Abstract
The frequent expression of latent membrane proteins LMP2A and LMP2B in Epstein Barr virus (EBV)-associated tumors suggests that these proteins play a role in EBV-induced epithelial cell growth transformation. Expression of LMP2A and LMP2B had no effect on the morphology of squamous epithelial cells in monolayer culture, but their expression was associated with an increased capacity to spread and migrate on extracellular matrix. Although the mechanisms by which LMP2A and LMP2B promote cell spreading and motility are unclear, the use of selective pharmacological inhibitors has established a role for tyrosine kinases in this phenotype but ruled out contributions of phosphatidylinositol 3-kinase, extracellular signal-regulated kinase/mitogen-activated protein kinase, and protein kinase C. The ability of LMP2B to induce a phenotype that is virtually indistinguishable from that of LMP2A suggests that regions of the LMP2 protein in addition to the cytosolic amino terminus are capable of inducing phenotypic effects in epithelial cells. Thus, rather than serving to modulate the activity of LMP2A, LMP2B may directly engage signaling pathways to influence epithelial cell behavior such as cell adhesion and motility.
Epstein-Barr virus (EBV) is a ubiquitous human herpesvirus that is carried by greater than 90% of the population. Scientific interest in EBV stems from the finding that it is causally associated with a variety of B-cell malignancies (Burkitt's lymphoma, immunoblastic lymphoma, and Hodgkin's disease [HD]) and epithelial cell malignancies (nasopharyngeal carcinoma [NPC] and gastric adenocarcinomas) (54). The exact contribution of EBV to the development of NPC and other carcinomas is unclear, although emerging data suggest that viral infection may be a secondary event in tumor pathogenesis (40). Although viral infection may constitute a relatively late event in carcinoma formation, the finding that both NPC and EBV-positive gastric carcinoma tumor cells carry monoclonal viral genomes indicates that EBV infection must have occurred prior to the expansion of the malignant cell clone (33, 52).
A novel form of virus-cell interaction has been demonstrated in NPC and HD tumor cells, with the pattern of viral gene expression restricted to Epstein-Barr nuclear antigen 1 and variable but consistent expression of the latent membrane proteins (latent membrane protein 1 [LMP1], LMP2A, and LMP2B) (7, 9, 13). The consistent detection of LMP2A and LMP2B in EBV-associated malignancies such as HD, NPC, and gastric adenocarcinoma suggests that these viral proteins may participate in disease pathogenesis.
Unlike LMP1, much less is known about the function of LMP2A and LMP2B in epithelial cells. LMP2 is a hydrophobic membrane protein that exists as two alternative forms, LMP2A and LMP2B (42). These forms are transcribed across the fused terminal repeats of the EBV episome from promoters 3 kb apart which generate mRNAs with eight common exons and a 5′ exon unique to each type. The 5′ exon of LMP2B is noncoding, whereas the 5′ exon of LMP2A encodes a 119-amino-acid cytoplasmic domain which is implicated in cell signaling (2). The proteins share other structural properties including 12 hydrophobic membrane-spanning domains and a 27-amino-acid cytosolic carboxy terminus.
Although initial studies indicated that LMP2A and LMP2B target the plasma membrane in lymphoblastoid cell lines (44), further investigation revealed that they are broadly distributed among intracellular membranes (43). This intracellular localization has been substantiated with recent findings in non-B cells, where most, if not all, LMP2A and LMP2B localizes to perinuclear endosomes (17, 46). Most of the functional investigations of LMP2A and LMP2B have been performed in B cells by using recombinant EBV and indicate that LMP2A and LMP2B are dispensable for B-cell transformation in vitro (44, 45, 58). Although LMP2A and LMP2B do not appear to play significant roles in B-cell transformation in vitro, LMP2A plays a critical role in maintaining EBV latency. In latently infected B cells, the switch from a latent to a lytic infection program is regulated by engagement of the B-cell receptor (BCR). LMP2A negatively regulates BCR signaling by (i) excluding the BCR from “lipid rafts” (19) and (ii) targeting Src family members Lyn and Syk protein tyrosine kinases (PTKs) for ubiquitin-mediated degradation (32). In doing so, LMP2A blocks BCR-mediated intracellular calcium release and PTK cascades, the net effect of which is to block B-cell differentiation (42).
Extensive mutational analysis has identified particular signatures and motifs within the cytosolic amino terminus of LMP2A that are essential for function (25, 26, 59). These motifs include tyrosines located at positions 74 and 85 (Y74/Y85), which constitute a putative immunoreceptor tyrosine activation motif, and a tyrosine at position 112 (Y112), which forms a consensus Src binding site. Once phosphorylated, the immunoreceptor tyrosine activation motif (Y74/Y85) recruits and activates Src family and Syk PTKs. LMP2A is also a substrate of these kinases including the Src family PTKs, especially Lyn (2, 8). This subsequently results in the recruitment of PTKs to LMP2A, possibly sequestering these enzymes away from the BCR signaling complex and blocking downstream signaling (25). LMP2A is also phosphorylated on serine and threonine residues by mitogen-activated protein kinase (MAPK) and interacts with extracellular signal-regulated kinase 1 (ERK1) directly (51). LMP2A has been shown to engage the phosphatidylinositol 3-kinase (PI3-K)/Akt pathway in both B cells and epithelial cells (55, 60), a consequence of which is the promotion of cell survival (27). Recently, LMP2A has been shown to induce beta-catenin/Tcf activity through a PI3-K/Akt-dependent pathway, although the physiological consequences of this activation, as it relates to effects on epithelial cell growth control, are still to be elucidated (48).
Recent evidence has shown that the expression of LMP2A in the B cells of transgenic mice provides a developmental and survival signal that bypasses the requirement for BCR signaling (10, 11). Unlike the situation in B cells, targeting of LMP2A to the epidermis of transgenic mice is not associated with gross alterations in tissue architecture (41). Despite the lack of a phenotype in normal differentiating epithelium, expression of LMP2A in an immortalized epithelial cell line, HaCat, is associated with differentiation blockade and growth transformation (55).
Comparatively little is known about the effect of LMP2A or LMP2B on epithelial cell biology. The phosphorylation of LMP2A in epithelial cells is triggered by cell adhesion, but this does not appear to be mediated via Src kinases (56). It is speculated that this phosphorylation is due to C-terminal c-Src kinase (Csk, a negative regulator of Src kinases). Csk has been shown to regulate the activity of focal adhesion kinase (FAK) and paxillin, providing a mechanism whereby LMP2A may influence cell spreading (62). In addition, both LMP2A and LMP2B have been shown to alter the expression of keratinocyte adhesion molecules, although the effects of these findings, as they relate to effects on epithelial cell behavior, are poorly understood (20, 56). By using epithelial cell lines in which LMP2A and LMP2B are stably expressed, we demonstrate that these two proteins can alter the adhesive and migratory properties of epithelial cells. These data have important implications for our understanding of the role of LMP2A and LMP2B in the pathogenesis of EBV-associated malignancies and may provide better understanding of the mechanisms involved in this process.
MATERIALS AND METHODS
Cell lines and tissue culture.
The A431 cell line, derived from an epidermal carcinoma, was a kind gift from F. Berditchevski, Cancer Research UK Institute for Cancer Studies, University of Birmingham, Birmingham, United Kingdom. The HaCat cell line is a spontaneously immortalized nontumorigenic epidermal keratinocyte cell line (6). The SCC12F cell line is an immortalized nontumorigenic subclone established from a squamous cell carcinoma of facial epidermis (53). The A431 and HaCat cell lines were grown in Dulbecco's modified Eagle's medium (DMEM; Gibco) supplemented with 5% fetal calf serum (Gibco), 2 mM glutamine, and the antibiotics penicillin (1,000 U/ml) and streptomycin (1 mg/ml) (Sigma). For routine culture, A431 and HaCat cells were split once per week at a 1:10 ratio. The SCC12F cell line was grown in DMEM-F12 (3:1 ratio; Gibco) supplemented with a solution of 5% fetal calf serum (Gibco), 2 mM glutamine, hydrocortisone (0.4 μg/ml; Up-John Ltd.), and the antibiotics penicillin (1,000 U/ml) and streptomycin (1 mg/ml) (Sigma). For routine culture, SCC12F cells were split once per week at a 1:5 ratio. At this cell density, SCC12F cells do not require NIH 3T3 feeder support (18).
Pharmacological inhibitors and growth factors.
The pharmacological inhibitors PP2, PP3, LY294002, and calphostin C were purchased from Calbiochem. UO126 was purchased from Promega. Tetradecanoyl phorbol acetate (TPA) was purchased from Sigma. All compounds were reconstituted in dimethyl sulfoxide (DMSO; Fischer Scientific) and diluted to an appropriate concentration in serum-free medium immediately prior to use. Epidermal growth factor (EGF; Sigma) was reconstituted in water to give a stock solution of 1 μg/ml; this solution was then further diluted in serum-free medium to a final concentration of 25 ng/ml.
Transduction of established cell lines with amphotrophic retroviruses.
Retroviral packaging cell lines producing LMP2A, LMP2B, or the neomycin control retrovirus were obtained from G. Winberg (Karolinska Institute, Stockholm, Sweden). The construction of the retroviral vectors and the generation of the producer cell lines (PA317) have been detailed previously (17). The transduction of established epithelial cell lines was achieved through cocultivation of retrovirus-producing packaging cells and the cell line of interest. Briefly, 2 × 106 γ-irradiated packaging cells were seeded onto 9-cm petri dishes containing actively growing cells (10% confluent) in growth medium containing 10 μg of hexadimethrine bromide/ml. After an overnight incubation, the medium was removed, and fresh growth medium was added. The γ-irradiated packaging cells were removed by EDTA washing 48 h after cocultivation, and growth medium containing G418 (400 μg/ml) was added. After 10 days of drug selection, drug-resistant polyclonal populations were screened for expression of LMP2A and LMP2B by reverse transcription (RT)-PCR and indirect immunofluorescence. Drug-resistant, polyclonal populations were then expanded in the presence of G418 (400 μg/ml).
RT-PCR analysis of LMP2A and LMP2B expression.
Cells were grown until ∼70% confluent and then harvested for analysis. Between 2× 106 and 5 × 106 cells were recovered by trypsinization and washed with two to three changes of phosphate-buffered saline (PBS) prior to RNA extraction. Total RNA was extracted from cell lines by using a NucleoSpin RNA II kit (Macherey-Nagel) according to the protocol of the manufacturer. Three micrograms of RNA was used in a cDNA synthesis reaction with a reverse gene-specific primer (ATA AAA ACT GGA CCG TAT CTT CTA TTT CCA) using AMV reverse transcriptase (Roche). LMP2A and LMP2B transcripts were amplified by using the forward and reverse primers ACT GAT TTT GGG CAC ACT TA and ATT CGG TCA GGA TAG CAA GA, respectively. PCR was performed by using Red Hot Taq DNA polymerase (Abgene) and involved an initial 3-min denaturation step at 94°C followed by 35 cycles consisting of a denaturing step for 30 s at 94°C, an annealing step for 1 min at 50°C, and an extension step for 2 min at 72°C. PCR products (303 bp) were visualized on 1% agarose gels.
