Memory consists of several separate entities that depend on various brain systems [1]. Clinical and behavioral evidence suggests that the hippocampus and the surrounding anatomically associated regions serve a critical role in learning and memory [2]. In the 1960s, the outlines of the essential neural substrates of memory were gradually elucidated based on analyzing the effects of therapeutic surgical lesions of the bilateral medial temporal lobes to suppress uncontrollable epilepsy in a patient [3]. Although the operation was effective in controlling the patient’s epilepsy, one unexpected consequence was that he became profoundly amnesic while retaining his intelligence and perceptual and motor functions. Similar cases were also seen in other patients with damage to the hippocampal formation and surrounding medial temporal lobe structures [4]. These individuals had severe amnesia for episodic events, although other forms of learning and memory—semantic, perceptual, procedural, and simple forms of conditioning—were spared. It is now believed that the hippocampal formation has a central role in declarative memory, the ability to recollect everyday facts and events consciously [5]. The unique anatomy, electrophysiologic characteristics, and key roles in memory formation have made the hippocampus an attractive target of research for neuroscientists.
For decades, it was believed that neurogenesis only occurred during embryonic stages in the mammalian central nervous system (CNS), making the brain one of the few mammalian organs incapable of replenishing its functional cell population throughout life [6]. In the 1960s, seminal studies by Altman and Das [7–9] provided the first evidence that new neurons were generated in the postnatal mammalian brain. In 1992, Reynolds and Weiss [10] isolated multipotent neural stem/progenitor cells (NSCs) from the adult rodent brain and characterized them in vitro. Studies in the 1990s confirmed that, contrary to long-held dogma, NSCs reside in the adult CNS and active neurogenesis occurs in discrete regions of the adult brain across various mammalian species, including mice, rats, monkeys, and humans [11–14]. Only recently has it been recognized that adult neurogenesis replicates the complex process of neuronal development to generate functionally integrated new neurons (Fig. 1) [15–17]. A role for these postnatally generated cells in learning was first suggested by Altman and Das in the 1960s [7–9,18]. Later, Nottebohm [19] directly tested the role of adult-generated neurons in song learning in birds. Subsequent work in rodents has led to the idea that adult neurogenesis is important for learning and memory of spatial information.
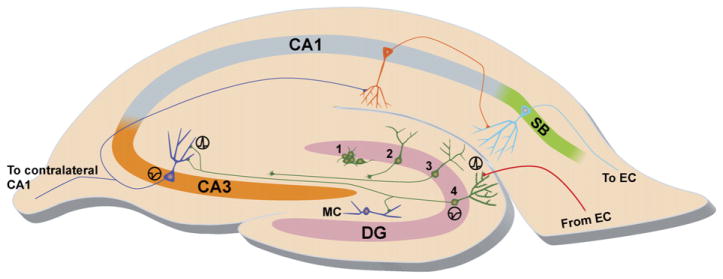
Fig. 1.
DG neurogenesis incorporation of new neurons. Neurogenesis in the hippocampus occurs in the SGZ of the DG, wherein neural progenitor cells reside (1). Within the first week after birth, these progenitors undergo a fate choice, in this case, becoming an immature neuron (2) with developing neurites. The axons of these new granule neurons are guided through the hilus of the DG, with long-distance branches targeting the CA3 (3) and the dendrites extending into the molecular layer (3). After 3 to 4 weeks, these cells form a mature phenotype, with their dendrites containing spines that receive input primarily from the entorhinal cortex (EC) by way of the perforant pathway (red axon) and their highly branched axons that output to CA3 pyramidal neurons (4) and hilar mossy cells (MC) by way of the mossy fiber pathway (green axon). Continuing the hippocampal circuitry, the CA3 pyramidal neurons output to the CA1 ipsilateral pyramidal neurons by way of the Schaffer collateral pathway or contralaterally by way of the associational commissural pathway (blue axon). The CA1 pyramidal neurons output (orange axon) to pyramidal neurons of the subiculum (SB), which output (cyan axon) to the EC, and these neurons eventually output to the parahippocampal and perirhinal cortex. Ultimately, the circuitry connects to the association cortices.
The discovery of adult neurogenesis has generated significant interest, especially in regard to the hippocampus, not only for neuroscientists but for physicians who are engaged in treating various neurologic diseases. Although interest in adult neurogenesis has grown exponentially in recent years, evidence for a role of adult-generated granule cells in learning and memory remains limited and, in most cases, indirect. In this review, the authors summarize the current body of research on mechanisms of adult neurogenesis in the hippocampus and then discuss research in the field, focusing on possible functions of adult neurogenesis in memory and learning, with comments on future directions.
