Abstract
Background: Studies have shown that influenza vaccination during pregnancy reduces the risk of influenza disease in pregnant women and their offspring. Some have proposed that maternal vaccination may also have beneficial effects on birth outcomes. In 2014, we conducted an observational study to test this hypothesis using data from two large hospitals in Managua, Nicaragua.
Methods: We conducted a retrospective cohort study to evaluate associations between influenza vaccination and birth outcomes. We carried out interviews and reviewed medical records post-partum to collect data on demographics, influenza vaccination during pregnancy, birth outcomes and other risk factors associated with adverse neonatal outcomes. We used influenza surveillance data to adjust for timing of influenza circulation. We assessed self-reports of influenza vaccination status by further reviewing medical records of those who self-reported but did not have readily available evidence of vaccination status. We performed multiple logistic regression (MLR) and propensity score matching (PSM).
Results: A total of 3268 women were included in the final analysis. Of these, 55% had received influenza vaccination in 2014. Overall, we did not observe statistically significant associations between influenza vaccination and birth outcomes after adjusting for risk factors, with either MLR or PSM. With PSM, after adjusting for risk factors, we observed protective associations between influenza vaccination in the second and third trimester and preterm birth (aOR: 0.87; 95% confidence interval (CI): 0.75–0.99 and aOR: 0.66; 95% CI: 0.45–0.96, respectively) and between influenza vaccination in the second trimester and low birth weight (aOR: 0.80; 95% CI: 0.64–0.97).
Conclusions: We found evidence to support an association between influenza vaccination and birth outcomes by trimester of receipt with data from an urban population in Nicaragua. The study had significant selection and recall biases. Prospective studies are needed to minimize these biases.
Keywords: Influenza vaccination, Pregnant women, Birth outcomes, Small for gestational age, Preterm birth, Low birth weight
1. Introduction
Infection with influenza during pregnancy and its association with adverse birth outcomes has been documented since the early 1900s [1], [2]. For instance, infection with influenza virus during pregnancy may prompt an inflammatory [cascade] response that is associated with preterm birth [3], [4]. Influenza vaccination has been shown to reduce the risk of influenza virus disease and its complications among pregnant women and their infants [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15]. Furthermore, some studies have found a beneficial effect of influenza vaccination on birth outcomes, specifically a decreased likelihood of delivering a baby preterm or small for gestational age [16], [17], [18], [19], [20], [21], [22]. Other studies, however, have found no evidence to support this hypothesis [23], [24], [25], [26], [27], [28], [29], [30]. Published studies have differed substantially from one another in design and in ability to account for variations in circulating influenza viruses, vaccine components, target population, and place of study, making cross-study comparisons difficult [31].
Historic influenza surveillance data from Nicaragua shows that influenza circulates between June and November [32]. Since 2007, the Nicaraguan Government has provided influenza vaccination to women with high-risk pregnancies in the month of May, following the vaccination schedule for countries with southern hemisphere circulation of influenza viruses [33], [34]. In May 2014, Nicaragua expanded influenza vaccination to all pregnant women in the country. With the aim of evaluating the hypothesis that influenza vaccination has a beneficial effect on birth outcomes, we collected birth outcome data, current influenza vaccination status, and risk factors for adverse pregnancy outcomes by interviewing post-partum women at two large hospitals in Managua.
2. Methods
2.1. Survey
2.1.1. Sample size calculation
Sample size planning was informed by calculating sample needs in order to detect a 40% reduction of low birth weight among newborns of vaccinated (regardless of vaccination trimester) compared to unvaccinated mothers [20], [21], assuming a two-sided 95% confidence level, 80% power, and a ratio of unvaccinated to vaccinated of 2:5 [33]. A design effect of 1.5 was considered to account for any potential biases. The sample size required was estimated to be 2769 (1938 vaccinated and 831 unvaccinated pregnant women).
2.1.2. Inclusion criteria
We selected two large public hospitals, Hospital Aleman Nicaragüense and Hospital Bertha Calderon Roque. Inclusion criteria were being a resident of the Department of Managua and having a singleton birth. Exclusion criteria were delivering a stillborn or newborn with congenital or neonatal anomalies, receiving influenza vaccination before May 1st, 2014 -the start date for the national influenza vaccination campaign [32]- or less than 14 days before delivery, and having a gestational age <28 or >42 weeks at delivery which are the viability and post-term cut-offs, respectively [35], [36]. The study was designed as an observational study where a cohort of women who provided informed consent were interviewed between July 21st and December 4th 2014, within 48 h after delivery. Participants were selected in a convenience manner.
2.1.3. Questionnaire
The questionnaire included demographic information (age, race, living environment, education level, type of fuel used for cooking, number of persons in the household, delivery hospital), antenatal care (number of antenatal visits, vaccinations received during pregnancy, consumption of antenatal supplements), presence of medical conditions prior to pregnancy (obesity, diabetes, asthma, renal disease, liver disease, blood disease, neurologic disease), any complications during pregnancy (hospitalization for any specific complication such as preeclampsia, eclampsia, hemorrhage, sepsis, urinary infection, diabetes, severe acute respiratory infection, or influenza-like illness), other risk factors for adverse pregnancy outcomes (alcohol consumption and smoking before and during pregnancy), obstetric characteristics of the mother (number of parturitions, number of abortions, number of livebirths and stillbirths, type of delivery), and characteristics of the offspring (sex, weight, gestational age at delivery). Pregnancy and birth outcome data were obtained from antenatal and hospital medical records and included date of last menstrual period (LMP), gestational age calculated by ultrasound when LMP was not available from antenatal medical records, delivery date, and birth weight. Influenza vaccination was self-reported; however, interviewers validated this information using vaccination cards and/or antenatal medical records if available.
2.1.4. Assessing self-reported influenza vaccination
We assessed the validity of self-reporting influenza vaccination or non-vaccination through random sampling of the self-reported vaccinated and unvaccinated group. Sample size of the random sample, per group, was calculated using the formula to estimate a proportion assuming that 90% of those who self-reported being vaccinated or unvaccinated status were truly vaccinated or unvaccinated, respectively [37]. The sample size in each group was 123 and 128 for the vaccinated and unvaccinated groups, respectively. For each selected mother, we reviewed vaccination records of the Expanded Program on Immunization at the Health Unit where the mother received antenatal care and/or we contacted the mother and documented the vaccination date directly from the vaccination card (during a home visit) or as reported by the mother reading from the card (by telephone); influenza vaccination was considered confirmed if an exact date for vaccination could be provided by any of these methods. Likewise, non-vaccination status was corroborated if no information on vaccination including specific date for vaccination was identified from these sources. The positive predictive value (PPV) and negative predictive value (NPV) of self-reporting vaccination and non-vaccination status were then calculated. Out of all participants randomly selected for this assessment who self-reported receiving influenza vaccination (n = 123), 61% (75) had evidence of vaccination. Of the total (n = 128) of participants who self-reported not receiving influenza vaccination, only 2 (2%), had evidence of influenza vaccination. Thus, PPV was 61% (75/123) for self-reporting receiving influenza vaccination, and NPV was 98% (126/128) for self-reports of being unvaccinated. Due to the low PPV and high NPV, we excluded from further analyses women who self-reported vaccination status but did not present evidence of vaccination, and we included all participants who self-reported non-vaccination status.