Immunofluorescent staining in situ.
Cells were recovered by trypsinization, resuspended in normal growth medium, and seeded out at between 2 × 104 and 5 × 104 cells/well onto Teflon-coated microscope slides (Hendley-Essex). Twenty-four hours later, the cells were fixed in 4% paraformaldehyde followed by permeabilization in 0.5% Triton X-100. After blocking in 20% heat-inactivated normal goat serum, cells were incubated with a 1:50 dilution of the human NPC reference serum Ba (17) or a 1:1 dilution of 8C3, a rat monoclonal antibody (MAb) specific for the amino terminus of LMP2A (25). LMP2 staining was visualized by using fluorescein isothiocyanate-conjugated goat anti-human immunoglobulin G (IgG) (Sigma) or, for 8C3 staining, Oregon Green-conjugated anti-rat IgG (Molecular Probes). After washing, slides were mounted in DABCO antifading agent and viewed under a light microscope.
For adhesion studies, 104 cells were plated onto Teflon-coated slides precoated overnight with fibronectin (10 μg/ml), poly-l-lysine (10 μg/ml), or laminin-5 matrix (LN-5M)-enriched slides (1). Cells were allowed to adhere for various time points and then fixed in 4% paraformaldehyde, permeabilized in 0.5% Triton X-100 (ICN Biochemicals) in PBS, and stained with primary antibodies to vinculin (Sigma), antiphosphotyrosine (p-Tyr) clone 4G10 (Upstate), or anti-FAK397 (Biosource International) followed by AlexaFluor 594-conjugated anti-mouse or anti-rabbit antibodies (Molecular probes). To visualize cellular actin, fixed and permeabilized cells were incubated with 0.5 μg of tetramethyl rhodamine isothiocyanate-conjugated phalloidin (Sigma)/ml for 60 min. Slides were mounted with DABCO and viewed at ×400 magnification with a Nikon Eclipse E600 microscope and the Nikon ACT-1 program.
Transwell migration assays.
Serum-starved cells were recovered as single-cell suspensions and seeded into the upper well of a transwell migration chamber (BD Biosciences) whose underside had been coated with 10 μg of fibronectin (Sigma)/ml. Eighteen hours after incubation at 37°C, the wells were fixed in 30% methanol and stained with 1% crystal violet, and the percentage of cell migration was determined after photographing representative fields and counting the number of stained (migrating) cells. In each case, the number of cells per field was scored, and the mean was determined from at least six representative fields.
Transient adhesion assays.
Cells expressing LMP2A, LMP2B, or the Neo control were serum starved for 24 h and recovered as single-cell suspensions. A total of 2.5 × 104 cells were plated out in triplicate into 96-well plates coated with fibronectin or poly-l-lysine (10 μg/ml; Sigma). At various times after incubation at 37°C, cells were washed extensively with serum-free DMEM and fixed in 100% ethanol. The number of adherent cells were analyzed after staining with 0.1% crystal violet followed by solubilization in 0.2% Triton X-100, and the absorbance was read at 550 nm. The degree of cell attachment is expressed as raw optical density (OD) values (OD at 550 nm [OD550]) on an arbitrary scale. One-way analysis of variance with Tukey's multiple-comparison posttest was performed on the data set by using Graphpad Prism (San Diego, Calif.) software.
Immunoblotting analysis.
Serum-starved cells were recovered by light trypsinization and held in suspension for 60 min prior to plating onto fibronectin-coated dishes. Cells (2 × 106 to 3 × 106 cells/plate) were allowed to adhere and were lysed in RIPA buffer (50 mM Tris-HCl [pH 7.4], 1% NP-40, 0.25% Na-deoxycholate, 150 mM NaCl, 1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, 1 mM Na3VO4, 1 mM NaF, 1 μg [each] of aprotinin, leupeptin, and pepstatin/ml) at the indicated time points. Total cellular phosphorylated proteins induced in response to adhesion was assessed by using standard immunoblotting procedures. Briefly, a total protein extract from each time point (equivalent to ∼106 cells) was separated on a sodium dodecyl sulfate-10% polyacrylamide gel electrophoresis gel. Separated proteins were immunoblotted onto nitrocellulose membranes and, after blocking with Tris-buffered saline-Tween (0.1%) containing 5% bovine serum albumin, incubated with primary antibodies specific for phosphotyrosine using the 4G10 MAb (Upstate Biotechnology) or p-ERK-MAPK/p-Akt (Cell Signaling Technologies). After several washes in TBS-Tween (0.1%), the nitrocellulose membrane was incubated with either horseradish peroxidase (HRP)-conjugated goat anti-mouse immunoglobulin (Sigma) or HRP-conjugated goat anti-rabbit immunoglobulin (Sigma). After an additional three 15-min washes in TBS-Tween (0.1%), antibody-protein complexes were visualized by enhanced chemiluminescence (Amersham). In the case of p-ERK-MAPK and p-Akt, blots were stripped and reprobed with polyclonal rabbit antibodies to total ERK-MAPK and Akt (Cell Signaling Technologies). To ensure equal loading of samples, blots were reprobed with a mouse monoclonal antibody to β-actin followed by HRP-conjugated goat anti-mouse immunoglobulin (Sigma); antibody-protein complexes were then visualized by enhanced chemiluminescence.
Preparation of LN-5M.
LN-5M was prepared according to the method described by Baker et al. (1). A431 cells were grown to confluence on either Teflon-coated microscope slides or 6-cm petri dishes. Thereafter, cells were rinsed in PBS and removed after treatment with 20 mM ammonium hydroxide. The treated plates or slides were subjected to extensive washing in sterile distilled water to remove all traces of ammonium hydroxide and stored at 4°C prior to use.
Flow cytometry.
Subconfluent cultures of Neo control cells and LMP2A- and LMP2B-expressing cells (both A431 and SCC12F) were recovered by mild trypsinization (0.0125% trypsin plus 0.02% EDTA). Single-cell suspensions were collected by centrifugation, counted, and distributed into the wells of a 96-well microtiter plate at 2.5 × 105 cells/well. Cell pellets were resuspended in 50 μl of antibody and incubated on ice for 45 min. Monoclonal antibodies used in this study included P1E6, P1B5, P1D6, and GoH3, directed against the α2, α3, α5, and α6 integrin subunits (Telios), and 3E1, directed against the β4 integrin subunit (Immunotech). After three washes in cold PBS (4°C), the cells were resuspended in fluorescein isothiocyanate-conjugated goat anti-mouse IgG (Sigma) and incubated on ice for an additional 45 min. After an additional three washes, the cell suspensions were fixed in 1% paraformaldehyde and processed for fluorescence-activated cell sorter (FACS) analysis. Fluorescent staining of cell surface integrins was analyzed by flow cytometry with a Coulter EPICS XL FACSCAN. Values are presented as mean fluorescence intensity values on an arbitrary log scale.
PKC translocation assay.
A total of 104 SCC12F cells were seeded onto Teflon-coated slides and serum starved for 18 h prior to stimulation. Where appropriate, cells were incubated in the presence of 30 μM calphostin C or DMSO vehicle control for 30 min prior to TPA stimulation. Cells were stimulated with 100 ng of TPA/ml for 30 min, fixed in 4% paraformaldehyde, and permeabilized with 0.1% Triton X-100. Protein kinase C (PKC) activation was assessed after immunostaining with a polyclonal rabbit antiserum specific for PKCα (Sigma) and detection with AlexaFluor 488-conjugated goat anti-rabbit antibodies (Molecular Probes). When activated, PKCα undergoes translocation from the cytosol to the membrane, which is clearly visible after immunostaining (4).
RESULTS
Stable expression and cellular localization of LMP2A and LMP2B in epithelial cells.
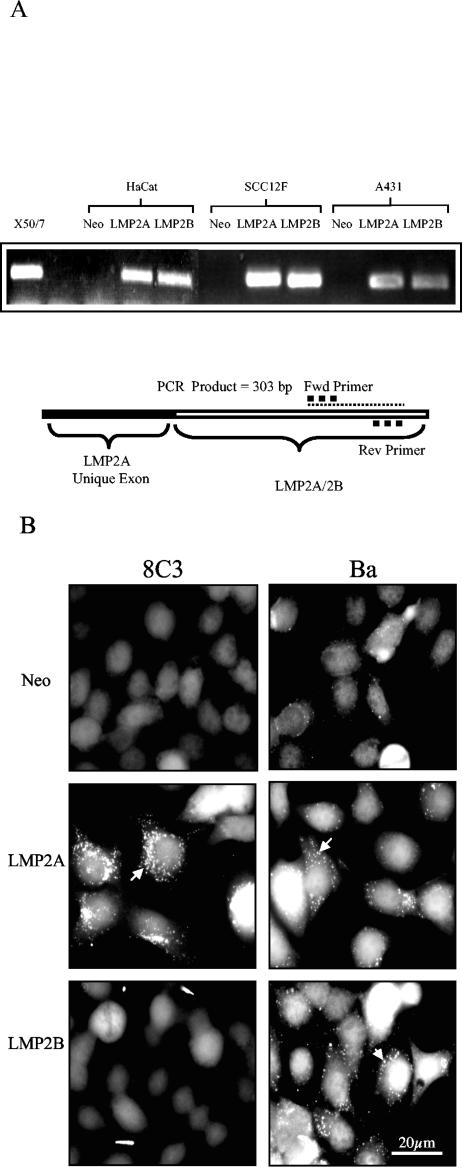
To examine the effects of LMP2A and LMP2B expression on epithelial cell adhesion, A431, SCC12F, and HaCat cells were transduced with recombinant neomycin-resistant retroviruses containing cDNAs for LMP2A and LMP2B or the neomycin drug resistance cassette alone (17). Polyclonal, drug-resistant populations were expanded and subjected to further experimental analysis. To confirm expression of LMP2A and LMP2B in transduced epithelial cell lines, RNA was isolated from representative panels of cells, and RT-PCR analysis was performed with a set of primers that amplify both LMP2A and LMP2B mRNA. As shown in Fig. 1A, LMP2A and LMP2B expression was confirmed in cell lines transduced with the relevant retroviruses. Amplification of purified mRNA with this set of primers generated a 303-bp fragment, confirming expression of LMP2A and LMP2B mRNA in A431, SCC12F, and HaCat epithelial cells with the absence of any signal in the Neo control cells. To confirm expression of LMP2A and LMP2B at the protein level, representative cell lines were screened for LMP2A and LMP2B expression with either 8C3, a rat monoclonal antibody specific for LMP2A (25), or Ba serum, an NPC serum with reactivity for both LMP2A and LMP2B (17). As shown in Fig. 1B, immunostaining with 8C3 confirmed expression of LMP2A in SCC12F cells transduced with the LMP2A retrovirus, whereas Neo control and LMP2B-expressing cells gave only background levels of staining. In keeping with previous reports, the majority of LMP2A localized to intracellular vesicles (17, 46). Immunostaining of epithelial cells with Ba serum confirmed expression of LMP2A and LMP2B in SCC12F cells transduced with the LMP2A and LMP2B retroviruses, respectively, with the majority of LMP2A and LMP2B localizing to perinuclear vesicles. Immunostaining of SCC12F cells transduced with the neomycin resistance gene alone gave only background levels of staining with Ba serum. Identical results were obtained for both A431 and HaCat epithelial cells (data not shown).
