Abstract
Burkitt's lymphoma (BL) is an aggressive B cell neoplasm, which is one of the most common neoplasms of childhood. It is highly widespread in East Africa, where it appears in endemic form associated with Epstein–Barr virus (EBV) infection, and around the world in a sporadic form in which EBV infection is much less common. In addition to being the first human neoplasm to be associated with EBV, BL is associated with a characteristic translocation, in which the Ig promoter is translocated to constitutively activate the c-myc oncogene. Although many BLs respond well to chemotherapy, a significant fraction fails to respond to therapy, leading to death. In this article, we demonstrate that EBV-positive BL expresses high levels of activated mitogen-activated protein kinase and reactive oxygen species (ROS), and that ROS directly regulate NF-κB activation. EBV-negative BLs exhibit activation of phosphoinositol 3-kinase, but do not have elevated levels of ROS. Elevated reactive oxygen may play a role in diverse forms of viral carcinogenesis in humans, including cancers caused by EBV, hepatitis B, C, and human T cell lymphotropic virus. Our findings imply that inhibition of ROS may be useful in the treatment of EBV-induced neoplasia.
Keywords: angiogenesis
Burkitt's lymphoma (BL) is a distinct neoplasm of B lymphocytes characterized by a translocation of the Ig heavy- or light-chain promoter and enhancer elements into the c-myc oncogene (1). This neoplasm is also of historic interest, because a technician who developed infectious mononucleosis became seropositive for Epstein–Barr virus (EBV), thus establishing a role of EBV in both acute infectious mononucleosis and BL (1). Since the recognition of a causative role of EBV in infectious mononucleosis, EBV has been implicated in other neoplastic disorders, including nasopharyngeal carcinoma, transplant-related lymphoproliferative disorder, Hodgkins disease, gastric cancer, and cutaneous leiomyosarcoma in patients with AIDS (2–9).
BL is characterized by EBV-positive and -negative phenotypes, which occur in distinct epidemiological subgroups. Endemic BL, which was first characterized as a childhood neoplasm in East Africa, is characterized by rapidly growing tumors of the mandible. EBV-positive BL also occurs at a high frequency in iatrogenically immunosuppressed patients as well as patients who are positive for HIV. Although BLs are often highly responsive to conventional chemotherapy, severe preexisting immunosuppression and other comorbid conditions often prevent full use of chemotherapy in these patients (10).
We have previously hypothesized that tumors that exhibit loss of the tumor suppressor genes p16ink4a and/or p53 also exhibit distinct and predictable patterns of signal transduction pathway dependence (11, 12). Based on prior results from our laboratory, we have demonstrated synergy between inactivation of p16ink4a and activation of mitogen-activated protein kinase (MAPK) (13–15). Also, we have found that cancers caused by reactive oxygen species (ROS) demonstrate high levels of hypermethylation of the tumor suppressor p16ink4a and activation of MAPK (16). Last, we have demonstrated that genes that generate reactive oxygen also activate angiogenesis and transform p16ink4a-deficient NIH 3T3 cells (17). Several groups have demonstrated a high level of hypermethylation of p16ink4a in EBV-positive tumors, including BL, nasopharyngeal carcinoma, and gastric carcinoma (5–9). Thus, we predicted that EBV-positive BL would demonstrate high levels of MAPK activation and high levels of ROS compared with EBV-negative BL. In this article, we demonstrate that EBV-positive BL cells use MAPK and ROS as major signaling pathways, and these pathways are not activated in EBV-negative BL. Increased ROS is found in both type I and III latency. Inhibition of reactive oxygen may be of therapeutic benefit in the treatment of EBV-induced neoplasia.
Materials and Methods
Cell Lines. The EBV-positive and -negative cell lines Ramos, Raji, Seraphine, LY91, BL2, BL60, DG75, Akata, and Mutu were grown in RPMI medium 1640 (Sigma) supplemented with 100 units/ml penicillin–streptomycin/2 mM l-glutamine/10% FBS. The LG2 lymphoblastoid cell line was provided by Peter Jensen (Department of Pathology, Emory University School of Medicine) and grown in RPMI medium 1640 supplemented with 100 units/ml penicillin–streptomycin/2 mM l-glutamine/10% FBS. EREB 2–5 cell line, which was provided by George Bornkamm (Institute for Clinical Molecular Biology and Tumor Genetics, Munich), was grown in RPMI medium 1640 supplemented with 20% FBS/1 μM estrogen. EREB 2–5 cells express EBNA2 directed by an estrogen-inducible promoter (18). SCC12F/tet and SCC12F/tetLMP1 were grown in DMEM supplemented with 100 units/ml penicillin–streptomycin/2 mM l-glutamine/10% FBS, and 1 μg/ml doxycycline was used to suppress the expression of LMP1 (19). PD98059 and LY294002 were obtained from Calbiochem, and ebselen was obtained from Sigma–Aldrich. Cells were treated with 25 μM ebselen, 25 μM PD98059, or 25 μM LY294002 for 24 h before harvesting for protein or RT-PCR.