2.2. Data analysis
2.2.1. Variable definitions
Age was categorized into three groups: <18, 18–34 and ≥35 years old. A proxy variable was calculated for cumulative influenza exposure using influenza surveillance data from Nicaragua [38]. This variable was calculated in stages, first by dividing the number of positive influenza samples by the total number of samples tested per week from the surveillance data and then assigning the cumulative sum of the influenza positive proportions per week to the weeks that women were pregnant; we dichotomized this variable by high or low exposure using the median cumulative influenza exposure as a cutoff. Birth outcome data were defined and calculated as follows: (1) gestational age at delivery was calculated using LMP, if available, or gestational age at any ultrasound obtained from antenatal medical records (see Supplementary material A1 for gestational age calculation algorithm); (2) small for gestational age (SGA) was calculated from weight and gestational age at delivery according to the International Fetal and Newborn Growth Consortium for the 21st Century, or intergrowth-21st standards [36]; (3) preterm birth (PTB) was defined as born with <37 weeks of gestational age; and (4) low birth weight (LBW) was defined as born weighing <2500 g.
2.3. Statistical analysis
We compared all variables by vaccination status using chi-square test to compare differences between vaccinated and unvaccinated women regardless of trimester of receipt of influenza vaccination. We performed multivariable logistic regression (MLR) analyses per birth outcome (SGA, PTB and LBW) as the variable of interest and influenza vaccination as the exposure variable. We used the Akaike Information criterion (AIC) to identify the most parsimonious model. In addition, we performed propensity score matching (PSM) to control for the individual differences in probability of being vaccinated. Variables included in the PSM model that could explain probability of receiving vaccination were selected a priori (age, race, education level, number of antenatal visits, reporting at least one chronic condition (obesity, diabetes, asthma, renal disease, liver disease, blood disease, neurologic disease) and because of a crude association with vaccination and/or birth outcomes (type of fuel used for cooking, number of people in the household, reporting receiving at least one tetanus vaccine, iron and vitamin B12 consumption during pregnancy, albendazole consumption during pregnancy, calcium consumption during pregnancy, aspirin consumption during pregnancy, alcohol consumption before pregnancy, body mass index (BMI) calculated from height and weight measurements taken at first antenatal visit, number of parturitions). For PSM, we used the nearest neighbor approach that requires one-to-two vaccinated-to-unvaccinated proportion for good propensity score matching [39]. When the requirement was not met, we randomly sampled from the vaccinated cases to obtain an unvaccinated to vaccinated ratio greater than one. We repeated the random sample selection a thousand times; point estimate and lower and upper 95% confidence intervals represent 50th, 2.5th and 97.5th percentiles, respectively [40]. PSM matched one vaccinated case to one unvaccinated case for the probability of vaccination. After PSM, we evaluated these variables for significant differences between the vaccination status groups in order to know how well matching occurred. We then evaluated the association of influenza vaccination and birth outcomes by MLR models, including variables measured after-vaccination: delivery hospital, hospitalization during pregnancy for any specific complication (preeclampsia, eclampsia, hemorrhage, sepsis, urinary infection, diabetes, severe acute respiratory infection), reporting at least one episode of influenza-like illness during pregnancy (ILI), alcohol consumption before pregnancy, number of parturitions, sex of the baby, and influenza circulation.
As a secondary investigation, we assessed the association of influenza vaccination and birth outcomes by trimester in which women received influenza vaccination; the unvaccinated group was used as a control for all models. We present adjusted odds ratios (aORs), as well as 95% confidence intervals and p-values. As a sensitivity analysis, we repeated the analyses including all women who self-reported vaccination, regardless of proof of evidence. All analyses were performed in R software (R version 3.2.2).
2.3.1. Ethics statement
The Institutional Review Board at the Nicaraguan Ministry of Health reviewed and approved the protocol. The protocol was also reviewed at the Centers for Disease Control and Prevention and was determined to be public health practice-program evaluation.
3. Results
3.1. Descriptive analysis
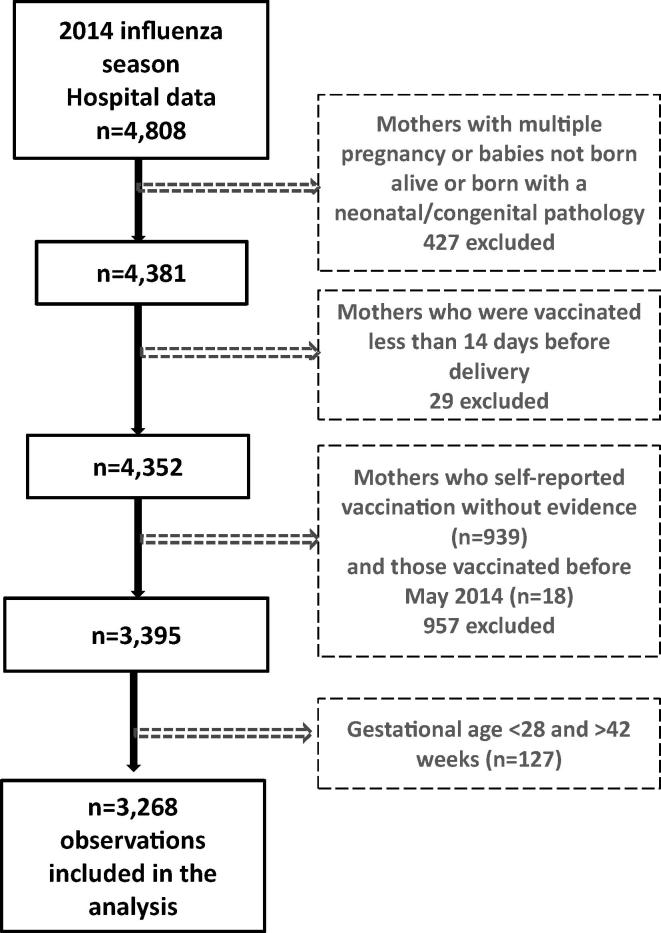
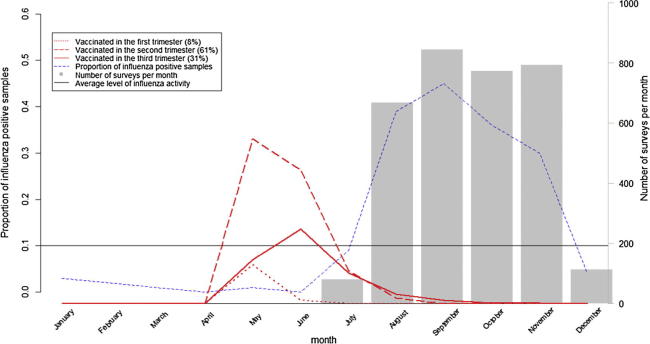
From July 21st through December 4th 2014, we interviewed and collected surveys on 4808 post-partum women. After exclusions, a final sample of 3268 women was included in the analysis (Fig. 1). Fig. 2 shows timing of influenza vaccination (Trivalent inactivated influenza vaccine, southern hemisphere formulation) with respect to influenza circulation in the country; most participants (96%) received influenza vaccination before the peak of influenza circulation in August through November 2014.
