Abstract
The membrane fusion function of murine leukemia virus (MLV) is carried by the Env protein. This protein is composed of three SU-TM subunit complexes. The fusion activity is loaded into the transmembrane TM subunit and controlled by the peripheral, receptor-binding SU subunit. It is assumed that TM adopts a metastable conformation in the native Env and that fusion activation involves the folding of TM into a stable form. Activation is suppressed by the associated SU and triggered by its dissociation, which follows receptor binding. Recently we showed that the two subunits are disulfide linked and that SU dissociation and triggering of the fusion function are caused by a switch of the intersubunit disulfide into an intrasubunit disulfide isomer using an isomerization-active CWLC motif in SU (M. Wallin, M. Ekstrom, and H. Garoff, EMBO J. 23:54-65, 2004). In the present work we address how the SU disulfide isomerase is activated. Using Moloney MLV, we show that isomerization of the SU-TM disulfide bond can be triggered by heat, urea, or guanidinium hydrochloride. Such protein perturbation treatments also significantly increase the kinetics and efficiency of viral fusion. The threshold conditions for the effects on isomerization and fusion are virtually the same. This finding indicates that destabilization of interactions in the SU oligomer induces the disulfide bond isomerase and the subsequent activation of the fusion function in TM.
Retroviruses enter cells by membrane fusion at the plasma membrane (PM) or in the endosome (14, 24). The fusion function is carried by the viral Env protein and triggered by receptor binding or by a combination of receptor binding and low pH. The Env protein is an oligomer composed of three SU-TM subunit complexes (16). TM has a transmembrane topology, whereas SU adopts a peripheral location. Consequently, SU mediates receptor binding and TM mediates membrane fusion. The fusion mechanism has not yet been elucidated, but it has been suggested to resemble that of influenza virus (31).
In the trimeric hemagglutinin (HA) of influenza virus, the peripheral HA1 subunits will bind to the receptor whereas the transmembrane HA2 subunits perform fusion after triggering by low pH in the endosome (15, 36). HA2 persists in a metastable form in the native HA1-HA2 complex but folds into a stable form after low-pH-induced dissociation of the HA1-HA2 interaction (4, 6, 7, 9, 37). Notably, the membrane fusion reaction is mediated by intermediates in the folding process and not by the stable form, which is fusion inactive. The folding involves exposure of a fusion peptide that targets the cell membrane and a jackknife-like chain reversal, which brings the viral and cell membranes together. This transforms the core structure in the HA2 trimer from a triple-stranded α-helical coiled-coil into a very stable double-layered six-helix-bundle. As expected for metastable structures, HA2 folding can also be induced by nonspecific protein destabilization. Thus, treatment of influenza virus with heat or urea activates the fusion function in HA (6, 29).
There are several studies suggesting that retrovirus TM also is metastable in the native Env and that it undergoes HA2-like changes upon fusion activation. For instance, structure analyses of complexes formed by fragments of several retrovirus fusion subunits have revealed stable double-layered six-helix-structures (10, 19, 35). Furthermore, peptides corresponding to the outer helix layer have been found to inhibit human immunodeficiency virus type 1 (HIV-1) fusion, probably by interfering with chain reversal (8, 23). Finally, the TM subunits of avian leukosis and sarcoma virus (ALSV) Env have been shown to form sodium dodecyl sulfate (SDS)-resistant oligomers upon fusion activation (24). Similar structures were also seen after treatment of avian leukosis virus (ALV) with heat or urea (32).
Although the HA2 of influenza virus and the TM of retroviruses seem to operate in a similar fashion in fusion, they differ in their modes of activation. Thus, ALSV is triggered for fusion by a combination of receptor binding and low pH, and most other retroviruses are triggered solely by the interaction with the cell receptor (14, 24). Furthermore, we recently demonstrated that the SU and TM subunits of murine leukemia virus (MLV) Env were disulfide bonded and that membrane fusion and infection of MLV are controlled by isomerization of the intersubunit disulfide bond using an internal switch motif, CWLC, in the C-terminal part of SU (33). In this motif one Cys residue participates in the formation of the SU-TM disulfide bond and the other is free (27). The isomerization reaction is initiated by activation of the free thiol. This then attacks the SU-TM disulfide bond and forms a disulfide isomer within the motif instead. The isomerization results in dissociation of the SU subunits from the Env complex and activation of the fusion function in TM.
The binding of the SU subunit of MLV Env to its receptor at the PM of the cell provides the primary trigger for isomerization of the intersubunit disulfide bond. An important question is how SU mediates this activation. It is well established that the SU of MLV consists of a receptor-binding N-terminal domain (RBD) and a TM-associating C-terminal domain. This modular organization was indicated by sequence analyses (18, 26, 34). It was directly proven by separate expression of the RBD and Env with RBD deleted and the demonstration of their capacity to complement each other in virus infection (3, 13). Therefore, it appears reasonable to assume that binding of the RBD to its cell receptor will convey a signal to the C-terminal domain of SU, which will activate the isomerization of the intersubunit disulfide bond. The signaling could involve receptor-induced conformational changes in, e.g., the RBD or simply destabilization of the SU subunit oligomer in Env. Here we have tested the sensitivity of MLV Env to be triggered for fusion by protein destabilization. We found that treatment of the virus with heat, urea, or guanidinium hydrochloride (Gnd HCl) activated SU-TM disulfide bond isomerization in Env and facilitated virus-mediated cell-cell fusion and infectivity. This suggests that natural triggering of the isomerization activation signal in SU might involve receptor-induced destabilization of the SU oligomer rather than the induction of a new conformation of, e.g., RBD. Interestingly, the threshold conditions for the effects were much lower than those found previously to trigger influenza virus HA and ALSV Env. As the latter cannot induce fusion activation by isomerization of their intersubunit disulfide bond, but require low pH in the endosome, we suggest that the SU isomerase has evolved as a mechanism to facilitate receptor-triggered cell surface entry of a retrovirus with covalently linked Env subunits.
MATERIALS AND METHODS
Cells and virus.
BHK-21 cells were grown as described elsewhere (25). XC and 3T3 cells (American Type Culture Collection, Manassas, Va.) and MOV-3 cells (G. Schmidt, GSF-National Research Center for Environment and Health, Neuherberg, Germany) were maintained in Dulbecco's modified Eagle's medium (DMEM; GIBCO BRL) supplemented with 10% fetal calf serum (FCS), 20 mM HEPES, and l-glutamine. Moloney MLV (Mo-MLV) was prepared in MOV-3 cells by incubation for 14 h in DMEM containing 5% FCS. Radioactively labeled Mo-MLV was produced similarly but including [35S]cysteine (Cys) (Amersham Pharmacia Biotech, Amersham, United Kingdom) as described elsewhere (25).
