Abstract
Objective:
To examine whether vagotomy decreases the risk of Parkinson disease (PD).
Methods:
Using data from nationwide Swedish registers, we conducted a matched-cohort study of 9,430 vagotomized patients (3,445 truncal and 5,978 selective) identified between 1970 and 2010 and 377,200 reference individuals from the general population individually matched to vagotomized patients by sex and year of birth with a 40:1 ratio. Participants were followed up from the date of vagotomy until PD diagnosis, death, emigration out of Sweden, or December 31, 2010, whichever occurred first. Vagotomy and PD were identified from the Swedish Patient Register. We estimated hazard ratios (HRs) with 95% confidence intervals (CIs) using Cox models stratified by matching variables, adjusting for country of birth, chronic obstructive pulmonary disease, diabetes mellitus, vascular diseases, rheumatologic disease, osteoarthritis, and comorbidity index.
Results:
A total of 4,930 cases of incident PD were identified during 7.3 million person-years of follow-up. PD incidence (per 100,000 person-years) was 61.8 among vagotomized patients (80.4 for truncal and 55.1 for selective) and 67.5 among reference individuals. Overall, vagotomy was not associated with PD risk (HR 0.96, 95% CI 0.78–1.17). However, there was a suggestion of lower risk among patients with truncal vagotomy (HR 0.78, 95% CI 0.55–1.09), which may be driven by truncal vagotomy at least 5 years before PD diagnosis (HR 0.59, 95% CI 0.37–0.93). Selective vagotomy was not related to PD risk in any analyses.
Conclusions:
Although overall vagotomy was not associated the risk of PD, we found suggestive evidence for a potential protective effect of truncal, but not selective, vagotomy against PD development.
Gastrointestinal symptoms, in particular constipation, are among the most common nonmotor symptoms of Parkinson disease (PD).1,2 Furthermore, Braak and others3–5 hypothesized that in PD the Lewy pathology may start in peripheral nerves such as the enteric nervous system and later spread to the CNS through the vagus nerve via prion-like mechanisms.3–5 In support of this, recent pathologic studies found Lewy-type deposition in the gut of individuals with prodromal PD.6,7 Furthermore, cell-to-cell α-synuclein transmission has been observed in both grafted neurons in patients with PD8,9 and transplanted neurons in a transgenic mouse model of PD.10 In murine models, injection of human α-synuclein into the intestinal wall led to a later α-synuclein observation in the vagus nerve.11 Intragastric injection of rotenone initiated α-synuclein accumulation in the enteric nerve system that subsequently spread to the vagal dorsal motor nuclei and substantia nigra pars compacta.12 Resection of the vagus nerve before rotenone treatment stopped the spread of PD-like pathology.13 Taken together, these data support the intriguing Braak and prion hypotheses of PD development.
These observations also led to the hypothesis that vagotomy surgery in human reduces the risk of PD. Empiric evidence to date, however, is scarce and inconsistent.14,15 We therefore evaluated the association of vagotomy with PD risk using data from Swedish nationwide registers.
METHODS
Databases.
Analytic data were derived from several nationwide Swedish registers that were linked via the unique personal identification number of Swedish residents. In brief, the Swedish Patient Register was first established in 1964 to 1965 to collect information about dates of admission to and discharge from hospitals, surgical procedures, and medical diagnoses at discharge. Coverage increased gradually to 76% in 1977 and became 100% in 1987.16,17 In 1997, the Patient Register started to cover surgical day care procedures, and in 2001, it was expanded to outpatient visits, covering ≈80% of all hospital visits and almost 100% of public clinics.18 Primary health care visits are not yet included in the Patient Register.18 Swedish Population and Housing Censuses were conducted every 5 years from 1960 to 1990 by Statistics Sweden19 and were mandatory for Swedish residents ≥16 years of age. The censuses contain detailed information on housing, civil status, highest attained level of education, income, occupation, and social class. The Cause of Death Register, available since 1961, contains nationwide information about dates of death, places of residence, underlying causes of death, and contributing causes of death. Information about dates of immigration into (from 1969) and emigration out of (from 1961) Sweden is recorded in the Total Population Register.20
Ascertainment of vagotomy and patients with PD.