FIG. 1.
Expression of LMP2A and LMP2B in HaCat, SCC12F, and A431 epithelial cell lines. (A) RT-PCR analysis confirming expression of LMP2A and LMP2B mRNA transcripts in epithelial cells transduced with LMP2A and LMP2B retroviruses but not with the Neo control retrovirus. (B) Immunofluorescence staining of Neo control and LMP2A- and LMP2B-expressing SCC12F epithelial cells with the LMP2A-specific rat MAb 8C3 shows expression of LMP2A only, whereas the NPC reference serum Ba recognizes both LMP2A and LMP2B. Arrows denote vesicular localization of LMP2A and LMP2B. Bar, 20 μm.
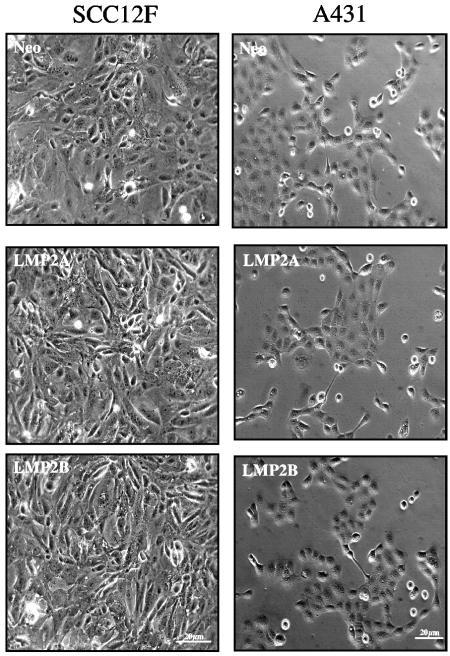
LMP2A and LMP2B do not induce morphological changes in squamous epithelial cells.
Epithelial cell cultures transduced with the LMP2A and LMP2B retroviruses were similar in appearance to Neo control cells. As shown in Fig. 2, SCC12F and A431 cells expressing LMP2A or LMP2B were indistinguishable from Neo control cells. LMP2A- and LMP2B-expressing cells displayed a flat cuboidal morphology, maintained tight intercellular contacts, and, in the case of SCC12F cells, showed evidence of stratification at high cell densities.
FIG. 2.
LMP2A and LMP2B do not alter the morphology of squamous epithelial cell lines in monolayer culture. Phase-contrast micrographs of Neo control and LMP2A- and LMP2B-expressing epithelial cells growing in monolayer culture. Transduced SCC12F and A431 cells are shown. Bar, 20 μm.
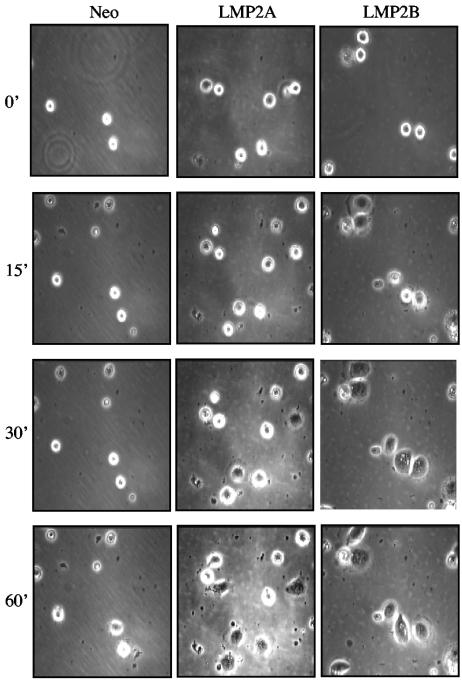
LMP2A and LMP2B enhance cell spreading on ECM.
Previous studies identified a link between epithelial cell adhesion and LMP2A phosphorylation, suggesting that signals generated as a consequence of cell adhesion are required for LMP2A phosphorylation (56). Although these initial studies highlighted the requirement for integrin activation in this process, they did not address any possible contribution of LMP2A to the adhesion process itself. Observations made through the course of our studies identified a direct effect of LMP2A and LMP2B on epithelial cell attachment and spreading, raising the possibility that LMP2A and LMP2B influence the adhesive process directly. To investigate this phenomenon in more detail, time-lapse video microscopy was performed to analyze the kinetics of attachment and cell spreading on extracellular matrix (ECM). HaCat cells were serum starved for 24 h, recovered as single-cell suspensions, and plated onto petri dishes coated with purified extracellular matrix proteins (fibronectin or collagen type I) or LN-5M. As shown in Fig. 3, relative to Neo control cells, the kinetics of cell spreading on fibronectin was clearly accelerated in LMP2A- and LMP2B-expressing cells. In typical experiments, Neo control cells remained rounded and “phase bright” up to 60 min after plating, after which time they started to spread. This contrasted with LMP2A- and LMP2B-expressing cells which, although clearly round and phase bright at the time of plating, began to spread within 15 min and had become fully spread within 60 min. This accelerated cell spreading was more pronounced for LMP2B-expressing cells, which attached and became fully spread within 30 to 45 min after plating. Similar results were obtained when cells were plated onto LN-5M or collagen type I (data not shown), suggesting that the accelerated spreading displayed by LMP2A- and LMP2B-expressing cells was not confined to a particular ECM substrate.
FIG. 3.
Epithelial cells expressing LMP2A and LMP2B show increased rates of attachment and spreading on extracellular matrix. Neo control and LMP2A- and LMP2B-expressing HaCat epithelial cells were serum starved, collected as single cells, and plated onto fibronectin-coated petri dishes. Time-lapse video microscopy was used to analyze the kinetics of cell attachment and spreading over a 60-min time frame, with frames taken every 15 min. Magnification, ×100. Serum starvation and suspension culture resulted in a loss of cell viability that was 18% (±1.7%) in Neo control cells, 31% (±2.6%) in LMP2A-expressing cells, and 29% (±1.5%) in LMP2B-expressing cells (data are from three independent experiments).
To ensure that the ability of LMP2A and LMP2B to promote cell spreading was not due to a loss of viability in Neo control cells after serum starvation and suspension, cell viability was assayed by trypan blue dye exclusion. In three individual experiments, serum starvation and suspension resulted in an 18% reduction of viability in Neo control cells, whereas this increased to 31% in LMP2A-expressing cells and 29% in LMP2B-expressing cells. This analysis ruled out the possibility that LMP2A and LMP2B promote cell spreading by increasing viability after serum starvation or suspension.
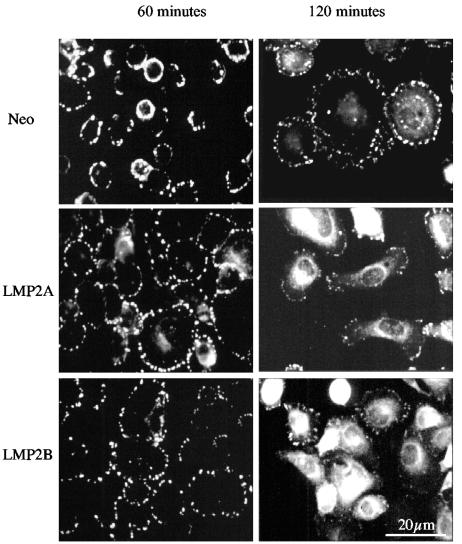
LMP2A and LMP2B accelerate cell spreading and focal adhesion assembly in response to adhesion.
To determine whether LMP2A and LMP2B influenced cell spreading by accelerating the kinetics of focal adhesion formation, cells were plated onto ECM-coated slides (fibronectin or LN-5M), and after various times, the degree of focal adhesion formation was assessed after staining for vinculin, a known component of focal adhesions. As shown for SCC12F cells in Fig. 4, 60 min after plating, Neo control cells, although attached, failed to spread and to establish prominent focal adhesions on ECM. This was in marked contrast to cells expressing LMP2A or LMP2B, which not only had attached and spread at this time point but also had established large prominent focal adhesions. The ability of the Neo control cells to initiate the formation of these structures at the later 120-min time point suggests that LMP2A and LMP2B accelerate the normal processes of cell spreading and focal adhesion formation. Interestingly, at this later time point, focal adhesion formation in LMP2A- and LMP2B-expressing cells was less prominent and accompanied by an alteration in cell shape indicative of motile cells.
FIG. 4.
LMP2A and LMP2B accelerate the process of cell spreading on ECM and promote focal adhesion formation. Neo control and LMP2A- and LMP2B-expressing SCC12F cells were serum starved, recovered as single cells, and plated onto fibronectin-coated slides. The extent of focal adhesion formation was assessed after 60 and 120 min using an antibody specific for vinculin. Bar, 20 μm.
LMP2A and LMP2B promote cell migration on ECM.
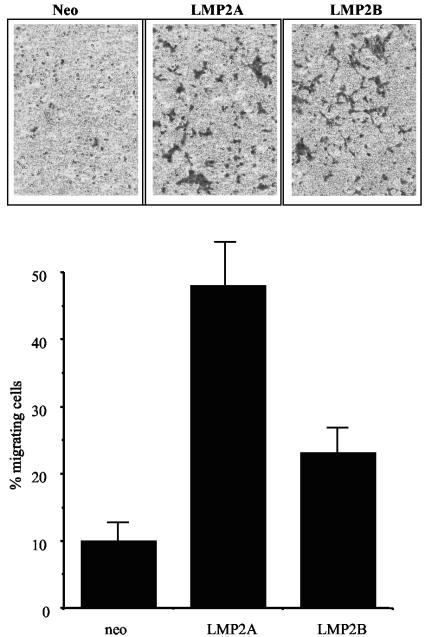
Our finding that LMP2A and LMP2B both promoted cell spreading and focal adhesion formation upon adhesion to ECM prompted us to examine whether LMP2A and LMP2B could also stimulate cell migration. Transwell migration assays were employed to assay the migratory properties of cells in the absence of a chemotactic stimulus. Serum-starved SCC12F cells were recovered as single-cell suspensions and seeded into the upper chamber of a transwell migration chamber. The ability of cells to migrate in response to fibronectin, so-called “haptotactic” migration, was examined 18 h later by measuring the number of cells that had migrated through the fibronectin matrix. As shown in Fig. 5, compared to Neo control cells, of which only 10 to 15% of cells migrated over 18 h, between 45 and 50% of LMP2A-expressing cells migrated over the same time period. Interestingly, LMP2B-expressing cells showed a response that was appreciably lower than that of LMP2A-expressing cells, with between 20 and 25% of cells migrating over the same time frame. Similar findings were observed with A431 and HaCat epithelial cells (data not shown).