Fig. 1.
Exclusion criteria and data cleaning algorithm.
Fig. 2.
Number of surveyed women at delivery, number of pregnant women vaccinated for month stratified by trimester of vaccination, and proportion of influenza positive samples from routine surveillance*, Nicaragua 2014 *Proportions of influenza positive samples from routine surveillance correspond to year 2014. Average level of influenza activity corresponds to years 2011–2013 (Nicaragua, national influenza surveillance data, Severe Acute Respiratory Infections network, SARInet, PAHO).
Of the 3268 women included in the analysis, 55% (1789) had documented influenza vaccination in 2014. Of those, 8% (137) were vaccinated in their first trimester, 61% (1093) in their second trimester, and 31% (559) in their third trimester.
Overall, 13% (415) were younger than 18 years (range: 12–17), 80% (2620) were between 18 and 34 years, and 7% (233) were 35 years and older (range: 35–45; Table 1). Ninety-seven percent (3172) reported having at least one antenatal visit during pregnancy, 78% (2549) reported four or more antenatal visits, and 91% (2984) reported having at least one dose of tetanus vaccine; all of these antenatal care indicators were significantly more common among vaccinated compared to unvaccinated women.
Table 1.
Selected characteristics of participant pregnant women (n = 3268), post-partum interview, July–December 2014, Managua Province, Nicaragua.
| All | Vaccinated | Unvaccinated | p-value† | |
|---|---|---|---|---|
| n = 3268 | n = 1789 | n = 1479 | ||
| n (%) | n (%) | n (%) | ||
| Age | ||||
| <18 years | 415 (13) | 232 (13) | 183 (12) | 0.80 |
| 18–34 years | 2620 (80) | 1433 (80) | 1187 (80) | |
| ≥35 years | 233 (7) | 124 (7) | 109 (7) | |
| Number of persons in household | ||||
| 2–4 | 898 (27) | 528 (30) | 370 (25) | 0.02 |
| 5–6 | 1070 (33) | 580 (32) | 490 (33) | |
| 7–8 | 707 (22) | 381 (21) | 326 (22) | |
| >8 | 593 (18) | 300 (17) | 293 (20) | |
| Delivered at reference*obstetric hospital | 1722 (53) | 1083 (61) | 639 (43) | <0.01 |
| Education level | ||||
| No education/primary school incomplete | 421 (13) | 231 (13) | 190 (13) | 0.01 |
| Primary school complete | 337 (10) | 195 (11) | 142 (10) | |
| Secondary school incomplete | 1372 (42) | 771 (43) | 601 (41) | |
| Secondary school complete | 637 (19) | 353 (20) | 284 (19) | |
| Technical/university studies | 501 (15) | 239 (13) | 262 (18) | |
| Received at least one dose of tetanus vaccine | 2984 (91) | 1759 (98) | 1225 (83) | <0.01 |
| At least one antenatal visits | 3172 (97) | 1786 (100) | 1386 (94) | <0.01 |
| Four or more antenatal visits | 2549 (78) | 1542 (86) | 1007 (68) | <0.01 |
| Gestational age at first antenatal visit (median, quantiles in weeks) | 12 (9, 19) | 12 (9, 17) | 13 (9, 22) | <0.01‡ |
| Consumption of iron and folic acid | 1485 (45) | 805 (45) | 680 (46) | 0.59 |
| Consumption of albendazol | 703 (22) | 482 (27) | 221 (15) | <0.01 |
| Consumption of calcium | 1243 (38) | 722 (40) | 521 (35) | <0.01 |
| Consumption of aspirin | 938 (29) | 600 (34) | 338 (23) | <0.01 |
| BMI (height and weight measured at 1st antenatal visit) | ||||
| Underweight (<18.5) | 315 (10) | 116 (6) | 199 (13) | <0.01 |
| Normal weight (18.5–24.9) | 1338 (41) | 808 (45) | 530 (36) | |
| Overweight (25–29.9) | 928 (28) | 510 (29) | 418 (28) | |
| Obesity I (30–34.9) | 408 (12) | 224 (13) | 184 (12) | |
| Obesity II (35–39.9) | 163 (5) | 87 (5) | 76 (5) | |
| Extreme obesity (>40) | 88 (3) | 41 (2) | 47 (3) | |
| Alcohol consumption before pregnancy | 441 (13) | 220 (12) | 221 (15) | 0.05 |
| Renal Disease | 1266 (39) | 734 (41) | 532 (36) | <0.01 |
| Blood Disease | 743 (23) | 445 (25) | 298 (20) | <0.01 |
| At least one chronic condition** | 1855 (57) | 1069 (60) | 786 (53) | <0.01 |
| Number of parturitions | ||||
| 1 | 1519 (46) | 879 (49) | 640 (43) | <0.01 |
| 2–3 | 1447 (44) | 768 (43) | 679 (46) | |
| >3 | 302 (9) | 142 (8) | 160 (11) | |
| Female | 1585 (49) | 804 (45) | 781 (53) | <0.01 |
| Preterm birth | 175 (5) | 95 (5) | 80 (5) | 0.96 |
| Small for gestational age | 343 (10) | 198 (11) | 145 (10) | 0.26 |
| Low birth weight | 195 (6) | 99 (6) | 96 (6) | 0.28 |
X2 test.
Wilcoxon test.
Reference obstetric hospital in Nicaragua: Hospital Bertha Calderon Roque.
At least one chronic condition of the following: obesity, diabetes, asthma, renal disease, liver disease, blood disease, neurologic disease.
More than half of the surveyed women (57%) reported having at least one chronic condition (obesity, diabetes, asthma, renal disease, liver disease, blood disease, neurologic disease); conditions were more common among vaccinated (60%) than unvaccinated women (53%) (p-value < 0.01). Overall, for approximately half of the participants (46%) the baby included in the study was their first; however, being a first-time mother was more frequent among those vaccinated than unvaccinated (49% vs. 43%, respectively). Finally, adverse birth outcomes were infrequent among the participants and did not differ by vaccination status (p-value > 0.05); the percentage with preterm birth was 5%, small for gestational age was 10% and low birth weight was 6% (Table 1). However, only among participants vaccinated in the first trimester, the percentage with preterm birth was 12%, significantly higher than the unvaccinated group (6%; p-value < 0.05; Supplementary material A2).