Vagotomized patients were identified from the Patient Register with the use of codes from the Swedish Classification of Operations and Major Procedures: 4471 to 4478, 4411 to 4416, 4418 to 4419, 4451, and 4453 during the period of 1964 to 1996, and JDG00, JDG01, JDG10, JDG11, JDG96, and JDG97 from 1997 onward. Vagotomy was further classified as truncal or selective (including both selective and highly selective) subtypes after review of the surgical codes by the gastroenterologic surgeon in our team (A.E.) (table e-1 at Neurology.org). The date of hospital discharge was used as the date of surgery. Cases with PD were individuals with PD diagnosis from any of the inpatient or outpatient hospital visits with the Swedish revisions of ICD codes (ICD-7: 350, 1964–1968; ICD-8: 342, 1969–1986; ICD-9: 332.0, 1987–1996; and ICD-10: G20, 1997 onward). The date of first hospital admission or outpatient contact was used as the date of identification. In a previous study, the positive predictive value of any PD diagnosis (primary or secondary) from the inpatient hospital care compared to standardized clinical evaluation was 70.8%.21
Study design.
We applied a matched-cohort study design. A total of 10,006 vagotomized patients were identified from the Patient Register between 1970 and 2010, among whom 9,430 vagotomized patients who met the criteria of being born before 1970 and living in Sweden without PD diagnosis before vagotomy were eligible for inclusion in the study. The date of vagotomy was defined as the index date. For each patient with vagotomy, 40 reference individuals who were living in Sweden without previous vagotomy and PD diagnosis were randomly selected from the Swedish Population and Housing Census in 1970 and individually matched on sex and year of birth on the index date. To account for incomplete coverage of the Patient Register before 1987, potential reference individuals were required to live in the counties covered by the Patient Register according to their residence information in the 1970 census (n = 6,018,543). A total of 9,430 vagotomized patients and 377,200 reference individuals were identified and followed up from the index date to the date of PD identification, death, or emigration out of Sweden or December 31, 2010, whichever occurred first.
Covariates.
We categorized age on the index date in the categories of 13 to 39, 40 to 44, 45 to 49, 50 to 54, 55 to 59, 60 to 64, 65 to 69, and ≥70 years and the index year of vagotomy into 5-year intervals. Country of birth was defined as Sweden, not Sweden, or unknown. Comorbidities were identified by ICD codes (see table e-2 for details) and included diabetes mellitus, vascular diseases, and rheumatologic disease. We used osteoarthritis and rheumatologic disease as indicators for use of nonsteroidal anti-inflammatory drugs (NSAIDs). Lifetime chronic obstructive pulmonary disease (COPD) was derived as a proxy for smoking status. Furthermore, we also created a comorbidity index for analytic purposes according to the Deyo et al22 modification of Charlson Comorbidity Index.23 We excluded the above-referenced specific diseases from the index because they were adjusted for individually. The comorbidity index was weighted according to the severity of the diseases and categorized into 3 groups: 0, 1 to 2, or ≥3 points.24 Peptic ulcer, which was the main indication of vagotomy, was not considered as a covariate because of concern about overadjustment.
Statistical analysis.
We estimated hazard ratios (HRs) with 95% confidence intervals (CIs) for PD from Cox proportional hazard models using time since the index date as an underlying time scale. Cumulative incidence of PD was calculated for vagotomized patients and their matched controls, accounting for death as a competing risk. We first analyzed vagotomy overall as the primary outcome of interest and further analyzed truncal and selective vagotomy separately. To explore the temporal relationship between vagotomy and PD and to minimize concern about reverse causation, we analyzed the association among individuals followed up at least 5, 10, or 20 years by including an interaction between vagotomy and time-band variables splitting at respective follow-up years.