Basic processes of adult neurogenesis
Adult neurogenesis is an evolutionary conserved process in various species, including birds [20], rodents [21], primates [22,23], and human beings [24]. In mammals, under normal conditions, active adult neurogenesis is primarily restricted to two brain regions, the subgranular zone (SGZ) of the hippocampal dentate gyrus (DG) and the subventricular zone (SVZ) of the lateral ventricles [16]. The SGZ is located at the interface between the granule cell layer (GCL) and the hilus of the DG, deep within the parenchyma (see Fig. 1). A subpopulation of glial fibrillary acidic protein (GFAP)–expressing cells in this region has been proposed to be the resident NSCs [25,26]. These NSCs proliferate and give rise to new granule cells through five developmental stages (see Fig. 1): proliferation, fate specification, migration, axon/dendrite targeting, and synaptic integration [16]. These stages can be readily identified on the basis of cell morphology, mitotic capability, electrophysiological characteristics, and expression of developmentally regulated markers [27]. NSCs generate significant numbers of progeny in young adult rats, with approximately 9000 new cells, or 0.1% of the granule cell population, being replaced daily [28]. Approximately 50% of the neuronal progeny survive, and new granule cells populate the inner third of the GCL. Within 4 to 10 days after birth, new granule cells send their axonal projections toward the CA3 region and spineless dendritic arbors into the molecular layer (see Fig. 1). Their dendrites concomitantly become more complex and extend deeper into the molecular cell layer as new granule cells differentiate [29,30]. As the dendrites grow, they receive synaptic contacts and become integrated into the preexisting circuitry within 2 to 4 weeks after their birth (see Fig. 1). These new granule neurons continue their maturation process for at least another 2 months and are then maintained for a long duration in the hippocampus [31].
Regulation of adult neurogenesis by learning
The basic rate of neurogenesis in the DG is thought to be genetically determined [28] but can be dramatically regulated under various conditions, including aging [32]; gender [33]; steroids [34,35]; stress [36]; enriched environment [37]; voluntary exercise [38]; and pathologic conditions, such as seizures [39] or cerebral ischemia [40].
It is widely accepted that medial temporal lobe structures, including the hippocampus and surrounding cortical areas, are critical to the processes of learning and memory [5]. Interestingly, specific learning paradigms involving the hippocampus have been shown to regulate hippocampal neurogenesis in several animal models combined with various behavioral tasks (Table 1). In particular, the survival of the newborn cells is enhanced by spatial learning tasks, and there is a correlation between an individual’s learning and newborn cell survival [41–43]. For example, trace eye-blink conditioning, which depends on hippocampal function, increases the number of newly generated neurons in the DG, whereas learning that does not require the hippocampus, including delayed eye-blink conditioning and cue-maze training, does not alter the number of new granule neurons compared with naive controls [44]. This phenomenon was not observed in some experiments [38,45] and these discrepancies may be attributable to different experimental designs of the learning tasks [38,44] or to stress associated with the training possibly causing downregulation in adult neurogenesis [46]. Associative long-term potentiation (LTP), an attractive model for certain forms of learning and memory [47], has been shown to enhance neurogenesis in the adult DG [48], supporting the hypothesis that activity-dependent synaptic plasticity in the DG during learning may provide signals for promoting learning-induced neurogenesis.
Table 1.
Influences of learning on the hippocampal neurogenesis
| Tasks | Proliferation | Survival | References | |
|---|---|---|---|---|
| Water maze | (2 trials per day × 4 days) | nc | Gould, et al [44] | |
| (4 trials per day × 4 days) | + | |||
| Water maze | (10 trials per day × 5 days) | + | Ambrogini, et al [41] | |
| Water maze | (6 trials per day × 3 days) | + | Rola, et al [43] | |
| Water maze | (8 trials per day × 4 days) | + | Hairston, et al [42] | |
| Water maze | (2 trials per day × 30 days) | nc | nc | van Praag, et al [58] |
| Water maze | (4 trials per day × 8 days) | Dobrossy, et al [78] | ||
| (early phase) | nc, then decreased by later learning | |||
| (late phase) | + | + | ||
| Water maze | (4 trials per day) | Drapeau, et al [55] | ||
| (old animals) | + | + | ||
| (young animals) | nc | |||
| Water maze | (1 or 2 trials per day × 10 days) | nc | Merrill, et al [45] | |
| Water maze | (6 trials per day × 4 days) | Ehninger and Kempermann [46] | ||
| (type 3 cells) | − | |||
| (type 2 cells) | −, then + | |||
| Trace eye-blink conditioning | nc | + | Gould, et al [44] | |
| Trace eye-blink | + | Leuner, et al [79] | ||
| STFP task | (1 day of training) | + | ||
| (2 days of training) | − | Olariu, et al [80] | ||
| Contextual fear conditioning | − | nc | Pham, et al [81] |
Unmarked indicates not examined in proliferation column.
Abbreviations: nc, no change; STFP, social transmission of food preference; +, increase; −, decrease.
Potential involvement of adult neurogenesis in learning and memory
The functional relevance of adult neurogenesis in memory processes was first suggested in studies looking at the neural basis of song learning in birds [19]. In adult song birds, the volume of song-related nuclei showed seasonal and hormonal changes, with thousands of new neurons being added daily. These putative neurons responded to sound with action potentials, and neurogenesis in the avian hippocampus was modulated by the environmental complexity and learning experience [19,49]. Since then, various studies of rodent behaviors with various manipulations to eliminate or increase adult neurogenesis have provided substantial evidence for a role of newborn neurons in learning and memory (Table 2).
Table 2.