FIG. 5.
LMP2A and, to a lesser extent, LMP2B promote epithelial cell migration on ECM. Neo control and LMP2A- and LMP2B-expressing SCC12F cells were assessed for their ability to migrate in response to ECM (in the absence of serum growth factors) in transwell migration assays. The upper panel shows representative transwell membranes stained after 18 h. The lower graph shows the percentage of total cells migrating through fibronectin over an 18-h time interval. Data show the means ± standard deviations from triplicate wells and are representative of six experiments.
The ability of LMP2A and LMP2B to promote cell spreading and migration on extracellular matrix is integrin dependent.
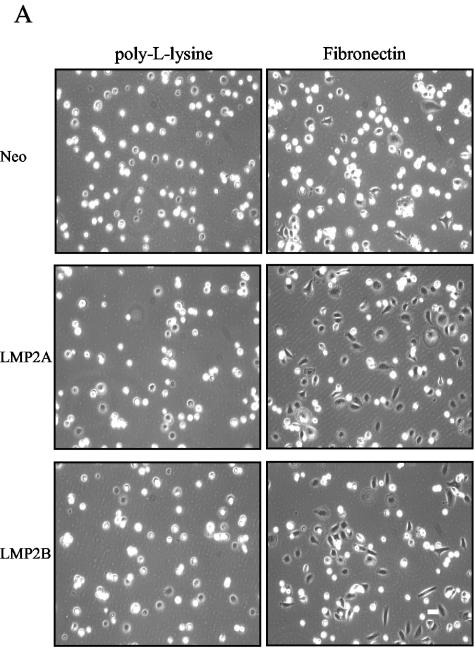
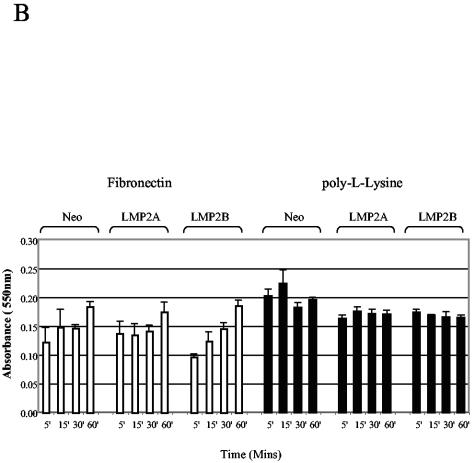
To establish whether the ability of LMP2A and LMP2B to promote epithelial cell spreading was integrin dependent, we compared the rates of epithelial cell attachment and spreading on fibronectin to rates on poly-l-lysine. Unlike adhesion to ECM, which requires integrins, adhesion to poly-l-lysine is mediated through nonspecific electrostatic interactions. Serum-starved SCC12F cells were recovered as single-cell suspensions and plated onto petri dishes coated with either fibronectin or poly-l-lysine. At various times after plating, the degree of cell attachment and spreading was assessed by phase-contrast microscopy. As shown in Fig. 6A, the rate of cell attachment to poly-l-lysine was almost identical for all three cell lines, with Neo control and LMP2A- and LMP2B-expressing cells showing maximal attachment 30 min after plating. This was in marked contrast to cells plated onto fibronectin where, at the same 30-min time point, LMP2A- and LMP2B-expressing cells had attached and spread, whereas Neo control cells, although clearly attached, were still rounded and had failed to spread. Similar results were obtained by using collagen type I and LN-5M, findings which suggest that the ability of LMP2A and LMP2B to promote cell spreading was not confined to a particular ECM substrate (data not shown). To rule out the possibility that LMP2A and LMP2B promote cell spreading by accelerating the rate of cell attachment to ECM, we analyzed the rates of cell attachment to ECM in more detail. Single-cell suspensions were plated onto fibronectin and poly-l-lysine, and the number of adherent cells was quantified after 5, 15, 30, and 60 min. As shown in Fig. 6B, the rates of cell attachment to fibronectin and poly-l-lysine were broadly similar over the 60-min time course. These data indicate that LMP2A and LMP2B do not affect cellular processes that are important for cell attachment but rather influence pathways that regulate cell spreading.
FIG. 6.
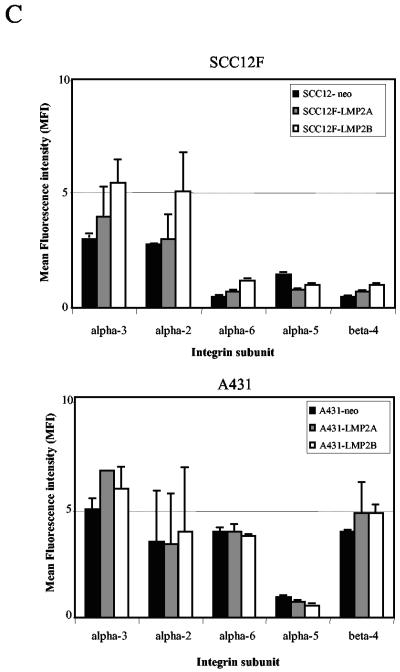
LMP2A and LMP2B promote cell spreading rather than attachment on extracellular matrix. (A) Neo control and LMP2A- and LMP2B-expressing SCC12F cells were serum starved, collected as single cells, and plated onto petri dishes coated with fibronectin or poly-l-lysine. The extent of cell attachment and spreading was visualized after 60 min by phase-contrast microscopy. Magnification, ×50. (B) Attachment of Neo control and LMP2A- and LMP2B-expressing cells to extracellular matrix (fibronectin and poly-l-lysine) are similar, indicating that LMP2A and LMP2B promote cell spreading rather than attachment. Serum-starved cells were plated onto 96-well plates precoated with fibronectin or poly-l-lysine, and the number of adherent cells were quantitated at various times after plating. Data are presented as raw values (OD550) from triplicate determinations (one-way analysis of variance showed no significant differences in the rates of adhesion between Neo control cells, LMP2A-expressing cells, and LMP2B-expressing cells at the time points indicated). (C) The integrin profiles of Neo control and LMP2A- and LMP2B-expressing SCC12F and A431 cells were analyzed by cytofluorimetric (FACS) analysis using a panel of MAbs specific for the α2, α3, α5, α6, and β4 integrin subunits. Data are presented as mean fluorescence intensity (MFI) on an arbitrary scale. Data shown are the means ± standard deviations from three independent experiments.
We next chose to examine cells for expression of individual integrin family members to determine whether the increased adhesive properties of LMP2A- and LMP2B-expressing cells was due to an increase in the levels of cell surface integrins. The integrin profiles of Neo control and LMP2A- and LMP2B-expressing cells were analyzed by flow cytometry (FACS). As shown in Fig. 6C, the integrin profiles of LMP2A- and LMP2B-expressing cells were broadly similar to that of Neo control cells. SCC12F cells expressed high levels of the collagen and fibronectin/LN-5 receptors α2β1 and α3β1 and lower levels of the LN-5 and fibronectin receptors α6β4 and α5β1. Essentially similar findings were observed with A431 cells, although much higher levels of the laminin-5 receptor α6β4 were expressed in this background. Taken together, these findings suggest that the ability of LMP2A and LMP2B to promote cell spreading on ECM is not attributable to increased levels of cell surface integrins.
LMP2A and LMP2B accelerate focal adhesion assembly and turnover—a role for tyrosine kinases.
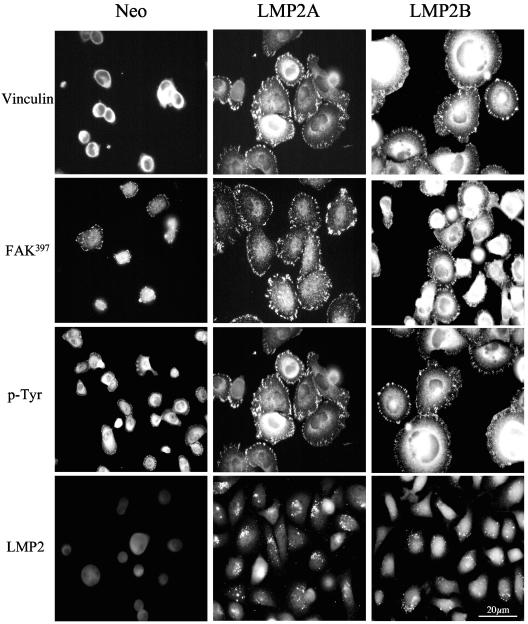
Attachment and spreading of cells on ECM requires the recruitment of specific structural proteins to sites of focal adhesion. These proteins participate in focal adhesion assembly but also serve to recruit and activate proteins that transduce signals to promote proliferation and survival (31). Key molecules that regulate focal adhesion formation and intracellular signaling are nonreceptor tyrosine kinases such as Src and FAK. To examine the distribution of FAK and other tyrosine-phosphorylated proteins in adhering cells, serum-starved, single-cell suspensions were plated onto fibronectin-coated slides and fixed and stained for vinculin, FAK, and phosphotyrosine 60 min after plating. As shown in Fig. 7, immunostaining with an antibody to vinculin revealed diffuse cytoplasmic staining in Neo control cells at this time point, whereas intense focal adhesion formation was observed in LMP2A- and LMP2B-expressing cells. To determine whether FAK activity was elevated in adhering LMP2A- and LMP2B-expressing cells, immunostaining was performed with a polyclonal antiserum specific for the active phosphorylated form of FAK, Tyr397-FAK. Whereas immunostaining for Tyr397-FAK in Neo control cells gave only diffuse cytosolic staining and weak staining of focal adhesions, both LMP2A- and LMP2B-expressing cells showed intense staining for Tyr397-FAK at focal adhesions (Fig. 7). Essentially similar patterns of staining were observed when cells were stained with a phosphotyrosine-specific antibody (4G10). Again, diffuse cytosolic staining was observed in loosely attached Neo control cells that had failed to assemble strong focal adhesions, whereas intense staining was observed at focal adhesions in strongly adherent LMP2A- and LMP2B-expressing cells. These findings are consistent with published reports demonstrating increased tyrosine phosphorylation of structural proteins at focal adhesions in strongly adherent cells. To determine whether LMP2A and LMP2B were themselves targeted to focal adhesions in response to adhesion, the subcellular localization of LMP2A and LMP2B was investigated in fully spread cells. Unexpectedly, immunostaining with the Ba serum showed that the bulk of LMP2A and LMP2B remained perinuclear at all time points analyzed, with no evidence of localization to focal adhesions (Fig. 7).