The association between vagotomy and PD risk was first evaluated conditionally on matching variables only and then additionally adjusted for country of birth, COPD, diabetes mellitus, vascular diseases, rheumatologic disease, osteoarthritis, and comorbidity index. Selection of potential confounders was based on previous literature.14,25–27 Potential effect modification by sex was examined by including an interaction term between sex and type of vagotomy. Participants with missing data on country of birth (0.02% of total) were excluded from the adjusted models. The proportional hazard assumption for Cox models was tested and fulfilled over the entire follow-up period.
To control for confounding by indication for surgery and factors related to the indication, we conducted a sensitivity analysis by comparing PD risk in individuals with truncal and selective vagotomy. While truncal vagotomy and selective vagotomy differ by extent of surgical procedure, indications for the surgery (i.e., peptic ulcer with complications) and factors related to the indication (i.e., smoking and NSAID use) are often similar. Thus, if differential risks were observed, it would further suggest that the risk difference was due to the procedure itself rather than an indication for surgery or related factors. We used Stata 13 for statistical analyses with a 2-sided α of 0.05.
Standard protocol approvals, registrations, and patient consents.
The study was approved by the Regional Ethics Review Board in Stockholm. Because this study included analyses of deidentified data, written consent from participants was not required.
RESULTS
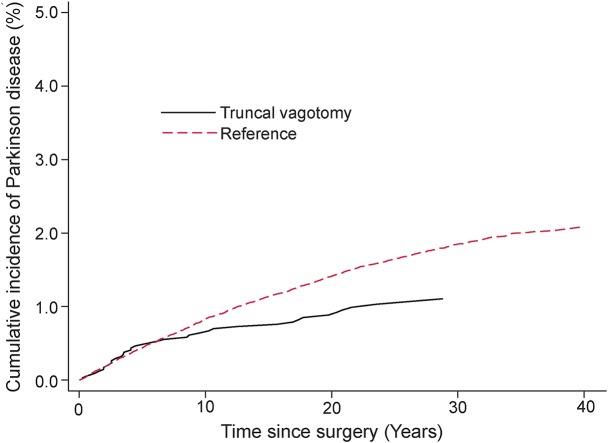
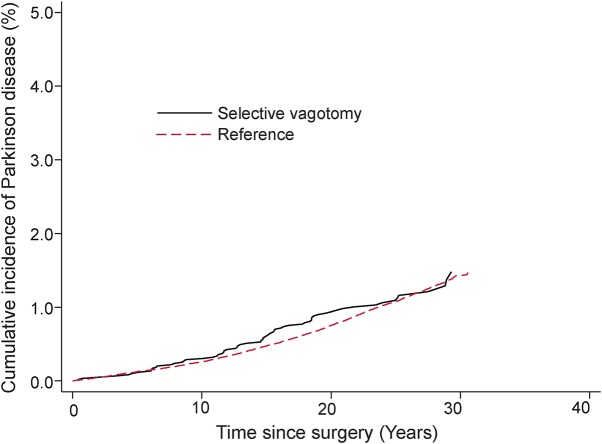
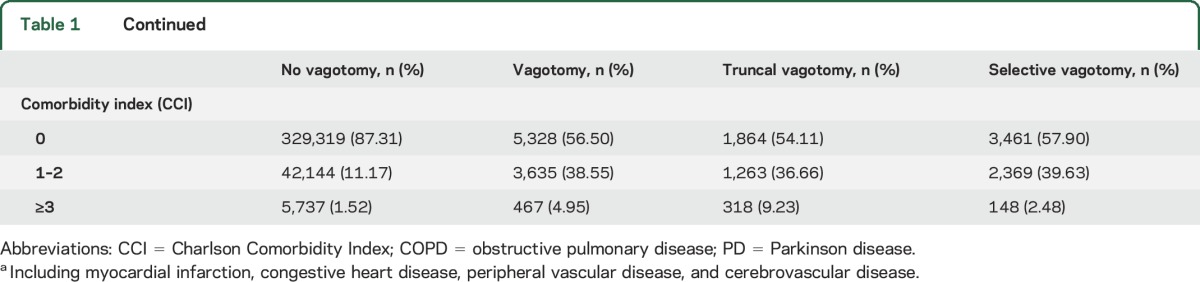
The analyses included 9,430 vagotomized patients (3,445 truncal, 5,978 selective, and 7 unknown) and 377,200 reference individuals. Mean ± SD age at index date was 54.3 ± 15.2 years. A total of 4,930 cases of PD were identified during 7.3 million person-years of follow-up. Mean ± SD age at PD diagnosis in the entire cohort was 75.6 ± 8.4 years. The crude incidence rate (per 100,000 person-years) for PD was 61.8 among vagotomized patients (80.4 for truncal and 55.1 for selective) and 67.5 among reference individuals. The cumulative incidence of PD in truncal and selective vagotomized patients compared to the matched reference individuals is shown in figures 1 and 2.