In vitro induction of isomerization in Env and analyses of virus structure.
[35S]Cys-labeled virus in DMEM was taken up into TN (17 mM Tris, 8 mM HEPES [pH 7.45], 150 mM NaCl) containing 1.8 mM Ca2+ (control buffer) by ultrafiltration (Nanose OMEGA 300-kDa MW cutoff filters; Pall Corporation, Ann Arbor, Mich.) at 4°C and incubated either (i) at 37 to 56°C for 60 min, (ii) at 37°C for 30 min in the presence of 0 to 2.5 M urea, or (iii) at 30°C for 30 min in the presence of 0 to 1 M Gnd HCl. Additional conditions (indicated in Results) were also used. Heat, urea, and Gnd HCl treatments were carried out by adding precalibrated volumes of heated TN-1.8 mM Ca2+ or TN-1.8 mM Ca2+ with concentrated urea or Gnd HCl. The isomerization reactions were terminated by adding N-ethylmaleimide (NEM) (SIGMA-Aldrich Chemie, Munich, Germany) to 20 mM and NP-40 lysis-buffer (50 mM Tris [pH 7.5], 150 mM NaCl, 2 mM EDTA, 1% NP-40) containing 20 mM NEM. After incubation at 30°C for 30 min, viral proteins were immunoprecipitated with goat polyclonal antibodies HE863 against MLV proteins (Viromed Biosafety Laboratories, Camden, N.J.). The recovered viral proteins were analyzed by nonreducing SDS-12% polyacrylamide gel electrophoresis (PAGE). To estimate the degree of isomerization, we measured (i) the decrease in the level of the covalently linked SU-TM complex and the increases in levels of (ii) unlinked SU and (iii) TM subunits compared to levels for control samples that received NEM before induction of isomerization. The three estimates, given as percentages of control levels, agreed within 15% and their mean value was used to describe the degree of isomerization The procedures for immunoprecipitation, SDS-PAGE, autoradiography, and quantification of labeled protein bands on gels have been described elsewhere (25). Flotation centrifugation analysis of the virus was done in a step gradient, composed of 5 ml of 55% (wt/wt) sucrose (loading zone), 2 ml of 40% sucrose, 2 ml of 35% sucrose, 1 ml of 30% sucrose, and 1 ml of 10% sucrose in 50 mM Tris (pH 7.4)-150 mM NaCl. Centrifugation was carried out for 16 h at 160,000 × g and 4°C in a Beckman SW41 rotor.
Assays for virus functions.
Mo-MLV (4 × 106 infectious units per ml) was bound to confluent XC cells in 6-well (35-mm-diameter) dishes for 1 h at 4°C in DMEM with Polybrene (8 μg/ml) and subjected to fusion incubation (i) in control buffer at 37 to 58°C for 2 min, (ii) in control buffer containing 0 to 2 M urea for 2 min at 37°C, or (iii) in control buffer containing 0 to 0.8 M Gnd HCl for 105 s at 37°C. Additional conditions (described in Results) were also used. The heat, urea, and Gnd HCl treatments were preceded and followed by 30-s washes with control buffer at room temperature. In some fusion experiments, the virus (in 2 ml of medium) was dialyzed against 2 liters of HN buffer (15 mM HEPES [pH 7.4], 150 mM NaCl) for 24 h at 4°C by using a 15-kDa MW cutoff membrane (Spectrum Laboratories Inc., Rancho Dominiguez, Calif.) and then subjected to isomerization-inducing treatments before cell binding and fusion. These treatments included heating in the presence of 1.8 mM Ca2+ and incubations at 37°C in the presence of different concentrations of Ca2+ or Mg2+. The latter samples were adjusted to, or close to, control buffer conditions after incubation. The fusion incubations were terminated by incubation in 40 mM sodium citrate (pH 3)-10 mM KCl-135 mM NaCl for 1 min at 20°C. Virus-fused cells were allowed to rearrange into polykaryons by further incubation in DMEM for 3 h. The cells were stained in methanol-methylene blue (0.5%), the numbers of cells (ncell) and nuclei (nnuclei) were counted, and the fusion index (Fi = 1 − ncell/nnuclei) was calculated. Parallel 1.5- to 3-fold dilution series under test and control conditions were measured, and the relative fusion efficiency (as a percentage of fusion by the control), obtained by matching the Fi values of the two series, was given. Only Fi values in the range of 0.05 to 0.85, which have been shown to be directly proportional to virus amount, were used (33). Standard deviations were based on data from three to five experiments. 3T3-cells (30 to 40% confluent) were infected with Mo-MLV by using the same protocols as for XC cell fusion. However, after virus inactivation with a low-pH buffer, 3T3 cells were incubated for 33 h and scored for infected cells by immunofluorescence using the anti-SU monoclonal antibody (MAb) 273 essentially as described previously (30). Duplicate samples were used for each condition, and relative infectivities were calculated and expressed as percentages of the infectivity of the control.
RESULTS
Heat, urea, and Gnd HCl induce SU-TM disulfide bond isomerization and enhance the fusion activity of Env.