Influences of altered neurogenesis on learning performance
| Factors | Neurogenesis | Learning task | Learning | References |
|---|---|---|---|---|
| Aging | Water maze | Positive correlation | Bizon and Gallagher [54] | |
| Strain | Water maze | Positive correlation | Kempermann and Gage [53] | |
| Running | + | Water maze | Improved | van Praag, et al [58] |
| Enriched environment | + | Water maze | Improved | Nilsson, et al [37] |
| Stress | − | Water maze | Impaired | Lemaire, et al [82] |
| Irradiation | − | Water maze | nc | Madsen, et al [64] |
| Object recognition task | nc | |||
| Place recognition task | Impaired | |||
| Barnes maze | Impaired | |||
| Irradiation | − | Water maze | nc | Raber, et al [65] |
| Elevated plus maze | nc | |||
| Irradiation | − | Water maze | Impaired | Rola, et al [43] |
| Barnes maze | nc | |||
| Irradiation | − | Water maze | Impaired | Snyder, et al [68] |
| Irradiation + enrichment | − | Water maze | nc | Meshi, et al [57] |
| Irradiation | − | NMTS task | Impaired | Winocur, et al [66] |
| Contextual fear conditioning | Impaired | |||
| MAM | − | Trace-eyeblink | Impaired | Shores, et al [60] |
| MAM | − | Water maze | nc | Shores, et al [61] |
| − | Elevated plus maze | nc | ||
| Trace fear conditioning | Impaired | |||
| MAM | − | Novel object recognition task | nc | Bruel-Jungerman, et al [59] |
| Enriched environment | + | Novel object recognition task | Improved | |
| MAM+enriched environment | − | Novel object recognition task | Impaired | |
| Deletion of MBD1 | − | Water maze | Impaired | Zhao, et al [71] |
| Deletion of NT-3 | − | Water maze | Impaired | Shimazu, et al [76] |
| VEGF overexpression | + | Water maze | Improved | Cao, et al [70] |
| Passive avoidance | Improved | |||
| Immunedeficiency | − | Water maze | Impaired | Ziv, et al [83] |
Abbreviations: MAM, methylazoxymethanol acetate; MBD1, methyl-CpG binding protein 1; nc, no change; NMTS, non–matching-to-sample; NT-3, neurotrophin-3; VEGF, vascular endothelial growth factor; +, increase; −, decrease.
The basic level of proliferation and the survival rate of newborn neurons in rodents are influenced by genetic background [50,51]. For example, among different strains of mice, including C57BL/6, BALB/c, CD1(ICR), and 129Sv/J strains, the proliferation rate of NSCs was highest in C57BL/6 mice, whereas the survival rate of newborn neurons was highest in CD1 mice [51]. Comparing differences in the ability to learn the Morris water maze task between various strains of mice, it was shown that the strain with the highest baseline level of neurogenesis performed best in the learning task and that the strain with the lowest rate of basal neurogenesis performed the poorest [52,53]. Such a positive correlation between an increased rate in the number of newly generated neurons and better efficiency at completing a learning task was supported by another study that observed a quantitative relation between spontaneous individual differences in aged subjects performing a hippocampal-dependent task and the number of newly generated neurons [54,55].
To examine the influence of new neurons on the learning process, alterations of adult neurogenesis have been induced intentionally in various animal models (see Table 2). Environmental enrichment [56] and increased physical activity [38,56] enhance neurogenesis in the DG, and combining both stimuli leads to improved performance in a water maze test [37,57,58]. Conversely, administration of antimitotic agents and irradiation are two of the approaches to reduce the number of adult-generated cells [59–61]. Treatment with a toxin for proliferating cells, the DNA methylating agent methylazoxymethanol acetate (MAM), reduced the number of newly generated cells in the DG without impairing overall health. Ionizing irradiation of the adult hippocampus also caused deletion of proliferating cells in the DG, leaving other cells apparently unchanged [62]. The effect of radiation was dose dependent, with cell death in the proliferating cells occurring within 3 to 6 hours after treatment, and lasted at least up to 120 days after irradiation [63]. In the learning tasks that are known to require hippocampus-controlled memory function (eg, place-recognition task [64], spatial learning in the Barnes maze [65]), the mice with reduced hippocampal neurogenesis performed more poorly than controls, whereas they were not impaired in hippocampus-independent learning (eg, object-recognition task [64], elevated plus maze [65]). Similar dependency on hippocampus-related function was observed in trace conditioning tasks [60,61] and in the basic non–matching-to-sample (NMTS) task, in which an animal must associate stimuli that are separated in time. Reduced neurogenesis caused no impairment in these tasks when the interval between the cue and the test trials were short. However, when the interval was relatively long, thereby increasing the demand on hippocampus-associated memory function, the decreased neurogenesis caused a significant impairment on learning [66].
Although these studies suggest a significant role for adult neurogenesis in some types of learning, this finding is nowhere conclusive because of the nonspecific nature of these manipulations. Irradiation can cause an inflammatory response despite no obvious morphologic changes or alterations in the tissue. Activated microglia and infiltrating peripheral monocytes seen in the tissue of irradiated animals indicate that reduced neurogenesis may be associated with alterations in the neurogenic microenvironment, leading to persistent inflammation [43,67]. This prolonged inflammation can cause side effects that do not seem to be directly involved in cell death, such as weight loss [68]. MAM treatment, per se, does not alter general activity, pain sensitivity, or stress levels or cause structural changes in the hippocampus other than a reduction in the number of new cells, but MAM reduces cell proliferation systemically; thus, its influence is not exclusively limited to the hippocampus. Despite no side effects at low doses, which were often used, slightly higher doses caused weight loss and reduced locomotion [69].