3.2. Regression analysis
After adjusting for risk factors, we did not find an association between receiving influenza vaccination and birth outcomes with either of the two different analytical approaches. With the MLR models, the adjusted ORs and their respective 95% CIs for SGA, PTB, and LWB were 1.03 (0.82–1.31), 0.95 (0.75–1.20), and 0.89 (0.66–1.22). We obtained similar results with PSM (Table 2).
Table 2.
Evaluation of the association of influenza vaccination and birth outcomes among pregnant women by trimester of vaccination (n = 3268), post-partum interview, July–December 2014, Managua Province, Nicaragua.
| Birth outcomes | Influenza vaccine 2014 season | Influenza vaccine 2014 season | ||
|---|---|---|---|---|
| Multiple Logistic Regression (MLR) | Propensity score Model (PSM)† + MLRǂ n = 1600 × 1000 simulations¥ | |||
| aOR (95% CI) | aOR (95% CI) | |||
| Small for gestational age (SGAi) | 1.03* | (0.82; 1.31) | 1.02 | (0.83; 1.24) |
| Preterm birth (PTB) | 0.95** | (0.75; 1.20) | 0.88 | (0.73; 1.06) |
| Low birth weight (LBW) | 0.89*** | (0.66; 1.22) | 0.83 | (0.62; 1.06) |
aOR = Adjusted Odds Ratio.
Model adjusted for reporting at least one chronic condition (obesity, diabetes, asthma, renal disease, liver disease, blood disease, neurologic disease), age, race, calcium, iron, and folic acid consumption during pregnancy, education level, number of parturitions, body mass index (BMI) at first antenatal visit, and sex of the baby.
Model adjusted for reporting at least one chronic condition (obesity, diabetes, asthma, renal disease, liver disease, blood disease, neurologic disease), age, albendazol and aspirin consumption during pregnancy, hospitalization during pregnancy for any specific complication (preeclampsia, eclampsia, hemorrhage, sepsis, urinary infection, diabetes, severe acute respiratory infection), delivery hospital, number of parturitions, body mass index (BMI) at first antenatal visit, alcohol consumption before pregnancy, number of antenatal visits, and sex of the baby.
Model adjusted for delivery hospital, number of parturitions, education level, alcohol consumption before pregnancy, hospitalization during pregnancy for any specific complication (preeclampsia, eclampsia, hemorrhage, sepsis, urinary infection, diabetes, severe acute respiratory infection), body mass index (BMI) at first antenatal visit, sex of the baby, and number of antenatal visits.
Propensity Score Models adjusted for age, race, education level, type of fuel used for cooking, number of people in the household, reporting receiving at least one tetanus vaccine, number of antenatal visits, Fe and vitamin B12 consumption during pregnancy, albendazol consumption during pregnancy, calcium consumption during pregnancy, aspirin consumption during pregnancy, alcohol consumption before pregnancy, body mass index (BMI) at first antenatal visit, number of parturitions, and reporting at least one chronic condition (obesity, diabetes, asthma, renal disease, liver disease, blood disease, neurologic disease).
Models adjusted for delivery hospital, hospitalization during pregnancy for any specific complication (preeclampsia, eclampsia, hemorrhage, sepsis, urinary infection, diabetes, severe acute respiratory infection), reporting at least one episode of influenza-like illness during pregnancy (ILI), alcohol consumption before pregnancy, number of parturitions, sex of the baby, and influenza circulation.
Based on 1000 simulations when requirement one-to-two vaccinated-to-unvaccinated proportion for good propensity score matching was not met; point estimate and lower and upper confidence interval represent 50th, 25th and 97.5th percentiles of point estimates (n = 1000), respectively.
Table 3, Table 4 present results by trimester of influenza vaccination for the MLR and PSM models, respectively. In both models, we observed a trend whereby the later in pregnancy a women received vaccination the lower her adjusted odds of PTB. This was not statistically significant in the MLR models. However, the protective effect of influenza vaccination on PTB among those receiving vaccination in their second and third trimester was significant with PSM (aOR: 0.87; 95% CI: 0.75–0.99 and aOR: 0.66; 95% CI: 0.45–0.96, respectively; Table 4). In addition, we found a protective effect of influenza vaccination on LBW among those vaccinated in the second trimester (aOR: 0.80; 95%CI: 0.64–0.97) using PSM. Further analyses including those who self-reported vaccination showed similar trends (see Supplementary materials A3-A5). No other significant associations between birth outcomes and vaccination by trimester were noted.
Table 3.
Evaluation of the association of influenza vaccination and birth outcomes among pregnant women by trimester of vaccination (n = 3268) with multiple logistic regression (MLR) method, post-partum interview, July–December 2014, Managua, Nicaragua.
| Birth outcomes | Influenza vaccine 2014 season |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Vaccinated in first trimester (n = 140) |
Vaccinated in second trimester (n = 1142) |
Vaccinated in third trimester (n = 581) |
|||||||
| aOR (95% CI) | p-value | aOR (95% CI) | p-value | aOR (95% CI) | p-value | ||||
| Small for gestational age* (SGAi) | 1.13 | (0.66; 1.94) | 0.66 | 1.03 | (0.79; 1.34) | 0.82 | 0.92 | (0.65; 1.30) | 0.64 |
| Preterm birth** (PTB) | 1.43 | (0.86; 2.39) | 0.17 | 0.91 | (0.70; 1.17) | 0.45 | 0.86 | (0.62; 1.18) | 0.35 |
| Low birth weight (LBW)*** | 1.56 | (0.81; 3.02) | 0.19 | 0.88 | (0.61; 1.26) | 0.48 | 0.85 | (0.55; 1.32) | 0.47 |
aOR = Adjusted Odds Ratio.
Models adjusted for race, education level, iron and folic acid consumption during pregnancy, number of parturitions, body mass index (BMI) at first antenatal visit, number of antenatal visits and sex of the baby for mothers vaccinated in the first trimester; age, race, education level, iron and folic acid consumption during pregnancy, number of parturitions, body mass index (BMI) at first antenatal visit, number of antenatal visits, number of people in the household and sex of the baby for mothers vaccinated in the second trimester; number of parturitions, body mass index (BMI) at first antenatal visit, reporting at least one chronic condition (obesity, diabetes, asthma, renal disease, liver disease, blood disease, neurologic disease) and sex of the baby for mothers vaccinated in the third trimester.
