Abstract
The role of infection with Mycoplasma hominis following cardiothoracic organ transplantation and its source of transmission have not been well-defined. Here, we identify and describe infection with M. hominis in patients following cardiothoracic organ transplantation after reviewing all cardiothoracic transplantations performed at our center between 1998 and July 2015. We found seven previously unreported cases of M. hominis culture positive infection all of whom presented with pleuritis, surgical site infection, and/or mediastinitis. PCR was used to establish the diagnosis in four cases. In two instances, paired single lung transplant recipients manifested infection, and in one of these pairs, isolates were indistinguishable by multilocus sequence typing (MLST). To investigate the prevalence of M. hominis in the lower respiratory tract, we tested 178 bronchoalveolar lavage (BAL) fluids collected from immunocompromised subjects for M. hominis by PCR; all were negative. Review of the literature revealed an additional 15 cases of M. hominis in lung transplant recipients, most with similar clinical presentations to our cases. We recommend that M. hominis should be considered in post-cardiothoracic transplant infections presenting with pleuritis, surgical site infection, or mediastinitis. M. hominis PCR may facilitate early diagnosis and prompt therapy. Evaluation for possible donor transmission should be considered.
Keywords: Mycoplasma hominis, Cardiothoracic transplantation, Infection
Highlights
-
•
M. hominis is a cause of cardiothoracic transplant infection, including pleuritis, mediastinitis or wound infection.
-
•
M. hominis cardiothoracic transplant infection may be donor-derived.
-
•
Most transplant prophylactic antibiotic regimes to prevent empirical infections do not cover M. hominis.
-
•
PCR is useful to detect M. hominis in cardiothoracic transplant donors and patients as the organism is difficult to culture.
M. hominis is a distinct cause of cardiothoracic transplant infection, presenting with pleuritis, surgical site infection, and/or mediastinitis. The infection is probably donor-derived. Immune defense against and airway colonization with mollicutes in healthy populations is poorly understood. Rapid PCR is an expedient method of detection but does not replace culturing the organism to determine the susceptibility profile. Currently, most antibiotic prophylaxis regimens, and regimens for empirical therapy of post-transplant infectious complications do not cover M. hominis. Prospective studies are needed to assess the correlation between donor airway positivity for M. hominis and clinical infection in transplant recipients.
1. Introduction
Mycoplasma hominis (M. hominis) is a mollicute that colonizes the urogenital tract and occasionally causes invasive disease. Extra-genital infections with this organism occur primarily in immunosuppressed persons. M. hominis has been linked to pregnancy-related complications and causes meningitis and pneumonia in neonates (Cassell et al., 1991, Samra et al., 2002, Waites et al., 1988). M. hominis is not visualizable by gram stain due to its lack of a cell wall, and although it may grow on standard aerobic or anaerobic bacterial culture plates, this method is insensitive and requires highly experienced laboratory personnel to recognize colonies of M. hominis. M. hominis-specific culture may be performed, but are not widely available, and even if they are available, is not rapid. The route of acquisition of M. hominis in patients who undergo cardiothoracic transplantation has not been defined. In two prior reports in Chest the authors speculated 1) the organism entered the bloodstream (though blood cultures were negative) of a lung transplant recipient who developed pleural and pulmonary infection with M. hominis. These authors speculated manipulation of the urinary tract with a Foley catheter elicited invasion of lung tissue damaged by transplantation (Lyon et al., 1997) and 2) an 18 year old women developed diffuse alveolar hemorrhage following bone marrow transplant due to unproven airway or urinary tract colonization (Kane et al., 1994). Herein, we describe seven new cases of M. hominis infection in cardiothoracic transplant recipients and review the literature on the topic. We highlight the unique clinical presentation of M. hominis in this patient population and present evidence suggesting that this infection may be donor-transmitted. M. hominis should be considered in the etiological diagnosis of surgical site infections, mediastinitis and pleuritis after lung or heart-lung transplantation; use of M. hominis-specific PCR may expedite diagnosis.
2. Methods
The study was reviewed and approved by the Mayo Clinic Institutional Review Board (14-007214). Individual patient consent was not needed.
2.1. Case Detection
Using our heart and lung transplant database and chart review (between 1998 and July 2015) we found seven (among 182 lung transplants and heart-lung performed and 453 heart transplants) previously unreported cases of culture positive M. hominis infection in cardiothoracic transplant recipients (Table 1). In each case routine cultures grew M. hominis and no other bacterial pathogens were identified. PCR for M. hominis (beginning January 2014), Ureaplasma urealyticum, and Ureaplasma parvum (beginning January 2015) were requested when suspected infection sites were gram stain negative, and early bacterial cultures were negative with the presence of white blood cells. M. hominis, Ureaplasma urealyticum, and Ureaplasma parvum PCR were specifically performed prospectively on all donor airway specimens beginning in 2015. Importantly, in each case reported herein M. hominis was the only infectious agent identified. None of these M. hominis cases developed hyperammonemia and none of these cases tested positive for Ureaplasma sp. However, we have recently identified a single case of donor bronchus which tested as M. hominis PCR negative and Ureaplasma urealyticum PCR positive as well as a single case of donor bronchus which tested as M. hominis PCR positive and Ureaplasma urealyticum PCR negative. Both cases were treated prophylactically without evidence of disease. All M. hominis cases and donor surveillance cases were reported to the host organ procurement organization as required by the Organ Procurement Transplant Network. Prior to 2009 all patient received cytolytic induction, after 2009 only heart recipients received cytolytic induction. All patients received intraoperative methylprednisolone. Post-transplant immunosuppression is listed in Table 1.