FIG. 7.
LMP2A and LMP2B promote tyrosine phosphorylation of FAK and other proteins at sites of focal adhesion. Neo control and LMP2A-and LMP2B-expressing SCC12F cells were serum starved, recovered as single cells, and plated onto fibronectin-coated slides. Sixty minutes after plating, the cells were fixed and stained with antibodies to vinculin, FAK397, phosphotyrosine, and LMP2. Bar, 20 μm.
LMP2A- and LMP2B-mediated cell spreading requires tyrosine kinase but not PI3-K, ERK-MAPK, or PKC activity.
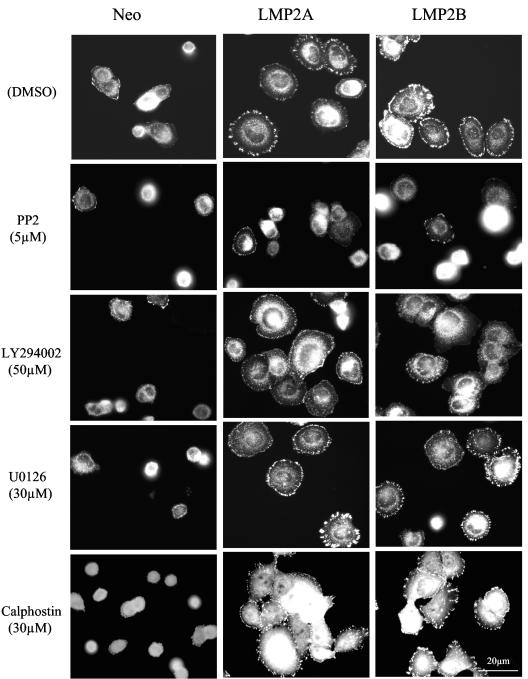
Members of the Src family of tyrosine kinases are known to play an important role in cell attachment and spreading by modulating the activity of FAK and other kinases that localize to focal adhesions. Similarly, PI3-K, ERK-MAPK, and PKC have been shown to play key roles in promoting cell spreading and motility induced by EGF, scatter factor, or insulin-like growth factor or via engagement of the α3β1 and α6β4 integrin receptors (36, 61, 66). A role for these signaling effectors in LMP2A- and LMP2B-mediated cell spreading was investigated by using pharmacological inhibitors specific for these families of kinases. Included in this study were (i) the broad-spectrum tyrosine kinase inhibitor PP2 and its inactive analogue PP3, (ii) the selective PI3-K inhibitor LY294002, (iii) the MEK inhibitor UO126, and (iv) the broad-spectrum PKC inhibitor calphostin C.
Serum-starved cells were recovered as single-cell suspensions and incubated in the presence of various pharmacological inhibitors for 30 min prior to plating. Treated cells were then plated onto fibronectin-coated slides and allowed to attach for 60 min prior to fixation. The degree of cell spreading and focal adhesion formation was then assessed in cells after staining for vinculin. As shown in Fig. 8, compared to solvent control or PP3-treated cells (data not shown), the ability of Neo control and LMP2A- and LMP2B-expressing cells to attach and spread on fibronectin was severely inhibited in the presence of 5 μM PP2. Closer inspection revealed that relative to Neo control cells, LMP2A- and LMP2B-expressing cells were clearly more resistant to the inhibitory effects of PP2, and although cell spreading was somewhat impaired, these cells were clearly able to establish focal adhesions. Titration studies showed that at lower concentrations of PP2 (0.05 to 1 μM), LMP2A- and LMP2B-expressing cells were able to overcome the inhibitory effects of this compound and initiate cell spreading and focal adhesion formation (data not shown). In marked contrast to PP2, treatment of cells with 50 μM LY294002, 30 μM UO126, or 30 μM calphostin C had little if any effect on the ability of LMP2A- or LMP2B-expressing cells to attach and spread on fibronectin. These findings suggest that, unlike tyrosine kinase activity, PI3-K, ERK-MAPK, and PKC activity are not required by LMP2A and LMP2B to promote the cell spreading on ECM.
FIG. 8.
Tyrosine kinase but not PI-3 kinase, ERK-MAP kinase, or PKC is required by LMP2A and LMP2B to initiate cell spreading on ECM. (A) Neo control and LMP2A- and LMP2B-expressing SCC12F cells were serum starved, collected as single cells, and treated with DMSO, 5 μM PP2, 50 μM LY294002, 30 μM U0126, or 30 μM calphostin C for 30 min prior to plating onto fibronectin-coated slides. Sixty minutes after plating, cells were fixed and stained with an antibody to vinculin to assess the degree of focal adhesion formation. Bar, 20 μm.
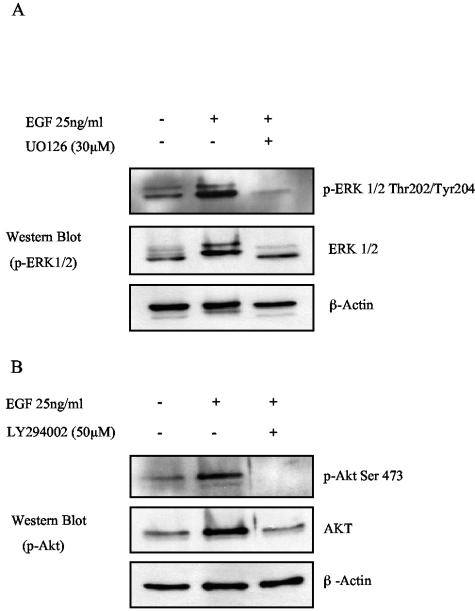
To establish that the pharmacological inhibitors used in these studies were effective at blocking the biological activity of their respective target kinases, each compound was assayed for its ability to block ERK-MAPK, PI3-K/Akt, and tyrosine kinase activation in response to EGF stimulation (Fig. 9). EGF was chosen primarily because the EGF receptor is known to activate these kinases in epithelial cells (66). SCC12F cells were serum starved for 18 h prior to stimulation with EGF (25 ng/ml). Where appropriate, cells were incubated for 30 min with the selective compound prior to stimulation. Cell lysates were then analyzed for ERK-MAPK, Akt, and tyrosine kinase activity using reagents specific for phospho-ERK (p-ERK), phospho-Akt (p-Akt), and phosphotyrosine (p-Tyr). As shown in Fig. 9A, stimulation of SCC12F cells with EGF resulted in robust ERK-MAPK phosphorylation that was completely blocked by pretreatment with 30 μM U0126. Similarly, EGF stimulation resulted in marked Akt phosphorylation (Fig. 9B), a response that was completely inhibited by pretreatment with 50 μM LY294002. Treatment of SCC12F cells with the broad-spectrum tyrosine kinase inhibitor PP2 significantly reduced EGF-induced tyrosine phosphorylation, with the extent of the inhibition increasing as the dose of PP2 was increased from 5 to 50 μM (Fig. 9C). In contrast, the inactive analogue PP3 was significantly less effective at blocking EGF-induced tyrosine phosphorylation over the same concentration range. To assess the ability of calphostin C to block PKC activity, serum-starved SCC12F cells were treated with TPA, and its ability to block membrane translocation of PKCα was assessed by UV microscopy. As shown in Fig. 9D, treatment of SCC12F cells with 100 ng of TPA/ml resulted in a marked redistribution of PKCα from the cytosol to large membranous aggregates and to the leading edge of membrane ruffles. In marked contrast, 30 μM calphostin C completely blocked TPA-induced PKCα membrane translocation, whereas pretreatment with DMSO was ineffective; here, PKCα translocation was identical to TPA treatment alone. These data confirmed the biological potency of the pharmacological inhibitors used in this study and substantiate the findings that LMP2A and LMP2B do not utilize PI3-K, ERK-MAPK, and PKC activity to promote epithelial cell spreading on ECM.
FIG. 9.
Validation of pharmacological inhibitors. SCC12F cells were serum starved in the presence or absence of various pharmacological inhibitors for 30 min prior to stimulation with EGF (25 ng/ml). The ability of 30 μM U0126, 50 μM LY294002, and 5 to 50 μM PP2 to block EGF-induced ERK, Akt, and tyrosine phosphorylation was then analyzed by Western blotting using antibodies specific for phosphorylated forms of ERK and Akt and phosphotyrosine. The ability of 30 μM calphostin C to block PKC activation was assessed in SCC12F cells treated for 30 min with 100 ng of TPA/ml. PKC activation was assessed by determining the extent of PKCα translocation in response o TPA stimulation. Arrows denote plasma membrane-associated PKCα. Bar, 10 μm.
LMP2A augments tyrosine phosphorylation of cellular proteins in response to adhesion.
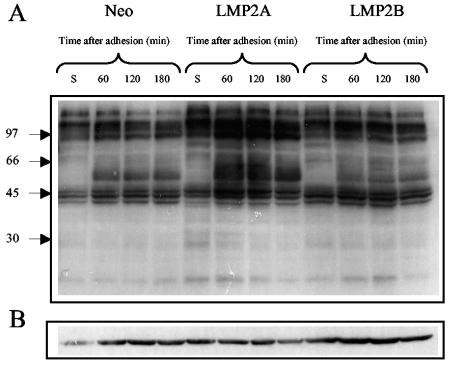
As integrins are coupled to intracellular signaling pathways, we next examined the effects that LMP2A and LMP2B expression may have on integrin signaling. As adhesion of cells to ECM is associated with changes in tyrosine phosphorylation, we chose to examine the effects of cell adhesion on “global” tyrosine phosphorylation over a 180-min time course. Serum-starved SCC12F cells were recovered as single-cell suspensions, held in suspension for 60 min, and then plated onto fibronectin-coated petri dishes. At various times after plating, cells were lysed in situ and then subjected to immunoblotting with a phosphotyrosine-specific antibody to identify changes in protein tyrosine phosphorylation that occur as a consequence of cell adhesion. As shown in Fig. 10, significant differences were observed in the pattern of tyrosine-phosphorylated proteins in the adhesion of Neo control and LMP2A- and LMP2B-expressing cells. Adhesion of cells to fibronectin resulted in the appearance of multiple tyrosine-phosphorylated cellular proteins that increased over time. A band migrating at approximately 125 kDa became hyperphosphorylated in response to adhesion, but in addition, abundant proteins migrating between 56 and 60 kDa were also hyperphosphorylated. Of particular interest was the finding that the kinetics and amplitude of tyrosine phosphorylation of both common and novel proteins were significantly elevated in adhering LMP2A-expressing cells. This was in marked contrast to LMP2B-expressing cells, where slight increases in the phosphorylation of certain common proteins was evident relative to Neo control cells but the extent of tyrosine phosphorylation was significantly lower than that observed in LMP2A-expressing cells.
