Significance
Vitamin B12 deficiency causes hematological and neurological pathologies by impairing DNA synthesis. The nucleus is shown to be highly sensitive to 5-methyltetrahydrofolate (5-methylTHF) accumulation induced by vitamin B12 depletion in the cytosol, leading to impaired nuclear de novo thymidylate synthesis and genome instability. These effects of the 5-methylTHF trap are exacerbated by folate depletion in nitrous oxide-treated HeLa cells and methionine synthase loss-of-function fibroblasts. These results further our understanding of mechanisms underlying vitamin B12–folate interrelationships in pathologies, including megaloblastic anemia and potentially neural tube defects.
Keywords: vitamin B12, folate, methyl trap, thymidylate, genome instability
Abstract
Clinical vitamin B12 deficiency can result in megaloblastic anemia, which results from the inhibition of DNA synthesis by trapping folate cofactors in the form of 5-methyltetrahydrofolate (5-methylTHF) and subsequent inhibition of de novo thymidylate (dTMP) biosynthesis. In the cytosol, vitamin B12 functions in the remethylation of homocysteine to methionine, which regenerates THF from 5-methylTHF. In the nucleus, THF is required for de novo dTMP biosynthesis, but it is not understood how 5-methylTHF accumulation in the cytosol impairs nuclear dTMP biosynthesis. The impact of vitamin B12 depletion on nuclear de novo dTMP biosynthesis was investigated in methionine synthase-null human fibroblast and nitrous oxide-treated HeLa cell models. The nucleus was the most sensitive cellular compartment to 5-methylTHF accumulation, with levels increasing greater than fourfold. Vitamin B12 depletion decreased de novo dTMP biosynthesis capacity by 5–35%, whereas de novo purine synthesis, which occurs in the cytosol, was not affected. Phosphorylated histone H2AX (γH2AX), a marker of DNA double-strand breaks, was increased in vitamin B12 depletion, and this effect was exacerbated by folate depletion. These studies also revealed that 5-formylTHF, a slow, tight-binding inhibitor of serine hydroxymethyltransferase (SHMT), was enriched in nuclei, accounting for 35% of folate cofactors, explaining previous observations that nuclear SHMT is not a robust source of one-carbons for de novo dTMP biosynthesis. These findings indicate that a nuclear 5-methylTHF trap occurs in vitamin B12 depletion, which suppresses de novo dTMP biosynthesis and causes DNA damage, accounting for the pathophysiology of megaloblastic anemia observed in vitamin B12 and folate deficiency.
Vitamin B12 (cobalamin) deficiency disproportionately affects older adults and pregnant women (1). It can arise from inadequate dietary intake or impaired absorption, with the latter accounting for the majority of cases in developed countries (2, 3). Vitamin B12 is essential for maintaining nervous system function as well as hematopoiesis, as the classic sequelae of deficiency include irreversible neurological damage and/or reversible hematological changes (2, 4). The clinical manifestations of vitamin B12 deficiency have been known for decades, and it is known that high levels of folate intake can mask megaloblastic anemia (5), indicating that increased intracellular folate levels can rescue some metabolic consequences of vitamin B12 deficiency. Conversely, data from cross-sectional studies suggest that low vitamin B12 and elevated folate status exacerbate symptoms of cognition and anemia (6, 7) as well as biochemical indicators of vitamin B12 deficiency (7, 8); however, there is no mechanistic understanding to support these observations.
There are two enzymes that require vitamin B12 as a cofactor in mammals: methionine synthase (MTR), which functions in the cytosol, and l-methylmalonyl-CoA (coA) mutase (MUT), which functions in the mitochondria (4). MTR is both vitamin B12 and folate dependent and catalyzes the conversion of homocysteine to methionine in a two-step process. In the first step, the methyl group from 5-methyltetrahydrofolate (5-methylTHF) is transferred to cobalamin, thereby creating methylcobalamin and releasing THF. In the second step, the methyl group from methylcobalamin is transferred to homocysteine for methionine synthesis (4). Consequences of reduced MTR activity include elevated homocysteine in tissue and plasma, a biomarker associated with adverse health outcomes, including risk for neural tube defects (NTDs) (9) and impaired methylation status, as methionine is required for the synthesis of S-adenosylmethionine (AdoMet), the universal methyl donor required for over 100 cellular methylation reactions (10). Reduced MTR activity also leads to the trapping of cellular folate as 5-methylTHF, as the methylenetetrahydrofolate reductase (MTHFR)-catalyzed conversion of 5,10-methyleneTHF to 5-methylTHF is irreversible in vivo (Fig. 1) (11). The “5-methylTHF trap” is associated with the onset of megaloblastic anemia, which is characterized by erythroid precursors with enlarged cytosol, immature nuclei, and incomplete DNA replication and cell division due to inadequate de novo thymidylate (dTMP) biosynthesis (Fig. 1) (2, 5, 12).
