information that is integrated in service of the simulation (i.e., an event with fewer or more episodic details).
In the current study, participants imagined future events that included specific places, people, and objects. We manipulated the amount of simulated information by varying the number of people and objects that participants were cued to incorporate in their simulation (3, 4, or 5 details). In addition to manipulating the number of details, we also varied the duration for which participants simulated the future event (8, 10, or 12 s). Through this manipulation, we attempt to dissociate core network regions that demonstrate a sustained1 profile (i.e., neural activity that covaries with the period during which participants simulated a future episode) and core network regions that demonstrated a transient profile (i.e., neural activity that is unaffected by the delay; Vilberg & Rugg, 2012, 2014).
1.4. Contributions of Core Regions to Episodic Memory
Our rationale for examining the factors of amount of information and timecourse comes from findings in the episodic memory literature. A number of studies examining the neural correlates of episodic memory have demonstrated that neural activity in several core network regions covaries with the amount of information recollected (for a review, see Rugg, Johnson, & Uncapher, 2015). For example, activity in the lateral parietal cortex is greater under conditions of full relative to partial recollection (e.g., recall of a studied episode and its contextual features relative to recall of the studied episode alone; Hayama, Vilberg, & Rugg, 2012; Vilberg & Rugg, 2007; see also, Thakral, Wang, & Rugg, 2015; Yu, Johnson, & Rugg, 2012). These findings have been taken to support the idea that these regions contribute to the maintenance or representation of recollected information (Rugg et al., 2015; Vilberg & Rugg, 2008).
Regarding the timecourse of activation, Vilberg and Rugg (2012, 2014) evaluated whether episodic memory-related activity in regions of the core network is sustained across a delay over which recollected information is maintained in working memory. They reasoned that regions where activity is transient likely support processes associated with the initiation of episodic memory. In contrast, regions where activity is sustained across the delay support the representation and/or maintenance of episodic information. Within the core network, neural activity was sustained in the left lateral parietal cortex and lateral temporal cortex. Transient episodic memory effects were observed in the hippocampus, among other regions. The hippocampal effects are consistent with the proposal that one way that the hippocampus supports episodic memory is by initially reinstating the episodic details of a past experience (see, Rugg et al., 2015; Schacter & Addis, 2007). The observation of sustained activation profiles in the left lateral parietal cortex, together with the observation that activity within this region is sensitive to the amount of recollected information (see above), provide strong support for the interpretation the left lateral parietal cortex supports the maintenance of episodic information (Rugg et al., 2015).
1.5. Hypotheses
1.5.1. Common Simulation and Memory Processes
To the extent that episodic simulation is supported by similar processes as episodic memory (Schacter & Addis, 2007), we predicted to observe similar effects to those during the simulation of future episodes. For example, the left lateral parietal cortex may serve a similar role during episodic simulation – to actively maintain and represent episodic information – and therefore exhibit amount-sensitive and sustained activation profiles like those observed previously in studies of episodic memory (see, Rugg et al., 2015). In addition, if the hippocampus provides the initial access to episodic details not only during memory but also during simulation (see, Addis & Schacter, 2012; Schacter & Addis, 2007), its activity should demonstrate a transient profile. In support of this prediction, previous studies have shown that hippocampal activity increases during the initial construction of an imagined event (Addis, Wong, & Schacter, 2007; Addis, Cheng, Roberts, & Schacter, 2011a; Gaesser et al., 2013; Madore, Szpunar, Addis, & Schacter, 2016). These findings provide support for the idea that at least part of the hippocampal contribution to episodic simulation involves processes that support access to and/or retrieval of episodic details.
We also predicted to observe sustained effects in regions of the frontoparietal control network (Vincent, Kahn, Snyder, Raichle, & Buckner, 2008), such as the lateral prefrontal cortex. These regions have been implicated in various control processes recruited during the maintenance of information during working memory (e.g., Curtis & D’Esposito, 2003; Jonides et al., 2008; Wager & Smith, 2003). Thus, we expected that such regions would be recruited during the active maintenance of a simulated episode. In support of this prediction, prior studies have observed sustained activity in regions of the frontoparietal control network during the maintenance of recollected information (Vilberg & Rugg, 2012, 2014).
1.5.2. Distinct Simulation and Memory Processes
In contrast to the above commonalities between episodic memory and simulation, a number of studies have revealed differences between the two (e.g., Addis et al., 2007; Addis, Pan, Vu, Laisser, & Schacter, 2009; Gilmore, Nelson, & McDermott, 2014; Szpunar, Chan, & McDermott, 2009; Weiler, Suchan, & Daum, 2010; for a review, see Benoit & Schacter, 2015). These studies have shown that some regions of the core network (such as the lateral and medial parietal cortex and hippocampus), as well as some “non-core” regions (such as the right lateral prefrontal cortex) are more strongly engaged during episodic simulation than during episodic memory. Greater recruitment of activity within core regions during simulation has been taken to reflect greater constructive processing during simulation arising from the necessity to recombine elements of past experience into a novel event (Addis & Schacter, 2012; Benoit & Schacter, 2015; Schacter & Addis, 2007). The greater recruitment of activity within non-core regions, particularly those within the frontoparietal control network, may reflect the sampling of a number of disparate past episodic details that requires further control and selection processes (Benoit & Schacter, 2015). In the present study, we provided a direct test of these interpretations. We reasoned that when a simulation is comprised of more relative to fewer details, greater constructive and control processes should be recruited. Thus, neural activity that varies with the amount of simulated information should be evident in regions previously found to be modulated as a function of future relative to past episodes (e.g., Benoit & Schacter, 2015).
2. Materials and Methods
2.1. Participants
The experimental protocol was approved by the Institutional Review Board of Harvard University. Informed consent was obtained prior to participation. Twenty-eight participants completed the experiment. All self-reported to be right handed, have normal or corrected-to-normal vision, and have no history of psychiatric or neurological disorder. Two participants were excluded from all analyses because their MRI data were acquired using a different imaging sequence than that used for subsequent participants. One additional participant was excluded because of excessive head movement (> 5 mm). The remaining 25 participants (18 female) had a mean age of 22.4 years (range 18–30).
2.2. Stimuli and Task
The study comprised two sessions. In Session 1, participants provided a list of 162 people and 108 places with which they were personally familiar. We instructed participants to provide people and places that they have come across in the past and would also come across sometime in the near future. We asked participants to generate places for which they could instantaneously imagine themselves at an exact location (e.g., a specific table they may sit at in their regularly visited coffee shop). For each person and place, participants provided a familiarity rating (on a scale of 1 to 9, ranging from very unfamiliar to very familiar) and a pleasantness rating (on a scale of 1 to 9, ranging from very unpleasant to very pleasant; see Benoit et al., 2014). In addition to providing the person and place names, participants were familiarized with a list of 378 object words. These words were drawn from Hemera Photo Objects 50,000 Volume III (http://www.hemera.com/index.html). Their names ranged in length from 3 to 10 letters and in frequency from 1 to 100 counts/million (Kucera & Francis, 1967). Participants read each word and made the same pleasantness and familiarity rating as they did for the places and people. In addition, we instructed participants to imagine performing an action with the object denoted by the word and to provide a rating for how vivid the image/action was (on a scale of 1 to 9, ranging from not vivid to very vivid).
Participants returned for Session 2 (median delay of 6 days, range 1–17 days) for the MRI portion of the study. In Session 2, participants randomly alternated on a trial-by-trials basis between performing 2 tasks: an episodic simulation task and a sentence task (i.e., the non-episodic control task; for prior uses of this control task, see also Addis et al., 2009; Addis, Roberts, & Schacter, 2011b; Gaesser et al., 2013; Martin et al., 2011; van Mulukom, Schacter, Corballis, & Addis, 2013). In the simulation task, participants were presented with trial-unique combinations of 3, 4, or 5 episodic details (i.e., person, place, and object words from Session 1). On each simulation trial, participants were shown one previously provided place, and a random combination of people and objects (with the constraint that there was always at least one person and at least one object). These details were presented in the same order on the screen from top to bottom: place, people, and then objects (see Figure 1A). The use of 3, 4, and 5 details was motivated by our previous experiments that have used a minimum of 3 cued details (i.e., place, person, and object cues; see, Addis et al., 2009; Gaesser et al., 2013; Martin et al., 2011; Szpunar et al., 2015). The use of three details allowed us to replicate prior findings, and adding people and/or objects allowed us to manipulate the number of details within a given simulation. In the sentence task, participants were presented with trial-unique combinations of 3, 4, or 5 object words from Session 1.
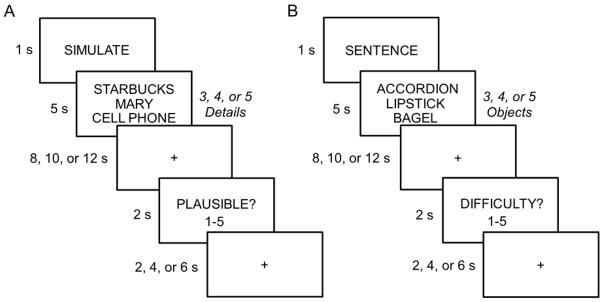
Figure 1.
A. Simulation task. Participants were instructed to simulate a hypothetical future episode that involved all of the cued episodic details (i.e., 3, 4, or 5 person, place and object details; every simulation trial consisted of only one place cue and a varying combination of person and object cues, with the restriction that there was always at least one person and object). Participants were instructed to continually simulate until the onset of the probe question. B. Sentence task. Participants were instructed to construct a sentence that ranked the cued objects by their respective size. Trials varied with respect to the number of objects to be included in their sentence (i.e., 3, 4, or 5 objects). Participants were instructed to continually think about the meanings of the word or repeat the sentence until the onset of the probe question. Durations are shown to the left of each frame.
As illustrated in Figure 1, participants were first presented a task cue (‘SIMULATE’ or ‘SENTENCE’) for 1 s. Following the task cue, the task-specific stimuli were presented for 5 s, followed by a variable fixation/delay period (8, 10, or 12 s). The trial ended with the probe question presented for 2 s (i.e., plausibility or difficulty for the simulation and sentence tasks, respectively), followed by a variable fixation period (i.e., inter-trial interval of 2, 4 or 6 s). All stimuli were presented on a black background in 25 point Arial font.