Genetic modifications, including gene knockout and transgenic techniques, have been used to study the involvement of adult neurogenesis in learning (see Table 2). Overexpression of vascular endothelial growth factor (VEGF) in the hippocampus using recombinant adenoassociated viral vectors led to a twofold increase in neurogenesis, and inhibition of VEGF expression by RNA interference completely blocked the environmental induction of neurogenesis [70]. This animal model of VEGF overexpression also showed a positive correlation between increases in adult neurogenesis and improved cognitive function in the water maze and passive avoidance tasks [70]. In another example, deletion of methyl-CpG binding protein 1 (MBD1), a member of the methylated DNA-binding protein family, increased genomic instability in adult NSCs and caused a reduction in hippocampal DG neurogenesis. MBD1 knockout mice exhibited impaired spatial learning and had a significant reduction in LTP [71]. In another example, the conditional knockout mice of presenilin-1 (PS1), which is known to be associated with the early onset of Alzheimer’s disease, showed a pronounced deficiency in enrichment-induced neurogenesis in the DG, and this phenomenon was accompanied by prolonged long-term memory retention as seen with the contextual fear-conditioning task. This study suggests an association between hippocampal neurogenesis and the clearance of old memory traces after cortical memory consolidation [72].
Animal models with genetic modifications can provide new clues to discover associations with other biologic activities that originally seemed unrelated and have become powerful tools for studying adult neurogenesis. Current animal models are not specific enough to select for newborn neurons exclusively and the influence of genetic mutation on other tissues or cell types and compensation mechanisms cannot be excluded. Future development of more cell type–specific and inducible animal models is essential to provide definitive evidence for the role of adult neurogenesis in mammals.
Potential mechanisms underlying the contribution of adult neurogenesis on learning and memory
Despite some unresolved issues, if adult hippocampal neurogenesis can be assumed to play a critical role in learning and memory, how do these new cells contribute to the process? It takes approximately 2 to 4 weeks before the newly generated neurons are functionally integrated and start modifying active hippocampal circuits. Thus, it seems that mere replacement of the old neuronal population with newly generated cells is too slow and cannot explain the mechanism of plasticity alone. In addition to their morphologic changes, single cell–recording studies have revealed that newly formed neurons have electrophysiological properties that are distinct from those of mature neurons but resemble immature neurons formed during embryonic development [73,74]. At 1 to 3 weeks after mitosis, these cells showed a higher input resistance, a markedly lower threshold for triggering action potentials, and a much slower membrane time constant that favored action potential generation with extremely small current stimuli [73]. This enhanced excitability may be important for young neurons, because only a few excitatory contacts have been formed early in their incorporation process. More significantly, newborn immature granule cells at 1 to 3 weeks after birth exhibit a lower threshold for LTP induction than mature neurons [73,74]. Recent studies have further demonstrated that a critical period exists up to 6 weeks after birth, when newborn neurons exhibit significantly greater LTP and a lower threshold for induction than those of new neurons after maturation (S. Ge, H. Song, unpublished data, 2006). These studies suggest that newly generated neurons in the adult hippocampus can enhance the synaptic plasticity and modulate the neural network through their unique physiologic properties. Enhanced LTP that causes an increase in hippocampal neurogenesis has also been observed in several animal models [58,75]. Conversely, inhibition of neurogenesis leads to reduced LTP [71,76,77] (also, see the article by Shors and colleagues [60]). Hence, promoting the survival of newly generated neurons in the process of learning may be useful and effective to shift the neural population from the existing status, in which old neurons are tightly incorporated with each other, to a more flexible condition, with increased excitability and synaptic plasticity (Fig. 2). It can be reasoned that it is faster, and more biologically cost-effective, to reconstruct the neural network by increasing the “plasticity index” of the hippocampus by increasing the number of young neurons causing elevated regional plasticity rather than by remodeling the developmentally born, old established network.
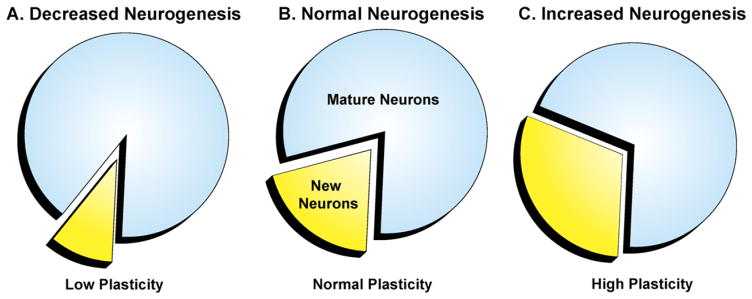
Fig. 2.
Theory of hippocampal neurogenesis in learning and memory. In normal neurogenesis (B), it appears that new neurons are constantly replacing a small portion of the existing population at a basal rate without a significant overall increase in the number of total granule neurons in the DG over time. Under certain conditions that cause a decrease in neurogenesis (A), it is assumed that this rate of replacement is significantly decreased and that with stimulation, this rate of replacement increases (C). Compared with the mature neuron population, the proportion of new neurons may cause a shift into an elevated plastic state. An increase in neurogenesis, and thus an increase in plasticity, may cause improvement in learning and memory tasks. Therefore, conversely, blocking neurogenesis and decreasing the plasticity index may be the cause of the observable decline in performance with various learning- and memory-related tasks.