Table 1.
Summary of 7 Mayo Clinic cases of Mycoplasma hominis infection in thoracic transplant recipients.
| Case tran-plant (year) |
Age (years) Sex | Transplant indication | Transplant type Immune suppression |
Signs or symptoms (days after transplant) | Clinical presentation | Method used to diagnose M. hominis infection | Surgical management | Antimicrobial therapy | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| A (2000) | 44M | Secondary pulmonary hypertension | HL ATG/CSA/AZA/Pred |
Sternal site infection with sternal dehiscence (15) | Sternal wound infection | Culture-positive surgical debridement specimens | Debridement and sternectomy | Doxycycline and ciprofloxacin × 10 years | Recovered |
| B (2009) | 69M | Idiopathic pulmonary fibrosis | SL ATG/Tacro/AZA/Pred |
Dyspnea, unilateral pleural effusion (14) | Pleuritis | Culture positive pleural fluid | Catheter drainage of pleural fluid | Levofloxacin for 3 weeks | Recovered |
| C (2011) | 41F | Pulmonary hypertension | HL ATG/Tacro/Mycop/Pred |
Dyspnea, cough with progression to circulatory shock in 48 h (19) | Mediastinitis with aortic anastomotic leak | Culture positive pericardial fluid, periaortic tissue and BAL fluid | Mediastinal debridement (3) | Clindamycin for 4 weeks then doxycycline (lifelong) | Recovered |
| D (2014) | 64M | Idiopathic pulmonary fibrosis | SL Tacro/AZA/Pred |
Dyspnea, loculated pleural effusion (16) | Pleuritis | PCR- and culture-positive pleural fluid | Thoracotomy and debridement | Levofloxacin and doxycycline for 6 weeks, followed by doxycycline for 6 months | Slow recovery with multiple subsequent infectious complications |
| E (2015) | 64M | Chronic obstructive lung disease | SL Tacro/AZA/Pred |
Fever, leukocytosis and a loculated hydropneumothorax (7) | Pleuritis | PCR- and culture-positive pleural fluid; culture positive debridement specimens (multiple) | Thoracotomy and debridement, wound VAC | Doxycycline (lifelong) | Recovered |
| F (2015) | 70M | Idiopathic pulmonary fibrosis | SL (same donor as D) Tacro/AZA/Pred |
Dyspnea, and loculated pleural effusion (21) | Pleuritis | PCR- and culture-positive pleural fluid; culture-positive central venous catheter tip | Pleural fluid aspiration | Doxycycline (lifelong) | Recovered |
| G (2015) | 63M | Idiopathic pulmonary fibrosis | BL Tacro/Myco/Pred |
Subcutaneous emphysema (63) | Right bronchial anastomotic leak | PCR positive from both pretransplant donor bronchi and recipient pleural fluid; culture positive from both pretransplant donor brochi and later bronchial anastomotic eschar | Delayed bronchial anastomotic healing | Levofloxacin and doxycycline (lifelong) | Recovered |
M, male; F, female; HL, heart-lung transplant; BL, bilateral lung transplant; SL, single lung transplant; BAL, bronchoalveolar lavage; ATG, antithymocyte globulin; Tacro, tracrolimus; AZA, azathioprine; Pred, prednisone; Myco, mycophenolate mofetil.
2.2. Literature Review
PubMed was queried beginning in 1950 for M. hominis infection and cardiothoracic transplantation.
2.3. Surveillance Detection of M. hominis DNA in BAL Fluid from Non-cardiothoracic Transplant Immunocompromised Patients
BAL samples submitted for detecting organisms commonly found in immunocompromised hosts undergoing clinically indicated bronchoscopy were tested for M. hominis DNA. We used a previously-described real-time PCR assay targeting M. hominis tuf (Cunningham et al., 2013). DNA was extracted on the MagNA Pure LC instrument using the MagNA Pure total nucleic acid isolation kit (Roche Applied Science, Indianapolis, IN).
3. Results
3.1. Patient Evaluation and Donor Characteristics
All patients received standard immunosuppression. All patients had pre-transplant sputum cultures which were negative for M. hominis. Routine perioperative bacterial prophylaxis included Vancomycin for 48 h and Cefepime for 48–72 h. Trimethoprim/sulfamethoxasole was typically begun on post-operative day 2 or 3. Any additional bacterial prophylaxis was based upon donor and recipient airway cultures. In all cases clinical signs, including prolonged pleural effusion, prompted routine bacterial and fungal cultures of suspected sites of infection. M. hominis was consider etiologic if routine cultures were negative and M. hominis was identified by culture or PCR in presumed sterile sites. Donor characteristics are summarized in Table 2. Although our observations are limited to seven distinct donors among eight recipient cases (including a previously reported case Wylam et al., 2013), several characteristics are noted. The average donor age was 19.7 years (16–23). Six of seven donors had a history of, or tested positive for, drugs of abuse, including methamphetamine and marijuana. In addition, 5 of 7 donors were known tobacco users.
Table 2.