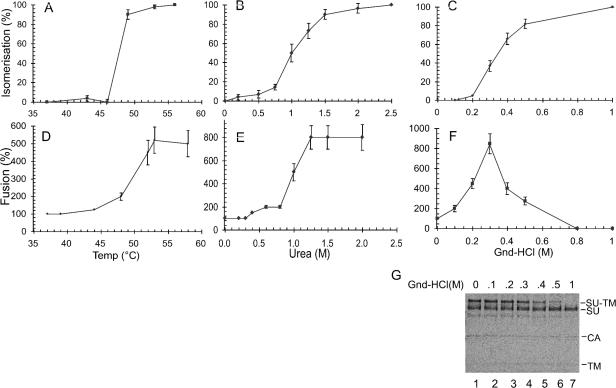
Our previous screening of conditions that induce isomerization of the SU-TM disulfide bond in Mo-MLV Env indicated that the reaction could be triggered by heat (48 and 53°C) or 2 M urea (33). However, the relation of this artificially triggered isomerization reaction to fusion activation in Env remained unresolved. Therefore, we first determined the temperature and urea concentration thresholds for isomerization of the SU-TM disulfide bond and then analyzed whether the corresponding conditions triggered an enhancement of virus fusion with cells. Another protein perturbant, Gnd HCl, was also included in these studies. For the isomerization analyses, Mo-MLV was incubated in control buffer (TN-1.8 mM Ca2+) at 37 to 56°C for 60 min (Fig. 1A), in control buffer containing 0 to 2.5 M urea at 37°C for 30 min (Fig. 1B), or in control buffer containing 0 to 1 M Gnd HCl at 30°C for 30 min (Fig. 1C). The viral proteins were immunoprecipitated and analyzed by nonreducing SDS-PAGE. The thiol-alkylating agent NEM was added to each sample after the incubation to avoid isomerization during subsequent lysis. The degree of SU-TM disulfide bond isomerization during protein perturbation treatments was determined by measuring the relative decrease and increase in levels of covalently linked and free SU and TM subunits, respectively, compared to levels in control samples that received NEM before the start of incubation. As shown previously, the presence of NEM during protein perturbation treatments completely blocks SU-TM disulfide bond isomerization (33). The experiment demonstrated thresholds for triggering isomerization in the ranges of 46 to 48°C, 0.75 to 1.2 M urea, and 0.2 to 0.4 M Gnd HCl (Fig. 1A to C). As an example of the isomerization assay used in the experiment, we show in Fig. 1G the results of nonreducing SDS-PAGE of virus treated with Gnd HCl at increasing concentrations. The kinetics of the heat- and urea-induced isomerization were also analyzed. The heat data are shown in Fig. 6A and discussed in relation to virus inactivation. It should be noted here that heat-induced isomerization at temperatures above the threshold (e.g., 53°C) is a rapid event occurring within a few minutes of incubation, while isomerization induced by lower temperatures is considerably slower. Similar results were observed with urea-induced isomerization (data not shown).
FIG. 1.
Heat, urea, and Gnd HCl activation-thresholds for SU-TM disulfide bond isomerization and enhanced membrane fusion. (A) Heat-induced isomerization. [35S]Cys-labeled Mo-MLV was incubated at 37 to 56°C for 1 h in TN-1.8 mM Ca2+. NEM was added to 20 mM, and the samples were lysed. The viral proteins were immunoprecipitated by using a polyclonal anti-MLV antiserum and analyzed by nonreducing SDS-12% PAGE. Isomerization of the SU-TM disulfide bond is expressed as a percentage of control samples that received NEM before heat treatment. (B) Urea-induced isomerization. Mo-MLV was incubated for 30 min at 37°C in TN-1.8 mM Ca2+ containing 0 to 2.5 M urea. NEM was added, and the samples were analyzed for SU-TM disulfide bond isomerization as described for panel A. (C) Gnd HCl-induced isomerization. Mo-MLV was incubated for 30 min at 30°C in TN-1.8 mM Ca2+ containing 0 to 1 M Gnd HCl. (D) Heat-facilitated fusion. XC cell-bound Mo-MLV was subjected to a 2-min fusion incubation at 37 to 58°C in TN-1.8 mM Ca2+. This was terminated by inactivating Env with a pH 3 buffer. The efficiency of virus fusion was estimated from the extent of formation of XC cell polykaryons during a subsequent incubation at 37°C for 2 h and was expressed as a percentage of fusion by the control, i.e., with fusion incubation at 37°C (set at 100%). (E) Urea-facilitated fusion. Cell-bound virus was subjected to fusion incubations for 2 min at 37°C in TN-1.8 mM Ca2+ containing 0 to 2 M urea. Fusion efficiency was estimated as described for panel D. (F) Gnd HCl-facilitated fusion. Cell-bound virus was subjected to fusion incubations for 105 s at 37°C in TN-1.8 mM Ca2+ containing 0 to 0.8 M Gnd HCl. (G) SDS-12% PAGE of Gnd HCl-treated radiolabeled virus. The Gnd HCl treatments and protein analyses were done as described for panels C and D, respectively. Gnd HCL concentrations (0 to 1 M) are indicated above the lanes. Viral proteins, including the covalently linked SU-TM complexes, are indicated. Note the significant isomerization of the SU-TM disulfide bond in virus treated with ≥0.3 M Gnd HCl. Note also that the untreated sample (lane 1) contains a significant amount of free SU, which has, in addition to the virus, been released from the infected cells. The figure represents an autoradiograph of the dried gel.
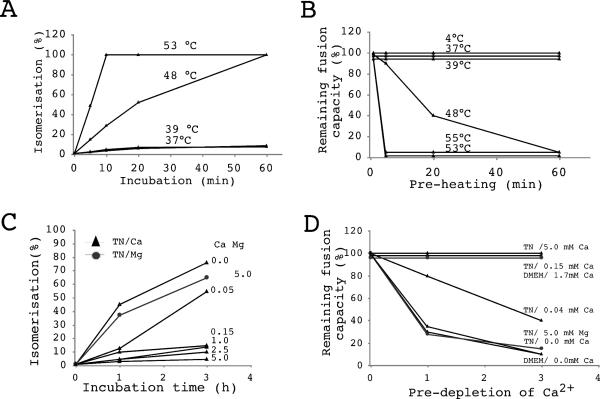
FIG. 6.
Heat- and Ca2+ depletion-induced isomerization inactivates free virus. Mo-MLV was incubated at 4 to 53°C in TN-1.8 mM Ca2+ for 0 to 60 min or at 37°C in TN containing 0 to 5 mM Ca2+ for 0 to 3 h and then tested for SU-TM disulfide bond isomerization (A and C) and fusion on XC cells (B and D). Isomerization and remaining fusion activity were determined as described for Fig. 1A and D and were expressed as a percentage of the activity of the control virus that was not subjected to preincubation. Note that the Ca2+ predepletion treatments included treatments with TN-5.0 mM Mg2+- and Ca2+-free DMEM. Isomerization data for the Ca2+ depletion treatments are from reference 33. Panel C reprinted by permission from EMBO Journal (33), copyright 2004 Macmillan Publishers Ltd.