For the simulation task, participants were instructed to imagine a specific future episode where they were interacting with the cued people and objects in a location-specific manner. They were instructed to always imagine a novel episode (i.e., not to simply recall an episode that they had experienced before with the person(s) and object(s) at the given location). Person, place, and object details were randomly combined to maximize the probability that simulated episodes would be novel. Participants were instructed to begin imagining the episode as quickly as possible. They were further instructed to imagine the episode through their own eyes (e.g., as if they were experiencing the episode at that specific location). Critically, participants were instructed to continually imagine and simulate their episode throughout the fixation/delay period until the probe question appeared on the screen. The probe asked participants to rate how plausible the simulated episode would be if it were to occur (on a scale from 1 to 5, ranging from not plausible to very plausible).
For the sentence task, participants were instructed to covertly create a sentence that ranked 3, 4, or 5 object words according to the size of the objects (see Figure 1B, e.g., “the accordion is bigger than the bagel which is bigger than the lipstick”). Participants were instructed to be as quick as possible in generating the sentence. Once they had generated the sentence, for the remainder of the trial, participants elaborated on the representation of the nouns, generating as much detail about the meaning on the objects (including imagining the objects), or covertly repeated the sentence. Analogous to the simulation task, participants were thus instructed to continually perform the task throughout the fixation/delay period until the probe question appeared. Here, they were asked to rate how difficult it was to create the sentence (on a scale from 1 to 5, ranging from very easy to very difficult). Adopting the logic of our prior studies that also employed a similar sentence task as a non-episodic control (Addis et al., 2009, 2011b; Gaesser et al., 2013; Martin et al., 2011; van Mulukom et al., 2013), the sentence task is similar to the simulate task in that it requires the integration of information, and generation of semantic and visual details, with the critical exception of the requirement to generate a coherent imagined event.
Participants completed 6 fMRI scans (for one participant only 5 scans were collected due to technical difficulty). Across the 6 fMRI scans, participants completed 108 simulation trials and 54 sentence trials. Simulation and sentence trials were subdivided equally across the respective 3, 4, and 5 amount conditions (i.e., 36 trials for each amount of episodic details for the simulation task and 18 trials for each amount of object words for the sentence task). An equal proportion of each fixation delay period (8, 10, and 12 s fixation periods) and inter-trial interval (2, 4, and 6 s fixation periods) occurred for each task type. Each fMRI scan comprised 27 trials within which the ordering of simulation and sentence trials was pseudo-randomized such that no more than 4 trials of a given task occurred in succession. Participants responded to probe questions using a button box in their right hand. Before entering the scanner participants practiced both tasks to ensure that they understood and complied with the instructions (e.g., performed tasks for entirety of the trial). After exiting the scanner, we assessed memory for simulations and sentences. Analyses pertaining to this final memory test phase are not currently reported.
2.3. Image acquisition and analysis
Functional and anatomic images were acquired at the Harvard Center for Brain Science using a 3 Tesla Siemens Prisma scanner equipped with a 32-channel head coil. Anatomic images were acquired with a magnetization-prepared rapid gradient echo sequence (matrix size of 256 × 256, voxel size of 1 mm3, 176 slices). Functional images were acquired with a multiband echo-planar imaging sequence (University of Minnesota C2P sequence: TR = 2 s, TE = 30 ms, matrix size of 136 × 136, 84 slices (3 slices acquired simultaneously), 1.5 mm3 resolution, multiband factor of 3, in-plane GRAPPA acceleration factor of 2; Moeller et al., 2009; Feinberg et al., 2010; Xu et al., 2013). Slices were auto-aligned to an angle 20o towards coronal from anterior-posterior commissure alignment (van der Kouwe et al., 2005). For each fMRI scan, 309 images were acquired. To allow equilibration of tissue magnetization, each scan began with a 10 s fixation period.
fMRI data were analyzed using Statistical Parametric Mapping (SPM12, Wellcome Department of Cognitive Neurology, London, UK). Functional image preprocessing included three steps: 1) slice-time correction (using the first slice as reference), 2) two-pass spatial realignment (first to mean image within sessions and then to the mean image across sessions), and 3) normalization (into Montreal Neurological Institute (MNI) space using the TPM template supplied by SPM12, no resampling). Functional images were smoothed with a 3 mm full-width-half-maximum Gaussian smoothing kernel applied to the normalized images. Anatomic images were normalized using an analogous procedure as that used for the functional images.
Univariate analysis was conducted in a two stage mixed effects general linear model (GLM). In the first stage, a 5 s boxcar function that onset concurrently with the episodic details/object words was used to model stimulus/transient neural activity. Sustained activity was modeled with a boxcar that onset concurrently with the fixation following stimulus presentation (see also, Vilberg & Rugg, 2012, 2014). This boxcar varied in length with the duration of the fixation period (8, 10, or 12 s following the offset of the episodic details/object words). The associated BOLD response was modeled by convolving the boxcar functions with a canonical hemodynamic response function to yield regressors in a GLM that modeled the BOLD response for each event type.
There were six events of interest, comprising trials associated with each task (simulation and sentence) and each amount (3, 4, or 5). The model thus contained 6 events of interest for each transient and sustained regressor (for a total of 12 events of interest in the model). There were three additional events of no interest, including trials without a response (participants failed to respond to < 1 % of all trials), the 1 s task cue period, and the subsequent 2 s probe. Six regressors representing movement related variance (three for rotation and three for rigid-body translation) and regressors modeling each scan session were also entered into the design matrix. An AR(1) model was used to estimate and correct for nonsphericity of the error covariance (Friston et al., 2002). As detailed above, the delay period and the inter-trial interval varied across both sentence and simulation trials. The purpose of this jitter was to reduce the collinearity between the transient and sustained regressors in the GLM (see also, Vilberg & Rugg, 2012, 2014). Temporal smoothing was conducted before estimation of the parameter estimates (i.e., the default high-pass filter of 128 s in SPM12).
The participant-specific parameter estimates for the 12 events of interest were carried forward to a second analysis stage where they were entered into a repeated measures ANOVA with participants modeled as a random effect. The ANOVA model employed factors of task (sentence or simulation), amount (3, 4, or 5), and regressor (transient or sustained). Unless otherwise noted, an individual voxel threshold of p < 0.001 was employed corrected for multiple comparisons to p < 0.05 with a cluster extent threshold of 19 voxels (Slotnick, Moo, Segal, & Hart, 2003; Slotnick, 2016, see also, Thakral et al., 2015; Vilberg & Rugg, 2012, 2014). The cluster extent threshold was computed using a Monte Carlo simulation with 10,000 iterations with an estimated spatial autocorrelation of 11.13 mm (i.e., the full-width-half-maximum of the image corresponding to the standard error of the ANOVA model). Our method for computing the cluster extent threshold (for a similar approach, see also, Ford & Kensinger, 2016; Gu et al., 2015; Hoffman, Binney, Lambon Ralph, 2015; Shigemune, Tsukiura, Kambara, & Kawashima, 2014; Slotnick & Schacter, 2004; Thakral & Slotnick, 2009) does not employ a random-field theory approach and therefore does not make assumptions which may or may not be met with respect to the data (e.g., that error be normally distributed).
In addition to the GLM analysis described above, we employed a finite-impulse response (FIR) model to estimate the timecourses associated with each of the events described above (see also, Vilberg & Rugg, 2012, 2014). The FIR model was used to evaluate the timecourse of neural activity associated with activity identified by the GLM analysis. The timecourses were separately modeled for the 8, 10, and 12 s delay periods across 12 TRs (i.e., from item onset to 24 seconds after stimulus onset). This time window was selected a priori on the basis of the predicted timecourse after convolving the canonical hemodynamic response function with the combined stimulus and variable delay periods (i.e., 13, 15, and 17 s windows; Slotnick, 2005; see also, Thakral and Slotnick, 2009). To avoid signal contamination from the preceeding trial, prior to averaging, the FIR timecourses were truncated at the 16, 18, and 20 s post stimulus onset for the 8, 10, and 12 s delay periods, respectively. While the FIR timecourses were initially examined in parallel to the extracted parameter estimates from the GLM, although qualitatively similar, they did not fully replicate the results of the GLM. These differential results suggest that the individual impulse response functions used in the FIR analysis may not best characterize the neural activity relative to the extended boxcar functions (for related disparate findings across GLM and timecourse analyses, see Slotnick and Schacter (2006) and Thakral and Slotnick (2009)).
3. Results
3.1. Behavioral results
Mean difficulty and plausibility ratings, and associated reaction times for each task are listed in Table 1. A one-way nonparametric test revealed that difficulty ratings associated with the sentence task significantly differed as a function of the number of objects (Friedman test, Χ2(2) = 40.56, p < 0.001). As expected, follow-up comparisons revealed greater difficulty with greater number of objects (5 > 4 > 3, Wilcoxon Signed Rank tests, ps < 0.001). Analyses of the reaction times associated with the difficulty responses also revealed a significant difference amongst the three conditions (F(2, 48) = 5.39, p < 0.05). Follow-up pairwise comparisons, revealed that reaction times in making the difficulty response were significantly slower for sentence trials with 4 objects relative to 3 objects (t(24) = 3.16, p < 0.01). No other tests were significant (ps > 0.05).
Table 1.
Mean reaction times (± 1 standard error of the mean) and ratings (± 1 standard error of the mean) as a function of task (sentence and simulate) and amount (3, 4, and 5).
| Reaction times | Ratings | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| 3 | 4 | 5 | 3 | 4 | 5 | |
| Sentence (Difficulty) | 1156 (78) | 1285 (82) | 1242 (76) | 1.54 (0.11) | 2.15 (0.14) | 3.42 (0.17) |
| Simulate (Plausibility) | 1260 (61) | 1240 (76) | 1434 (62) | 3.26 (0.10) | 2.81 (0.10) | 2.48 (0.09) |
Analogous analyses conducted on the simulation trials revealed that plausibility also differed across the three conditions (Friedman test, Χ2(2) = 42.32, p < 0.001). Follow-up Wilcoxon Signed Rank tests revealed plausibility in the future episode decreased with the amount of details simulated (3 > 4 > 5, ps < 0.001). Analyses of the reaction times associated with the plausibility response also revealed a significant difference (F(2, 48) = 9.47, p < 0.001). Follow-up comparisons revealed slower reaction times for episodes containing 5 details relative to episodes containing both 3 and 4 details (ts(24) > 3.63, ps > 0.01), which did not significantly differ from one another (p > 0.20). Of most importance, across all experimental conditions and for both tasks, mean plausibility and difficulty ratings did not approach the extreme ratings (i.e. least plausible and very difficult), which indicates that the participants could perform the tasks in an appropriate manner.
3.2. fMRI results
We first identified sustained simulation effects and then went on to identify transient simulation effects. For both classes of effects, we also identified neural regions that varied as a function of the amount of simulated details2.