Donor characteristics associated with the cases described in table and our case described in Reference (Wylam et al., 2013).
| Case | Donor age (years) Sex | Donor race | Donor serologies | Drug screen | Other drug history | Tobacco | Donor mechanism of death |
|---|---|---|---|---|---|---|---|
| A | 22 F | Caucasian | CMV +/EBV + | Methampetamine, tricyclic antidepressant, acetaminophen, aspirin | Alcohol | No | Drug overdose |
| B | 23 M | Hispanic | CMV +/EBV + | Marajuana, ETOH | Alcohol | Yes | CHI |
| C | 19 M | Caucasian | CMV +/EBV + | Negative | Marajuana | Not known | CHI |
| D/E | 16 M | African American | CMV +/EBV + | Negative | Marajuana | Yes | GSW to head |
| F | 23 M | Caucasian | CMV −/EBV + | Negative | Alcohol | Yes, chewing | CHI/MVA |
| G | 19 F | Caucasian | CMV +/EBV + | Methampetamine | Alcohol | Yes | CHI/MVA |
| Ref (Wylam et al., 2013) | 16 M | African American | CMV +/EBV + | Marajuana | Alcohol | Yes | CHI/MVA |
M, male; F, female; CMV, cytomegalovirus; EBV, Epstein-Barr virus; GSW, gun shot wound; CHI, closed head injury; MVA, motor vehicle accident.
3.2. Literature Review
Our review of the literature since 1950 identified 15 cases of M. hominis infection in lung, heart or heart-lung transplant recipients (Table 3), one of which was reported from our institution (Wylam et al., 2013). Including our seven new cases, the mean age of the 22 recipients was 47.9 years with 72.7% being male. Pulmonary hypertension, chronic obstructive pulmonary disease (COPD) and pulmonary fibrosis were the most frequent indications for transplantation. Patients developed symptoms a mean of 17.1 days (range 4–63 days) following transplantation. Fever was reported in 8/22 (36%). Pleural/pericardial effusion, chest wall/sternal wound infection, or M. hominis bacteremia was evident in 13 (59%) and 12 (55%), and 2 (9%), respectively. All 13 patients with pleural effusions had positive pleural fluid cultures for M. hominis. Twelve (55%) patients had M. hominis cultured from more than one site; M. hominis was isolated from BAL fluid in seven cases.
Table 3.
Literature review of thoracic transplant recipient infection with Mycoplasma hominis.
| Case (Ref) |
Age (years) Sex | Transplant indication | Transplant type | Signs or symptoms (days after transplant) | Cultures positive for M. hominis | Surgical management | Antimicrobial therapy | Outcome |
|---|---|---|---|---|---|---|---|---|
| 1 (Hopkins et al., 2002) | 52 M | Bronchiectasis | BL | Bilateral pleural effusions, thoracotomy dehiscence, bronchial anastomostic dehiscence (14) | Wound swab and debrided tissue | Bronchial stent placement | Clindamycin and ciprofloxacin followed by oral doxycycline for 6 weeks | Recovered |
| 2 (Hopkins et al., 2002) | 55 M | Ischemic cardiomyopathy | H | Sternal dehiscence, fever (8) | Sternal wound swab, pleural fluid | Surgical debridement, drainage | Ciprofloxacin and doxcycline for 14 days | Recovered |
| 3 (Hopkins et al., 2002) | 21 M | Dilated cardiomyopathy | H | Sternal wound infection (14) | Sternal wound, pericardial and pleural fluid | Surgical debridement, drains | Ciprofloxacin and doxcycline | Recovered |
| 4 (Hopkins et al., 2002) | 17 M | Becker cardiomyopathy | H | Sternal wound, pleural effusion (21) | Sternal wound, pericardial and pleural fluid | Surgical irrigation | Ciprofloxacin and doxcycline for 3 weeks | Recovered |
| 5 (Lyon et al., 1997) | 34 M | APL and pulmonary hypertension | BL | Pulmonary infiltrates, pleural effusions (5) | Pleural fluid, bronchial brush specimen, lung biopsy | Surgical debridement | Doxycycline for 4 weeks | Recovered, replased M. hominis sternal wound infection on day 108 |
| 6 (Mitsani et al., 2010) | 64 M | Emphysema | BL | Tachycardia, tachypnea (36) | Pericardial fluid | Pericardiocentesis | Doxycycline for 6 week | Recovered |
| 7 (Steffenson et al., 1987) | 30 F | Pulmonary hypertension | HL | Cough (3), unstable sternum, fever (24) | Sternum, BAL | Surgical debridement + muscle flap | Clindamycin and doxycycline and gentamicin; duration unknown | Died, day 69 post-transplant |
| 8 (Steffenson et al., 1987) | 43 F | Pulmonary hypertension | HL | Dyspnea, pulmonary infiltrates (13), erythema sternal tenderness (17) | Sternal wound, BAL |
Superficial I&D with wound packing | Doxycycline and clindamycin and gentamicin | Died, day 37 post-transplant |
| 9 (Boyle et al., 1993) | 48 M | Eisenmenger syndrome | HL | Unstable sternum, fever (30) | Sternal wound | Surgical debridement + muscle flap over sternum | Doxycycline for 6 weeks | Relapse of M. hominis empyema seven months later. Placed on doxycycline suppression |
| 10 (Mossad et al., 1996) | 41 M | Cystic fibrosis | BL | Sternal wound drainage (22) | Sternal wound culture | Surgical debridement and omental muscle flap | Erythromycin for 14 weeks | Recovered |
| 11 (Gass et al., 1996) | 65 M | Alpha-1 antitrypsin deficiency | BL | Pulmonary infiltrates, pleural effusion, fever (7) | Pleural fluid, BAL | Not available | Ciprofloxacin for 4 weeks | Recovered |
| 12 (Gass et al., 1996) | 50 M | Emphysema | SL | Pleural effusion, dyspnea (5) | Pleural fluid | None | Ciprofloxacin for 4 weeks | Recovered |
| 13 (Mcmahon et al., 1990) | 31 F | Not available | HL | Sternal wound drainage, fever (6) | BAL, sternal wound culture | Debridement + muscle flaps | Clindamycin and doxycycline for 3 weeks | Dieda |
| 14 (Mcmahon et al., 1990) | 43 F | Not available | HL | Sternal wound drainage, fever (13) | BAL, sternal wound | Unknown | Clindamycin and doxycycline for 3 weeks | Diedb |
| 15 (Wylam et al., 2013) | 64 F | Idiopathic pulmonary fibosis | BL | Confusion, fever, pulmonary infiltrates, coma, hyperammon-emia (4) | Catheter tip (multiple post-mortem tissues PCR positive for M. hominis) | None | Ciprofloxacin | Died |
M, male; F, female; HL, heart-lung transplant; BL, bilateral lung transplant; SL, single lung transplant; BAL, bronchoalveolar lavage; H, heart; APL, antiphospholipid antibody syndrome.