Western Blotting. Cells were lysed in lysis buffer containing 20 mmol/liter Tris·HCl (pH 7.5), 150 mmol/liter NaCl, 1% (vol/vol) Triton X-100, 10% glycerol, 1 mmol/liter EDTA, 10 μg/ml leupeptin, 10 μg/ml aprotinin, 1 mmol/liter benzamidine, 1 mmol/liter phenylmethylsulfonyl fluoride, and 1 mmol/liter Na3VO4. Protein concentration was determined by the Bradford assay using BSA as a standard. Samples were treated with Laemmli sample buffer and heated to 90°C for 5 min before SDS/PAGE (Bio-Rad) and transfer to PVDF membranes. The membranes were then blocked with 5% nonfat dry milk in Tris-buffered saline containing 0.1% Triton X-100 and incubated subsequently with the appropriate Ab for immunoblotting. Polyclonal anti-phospho-MAPK Ab, polyclonal anti-phospho-AKT Ab, and polyclonal anti-phospho-IκBα Ab were obtained from Cell Signaling Technology (Beverly, MA), and anti-LMP 1 mAb was obtained from DAKO.
Measurement of H2O2. Confluent cells in 100-mm dishes (≈5–6 × 106 cells) were washed with 6 ml of Hanks' balanced salt solution (HBSS) and released by using 0.25% trypsin (wt/vol)/1 mM EDTA, followed by the addition of 5% (vol/vol) FBS in HBSS. After pelleting, cells were resuspended in 5% (vol/vol) FBS in HBSS and counted. Dichlorofluorescein (DCF) diacetate was added to a final concentration of 2 μM and incubated for 1 h in the dark at room temperature (17). DCF fluorescence was determined by using 0.5 × 106 cells per 3 ml of 5% (vol/vol) FBS in HBSS with a FACSCalibur (excitation wavelength, 488 nm; emission wavelength, 515–545 nm; Becton Dickinson).
Real Time RT-PCR. Total RNA was isolated with TriReagent (Sigma) according to the manufacturer's instructions. To ensure that no contaminating DNA was present, all samples were incubated for 1 h at room temperature with RNase-free DNase, followed by heat inactivation of the enzyme for 30 min at 75–80°C. cDNA was prepared from 2 μg of total RNA by using the Superscript II preamlification system for First-Strand cDNA synthesis (Invitrogen) by using the random-primer protocol.
We used the following primers: VEGF-F, 5′-TACCTCCACCATGCCAAGTG; VEGF-R, 5′-GATGATTCTGCCCTCCTC; GAPDH-F, 5′-GTGGTCTCCTCTGACTTCAACA; and GAPDH-R, 5′GCTTGACAAAGTGGTCGTCGTTGAG. Real-time PCR was performed by using SYBR Green technology with the Gene-Amp 5700 sequence-detection system (Applied Biosystems). Briefly, SYBR Green is a passive dye that binds to the minor groove of double stranded DNA complexes (17). The intensity of fluorescence, which is a direct measure of the amount of amplified product, is measured with each cycle. The threshold at which significant application is first detected is determined, and all samples are evaluated by determining how quickly each sample reaches this threshold. The cycle at which this threshold is achieved is recorded as the Ct value. Data were normalized with primers for a housekeeping gene, GAPDH. A sample with no template was included to ensure the absence of primer–dimer.
Lentiviral Infection of BL Cells with akt Constructs. pLENTI6-DNAkt was created by digesting pMIG-DNAkt with BglII and SalI to release the DNAkt-IRES-GFP cassette and by ligating the cassette to pLenti6/V5-D-TOPO (Invitrogen) digested with BamHI and XhoI. Lentiviral supernatants were generated by cotransfecting 293T cells with pClAmpho and pLENTI6-DNAkt by using FuGENE 6 transfection reagent (Roche). The lentiviral supernatants were then added to Raji, Ramos, and Seraphine cells in the presence of 10 μg/ml protamine sulfate for 3 h. The resultant cells were then cultured in DMEM with 10% FCS, and after 24 h, 5–10% of the cells were GFP-positive by fluorescence microscopy. At that time, 2 μg/ml blastocydin was added to both infected and parental cells. After 24 h of blasticidin treatment, 100% of both parental and infected cells were dead. The infections were then repeated, and the cells were expanded after infection for 3 days to have sufficient cells to sort for GFP positivity by fluorescence-activated cell sorting (FACS). After 3 days, there were <1% GFP-positive cells remaining, as detected by fluorescence microscopy.