The effects of heat, urea, and Gnd HCl on fusion activation in Env were studied by using an assay for Mo-MLV-induced XC cell-cell fusion “from without” (33). Accordingly, cell-bound virus was subjected to fusion incubation under various conditions of protein perturbation. The fusion was terminated by a wash with a pH 3 buffer, which inactivates Env. Fusion was scored by quantification of polykaryons that were formed by further incubation. Preliminary analyses showed that XC cells resisted a 2-min incubation at 57°C in control buffer or at 37°C in control buffer with 3 M urea or 2 M Gnd HCl. We observed that a short (2-min or 105-s) treatment with heat (52 to 58°C), urea (1 to 2 M), or Gnd HCl (0.2 to 0.3 M) resulted in a severalfold enhancement of fusion activity over that observed in parallel samples under control conditions (Fig. 1D to F). Treatments with more than 0.3 M Gnd HCl inhibited fusion. Most significantly, the treatment thresholds for triggering fusion enhancement correlated with those for inducing isomerization; they were in the range of 48 to 52°C for heat treatment, 0.75 to 1.2 M for urea, and 0.1 to 0.3 M for Gnd HCl treatment. The slightly lower activation threshold for Gnd HCl-induced fusion enhancement compared to Gnd HCl-induced isomerization (0.2 to 0.4 M) (Fig. 1C) could be due to the fact that the latter incubations were carried out at 30°C and not at 37°C as were the fusions. However, originally we analyzed urea-induced isomerization at 30°C, and these results were very similar to the 37°C values, which are shown in Fig. 1B. An alternative explanation for the slight difference in Gnd HCl effects is that they reflect cooperation of Gnd HCl and the receptor in fusion induction. Notably, all of the protein perturbation-enhanced fusion reactions appeared to be receptor dependent, as Mo-MLV receptor-negative cells (chicken DF-1 cells) could not be fused by Mo-MLV by using any of these treatments (data not shown). Altogether, these results suggest that the fusion enhancement is due to a protein perturbation-forced SU-TM disulfide bond isomerization increase in receptor-bound Env proteins of Mo-MLV.
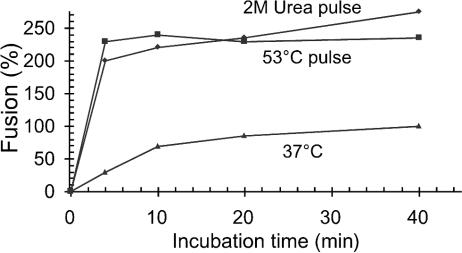
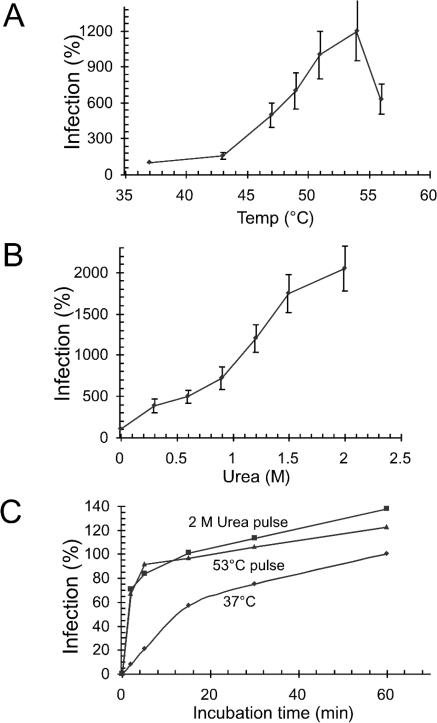
In additional experiments we analyzed the effect of an initial 2-min heat pulse (53°C) on fusion kinetics during a 40-min incubation under control conditions. As shown before, fusion at 37°C, without a heat pulse, displays half-maximal fusion after about 8 min, and maximal fusion after about 40 min, of incubation (Fig. 2, bottom curve) (33). If the incubation was initiated with the heat pulse, we observed an immediate release of fusion efficiency, which was almost 2.5-fold that of the maximal control fusion (Fig. 2). Similar fusion incubation, but using a 2-min pulse with 2 M urea, resulted in equally rapid, about 2.5-fold fusion enhancement (Fig. 2). We conclude that protein perturbation treatments result in a drastic enhancement of fusion kinetics and also a significant increase in total fusion capacity. This is in accord with the similar effects on isomerization kinetics described above and supports the notion that the fusion effects are caused by enhanced isomerization due to the protein perturbation treatment.
FIG. 2.
Kinetics of heat- and urea-facilitated fusion. XC cell-bound virus was subjected to a 2-min heat (53°C) or 2 M urea pulse and then incubated further at 37°C for 2 to 38 min before Env inactivation treatment and subsequent polykaryon incubation. Parallel samples without an enhancement pulse were used as controls. Fusion efficiencies were measured as described for Fig. 1D and given as a percentage of fusion by the control after a 40-min incubation (set at 100%).
Heat-facilitated fusion is alkylation sensitive.
To further study the relationship between the fusion facilitation obtained with protein perturbation treatments and the SU-TM disulfide bond isomerization, we analyzed the dependence of heat-facilitated fusion on alkylation of the isomerization-active thiol in the CXXC-motif of SU. If heat induces a natural activation pathway for isomerization and subsequent fusion, then these functions should be blocked by alkylation of the CXXC thiol in SU. Previously we showed that both functions could be blocked by 0.8 mM 4-(N-maleimido)benzyl-α-trimethylammonium iodide (M135), a membrane-impermeant alkylating agent (33). Analysis of heat-induced isomerization in the presence of M135 demonstrated that the corresponding concentration was effective at 48°C but only partially effective at 53°C (data not shown). Under the latter conditions about half of the SU-TM complexes still isomerized. Complete isomerization inhibition at the higher temperature was achieved by using 2 mM M135. When the M135 effects on heat-facilitated fusion were tested in a 2-min fusion incubation, we found that 0.8 mM M135 blocked fusion at 48°C as well as under control conditions but was only partially effective (about 40% inhibition) at 53°C. In contrast, 2 mM M135 blocked all fusion (data not shown; but see Fig. 3, columns 2 and 3). Thus, alkylation inhibited both heat-induced isomerization and heat-facilitated fusion under corresponding conditions. This suggests that heat activates a natural CXXC thiol-mediated isomerization/fusion reaction pathway in Mo-MLV Env.
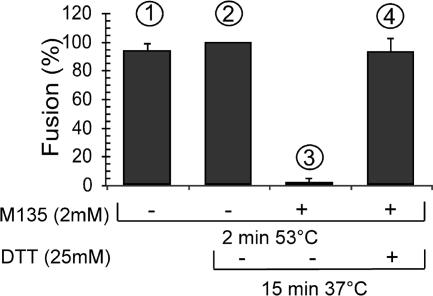
FIG. 3.