3.2.1. Sustained effects
Sustained simulation effects were identified by the outcome of the simulate > sentence contrast for the sustained regressor (collapsed across amount; threshold of p < 0.001 corrected for multiple comparisons to p < 0.05, see Section 2.3). Note that this procedure does not identify simulation effects specific to the delay period (e.g., via exclusively masking of transient simulation effects). Such an analysis would identify sustained effects with a delayed onset, which likely reflect processes other than those associated with the maintenance of episodic content (e.g., Curtis & D’Esposito, 2003) and are distinct from what we refer to as ‘sustained’ (see Introduction; and for a similar approach, see Vilberg & Rugg, 2012, 2014).
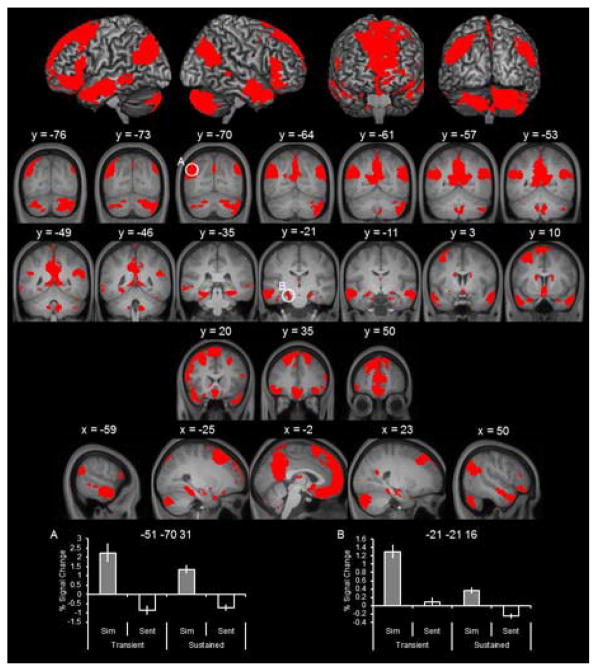
As detailed in Figure 2 and Table 2, sustained effects were observed in every region of the core network including lateral parietal cortex, medial prefrontal cortex, lateral temporal cortex, and hippocampus. As depicted in the bottom of Figure 2, parameter estimates extracted from representative regions illustrate that the clusters identified exhibited simulation effects (simulate > sentence) for the sustained regressor. We also provide the parameter estimates for the transient regressor to illustrate that neural activity within these regions was persistently elevated across the trial.
Figure 2.
Sustained simulation effects. Sustained simulation effects identified with the contrast of simulate > sentence of the sustained regressors (collapsed across amount). In this and subsequent figures, results are also are overlaid onto the coronal and sagittal sections of the across-participants mean T1-weighted anatomical image (results are also projected onto a cortical surface using the skull-stripped template of MRIcron; Rorden, Karnath, & Bonilha, 2007). Parameter estimates extracted from peak voxels within representative regions are denoted by white circles with corresponding MNI coordinates.
Table 2.
Loci of sustained simulation effects
| MNI Coordinates |
Peak Z | Number of above-threshold voxels | Region | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Sustained simulation effects | |||||
| −2 | 49 | −13 | Inf | 35118 | L ventromedial prefrontal cortex |
| −2 | 35 | −25 | Inf | L orbitofrontal cortex | |
| −15 | 37 | 54 | Inf | L superior frontal gyrus | |
| −4 | 66 | 18 | Inf | L anterior prefrontal cortex | |
| 6 | 44 | −16 | Inf | R ventromedial prefrontal cortex | |
| 23 | 40 | 50 | Inf | R superior frontal gyrus | |
| −24 | 31 | 52 | Inf | L superior frontal sulcus | |
| −32 | 20 | 59 | Inf | L middle frontal gyrus | |
| −62 | −7 | −13 | Inf | L superior temporal sulcus | |
| −65 | −13 | −12 | Inf | L middle temporal gyrus | |
| −56 | −63 | 20 | Inf | L superior temporal gyrus | |
| −41 | 20 | −22 | 7.69 | L temporal pole | |
| −50 | 37 | −10 | 7.67 | L inferior frontal gyrus | |
| 2 | 62 | 11 | 7.62 | R anterior prefrontal cortex | |
| −21 | −21 | −16 | 7.33 | L hippocampus | |
| 29 | −34 | −15 | 7.31 | R parahippocampal cortex | |
| −32 | −39 | −13 | 7.00 | L parahippocampal cortex | |
| 9 | 55 | 11 | 6.77 | R medial prefrontal cortex | |
| 23 | 28 | 41 | 6.59 | R superior frontal sulcus | |
| 27 | 20 | 41 | 5.81 | R middle frontal gyrus | |
| 24 | −22 | −15 | 4.68 | R hippocampus | |
| −44 | 43 | −9 | 4.63 | L inferior frontal gyrus | |
| −51 | −70 | 30 | Inf | 4678 | L angular gyrus |
| 12 | −84 | −40 | Inf | 5282 | R cerebellum |
| −2 | −55 | 37 | Inf | 8093 | L precuneus |
| −2 | −54 | 19 | Inf | L retroslpenial/posterior cingulate cortex | |
| −12 | −58 | 14 | Inf | L parietooccipital sulcus | |
| 63 | −4 | −22 | Inf | 4686 | R middle temporal gyrus |
| 56 | −6 | −18 | Inf | R superior temporal sulcus | |
| 53 | −66 | 35 | Inf | 3268 | R angular gyrus |
| −14 | −85 | −42 | 7.73 | 1873 | L cerebellum |
| 6 | −55 | −45 | 7.61 | 989 | R cerebellum |
| −15 | 10 | 17 | 5.61 | 258 | L caudate |
| −17 | −72 | −28 | 5.53 | 105 | L cerebellum |
| 17 | 11 | 10 | 5.28 | 179 | R caudate |
| 63 | −39 | 1 | 5.15 | 182 | R superior temporal sulcus |
| 71 | −36 | 1 | 5.09 | R middle temporal gyrus | |
| −41 | −18 | −27 | 4.74 | 105 | L fusiform gyrus |
| 0 | −15 | 11 | 4.72 | 42 | L caudate |
| 38 | −42 | −25 | 4.17 | 58 | L fusiform gyrus |
| 42 | 7 | 50 | 3.98 | 45 | L middle frontal gyrus |
| Sustained simulation effects, amount dependent (5 > 3) | |||||
| 0 | −58 | 41 | Inf | 1038 | L precuneus |
| 0 | −61 | 49 | Inf | L medial superior parietal lobule | |
| −46 | −61 | 22 | Inf | 103 | L superior temporal sulcus |
| −45 | −70 | 25 | 7.24 | L angular gyrus | |
| 14 | −82 | −39 | Inf | 144 | R cerebellum |
| 15 | −70 | −30 | Inf | 49 | R cerebellum |
| −21 | 20 | 46 | 7.64 | 723 | L superior frontal sulcus |
| −29 | 20 | 58 | 7.41 | L middle frontal gyrus | |
| 0 | 17 | 59 | 7.19 | 100 | L medial superior frontal gyrus |
| 42 | −52 | 26 | 7.16 | 100 | R superior temporal gyrus |
| 8 | −52 | −43 | 7.15 | 32 | R cerebellum |
| 41 | −73 | −43 | 7.04 | 60 | R cerebellum |
| −26 | −39 | −15 | 6.98 | 97 | L parahippocampal cortex |
| 9 | −61 | 17 | 6.48 | 37 | R parietooccipital sulcus |
| −26 | −78 | 53 | 6.34 | 75 | L extrastriate cortex |
| 24 | 23 | 44 | 6.03 | 131 | R superior frontal sulcus |
| −56 | 26 | 10 | 6.02 | 310 | L inferior frontal gyrus |
| −50 | 20 | 37 | 4.87 | L middle frontal gyrus | |
| 35 | −61 | −30 | 5.43 | 35 | R cerebellum |
| 38 | −67 | −54 | 5.25 | 24 | R cerebellum |
| −2 | 28 | 38 | 3.84 | 57 | L medial superior frontal gyrus |
Coordinates for cluster sub-peaks which lay in distinct cortical regions are listed directly below relevant peak cluster.
3.2.2. Sustained effects, amount-dependent
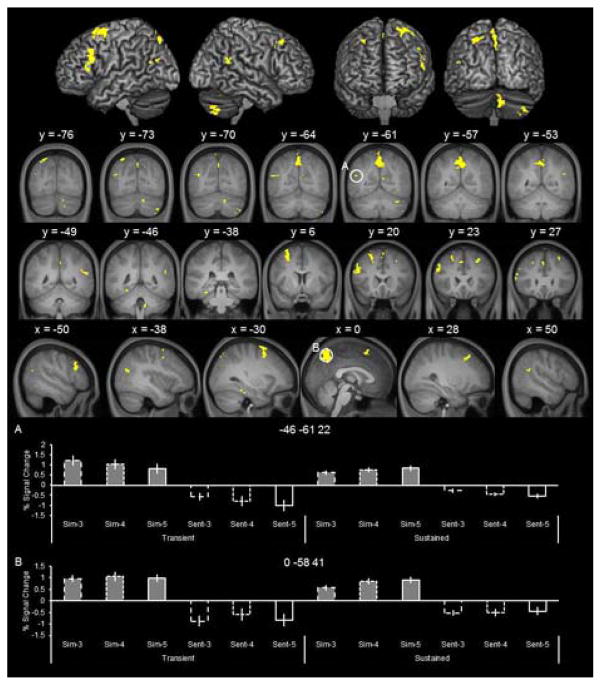
To identify sustained effects that modulated as a function of the amount of simulated details, we inclusively masked the outcome of the simulate > sentence contrast (threshold of p < 0.001, see above) with the 5 > 3 contrast for the sustained regressor (mask threshold of p < 0.01, giving an estimated conjoint significance of p < 0.0001; Fisher, 1950; see also, Thakral et al., 2015; Thakral, Yu, & Rugg, 2015). This contrast identifies regions maximally sensitive to differences in the amount of simulated information (for control analyses including the 4 condition, see below). Because the 5 relative to the 3 condition places greater demands on maintenance-related cognitive processes this analysis identifies neural activity underlying the maintenance of simulated information across the delay period. Figure 3 illustrates the sustained simulation effects that varied as a function of the amount of simulated details. As detailed in Table 2, amount-dependent effects (i.e., 5 > 3), were observed in, among other regions, medial superior parietal lobule, left angular gyrus, left parahippocampal cortex, and bilateral superior and medial frontal regions. Parameter estimates extracted from these regions (Figure 3, bottom) indicate that the amount-dependent effects (5 > 3) only emerged during the delay period (i.e., the sustained regressor; bars 9 versus 7). These amount-dependent effects cannot be accounted for by differences in visual presentation (i.e., 5 cue words versus 3 cue words), as the delay period, during which amount-dependent effects were evident, was perceptually identical in all conditions (i.e., a fixation screen).