FIG. 10.
LMP2A enhances global tyrosine phosphorylation of cellular proteins in response to adhesion. Neo control and LMP2A- and LMP2B-expressing SCC12F cells were serum starved, collected as single cells, and held in suspension for 60 min prior to plating onto fibronectin-coated petri dishes. At various times after plating, cells were harvested and subjected to immunoblotting with an antibody to (A) p-Tyr (4G10) to assess the degree of tyrosine phosphorylation and (B) β-actin to ensure equal loading.
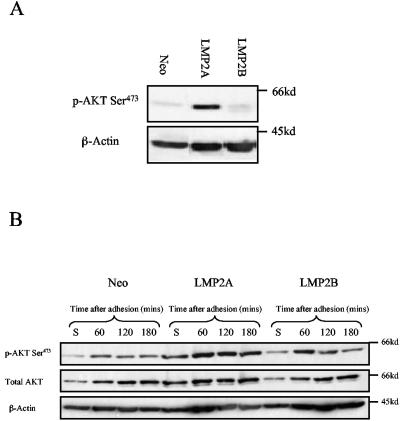
LMP2A induces Akt activity in the absence of integrin signals.
Integrin-dependent signaling pathways are linked not only to cell motility but also to cell survival. By virtue of their ability to activate receptor tyrosine kinases such as Src and FAK, integrins transduce signals to ERK-MAPK and PI3-K, signaling pathways that provide both growth and survival signals. To establish whether expression of LMP2A and LMP2B had any effect on integrin-regulated signaling, serum-starved SCC12F cells were placed in suspension for 60 min prior to plating on fibronectin. At various times after plating, cells were lysed in situ and processed for immunoblotting. The degree of ERK-MAPK and Akt activation was then assessed after immunoblotting with antibodies specific for phosphorylated forms of ERK-MAPK and Akt. As illustrated in Fig. 11A, when serum-starved Neo control or LMP2B-expressing cells were placed in suspension, basal Akt phosphorylation was markedly reduced, presumably due to the lack of growth factor- and/or integrin-generated signals. This was in marked contrast to LMP2A-expressing cells, where Akt phosphorylation remained high, presumably through a direct ability of LMP2A to activate PI3-K. In response to adhesion, Akt phosphorylation increased with time in Neo control and LMP2A- and LMP2B-expressing cells (Fig. 11B), but interestingly, the amplitude of Akt phosphorylation was clearly much greater in LMP2A-expressing cells. Although the kinetics of Akt phosphorylation started to decline over time in Neo control and LMP2B-expressing cells, Akt phosphorylation remained high in LMP2A-expressing cells for the full duration of the time course. Interestingly, and unlike Akt, little or no effect was observed with ERK-MAPK activation in response to cell adhesion, with basal ERK-MAPK being constitutively elevated even in serum-starved SCC12F cells (data not shown).
FIG. 11.
LMP2A but not LMP2B induces constitutive Akt phosphorylation in the absence of growth factor and integrin signals. (A) Neo control and LMP2A- and LMP2B-expressing SCC12F cells were serum starved, collected as single cells, and held in suspension for 60 min in the absence of serum. The degree of Akt phosphorylation was assessed after immunoblotting total cell lysates with an antibody recognizing the serine 473-phosphorylated form of active Akt. (B) The same cells were then plated onto fibronectin-coated dishes and harvested for immunoblotting at the indicated times. Total cell lysates were assayed for Akt phosphorylation using an antibody recognizing the serine 473-phosphorylated form of active Akt. The same blots were then stripped and reprobed with antibodies for total Akt and β-actin to ensure equal loading.
DISCUSSION
In contrast to LMP1, NPC tumors show consistent expression of LMP2A and LMP2B (7, 9, 13). Although speculative, these findings suggest that these membrane proteins may contribute to epithelial cell growth transformation and may ultimately play a role in the pathogenesis of NPC. Although studies have started to address the effects of LMP2A on epithelial cell growth and phenotype, functional roles for LMP2A and LMP2B in this cell type are yet to be fully elucidated (52). Unlike LMP1, targeting LMP2A to the epidermis of transgenic mice is not associated with gross alterations in tissue architecture. Indeed, LMP2A fails to induce any histomorphological changes in mouse epidermis (41). Despite the lack of a phenotype for LMP2A in normal epithelial cells, at least one study has demonstrated a “transforming” function for LMP2A in an immortalized epithelial cell line. In an original study, Scholle et al. (55) demonstrated that LMP2A induced not only a differentiation blockade but also full malignant transformation of the HaCat epithelial cell line. These observations suggest that phenotypes associated with LMP2A expression are more subtle than those of LMP1 and, perhaps more interestingly, are only manifest in a particular cellular context.
In our study, expression of LMP2A and LMP2B in A431, HaCat, or SCC12F cells was not associated with a striking alteration in cell morphology. Cells retained a flat cuboidal morphology, formed clearly visible tight junctions, and, in the case of SCC12F cells, stratified when confluent. Despite the lack of an effect on epithelial cell morphology, we found that expression of LMP2A and LMP2B was associated with a striking change in the behavior of epithelial cells. A novel phenotype which relates to the ability of these proteins to promote epithelial cell spreading and motility has been identified. This observation is clearly relevant to NPC pathogenesis, as deregulated cell movement may facilitate tumor cell spread and invasion (36). Of particular interest is the finding that LMP2B is able to exert biological effects, a finding that is intriguing given that LMP2B lacks the cytosolic amino terminus that possesses most of LMP2A's signaling capabilities (42). The mechanisms by which LMP2A and LMP2B promote this particular phenotype remain unknown. Both proteins localize to perinuclear endosomes in adherent cells and do not translocate to focal adhesions in response to adhesion. Indeed, attempts to demonstrate an association of LMP2A or LMP2B with structural components of focal adhesions by confocal imaging have proved unsuccessful. In view of these findings, it is more likely that these two proteins engage signaling pathways that directly promote cell spreading and motility. Such a scenario is not without precedent, as many growth factor, cytokine, and immunoglobulin receptors engage signaling pathways that promote cell adhesion and motility in addition to cell proliferation (12, 28, 66).
Although the processes by which LMP2A and LMP2B influence cell spreading are unknown, it is likely that these proteins modulate the activity of integrins or influence integrin-signaling pathways. The initial engagement of integrins with their substrate triggers signaling pathways that regulate changes in morphology and cell spreading. That LMP2A and LMP2B promote cell spreading rather than attachment suggests that they may modulate integrin signaling directly. Integrin engagement activates a variety of intracellular signaling molecules. These molecules include tyrosine kinases, serine/threonine kinases, lipid kinases, and the small Rho GTPases (49). Rho GTPases play an essential role in cell shape change, as cell spreading requires the polymerization and reorganization of actin filaments into lamellopodia and filopodia and the formation of new integrin-substrate adhesions (3, 57, 67). The ability of LMP2A and LMP2B to regulate activity of the small Rho GTPases is clearly an area for future study.
Through the use of specific pharmacological inhibitors, we have ruled out contributions of a number of signaling molecules as key effectors in the cell spreading phenotype induced by LMP2A and LMP2B. We found that high concentrations of the tyrosine kinase inhibitor PP2 partially inhibited the spreading of both LMP2A- and LMP2B-expressing cells on ECM. These results are not surprising given that Src and Src-related tyrosine kinases regulate focal adhesion assembly and turnover by modulating the activity of FAK and other focal adhesion-associated proteins (22, 23). However, the fact that lower doses of PP2 (0.05 to 5 μM) were less effective at inhibiting the spreading of LMP2A- and LMP2B-expressing cells suggests that LMP2A and LMP2B are able to overcome the inhibitory effects of this compound. Whether this is a direct effect on tyrosine kinase activity or is mediated indirectly via inhibition of a negative regulator such as Csk or a protein tyrosine phosphatase remains to be elucidated.
The inability to block adhesion and spreading of LMP2A-and LMP2B-expressing cells with LY294002 suggests that LMP2A and LMP2B bypass the requirement for PI3-K in the initial phase of cell spreading. This is particularly interesting, as PI3-K along with c-Src appear critical for cell spreading and focal adhesion formation in many cell types (3, 38, 39). The lipid products generated as a consequence of PI3-K activation are known to activate guanine nucleotide exchange factors for Rho GTPases such as Rac. D3-phosphorylated lipids sensitize guanine nucleotide exchange factors such as Vav and Tiam-1 to further activation by tyrosine kinases (29). Although it is possible that LMP2A and LMP2B may promote Rac activity through a pathway independent of PI3-K, it is also possible that they elevate the basal levels of these D3 lipid products to overcome PI3-K inhibition, possibly as a consequence of inhibiting PTEN activity.
In a number of cell types, ERK-MAPK appears to be important for cell migration (63, 65). MEK-ERK activation appears to play an important role in the later stages of cell spreading by facilitating filopodia and microspike formation at the leading edge of spreading epithelial cells; indeed, phosphorylated ERK is targeted to focal adhesions in spreading cells (21, 65). Our finding that the MEK inhibitor UO126 was ineffective at blocking the attachment and spreading of LMP2A- and LMP2B-expressing cells on ECM suggests that ERK activity is not required by LMP2A and LMP2B to promote cell spreading. However, the fact that ERK-MAPK is required for phosphorylation of myosin light chain kinase, which functions to coordinate actin-myosin contractility, suggests that sustained ERK-MAPK activity may be required for LMP2A and LMP2B to promote protracted cell movement (65). A recent study by Chen et al. (15) supports such a notion, as sustained ERK-MAPK activation is essential for LMP2A to promote tubulogenesis and motility in embedded matrices. Although basal ERK-MAPK activity was high in SCC12F cells expressing LMP2A and LMP2B, this level of ERK-MAPK activity did not appear to be significantly greater than that observed in Neo control cells. Further work will examine the effects of stable LMP2A expression on ERK-MAPK activation in other epithelial cell lines and, more specifically, whether LMP2B is able to engage the ERK-MAPK pathway.
Inhibitor studies also showed that LMP2A and LMP2B do not require PKC activity to induce cell spreading, as treatment of cells with the PKC inhibitor calphostin C had little effect on the attachment and spreading of LMP2A- and LMP2B-expressing SCC12F cells on ECM. Accumulating evidence indicates that members of the PKC superfamily influence cell attachment and spreading by modulating integrin avidity (35, 49). Both PKCα and PKCɛ are activated in response to cell attachment (35) and appear to promote cell spreading (16, 64). EGF, a growth factor with proven ability to promote epithelial cell migration, does so in part through activation of phospholipase C γ (PLC-γ) and PKC (61). Although PLC-γ and PKC activities appear to be essential for EGF-mediated cell spreading and motility (14, 66), this activity does not appear to be required by LMP2A and LMP2B. This finding is particularly interesting, as it suggests that LMP2A and LMP2B affect cell adhesion and motility through pathways that are distinct from those of EGF and possibly other receptor tyrosine kinases. In a recent study, Kassis et al. (37) demonstrated that EBV-infected AGS cells were more motile than their noninfected counterparts in an in vitro Matrigel invasion assay. This study ruled out contributions of classical receptor tyrosine kinase signaling cascades, as EBV-infected cells were refractory to inhibitors of the EGF receptor, PLC-γ, PI3-K, and ERK-MAPK. Although EBV-infected gastric epithelial cells do not express LMP1, they do express low levels of LMP2A (34, 50). These findings lend weight to our observations and suggest that LMP2A and/or LMP2B is the probable effector of this phenotype.