After complications with rejection & respiratory failure. Cultures for M. hominis were negative at that time.
Developed an unstable sternum and CMV pneumonitis.
3.3. Detection of M. hominis DNA in BAL Fluid from Immunocompromised Patients
In 178 BAL samples obtained from random immunocompromised non-cardiothoracic transplant patients submitted for detecting organisms commonly found in immunocompromised hosts M. hominis DNA was not detected in any specimen.
3.4. Assessment for Donor-derived Infection
In our Case B of culture proven M. hominis single lung transplant associated pleuritis, the donor's other lung was transplanted into a 67 year old female (not included in this report) at our institution who developed superficial wound cellulitis, chest pain and a right-sided hydropneumothorax in the second week post-surgery. A pleural pigtail catheter was placed and routine bacterial cultures on standard media were negative. M. hominis PCR testing was not performed. However, imaging showed persistent pleural thickening with fluorodeoxyglucose uptake in the right thoracic cage. She received levofloxacin for diverticulitis and improved clinically. Together, this data suggest the possibility of undiagnosed M. hominis infection.
In case G, the donor bronchus culture swab was PCR positive for M. hominis. Levofloxacin was given for 2 weeks and doxycycline (lifelong) in addition to standard prophylaxis with Vancomycin and Cefepime. Recently, (data not included) we have prophylactically treated one patient whose donor bronchus culture swab was PCR positive for M. hominis and one patient who's donor bronchus culture swab was PCR positive for Ureaplasma urealyticum. Each patient was treated with doxycycline for 28 days.
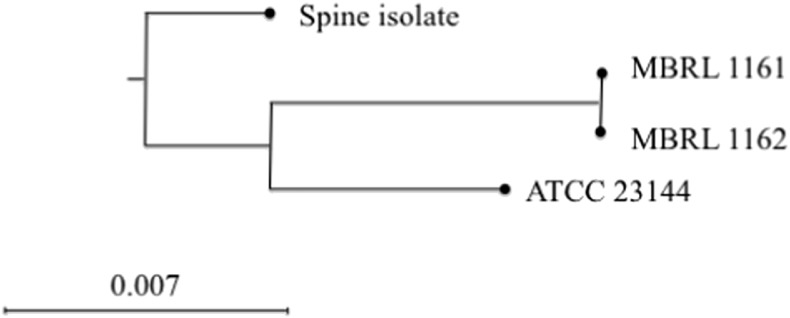
Cases E and F (Table 1, Fig. 1) had received single lung transplants from the same donor and each case had transplant site specific infection. Multilocus sequence typing (MLST) was performed on isolates recovered from these two cases and two additional reference isolates (a clinical isolate and the type strain ATCC 23114), using methods described by Sogaard et al. (Sogaard et al., 2002). Briefly, PCR was performed targeting housekeeping genes, tuf, ftsY, hitB-hitL, uvrA and gap, followed by bidirectional Sanger sequencing. Sequencing data were analyzed (trimming and contig assembly) with the CLC Genomics Workbench (CGW) version 8.0.3 (CLC bio, Cambridge, MA) sequence analysis module. For each target, sequences were aligned and identities calculated using the CGW alignment and tree module. Alignments of the housekeeping genes were joined and a phylogenetic tree constructed using the CGW alignment and tree module. Maximum-likelihood and neighbor joining trees with Jukes-Cantor correction and bootstrap analysis (1000 replicates) were constructed with the CGW software. Isolates recovered from cases D and E were indistinguishable by MLST, with no base pair differences across the five sequenced housekeeping genes (3094 bases). These results suggest common source infection, such as donor-derived infection. No other patients on their hospital unit, other hospital units, or under their surgeons care were infected by M. hominis.
Fig. 1.
Phylogenetic tree based on multilocus sequence typing showing the M. hominis isolates from case D (MBRL 1161) and case E (MBRL 1162) to be indistinguishable. The spine isolate represents an isolated from a contemporaneous clinical case of M. hominis spinal osteomyelitis; ATCC 23114 is the reference strain.