Results
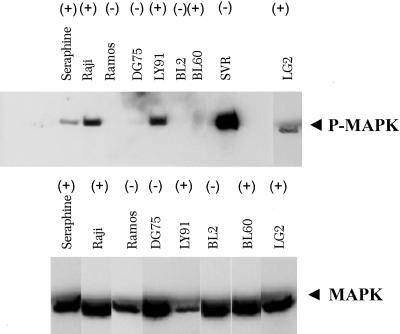
EBV-Positive BL Cell Lines Show Activation of MAPK Signaling. Several BL cell lines have been characterized in terms of EBV status and inactivation of p16ink4a and p53 (4). We hypothesized that MAPK is activated in tumors that are deficient in p16ink4a (11–16). To test this hypothesis, we assessed the status of MAPK activation in BL cell lines. Fig. 1 shows a strong correlation of EBV status with activation of MAPK. Virtually all EBV-positive cell lines show activation of MAPK, whereas none of the EBV-negative cell lines show activation of MAPK.
Fig. 1.
MAPK activation in EBV-positive BL cell lines. (Upper) Western blot analysis of BL-positive and -negative cell lines for phospho-MAPK (P-MAPK). SVR is an angiosarcoma cell line that was used as positive control for MAPK. EBV-positive (+) and -negative (-) cell lines are indicated. (Lower) Total MAPK was used to normalize the amount of total protein in each preparation.
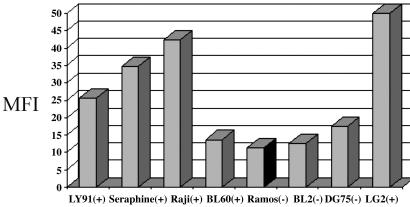
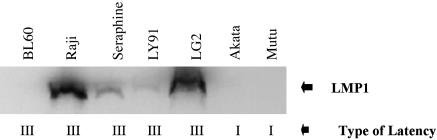
EBV-Positive BL Cell Lines Show Elevated Levels of Reactive Oxygen Compared with EBV-Negative BL Cell Lines. We have previously shown that EBV is associated with high levels of MAPK activation, but we have hypothesized also that these cell lines would exhibit high levels of ROS as well. Examination of EBV-positive and -negative cell lines showed that ROS is associated with EBV positivity (Fig. 2). Note that both type I latency BL cells, which are associated with absence of the EBV oncoprotein LMP1, and type III latency BL cells, which are associated with LMP1 expression, exhibit high levels of reactive oxygen expression (Fig. 3). The single outlier to the association of high levels of reactive oxygen and active MAPK expression with EBV status is the BL60 cell line. Of interest, the BL60 cell line, although derived from an EBV-positive BL, lost nearly all EBV expression (18). Note that no expression of LMP1 is detected in BL60 cells, consistent with BL60 being functionally EBV-negative (Fig. 3).
Fig. 2.
ROS expression levels correlate with EBV positivity in BL cell lines. DCF fluorescence was monitored by flow cytometry in BL cells. Fluorescence was much higher in all EBV-positive cell lines, except BL60, when compared with EBV-negative cell lines. EBV-positive (+) and -negative (-) cell lines are indicated. MFI, median fluorescence intensity.
Fig. 3.
LMP1 expression in EBV cell lines. Western blot analysis shows expression in EBV-positive cell lines associated with type III latency, whereas lack of LMP1 expression is associated with type I latency. All cell lines are EBV-positive.
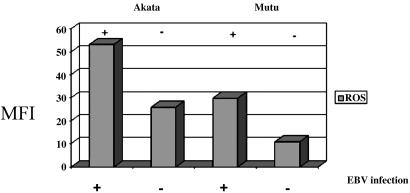
EBV Infection Results in Induction of ROS. The finding that ROS is increased in both type I and III latency suggests that EBV infection may lead to increased ROS. To test this hypothesis, we used the Akata and Mutu cell lines. Akata and Mutu are BL cells derived from patients with EBV-positive BL and represent type I latency (20). Clones that have lost EBV have been derived from each of these cell lines, and they can be used for comparison. We tested Akata and Mutu clones infected with EBV and compared them with EBV-negative clones of Akata and Mutu. We found that the EBV-positive clones produced significantly higher levels of ROS than the EBV-negative clones, thus demonstrating that EBV infection results in induction of ROS (Fig. 4).
Fig. 4.
EBV infection results in induction of ROS. EBV-positive and -negative Akata and Mutu cell lines were tested by flow cytometry for ROS. EBV-infected (+) and EBV-negative (-) cell lines are indicated. In both cell lines, ROS production was higher in the EBV-positive clones.