Models adjusted for alcohol consumption before pregnancy, BMI at first antenatal visit, number of antenatal visits, albendazol consumption during pregnancy, hospitalization during pregnancy for any specific complication (preeclampsia, eclampsia, hemorrhage, sepsis, urinary infection, diabetes, severe acute respiratory infection), delivery hospital, sex of the baby, type of fuel used for cooking, reporting receiving at least one tetanus vaccine, number of parturitions, education level, age, reporting at least one episode of influenza-like illness during pregnancy (ILI), race and reporting at least one chronic condition for mothers vaccinated in the first trimester; alcohol consumption before pregnancy, BMI at first antenatal visit, influenza circulation, aspirin consumption during pregnancy, hospitalization during pregnancy for any specific complication (preeclampsia, eclampsia, hemorrhage, sepsis, urinary infection, diabetes, severe acute respiratory infection), delivery hospital, and, number of antenatal visits for mothers vaccinated in the second trimester; alcohol consumption before pregnancy, BMI at first antenatal visit, number of antenatal visits, albendazol consumption during pregnancy, hospitalization during pregnancy for any specific complication (preeclampsia, eclampsia, hemorrhage, sepsis, urinary infection, diabetes, severe acute respiratory infection), delivery hospital, sex of the baby, race and reporting at least one chronic condition for mothers vaccinated in the third trimester.
Models adjusted for alcohol consumption before pregnancy, BMI at first antenatal visit, number of antenatal visits, hospitalization during pregnancy for any specific complication (preeclampsia, eclampsia, hemorrhage, sepsis, urinary infection, diabetes, severe acute respiratory infection), delivery hospital, sex of the baby, and ILI for mothers vaccinated in the first trimester; education level, alcohol consumption before pregnancy, BMI at first antenatal visit, number of antenatal visits, hospitalization during pregnancy for any specific complication (preeclampsia, eclampsia, hemorrhage, sepsis, urinary infection, diabetes, severe acute respiratory infection), delivery hospital, sex of the baby, number of parturitions, and ILI for mothers vaccinated in the second trimester; alcohol consumption before pregnancy, BMI at first antenatal visit, number of antenatal visits, hospitalization during pregnancy for any specific complication (preeclampsia, eclampsia, hemorrhage, sepsis, urinary infection, diabetes, severe acute respiratory infection), and sex of the baby for mothers vaccinated in the third trimester.
Table 4.
Evaluation of the association of influenza vaccination and birth outcomes among pregnant women by trimester of vaccination with propensity score matching (PSM) analysis*, post-partum interview, July–December 2014, Managua, Nicaragua.
| Birth outcomes | Influenza vaccine 2014 season |
|||||
|---|---|---|---|---|---|---|
| Vaccinated in first trimester (n = 280) | Vaccinated in second trimester (n = 1600) | Vaccinated in third trimester (n = 1162) | ||||
| aOR (95% CI) | aOR (95% CI) ×1000sim*** | aOR (95% CI) | ||||
| Small for gestational age (SGA) | 0.90 | (0.40; 2.03) | 1.00 | (0.86; 1.17) | 0.83 | (0.55; 1.25) |
| Preterm birth** (PTB) | 1.67 | (0.73; 3.83) | 0.87 | (0.75; 0.99) | 0.66 | (0.45; 0.96) |
| Low birth weight (LBW)** | 1.71 | (0.55; 5.26) | 0.80 | (0.64; 0.97) | 0.64 | (0.38; 1.09) |
aOR = Adjusted Odds Ratio.
Propensity Score Models adjusted for age, race, education level, type of fuel used for cooking, number of people in the household, reporting receiving at least one tetanus vaccine, number of antenatal visits, Fe and vitamin B12 consumption during pregnancy, albendazol consumption during pregnancy, calcium consumption during pregnancy, aspirin consumption during pregnancy, alcohol consumption before pregnancy, body mass index (BMI) at first antenatal visit, number of parturitions, and reporting at least one chronic condition (obesity, diabetes, asthma, renal disease, liver disease, blood disease, neurologic disease).
Models adjusted for delivery hospital, hospitalization during pregnancy for any specific complication (preeclampsia, eclampsia, hemorrhage, sepsis, urinary infection, diabetes, severe acute respiratory infection), reporting at least one episode of influenza-like illness during pregnancy (ILI), alcohol consumption before pregnancy, number of parturitions, sex of the baby, and influenza circulation.
Based on 1000 simulations when requirement one-to-two vaccinated-to-unvaccinated proportion for good propensity score matching was not met; point estimate and lower and upper confidence interval represent 50th, 25th and 97.5th percentiles of point estimates (n = 1000), respectively.
4. Discussion
The present study did not find an association between maternal influenza vaccination administered at any point in pregnancy and adverse birth outcomes among their babies. However, in secondary analyses stratifying influenza vaccination by trimester we observed a protective effect of vaccination on PTB in the second and third trimester and on LBW in the second trimester. Although there is a possibility that vaccination can explain these protective effects, observational studies have inherent biases that preclude them from establishing causality [41], [42].
Using PSM, we observed a protective effect of influenza vaccination on PTB and LBW among those vaccinated in the second and third trimester. Although not statistically significant, the MLR approach also trended in the same direction. Likely, the analysis with PSM corrected for biases related to the probability of influenza vaccination by controlling for differences between vaccinated and unvaccinated women that we were not able to control for in MLR [43]. Our findings are consistent with a number of studies that found an association between receiving influenza vaccination and reduced likelihood of delivering a baby PTB or with LBW [16], [17], [18], [19], [20], [21], [22]. In addition, a recent meta-analysis found a strong association between receipt of influenza vaccination and reduced likelihood of PTB and LBW, and the magnitude of the association that Nunes et al. reported was comparable to our findings [44].
On the other hand, numerous other studies report a lack of association between influenza vaccination and birth outcomes [23], [24], [25], [26], [27], [28], [29], [30]. Two of these studies used similar methods to our study. Nordin et al. analyzed the large Vaccine Safety Datalink dataset (n = 57,554 pregnant women in the United States) to assess these associations and after using propensity score to minimize bias introduced by the probability of being vaccinated found no association between influenza vaccination during pregnancy and risk of SGA or PTB [26]. In Finland, Baum et al. also did not find an association when analyzing retrospective data by trimester of vaccination and applying propensity score to account for the probability of vaccination; however, this study was performed during the 2009 pandemic so may not be directly comparable [29]. Overall, differences in study design, populations, influenza virus and circulation variability make it a challenge to compare results [31].
Observational studies like ours are subject to potential residual confounding, and Vazquez-Benitez et al. [43] demonstrated that not accounting for biases in observational data can mislead the results. In our study, in addition to social desirability or recall bias on key variables like chronic conditions, there may have been residual confounding as we did not collect information on other risk factors known to be associated with adverse neonatal outcomes, such as previous history of adverse birth outcomes, domestic violence, harsh working conditions, or exposure to pollutants or chemicals [45], [46], [47], [48], [49], [50]. In addition, we lacked specific information regarding the timing of antenatal supplements or health complications during pregnancy, and the literature shows that the timing of these events with respect to trimester of gestation could be associated with birth outcomes [51], [52].