4. Discussion
M. hominis infection in cardiothoracic transplant recipients has been uncommonly reported. The true infection rate may be underestimated in view of difficulties in detecting the organism in clinical specimens and in the use of antibiotic prophylaxis and empiric antimicrobial regimens, such as fluoroquinolones, that do target M. hominis. Cardiothoracic transplant patients sustain all of the risks for infection generally encountered in the routine practice of cardiovascular surgery, including Staphylococcus species, Pseudomonas, and Serratia, but fungi especially Aspergillus species and Candida contribute importantly (Stinson and Oyer, 1995). Donor transmission of infection remains important. Surveillance cultures of donor and recipient, and antimicrobial prophylaxis remain the cornerstone of infection prevention. In the report herein, we note infection with M. hominis in the absence of other known infection. The manifestations were often delayed after transplantation by several weeks, and included pleuritis with cellulitis, trapped lung, delayed airway anastomotic healing, and aortic and sternal dehiscence. Treatment included surgical intervention and long-term antibiotics.
M. hominis is not stainable by Gram stain and recognition of its pinpoint-sized colonies requires experienced laboratory personnel. BacT/ALERT and BACTEC media (used for blood cultures) contain sodium polyanethol sulfonate, which may inhibit Mycoplasma growth (Waites and Canupp, 2001), and growth may not exceed the threshold value for positivity (Waites and Canupp, 2001). Selective M. hominis culture media may be useful but remain laborious and expensive.
Since the mid-1990s DNA probe methods using PCR to identify the nucleotide sequences of M. hominis have been developed which seek to avoid diagnostic time delays while offering the sensitivity and specificity of conventional culture methods (Yoshida et al., 2003). PCR assays have been designed to target the glyceraldehyde-3-phosphate dehydrogenase gene (gap) (Waites et al., 2009), yidC (Zhang and Wise, 1996), the M. hominis 16S ribosomal RNA gene (Schwab et al., 2012), and, as used herein, tuf (Cunningham et al., 2013). We previously reported (in genitourinary specimens) a clinical sensitivity and specificity of 90.7% (95% CI: 77.4%, 97.3%) and 99.2% (95% CI: 97.0%, 99.9%), respectively (Cunningham et al., 2013). For the detection of M. hominis PCR tests are faster than culture tests using media supplemented with arginine, allowing the time to diagnosis to be reduced from two to five days to < 24 h (Abele-Horn et al., 1996). To date the Federal Drug Administration Center for Devices and Radiologic Health has not approved a nucleic acid based test for the detection of M. hominis. Thus, in the U.S. transplant physician will need to consider reference laboratories to perform PCR testing as well as dedicated M. hominis cultures and empiric treatment for suspected cases of M. hominis infection.
The mode of acquisition of M. hominis in cardiothoracic transplant recipients has been uncertain. Colonization rates of donors and recipients have hitherto been uncharacterized (Waites and Canupp, 2001). Lacking the genes coding for a cell wall appears to limit M. hominis to a parasitic and saprophytic existence. There are no known natural reservoirs for M. hominis though it has been recovered from random public toilet rims (Potasman et al., 1999). M. hominis is part of the normal urogenital flora, suggesting a potential endogenous source. Thus, others have speculated without data that M. hominis enters the bloodstream from manipulation of the urethra by Foley catheter placement in colonized recipients (Lyon et al., 1997). This rationale is supported by the finding of M. hominis sternal wound infection in non-immunocompromised patients undergoing coronary artery bypass grafting without transplantation (Steffenson et al., 1987). A case cluster involving four cases alluded to a common environmental source (Hopkins, 2001, Hopkins et al., 2002), with a suspicion of nosocomial transmission in the four reported cases of M. hominis infection occurring over three weeks in the same hospital (Hopkins et al., 2002).
Primary immunodeficiencies are associated with sepsis and septic arthritis due to M. hominis. Most reports include patients with common variable immunodeficiency or X-linked agammaglobulinemia (Bloom et al., 2008). Though postpartum septicemia caused by M. hominis may be due to genitourinary bacterial translocation (Tully and Smith, 1968), immunodeficiency cases commonly are not associated with urinary tract manipulation. This suggests that dissemination of M. hominis may be from acquired from sites outside the genitourinary tract. Though the mechanism(s) required for immunocompetence against M. hominis is not clear, we note that in all of our cases pre-transplant immunoglobulin IgA, IgM, IgG and IgG subclasses were determined and values were normal (data not shown) in all cases.
The colonization rate of heart and lung transplant donors with M. hominis is not known and may be different from the general population. M. hominis may induce exudative pharyngitis (Mufson et al., 1965) and older reports using culture suggest reported it to be recoverable from the respiratory secretions of 1–3% of healthy persons (Hendley and Jordan, 1968) and of ≤ 8% of persons with chronic respiratory disease (Mufson, 1983). Thus, there is the possibility of transmission related to donor airway colonization, or organ contamination during organ procurement. Donor oral sexual practice may be a risk factor for M. hominis airway colonization as oral sex has been noted as an important route of transmission for M. hominis (Edwards and Carne, 1998, Sackel et al., 1979). Our review of the literature (Table 3, cases 11 and 12) describes two recipients who had received lungs from the same donor, supporting the possibility of a donor-derived infection (Gass et al., 1996). Two of their cases (heart and bilateral lung recipients) shared the same donor, although cultures of donor bronchus did not grow M. hominis; it is unknown whether M. hominis-specific cultures were performed.