Figure 3.
Sustained and amount-dependent simulation effects. These effects were identified by inclusively masking the outcome of the simulate > sentence contrast with the 5 > 3 contrast of the sustained regressors.
The parameter estimates illustrated in Figure 3 show that activity associated with the 4 condition was at an intermediate level relative to the 3 and 5 conditions, supporting the observation that the regions were modulated by the amount of simulated information (see bars 7, 8, and 9). To confirm that activity associated with the 4 condition did not exceed the 5 condition or, conversely was significantly lower than the 3 condition (both patterns violating the amount-dependent nature of the activity), we conducted two follow-up analyses. We performed the same analysis as that described above but replaced the 5 > 3 contrast with, in the first analysis, the 4 > 5 contrast and, in the second analysis, the 3 > 4 contrast. Neither contrast identified clusters of activity that overlapped with those illustrated in Figure 3 (i.e., 5 > 3). This was true even when the threshold for these contrasts was relaxed to p < 0.05. These analysis support the interpretation that the clusters identified by the 5 > 3 contrast were sensitive to the amount of information simulated.
3.2.3. Transient effects
Transient simulation effects were identified in a two-stage procedure. First, we identified regions demonstrating simulation > sentence effects for the transient regressor (collapsed across amount; threshold of p < 0.001). Then, the outcome of this contrast was exclusively masked with the analogous contrast for the sustained regressor (i.e., we identified simulation > sentence effects for the sustained regressor at the threshold of p < 0.05 and the resulting map was used as the exclusive mask; note the more liberal threshold the more conservative the analysis; see also, Thakral et al., 2015; Yu et al., 2012). The outcome of this procedure yielded clusters of activity where simulation effects were uniquely associated with the stimulus/transient regressor (see also, Vilberg & Rugg, 2012, 2014). We note that the exclusive masking procedure is necessary as the contrast of the simulate > sentence effect for the transient regressor alone would not dissociate between activity associated with the stimulus or delay period.
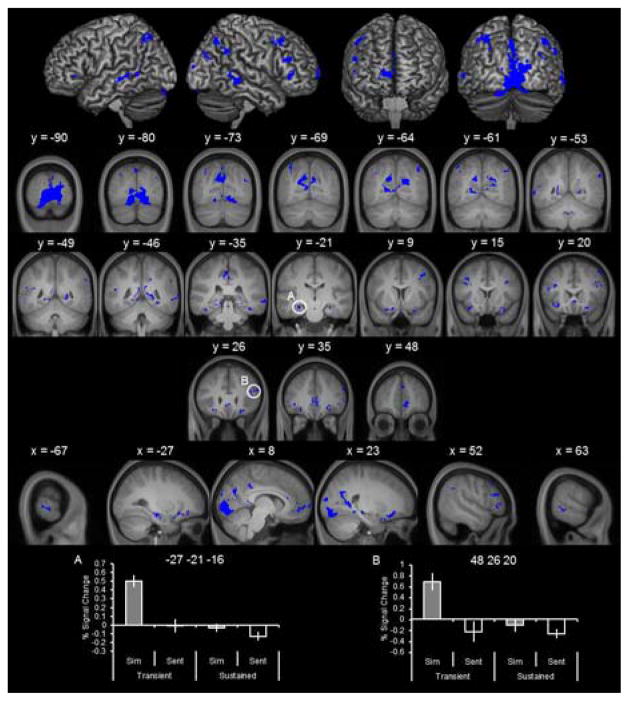
As detailed in Figure 4 (top) and Table 3, transient effects were identified in many brain regions, including extrastriate cortex, lateral parietal cortex, middle and inferior frontal regions, lateral temporal cortex in superior and middle temporal gyri, medial prefrontal cortex, and bilateral hippocampus. Of note, the majority of these effects fell within regions of the core simulation network such as the hippocampus. Parameter estimates for representative regions are illustrated in the bottom of Figure 4. In accordance with how these clusters were identified, the extracted parameter estimates demonstrate that the simulation effects (simulate > sentence) were uniquely associated with the transient regressor.
Figure 4.
Transient simulation effects. Transient simulation effects were identified with the contrast simulate > sentence of the transient regressors (collapsed across amount), exclusively masked with the identical contrast for the sustained regressors.
Table 3.
Loci of transient simulation effects
| MNI Coordinates |
Peak Z | Number of above-threshold voxels | Region | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Transient simulation effects | |||||
| −15 | −64 | 23 | Inf | 2101 | L parietooccipital sulcus |
| 18 | −91 | 4 | Inf | 5827 | R extrastriate cortex |
| −6 | −94 | −6 | Inf | L extrastriate cortex | |
| 29 | 35 | −15 | 6.94 | 250 | R orbital gyrus |
| 29 | 13 | −25 | 5.26 | R inferior frontal gyrus | |
| 2 | 19 | −7 | 6.49 | 64 | R anterior cingulate cortex |
| −6 | −40 | 43 | 6.26 | 335 | L precuneus |
| −5 | −31 | 40 | 5.39 | L posterior cingulate cortex | |
| 2 | −39 | 40 | 5.15 | R precuneus | |
| −27 | 28 | −15 | 6.23 | 181 | L orbital gyrus |
| −26 | 11 | −21 | 4.95 | L inferior frontal gyrus | |
| −30 | 16 | −18 | 5.28 | L insula | |
| −16 | 8 | −21 | 6.16 | 23 | L orbital gyrus |
| −39 | 17 | 35 | 5.97 | 105 | L middle frontal gyrus |
| −36 | 20 | 22 | 4.45 | L inferior frontal sulcus | |
| −27 | −21 | −16 | 5.76 | 196 | L hippocampus |
| −41 | 16 | 29 | 5.69 | 33 | L inferior frontal sulcus |
| −35 | −69 | 52 | 5.61 | 225 | L angular gyrus/intraparietal sulcus |
| 63 | −39 | −6 | 5.53 | 227 | R middle temporal gyrus |
| 68 | −42 | 7 | 3.33 | R superior temporal sulcus | |
| 48 | 26 | 20 | 5.38 | 279 | L inferior frontal sulcus |
| 53 | 31 | −1 | 4.66 | L inferior frontal gyrus | |
| 56 | 32 | 22 | 4.65 | L middle frontal gyrus | |
| 30 | −43 | −12 | 4.84 | 145 | R parahippocampal cortex |
| 27 | −22 | −16 | 4.76 | R hippocampus | |
| −3 | −45 | 17 | 5.35 | 45 | L posterior cingulate cortex |
| −3 | 35 | 2 | 5.32 | 479 | L anterior cingulate cortex |
| 5 | 35 | −7 | 5.06 | R anterior cingulate cortex | |
| 5 | 49 | −7 | 4.98 | R medial prefrontal cortex | |
| 57 | −51 | 34 | 4.97 | 177 | R supramarginal/angular gyrus |
| 42 | −67 | 50 | 4.88 | R angular gyrus/intraparietal sulcus | |
| −38 | 32 | 2 | 4.91 | 85 | L inferior frontal gyrus |
| 38 | −34 | −19 | 4.90 | 30 | R fusiform gyrus |
| −50 | −48 | 17 | 4.64 | 33 | L superior temporal gyrus |
| −68 | −42 | −3 | 4.63 | 88 | L middle temporal gyrus |
| −68 | −28 | −3 | 4.40 | L superior temporal sulcus | |
| 50 | 22 | 47 | 4.62 | 52 | R middle frontal gyrus |
| 33 | 7 | 35 | 4.45 | 91 | R inferior frontal sulcus |
| 41 | 11 | 46 | 4.28 | R middle frontal gyrus | |
| −47 | 22 | 13 | 4.43 | 39 | L inferior frontal gyrus |
| 11 | −48 | 22 | 4.25 | 24 | R posterior cingulate cortex |
| −2 | −55 | −36 | 4.20 | 23 | L cerebellum |
| 35 | 17 | 28 | 4.20 | 32 | R inferior frontal sulcus |
| −54 | −49 | −6 | 4.10 | 55 | L middle temporal gyrus |
| 2 | 47 | 31 | 4.08 | 36 | R medial prefrontal cortex |
| 5 | −88 | 29 | 4.06 | 36 | R extrastriate cortex |
| −42 | −33 | −21 | 4.02 | 55 | L fusiform gyrus |
| 42 | −75 | 26 | 4.01 | 39 | R angular gyrus |
| 35 | −82 | 34 | 3.57 | R extrastriate cortex | |
| −54 | −57 | 13 | 3.93 | 22 | L superior temporal sulcus |
| Transient simulation effects, amount dependent (5 > 3) | |||||
| 5 | −91 | −1 | Inf | 1408 | R striate cortex |
| −2 | −93 | −7 | 7.54 | L extrastriate cortex | |
| −5 | −72 | 34 | 6.86 | 43 | L precuneus |
| 21 | −85 | −7 | 5.35 | 144 | R extrastriate cortex |
| 17 | −96 | 8 | 4.75 | 52 | R extrastriate cortex |
| −18 | −82 | −18 | 4.33 | 29 | L extrastriate cortex |
Coordinates for cluster sub-peaks which lay in distinct cortical regions are listed directly below relevant peak cluster.