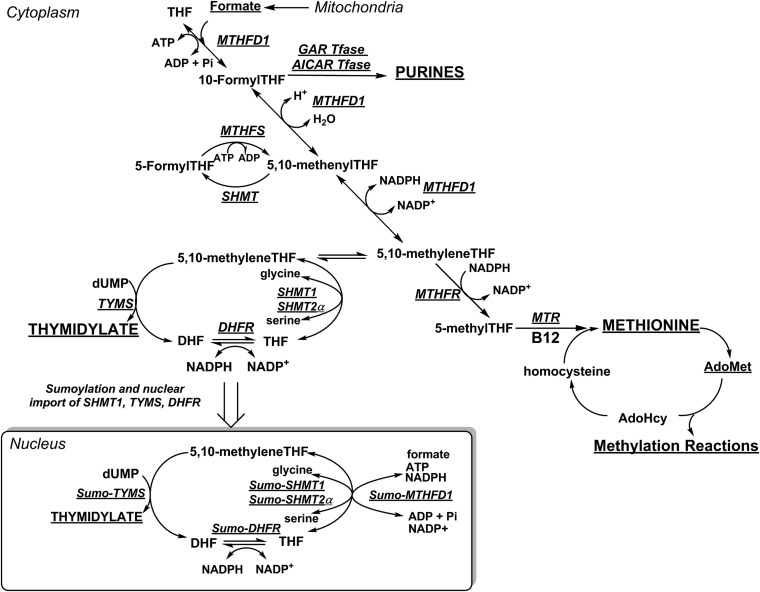
Fig. 1.
Folate- and vitamin B12-mediated one-carbon (1C) metabolism is compartmentalized within the cell. The hydroxymethyl group of serine is a primary source of 1C units and can enter the pool of activated 1C units by SHMT-catalyzed cleavage of serine. Otherwise, serine and glycine are converted to formate in mitochondria, which traverses to the cytosol and nucleus where it is condensed with THF by MTHFD1. One-carbon metabolism in the cytosol includes the de novo synthesis of purines and the remethylation of homocysteine to methionine. Vitamin B12 is a required cofactor for MTR. De novo dTMP biosynthesis occurs in the nucleus, catalyzed by the enzymes SHMT1, SHMT2α, TYMS, and DHFR, which undergo SUMOylation leading to nuclear import in S phase. The 5,10-methyleneTHF is synthesized by SHMT or MTHFD1 for de novo dTMP synthesis. AdoHcy, S-adenosylhomocysteine; AdoMet, S-adenosylmethionine; AICAR Tfase, aminoimidazolecarboxamide ribonucleotide transformylase; DHF, dihydrofolate; DHFR, dihydrofolate reductase; GAR Tfase, glycinamide ribonucleotide tranformylase; MTHFD1, methylenetetrahydrofolate dehydrogenase 1; MTHFS, methenyltetrahydrofolate synthetase; MTHFR, methylenetetrahydrofolate reductase; MTR, methionine synthase; SHMT, serine hydroxymethyltransferase; SUMO, small ubiquitin-like modifier; THF, tetrahydrofolate; and TYMS, thymidylate synthase. Figure is adapted from Field et al. (48).
The proposed mechanisms underlying vitamin B12 deficiency-induced megaloblastic anemia are based on an assumption that both the homocysteine remethylation and de novo dTMP biosynthesis pathways function in the cytosol (13, 14). However, there is increasing evidence that de novo dTMP biosynthesis occurs in the nucleus at sites of DNA synthesis (15). The enzymes that participate in de novo dTMP biosynthesis, serine hydroxymethyltransferase 1 and 2α (SHMT1, SHMT2α), dihydrofolate reductase (DHFR), methylenetetrahydrofolate dehydrogenase 1 (MTHFD1), and thymidylate synthase (TYMS) undergo modification by the small ubiquitin-like modifier (SUMO) protein, and translocate to the nucleus where they form a complex at sites of DNA replication and repair (Fig. 1) during S phase or in response to DNA damage (15–18). SHMT serves as an essential scaffold protein that connects this multienzyme complex to the nuclear lamina (15), but does not serve as a primary source of one-carbon units, as most one-carbons incorporated into dTMP are derived from formate via MTHFD1 (19). TYMS methylates deoxyuridylate (dUMP) using 5,10-methyleneTHF as the cofactor to produce dTMP and dihydrofolate (DHF). DHF is reduced to THF by DHFR, which is NADPH dependent (Fig. 1). Insufficient concentrations of intracellular folate or impairments in nuclear localization of the de novo dTMP biosynthesis complex can lead to elevated uracil levels in DNA (20, 21). Low intracellular vitamin B12 concentrations and/or status can increase markers of genome instability, including chromosomal abnormalities (22, 23), DNA strand breaks (24), and uracil content in DNA (25, 26). The mechanisms underlying the interactions between folate and vitamin B12 in maintaining genome stability, and their contributions to human disease, are unresolved. There is evidence that the cytosolic and nuclear folate cofactor pools are distinct. Unlike the cytosol, the nucleus is resistant to folate depletion when total cellular folates are depleted (27). Therefore, it is not clear whether an accumulation of 5-methylTHF in the cytosol during vitamin B12 depletion influences folate cofactor pools in the nucleus.