Our observations in cases B, E, F, and G suggest that in cardiothoracic transplant recipients, donor-derived acquisition of M. hominis is possible. The likelihood that the disease is of donor origin fulfills the term probable donor transmission established by Project NOTIFY (Garzoni and Ison, 2011) as (1) we cannot exclude pre-transplant disease in the recipient; but (2) the disease was documented in more than one recipient (cases E and F) and (3) we provide epidemiologic evidence reporting that in cases D and E there was strain as the isolates were indistinguishable via MLST. Due to a lack of residual specimen, it was not possible to isolate the organism from the donor respiratory tract via culture or detect it by PCR. However, in case G, a donor bronchus swab (the first swab we tested prospectively) tested positive PCR for M. hominis. Our surveillance data showing the absence of M. hominis DNA by PCR in the BAL samples of unselected immunocompromised hosts suggests that a positive test should be considered significant in the appropriate clinical context. Further studies are needed to evaluate the correlation between clinical infection in cardiothoracic transplant recipients and M. hominis positivity in the donor respiratory tract.
We have previously reported detection of M. hominis infection in a case of post-lung transplant hyperammonemia (Wylam et al., 2013); the patient had a positive central venous blood culture for M. hominis and PCR and immunofluorescence detection of M. hominis in multiple post-mortem tissues. Follow-up testing showed the patient to have been co-infected with Ureaplasma parvum (Bharat et al., 2015). In follow-up studies, Ureaplasma species, both U. parvum and Ureaplasma urealyticum (without M. hominis) were shown to be associated with hyperammonemia in five other lung transplant recipients (Bharat et al., 2015). Therefore, whether or not M. hominis plays a role in hyperammonemia in lung transplant patients is unclear. In our cases D, E, F, and G, blood ammonia determined and was not elevated, suggesting that non-systemic M. hominis infection is insufficient to cause hyperammonemia in lung transplant recipients.
As M. hominis does not produce peptidoglycan, cell wall active antibiotics like β-lactams and vancomycin, commonly used post-transplant, are ineffective. Moreover, M. hominis does not require folate and is resistant to folate antagonists (such as trimethoprim/sulfamethoxazole); it is also resistant to aminoglycosides (Sielaff et al., 1996). M. hominis is naturally resistant to 14- and 15-membered macrolides, including erythromycin and azithromycin, but is usually susceptible to clindamycin (Furneri et al., 2000). One study tested 110 clinical isolates of M. hominis and found levofloxacin to be inhibitory in all instances, with an MIC90 of 0.19 μg/ml (Samra et al., 2002). Treatment recommendations for M. hominis infection therefore include tetracyclines (doxycycline is preferred), fluoroquinolones and/or clindamycin. Importantly, tetracyclines, fluroquionolones and clindamycin are not typically included in standard post-transplant prophylactic regimens, nor are they typically included in empiric therapy regimens for surgical site infections. The tet(M) determinant confers tetracycline (and doxycycline) resistance; the prevalence of tetracycline resistance in M. hominis varies by geographic location and may be as high as 30% (Pogodina, 1975, Samra et al., 2002, Ye et al., 2014). Although susceptibility testing should be used when available, in the absence of definitive evidence, we suggest that combination therapy with a fluoroquinolone and doxycycline may be a preferred regimen for established M. hominis infection in cardiothoracic transplant recipients, given the possibility of tetracycline resistance and the potential for selection of fluoroquinolone resistance with fluoroquinolone monotherapy (Bebear et al., 2003). The optimal duration of therapy for M. hominis infection in cardiothoracic transplant recipients is not known.
In summary, M. hominis is a cause of cardiothoracic transplant infection, presenting with pleuritis, surgical site infection, and/or mediastinitis. We provide evidence that this infection is probably donor-derived. Rapid PCR is an expedient method of detection, used to diagnose four of seven of the cases reported, but does not replace culturing the organism to determine the susceptibility profile. Our current approach is to perform routine PCR for M. hominis, Ureaplasma urealyticum and U. parvum and to perform bacterial culture all donor airways. We perform the same PCR tests on any suspected surgical site infection which is gram stain negative. In addition, we reflexively culture any PCR-positive specimens (e.g., pleural fluid) from cardiothoracic transplant recipients for the purpose of susceptibility testing. Currently, most antibiotic prophylaxis regimens, and regimens for empirical therapy of post-transplant infectious complications do not cover M. hominis. Prospective studies are needed to assess the correlation between donor airway positivity for M. hominis and clinical infection in transplant recipients.
Funding Source
None.
Conflict of Interest
RS, RP, SAC, JJS, SA, RCD, ADB and MEW have no conflicts of interest.
Author Contributions
MEW, PR, RCD and ADB recognized the potential for investigation. SAC and SA performed the PCR and multilocus analysis. RS performed the literature review and database analysis. RS and MEW drafted and MEW, RP and ADB revised the manuscript. All authors approve of the final version of the manuscript.