Summary and future directions
Although profound progress has been made in understanding and characterizing the mechanisms of adult neurogenesis, current studies are still insufficient to establish the true functional relevance of newborn neurons in adult mammalian brain. Learning and memory consist of extremely complicated and tightly orchestrated functions causing complex higher order behavior. It seems that the classic assumption in learning-induced structural plasticity—”more neurons are better”—may not address this mechanism correctly. Adult neurogenesis seems to contribute to this critical function not only by increasing the number of neurons but by adding “immaturity” to the region, meaning more excitability (higher sensitivity to γ-aminobutyric acid [GABA] and a lower threshold for the activity) and more fate options (neuron, glia, or cell death). Identification of the stage of newborn neurons that may make special contributions to hippocampal function is critical.
Currently, however, all manipulations may directly or indirectly change the properties of these new neurons, and none of the experimental models are sufficient to investigate how, when, and to what extent adult neurogenesis contributes to this profound function. New animal models are needed to test a role of adult neurogenesis directly. In this regard, establishment of refined genetic models in which neurogenesis exclusively in the DG can be regulated at suitable time points and in the suitable region, so that learning and memory can be studied in its depth, is needed. In addition, behavior tests that have better sensitivity are needed to detect subtle changes in learning and memory with respect to changes in adult neurogenesis. Although it took a century to establish the existence of adult neurogenesis fully, recent rapid progress in the field has led to confidence that the true physiologic significance of this evolutionally conserved phenomenon is likely to be revealed in the near future.
Acknowledgments
Supported by a Postdoctoral Fellowship Award from the Sankyo Foundation of Life Science in Japan (Y. Kitabatake), the National Institute of Health (H. Song and G-l. Ming), Klingenstein Fellowship Award in the Neurosciences (G-l. Ming and H. Song), the Whitehall Foundation (G-l. Ming), McKnight Scholar Award (H. Song).
References
- 1.Squire LR, Zola SM. Structure and function of declarative and nondeclarative memory systems. Proc Natl Acad Sci USA. 1996;93:13515–22. doi: 10.1073/pnas.93.24.13515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Squire LR, Stark CE, Clark RE. The medial temporal lobe. Annu Rev Neurosci. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- 3.Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. 1957. J Neuropsychiatry Clin Neurosci. 2000;12:103–13. doi: 10.1176/jnp.12.1.103. [DOI] [PubMed] [Google Scholar]
- 4.Aggleton JP, Brown MW. Episodic memory, amnesia, and the hippocampal-anterior thalamic axis. Behav Brain Sci. 1999;22:425–44. [PubMed] [Google Scholar]
- 5.Squire LR, Zola SM. Episodic memory, semantic memory, and amnesia. Hippocampus. 1998;8:205–11. doi: 10.1002/(SICI)1098-1063(1998)8:3<205::AID-HIPO3>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 6.Ramon y, Cajal S. Degeneration and regeneration of the nervous system. London: Oxford University Press; 1913. [Google Scholar]
- 7.Altman J. Are new neurons formed in the brains of adult mammals? Science. 1962;135:1127–8. doi: 10.1126/science.135.3509.1127. [DOI] [PubMed] [Google Scholar]
- 8.Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124:319–35. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- 9.Altman J, Das GD. Autoradiographic and histological studies of postnatal neurogenesis. I. A longitudinal investigation of the kinetics, migration and transformation of cells incorporating tritiated thymidine in neonate rats, with special reference to postnatal neurogenesis in some brain regions. J Comp Neurol. 1966;126:337–89. doi: 10.1002/cne.901260302. [DOI] [PubMed] [Google Scholar]
- 10.Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–10. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- 11.Alvarez-Buylla A, Garcia-Verdugo JM. Neurogenesis in adult subventricular zone. J Neurosci. 2002;22:629–34. doi: 10.1523/JNEUROSCI.22-03-00629.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gross CG. Neurogenesis in the adult brain: death of a dogma. Nat Rev Neurosci. 2000;1:67–73. doi: 10.1038/35036235. [DOI] [PubMed] [Google Scholar]
- 13.Kempermann G, Wiskott L, Gage FH. Functional significance of adult neurogenesis. Curr Opin Neurobiol. 2004;14:186–91. doi: 10.1016/j.conb.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 14.Saghatelyan A, Carleton A, Lagier S, et al. Local neurons play key roles in the mammalian olfactory bulb. J Physiol (Paris) 2003;97:517–28. doi: 10.1016/j.jphysparis.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 15.Lledo PM, Alonso M, Grubb MS. Adult neurogenesis and functional plasticity in neuronal circuits. Nat Rev Neurosci. 2006;7:179–93. doi: 10.1038/nrn1867. [DOI] [PubMed] [Google Scholar]