We investigated the impact of vitamin B12 depletion on nuclear dTMP synthesis in two models of vitamin B12 deficiency: human fibroblasts with loss-of-function mutations in MTR that comprise the inborn errors of cobalamin metabolism complementation group G (cblG) (28), and HeLa cells treated with nitrous oxide (N2O). The nucleus was observed to be highly sensitive to 5-methylTHF accumulation in vitamin B12 depletion, which led to depressed rates of de novo dTMP biosynthesis and increased genome instability. We also demonstrate that the nucleus is enriched in 5-formylTHF cofactors, which are slow, tight-binding inhibitors of SHMT (29). This observation accounts for the lack of nuclear SHMT catalytic activity in de novo dTMP biosynthesis. These findings link vitamin B12 depletion to nuclear 5-methylTHF accumulation, impaired DNA synthesis, and genome instability, and provide a mechanism underpinning vitamin B12-associated pathologies.
Results
Five-MethylTHF Is Enriched in Nuclei of Vitamin B12-Depleted HeLa Cells and in CblG Patient Fibroblasts.
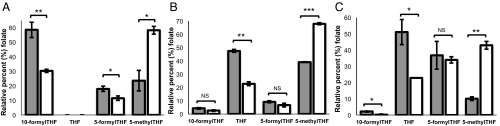
Intracellular 5-methylTHF was 2.5-fold higher in cblG (WG4215) compared with control [Montreal Children’s Hospital (MCH)058] fibroblasts (P = 0.01), with the elevation in 5-methylTHF occurring at the expense of 10-formylTHF (P = 0.009) and 5-formylTHF (P = 0.03) (Fig. 2A). THF was not detected in either MTR-null or control fibroblasts. HeLa cells cultured in vitamin B12-depleted conditions exhibited a 1.75-fold increase in intracellular 5-methylTHF (P = 0.0002) with a more than 50% reduction in THF (P = 0.001) compared with HeLa cells maintained in vitamin B12-replete media (Fig. 2B). Nuclei isolated from vitamin B12-depleted HeLa cells exhibited a more than fourfold enrichment of 5-methylTHF (P = 0.002), whereas THF levels decreased by ∼50% (P = 0.02) (Fig. 2C) compared with vitamin B12-replete HeLa cells. Nuclear 10-formylTHF pools were also lower in nuclei isolated from HeLa cells maintained in vitamin B12-depleted conditions compared with vitamin B12-replete cells (P = 0.02). Interestingly, 5-formylTHF was enriched in nuclei compared with total cellular 5-formylTHF levels in both vitamin B12-replete and -depleted HeLa cells (P = 0.002) (Fig. 2 B and C).
Fig. 2.
Vitamin B12 depletion or MTR disruption results in elevated 5-methylTHF in cells (A and B) and nuclei (C). (A) Distribution of folate one-carbon forms in human fibroblasts. The percentages of folate forms are shown for one cell line of each cblG (WG4215, white bars) and control (MCH058, gray bars). (B and C) Nitrous oxide-induced changes in folate one-carbon distribution in HeLa cells (B) and nuclei (C). The percentages of folate forms present in HeLa cells and nuclei maintained in vitamin B12-depleted (white bars) or -replete (gray bars) conditions are shown. All measurements were made in duplicate. Data are shown as mean ± SD. Differences in folate forms between replete and depleted conditions were determined by a Student’s two-tailed t test. For C, a Western blot confirmed that nuclei were free of cytoplasmic contamination (SI Appendix, Fig. S1). In A–C, statistical significance is denoted as follows: NS, not significant (P > 0.05); *P = 0.01 < P < 0.05; **P = 0.01 < P < 0.001; ***P < 0.001.
Folate and Vitamin B12 Depletion Interact to Increase DNA Damage in HeLa Cells.