DTT rescue of heat-facilitated fusion that has been blocked by alkylation. XC cell-bound Mo-MLV was subjected to fusion incubation for 2 min at 53°C in TN-1.8 mM Ca2+ with (columns 3 and 4) or without (columns 1 and 2) 2 mM M135 and then, after a wash, was either further incubated at 37°C for 15 min in TN-1.8 mM Ca2+ with (column 4) or without (columns 2 and 3) 25 mM DTT or kept on ice (column 1). The fusion incubations were terminated by treatment with a pH 3 buffer. Fusion efficiencies were determined by monitoring the extent of polykaryon formation as described for Fig. 1D. The efficiencies were expressed as percentages of that obtained by the initial and second incubations under drug-free conditions (column 2; set at 100%).
It remained possible that the alkylation-mediated fusion inhibition at elevated temperatures was caused by nonspecific alkylation of virus or cell proteins rather than by alkylation of the CXXC thiol. This possibility was tested by dithiothreitol (DTT) rescue. In this experiment we complemented a 2-min fusion incubation at 53°C, in the presence of 2 mM M135, with a second, alkylator-free incubation at 37°C for 15 min in the presence of 25 mM DTT. Based on our findings described above, we expected that all Env molecules of the virus should be induced to isomerize at 53°C but should be blocked by the alkylation. However, as we demonstrated before for alkylation-blocked fusion under control conditions (33), DTT should cleave the isomerization-blocked SU-TM disulfide bond and rescue the fusion, providing that the inhibition was not caused by nonspecific alkylation of virus or cell proteins. A control experiment performed without drugs showed that a second 15-min incubation at 37°C did not significantly enhance the fusion obtained by the 2-min heat treatment alone (Fig. 3, columns 1 and 2). This result indicated that the initial heat treatment released the total temperature-inducible fusion capacity of the cell-bound virus and was in accordance with the kinetics of the heat-facilitated fusion shown in Fig. 2. In agreement with these findings, we observed that fusion, blocked with 2 mM M135 during the first incubation, could not be rescued by a subsequent drug-free incubation (Fig. 3, column 3). However, if the second incubation was carried out in the presence of 25 mM DTT, fusion was almost totally rescued (column 4), apparently due to SU-TM disulfide bond disruption by reduction. Previously we showed that DTT does not facilitate Mo-MLV fusion in a nonspecific way (33). Indeed, incubation, in the presence of DTT, of cell-bound virus that had not been M135 modified inhibited the fusion activity of Env. We speculated that in this case DTT causes premature uncoupling of the subunits. Therefore, the above data suggest that alkylation at 53°C targets the isomerization-active CXXC thiol in SU and that this blocks fusion by inhibiting SU-TM disulfide bond isomerization.
Heat and urea treatments increase the kinetics of Mo-MLV cell entry.
As nonspecific destabilization of the Env of Mo-MLV resulted in isomerization-mediated facilitation of fusion, we expected that it should also facilitate virus infection. This possibility was tested by subjecting cell-bound virus to short (2-min) incubations at elevated temperatures (43 to 56°C) or treatments with 0.3 to 2 M urea at 30°C. The virus was then inactivated by washing with a pH 3 buffer, and cells were further incubated for 33 h. Infectivity was scored by using an immunofluorescence assay for Env production in infected cells. We found that heat treatments increased infectivity (Fig. 4A). Analysis of temperature dependence showed a threshold in the range of 47 to 51°C, above which infectivity increased to a level which was up to 12-fold that of the control infectivity obtained by a 2-min incubation at 37°C. Treatment with urea increased infectivity as well. Analysis of urea concentration dependence showed a threshold concentration in the range of 0.9 to 1.5 M, above which infectivity increased to a level which was up to 20 times that of the control (Fig. 4B). We conclude that heat and urea treatments that facilitate isomerization and fusion also facilitate infection. In a further experiment we demonstrated that the heat-facilitated infectivity of Mo-MLV was almost completely blocked if the infection was carried out in the presence of 2 mM M135 (data not shown). This effect of alkylation confirms the dependence of heat-facilitated infectivity on the CXXC-driven isomerization reaction in Env. Finally, we analyzed the effect of an initial heat (53°C) or urea (2 M) pulse on cell-entry kinetics during a 60-min incubation under control conditions. We found that an initial 2-min heat pulse resulted in a rapid release of most of the infectivity of the cell-bound virus (Fig. 4C). The rapidly released infectivity corresponded to about 90% of the infectivity of an untreated control virus after a 60-min incubation (Fig. 4C, lower curves). Continued incubation under control conditions for up to 60 min after the heat pulse resulted in about 20%-higher infectivity than in the control. An initial treatment with 2 M urea at 30°C for 2 min had a rapid effect similar to that the heat pulse. In this case, continuation of incubation for up to 60 min resulted in about 40%-higher infectivity than in the control. Incubations for more than 60 min did not significantly increase infectivity in control, heat-pulsed, or urea-pulsed samples (data not shown). We conclude that the major effect of the protein perturbation treatments on infectivity is increased kinetics of viral entry (cell penetration).
FIG. 4.
Heat and urea treatments facilitate the infectivity of Mo-MLV. (A and B) 3T3 cell-bound virus was incubated for 2 min at control (37°C) or elevated temperatures in TN-1.8 mM Ca2+ (A) or at 30°C in TN-1.8 mM Ca2+ containing 0 to 2 M urea (B). Fusion incubations were terminated by washing with a pH 3 buffer, the cultures were further incubated at 37°C for 33 h, and infectivity was measured by scoring Env-positive cells by using immunofluorescence. Infection efficiency is given as a percentage of that of the control virus incubated at 37°C for 2 min (A) or at 30°C for 2 min in the absence of urea (B). (C) Kinetics of cell entry under conditions of protein perturbation. 3T3 cell-bound virus was subjected to a 2-min heat (53°C) or a urea (2 M) pulse and then incubated further at 37°C for 3 to 58 min before Env inactivation treatment. Infection efficiency was measured as described above and given as a percentage of the level of a 60-min control infection without an enhancement pulse.
Protein perturbation-induced isomerization results in SU dissociation.