Figure 1. Cumulative incidence of PD among truncal vagotomized patients and their matched reference individuals.
PD = Parkinson disease.
Figure 2. Cumulative incidence of PD among selective vagotomized patients and their matched reference individuals.
PD = Parkinson disease.
Characteristics of the study participants are shown in table 1. The distributions of sex and year of birth were the same in vagotomized patients and reference individuals as a result of matching. Proportionally, more vagotomized patients were born outside Sweden. Individuals with truncal vagotomy were older than those with selective vagotomy or reference individuals. During 1970 to 1979, more truncal vagotomies were performed than selective vagotomies, whereas in 1980 and after, selective vagotomies were more common. As expected, >85% of vagotomized patients had a history of peptic ulcer, which is the main indication for vagotomy. More COPD was observed in both the truncal and selective vagotomy groups compared to the reference group. Furthermore, vagotomized patients, especially those with truncal vagotomy, were more likely to have diabetes mellitus, vascular diseases, rheumatologic disease, osteoarthritis, and a higher comorbidity index than reference individuals.
Table 1.
Baseline characteristics of study participants
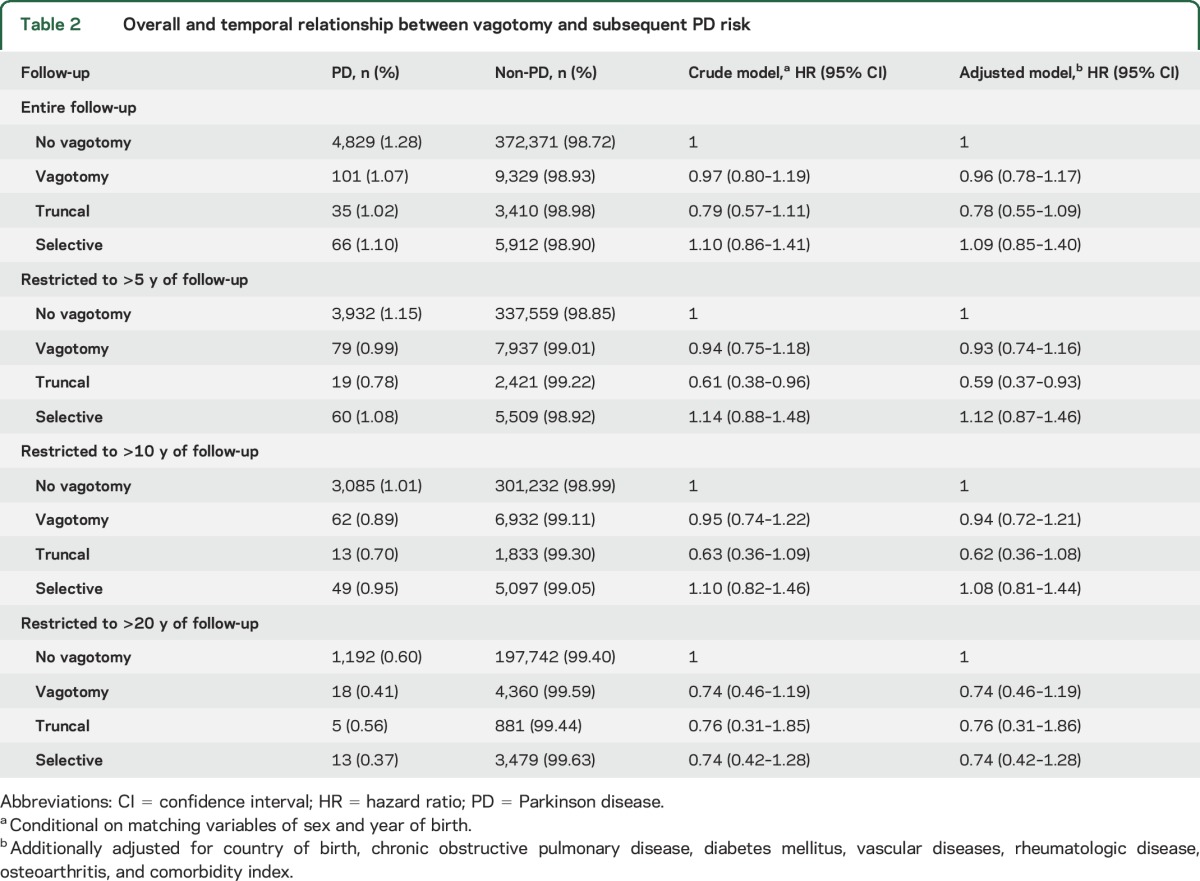
Overall, we did not observe an association of vagotomy with PD (HR 0.96, 95% CI 0.78–1.17) (table 2). However, patients with truncal vagotomy appeared to have a lower risk than reference individuals, although the difference was not statistically significant. In the analysis that split the follow-up at year 5, we found a lower risk of PD >5 years after truncal vagotomy (HR 0.59, 95% CI 0.37–0.93), and similar results were observed for >10 years after truncal vagotomy (HR 0.62, 95% CI 0.36–1.08). In the analysis that restricted to follow-up >20 years, although there appeared to be a lower PD risk after truncal vagotomy, the risk reduction was not statistically significant. Selective vagotomy was not associated with PD risk in any of the analyses. In the analyses that compared truncal and selective vagotomy > 5 years after the surgery, we observed a lower PD risk among patients with truncal vagotomy (HR = 0.54, 95% CI = 0.32–0.91) (table e-3).