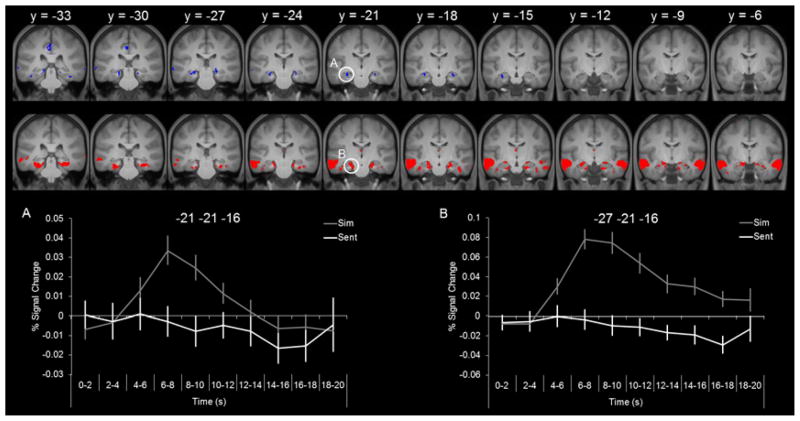
Notably, the transient hippocampal effects were spatially distinct from those that were sustained which were observed relatively more anterior (compare Figure 2 and Figure 4). Although the peak voxel of each effect fell at the same y coordinate in each hemisphere (-21 in the left hemisphere and -22 in the right hemisphere), the clusters of transient and sustained activity extended more posteriorly and anteriorly, respectively. Figure 5 illustrates the temporal dissociation within each hemisphere. The sustained hippocampal effect extended from y = -6 to y = -25 and the transient hippocampal effect extended from y = -16 to y = -33 (in the left hemisphere, the transient effect extended slightly more anterior to y = -13). The timecourses are shown for illustrative purposes only as they are not independent from the analysis employed to identify the transient and sustained effects (i.e., GLM analysis).
Figure 5.
Anterior-posterior hippocampal simulation effects. Neural activity in blue signifies transient-specific simulation effects and neural activity in red signifies the sustained simulation effects. Timecourses extracted from the peak voxel within the left hemisphere for the transient effect (left) and sustained effect (right).
3.2.4. Transient effects, amount-dependent
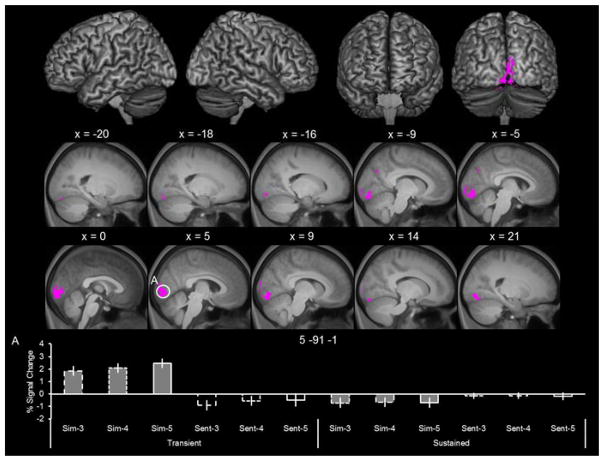
To identify transient effects that varied as a function of the amount of simulated details, we inclusively masked the selective transient effects (as depicted in Figure 4) with the 5 > 3 contrast for the transient regressor (employing the same mask threshold of p < 0.01 as in the analysis of sustained effects). Figure 6 illustrates the transient effects that varied as a function of the amount of simulated details. As detailed in Table 2, amount-dependent effects (i.e., 5 > 3), were largely restricted to occipital cortex within striate and extrastriate cortex. The bottom of Figure 4 illustrates the parameter estimates extracted from the peak voxel within the striate cluster. As can be seen from the figure, the profile across the three simulate events for the transient regressor demonstrates that these transient effects varied as a function of the amount of simulated information (i.e., 5 > 3; bar 3 versus bar 1), with no corresponding simulation effects for the sustained regressor.
Figure 6.
Transient and amount-dependent simulation effects. Effects were identified by inclusively masking the transient-specific simulation effects with the 5 > 3 contrast for the transient regressors.
Analogous to the sustained analyses, we performed the amount-dependent transient analyses described above with the contrasts of 4 > 5 and 3 > 4, to corroborate the amount-dependent nature of the regions identified. No overlapping clusters of activity were identified in these subsidiary analyses even at the liberal threshold of p < 0.05.
4. Discussion
In the current study, we aimed to determine whether regions within the core network dissociate as a function of the amount of simulated information and also their timecourse of engagement. Relative to a non-episodic control task, sustained simulation-related activity was identified in the majority of regions in the core network. Additional analyses revealed that activity in the medial and lateral parietal cortex and bilateral frontal cortex was further modulated as a function of the amount of simulated information (i.e., greater activity when a simulated event contained more relative to fewer episodic details). Of particular interest, we observed a temporal dissociation within the hippocampus. Specifically, transient-specific simulation effects were identified in relatively posterior portions of the hippocampus and sustained simulation effects (i.e., those that persisted across the trial) were identified in more anterior portions of the hippocampus. We discuss the implications of these findings below.
4.1.2. Hippocampal Findings
In line with one of our initial predictions, we observed transient simulation-related activity within the hippocampus. That is, these effects are analogous to findings of transient episodic memory-related activity within the hippocampus (Vilberg & Rugg, 2012). Because these simulation effects closely correspond to those observed during memory, we adopt a similar interpretation and suggest that the transient hippocampal effects reflect the initial retrieval and reinstatement of episodic details (see, Addis et al., 2007; Madore et al., 2016; Rugg et al., 2015; Schacter & Addis, 2007).
The current findings are relevant to recent findings regarding hippocampal involvement during the initial construction and subsequent elaboration of simulated episodes (Addis et al., 2007; Madore et al., 2016; see also, Gaesser et al., 2013). Addis et al., (2007) observed hippocampal activity, during both the construction and elaboration phases, in a cluster that overlapped with the present anterior/sustained effect. Under the assumption that that current sustained activity reflects both construction-related and elaboration processes, the current findings corroborate those reported by Addis et al., (2007). Unique to the present study is the observation of more posterior hippocampal activity that was exclusively transient. With respect to prior studies, we highlight an important difference in the analysis procedures employed. In the present study, we exclusively masked out neural activity associated with the sustained regressor to isolate neural activity that was transient (see also, Vilberg & Rugg, 2012, 2014). In contrast, Addis et al., (2007) simply identified activity associated with each phase of the simulation trial (i.e., construction and elaboration; a similar analysis was conducted in Gaesser et al., 2013 and in Madore et al., 2016). Without the use of an exclusive mask, activity associated with the early phase of the trial, although assumed to be ‘construction-related’, may extend into the elaboration period and exhibit a sustained profile. Thus, it will be important for future studies to utilize a similar analysis to the current study when identifying construction-related processes during simulation. An additional difference comes from the fact that in these prior studies, participants were required to make a motor response when they had successfully constructed an event. By contrast, in the current study there was no requirement to signal when the simulated event was constructed. Thus, the procedure used in the current study is different in that it allows for the identification of transient-specific simulation effects devoid of any of the cognitive operations associated with making a motor response (e.g., decision making and motor planning). Lastly, we highlight that we employed a relatively higher resolution imaging protocol than these previous studies of episodic simulation (i.e., 1.5 mm relative to 2–3 mm, e.g., Gaesser et al., 2013; Madore et al., 2016). It will be critically important for future studies to employ similar high-resolution imaging protocols to replicate and extend the current findings (for further discussion, see Schacter, Addis, & Szpunar, in press).
Before discussing possible interpretations of the spatial dissociation within the hippocampus, we highlight that both the sustained and transient effects in this region were insensitive to the amount of simulated details. As stated in the Introduction, a number of studies directly comparing episodic memory and episodic simulation have observed greater activity in the hippocampus during simulation than during memory (for reviews, see Addis & Schacter, 2012; Benoit & Schacter, 2015; Schacter et al., in press), with this difference possibly reflecting greater recruitment of hippocampally-mediated construction and relational (Eichenbaum & Cohen, 2014) processes. Thus we predicted that hippocampal activity would modulate as a function of the amount of detail simulated, given the greater construction/relational processing demands in the 5 relative to the 3 condition. As with any null finding, the lack of amount-dependent hippocampal effects can be accounted for in any number of ways (e.g., low statistical power)3. One notable difference from prior studies is that Addis and Schacter (2008) operationalized amount of detail as subjectively rated vividness. This approach stands in contrast to the present study, where the amount of detail was operationalized as the number of objectively cued details. Future research is necessary to further examine the nature of differences between objective and subjective measures of simulated content. For example, it may be the case that, akin to episodic memory, episodic simulations with more objective details are experienced as more subjectively vivid (cf., Slotnick, 2010).
There are a number of prior theoretical proposals that can account for the present anterior-posterior temporal dissociation of hippocampal function during episodic simulation. According to Addis and Schacter (2012; see also Gaesser et al., 2013), posterior hippocampal activity supports the retrieval of previously experienced details, particularly those spatial in nature. Anterior hippocampal activity, by contrast, reflects the relational processing (i.e. recombination) of accessed details into a coherent scenario and the encoding of the episode for later use. As we noted above, the posterior and transient hippocampal effect replicates previous episodic memory findings (Vilberg & Rugg, 2012, 2014), and thus support the retrieval interpretation of Addis and Schacter (2012). However, the posterior effects may also reflect the initial recruitment of spatial processing required in configuring the episodic details within a given episode (e.g., where people are seated, where is the object, etc.). Interestingly, activity in sensory cortical regions (in occipital cortex), similar to the posterior hippocampal activity, was also transient. As these regions have been implicated in visual-spatial memorial processing (for a review, see Slotnick, 2004), these findings, together with the posterior hippocampal effects, may reflect the retrieval of the visual spatial information associated with the cued details. In contrast, the anterior hippocampal effects may reflect the continual recruitment of encoding and relational processing associated with the novel episodic simulation (see also Martin et al., 2011).
There are alternative proposals of hippocampal function that may also be compatible with the current hippocampal temporal dissociation (e.g., Sheldon & Levine, 2016; Zeidman, Mullally, & Maguire, 2016). Regardless of which proposal is correct, however, the current findings have implications for any theoretical proposal of hippocampal function during episodic simulation because they highlight the necessity of considering when hippocampal processes are engaged and not simply what processes are engaged. The present findings clearly add to the evidence of a functional dissociation within the hippocampus, and are the first to indicate that this dissociation extends to the temporal domain during episodic simulation.
The current hippocampal findings add to the growing interest in research examining the temporal properties of hippocampal function. Such research includes experiments examining how the hippocampus codes the passage of time (i.e., ‘time cells’), the role of the hippocampus in encoding temporal context information, and the role of the hippocampus in retrieving temporal sequences (for reviews, see Howard & Eichenbaum, 2015; Ranganath & Hsieh, 2016). Of direct relevance to the present results, D’Argembeau, Jeunehomme, Majerus, Bastin, and Salmon (2014) found that activity in the posterior hippocampus was recruited when participants judged the temporal order of both past and future events (relative to a judgment concerning the content of past and future events). These authors suggested that the posterior hippocampal activity reflects its role in temporal order processing (e.g., representing the temporal context within which events occur). In light of these results, one possibility is that the transient and posterior hippocampal activity observed here reflects the retrieval of the different temporal contexts associated with the cued details (e.g., differentiating between the prior times one went to Starbucks, see Figure 1). The present findings highlight the need for future work in understanding the temporal properties of hippocampal function not only during episodic simulation but across domains.