We acknowledge the following limitations. First, from 2007–2013, the Government of Nicaragua provided influenza vaccination only for pregnant women with chronic conditions [34]. In 2013, the influenza vaccination recommendation was extended to all pregnant women in the Municipality of Managua, and in 2014, the Government of Nicaragua extended influenza vaccination to all pregnant women in the country. However, little is known about how well the recommendations were implemented. Indeed, vaccine uptake remained somewhat higher among pregnant women with pre-existing chronic medical conditions in our study year suggesting that the recommendation may have been slow to take effect. Higher vaccination among pregnant women with high-risk pregnancies could have caused a selection bias as those vaccinated women would have been at higher risk for adverse pregnancy outcomes compared to unvaccinated women. Second, the sample was not randomly selected. Women who agreed to participate in the study could have been different from women who did not participate in the study, and we did not collect characteristics on women who refused to participate in the study to ascertain this bias. Third, we did not power the sample size to analyze effects by trimester of vaccination and so may have missed important effects. Fourth, we based our calculation of gestational age at delivery on LMP which is known to be subject to recall bias and could have introduced errors in our calculations [53]. However, when LMP was determined to be unreliable, we calculated gestational age at delivery using data from an ultrasound. Approximately 6% of the gestational age estimates came from ultrasounds in the last trimester, which have higher rates of estimate error than estimates calculated in early pregnancy. Finally, we were not able to obtain information on previous pregnancy outcomes; previous history of PTB or abortion in the second trimester has been associated with an increased frequency of PTB [54].
Despite these limitations, the strengths of our study include having access to birth outcomes from medical records as well as having available influenza surveillance data. Studies suggest that the effect of influenza vaccination on birth outcomes may be better observed during high influenza circulation period, and there was a substantial influenza circulation in Nicaragua in 2014 [20], [21], [55]. Furthermore, the propensity score analyses minimized biases introduced by differences in baseline characteristics between unvaccinated and vaccinated pregnant women by accounting for the probability of vaccination based on these characteristics [43]. We were also able to perform sensitivity analysis for self-reported vaccination. Moreover, we used international standards to define the variable small for gestational age [36] and validated it with a World Health Organization calculator to estimate SGA [56]; results were consistent for both (data not shown).
In summary, we found some evidence of a protective effect of influenza vaccination by trimester on birth outcomes; however, we encourage further investigation by prioritizing prospective cohort designs over retrospective studies to minimize selection and recall biases. In addition, it may be helpful for countries to assess knowledge, attitudes and practices of influenza vaccination recommendations among health personnel, especially in countries where influenza vaccination recommendations and practices have recently been implemented or expanded.
Funding
This study was supported by the Centers for Disease Control and Prevention, United States (CDC) (Grant No. U51GH001191) and the Pan American Health Organization (PAHO) (NIC 15010973).
Disclaimer
The findings and conclusions in this publication are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention and the Pan American Health Organization (PAHO). The content of this article has not been previously presented.
Conflict of interest statement
The authors do not have an association that might pose a conflict of interest.
Acknowledgement
We would like to thank the Nicaraguan Ministry of Health, the Public Health Surveillance Directorate, the Expanded Program on Immunizations, the Local System of Primary Care in Managua (SILAIS Managua), members of the evaluation team; the participant hospitals in Managua, Hospital Bertha Calderón Roque and Hospital Alemán Nicaragüense; participant women who agreed to be interviewed; and Dr. Pablo Durán for his valuable insight.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.vaccine.2017.04.045.
Appendix A. Supplementary material
References
- 1.Harris J. Influenza occurring in pregnant women. JAMA. 1919:978–980. [Google Scholar]
- 2.Pierce M., Kurinczuk J.J., Spark P., Brocklehurst P., Knight M., Ukoss Perinatal outcomes after maternal 2009/H1N1 infection: national cohort study. BMJ. 2011;342:d3214. doi: 10.1136/bmj.d3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fell D.B., Bhutta Z.A., Hutcheon J.A., Karron R.A., Knight M., Kramer M.S. Report of the WHO technical consultation on the effect of maternal influenza and influenza vaccination on the developing fetus: Montreal, Canada, September 30-October 1, 2015. Vaccine. 2017;35:2279–2287. doi: 10.1016/j.vaccine.2017.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Romero R., Espinoza J., Goncalves L.F., Kusanovic J.P., Friel L., Hassan S. The role of inflammation and infection in preterm birth. Semin Reprod Med. 2007;25:21–39. doi: 10.1055/s-2006-956773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dodds L., McNeil SA., Fell DB., Allen VM., Coombs A., Scott J. Impact of influenza exposure on rates of hospital admissions and physician visits because of respiratory illness among pregnant women. CMAJ: Can Med Assoc J = journal de l'Association medicale canadienne. 2007;176:463–468. doi: 10.1503/cmaj.061435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eick A.A., Uyeki T.M., Klimov A., Hall H., Reid R., Santosham M. Maternal influenza vaccination and effect on influenza virus infection in young infants. Arch Pediatr Adolesc Med. 2011;165:104–111. doi: 10.1001/archpediatrics.2010.192. [DOI] [PubMed] [Google Scholar]
- 7.Irving W.L., James D.K., Stephenson T., Laing P., Jameson C., Oxford J.S. Influenza virus infection in the second and third trimesters of pregnancy: a clinical and seroepidemiological study. BJOG: Int J Obstet Gynaecol. 2000;107:1282–1289. doi: 10.1111/j.1471-0528.2000.tb11621.x. [DOI] [PubMed] [Google Scholar]
- 8.Lindsay L., Jackson L.A., Savitz D.A., Weber D.J., Koch G.G., Kong L. Community influenza activity and risk of acute influenza-like illness episodes among healthy unvaccinated pregnant and postpartum women. Am J Epidemiol. 2006;163:838–848. doi: 10.1093/aje/kwj095. [DOI] [PubMed] [Google Scholar]
- 9.Madhi S.A., Nunes M.C., Cutland C.L. Influenza vaccination of pregnant women and protection of their infants. New Engl J Med. 2014;371:2340. doi: 10.1056/NEJMc1412050. [DOI] [PubMed] [Google Scholar]