Table 2.
Overall and temporal relationship between vagotomy and subsequent PD risk
DISCUSSION
In this large population-based study, although we did not observe an association between overall vagotomy and PD risk, patients with truncal vagotomy appeared to have a lower risk of PD. Further sensitivity analysis comparing truncal and selective vagotomy (i.e., selective and highly selective subtypes) argues against confounding by indication of surgery or factors related to indication such as smoking and NSAID use. In contrast, we found no evidence for lower PD risk among patients with selective vagotomy.
Although controversial, one implication of the Braak hypothesis is that, in some cases of PD, the Lewy pathology may initiate from the Meissner and Auerbach plexuses of the gut and then spread retrogradely along the vagus nerve to the dorsal motor nucleus in the lower brainstem via prion-like mechanisms.4,28 If this is true, resection of the vagus nerve may stop or delay the spreading of Lewy pathology and thus prevent or delay the clinical onset of PD. Indeed, in murine models of PD, vagotomy slowed the spreading of synucleinopathy to the brainstem that was initiated by gastrointestinal administration of rotenone, a pesticide known to induce parkinsonism.13
In clinical practice, there are 3 types of vagotomy. The truncal vagotomy denervates multiple organs, including the stomach, liver, gall bladder, pancreas, small intestine, and proximal colon; selective vagotomy eliminates innervation to the stomach; and highly selective vagotomy eliminates only innervation to the stomach body while preserving innervation to the antrum and pylorus.29 Therefore, in the case of selective or highly selective vagotomy, synucleinopathy initiated at other gastrointestinal sites may still find its way to the vagus nerve and then to the brainstem. Because Lewy pathology was found at multiple gastrointestinal sites in patients with PD,30 this reasoning may in part explain our observation that only truncal vagotomy, but not selective or highly selective vagotomy, was associated with a lower risk of PD.