References
- Abele-Horn M., Wolff C., Dressel P., Zimmermann A., Vahlensieck W., Pfaff F., Ruckdeschel G. Polymerase chain reaction versus culture for detection of Ureaplasma urealyticum and Mycoplasma hominis in the urogenital tract of adults and the respiratory tract of newborns. Eur. J. Clin. Microbiol. Infect. Dis. 1996;15:595–598. doi: 10.1007/BF01709369. [DOI] [PubMed] [Google Scholar]
- Bebear C.M., Renaudin H., Charron A., Clerc M., Pereyre S., Bebear C. DNA gyrase and topoisomerase IV mutations in clinical isolates of Ureaplasma spp. and Mycoplasma hominis resistant to fluoroquinolones. Antimicrob. Agents Chemother. 2003;47:3323–3325. doi: 10.1128/AAC.47.10.3323-3325.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharat A., Cunningham S.A., Scott Budinger G.R., Kreisel D., Dewet C.J., Gelman A.E., Waites K., Crabb D., Xiao L., Bhorade S., Ambalavanan N., Dilling D.F., Lowery E.M., Astor T., Hachem R., Krupnick A.S., Decamp M.M., Ison M.G., Patel R. Disseminated Ureaplasma infection as a cause of fatal hyperammonemia in humans. Sci. Transl. Med. 2015;7:284re3. doi: 10.1126/scitranslmed.aaa8419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom K.A., Chung D., Cunningham-Rundles C. Osteoarticular infectious complications in patients with primary immunodeficiencies. Curr. Opin. Rheumatol. 2008;20:480–485. doi: 10.1097/BOR.0b013e3282fd6e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle E.M., Jr., Burdine J., Bolman R.M., III Successful treatment of Mycoplasma mediastinitis after heart-lung transplantation. J. Heart Lung Transplant. 1993;12:508–512. [PubMed] [Google Scholar]
- Cassell G.H., Waites K.B., Crouse D.T. Perinatal mycoplasmal infections. Clin. Perinatol. 1991;18:241–262. [PubMed] [Google Scholar]
- Cunningham S.A., Mandrekar J.N., Rosenblatt J.E., Patel R. Rapid PCR detection of Mycoplasma hominis, Ureaplasma urealyticum, and Ureaplasma parvum. Int. J. Bacteriol. 2013;2013:168742. doi: 10.1155/2013/168742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S., Carne C. Oral sex and transmission of non-viral STIs. Sex. Transm. Infect. 1998;74:95–100. doi: 10.1136/sti.74.2.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furneri P.M., Rappazzo G., Musumarra M.P., Tempera G., Roccasalva L.S. Genetic basis of natural resistance to erythromycin in Mycoplasma hominis. J. Antimicrob. Chemother. 2000;45:547–548. doi: 10.1093/jac/45.4.547. [DOI] [PubMed] [Google Scholar]
- Garzoni C., Ison M.G. Uniform definitions for donor-derived infectious disease transmissions in solid organ transplantation. Transplantation. 2011;92:1297–1300. doi: 10.1097/TP.0b013e318236cd02. [DOI] [PubMed] [Google Scholar]
- Gass R., Fisher J., Badesch D., Zamora M., Weinberg A., Melsness H., Grover F., Tully J.G., Fang F.C. Donor-to-host transmission of Mycoplasma hominis in lung allograft recipients. Clin. Infect. Dis. 1996;22:567–568. doi: 10.1093/clinids/22.3.567. [DOI] [PubMed] [Google Scholar]
- Hendley J.O., Jordan W.S., Jr. Mycoplasma pharyngeal flora in civilians. Am. Rev. Respir. Dis. 1968;97:524–532. doi: 10.1164/arrd.1968.97.4.524. [DOI] [PubMed] [Google Scholar]
- Hopkins P. A cluster of mycoplasma hominis infection in heart-lung transplantation. J. Heart Lung Transplant. 2001;20:223–224. doi: 10.1016/s1053-2498(00)00492-7. [DOI] [PubMed] [Google Scholar]
- Hopkins P.M., Winlaw D.S., Chhajed P.N., Harkness J.L., Horton M.D., Keogh A.M., Malouf M.A., Glanville A.R. Mycoplasma hominis infection in heart and lung transplantation. J. Heart Lung Transplant. 2002;21:1225–1229. doi: 10.1016/s1053-2498(02)00427-8. [DOI] [PubMed] [Google Scholar]
- Kane J.R., Shenep J.L., Krance R.A., Hurwitz C.A. Diffuse alveolar hemorrhage associated with Mycoplasma hominis respiratory tract infection in a bone marrow transplant recipient. Chest. 1994;105:1891–1892. doi: 10.1378/chest.105.6.1891. [DOI] [PubMed] [Google Scholar]
- Lyon G.M., Alspaugh J.A., Meredith F.T., Harrell L.J., Tapson V., Davis R.D., Kanj S.S. Mycoplasma hominis pneumonia complicating bilateral lung transplantation: case report and review of the literature. Chest. 1997;112:1428–1432. doi: 10.1378/chest.112.5.1428. [DOI] [PubMed] [Google Scholar]
- Mcmahon D.K., Dummer J.S., Pasculle A.W., Cassell G. Extragenital Mycoplasma hominis infections in adults. Am. J. Med. 1990;89:275–281. doi: 10.1016/0002-9343(90)90338-e. [DOI] [PubMed] [Google Scholar]
- Mitsani D., Nguyen M.H., Silveira F.P., Bermudez C., Toyoda Y., Pasculle A.W., Clancy C.J. Mycoplasma hominis pericarditis in a lung transplant recipient: review of the literature about an uncommon but important cardiothoracic pathogen. Transpl. Infect. Dis. 2010;12:146–150. doi: 10.1111/j.1399-3062.2009.00457.x. [DOI] [PubMed] [Google Scholar]