- 10.Meijer W.J., van Noortwijk A.G., Bruinse H.W., Wensing A.M. Influenza virus infection in pregnancy: a review. Acta Obstet Gynecol Scand. 2015;94:797–819. doi: 10.1111/aogs.12680. [DOI] [PubMed] [Google Scholar]
- 11.Thompson M.G., Li D.K., Shifflett P., Sokolow L.Z., Ferber J.R., Kurosky S. Effectiveness of seasonal trivalent influenza vaccine for preventing influenza virus illness among pregnant women: a population-based case-control study during the 2010–2011 and 2011–2012 influenza seasons. Clin Infect Dis: Off Publ Infect Dis Soc America. 2014;58:449–457. doi: 10.1093/cid/cit750. [DOI] [PubMed] [Google Scholar]
- 12.Zaman K., Roy E., Arifeen S.E., Rahman M., Raqib R., Wilson E. Effectiveness of maternal influenza immunization in mothers and infants. New Engl J Med. 2008;359:1555–1564. doi: 10.1056/NEJMoa0708630. [DOI] [PubMed] [Google Scholar]
- 13.Benowitz I., Esposito D.B., Gracey K.D., Shapiro E.D., Vazquez M. Influenza vaccine given to pregnant women reduces hospitalization due to influenza in their infants. Clin Infect Dis: Off Publ Infect Dis Soc America. 2010;51:1355–1361. doi: 10.1086/657309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Myers E.R., Misurski D.A., Swamy G.K. Influence of timing of seasonal influenza vaccination on effectiveness and cost-effectiveness in pregnancy. Am J Obstet Gynecol. 2011;204:S128–S140. doi: 10.1016/j.ajog.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 15.Fell DB., Savitz DA., Kramer MS., Gessner BD., Katz MA., Knight M. Maternal influenza and birth outcomes: systematic review of comparative studies. BJOG: Int J Obstet Gynaecol. 2016 doi: 10.1111/1471-0528.14143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dodds L., MacDonald N., Scott J., Spencer A., Allen V.M., McNeil S. The association between influenza vaccine in pregnancy and adverse neonatal outcomes. J Obstet Gynaecol Can. 2012;34:714–720. doi: 10.1016/S1701-2163(16)35336-1. [DOI] [PubMed] [Google Scholar]
- 17.Fell D.B., Sprague A.E., Liu N., Yasseen A.S., 3rd, Wen S.W., Smith G. H1N1 influenza vaccination during pregnancy and fetal and neonatal outcomes. Am J Public Health. 2012;102:e33–e40. doi: 10.2105/AJPH.2011.300606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kallen B., Olausson P.O. Vaccination against H1N1 influenza with Pandemrix((R)) during pregnancy and delivery outcome: a Swedish register study. BJOG: Int J Obstet Gynaecol. 2012;119:1583–1590. doi: 10.1111/j.1471-0528.2012.03470.x. [DOI] [PubMed] [Google Scholar]
- 19.Olsen SJ., Mirza SA., Vonglokham P., Khanthamaly V., Chitry B., Pholsena V. The effect of influenza vaccination on birth outcomes in a cohort of pregnant women in Lao PDR, 2014–2015. Clin Infect Dis: Off Publ Infect Dis Soc America. 2016 doi: 10.1093/cid/ciw290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Omer S.B., Goodman D., Steinhoff M.C., Rochat R., Klugman K.P., Stoll B.J. Maternal influenza immunization and reduced likelihood of prematurity and small for gestational age births: a retrospective cohort study. PLoS Med. 2011;8:e1000441. doi: 10.1371/journal.pmed.1000441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steinhoff M.C., Omer S.B., Roy E., El Arifeen S., Raqib R., Dodd C. Neonatal outcomes after influenza immunization during pregnancy: a randomized controlled trial. CMAJ: Can Med Assoc J = journal de l'Association medicale canadienne. 2012;184:645–653. doi: 10.1503/cmaj.110754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sheffield J.S., Greer L.G., Rogers V.L., Roberts S.W., Lytle H., McIntire D.D. Effect of influenza vaccination in the first trimester of pregnancy. Obstet Gynecol. 2012;120:532–537. doi: 10.1097/AOG.0b013e318263a278. [DOI] [PubMed] [Google Scholar]
- 23.Ahrens K.A., Louik C., Kerr S., Mitchell A.A., Werler M.M. Seasonal influenza vaccination during pregnancy and the risks of preterm delivery and small for gestational age birth. Paediatr Perinat Epidemiol. 2014;28:498–509. doi: 10.1111/ppe.12152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cleary B.J., Rice U., Eogan M., Metwally N., McAuliffe F. 2009 A/H1N1 influenza vaccination in pregnancy: uptake and pregnancy outcomes – a historical cohort study. Eur J Obstet Gynecol Reprod Biol. 2014;178:163–168. doi: 10.1016/j.ejogrb.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 25.Fell D.B., Platt R.W., Lanes A., Wilson K., Kaufman J.S., Basso O. Fetal death and preterm birth associated with maternal influenza vaccination: systematic review. BJOG: Int J Obstetr Gynaecol. 2015;122:17–26. doi: 10.1111/1471-0528.12977. [DOI] [PubMed] [Google Scholar]
- 26.Nordin J.D., Kharbanda E.O., Vazquez Benitez G., Lipkind H., Vellozzi C., Destefano F. Maternal influenza vaccine and risks for preterm or small for gestational age birth. J Pediatr. 2014;164(1051–7):e2. doi: 10.1016/j.jpeds.2014.01.037. [DOI] [PubMed] [Google Scholar]
- 27.Ludvigsson J.F., Zugna D., Cnattingius S., Richiardi L., Ekbom A., Ortqvist A. Influenza H1N1 vaccination and adverse pregnancy outcome. Eur J Epidemiol. 2013;28:579–588. doi: 10.1007/s10654-013-9813-z. [DOI] [PubMed] [Google Scholar]
- 28.Pasternak B., Svanstrom H., Molgaard-Nielsen D., Krause T.G., Emborg H.D., Melbye M. Risk of adverse fetal outcomes following administration of a pandemic influenza A(H1N1) vaccine during pregnancy. JAMA. 2012;308:165–174. doi: 10.1001/jama.2012.6131. [DOI] [PubMed] [Google Scholar]
- 29.Baum U., Leino T., Gissler M., Kilpi T., Jokinen J. Perinatal survival and health after maternal influenza A(H1N1)pdm09 vaccination: a cohort study of pregnancies stratified by trimester of vaccination. Vaccine. 2015;33:4850–4857. doi: 10.1016/j.vaccine.2015.07.061. [DOI] [PubMed] [Google Scholar]
- 30.Fabiani M., Bella A., Rota M.C., Clagnan E., Gallo T., D'Amato M. A/H1N1 pandemic influenza vaccination: a retrospective evaluation of adverse maternal, fetal and neonatal outcomes in a cohort of pregnant women in Italy. Vaccine. 2015;33:2240–2247. doi: 10.1016/j.vaccine.2015.03.041. [DOI] [PubMed] [Google Scholar]
- 31.Savitz D.A., Fell D.B., Ortiz J.R., Bhat N. Does influenza vaccination improve pregnancy outcome? Methodological issues and research needs. Vaccine. 2015;33:6430–6435. doi: 10.1016/j.vaccine.2015.08.041. [DOI] [PubMed] [Google Scholar]
- 32.Durand L.O., Cheng P.Y., Palekar R., Clara W., Jara J., Cerpa M. Timing of influenza epidemics and vaccines in the American tropics, 2002–2008, 2011–2014. Influenza Other Respir Viruses. 2016;10:170–175. doi: 10.1111/irv.12371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arriola C.S., Vasconez N., Thompson M., Mirza S., Moen A.C., Bresee J. Factors associated with a successful expansion of influenza vaccination among pregnant women in Nicaragua. Vaccine. 2016 doi: 10.1016/j.vaccine.2015.12.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Republica de Nicaragua. Plan Multianual: Programa Nacional de Inmunizaciones 2009–2015. Minsiterio de Salud, Programa Nacional de Inmunizaciones; 2009.