4.1.3. Lateral Parietal Findings
In line with another of our predictions, we observed amount-dependent and sustained effects in the left lateral parietal cortex, within the left angular gyrus (see Figure 5A). These results mirror the amount-sensitive and sustained effects observed in the angular gyrus during episodic memory (see Introduction). Therefore, the results are in line with the proposal that the lateral parietal cortex, particularly the left angular gyrus, actively maintains/represents episodic content (Rugg et al., 2015; Vilberg & Rugg, 2008). Critically, the present findings extend this function to episodic simulation, and therefore lend further support to the idea that episodic simulation is supported, in part, by episodic memory processes (Schacter & Addis, 2007).
In addition to the sustained effects observed within the lateral parietal cortex, we also observed transient effects within this region (see Figure 4). On inspection of their respective spatial locations, the sustained effects were more ventral relative to the transient effects, which were observed near the intraparietal sulcus. A recent meta-analysis examining lateral parietal involvement across a number of cognitive domains (including episodic memory, attention, semantic memory, numerical processing, sentence processing, and phonology), concluded that executive processes (e.g., top-down attention and executive semantic decisions) and automatic processes (e.g., episodic retrieval, automatic semantic retrieval, and sentence level processing) cluster around dorsal and ventral parietal regions, respectively (Humphries & Ralph, 2015). In light of these findings, the transient/dorsal parietal effects may reflect the recruitment of transient executive processing to assist construction of the episodic simulation (e.g., top-down attention to the cued episodic details). The relatively more ventral parietal effects, which were sustained, may reflect the relatively automatic maintenance of the episodic content during simulation, consistent with proposals from the episodic memory literature (Rugg et al., 2015; Vilberg & Rugg, 2008). This interpretation is also consistent with a recent meta-analysis showing that more ventral lateral parietal cortex is commonly engaged during both episodic memory and simulation, whereas simulation (requiring greater cognitive control) compared to memory (requiring lesser cognitive control) also engages more dorsal lateral parietal cortex (Benoit & Schacter, 2015).
4.1.4. Lateral Frontal and Medial Parietal Findings
In addition, regions of the lateral frontal cortex (e.g., in the middle and superior frontal gyri) as well as superior medial parietal cortex/precuneus were modulated by the amount of simulated information (see Figure 3). The lateral prefrontal regions correspond well to prior working memory findings, which have implicated these regions in various control processes that support the maintenance of information held in working memory (for reviews, see Curtis & D’Esposito, 2003; Jonides et al., 2008; Wager & Smith, 2003). As participants were required to maintain simulated information across a variable delay, it is unsurprising that such control process were engaged. The medial parietal cortex has also been associated with executive processing during working memory (Wager and Smith, 2003). More recent findings have indicated that this region supports domain-independent control processes (Chiu & Yantis, 2009; Tamber-Rosenau, Esterman, Chiu, & Yantis, 2011). In these studies, common activity in the medial superior parietal lobule has been observed across various tasks that require cognitive ‘shifts’, such as shifts of attention between spatial locations, shifts between categorization rules, and shifts between elements held in working memory. The present medial parietal activity, which overlapped with those reported by Chiu and Yantis (2009), may thus reflect the recruitment of a control process associated with shifting between the episodic details comprising the simulation. As participants were instructed to continuously interact with all the cued details during simulation, such a shift process would have been recruited to a greater extent under conditions with more relative to fewer details. These lateral prefrontal and medial parietal regions also overlap with those reported in the meta-analysis of Benoit & Schacter (2015), who reported that these regions were recruited to a greater extent during simulation relative to remembering (see Introduction). The overlapping nature of the current and past findings adds support to the idea that some processes are more strongly associated with episodic simulation than episodic memory (Addis & Schacter, 2012; Benoit & Schacter, 2015; Schacter & Addis, 2007). The present findings provide specific clues concerning the nature and role of these processes (i.e., maintenance of information and shift-related control processes).
4.2. Possible Challenges to the Current Study
With respect to the amount-dependent effects described above, there are two possible confounds that are important to consider. First, it is possible that as the number of details increased (e.g., 3 versus 5), the phenomenal quality of the simulated event decreased because of more effortful demands to create a coherent simulation (e.g., a less vivid simulation when cued with 5 relative to 3 details). Second, an event with more details may have been more difficult to simulate. Although both of these differences could be considered as confounds that covary with our manipulation of amount, it is possible that these sorts of differences are defining characteristics of simulations with more versus less information. An important avenue for future research is to independently manipulate different factors such as difficulty and vividness to determine if regions of the core network are differentially sensitive to them (see also, Gaesser et al., 2013).
An additional challenge to our interpretation of the present results concerns the use of the sentence task as a non-episodic control. We attempted to match the demands of the sentence and simulation tasks as closely as possible except for the requirement of generating a coherent imagined event (see, 2.2. Stimuli and Task). However, it is still important to directly address whether the sentence task is an appropriate control for the episodic simulation task. First, we note that the simulation and sentence tasks have been shown to be generally matched with respect to construction times. Across two studies that employed the similar sentence task (with the same number of 3 cue words; Addis et al., 2009, 2011b), either construction times were slightly albeit significantly faster (on the order of only 1 sec) for the sentence relative to the simulate tasks (Addis et al., 2009), or construction times did not significantly differ for the two tasks (Addis et al., 2011b). Second, we think that the sentence task as a control is empirically validated in so far as it has yielded very similar results in the present study to other neuroimaging studies that have identified activity in the core network using the same control task as well as different control tasks (for a review, see Benoit & Schacter, 2015). Of particular importance, we replicate core network activity relative to studies that could be argued to have employed relatively stricter control tasks (i.e., semantic memory and visual imagery tasks that required the generation of information not visually cued; see Addis et al., 2007; Addis et al., 2011a). These findings demonstrate that our results do not critically depend on our choice of control task.
Although we identified regions of the core network where activation varied with the amount of simulated information (e.g., left angular gyrus), the large majority of regions did not show such an effect (e.g., hippocampus and medial prefrontal cortex; compare Figure 2 versus Figure 3)4. As noted earlier, any null finding should of course be treated with caution, but our findings nevertheless suggest a possible dissociation between these two classes of simulation effects (cf., Vilberg & Rugg, 2007). Regions demonstrating amount-dependent effects may support processes that contribute to the maintenance or representation of simulated information (cf., Hayama et al., 2012). In contrast, amount-invariant effects may reflect processes engaged during initial construction of a simulation or during post-simulation-guided decision processes, respectively (e.g., Benoit et al., 2014; Gerlach et al., 2014). It will be critical for future research to clarify the nature of these processes.
One final limitation of the current study comes from the novelty of our analytic procedure and associated findings relative to the episodic simulation literature. This is the first application of the transient/sustained analysis approach to the episodic simulation literature, and thus the assumptions underlying this approach, and the reliability of the current results, need to be examined in future studies. Until then, caution should be exercised when interpreting the present findings.
4.3. Conclusions
To conclude, the current findings add to the growing body of studies that have attempted to elucidate the contributions of specific brain regions within the core simulation network. Here, we demonstrated that some of these regions differentially respond with regard to their timecourse of engagement and also with the amount of simulated information. We provide the first evidence of a temporal dissociation along the long axis of the hippocampus during episodic simulation. While relatively more posterior portions of the hippocampus demonstrated a transient response, more anterior portions demonstrated a sustained profile. This dissociation should motivate further inquiries into its functional significance.
We think that the present findings are also relevant to the broader literature concerning the adaptive role of episodic simulation (for review, see Schacter, 2012). It is now clear that episodic simulation can positively impact a variety of psychological functions, including far-sighted decision making (e.g., Benoit et al., 2011; Peters & Büchel, 2010), planning (e.g., Taylor, Pham, Rivkin, & Armor, 1998), emotional wellbeing (e.g., Brown, Macleod, Tata, & Goddard, 2002; Jing, Madore, & Schacter, 2016) and prosocial intentions (e.g., Gaesser & Schacter, 2014). However, only a few studies have examined the neural underpinnings of these adaptive benefits (e.g., Benoit et al., 2011, Gerlach et al., 2014; Peters & Büchel, 2010). Advances in identifying the role of individual core network regions in specific aspects of episodic simulation should provide a foundation that supports the development of a deeper understanding of how episodic simulation enhances cognitive functioning in everyday life.
Acknowledgments
We thank Haley Dodds for assistance in data collection and Scott Slotnick for statistical advice. We thank the Center for Magnetic Resonance Research at the University of Minnesota for use of the functional imaging sequence. This research was supported by NIMH Grant RO1MH60941 (to DLS) and NIH Shared Instrumentation Grant S10OD020039.
Footnotes
Here, the term ‘sustained’ refers to persistent neural activity across a given trial (i.e., across a variable delay until behavioral response; cf., Vilberg and Rugg, 2012, 2014; see also, Curtis and D’Esposito, 2003). Note that this characterization differs from others who have used the term to refer to sustained activity across a block of different trials and responses (e.g., Donaldson et al., 2001; Dosenbach et al., 2006; Visscher et al., 2003).
We chose to perform our analyses in an identical fashion to prior studies that have dissociated recollection-related neural activity as a function of timecourse (Vilberg and Rugg, 2012, 2014) and the amount of information recollected (for a review, see Rugg et al., 2015). This approach was taken so as to directly compare our episodic simulation effects with prior episodic memory effects (see Introduction). We do note that an alternative approach would have been to report every simple effect and interaction resulting from our 2 × 3 × 2 experimental design (i.e., factors of task (simulation and sentence), detail (3, 4, and 5) and time (transient and sustained)). We highlight that our approach was hypothesis-driven and therefore does not entail the increase in multiple comparisons and post-hoc interpretation of statistical tests that would arise from the alternative approach.
One may want to consider another reason for the absence of amount-dependent hippocampal effects. Based on prior findings indicating that the hippocampus contributes to the successful encoding of simulations (e.g., Gaesser et al., 2013; Martin et al., 2011), together with the possibility that the number of event details simulated (e.g., 5 versus 3) is negatively correlated with later subsequent memory, one may suggest that the absence of any amount-dependent hippocampal effects reflects an absence of successful encoding for the events comprised of more details. To assess this possibility, we conducted a follow-up subsequent memory analysis (Thakral et al., 2016). Although the analysis identified successful encoding effects within the hippocampus (i.e., subsequently remembered simulations > subsequently forgotten simulations), the hippocampal effects were not modulated as a function of the amount of simulated information. These findings fail to support the possibility that differences in memorability can account for the failure to identify amount-dependent hippocampal effects.