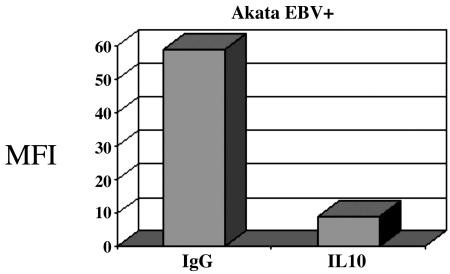
Type I Latency BL Induces ROS Through Autocrine Production of IL-10. Type I BL cells express a poly(A)-mRNA (EBER) and EBNA1 nuclear antigen, which is involved in proliferation of BL cells. Recent studies demonstrate a role of EBER mRNA in the activation of several cellular genes, including IL-10 (21). IL-10 has been implicated in the proliferation and immune evasion of several malignancies and, of interest, is strongly expressed in EBV-positive BL. IL-10 has also been implicated in activation of mitochondrial ROS in lupus erythematosus (22). Therefore, we selected IL-10 as a potential inducer of ROS in type I latency BL. We treated Akata cells with Abs to IL-10, and we found that inactivation of IL-10 led to inhibition of ROS in type I latency BL (Fig. 5).
Fig. 5.
Autocrine IL-10 production causes increased ROS type I latency BL cells. The EBV-positive Akata cells were treated with purified rat anti-human IL-10 Ab (Pharmingen) at a concentration of 10 μg/ml. Normal rat IgG isotype controls were used. After 72 h, the cells were harvested and DCF fluorescence was monitored by flow cytometry. IL-10 blockade results in significant decrease in ROS production.
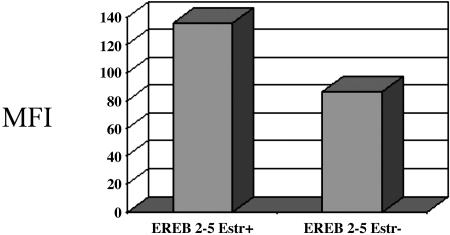
Type III Latency BL Induces ROS Through an EBNA2-Dependent Pathway. EBNA2 is a transcription factor that induces transcription of several EBV genes, including the LMP1 oncoprotein (16). To determine whether genes induced by EBNA2 may be involved in the induction of ROS in type III latency, we used a BL cell line expressing an estrogen-inducible EBNA2 gene. Induction of EBNA2 by estradiol led to induction of LMP1, as well as induction of ROS. Thus, we found that EBNA2 target genes may stimulate ROS in type III latency (Fig. 6).
Fig. 6.
EBNA2 expression induces ROS production. EREB 2–5 is a cell line that contains an estrogen-inducible EBNA2. We cultured the cells in the presence and absence of estrogen. After 48 h, the cells were harvested and DCF fluorescence was monitored by flow cytometry.
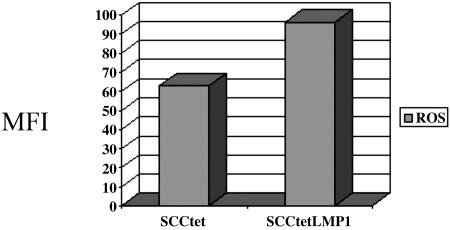
LMP1 Induces ROS. Of the genes downstream of EBNA2, LMP1 is a likely candidate for induction of ROS, given its strong oncogenic function in multiple cell types (20). We used a tetracycline-inducible epithelial cell line to determine whether LMP1 induces ROS. LMP1 was expressed in a tet-off system in the human epithelial cell line SCC12F, and it is constitutively activated unless exogenous tetracycline is added to the medium (19). Addition of tetracycline led to a significant decrease in LMP1 expression, as well as ROS production, thus implicating LMP1 as a major inducer of ROS in type III latency (Fig. 7).
Fig. 7.
LMP1 induces ROS in type III latency. The squamous cell carcinoma cell line SCC12F was transfected with an LMP1 expressing vector in a tet-off system. The LMP1 gene is silenced by adding 1 μg/ml doxycyclin to the growth media. We cultured the LMP1-transfected cells for 48 h in the presence and absence of doxycycline. Cells were then harvested and DCF fluorescence was monitored by flow cytometry. SCC12F transfected with a vector control was also tested for ROS production.
Ebselen Inhibits Production of ROS in EBV-Positive BL. Ebselen is a nontoxic selenoorganic antioxidant drug with superoxide dismutase and glutathione peroxidase-like activity (23–25). We treated the EBV-positive BL cell line Raji with ebselen at a concentration of 25 μM for 16 h, and we observed that ROS production was completely inhibited (data not shown).