- Mossad S.B., Rehm S.J., Tomford J.W., Isada C.M., Taylor P.C., Rutherford I., Sorg S., Mchenry M.C. Sternotomy infection with Mycoplasma hominis: a cause of “culture negative” wound infection. J. Cardiovasc. Surg. 1996;37:505–509. [PubMed] [Google Scholar]
- Mufson M.A. Mycoplasma hominis: a review of its role as a respiratory tract pathogen of humans. Sex. Transm. Dis. 1983;10:335–340. [PubMed] [Google Scholar]
- Mufson M.A., Ludwig W.M., Purcell R.H., Cate T.R., Taylor-Robinson D., Chanock R.M. Exudative pharyngitis following experimental Mycoplasma hominis: type 1 infection. JAMA. 1965;192:1146–1152. doi: 10.1001/jama.1965.03080260034010. [DOI] [PubMed] [Google Scholar]
- Pogodina V.V. Elizaveta Nilolaevna Levkovich-75th birthday. Acta Virol. 1975;19:509. [PubMed] [Google Scholar]
- Potasman I., Oren A., Srugo I. Isolation of Ureaplasma urealyticum and Mycoplasma hominis from public toilet bowls. Infect. Control Hosp. Epidemiol. 1999;20:66–68. doi: 10.1086/501545. [DOI] [PubMed] [Google Scholar]
- Sackel S.G., Alpert S., Fiumara N.J., Donner A., Laughlin C.A., Mccormack W.M. Orogenital contact and the isolation of Neisseria gonorrhoeae, Mycoplasma hominis, and Ureaplasma urealyticum from the pharynx. Sex. Transm. Dis. 1979;6:64–68. doi: 10.1097/00007435-197904000-00004. [DOI] [PubMed] [Google Scholar]
- Samra Z., Rosenberg S., Soffer Y. In vitro susceptibility of Mycoplasma hominis clinical isolates to tetracyclines, quinolones and macrolides. Diagn. Microbiol. Infect. Dis. 2002;44:359–361. doi: 10.1016/s0732-8893(02)00459-5. [DOI] [PubMed] [Google Scholar]
- Schwab J.J., C. S.A., Zomok C.D., Roden A.C., Patel R. 2012. Is Tropheryma whipplei or Mycoplasma hominis DNA Present in Bronchoalveolar Lavage Fluid from Immunocompromised Hosts? American Society for Microbiology General Meeting in San Francisco, June 16–19, 2012. [Google Scholar]
- Sielaff T.D., Everett J.E., Shumway S.J., Wahoff D.C., Bolman R.M., III, Dunn D.L. Mycoplasma hominis infections occurring in cardiovascular surgical patients. Ann. Thorac. Surg. 1996;61:99–103. doi: 10.1016/0003-4975(95)00826-8. [DOI] [PubMed] [Google Scholar]
- Sogaard I.Z., Boesen T., Mygind T., Melkova R., Birkelund S., Christiansen G., Schierup M.H. Recombination in Mycoplasma hominis. Infect. Genet. Evol. 2002;1:277–285. doi: 10.1016/s1567-1348(02)00036-9. [DOI] [PubMed] [Google Scholar]
- Steffenson D.O., Dummer J.S., Granick M.S., Pasculle A.W., Griffith B.P., Cassell G.H. Sternotomy infections with Mycoplasma hominis. Ann. Intern. Med. 1987;106:204–208. doi: 10.7326/0003-4819-106-2-204. [DOI] [PubMed] [Google Scholar]
- Stinson E.B., Oyer P.E. Thoracic transplantation. In: Shumway S.J., Shumway N.E., editors. Thoracic Transplantation. Blackwell Science, Inc.; Cambridge: 1995. [Google Scholar]
- Tully J.G., Smith L.G. Postpartum septicemia with Mycoplasma hominis. JAMA. 1968;204:827–828. [PubMed] [Google Scholar]
- Waites K.B., Canupp K.C. Evaluation of BacT/ALERT system for detection of Mycoplasma hominis in simulated blood cultures. J. Clin. Microbiol. 2001;39:4328–4331. doi: 10.1128/JCM.39.12.4328-4331.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waites K.B., Rudd P.T., Crouse D.T., Canupp K.C., Nelson K.G., Ramsey C., Cassell G.H. Chronic Ureaplasma urealyticum and Mycoplasma hominis infections of central nervous system in preterm infants. Lancet. 1988;1:17–21. doi: 10.1016/s0140-6736(88)91002-1. [DOI] [PubMed] [Google Scholar]
- Waites K.B., Schelonka R.L., Xiao L., Grigsby P.L., Novy M.J. Congenital and opportunistic infections: Ureaplasma species and Mycoplasma hominis. Semin. Fetal Neonatal Med. 2009;14:190–199. doi: 10.1016/j.siny.2008.11.009. [DOI] [PubMed] [Google Scholar]
- Wylam M.E., Kennedy C.C., Hernandez N.M., Peters S.G., Maleszewski J.J., Cassivi S.D., Scott J.P. Fatal hyperammonaemia caused by Mycoplasma hominis. Lancet. 2013;382:1956. doi: 10.1016/S0140-6736(13)62115-7. [DOI] [PubMed] [Google Scholar]
- Ye G., Jiang Z., Wang M., Huang J., Jin G., Lu S. The resistance analysis of Ureaplasma urealyticum and Mycoplasma hominis in female reproductive tract specimens. Cell Biochem. Biophys. 2014;68:207–210. doi: 10.1007/s12013-013-9691-8. [DOI] [PubMed] [Google Scholar]
- Yoshida T., Maeda S., Deguchi T., Miyazawa T., Ishiko H. Rapid detection of Mycoplasma genitalium, Mycoplasma hominis, Ureaplasma parvum, and Ureaplasma urealyticum organisms in genitourinary samples by PCR-microtiter plate hybridization assay. J. Clin. Microbiol. 2003;41:1850–1855. doi: 10.1128/JCM.41.5.1850-1855.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Wise K.S. Molecular basis of size and antigenic variation of a Mycoplasma hominis adhesin encoded by divergent vaa genes. Infect. Immun. 1996;64:2737–2744. doi: 10.1128/iai.64.7.2737-2744.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]