Before our study, only 2 publications reported vagotomy in relation to PD in humans, and the evidence to date is very preliminary.14,15 Using Danish national registers from 1977 to 1995, Svensson et al.14 reported a 15% lower PD risk >5 years after truncal vagotomy compared to the general population; the effect appeared to be stronger when restricted to >20 years after the surgery (HR 0.53, 95% CI 0.53–0.99). This Danish study, however, did not differentiate truncal from selective vagotomy and thus might have underestimated the association of truncal vagotomy with PD.14 Tysnes et al.,15 who analyzed the same Danish population with extended follow-up (1977–2011), reported a nonsignificantly lower PD risk for truncal vagotomy overall (HR 0.88, 95% CI 0.55–1.21) and a nonsignificantly elevated PD risk >20 years after the surgery (HR 1.14, 95% CI 0.23–2.05). Therefore, although data on temporal relationship are inconsistent, available evidence, including that from our study, offers preliminary support that truncal vagotomy may decrease the risk of PD.
Reasons for different observations on the temporal relationship are speculative. Among others, all studies had relatively few exposed cases of PD; furthermore, potential confounding from unmeasured factors (e.g., use of NSAIDs or smoking) during the long follow-up is likely. In our study, we observed stronger evidence for PD risk reduction >5 or 10 years after truncal vagotomy, but the association was attenuated with >20 years of follow-up. This observation was supported by our sensitivity analysis comparing truncal and selective vagotomy that may, to certain extent, have accounted for unmeasured confounding. Our observation of the temporal relationship is consistent with the possibility that PD pathology may start at multiple sites of peripheral nervous system. Therefore, even truncal vagotomy may delay rather than eliminate the risk for PD. Indeed, abnormal α-synuclein accumulation has been found throughout the digestive tract of patients with PD with descending pattern of density and even in the submandibular gland in patients with preclinical PD.1,6,7 Furthermore, the Braak hypothesis also posits that the PD pathology may start in the olfactory bulb.31,32 In support of this, poor sense of smell is the most prevalent nonmotor symptom in prodromal PD2 and is strongly associated with future PD risk in the general population. Nevertheless, all 3 studies had limited statistical power to examine the potential long-term effect of truncal vagotomy with PD.
Both the strengths and limitations of our study stem from the use of nationwide registries. Because of the rarity of both vagotomy and PD, one has to resort to extremely large databases such as this one to examine the proposed hypothesis. Other strengths of the current study include the long follow-up of ≈40 years and carefully planned data analyses. We distinguished truncal from selective or highly selective vagotomy and separately assessed their associations with PD. Furthermore, the comparison of truncal and selective vagotomy, to some extent, alleviated concerns of confounding by indication or by other confounders such as smoking. Limitations of the current study include the limited statistical power despite the use of nationwide data. This is particularly true in the analyses of temporal relationships. Second, the use of nationwide registries likely induced misclassification, particularly for PD diagnosis and time of diagnosis. However, these misclassifications were likely nondifferential with respect to exposure assessment and might therefore have underestimated the true association. Furthermore, the analyses for cases diagnosed at least 5, 10, or 20 years after vagotomy largely alleviated concerns about reverse causality. In addition, although we attempted to control for major confounders using surrogate variables, we were unable to fully control for potential confounding due to individual-based risk factors for PD such as smoking, coffee intake, or genetics using register-based data. Finally, if the observed association is confirmed and proven to be biological, we expect the results could be generalized to populations in other parts of the world.
Although we did not observe a relationship of overall vagotomy and PD risk, we found preliminary evidence that truncal, but not selective, vagotomy was related to a lower risk of PD. These observations are largely consistent with those from the previous Danish study. Taken together, these data provide preliminary and indirect support for the Braak and prion-like hypotheses for PD prodromal development.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Arvid Sjölander for valuable discussions and suggestions on statistical analysis.