- 16.Ming GL, Song H. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci. 2005;28:223–50. doi: 10.1146/annurev.neuro.28.051804.101459. [DOI] [PubMed] [Google Scholar]
- 17.Overstreet-Wadiche LS, Westbrook GL. Functional maturation of adult-generated granule cells. Hippocampus. 2006;16:208–15. doi: 10.1002/hipo.20152. [DOI] [PubMed] [Google Scholar]
- 18.Altman J. Autoradiographic and histological studies of postnatal neurogenesis. IV. Cell proliferation and migration in the anterior forebrain, with special reference to persisting neurogenesis in the olfactory bulb. J Comp Neurol. 1969;137:433–57. doi: 10.1002/cne.901370404. [DOI] [PubMed] [Google Scholar]
- 19.Nottebohm F. The road we travelled: discovery, choreography, and significance of brain replaceable neurons. Ann NY Acad Sci. 2004;1016:628–58. doi: 10.1196/annals.1298.027. [DOI] [PubMed] [Google Scholar]
- 20.Goldman SA, Nottebohm F. Neuronal production, migration, and differentiation in a vocal control nucleus of the adult female canary brain. Proc Natl Acad Sci USA. 1983;80:2390–4. doi: 10.1073/pnas.80.8.2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Altman J, Das GD. Post-natal origin of microneurones in the rat brain. Nature. 1965;207:953–6. doi: 10.1038/207953a0. [DOI] [PubMed] [Google Scholar]
- 22.Gould E, Reeves AJ, Graziano MS, et al. Neurogenesis in the neocortex of adult primates. Science. 1999;286:548–52. doi: 10.1126/science.286.5439.548. [DOI] [PubMed] [Google Scholar]
- 23.Kornack DR, Rakic P. Continuation of neurogenesis in the hippocampus of the adult macaque monkey. Proc Natl Acad Sci USA. 1999;96:5768–73. doi: 10.1073/pnas.96.10.5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eriksson PS, Perfilieva E, Bjork-Eriksson T, et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–7. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 25.Alvarez-Buylla A, Lim DA. For the long run: maintaining germinal niches in the adult brain. Neuron. 2004;41:683–6. doi: 10.1016/s0896-6273(04)00111-4. [DOI] [PubMed] [Google Scholar]
- 26.Seri B, Garcia-Verdugo JM, McEwen BS, et al. Astrocytes give rise to new neurons in the adult mammalian hippocampus. J Neurosci. 2001;21:7153–60. doi: 10.1523/JNEUROSCI.21-18-07153.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kempermann G, Jessberger S, Steiner B, et al. Milestones of neuronal development in the adult hippocampus. Trends Neurosci. 2004;27:447–52. doi: 10.1016/j.tins.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 28.Cameron HA, McKay RD. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J Comp Neurol. 2001;435:406–60. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- 29.Stanfield BB, Trice JE. Evidence that granule cells generated in the dentate gyrus of adult rats extend axonal projections. Exp Brain Res. 1988;72:399–406. doi: 10.1007/BF00250261. [DOI] [PubMed] [Google Scholar]
- 30.Zhao C, Teng EM, Summers RG, Jr, et al. Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J Neurosci. 2006;26:3–11. doi: 10.1523/JNEUROSCI.3648-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Markakis EA, Gage FH. Adult-generated neurons in the dentate gyrus send axonal projections to field CA3 and are surrounded by synaptic vesicles. J Comp Neurol. 1999;406:449–60. [PubMed] [Google Scholar]
- 32.Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16:2027–33. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tanapat P, Hastings NB, Reeves AJ, et al. Estrogen stimulates a transient increase in the number of new neurons in the dentate gyrus of the adult female rat. J Neurosci. 1999;19:5792–801. doi: 10.1523/JNEUROSCI.19-14-05792.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cameron HA, Gould E. Adult neurogenesis is regulated by adrenal steroids in the dentate gyrus. Neuroscience. 1994;61:203–9. doi: 10.1016/0306-4522(94)90224-0. [DOI] [PubMed] [Google Scholar]
- 35.Cameron HA, McKay RD. Restoring production of hippocampal neurons in old age. Nat Neurosci. 1999;2:894–7. doi: 10.1038/13197. [DOI] [PubMed] [Google Scholar]
- 36.Mirescu C, Peters JD, Gould E. Early life experience alters response of adult neurogenesis to stress. Nat Neurosci. 2004;7:841–6. doi: 10.1038/nn1290. [DOI] [PubMed] [Google Scholar]
- 37.Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–5. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- 38.van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2:266–70. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- 39.Parent JM. Injury-induced neurogenesis in the adult mammalian brain. Neuroscientist. 2003;9:261–72. doi: 10.1177/1073858403252680. [DOI] [PubMed] [Google Scholar]