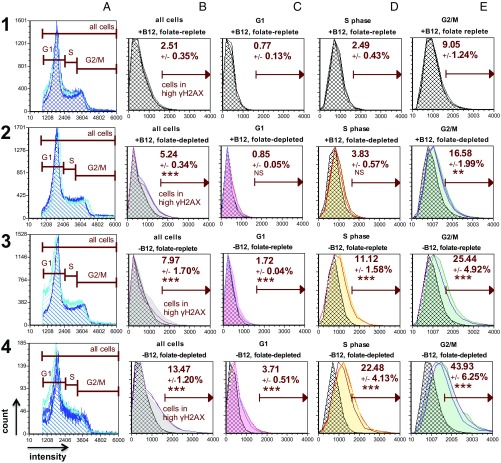
Both folate depletion and vitamin B12 depletion independently increased phosphorylated histone H2AX (γH2AX) immunostaining (Fig. 3, 2, B–E and 3, B–E). Intracellular folate concentrations were eightfold lower in cells cultured in folate-depleted media (5 nM) compared with cells cultured in folate-replete (25 nM) media at the time of γH2AX staining and quantification (P < 0.0001) (SI Appendix, Fig. S2). Vitamin B12 depletion significantly increased the percentage of cells with high γH2AX immunostaining, a marker of DNA double-strand breaks (DSBs), in all phases of the cell cycle compared with vitamin B12- and folate-replete conditions (Fig. 3, 1, B–E vs. 3, B–E). This effect was exacerbated by folate depletion (Fig. 3, 1, B–E vs. 4, B–E). Importantly, the difference in the percentage of cells with high γH2AX immunostaining between vitamin B12-depleted conditions was statistically significant (Fig. 3, 3, B–E vs. 4, B–E) (SI Appendix, Table S1, row 5 SI Appendix). Within treatment conditions, the greatest increase in the percentage of cells with high γH2AX immunostaining was observed during G2/M (Fig. 3, 1–4, E).
Fig. 3.
Vitamin B12 depletion induces changes in γH2AX, a marker of DNA damage in HeLa cells. Cells were stained for DNA content (Vybrant Violet; 1–4, A) and γH2AX (FITC; 1–4, B–E); individual plots depict the cell count (y axis) vs. fluorescence intensity (x axis). The high γH2AX parameter is a threshold defined by the mean top 2.5% of cells in G1, S, and G2/M (all cells) stained for γH2AX in the vitamin B12- and folate-replete condition (1, B), and this gate was uniformly applied to all conditions and cell cycle phases. Each plot shows the mean percent high γH2AX ± SD for triplicate measurements for each experimental condition and cell cycle phase. Individual triplicate measurements for each condition are plotted relative to the corresponding mean γH2AX values in the vitamin B12- and folate-replete condition (hatched histograms, 1, B–E). Asterisks designate statistical significance in percent high γH2AX values between treatment conditions and cell cycle phases compared with the corresponding phases in the vitamin B12- and folate-replete condition (SI Appendix, Table S1). The greatest percentage of cells with high γH2AX within conditions was observed in G2/M under vitamin B12- and folate-depleted conditions (P < 0.001; 4, E). The vitamin B12-depleted and folate-replete condition in row 3 contains duplicate measurements. The experiment was performed twice (SI Appendix, Fig. S3). Folate-replete, 25 nM (6S) 5-formylTHF in culture media; folate-depleted, 5 nM (6S) 5-formylTHF in culture media. The statistical significance is represented as follows: NS, not significant (P > 0.05); *P = 0.01 < P < 0.05; **P = 0.01 < P < 0.001; ***P < 0.001.
Folate Depletion, but Not MTR Loss of Function, Increases DNA Damage in Human Fibroblasts.
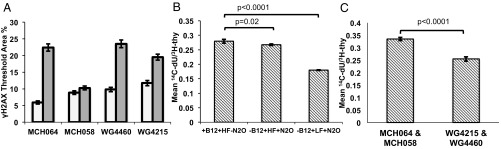
In fibroblasts, folate depletion significantly increased γH2AX immunostaining, as quantified by the percentage of nuclear area with γH2AX (P < 0.0001) (Fig. 4A). MTR loss of function did not significantly affect γH2AX levels when comparing cblG (WG4215 and WG4460) and control fibroblast lines (MCH058 and MCH064) cultured under either folate-replete or depleted culture conditions (P > 0.05) (Fig. 4A).
Fig. 4.