In response to cell adhesion, LMP2A promotes robust global tyrosine phosphorylation of many cellular proteins. Although it is likely that many of the tyrosine-phosphorylated species are structural proteins, it is also possible that some of these proteins represent signaling molecules such as protein kinases and phosphatases. The ability of LMP2A to promote Akt activation in the absence of growth factor-integrin signals is in agreement with previous observations (55, 60) and demonstrates that LMP2A is able to activate the PI3-K/Akt pathway directly. This ability may, under certain circumstances, inhibit or delay suspension-induced apoptosis (anoikis), a form of apoptosis that is induced as a consequence of epithelial cell detachment (24). The ability of LMP2A to engage this signaling pathway would provide LMP2A-expressing cells with a distinct survival advantage in vivo and would enhance the survival of EBV-infected carcinoma cells. Although our findings demonstrate that PI3-K activity remains elevated in LMP2A-expressing cells maintained in suspension, the same is not true for LMP2B. These findings suggest that PI3-K/Akt activation does not play a role in the adhesion and spreading phenotype, as LMP2B is able to promote cell spreading in the absence of sustained PI3-K/Akt activity.
The consistent expression of LMP2A and LMP2B in NPC tumors coupled with the highly metastatic rate of this tumor type suggest that like LMP1, LMP2A and LMP2B may contribute to the invasion and the metastatic spread of EBV-positive tumors. The precise mechanism(s) by which these proteins induce this phenotype is still unclear. Unlike LMP2A, LMP2B lacks the cytosolic amino terminus that contains putative binding sites for tyrosine kinases, ruling out a contribution of the amino terminus to this effect. However, a potential clue to their mode of action may lie in their subcellular localization. LMP2A localizes to cholesterol and sphingolipid-enriched lipid rafts in B cells (19, 30). Although data confirming the localization of LMP2B to lipid rafts are not available, it is likely that LMP2B also targets these structures. Immunofluorescent staining shows that LMP2B localizes to identical intracellular compartments as LMP2A in epithelial cells (17, 46), although whether these intracellular vesicles are glycolipid-rich microdomains remains to be determined. Whether lipid raft association is an important facet of LMP2A and LMP2B signaling and whether this association has any bearing on this particular phenotype are unknown. A domain within the C terminus common to both LMP2A and LMP2B which facilitates homo- or hetero-oligomerization of LMP2A and LMP2B has been identified (47). This domain contains motifs that interact with PDZ domain-containing proteins. PDZ binding proteins, by virtue of their ability to bind cellular proteins and receptors, regulate signal transduction (5). The identification of these interacting proteins may shed light on the signaling properties of both LMP2A and LMP2B.
Our finding that LMP2B is able to generate a phenotype in epithelial cells raises the possibility that this protein may be more functionally active than appreciated and, rather than serving to modulate the activity of LMP2A, may engage signaling pathways to promote changes in epithelial cell behavior. The notion that EBV-positive carcinomas possess a highly invasive phenotype suggests that expression of one or more viral proteins can have an impact on cell motility and invasion. In this respect, the vast majority of research has focused on LMP1, given its ability to induce profound alterations in epithelial cell growth. However, its low and variable expression in EBV-positive carcinomas suggests that in its absence, LMP2A and LMP2B may provide the stimulus to promote cell motility and invasion.
REFERENCES
- 1.Baker, S. E., A. P. DiPasquale, E. L. Stock, V. Quaranta, M. Fitchmun, and J. C. Jones. 1996. Morphogenetic effects of soluble laminin-5 on cultured epithelial cells and tissue explants. Exp. Cell Res. 228:262-270. [DOI] [PubMed] [Google Scholar]
- 2.Beaufils, P., D. Choquet, R. Z. Mamoun, and B. Malissen. 1993. The (YXXL/I)2 signalling motif found in the cytoplasmic segments of the bovine leukaemia virus envelope protein and Epstein-Barr virus latent membrane protein 2A can elicit early and late lymphocyte activation events. EMBO J. 12:5105-5112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berrier, A. L., A. M. Mastrangelo, J. Downward, M. Ginsberg, and S. E. LaFlamme. 2000. Activated R-ras, Rac1, PI 3-kinase and PKCɛ can each restore cell spreading inhibited by isolated integrin β1 cytoplasmic domains. J. Cell Biol. 151:1549-1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Besson, A., T. L. Wilson, and V. W. Yong. 2002. The anchoring protein RACK1 links PKCɛ to integrin β chains. J. Biol. Chem. 287:22073-22084. [DOI] [PubMed] [Google Scholar]
- 5.Bezprozvanny, I., and A. Maximov. 2001. PDZ domains: more than just a glue. Proc. Natl. Acad. Sci. USA 98:787-789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boukamp, P., R. T. Petrussevska, D. Breitkreutz, J. Hornung, A. Markham, and N. E. Fusenig. 1988. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J. Cell Biol. 106:761-771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brooks, L., Q. Y. Yao, A. B. Rickinson, and L. S. Young. 1992. Epstein-Barr virus latent gene transcription in nasopharyngeal carcinoma cells: coexpression of EBNA1, LMP1, and LMP2 transcripts. J. Virol. 66:2689-2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burkhardt, A. L., J. B. Bolen, E. Kieff, and R. Longnecker. 1992. An Epstein-Barr virus transformation-associated membrane protein interacts with src family tyrosine kinases. J. Virol. 66:5161-5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Busson, P., R. McCoy, R. Sadler, K. Gilligan, T. Tursz, and N. Raab-Traub. 1992. Consistent transcription of the Epstein-Barr virus LMP2 gene in nasopharyngeal carcinoma. J. Virol. 66:3257-3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caldwell, R. G., R. C. Brown, and R. Longnecker. 2000. Epstein-Barr virus LMP2A-induced B-cell survival in two unique classes of EmLMP2A transgenic mice. J. Virol. 74:1101-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caldwell, R. G., J. B. Wilson, S. J. Anderson, and R. Longnecker. 1998. Epstein-Barr virus LMP2A drives B cell development and survival in the absence of normal B cell receptor signals. Immunity 9:405-411. [DOI] [PubMed] [Google Scholar]
- 12.Cantrell, D. A. 2003. GTPases and T cell activation. Immunol. Rev. 192:122-130. [DOI] [PubMed] [Google Scholar]
- 13.Chen, F., L. F. Hu, I. Ernberg, G. Klein, and G. Winberg. 1995. Coupled transcription of Epstein-Barr virus latent membrane protein (LMP)-1 and LMP-2B genes in nasopharyngeal carcinomas. J. Gen. Virol. 76:131-138. [DOI] [PubMed] [Google Scholar]
- 14.Chen, P., K. Gupta, and A. Wells. 1994. Cell movement elicited by epidermal growth factor receptor requires kinase and autophosphorylation but is separable from mitogenesis. J. Cell Biol. 124:547-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen, S.-Y., J. Lu, Y.-C. Shih, and C.-H. Tsai. 2002. Epstein-Barr virus latent membrane protein 2A regulates c-Jun protein through extracellular signal-regulated kinase. J. Virol. 76:9556-9561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chun, J. S., M. J. Ha, and B. S. Jacobson. 1996. Differential translocation of protein kinase C epsilon during HeLa cell adhesion to a gelatin substratum. J. Biol. Chem. 281:13008-13012. [DOI] [PubMed] [Google Scholar]
- 17.Dawson, C. W., J. H. George, S. M. Blake, R. Longnecker, and L. S. Young. 2001. The Epstein-Barr virus encoded latent membrane protein 2A augments signaling from latent membrane protein 1. Virology 289:192-207. [DOI] [PubMed] [Google Scholar]
- 18.Dawson, C. W., and L. S. Young. 2001. In vitro assays to study epithelial cell differentiation. Methods Mol. Biol. 174:173-180. [DOI] [PubMed] [Google Scholar]
- 19.Dykstra, M. L., R. Longnecker, and S. K. Pierce. 2001. Epstein-Barr virus coopts lipid rafts to block the signaling and antigen transport functions of the BCR. Immunity 14:57-67. [DOI] [PubMed] [Google Scholar]
- 20.Farwell, D., J. McDougall, and M. Coltrera. 1999. Expression of Epstein-Barr virus latent membrane proteins leads to changes in keratinocyte cell adhesion. Ann. Otol. Rhinol. Laryngol. 108:851-859. [DOI] [PubMed] [Google Scholar]
- 21.Fincham, V. J., M. James, M. C. Frame, and S. J. Winder. 2000. Active ERK/MAP kinase is targeted to newly forming cell-matrix adhesions by integrin engagement and v-Src. EMBO J. 19:2911-2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frame, M. C., V. J. Fincham, N. O. Carragher, and J. A. Wyke. 2002. v-Src's hold over actin and cell adhesions. Nat. Rev. Mol. Cell Biol. 3:233-245. [DOI] [PubMed] [Google Scholar]
- 23.Frame, M. C., and V. G. Brunton. 2002. Advances in Rho-dependent actin regulation and oncogenic transformation. Curr. Opin. Genet. Dev. 12:36-43. [DOI] [PubMed] [Google Scholar]
- 24.Frisch, S. M., and H. Francis. 1994. Disruption of epithelial cell-matrix interactions induces apoptosis. J. Cell Biol. 124:619-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fruehling, S., S. K. Lee, R. Herrold, B. Frech, G. Laux, E. Kremmer, F. A. Grasser, and R. Longnecker. 1996. Identification of latent membrane protein 2A (LMP2A) domains essential for the LMP2A dominant-negative effect on B-lymphocyte surface immunoglobulin signal transduction. J. Virol. 70:6216-6226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fruehling, S., R. Swart, K. M. Dolwick, E. Kremmer, and R. Longnecker. 1998. Tyrosine 112 of latent membrane protein 2A is essential for protein tyrosine kinase loading and regulation of Epstein-Barr virus latency. J. Virol. 72:7796-7806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fukuda, M., and R. Longnecker. 2004. Latent membrane protein 2A inhibits transforming growth factor-β1-induced apoptosis through the phosphatidylinositol 3-kinase/Akt pathway. J. Virol. 78:1697-16705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fuller, C. L., V. L. Braciale, and L. E. Samelson. 2003. All roads lead to actin: the intimate relationship between TCR signaling and the cytoskeleton. Immunol. Rev. 191:220-236. [DOI] [PubMed] [Google Scholar]