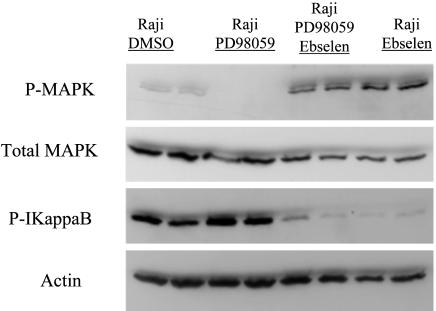
Differential Inhibition of NF-κB Signaling by Inhibition of PI3 Kinase Inhibition, MAPK Inhibition, and Reactive Oxygen Inhibition. Treatment of Raji cells with ebselen resulted in increased levels of the NF-κB inhibitor IkB, thus suggesting that ROS are a major regulator of NF-κB activation in EBV-positive BL cells (Fig. 8). Interestingly, MAPK was also activated by ebselen treatment, suggesting that a potential inverse relationship exists between reactive oxygen levels and MAPK activation, with antioxidant treatment resulting in increased levels of MAPK activation.
Fig. 8.
The effect of Ebselen on Raji (EBV-positive) cells is shown. Ebselen increases phosphorylation of MAPK and decreases phosphorylation of Ikb in Raji cells (EBV-positive). Cells were treated for 24 h before Western blot analysis.
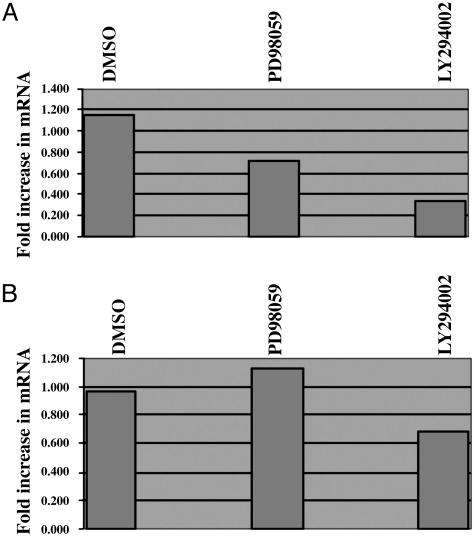
Regulation of VEGF mRNA by Signal Transduction Inhibition. Inhibition of PI3 kinase has been shown to be a major regulator of VEGF synthesis (12, 13). Treatment of both EBV-positive Raji and EBV-negative Ramos with the PI3 kinase inhibitor LY294002 led to reproducible decreases in VEGF mRNA (Fig. 9).
Fig. 9.
Effect of PI3 kinase and MAPK inhibition on VEGF production in EBV-positive (Raji) and EBV-negative (Ramos) cells. Total RNA was harvested from Raji (A) and Ramos (B) cells after 24 h of treatment with the PI3 kinase inhibitor LY294002 and the MAPK inhibitor PD98059. Levels of mRNA were measured by real-time PCR.
Discussion
EBV is a major cause of human neoplasia, associated with the development of both lymphoid and epithelial malignancy (1–10). EBV infection is associated with BL, Hodgkins disease, natural killer lymphoma, and transplant-associated lymphoproliferative disease, and EBV-associated lymphoid malignancy is common in individuals immunosuppressed by AIDS, organ transplantation, and congenital immunodeficiency (10, 26). Treatment of these lymphoid malignancies is compromised by the poor immune status in many of these individuals, because conventional chemotherapy results in further immune compromise. EBV-induced malignancy in the immunocompromised individual is usually fatal either because of progression of disease or treatment-associated sepsis (10, 26). EBV infection is also associated with solid epithelial tumors, including gastric carcinoma and nasopharyngeal carcinoma. Like EBV-induced lymphoid malignancy, the prognosis of EBV-induced solid tumors is also guarded.