To determine whether the majority of the core network was indeed insensitive to the amount of information simulated, we exclusively masked the outcome of the simulate > sentence contrast (for the sustained regressor) with the main effect of amount (i.e., an F-contrast across the three amount conditions of 3, 4, and 5; exclusive mask threshold of p < 0.05). This analysis identified the same peak clusters as those listed in Table 2 (top). Thus the majority of the sustained effects within the core network were amount-invariant. The same was true for the transient simulation effects (see Table 3, top). That is, when the contrast used to identify the transient simulation effects was exclusively masked with the main effect of amount (i.e., an F-contrast across the three amount conditions of 3, 4, and 5 for the transient regressor), the same clusters were identified, indicating that the large majority of the transient effects were also insensitive to the amount of simulated information.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Addis DR, Cheng T, Roberts R, Schacter DL. Hippocampal contributions to the episodic simulation of specific and general future events. Hippocampus. 2011a;21:1045–1052. doi: 10.1002/hipo.20870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addis DR, Pan L, Vu M-A, Laiser N, Schacter DL. Constructive episodic simulation of the future and the past: Distinct subsystems of a core brain network mediate imagining and remembering. Neuropsychologia. 2009;47:2222–2238. doi: 10.1016/j.neuropsychologia.2008.10.026. [DOI] [PubMed] [Google Scholar]
- Addis DR, Roberts RP, Schacter DL. Age-related neural changes in autobiographical remembering and imagining. Neuropsychologia. 2011b;4:3656–3669. doi: 10.1016/j.neuropsychologia.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addis DR, Schacter DL. Constructive episodic simulation: Transient distance and detail of past and future events modulate hippocampal engagement. Hippocampus. 2008;18:227–237. doi: 10.1002/hipo.20405. [DOI] [PubMed] [Google Scholar]
- Addis DR, Schacter DL. The hippocampus and imagining the future: where do we stand? Front Hum Neurosci. 2012;5:1–15. doi: 10.3389/fnhum.2011.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addis DR, Wong AT, Schacter DL. Remembering the past and imagining the future: Common and distinct neural substrates during event construction and elaboration. Neuropsychologia. 2007;45:1363–1377. doi: 10.1016/j.neuropsychologia.2006.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. Functional-anatomic fractionation of the brain's default network. Neuron. 2010;65:550–562. doi: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit RG, Gilbert SJ, Burgess PW. A neural mechanism mediating the impact of episodic prospection on farsighted decisions. J Neurosci. 2011;31:6771–6779. doi: 10.1523/JNEUROSCI.6559-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit RG, Schacter DL. Specifying the core network supporting episodic simulation and episodic memory by activation likelihood estimation. Neuropsychologia. 2015;75:450–457. doi: 10.1016/j.neuropsychologia.2015.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit RG, Szpunar KK, Schacter DL. Ventromedial prefrontal cortex supports affective future simulation by integrating distributed knowledge. Proc Natl Acad Sci. 2014;111:16550–16555. doi: 10.1073/pnas.1419274111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GP, Macleod AK, Tata P, Goddard L. Worry and the simulation of future outcomes. Anxiety Stress Coping. 2002;15:1–17. [Google Scholar]
- Buckner RL, Carroll DC. Self-projection and the brain. Trends Cogn Sci. 2007;11:49–57. doi: 10.1016/j.tics.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Chiu YC, Yantis S. A Domain-independent source of cognitive control for task sets: Shifting spatial attention and switching categorization rules. J Neurosci. 2009;29:3930–3938. doi: 10.1523/JNEUROSCI.5737-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis CE, D’Esposito M. Persistent activity in the prefrontal cortex during working memory. Trends Cogn Sci. 2003;7:415–423. doi: 10.1016/s1364-6613(03)00197-9. [DOI] [PubMed] [Google Scholar]
- D’Argembeau A, Jeunehomme O, Majerus S, Bastin C, Salmon E. The neural basis of temporal order processing in past and future thought. J Cogn Neurosci. 2015;27:185–197. doi: 10.1162/jocn_a_00680. [DOI] [PubMed] [Google Scholar]
- Donaldson DI, Petersen SE, Ollinger JM, Buckner RL. Dissociating state and item components of recognition memory using fMRI. NeuroImage. 2001;13:129–142. doi: 10.1006/nimg.2000.0664. [DOI] [PubMed] [Google Scholar]
- Dosenbach NUF, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, … Petersen SE. A core system for the implementation of task sets. Neuron. 2006;50:799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum HE, Cohen NJ. Can we reconcile the declarative memory and spatial navigation views on hippocampal function? Neuron. 2014;83:764–770. doi: 10.1016/j.neuron.2014.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg DA, Moeller S, Smith SM, Auerbach E, Ramanna S, Glasser MF, Miller KL, Uğurbil K, Yacoub E. Multiplexed echo planar imaging for sub-second whole brain fMRI and fast diffusion imaging. PLoS One. 2010;5:1–11. doi: 10.1371/journal.pone.0015710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher RA. Statistical Methods for Research Workers. Oliver and Boyd; London: 1950. [Google Scholar]
- Ford JH, Kensinger EA. Effects of internal and external vividness on hippocampal connectivity during memory retrieval. Neurobiology of Learning and Memory. 2016;134:78–90. doi: 10.1016/j.nlm.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Penny W, Phillips C, Kiebel S, Hinton G, Ashburner J. Classical and Bayesian inference in neuroimaging: theory. Neuroimage. 2002;16:465–483. doi: 10.1006/nimg.2002.1090. [DOI] [PubMed] [Google Scholar]
- Gaesser B, Spreng RN, Mclelland VC, Addis DR, Schacter DL. Imagining the future: Evidence for a hippocampal contribution to constructive processing. Hippocampus. 2013;23:1150–1161. doi: 10.1002/hipo.22152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaesser B, Schacter DL. Episodic simulation and episodic memory can increase intentions to help others. Proc Natl Acad Sci U S A. 2014;111:4415–20. doi: 10.1073/pnas.1402461111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach KD, Spreng RN, Madore KP, Schacter DL. Future planning: Default network activity couples with frontoparietal control network and reward-processing regions during process and outcome simulations. Soc Cogn Affect Neurosci. 2014;9:1942–1951. doi: 10.1093/scan/nsu001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore AW, Nelson SM, McDermott KB. The contextual association network activates more for remembered than for imagined events. Cereb Cortex. 2016;26:611–617. doi: 10.1093/cercor/bhu223. [DOI] [PubMed] [Google Scholar]
- Gu X, Eilam-stock T, Zhou T, Anagnostou E, Kolevzon A, Soorya L, … Fan J. Autonomic and Brain Responses Associated with Empathy Deficits in Autism Spectrum Disorder, Humm. Brain Map. 2015;3338:3323–3338. doi: 10.1002/hbm.22840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassabis D, Maguire EA. Deconstructing episodic memory with construction. Trends Cogn Sci. 2007;11:299–306. doi: 10.1016/j.tics.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Hassabis D, Spreng RN, Rusu AA, Robbins CA, Mar RA, Schacter DL. Imagine all the people: How the brain creates and uses personality models to predict behavior. Cereb Cortex. 2014;24:1979–1987. doi: 10.1093/cercor/bht042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayama HR, Vilberg KL, Rugg MD. Overlap between the neural correlates of cued recall and source memory: Evidence for a generic recollection network? J Cogn Neurosci. 2012;24:1127–1137. doi: 10.1162/jocn_a_00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman P, Binney RJ, Lambon MA. Differing contributions of inferior prefrontal and anterior temporal cortex to concrete and abstract conceptual knowledge. Cortex. 2014;63:250–266. doi: 10.1016/j.cortex.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard MW, Eichenbaum H. Time and space in the hippocampus. Brain Res. 2015;1621:345–354. doi: 10.1016/j.brainres.2014.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys GF, Lambon Ralph MA. Fusion and fission of cognitive functions in the human parietal cortex. Cereb Cortex. 2015;25:3547–3560. doi: 10.1093/cercor/bhu198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing H, Madore KP, Schacter DL. Worrying about the future: An episodic specificity induction impacts problem solving, reappraisal, and well-being. J Exp Psychol Gen. 145:402–418. doi: 10.1037/xge0000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonides J, Lewis RL, Nee DE, Lustig CA, Berman MG, Moore KS. The mind and brain of short-term memory. Annu Rev Psychol. 2008;59:193–224. doi: 10.1146/annurev.psych.59.103006.093615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucera H, Francis WN. Computational analysis of present-day American English. Brown University Press; Providence: 1967. [Google Scholar]
- Madore KP, Schacter DL. Remembering the past and imagining the future: Selective effects of an episodic specificity induction on detail generation. Q J Exp Psychol. 2016;69:285–298. doi: 10.1080/17470218.2014.999097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madore KP, Szpunar KK, Addis DR, Schacter DL. Episodic specificity induction impacts activity in a core brain network during construction of imagined experiences. Proc Natl Acad Sci U S A. 2016 doi: 10.1073/pnas.1612278113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin VC, Schacter DL, Corballis MC, Addis DR. A role for the hippocampus in encoding simulations of future events. Proc Natl Acad Sci U S A. 2011;108:13858–13863. doi: 10.1073/pnas.1105816108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller S, Yacoub E, Olman CA, Auerbach E, Strupp J, Harel N, Uğurbil K. Multiband multislice GE-EPI at 7 tesla, with 16-fold acceleration using partial parallel imaging with application to high spatial and temporal whole-brain fMRI. Magn Reson Med. 2009;63:1144–1153. doi: 10.1002/mrm.22361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palombo DJ, Hayes SM, Peterson KM, Keane MM, Verfaellie M. Medial temporal lobe contributions to episodic future thinking: Scene construction or future projection? Cereb Cortex. 2016 doi: 10.1093/cercor/bhw381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, Büchel C. Episodic future thinking reduces reward delay discounting through an enhancement of prefrontal-mediotemporal interactions. Neuron. 2010;66:138–148. doi: 10.1016/j.neuron.2010.03.026. [DOI] [PubMed] [Google Scholar]