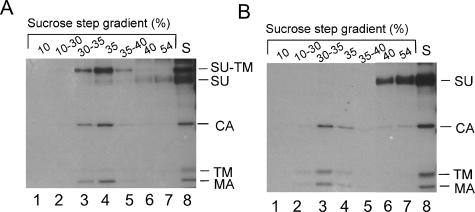
In order to monitor the effect of urea-induced isomerization of the SU-TM disulfide bond on the SU-TM association, Mo-MLV was treated with 2 M urea for 12 min and then subjected to flotation centrifugation in a sucrose step gradient. The treatment resulted in virtually complete isomerization of the SU-TM disulfide bond (Fig. 5B, lane 8). Analyses of the gradient fractions showed that the urea-treated particles floated to the 30-to-35% sucrose interface and that they were completely devoid of SU (Fig. 5B, lanes 3 and 4). The SU remained in the 40 and 54% sucrose layers of the gradient (Fig. 5B, lanes 6 and 7). In contrast, if isomerization was blocked by CXXC thiol alkylation with NEM during the urea incubation, virus particles, with disulfide-linked SU-TM subunits, floated to the 30-to-35% sucrose interface (Fig. 5A, lanes 3 and 4). The small amounts of unlinked subunits found in these fractions are probably the result of artificial reduction of covalently linked subunits during SDS-PAGE (33). The free SU at the bottom of the gradient (Fig. 5A, lanes 6 and 7) represents SU that has been released from the infected cells and was present in the original virus sample (25). Thus, the experiment shows that urea-induced isomerization results in SU dissociation. Previously we reported that isomerization activation by Ca2+ depletion also causes SU displacement and fusion enhancement (33).
FIG. 5.
Urea-induced isomerization results in SU dissociation. [35S]Cys-labeled Mo-MLV was incubated for 12 min at 37°C in TN-1.8 mM Ca2+ containing 2 M urea with (A) (control) or without (B) NEM (20 mM). After incubation, NEM was added to the urea-isomerized sample, and both samples were analyzed by flotation centrifugation in a sucrose step gradient. Fractions of the gradients were subjected to immunoprecipitation with anti MLV polyclonal antibodies, and the precipitates were analyzed by nonreducing SDS-12% PAGE. The figure shows autoradiographs of the gels. Viral proteins are indicated. The original, unfractionated sample (S) is shown in lane 8 of each panel.
Triggering of SU-TM disulfide bond isomerization in free virus correlates with virus inactivation.
If protein perturbation and Ca2+ depletion trigger an SU-TM disulfide bond isomerization-mediated fusion activation of Env, we expected that such treatments should inactivate the virus if conducted before cell binding and that the degree of inactivation should correlate with that of isomerization. To study this possibility, Mo-MLV was subjected to heat treatments or incubations at 37°C in TN containing low Ca2+ concentrations and was then analyzed for isomerization and tested for fusion activity. We found that the pretreatments inactivated the fusion capacity of Mo-MLV (Fig. 6B and D) in a way that correlated with the effects of the treatments on isomerization (Fig. 6A and C). In these studies we used our earlier data on the effects of Ca2+ depletion on isomerization (33). The Ca2+ depletion treatments included a treatment with 5 mM Mg2+ instead of Ca2+ and another with DMEM lacking Ca2+. These conditions, which earlier have been shown to induce isomerization (33), also caused inactivation of the fusion function (Fig. 6D). A trivial explanation of the inactivation would be loss of virus binding to cells due to SU dissociation. However, when tested, binding was not significantly affected (data not shown). Apparently nonspecific binding mediated by cellular proteins that had been incorporated into the viral envelope during budding provided for most of the attachment of the virus to the cells (12, 28). We conclude that induction of SU-TM disulfide bond isomerization in free virus results in virus inactivation, as expected for a metastable fusion protein.
DISCUSSION
According to the present model for MLV Env induced membrane fusion, binding of the SU subunit to the cell receptor results in isomerization of the SU-TM disulfide bond, SU dissociation, and folding of a metastable TM subunit into a stable form via fusion-active intermediates. The model predicts that receptor binding induces changes in the peripherally located SU subunit oligomer of Env, which can induce isomerization of the SU-TM disulfide bond. This could involve the induction of a new conformation in, e.g., the RBD or dissociation of domain interactions in the SU oligomer. Here we have tested the two models by subjecting MLV to treatments that cause protein destabilization nonspecifically. The expectation was that dissociation of domain interactions, but not the induction of new conformations, should be triggered by the treatments. We found that protein destabilization by heat, urea, or Gnd HCl caused isomerization of the intersubunit disulfide bond. The corresponding conditions also resulted in significantly increased kinetics of viral fusion and entry as well as a severalfold enhancement of viral fusion efficiency. The dependence of the functional effects on isomerization was confirmed by demonstrating inhibition of both fusion and infection under conditions where the isomerization-active thiol was blocked by alkylation. This suggests that triggering of the fusion-activating SU signal is coupled to destabilization of an isomerization-suppressing SU oligomer structure.
Our interpretation is supported by two earlier studies (20, 22). In the former work, Env chimeras were constructed by swapping regions of the RBD and/or the C-terminal domain of SU of amphotropic MLV with the corresponding domains of ecotropic MLV. It was found that many of the chimeras had an increased capacity to fuse. This phenotype was combined with a loss of most SU upon centrifugation. Although the effect of the region swapping on SU-TM disulfide bond isomerization was not analyzed, it is likely that the alterations of the SU structure caused an isomerization-facilitating destabilization in Env and thereby an increased fusion capacity. In the work of Lu and Roth (22), an SU point mutation in the amphotropic Env, which also resulted in enhancement of virus fusion and SU loss, is described.