Metabolic effects of vitamin B12 depletion are exacerbated by folate depletion. (A) Folate depletion (gray bars) significantly increases the formation of γH2AX in MTR-null and control fibroblasts compared with those maintained in folate-replete conditions (white bars). Data are shown as mean percent thresholded area ± SE. The number of cells analyzed per fibroblast line and treatment was: MCH064 25 nM folate: 648; MCH064 5 nM folate: 542; MCH058 25 nM folate: 623; MCH058 5 nM folate: 773; WG4460 25 nM: 884; WG4460 5 nM folate: 851; WG4215 25 nM folate: 645; and WG4215 5 nM folate: 625. Folate exposure predicted γH2AX response (P < 0.0001) in three of the four cell lines, but MTR loss of function did not (P > 0.05), as determined by a linear mixed-effects model. (B) Rates of de novo dTMP biosynthesis relative to salvage pathway synthesis in HeLa cells maintained in vitamin B12- and folate-replete or -depleted conditions. Vitamin B12 depletion and folate-replete conditions impaired de novo dTMP synthesis capacity by 5% compared with vitamin B12- and folate-replete conditions (P = 0.02). A combined vitamin B12 and folate depletion decreased rates of de novo dTMP synthesis by 35% (P < 0.0001). Data are shown as mean ± SD. (C) Rates of de novo dTMP biosynthesis relative to salvage pathway synthesis in cblG (WG4215 and WG4460) and control (MCH058 and MCH064) human fibroblasts maintained in vitamin B12-replete or -depleted media without folate. CblG fibroblasts exhibited a 25% reduction in de novo dTMP capacity compared with control fibroblasts (P < 0.0001). Data are shown as means ± SE (n = 12 per group). HF, 25 nM (6S) 5-formylTHF in culture media; LF, 5 nM (6S) 5-formylTHF in culture media; N2O, nitrous oxide.
Vitamin B12 and Folate Depletion Impair de Novo dTMP Biosynthesis.
In HeLa cells, vitamin B12 depletion impaired de novo dTMP synthesis by 5% compared with vitamin B12-replete cells (P = 0.02) (Fig. 4B). A combined folate and vitamin B12 depletion decreased de novo dTMP capacity by 35% (P < 0.0001). In cblG fibroblasts, MTR loss of function decreased the relative rate of de novo dTMP synthesis by ∼25% (P < 0.0001) (Fig. 4C).
MTR Loss of Function and Folate Depletion Do Not Impair de Novo Purine Biosynthesis.
Intracellular folate depletion did not significantly impair de novo purine biosynthesis in human fibroblasts (P > 0.05). One control and two cblG cell lines exhibited similar rates of de novo purine biosynthesis, as determined by the ratio of 14C-formate/3H-hypoxanthine incorporation into nuclear DNA (SI Appendix, Fig. S4). One control cell line showed reduced de novo purine biosynthesis relative to all other cell lines assayed (cblG and control), indicating heterogeneity between the control cell lines. The results clearly show that both cblG cell lines and one control cell line had identical rates of de novo purine synthesis relative to salvage pathway synthesis.
Vitamin B12 Depletion and Intracellular Folate Concentrations Influence the Source of One-Carbon Units for dTMP Biosynthesis.
The incorporation of one-carbons derived from l-[2,3,3-2H3]-serine into the de novo dTMP biosynthesis pathway either as formate (CD1 via MTHFD1) or the hydroxymethyl group of serine (CD2 via SHMT), as indicated by the ratio of CD1/(CD1+CD2), was increased by 12% in HeLa cells grown under vitamin B12-depleted and folate-replete conditions compared with HeLa cells cultured in vitamin B12- and folate-replete media (P = 0.007) (SI Appendix, Fig. S5A). The difference between vitamin B12-depleted conditions was significant (P = 0.002) (SI Appendix, Fig. S5A). These findings indicate that vitamin B12 depletion and folate-replete conditions decrease the contribution of SHMT-derived single carbons (CD2) compared with folate- and vitamin B12-depleted conditions. There was no difference in the CD1/(CD1+CD2) ratio between cblG (WG4215 and WG4460) and control (058MCH and MCH064) fibroblasts cultured in folate-replete conditions (SI Appendix, Fig. S5B).
Discussion
Previous studies have described the impact of the vitamin B12 deficiency-induced 5-methylTHF trap in terms of limiting available cytosolic THF cofactors for dTMP synthesis (5, 12). Our work was motivated by recent findings that the de novo dTMP biosynthesis pathway is present in the nucleus during S phase of the cell cycle and forms a complex at sites of DNA replication and repair (15, 17, 30). SHMT serves as an essential scaffold for the assembly of this complex but makes only minor contributions as a source of one-carbon units for dTMP synthesis (15, 19). Proper assembly of the metabolic complex and adequate levels of THF cofactors in the nucleus are required to maintain adequate dTMP synthesis and genome integrity (20, 21). Mice with reduced Shmt1 expression exhibit 2.5- to 4.5-fold elevations in levels of uracil in nuclear DNA (31), and elevated levels of uracil incorporation into DNA result in loss of DNA integrity (21).