- Ranganath C, Hsieh LT. The hippocampus: A special place for time. Ann N Y Acad Sci. 2016;1369:93–110. doi: 10.1111/nyas.13043. [DOI] [PubMed] [Google Scholar]
- Roberts RR, Schacter DL, Addis DR. Scene construction and relational processing: Separable constructs? Cereb Cortex. 2017 doi: 10.1093/cercor/bhx081. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorden C, Karnath H, Bonilha L. Improving lesion-symptom mapping. J Cogn Neurosci. 2007:1081–1088. doi: 10.1162/jocn.2007.19.7.1081. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Johnson JD, Uncapher MR. Encoding and retrieval in episodic memory: Insights from fMRI. In: Duarte A, Barense MD, Addis DR, editors. Handbook on the cognitive neuroscience of memory. Wiley-Blackwell; Oxford: 2015. pp. 84–107. [Google Scholar]
- Schacter DL. Adaptive constructive processes and the future of memory. Am Psychol. 2012;67:603–613. doi: 10.1037/a0029869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Madore KP. Remembering the past and imagining the future: Identifying and enhancing the contribution of episodic memory. Mem Stud. 2016;9:245–255. doi: 10.1177/1750698016645230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, Buckner RL. Episodic simulation of future events: Concepts, data, and applications. Ann N Y Acad Sci. 2008;1124:39–60. doi: 10.1196/annals.1440.001. [DOI] [PubMed] [Google Scholar]
- Schacter D, Addis DR. The cognitive neuroscience of constructive memory: remembering the past and imagining the future. Philos Trans R Soc B Biol Sci. 2007;362:773–786. doi: 10.1098/rstb.2007.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, Hassabis D, Martin VC, Spreng RN, Szpunar KK. The future of memory: Remembering, imagining, and the brain. Neuron. 2012;76:677–694. doi: 10.1016/j.neuron.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, Szpunar KK. Escaping the past: Contributions of the hippocampus to future thinking and imagination. In: Hannula DE, Duff MC, editors. The Hippocampus from cells to systems: Structure, connectivity, and functional contributions to memory and flexible cognition. Springer; New York: (in press) [Google Scholar]
- Sheldon S, Levine B. The role of the hippocampus in memory and mental construction. Ann N Y Acad Sci. 2016;1369:76–92. doi: 10.1111/nyas.13006. [DOI] [PubMed] [Google Scholar]
- Shigemune Y, Tsukiura T, Kambara T, Kawashima R. Remembering with gains and losses: Effects of monetary reward and punishment on successful encoding activation of source memories. Cereb Cortex. 2014;24:1319–1331. doi: 10.1093/cercor/bhs415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotnick SD. Visual memory and visual perception recruit common neural substrates. Behav Cogn Neurosci Rev. 2004;3:207–221. doi: 10.1177/1534582304274070. [DOI] [PubMed] [Google Scholar]
- Slotnick SD. Spatial working memory specific activity in dorsal prefrontal cortex? Disparate answers from fMRI beta-weight and timecourse analysis. Cogn Neuropsychol. 2005;22:905–20. doi: 10.1080/02643290442000455. [DOI] [PubMed] [Google Scholar]
- Slotnick SD. Does the hippocampus mediate objective binding or subjective remembering? NeuroImage. 2010;49:1769–76. doi: 10.1016/j.neuroimage.2009.09.039. [DOI] [PubMed] [Google Scholar]
- Slotnick SD. Cluster_threshold. 2016 Retrieved September 1, 2016 from Web site: http://www2.bc.edu/~slotnics/scripts.htm.
- Slotnick SD, Moo LR, Segal JB, Hart J. Distinct prefrontal cortex activity associated with item memory and source memory for visual shapes. Cogn Brain Res. 2003;17:75–82. doi: 10.1016/s0926-6410(03)00082-x. [DOI] [PubMed] [Google Scholar]
- Slotnick SD, Schacter DL. A sensory signature that distinguishes true from false memories. Nat Neurosci. 2004;7:664–672. doi: 10.1038/nn1252. [DOI] [PubMed] [Google Scholar]
- Slotnick SD, Schacter DL. The nature of memory related activity in early visual areas. Neuropsychologia. 2006;44:2874–2886. doi: 10.1016/j.neuropsychologia.2006.06.021. [DOI] [PubMed] [Google Scholar]
- Suddendorf T, Corballis MC. The evolution of foresight: What is mental time travel, and is it unique to humans? Behav Brain Sci. 2007;30:299–313-51. doi: 10.1017/S0140525X07001975. [DOI] [PubMed] [Google Scholar]
- Szpunar KK. Episodic future thought: An emerging concept. Perspect Psychol Sci. 2010;5:142–162. doi: 10.1177/1745691610362350. [DOI] [PubMed] [Google Scholar]
- Szpunar KK, Chan JCK, McDermott KB. Contextual processing in episodic future thought. Cereb Cortex. 2009;19:1539–1548. doi: 10.1093/cercor/bhn191. [DOI] [PubMed] [Google Scholar]
- Szpunar KK, Jacques PLS, Robbins CA, Wig GS, Schacter DL. Repetition-related reductions in neural activity reveal component processes of mental simulation. Soc Cogn Affect Neurosci. 2014;9:712–722. doi: 10.1093/scan/nst035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szpunar KK, Jing HG, Benoit RG, Schacter DL. Repetition-related reductions in neural activity during emotional simulations of future events. PLoS One. 2015;10:1–15. doi: 10.1371/journal.pone.0138354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szpunar KK, McDermott KB. Episodic future thought and its relation to remembering: Evidence from ratings of subjective experience. Conscious Cogn. 2008;17:330–334. doi: 10.1016/j.concog.2007.04.006. [DOI] [PubMed] [Google Scholar]
- Szpunar KK, Spreng RN, Schacter DL. A taxonomy of prospection: introducing an organizational framework for future-oriented cognition. Proc Natl Acad Sci U S A. 2014;111:18414–21. doi: 10.1073/pnas.1417144111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamber-Rosenau BJ, Esterman M, Chiu YC, Yantis S. Cortical mechanisms of cognitive control for shifting attention in vision and working memory. J Cogn Neurosci. 2011;23:2905–19. doi: 10.1162/jocn.2011.21608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SE, Pham LB, Rivkin ID, Armor DA. Harnessing the imagination. Mental simulation, self-regulation, and coping. Am Psychol. 1998;53:429–439. doi: 10.1037//0003-066x.53.4.429. [DOI] [PubMed] [Google Scholar]
- Thakral PP, Benoit RG, Schacter DL. Characterizing the role of the hippocampus during episodic simulation and episodic encoding. Poster presented at the annual meeting of the Psychonomic Society; Boston, MA. 2016. Nov, [Google Scholar]
- Thakral PP, Yu SS, Rugg MD. The hippocampus is sensitive to the mismatch in novelty between items and their contexts. Brain Res. 2015;1602:144–152. doi: 10.1016/j.brainres.2015.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakral PP, Wang TH, Rugg MD. Cortical reinstatement and the confidence and accuracy of source memory. Neuroimage. 2015;109:118–129. doi: 10.1016/j.neuroimage.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakral PP, Slotnick SD. The role of parietal cortex during sustained visual spatial attention. Brain Res. 2009;1302:157–166. doi: 10.1016/j.brainres.2009.09.031. [DOI] [PubMed] [Google Scholar]
- Tulving E. 2005 Episodic memory and autonoesis: uniquely human? In: Terrace HS, Metcalfe J, editors. In The missing link in cognition: origins of self-reflective consciousness. New York, NY: Oxford University Press; pp. 3–56. [Google Scholar]
- van der Kouwe AJW, Benner T, Fischl B, Schmitt F, Salat DH, Harder M, Sorensen AG, Dale AM. On-line automatic slice positioning for brain MR imaging. Neuroimage. 2005;27:222–230. doi: 10.1016/j.neuroimage.2005.03.035. [DOI] [PubMed] [Google Scholar]
- van Mulukom V, Schacter DL, Corballis MC, Addis DR. Re-imagining the future: Repetition decreases hippocampal involvement in future simulation. PLoS ONE. 2013;8:1–10. doi: 10.1371/journal.pone.0069596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilberg KL, Rugg MD. Dissociation of the neural correlates of recognition memory according to familiarity, recollection, and amount of recollected information. Neuropsychologia. 2007;45:2216–2225. doi: 10.1016/j.neuropsychologia.2007.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilberg KL, Rugg MD. Memory retrieval and the parietal cortex: A review of evidence from a dual-process perspective. Neuropsychologia. 2008;46:1787–1799. doi: 10.1016/j.neuropsychologia.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilberg KL, Rugg MD. The neural correlates of recollection: Transient versus sustained fMRI effects. J Neurosci. 2012;32:15679–15687. doi: 10.1523/JNEUROSCI.3065-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilberg KL, Rugg MD. Temporal dissociations within the core recollection network. Cogn Neurosci. 2014;5:77–84. doi: 10.1080/17588928.2013.860088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophys. 2008;100:3328–3342. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visscher KM, Miezin FM, Kelly JE, Buckner RL, Donaldson DI, McAvoy MP, … Petersen SE. Mixed blocked/event-related designs separate transient and sustained activity in fMRI. NeuroImage. 2003;19:1694–1708. doi: 10.1016/s1053-8119(03)00178-2. [DOI] [PubMed] [Google Scholar]
- Wager TD, Smith EE. Neuroimaging studies of working memory: a meta-analysis. Cogn Affect Behav Neurosci. 2003;3:255–274. doi: 10.3758/cabn.3.4.255. [DOI] [PubMed] [Google Scholar]
- Weiler JA, Suchan B, Daum I. When the future becomes the past: Differences in brain activation patterns for episodic memory and episodic future thinking. Behav Brain Res. 2010;212:196–203. doi: 10.1016/j.bbr.2010.04.013. [DOI] [PubMed] [Google Scholar]
- Xu J, Moeller S, Auerbach EJ, Strupp J, Smith SM, Feinberg DA, Yacoub E, Uğurbil K. Evaluation of slice accelerations using multiband echo planar imaging at 3T. Neuroimage. 2013;83:991–1001. doi: 10.1016/j.neuroimage.2013.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Yuan H, Lei X. Activation and connectivity within the default mode network contribute independently to future-oriented thought. Sci Rep. 2016;6:1–10. doi: 10.1038/srep21001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu SS, Johnson JD, Rugg MD. Dissociation of recollection-related neural activity in ventral lateral parietal cortex. Cogn Neurosci. 2012;3:142–149. doi: 10.1080/17588928.2012.669363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidman P, Mullally SL, Maguire EA. Constructing, perceiving, and maintaining scenes: Hippocampal activity and connectivity. Cereb Cortex. 2015;25:3836–3855. doi: 10.1093/cercor/bhu266. [DOI] [PMC free article] [PubMed] [Google Scholar]