GLOSSARY
- CI
confidence interval
- COPD
chronic obstructive pulmonary disease
- HR
hazard ratio
- ICD
International Classification of Diseases
- NSAID
nonsteroidal anti-inflammatory drug
- PD
Parkinson disease
Footnotes
Editorial, page 1982
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
J.F.L., P.S., H.C., and K.W. were responsible for study concept. K.W., F.F., and H.C. were responsible for study design and funding. B.L. performed data management and statistical analysis and drafted the manuscript. A.T. contributed to statistical analysis. All authors contributed to interpretation of results and critical revision of the manuscript.
STUDY FUNDING
The study was funded by the Swedish Research Council for Health, Working Life and Welfare, the Parkinson Research Foundation in Sweden, and the Karolinska Institutet–NIH Doctoral Partnership Program in Neuroscience. F.F. was supported by the Swedish Society for Medical Research and the Karolinska Institutet (Research Associate Grant and Strategic Research Program in Epidemiology). P.S. was supported by grants provided by the Stockholm County Council (ALF project).
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Fasano A, Visanji NP, Liu LWC, Lang AE, Pfeiffer RF. Gastrointestinal dysfunction in Parkinson's disease. Lancet Neurol 2015;14:625–639. [DOI] [PubMed] [Google Scholar]
- 2.Chen H, Zhao EJ, Zhang W, et al. Meta-analyses on prevalence of selected Parkinson's nonmotor symptoms before and after diagnosis. Transl Neurodegener 2015;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braak H, Ghebremedhin E, Rub U, Bratzke H, Del Tredici K. Stages in the development of Parkinson's disease-related pathology. Cell Tissue Res 2004;318:121–134. [DOI] [PubMed] [Google Scholar]
- 4.Braak H, Rub U, Gai WP, Del Tredici K. Idiopathic Parkinson's disease: possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J Neural Transm 2003;110:517–536. [DOI] [PubMed] [Google Scholar]
- 5.Angot E, Steiner JA, Hansen C, Li JY, Brundin P. Are synucleinopathies prion-like disorders? Lancet Neurol 2010;9:1128–1138. [DOI] [PubMed] [Google Scholar]
- 6.Stokholm MG, Danielsen EH, Hamilton-Dutoit SJ, Borghammer P. Pathological α-synuclein in gastrointestinal tissues from prodromal Parkinson disease patients. Ann Neurol 2016:940–949. [DOI] [PubMed] [Google Scholar]
- 7.Hilton D, Stephens M, Kirk L, et al. Accumulation of alpha-synuclein in the bowel of patients in the pre-clinical phase of Parkinson's disease. Acta Neuropathol 2014;127:235–241. [DOI] [PubMed] [Google Scholar]
- 8.Li JY, Englund E, Holton JL, et al. Lewy bodies in grafted neurons in subjects with Parkinson's disease suggest host-to-graft disease propagation. Nat Med 2008;14:501–503. [DOI] [PubMed] [Google Scholar]
- 9.Kordower JH, Chu Y, Hauser RA, Freeman TB, Olanow CW. Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson's disease. Nat Med 2008;14:504–506. [DOI] [PubMed] [Google Scholar]
- 10.Desplats P, Lee HJ, Bae EJ, et al. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proc Natl Acad Sci USA 2009;106:13010–13015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holmqvist S, Chutna O, Bousset L, et al. Direct evidence of Parkinson pathology spread from the gastrointestinal tract to the brain in rats. Acta Neuropathol 2014;128:805–820. [DOI] [PubMed] [Google Scholar]
- 12.Pan-Montojo F, Anichtchik O, Dening Y, et al. Progression of Parkinson's disease pathology is reproduced by intragastric administration of rotenone in mice. PLoS One 2010;5:e8762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pan-Montojo F, Schwarz M, Winkler C, et al. Environmental toxins trigger PD-like progression via increased alpha-synuclein release from enteric neurons in mice. Sci Rep 2012;2:898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Svensson E, Horvath-Puho E, Thomsen RW, et al. Vagotomy and subsequent risk of Parkinson's disease. Ann Neurol 2015;78:522–529. [DOI] [PubMed] [Google Scholar]