- 35.Glass H.C., Costarino A.T., Stayer S.A., Brett C.M., Cladis F., Davis P.J. Outcomes for extremely premature infants. Anesth Analg. 2015;120:1337–1351. doi: 10.1213/ANE.0000000000000705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Villar J., Cheikh Ismail L., Victora C.G., Ohuma E.O., Bertino E., Altman D.G. International standards for newborn weight, length, and head circumference by gestational age and sex: the Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet. 2014;384:857–868. doi: 10.1016/S0140-6736(14)60932-6. [DOI] [PubMed] [Google Scholar]
- 37.Regan A.K., Mak D.B., Moore H.C., Tracey L., Saker R., Jones C. Surveillance of antenatal influenza vaccination: validity of current systems and recommendations for improvement. BMC Publ Health. 2015;15:1155. doi: 10.1186/s12889-015-2234-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pan American Health Organization. Severe Acute Respiratory Infections network. <http://www.sarinet.org/?q=en:> PAHO; 2014..
- 39.Rubin D.B., Thomas N. Matching using estimated propensity scores: relating theory to practice. Biometrics. 1996;52:249–264. [PubMed] [Google Scholar]
- 40.Davison AC, Hinkley DV. Bootstrap methods and their application. New York: Cambridge; 1997.
- 41.Hutcheon J.A., Fell D.B., Jackson M.L., Kramer M.S., Ortiz J.R., Savitz D.A. Detectable risks in studies of the fetal benefits of maternal influenza vaccination. Am J Epidemiol. 2016 doi: 10.1093/aje/kww048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Remschmidt C., Wichmann O., Harder T. Frequency and impact of confounding by indication and healthy vaccinee bias in observational studies assessing influenza vaccine effectiveness: a systematic review. BMC Infect Dis. 2015;15:429. doi: 10.1186/s12879-015-1154-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vazquez-Benitez G., Kharbanda E.O., Naleway A.L., Lipkind H., Sukumaran L., McCarthy N.L. Risk of preterm or small-for-gestational-age birth after influenza vaccination during pregnancy: caveats when conducting retrospective observational studies. Am J Epidemiol. 2016;184:176–186. doi: 10.1093/aje/kww043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nunes M.C., Aqil A.R., Omer S.B., Madhi S.A. The effects of influenza vaccination during pregnancy on birth outcomes: a systematic review and meta-analysis. Am J Perinatol. 2016;33:1104–1114. doi: 10.1055/s-0036-1586101. [DOI] [PubMed] [Google Scholar]
- 45.Posthumus A.G., Birnie E., van Veen M.J., Steegers E.A., Bonsel G.J. An antenatal prediction model for adverse birth outcomes in an urban population: the contribution of medical and non-medical risks. Midwifery. 2015 doi: 10.1016/j.midw.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 46.Ebisu K., Holford T.R., Bell M.L. Association between greenness, urbanicity, and birth weight. Sci Total Environ. 2016;542:750–756. doi: 10.1016/j.scitotenv.2015.10.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alhusen J.L., Bullock L., Sharps P., Schminkey D., Comstock E., Campbell J. Intimate partner violence during pregnancy and adverse neonatal outcomes in low-income women. J Women’s Health. 2014;23:920–926. doi: 10.1089/jwh.2014.4862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alhusen J.L., Lucea M.B., Bullock L., Sharps P. Intimate partner violence, substance use, and adverse neonatal outcomes among urban women. J Pediatr. 2013;163:471–476. doi: 10.1016/j.jpeds.2013.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Croteau A., Marcoux S., Brisson C. Work activity in pregnancy, preventive measures, and the risk of delivering a small-for-gestational-age infant. Am J Publ Health. 2006;96:846–855. doi: 10.2105/AJPH.2004.058552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jones C.E., Munoz F.M., Spiegel H.M., Heininger U., Zuber P.L., Edwards K.M. Guideline for collection, analysis and presentation of safety data in clinical trials of vaccines in pregnant women. Vaccine. 2016 doi: 10.1016/j.vaccine.2016.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alwan N.A., Greenwood D.C., Simpson N.A., McArdle H.J., Cade J.E. The relationship between dietary supplement use in late pregnancy and birth outcomes: a cohort study in British women. BJOG: Int J Obstet Gynaecol. 2010;117:821–829. doi: 10.1111/j.1471-0528.2010.02549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mor G., Cardenas I. The immune system in pregnancy: a unique complexity. Am J Reprod Immunol. 2010;63:425–433. doi: 10.1111/j.1600-0897.2010.00836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barr W.B., Pecci C.C. Last menstrual period versus ultrasound for pregnancy dating. Int J Gynaecol Obstet. 2004;87:38–39. doi: 10.1016/j.ijgo.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 54.Mazaki-Tovi S., Romero R., Kusanovic J.P., Erez O., Pineles B.L., Gotsch F. Recurrent preterm birth. Semin Perinatol. 2007;31:142–158. doi: 10.1053/j.semperi.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Omer S.B., Richards J.L., Madhi S.A., Tapia M.D., Steinhoff M.C., Aqil A.R. Three randomized trials of maternal influenza immunization in Mali, Nepal, and South Africa: methods and expectations. Vaccine. 2015;33:3801–3812. doi: 10.1016/j.vaccine.2015.05.077. [DOI] [PubMed] [Google Scholar]
- 56.World Health Organization. Weight percentiles calculator. <http://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1&ved=0ahUKEwiX7KegkLnKAhXMbj4KHX3yB0YQFggcMAA&url=http%3A%2F%2Fwww.who.int%2Freproductivehealth%2Ftopics%2Fbest_practices%2Fweight_percentiles_calculator.xls&usg=AFQjCNFMbno4RDx5Sn2H7Pk8gU58JxXC7w&bvm=bv.112064104,d.dmo2016>.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.