- 40.Kokaia Z, Lindvall O. Neurogenesis after ischaemic brain insults. Curr Opin Neurobiol. 2003;13:127–32. doi: 10.1016/s0959-4388(03)00017-5. [DOI] [PubMed] [Google Scholar]
- 41.Ambrogini P, Cuppini R, Cuppini C, et al. Spatial learning affects immature granule cell survival in adult rat dentate gyrus. Neurosci Lett. 2000;286:21–4. doi: 10.1016/s0304-3940(00)01074-0. [DOI] [PubMed] [Google Scholar]
- 42.Hairston IS, Little MT, Scanlon MD, et al. Sleep restriction suppresses neurogenesis induced by hippocampus-dependent learning. J Neurophysiol. 2005;94:4224–33. doi: 10.1152/jn.00218.2005. [DOI] [PubMed] [Google Scholar]
- 43.Rola R, Raber J, Rizk A, et al. Radiation-induced impairment of hippocampal neurogenesis is associated with cognitive deficits in young mice. Exp Neurol. 2004;188:316–30. doi: 10.1016/j.expneurol.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 44.Gould E, Beylin A, Tanapat P, et al. Learning enhances adult neurogenesis in the hippocampal formation. Nat Neurosci. 1999;2:260–5. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- 45.Merrill DA, Karim R, Darraq M, et al. Hippocampal cell genesis does not correlate with spatial learning ability in aged rats. J Comp Neurol. 2003;459:201–7. doi: 10.1002/cne.10616. [DOI] [PubMed] [Google Scholar]
- 46.Ehninger D, Kempermann G. Paradoxical effects of learning the Morris water maze on adult hippocampal neurogenesis in mice may be explained by a combination of stress and physical activity. Genes Brain Behav. 2006;5:29–39. doi: 10.1111/j.1601-183X.2005.00129.x. [DOI] [PubMed] [Google Scholar]
- 47.Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–9. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 48.Bruel-Jungerman E, Davis S, Rampon C, et al. Long-term potentiation enhances neurogenesis in the adult dentate gyrus. J Neurosci. 2006;26:5888–93. doi: 10.1523/JNEUROSCI.0782-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barnea A, Nottebohm F. Recruitment and replacement of hippocampal neurons in young and adult chickadees: an addition to the theory of hippocampal learning. Proc Natl Acad Sci USA. 1996;93:714–8. doi: 10.1073/pnas.93.2.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kempermann G, Gage FH. Genetic influence on phenotypic differentiation in adult hippocampal neurogenesis. Brain Res Dev Brain Res. 2002;134:1–12. doi: 10.1016/s0165-3806(01)00224-3. [DOI] [PubMed] [Google Scholar]
- 51.Kempermann G, Kuhn HG, Gage FH. Genetic influence on neurogenesis in the dentate gyrus of adult mice. Proc Natl Acad Sci USA. 1997;94:10409–14. doi: 10.1073/pnas.94.19.10409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kempermann G, Brandon EP, Gage FH. Environmental stimulation of 129/SvJ mice causes increased cell proliferation and neurogenesis in the adult dentate gyrus. Curr Biol. 1998;8:939–42. doi: 10.1016/s0960-9822(07)00377-6. [DOI] [PubMed] [Google Scholar]
- 53.Kempermann G, Gage FH. Genetic determinants of adult hippocampal neurogenesis correlate with acquisition, but not probe trial performance, in the water maze task. Eur J Neurosci. 2002;16:129–36. doi: 10.1046/j.1460-9568.2002.02042.x. [DOI] [PubMed] [Google Scholar]
- 54.Bizon JL, Gallagher M. Production of new cells in the rat dentate gyrus over the lifespan: relation to cognitive decline. Eur J Neurosci. 2003;18:215–9. doi: 10.1046/j.1460-9568.2003.02733.x. [DOI] [PubMed] [Google Scholar]
- 55.Drapeau E, Mayo W, Aurousseau C, et al. Spatial memory performances of aged rats in the water maze predict levels of hippocampal neurogenesis. Proc Natl Acad Sci USA. 2003;100:14385–90. doi: 10.1073/pnas.2334169100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–5. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- 57.Meshi D, Drew MR, Saxe M, et al. Hippocampal neurogenesis is not required for behavioral effects of environmental enrichment. Nat Neurosci. 2006;9:729–31. doi: 10.1038/nn1696. [DOI] [PubMed] [Google Scholar]
- 58.van Praag H, Christie BR, Sejnowski TJ, et al. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci USA. 1999;96:13427–31. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bruel-Jungerman E, Laroche S, Rampon C. New neurons in the dentate gyrus are involved in the expression of enhanced long-term memory following environmental enrichment. Eur J Neurosci. 2005;21:513–21. doi: 10.1111/j.1460-9568.2005.03875.x. [DOI] [PubMed] [Google Scholar]
- 60.Shors TJ, Miesegaes G, Beylin A, et al. Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001;410:372–6. doi: 10.1038/35066584. [DOI] [PubMed] [Google Scholar]
- 61.Shors TJ, Townsend DA, Zhao M, et al. Neurogenesis may relate to some but not all types of hippocampal-dependent learning. Hippocampus. 2002;12:578–84. doi: 10.1002/hipo.10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Peissner W, Kocher M, Treuer H, et al. Ionizing radiation-induced apoptosis of proliferating stem cells in the dentate gyrus of the adult rat hippocampus. Brain Res Mol Brain Res. 1999;71:61–8. doi: 10.1016/s0169-328x(99)00170-9. [DOI] [PubMed] [Google Scholar]