We found previously that ROS-induced carcinogenesis causes hypermethylation of p16ink4a and activation of MAPK in experimental systems (16). Whereas acute elevations of ROS may be toxic to primary cells, chronic low levels of ROS cause induction of the angiogenic switch and subsequent tumorigenesis (17). P16ink4a and other tumor suppressor genes are commonly hypermethylated in other ROS-induced malignancies, including melanoma, squamous cell carcinoma of the head and neck, tobacco-induced lung carcinoma, and chronic inflammation-induced malignancies of the colon and bladder (26–29). Of interest, p16ink4a is hypermethylated in a many various EBV-induced tumors, including BL, natural killer lymphoma, nasopharyngeal carcinoma, and gastric carcinoma (5–8). Also, EBV gene expression has been shown to induce activation of DNA methyltransferase, a critical component of CpG promoter hypermethylation (30). These findings led us to inquire whether EBV infection contributes to oxidative carcinogenesis. We found that EBV infection results in increased ROS in lymphoid cells, and this induction occurs through two mechanisms. In type I latency, EBER induces IL-10, and IL-10 induces ROS. In type III latency, the oncoprotein LMP1 induces ROS, as a potential mechanism of tumorigenesis. Inhibition of ROS by the superoxide dismutase mimetic ebselen leads to decreased reactive oxygen signaling and as a downstream event, decreased NF-κB signaling. LMP1 has been shown to activate multiple pathways during transformation, including rac, PI3 kinase, and NF-κB (31–35). This article shows that reactive oxygen is a potential signaling mechanism downstream of LMP1. The induction of NF-κB by LMP1 is likely to be the most well studied, and inhibition of NF-κB has been shown to abrogate LMP1-mediated transformation (31). LMP1 is a transmembrane protein that contains two regions in its cytoplasmic tail that are capable of activating NF-κB. These regions are known as CTAR1 and CTAR2 (C terminus-activating regions 1 and 2), and deletions of these regions impair the ability of LMP1 to transform cells (31–35). The CTAR regions interact with members of the TNF receptor-associated factor (TRAF) families, and this interaction is required for optimal biologic activity of LMP1 (36). Whereas activation of ROS has not been associated previously with LMP1 signaling, TRAF activation by other ligands has been shown to induce ROS signaling, thus providing a potential mechanism for LMP1 mediated ROS induction (37). Thus, pharmacologic methods of decreasing NF-κB are likely benefit the treatment of EBV-induced neoplasia (31). In this study, we found that NF-κB activation can be inhibited pharmacologically by a superoxide dismutase mimic, ebselen.
Our data suggest that BL can be classified into two distinct disorders based on signaling pathways. EBV-positive BL relies on reactive oxygen and MAPK to regulate downstream events such as NF-κB activation. In contrast, EBV-negative BL demonstrates neither MAPK activation nor generation of reactive oxygen to activate NF-κB. Further, our data support the concept of recurrent associations of signaling pathways with inactivation of particular tumor suppressor genes (11, 12). We have shown (16, 38, 39) the association of p53 inactivation and PI3 kinase activation in malignant endothelial tumors and, conversely, between p16ink4a inactivation and MAPK activation in melanoma and fibrosarcoma. Here, we extend these findings to BL and suggest that these associations between tumor suppressor inactivation and signal transduction pathway activation may be a common feature of cancer.
We have demonstrated the involvement of reactive oxygen in EBV-mediated signal transduction. Like EBV, cancers caused by hepatitis B, C, and human T cell lymphotropic virus have a high frequency of p16ink4a hypermethylation (40, 41). Also, viral structural proteins, such as HBX of hepatitis B, HCV NS3, NS5, and human T cell lymphotropic virus tax protein, are known to induce reactive oxygen (42–45). Reactive oxygen may play a decisive role in carcinogenesis in potentially most virally induced human cancers, and it may serve as a pharmacologic target for chemoprevention and treatment.
Acknowledgments
J.L.A. was supported by National Institutes of Health Grants R01 AR47901 and P30 AR42687 (to Emory Skin Disease Research Core Center) and also by a Veterans Affairs Merit Award.
Author contributions: T.B., D.F., A.E., and J.L.A. designed research; F.C., T.B., R.L., E.M., C.C., N.M., J.W.S., R.S.W., and J.L.A. performed research; J.W.S., K.S., A.E., and B.S. contributed new reagents/analytic tools; F.C., R.L., D.F., B.S., and J.L.A. analyzed data; and J.L.A. wrote the paper.
Abbreviations: BL, Burkitt's lymphoma; DCF, dichlorofluorescein; EBV, Epstein–Barr virus; MAPK, mitogen-activated protein kinase; ROS, reactive oxygen species.