- 29.Han, J., K. Luby-Phelps, B. Das, X. Shu, Y. Xia, R. D. Mosteller, U. M. Krishna, J. R. Falck, M. A. White, and D. Broek. 1998. Role of substrates and products of PI 3-kinase in regulating activation of Rac-related guanosine triphosphatases by Vav. Science 279:558-560. [DOI] [PubMed] [Google Scholar]
- 30.Higuchi, M., K. M. Izumi, and E. Kieff. 2001. Epstein-Barr virus latent-infection membrane proteins are palmitoylated and raft-associated: protein 1 binds to the cytoskeleton through TNF receptor cytoplasmic factors. Proc. Natl. Acad. Sci. USA 98:4675-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Howe, A., A. E. Aplin, S. K. Alahari, and R. L. Juliano. 1998. Integrin signalling and cell growth control. Cell 110:673-687. [DOI] [PubMed] [Google Scholar]
- 32.Ikeda, M., A. Ikeda, L. C. Longan, and R. Longnecker. 2000. The Epstein-Barr virus latent membrane protein 2A PY motif recruits WW domain-containing ubiquitin-protein ligases. Virology 268:178-191. [DOI] [PubMed] [Google Scholar]
- 33.Imai, S., S. Koizumi, M. Sugiura, M. Tokunaga, Y. Uemura, N. Yamamoto, S. Tanaka, E. Sata, and T. Osato. 1994. Gastric carcinoma: monoclonal epithelial malignant cells expressing Epstein-Barr virus latent infection protein. Proc. Natl. Acad. Sci. USA 91:9131-9135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Imai, S., J. Nishikawa, and K. Takada. 1998. Cell-to-cell contact as an efficient mode of Epstein-Barr virus infection of diverse human epithelial cells. J. Virol. 72:4371-4378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ivaska, J., S. Kermorgant, R. Whelan, M. Parsons, T. Ng, and P. J. Parker. 2003. Integrin-protein kinase C relationships. Biochem. Soc. Trans. 31:90-93. [DOI] [PubMed] [Google Scholar]
- 36.Kassis, J., D. A. Lauffenburger, T. Turner, and A. Wells. 2001. Tumor invasion as dysregulated cell motility. Semin. Cancer Biol. 11:105-117. [DOI] [PubMed] [Google Scholar]
- 37.Kassis, J., A. Maeda, N. Teramoto, K. Takada, C. Wu, G. Klein, and A. Wells. 2002. EBV-expressing AGS gastric carcinoma cell sublines present increased motility and invasiveness. Int. J. Cancer 99:644-651. [DOI] [PubMed] [Google Scholar]
- 38.Khwaja, A., P. Rodriguez-Viciana, S. Wennstrom, P. H. Warne, and J. Downward. 1997. Matrix adhesion and Ras transformation both activate a phosphoinositide 3-OH kinase and protein kinase B/AKT cellular survival pathway. EMBO J. 16:2783-2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.King, W. G., M. D. Mattaliano, T. O. Chan, P. N. Tsichlis, and J. S. Brugge. 1997. Phosphatidylinositol 3-kinase is required for integrin-stimulated AKT and Raf-1/mitogen-activated protein kinase pathway activation. Mol. Cell. Biol. 17:4406-4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lo, K. W., and D. P. Huang. 2002. Genetic and epigenetic changes in nasopharyngeal carcinoma. Semin. Cancer Biol. 12:451-462. [DOI] [PubMed] [Google Scholar]
- 41.Longan, L., and R. Longnecker. 2000. Epstein-Barr virus latent membrane protein 2A has no growth-altering effects when expressed in differentiating epithelia. J. Gen. Virol. 81:2245-2252. [DOI] [PubMed] [Google Scholar]
- 42.Longnecker, R. 2000. Epstein-Barr virus latency: LMP2, a regulator or means for Epstein-Barr virus persistence? Adv. Cancer Res. 79:176-200. [DOI] [PubMed] [Google Scholar]
- 43.Longnecker, R., B. Drucker, T. M. Roberts, and E. Kieff. 1991. An Epstein-Barr virus protein associated with cell growth transformation interacts with a tyrosine kinase. J. Virol. 65:3681-3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Longnecker, R., C. Miller, X.-Q. Miao, B. Tomkinson, and E. Kieff. 1993. The last seven transmembrane and carboxy-terminal cytoplasmic domains of Epstein-Barr virus latent membrane protein 2 (LMP2) are dispensable for lymphocyte infection and growth transformation in vitro. J. Virol. 67:2006-2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Longnecker, R., C. L. Miller, B. Tomkinson, X.-Q. Miao, and E. Kieff. 1993. Deletion of DNA encoding the first five transmembrane domains of Epstein-Barr virus latent membrane proteins 2A and 2B. J. Virol. 67:5068-5074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lynch, D. T., J. S. Zimmerman, and D. T. Rowe. 2002. Epstein-Barr virus latent membrane protein 2B (LMP2B) co-localizes with LMP2A in perinuclear regions in transiently transfected cells. J. Gen. Virol. 83:1025-1035. [DOI] [PubMed] [Google Scholar]
- 47.Matskova, L., I. Ernberg, T. Pawson, and G. Winberg. 2001. C-terminal domain of the Epstein-Barr virus LMP2A membrane protein contains a clustering signal. J. Virol. 75:10941-10949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morrison, J. A., A. J. Klingelhutz, and N. Raab-Traub. 2003. Epstein-Barr virus latent membrane protein 2A activates beta-catenin signaling in epithelial cells. J. Virol. 77:12276-12284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mostafavi-Pour, Z., J. A. Askari, S. J. Parkinson, P. J. Parker, T. T. Ng, and M. J. Humphries. 2003. Integrin-specific signalling pathways controlling focal adhesion formation and cell migration. J. Cell Biol. 161:155-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nishikawa, J., S. Imai, T. Oda, T. Kojima, K. Okita, and K. Takada. 1999. Epstein-Barr virus promotes epithelial cell growth in the absence of EBNA2 and LMP1 expression. J. Virol. 73:1286-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Panousis, C. G., and D. T. Rowe. 1997. Epstein-Barr virus latent membrane protein 2 associates with and is a substrate for mitogen-activated protein kinase. J. Virol. 71:4752-4760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Raab-Traub, N. 2002. Epstein-Barr virus and nasopharyngeal carcinoma. Semin. Cancer Biol. 3:297-307. [PubMed] [Google Scholar]
- 53.Rheinwald, J. G., and M. A. Beckett. 1981. Tumorigenic keratinocyte lines requiring anchorage and fibroblast support cultures from human squamous cell carcinomas. Cancer Res. 41:1657-1665. [PubMed] [Google Scholar]
- 54.Rickinson, A. B., and E. Kieff. 1996. Epstein-Barr virus, p. 2397-2446. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology, 3rd ed. Lippincott-Raven Publishers, Philadelphia, Pa.
- 55.Scholle, F., K. M. Bendt, and N. Raab-Traub. 2000. Epstein-Barr virus LMP2A transforms epithelial cells, inhibits cell differentiation, and activates Akt. J. Virol. 74:10681-10689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Scholle, F., R. Longnecker, and N. Raab-Traub. 1999. Epithelial cell adhesion to extracellular matrix proteins induces tyrosine phosphorylation of the Epstein-Barr virus latent membrane protein 2: a role for C-terminal Src kinase. J. Virol. 73:4767-4775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Small, J. V., K. Rottner, and I. Kaverina. 1999. Functional design in the actin cytoskeleton. Curr. Opin. Cell Biol. 11:54-60. [DOI] [PubMed] [Google Scholar]
- 58.Speck, P., K. A. Kline, P. Cheresh, and R. Longnecker. 1999. Epstein-Barr virus lacking latent membrane protein 2 immortalizes B cells with efficiency indistinguishable from that of wild-type virus. J. Gen. Virol. 80:2193-2203. [DOI] [PubMed] [Google Scholar]
- 59.Swart, R., S. Fruehling, and R. Longnecker. 1999. Tyrosines 60, 64, and 101 of Epstein-Barr virus LMP2A are not essential for blocking B cell signal transduction. Virology 263:485-495. [DOI] [PubMed] [Google Scholar]
- 60.Swart, R., I. K. Ruf, J. Sample, and R. Longnecker. 2000. Latent membrane protein 2A-mediated effects on the phosphatidylinositol 3-kinase/Akt pathway. J. Virol. 74:10838-10845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thomas, S. M., F. M. Coppelli, A. Wells, W. E. Gooding, J. Song, J. Kassis, S. D. Drenning, and J. R. Grandis. 2003. Epidermal growth factor receptor-stimulated activation of phospholipase C γ-1 promotes invasion of head and neck squamous cell carcinoma. Cancer Res. 63:5629-5635. [PubMed] [Google Scholar]
- 62.Tremblay, L., W. Hauck, A. G. Aprikian, L. R. Begin, A. Chapdelaine, and S. Chevalier. 1996. Focal adhesion kinase (pp125FAK) expression, activation and association with paxillin and p50CSK in human metastatic prostate carcinoma. Int. J. Cancer 68:164-171. [DOI] [PubMed] [Google Scholar]
- 63.Vial, E., E. Sahai, and C. J. Marshall. 2003. ERK-MAPK signaling coordinately regulates activity of Rac1 and RhoA for tumor cell motility. Cancer Cell 4:67-79. [DOI] [PubMed] [Google Scholar]
- 64.Vuori, K., and E. Ruoslahti. 1993. Activation of protein kinase C precedes α5β1 integrin-mediated cell spreading on fibronectin. J. Biol. Chem. 268:1459-1462. [PubMed] [Google Scholar]
- 65.Webb, D. J., K. Donais, L. A. Whitmore, S. M. Thomas, C. E. Turner, J. T. Parsons, and A. F. Horwitz. 2004. FAK-Src signalling through paxillin, ERK and MLCK regulates adhesion disassembly. Nat. Cell Biol. 6:154-161. [DOI] [PubMed] [Google Scholar]
- 66.Wells, A., J. Kassis, J. Solava, T. Turner, and D. A. Lauffenburger. 2002. Growth factor-induced cell motility in tumor invasion. Acta Oncol. 41:124-130. [DOI] [PubMed] [Google Scholar]
- 67.Ylanne, J., Y. Chen, T. E. O'Toole, J. C. Loftus, Y. Takada, and M. H. Ginsberg. 1993. Distinct functions of integrin α and β subunit cytoplasmic domains in cell spreading and formation of focal adhesions. J. Cell Biol. 122:223-233. [DOI] [PMC free article] [PubMed] [Google Scholar]