This study demonstrates that vitamin B12 depletion in HeLa cells traps nuclear folate as 5-methylTHF, impairs rates of de novo dTMP biosynthesis, and leads to genome instability. These effects of vitamin B12 depletion were exacerbated by folate depletion. In vitamin B12-depleted HeLa cells, the elevations in 5-methylTHF were more pronounced in nuclei compared with whole cells, with a greater than fourfold increase in nuclear 5-methylTHF (Fig. 2 B and C). The sensitivity of the nucleus to 5-methylTHF accumulation is striking, given that 5-methylTHF is synthesized in the cytosol. CblG fibroblasts, which lack MTR activity, exhibit elevated levels of 5-methylTHF compared with control fibroblasts, and therefore serve as a model of severe vitamin B12 depletion (Fig. 2A). Loss of MTR activity seen in cblG fibroblasts was also associated with reduced de novo dTMP biosynthesis capacity compared with control fibroblasts (Fig. 4C). In contrast to HeLa cells, the reduction in de novo dTMP biosynthesis did not result in elevations in γH2AX immunostaining (Fig. 4A) in MTR loss-of-function fibroblasts.
The effect of impaired de novo dTMP biosynthesis on increasing genome instability as evidenced by increased γH2AX immunostaining could result from two potential mechanisms. γH2AX levels may increase as a result of reduced replication fork movement (32) or due to base excision repair pathways that remove uracil from DNA (33). Reduced replication fork movement has been reported in lymphocytes from patients with megaloblastic anemia (34). When stratified by cell cycle, the γH2AX immunostaining observed as a result of vitamin B12 and folate depletion occurred primarily during S phase and G2/M phase of the cell cycle (Fig. 3). The presence of a stalled replication fork may be responsible for the increased γH2AX immunostaining that is observed during S phase, as vitamin B12 depletion decreases the amount of nuclear THF available for subsequent nucleotide biosynthesis (Figs. 2C and 4 B and C). Alternatively, it is possible that the DSBs observed in S and G2/M phases of the cell cycle may arise as a result of base-excision repair of genomic uracil in DNA (33). In HeLa cells, folate depletion exacerbated genome instability associated with vitamin B12 depletion, with high rates of genome instability observed in all phases of the cell cycle, including G1 phase (Fig. 3, 4, B–E). Our findings are in contrast to observational studies reporting that high folate status worsens clinical symptoms (6, 7) and biochemical indicators of vitamin B12 deficiency (7, 8).
This study provides a role for 5-formylTHF in de novo dTMP synthesis, as it revealed that nuclear folate pools are enriched in 5-formylTHF. The role of 5-formylTHF in mammalian one-carbon metabolism has been elusive. It does not serve as a metabolic cofactor, but rather has been proposed to serve as a stable, storage form of folate, and is an inhibitor of folate-dependent enzymes, including SHMT (35). SHMT and MTHFS participate in a futile cycle that regulates cellular 5-formylTHF levels. SHMT synthesizes 5-formyTHF, whereas MTHFS converts 5-formylTHF back to a metabolically active cofactor (Fig. 1) (36). Five-formylTHF is a minor component of the total cellular folate pool (8%), but accounts for 35% in nuclei. The enrichment of 5-formylTHF in HeLa nuclei provides a mechanism for previous observations that the majority of one-carbon units incorporated into dTMP are derived from formate via MTHFD1 and not from serine via SHMT1 and/or SHMT2α (Fig. 1) (16, 19). The 5-formylTHF present in the nucleus is expected to inhibit SHMT1 and SHMT2α, as 5-formylTHF is a slow, tight-binding inhibitor of SHMT (29). The increase in incorporation of one-carbons from MTHFD1 into dTMP in folate-replete and vitamin B12-deficient HeLa cells is likely explained by the increased pool of 5-methylTHF, which also binds and inhibits SHMT (SI Appendix, Fig. S5A) (29). It is unclear whether nuclear SHMT activity and, hence, de novo dTMP biosynthesis is regulated by changes in nuclear 5-formylTHF levels.