References
- 1.Zech, L., Haglund, U., Nilsson, K. & Klein, G. (1976) Int. J. Cancer 17, 47-56. [DOI] [PubMed] [Google Scholar]
- 2.Raab-Traub, N., Flynn, K., Pearson, G., Huang, A., Levine, P., Lanier, A. & Pagano, J. (1987) Int. J. Cancer 39, 25-29. [DOI] [PubMed] [Google Scholar]
- 3.Dambaugh, T., Nkrumah, F. K., Biggar, R. J. & Kieff, E. (1979) Cell 16, 313-322. [DOI] [PubMed] [Google Scholar]
- 4.Lindstrom, M. S., Klangby, U. & Wiman, K. G. (2001) Oncogene 20, 2171-2177. [DOI] [PubMed] [Google Scholar]
- 5.Van Rees, B. P., Caspers, E., Zur, H. A., Van Den, B. A., Drillenburg, P., Weterman, M. A. & Offerhaus, G. J. (2002) Am. J. Pathol. 161, 1207-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kang, G. H., Lee, S., Kim, W. H., Lee, H. W., Kim, J. C., Rhyu, M. G. & Ro, J. Y. (2002) Am. J. Pathol. 160, 787-794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tong, J. H., Tsang, R. K., Lo, K. W., Woo, J. K., Kwong, J., Chan, M. W., Chang, A. R., van Hasselt, C. A., Huang, D. P. & To, K. F. (2002) Clin. Cancer Res. 8, 2612-2619. [PubMed] [Google Scholar]
- 8.Gulley, M. L., Nicholls, J. M., Schneider, B. G., Amin, M. B., Ro, J. Y. & Geradts, J. (1998) Am. J. Pathol. 152, 865-869. [PMC free article] [PubMed] [Google Scholar]
- 9.Lee, S., Lee, H. J., Kim, J. H., Lee, H. S., Jang, J. J. & Kang, G. H. (2003) Am. J. Pathol. 163, 1371-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Groopman, J. E., Sullivan, J. L., Mulder, C., Ginsburg, D., Orkin, S. H., O'Hara, C. J., Falchuk, K., Wong-Staal, F. & Gallo, R. C. (1986) Blood 67, 612-615. [PubMed] [Google Scholar]
- 11.Klafter, R. & Arbiser, J. L. (2000) J. Invest. Dermatol. Symp. Proc. 5, 79-82. [DOI] [PubMed] [Google Scholar]
- 12.Arbiser, J.L. (2004) Semin. Cancer Biol. 14, 81-91. [DOI] [PubMed] [Google Scholar]
- 13.Arbiser, J. L., Moses, M. A., Fernandez, C. A., Ghiso, N., Cao, Y., Klauber, N., Frank, D., Brownlee, M., Flynn, E., Parangi, S., et al. (1997) Proc. Natl. Acad. Sci. USA 94, 861-866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.LaMontagne, K. R., Jr., Moses, M. A., Wiederschain, D., Mahajan, S., Holden, J., Ghazizadeh, H., Frank, D. A. & Arbiser, J. L. (2000) Am. J. Pathol. 157, 1937-1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arbiser, J. L., Weiss, S. W., Arbiser, Z. K., Bravo, F., Govindajaran, B., Caceres-Rios, H., Cotsonis, G., Recavarren, S., Swerlick, R. A. & Cohen, C. (2001) J. Am. Acad. Dermatol. 44, 1-5. [DOI] [PubMed] [Google Scholar]
- 16.Govindarajan, B., Klafter, R., Miller, M. S., Mansur, C., Mizesko, M., Bai, X., LaMontagne, K., Jr., & Arbiser, J. L. (2002) Mol. Med. 8, 1-8. [PMC free article] [PubMed] [Google Scholar]
- 17.Arbiser, J. L., Petros, J., Klafter, R., Govindajaran, B., McLaughlin, E. R., Brown, L. F., Cohen, C., Moses, M., Kilroy, S., Arnold, R. S., et al. (2002) Proc. Natl. Acad. Sci. USA 99, 715-720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Delecluse, H. J., Bartnizke, S., Hammerschmidt, W., Bullerdiek, J. & Bornkamm, G. W. (1993) J. Virol. 67, 1292-1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eliopoulos, A. G., Stack, M., Dawson, C. W., Kaye, K. M., Hodgkin, L., Sihota, S., Rowe, M. & Young, L. S. (1997) Oncogene 14, 2899-2916. [DOI] [PubMed] [Google Scholar]
- 20.Razzouk, B. I., Srinivas, S., Sample, C. E., Singh, V. & Sixbey, J. W. (1996) J. Infect. Dis. 173, 529-535. [DOI] [PubMed] [Google Scholar]
- 21.Kitagawa, N., Goto, M., Kurozumi, K., Maruo, S., Fukayama, M., Naoe, T., Yasukawa, M., Hino, K., Suzuki, T., Todo, S. & Takada, K. (2000) EMBO J. 19, 6742-6750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gergely, P., Jr., Niland, B., Gonchoroff, N., Pullmann, R., Jr., Phillips, P. E. & Perl, A. (2002) J. Immunol. 169, 1092-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chandel, N. S., Maltepe, E., Goldwasser, E., Mathieu, C. E., Simon, M. C. & Schumacker, P. T. (1998) Proc. Natl. Acad. Sci. USA. 95, 11715-11720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao, R. & Holmgren, A. (2002) J. Biol. Chem. 277, 39456-39462. [DOI] [PubMed] [Google Scholar]