However, results of several other studies have been interpreted in favor of a receptor-induced fusion-activating conformational change in the RBD. In particular it has been shown that mutations of a conserved His residue in the N-terminal region of viral SU results in a receptor binding-competent but fusion-incompetent virus, which can be rescued in trans by wild-type RBD fragments (1-3, 21). Efficient complementation required that both the virus and the wild-type RBD fragments should be receptor bound. Under these conditions the wild-type RBD was apparently able to penetrate into the viral Env and transmit a fusion-activating signal to the C-terminal domain of the SU. It was suggested that receptor binding transforms the wild-type RBD from a fusion-inhibiting to a fusion-activating conformation (2). However, it is equally possible that receptor binding merely destabilizes and opens up the SU oligomer of the viral Env so much that the wild-type RBD, which has been correctly oriented by binding to the receptor, can diffuse into the SU oligomer and activate it. Thus, in this case there is no need to assume a new conformation of the receptor-bound RBD. In wild-type Mo-MLV, fusion activation would follow receptor-induced destabilization of Env. This might release the critical His residue so that it could induce triggering of the CXXC thiol to attack the SU-TM disulfide bond. In another study it was demonstrated that a MAb was able to bind to receptor-bound SU of the endogenous Fv-4r MLV at 4°C but was mostly released during the first few minutes of a subsequent incubation at 37°C (17). Apparently the incubation temperature triggered a change in the structure of the receptor-bound SU. This might correspond to a destabilization of, e.g., the interaction between the RBD and the C-terminal-SU domain. In still another study, the mechanism of neutralization was analyzed for two MAbs which reacted with the C-terminal region of the SU in AKR.623 Env (5). The antibodies inhibited MLV-induced cell-cell fusion from without, although they did not affect the binding of the virus to the receptor. It was suggested that the binding of the MAbs to MLV Env caused neutralization by preventing transduction of a fusion-activating signal from the RBD to the C-terminal region of SU. In view of our present results, we think that these MAb effects were caused by antibody binding-induced stabilization of Env and suppression of its isomerase activity.
The enhancement of the membrane fusion capacity of cell-bound MLV and the inactivation of free virus by nonspecific protein destabilization treatments are consistent with TM adopting a metastable state in native Env. According to this model, TM would fold via fusion-active intermediates into a stable and inactive form after a destabilization treatment-induced dissociation of SU. A corresponding behavior has previously been demonstrated for the HA of influenza virus. HA can be triggered to fuse with liposomes, not only by acid treatment, but also by treatment with heat or urea under neutral conditions (6, 29). Biochemical analyses of HA structure indicated that fusion activity was correlated with folding of the metastable HA2 into its stable form. Interestingly, the threshold conditions for activating the native HA with heat or urea were considerably higher (58 to 60°C or 3.5 to 3.75 M urea) than those required to activate MLV Env (48 to 52°C or 0.75 to 1.2 M urea). The reason for the difference in activation energies could be related to the isomerization capacity of the intersubunit disulfide bond. As is the case with MLV TM, influenza virus HA2 is disulfide bonded to the peripheral subunit (31). The Cys residue, which mediates the bond, is likewise located immediately C-terminal to the region constituting the loop of the back-folded, fusion-activated transmembrane subunit. Therefore, in the case of influenza virus HA, the chain reversal will probably also cause relocation of the covalently attached peripheral subunit. This drastic change in HA conformation might require a high activation energy. However, in the case of MLV Env, the intersubunit disulfide bond can be isomerized before chain reversal and therefore would require less activation energy. The explanation finds support in a recent study of fusion activation in ALV (32). ALV needs both receptor binding and low pH for efficient activation of its fusion function (11, 24). However, like influenza virus HA, ALV Env is lacking an isomerization-motif in SU and hence cannot rearrange its intersubunit disulfide bond (33). In the study of Smith et al. (32), it was found that receptor binding and low pH cooperated in triggering TM to fold into a stable form in vitro. Significantly, this could also be achieved by heat or urea treatment, but with considerably higher temperature (55 to 60°C) and concentration (2.5 to 3 M) thresholds, respectively, than with MLV. Indeed, it is possible that the MLV isomerase has evolved as a mechanism to provide efficient virus entry at the cell surface without compromising a stable SU-TM association in the native virus.
Acknowledgments
We acknowledge Maarit Suomalainen and Mathilda Sjöberg for critical reading of the manuscript.
Swedish Science Foundation grants 5107 and 13279 to H.G. supported this work.
REFERENCES
- 1.Bae, Y., S. M. Kingsman, and A. J. Kingsman. 1997. Functional dissection of the Moloney murine leukemia virus envelope protein gp70. J. Virol. 71:2092-2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnett, A. L., and J. M. Cunningham. 2001. Receptor binding transforms the surface subunit of the mammalian C-type retrovirus envelope protein from an inhibitor to an activator of fusion. J. Virol. 75:9096-9105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnett, A. L., R. A. Davey, and J. M. Cunningham. 2001. Modular organization of the Friend murine leukemia virus envelope protein underlies the mechanism of infection. Proc. Natl. Acad. Sci. USA 98:4113-4118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bullough, P. A., F. M. Hughson, J. J. Skehel, and D. C. Wiley. 1994. Structure of influenza haemagglutinin at the pH of membrane fusion. Nature 371:37-43. [DOI] [PubMed] [Google Scholar]
- 5.Burkhart, M. D., S. C. Kayman, Y. He, and A. Pinter. 2003. Distinct mechanisms of neutralization by monoclonal antibodies specific for sites in the N-terminal or C-terminal domain of murine leukemia virus SU. J. Virol. 77:3993-4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carr, C. M., C. Chaudhry, and P. S. Kim. 1997. Influenza hemagglutinin is spring-loaded by a metastable native conformation. Proc. Natl. Acad. Sci. USA 94:14306-14313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carr, C. M., and P. S. Kim. 1993. A spring-loaded mechanism for the conformational change of influenza hemagglutinin. Cell 73:823-832. [DOI] [PubMed] [Google Scholar]
- 8.Chan, D. C., D. Fass, J. M. Berger, and P. S. Kim. 1997. Core structure of gp41 from the HIV envelope glycoprotein. Cell 89:263-273. [DOI] [PubMed] [Google Scholar]