This study provides a mechanism whereby vitamin B12 depletion in the cytosol impairs de novo dTMP synthesis in the nucleus by trapping folate as 5-methylTHF. These studies were performed in cell culture model systems because of the challenges of measuring one-carbon metabolic pathways in humans and animal models in vitamin B12 deficiency. However, these findings extend our understanding of vitamin B12-associated human pathologies and inform the design of experiments in in vivo model systems. Megaloblastic anemia is characterized by impaired maturation and proliferation of red blood cell precursors during hematopoiesis that results in an accumulation of cells in S phase, as the G2-to-M transition cannot be completed for cell division because of inadequate THF pools (2). Furthermore, maternal vitamin B12 deficiency has been associated with NTDs (37–39). NTDs arise when the neural folds fail to fuse entirely during neurulation in early embryogenesis, a period of rapid growth that places demands on nucleotide pools for the cell division required to complete neural tube closure. Folic acid supplementation reduces the occurrence and recurrence of NTDs by up to 70% (40, 41). Impairments in de novo dTMP synthesis, have been implicated in the etiology of folate-responsive NTDs, both in human studies and in animal models (42–44). The role of a nuclear 5-methylTHF trap resulting from vitamin B12 depletion in neural tube closure warrants further investigation.
Materials and Methods
Cell Culture.
Two patient [WG4215 and WG4460 cblG (MTR loss of function)] and two MCH control fibroblast cell lines (MCH058 and MCH064) were maintained in MEM, α-modification (α-MEM; HyClone) supplemented with 10% (vol/vol) FBS (HyClone) and 1× penicillin–streptomycin (Mediatech). The WG4460 cells were derived from an 11-mo-old female patient and the molecular cause of the MTR deficiency is unknown. WG4215 cells were derived from a 6-mo-old female patient, and though these cells lack MTR activity, the causal mutation has not been characterized. The MCH058 and MCH064 control fibroblast cell lines were derived from healthy male patients aged 3 and 2 mo, respectively. For all analyses, control cell lines were used between passage 9 and 13 and cblG fibroblast cell lines were used at passages 14–18. For all studies, cells were maintained in custom-defined media (HyClone, no. SH3A4302.01) with 10% (vol/vol) dialyzed FBS (3 kDa molecular weight pore size) (HyClone). Culture medium contained 2 mM glycine (MP Biomedicals), 1 mg/L pyridoxine (Sigma), 250 μM methionine (Sigma), 1.35 μM cyanocobalamin (vitamin B12 replete, Sigma), and either 5 nM (6S) 5-formylTHF (folate deplete) or 25 nM (6S) 5-formylTHF (folate replete) (Schircks Laboratories).
N2O-Induced Inactivation of MTR.
To inactivate MTR activity, HeLa cells were placed in a hypoxia incubator chamber (Stemcell Technologies) and treated with a N2O gas mixture consisting of 50% N2O, 45% balance air, and 5% CO2 (Airgas). At time 0, the N2O gas mixture was delivered to the chamber at 20 psi for 5 min according to manufacturer’s instructions, and this step was repeated 1 h later; the chamber was flushed with the N2O gas mixture for 5 min twice every 24 h. Before initiating treatment with N2O, culture medium on appropriate plates was replaced with culture medium purged with N2O for 5 min, and fresh media was administered to plates that did not undergo N2O treatment.
Determination of Folate Cofactor One-Carbon Distribution.
HeLa cells (4.5 × 105) were seeded in 10-cm tissue culture plates (Corning), cultured in folate-free modified culture medium with or without cyanocobalamin, and cultured to 60–80% confluency. Plates were rinsed twice with 1× PBS (Cellgro) and replaced with 3 mL of medium containing 50 nM [3H]-folinic acid (Moravek Biochemicals). Following a 24-h incubation, 7 mL of vitamin B12-replete or -depleted medium purged with N2O gas was added to the appropriate plates, which were cultured for 24 h in the hypoxia chamber. Cell monolayers were washed, harvested, and intracellular folates were extracted as previously described (45). Fibroblasts were cultured with labeled folates as described elsewhere (45). Folate one-carbon forms were separated and quantified using reverse-phase HPLC as previously described (46).
Determination of Nuclear Folate Cofactor One-Carbon Distribution.
HeLa cells (4 × 105) were plated as described above. Following 48 h in culture, plates were labeled with 50 nM [3H]-folinic acid (Moravek Biochemicals) for 24 h and then maintained as previously described for another 48 h. Nuclei were extracted using the REAP method (47). The nuclear pellet was resuspended in 200 μL folate extraction buffer (45) and sonicated twice using a Branson Sonifier 150 on level V3 for 5 s on ice. Folate one-carbon forms were separated and quantified using reverse-phase HPLC as previously described (46).
Immunoblotting.