- 25.Weber, D. S., Taniyama, Y., Rocic, P., Seshiah, P. N., Dechert, M. A., Gerthoffer, W. T. & Griendling, K. K. (2004) Circ. Res. 94, 1219-1226. [DOI] [PubMed] [Google Scholar]
- 26.Raab-Traub, N., Flynn, K., Klein, C., Pizza, G., De Vinci, C., Occhiuzzi, L., Farneti, G., Caliceti, U. & Pirodda, E. (1987) J. Exp. Pathol. 3, 449-456. [PubMed] [Google Scholar]
- 27.Hsieh, C. J., Klump, B., Holzmann, K., Borchard, F., Gregor, M. & Porschen, R. (1998) Cancer Res. 58, 3942-3945. [PubMed] [Google Scholar]
- 28.Eissa, S., Ali-Labib, R. & Khalifa, A. (2000) Clin. Chim. Acta 300, 159-169. [DOI] [PubMed] [Google Scholar]
- 29.Vo, Q. N., Geradts, J., Gulley, M. L., Boudreau, D. A., Bravo, J. C. & Schneider, B. G. (2002) J. Clin. Pathol. 55, 669-675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsai, C. N., Tsai, C. L., Tse, K. P., Chang, H. Y. & Chang, Y. S. (2002) Proc. Natl. Acad. Sci. USA 99, 10084-10089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cahir-McFarland, E. D., Davidson, D. M., Schauer, S. L., Duong, J. & Kieff, E. (2000) Proc. Natl. Acad. Sci. USA 97, 6055-6060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sylla, B. S., Hung, S. C., Davidson, D. M., Hatzivassiliou, E., Malinin, N. L., Wallach, D., Gilmore, T. D., Kieff, E. & Mosialos, G. (1998) Proc. Natl. Acad. Sci. USA 95, 10106-10111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaye, K. M., Izumi, K. M., Mosialos, G. & Kieff, E. (1995) J. Virol. 69, 675-683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaye, K. M., Izumi, K. M., Li, H., Johannsen, E., Davidson, D., Longnecker, R. & Kieff, E. (1999) J. Virol. 73, 10525-10530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaye, K. M., Izumi, K. M. & Kieff, E. (1993) Proc. Natl. Acad. Sci. USA 90, 9150-9154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luftig, M., Prinarakis, E., Yasui, T., Tsichritzis, T., Cahir-McFarland, E., Inoue, J., Nakano, H., Mak, T. W., Yeh, W. C., Li, X., et al. (2003) Proc. Natl. Acad. Sci. USA 100, 15595-15600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chandel, N. S., Schumacker, P. T. & Arch, R. H. (2001) J. Biol. Chem. 276, 42728-42736. [DOI] [PubMed] [Google Scholar]
- 38.Cohen, C., Zavala-Pompa, A., Sequeira, J. H., Shoji, M., Sexton, D. G., Cotsonis, G., Cerimele, F., Govindarajan, B., Macaron, N. & Arbiser, J. L. (2002) Clin. Cancer Res. 8, 3728-3733. [PubMed] [Google Scholar]
- 39.Govindarajan, B., Bai, X., Cohen, C., Zhong, H., Kilroy, S., Louis, G., Moses, M. & Arbiser, J. L. (2003) J. Biol. Chem. 278, 9790-9795. [DOI] [PubMed] [Google Scholar]
- 40.Peng, C. Y., Chen, T. C., Hung, S. P., Chen, M. F., Yeh, C. T., Tsai, S. L., Chu, C. M. & Liaw, Y. F. (2002) Anticancer Res. 22, 1265-1271. [PubMed] [Google Scholar]
- 41.Yang, B., Guo, M. Z., Herman, J. G. & Clark, D. P. (2003) Am. J. Pathol. 163, 1101-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Waris, G., Livolsi, A., Imbert, V., Peyron, J. F. & Siddiqui, A. (2003) J. Biol. Chem. 278, 40778-40787. [DOI] [PubMed] [Google Scholar]
- 43.Trovato, R., Cereseto, A., Takemoto, S., Gessain, A., Watanabe, T., Waldmann, T. & Franchini, G. (2000) AIDS Res. Hum. Retroviruses 16, 709-713. [DOI] [PubMed] [Google Scholar]
- 44.Bureau, C., Bernad, J., Chaouche, N., Orfila, C., Beraud, M., Gonindard, C., Alric, L., Vinel, J. P. & Pipy, B. (2001) J. Biol. Chem. 276, 23077-23083. [DOI] [PubMed] [Google Scholar]
- 45.Schreck, R., Grassmann, R., Fleckenstein, B. & Baeuerle, P. A. (1992) J. Virol. 66, 6288-6293. [DOI] [PMC free article] [PubMed] [Google Scholar]