- 15.Tysnes OB, Kenborg L, Herlofson K, et al. Does vagotomy reduce the risk of Parkinson's disease? Ann Neurol 2015;78:1011–1012. [DOI] [PubMed] [Google Scholar]
- 16.Statistics Sweden. Täckningsgrad för den somatiska och psykiatriska slutenvården [online]. Available at: http://www.scb.se/sv_/Hitta-statistik/Regional-statistik-och-kartor/Regionala-indelningar/Lan-och-kommuner/Lan-och-kommuner-i-kodnummerordning/. Accessed April 9, 2016. [Google Scholar]
- 17.Statistics Sweden. The National Patient Register [online]. Available at: http://www.socialstyrelsen.se/register/halsodataregister/patientregistret/inenglish. Accessed May 9, 2015. [Google Scholar]
- 18.Ludvigsson JF, Andersson E, Ekbom A, et al. External review and validation of the Swedish national inpatient register. BMC Public Health 2011;11:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Statistics Sweden. Folk- och bostadsräkningen 1965–1990 [online]. Available at: http://www.scb.se/sv_/Hitta-statistik/Historisk-statistik/Statistik-efter-serie/Sveriges-officiella-statistik-SOS-utg-1912-/Folk-och-bostadsrakningarna-1860-1990/Folk-och-bostadsrakningen-1965-1990/. Accessed May 9, 2015. [Google Scholar]
- 20.Statistics Sweden. SCB-data för Forskning 2012/Microdata at Statistic Sweden for Research Purposes 2012 [online]. Available at: http://www.scb.se/statistik/_publikationer/OV9999_2012A01_BR_X104BR1201.pdf. Accessed May 9, 2015. [Google Scholar]
- 21.Feldman AL, Johansson AL, Gatz M, et al. Accuracy and sensitivity of parkinsonian disorder diagnoses in two Swedish national health registers. Neuroepidemiology 2012;38:186–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992;45:613–619. [DOI] [PubMed] [Google Scholar]
- 23.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005;43:1130–1139. [DOI] [PubMed] [Google Scholar]
- 24.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–383. [DOI] [PubMed] [Google Scholar]
- 25.Gao X, Chen H, Schwarzschild M, Ascherio A. Use of non-steroidal anti-inflammatory drugs and risk of Parkinson's disease: a prospective study and meta-analysis. Neurology 2010;74:A103. [Google Scholar]
- 26.Rugbjerg K, Friis S, Jørgensen TL, Ritz B, Korbo L, Olsen JH. Risk for Parkinson disease among patients with osteoarthritis: a Danish cohort study. Mov Disord 2010;25:2355–2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rugbjerg K, Friis S, Ritz B, Schernhammer ES, Korbo L, Olsen JH. Autoimmune disease and risk for Parkinson disease: a population-based case-control study. Neurology 2009;73:1462–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Braak H, de Vos RAI, Bohl J, Del Tredici K. Gastric α-synuclein immunoreactive inclusions in Meissner's and Auerbach's plexuses in cases staged for Parkinson's disease-related brain pathology. Neurosci Lett 2006;396:67–72. [DOI] [PubMed] [Google Scholar]
- 29.Glasgow RE, Mulvihill SJ. Surgery for peptic ulcer disease and postgastrectomy syndromes. In: Yamada T, editor. Textbook of Gastroenterology, 5th ed. Hoboken: Wiley; 2009:1054–1070. [Google Scholar]
- 30.Beach TG, Adler CH, Sue LI, et al. Multi-organ distribution of phosphorylated alpha-synuclein histopathology in subjects with Lewy body disorders. Acta Neuropathol 2010;119:689–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lemer A, Bagic A. Olfactory pathogenesis of idiopathic Parkinson disease revisited. Mov Disord 2008;23:1076–1084. [DOI] [PubMed] [Google Scholar]
- 32.Reichmann H. View point: etiology in Parkinson's disease: dual hit or spreading intoxication. J Neurol Sci 2011;310:9–11. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.