- 9.Chen, J., S. A. Wharton, W. Weissenhorn, L. J. Calder, F. M. Hughson, J. J. Skehel, and D. C. Wiley. 1995. A soluble domain of the membrane-anchoring chain of influenza virus hemagglutinin (HA2) folds in Escherichia coli into the low-pH-induced conformation. Proc. Natl. Acad. Sci. USA 92:12205-12209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fass, D., S. C. Harrison, and P. S. Kim. 1996. Retrovirus envelope domain at 1.7 angstrom resolution. Nat. Struct. Biol. 3:465-469. [DOI] [PubMed] [Google Scholar]
- 11.Gilbert, J. M., L. D. Hernandez, J. W. Balliet, P. Bates, and J. M. White. 1995. Receptor-induced conformational changes in the subgroup A avian leukosis and sarcoma virus envelope glycoprotein. J. Virol. 69:7410-7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hammarstedt, M., K. Wallengren, K. W. Pedersen, N. Roos, and H. Garoff. 2000. Minimal exclusion of plasma membrane proteins during retroviral envelope formation. Proc. Natl. Acad. Sci. USA 97:7527-7532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heard, J. M., and O. Danos. 1991. An amino-terminal fragment of the Friend murine leukemia virus envelope glycoprotein binds the ecotropic receptor. J. Virol. 65:4026-4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hernandez, L. D., L. R. Hoffman, T. G. Wolfsberg, and J. M. White. 1996. Virus-cell and cell-cell fusion. Annu. Rev. Cell Dev. Biol. 12:627-661. [DOI] [PubMed] [Google Scholar]
- 15.Huang, R. T., R. Rott, and H. D. Klenk. 1981. Influenza viruses cause hemolysis and fusion of cells. Virology 110:243-247. [DOI] [PubMed] [Google Scholar]
- 16.Hunter, E. 1997. Viral entry and receptors, p. 71-119. In J. M. Coffin, S. H. Hughes, and H. E. Varmus (ed.), Retroviruses. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y. [PubMed]
- 17.Ikeda, H., K. Kato, T. Suzuki, H. Kitani, Y. Matsubara, S. Takase-Yoden, R. Watanabe, M. Kitagawa, and S. Aizawa. 2000. Properties of the naturally occurring soluble surface glycoprotein of ecotropic murine leukemia virus: binding specificity and possible conformational change after binding to receptor. J. Virol. 74:1815-1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kayman, S. C., H. Park, M. Saxon, and A. Pinter. 1999. The hypervariable domain of the murine leukemia virus surface protein tolerates large insertions and deletions, enabling development of a retroviral particle display system. J. Virol. 73:1802-1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kobe, B., R. J. Center, B. E. Kemp, and P. Poumbourios. 1999. Crystal structure of human T cell leukemia virus type 1 gp21 ectodomain crystallized as a maltose-binding protein chimera reveals structural evolution of retroviral transmembrane proteins. Proc. Natl. Acad. Sci. USA 96:4319-4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lavillette, D., A. Ruggieri, B. Boson, M. Maurice, and F. L. Cosset. 2002. Relationship between SU subdomains that regulate the receptor-mediated transition from the native (fusion-inhibited) to the fusion-active conformation of the murine leukemia virus glycoprotein. J. Virol. 76:9673-9685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lavillette, D., A. Ruggieri, S. J. Russell, and F. L. Cosset. 2000. Activation of a cell entry pathway common to type C mammalian retroviruses by soluble envelope fragments. J. Virol. 74:295-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu, C. W., and M. J. Roth. 2003. Role of the mutation Q252R in activating membrane fusion in the murine leukemia virus surface envelope protein. J. Virol. 77:10841-10849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Melikyan, G. B., R. M. Markosyan, H. Hemmati, M. K. Delmedico, D. M. Lambert, and F. S. Cohen. 2000. Evidence that the transition of HIV-1 gp41 into a six-helix bundle, not the bundle configuration, induces membrane fusion. J. Cell Biol. 151:413-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mothes, W., A. L. Boerger, S. Narayan, J. M. Cunningham, and J. A. Young. 2000. Retroviral entry mediated by receptor priming and low pH triggering of an envelope glycoprotein. Cell 103:679-689. [DOI] [PubMed] [Google Scholar]
- 25.Opstelten, D. J., M. Wallin, and H. Garoff. 1998. Moloney murine leukemia virus envelope protein subunits, gp70 and Pr15E, form a stable disulfide-linked complex. J. Virol. 72:6537-6545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ott, D., R. Friedrich, and A. Rein. 1990. Sequence analysis of amphotropic and 10A1 murine leukemia viruses: close relationship to mink cell focus-inducing viruses. J. Virol. 64:757-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pinter, A., R. Kopelman, Z. Li, S. C. Kayman, and D. A. Sanders. 1997. Localization of the labile disulfide bond between SU and TM of the murine leukemia virus envelope protein complex to a highly conserved CWLC motif in SU that resembles the active-site sequence of thiol-disulfide exchange enzymes. J. Virol. 71:8073-8077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pizzato, M., S. A. Marlow, E. D. Blair, and Y. Takeuchi. 1999. Initial binding of murine leukemia virus particles to cells does not require specific Env-receptor interaction. J. Virol. 73:8599-8611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruigrok, R. W., S. R. Martin, S. A. Wharton, J. J. Skehel, P. M. Bayley, and D. C. Wiley. 1986. Conformational changes in the hemagglutinin of influenza virus which accompany heat-induced fusion of virus with liposomes. Virology 155:484-497. [DOI] [PubMed] [Google Scholar]
- 30.Sitbon, M., J. Nishio, K. Wehrly, D. Lodmell, and B. Chesebro. 1985. Use of a focal immunofluorescence assay on live cells for quantitation of retroviruses: distinction of host range classes in virus mixtures and biological cloning of dual-tropic murine leukemia viruses. Virology 141:110-118. [DOI] [PubMed] [Google Scholar]
- 31.Skehel, J. J., and D. C. Wiley. 2000. Receptor binding and membrane fusion in virus entry: the influenza hemagglutinin. Annu. Rev. Biochem. 69:531-569. [DOI] [PubMed] [Google Scholar]
- 32.Smith, J. G., W. Mothes, S. C. Blacklow, and J. M. Cunningham. 2004. The mature avian leukosis virus subgroup A envelope glycoprotein is metastable, and refolding induced by the synergistic effects of receptor binding and low pH is coupled to infection. J. Virol. 78:1403-1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wallin, M., M. Ekstrom, and H. Garoff. 2004. Isomerization of the intersubunit disulphide-bond in Env controls retrovirus fusion. EMBO J. 23:54-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weimin Wu, B., P. M. Cannon, E. M. Gordon, F. L. Hall, and W. F. Anderson. 1998. Characterization of the proline-rich region of murine leukemia virus envelope protein. J. Virol. 72:5383-5391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weissenhorn, W., A. Dessen, S. C. Harrison, J. J. Skehel, and D. C. Wiley. 1997. Atomic structure of the ectodomain from HIV-1 gp41. Nature 387:426-430. [DOI] [PubMed] [Google Scholar]
- 36.White, J., K. Matlin, and A. Helenius. 1981. Cell fusion by Semliki Forest, influenza, and vesicular stomatitis viruses. J. Cell Biol. 89:674-679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wilson, I. A., J. J. Skehel, and D. C. Wiley. 1981. Structure of the haemagglutinin membrane glycoprotein of influenza virus at 3 Å resolution. Nature 289:366-373. [DOI] [PubMed] [Google Scholar]