- 63.Tada E, Parent JM, Lowenstein DH, et al. X-irradiation causes a prolonged reduction in cell proliferation in the dentate gyrus of adult rats. Neuroscience. 2000;99:33–41. doi: 10.1016/s0306-4522(00)00151-2. [DOI] [PubMed] [Google Scholar]
- 64.Madsen TM, Kristjansen PE, Bolwig TG, et al. Arrested neuronal proliferation and impaired hippocampal function following fractionated brain irradiation in the adult rat. Neuroscience. 2003;119:635–42. doi: 10.1016/s0306-4522(03)00199-4. [DOI] [PubMed] [Google Scholar]
- 65.Raber J, Rola R, LeFevour A, et al. Radiation-induced cognitive impairments are associated with changes in indicators of hippocampal neurogenesis. Radiat Res. 2004;162:39–47. doi: 10.1667/rr3206. [DOI] [PubMed] [Google Scholar]
- 66.Winocur G, Wojtowicz JM, Sekeres M, et al. Inhibition of neurogenesis interferes with hippocampus-dependent memory function. Hippocampus. 2006;16:296–304. doi: 10.1002/hipo.20163. [DOI] [PubMed] [Google Scholar]
- 67.Monje ML, Mizumatsu S, Fike JR, et al. Irradiation induces neural precursor-cell dysfunction. Nat Med. 2002;8:955–62. doi: 10.1038/nm749. [DOI] [PubMed] [Google Scholar]
- 68.Snyder JS, Hong NS, McDonald RJ, et al. A role for adult neurogenesis in spatial long-term memory. Neuroscience. 2005;130:843–52. doi: 10.1016/j.neuroscience.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 69.Dupret D, Montaron MF, Drapeau E, et al. Methylazoxymethanol acetate does not fully block cell genesis in the young and aged dentate gyrus. Eur J Neurosci. 2005;22:778–83. doi: 10.1111/j.1460-9568.2005.04262.x. [DOI] [PubMed] [Google Scholar]
- 70.Cao L, Jiao X, Zuzga DS, et al. VEGF links hippocampal activity with neurogenesis, learning and memory. Nat Genet. 2004;36:827–35. doi: 10.1038/ng1395. [DOI] [PubMed] [Google Scholar]
- 71.Zhao X, Ueba T, Christie BR, et al. Mice lacking methyl-CpG binding protein 1 have deficits in adult neurogenesis and hippocampal function. Proc Natl Acad Sci USA. 2003;100:6777–82. doi: 10.1073/pnas.1131928100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Feng R, Rampon C, Tang YP, et al. Deficient neurogenesis in forebrain-specific presenilin-1 knockout mice is associated with reduced clearance of hippocampal memory traces. Neuron. 2001;32:911–26. doi: 10.1016/s0896-6273(01)00523-2. [DOI] [PubMed] [Google Scholar]
- 73.Schmidt-Hieber C, Jonas P, Bischofberger J. Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature. 2004;429:184–7. doi: 10.1038/nature02553. [DOI] [PubMed] [Google Scholar]
- 74.Wang S, Scott BW, Wojtowicz JM. Heterogenous properties of dentate granule neurons in the adult rat. J Neurobiol. 2000;42:248–57. [PubMed] [Google Scholar]
- 75.Farmer J, Zhao X, van Praag H, et al. Effects of voluntary exercise on synaptic plasticity and gene expression in the dentate gyrus of adult male Sprague-Dawley rats in vivo. Neuroscience. 2004;124:71–9. doi: 10.1016/j.neuroscience.2003.09.029. [DOI] [PubMed] [Google Scholar]
- 76.Shimazu K, Zhao M, Sakata K, et al. NT-3 facilitates hippocampal plasticity and learning and memory by regulating neurogenesis. Learn Mem. 2006;13:307–15. doi: 10.1101/lm.76006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Snyder JS, Kee N, Wojtowicz JM. Effects of adult neurogenesis on synaptic plasticity in the rat dentate gyrus. J Neurophysiol. 2001;85:2423–31. doi: 10.1152/jn.2001.85.6.2423. [DOI] [PubMed] [Google Scholar]
- 78.Dobrossy MD, Drapeau E, Aurousseau C, et al. Differential effects of learning on neurogenesis: learning increases or decreases the number of newly born cells depending on their birth date. Mol Psychiatry. 2003;8:974–82. doi: 10.1038/sj.mp.4001419. [DOI] [PubMed] [Google Scholar]
- 79.Leuner B, Mendolia-Loffredo S, Kozorovitskiy Y, et al. Learning enhances the survival of new neurons beyond the time when the hippocampus is required for memory. J Neurosci. 2004;24:7477–81. doi: 10.1523/JNEUROSCI.0204-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Olariu A, Cleaver KM, Shore LE, et al. A natural form of learning can increase and decrease the survival of new neurons in the dentate gyrus. Hippocampus. 2005;15:750–62. doi: 10.1002/hipo.20097. [DOI] [PubMed] [Google Scholar]
- 81.Pham K, McEwen BS, Ledoux JE, et al. Fear learning transiently impairs hippocampal cell proliferation. Neuroscience. 2005;130:17–24. doi: 10.1016/j.neuroscience.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 82.Lemaire V, Koehl M, Le Moal M, et al. Prenatal stress produces learning deficits associated with an inhibition of neurogenesis in the hippocampus. Proc Natl Acad Sci USA. 2000;97:11032–7. doi: 10.1073/pnas.97.20.11032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ziv Y, Ron N, Butovsky O, et al. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat Neurosci. 2006;9:268–75. doi: 10.1038/nn1629. [DOI] [PubMed] [Google Scholar]