Proteins (20 μg per lane) were separated on a 4–20% (vol/vol) SDS/PAGE gel (Novex) and transferred onto Immobilon-P PVDF membranes (Millipore). Membranes were blocked overnight in 5% (wt/vol) BSA (Sigma) in PBS with 0.2% Tween-20 (Amresco) and 0.02% sodium azide (Sigma). Full-length α-tubulin protein (52 kDa) was detected using mouse anti-human monoclonal primary antibody (mAb) at 1:1,000 dilution (Active Motif), and a 1:10,000 dilution of HRP-conjugated goat anti-mouse secondary antibody (Pierce). Full-length Lamin B1 protein (68 kDa) was detected using a rabbit anti-human monoclonal antibody (Cell Signaling) at 1:1,000 dilution and a 1:5,000 dilution of HRP-conjugated donkey anti-rabbit secondary (Pierce). Secondary antibodies were incubated in 10% (wt/vol) nonfat dry milk in PBS and 0.1% Nonidet P-40 (US Biologicals). The membranes were incubated in SuperSignal West Pico Chemiluminescent Substrate (Pierce) and exposed to autoradiography.
dU Suppression Assay.
The contributions of the de novo and salvage pathways to dTMP biosynthesis were measured in cells by exposure to [14C]-deoxyuridine and [3H]-thymidine, as described elsewhere (48). Briefly, fibroblasts (2 × 104 cells per well) were plated in triplicate in six-well plates (Corning) and allowed to undergo two doublings in vitamin B12-replete or -depleted modified media lacking folate and containing 500 nM [3H]-thymidine (Perkin-Elmer) and 10 μM [14C]-deoxyuridine (Moravek). HeLa cells were plated in modified media containing vitamin B12 and/or folate for 24 h. The appropriate treatment groups were exposed to nitrous oxide gas for 48 h, which represented two cell doublings. Cells were replated in triplicate (2 × 105 cells per well) in six-well plates containing [14C]-dU and [3H] thymidine. Cells in N2O treatment groups were returned to the chamber and exposed to N2O gas mixture for an additional 48 h. Cells were harvested and genomic DNA was extracted with a DNA Isolation Kit for Cells and Tissues (Roche). The radioisotope quantification was performed as described previously (48). Statistical analyses were performed on HeLa cell data using a Student’s two-tailed t test. Significance in fibroblasts was assessed using two-way ANOVA in JMP Pro-12 Software (SAS). Independent variables were fibroblast genotype, vitamin B12 (replete or depleted), and the interaction term; the dependent variable was the ratio of 14C-dU to 3H-thymidine in nuclear DNA.
Quantification of γH2AX by Flow Cytometry.
The impact of vitamin B12 and/or folate depletion on γH2AX formation in HeLa cells was assessed using flow cytometry (49). Briefly, cells (1 × 106) were seeded in 10-cm plates in modified media supplemented with folate and/or cyanocobalamin. Vitamin B12 depletion was induced with the N2O gas mixture at 24-h postplating and held for 48 h. Cells were reseeded at a 1:5 density in triplicate and treated with N2O gas mixture for an additional 48 h. One aliquot of cells per treatment group was saved at harvest for the microbiological assay to quantify intracellular folate.
For the positive control.
A total of 100 μM etoposide (Sigma) was added to culture medium in the positive control plate for 1 h before trypsinizing cells.
For preparation of all samples.
Cells were washed twice with 1× PBS and harvested with 1× trypsin-EDTA for no longer than 5 min. Cells were pelleted at 500 × g for 4 min at room temperature. Cell pellets were washed once with PBS before a final centrifugation. The supernatant was discarded, and pellets were incubated on ice until staining.
γH2AX staining.
A total of 150 μL of master mix containing Block-9 buffer, FITC-conjugated α-γH2AX antibody (1:3,000; Millipore), and Vybrant Violet DNA stain (1:1,000; Life Sciences) was added to each sample. Cell pellets were gently resuspended by vortexing and incubated on ice in the dark for 3 h. Samples were resuspended 1× PBS and strained immediately before each analysis. The samples were analyzed on a BD FACSAria flow cytometer using 488- and 405-nm lasers for excitation of FITC and Vybrant Violet Dye Cycle Stain, respectively. Data were recorded for ∼200,000 events per replicate, and initial gating excluded any debris and clumped cells. This population was further gated to exclude those cells with a large area and width to obtain the parent gated population for γH2AX analyses.
Quantification of High γH2AX.
All postrun analyses for flow cytometry data files were performed in FCS Express 5 (De Novo Software). The parent population of cells stained for both DNA content (Vybrant Violet) and γH2AX (FITC) was gated for cell cycle (G1, S, and G2/M). The gate for “all cells” comprised the G1, S, and G2/M phases analyzed together. Two gates for γH2AX included total γH2AX and high γH2AX. Total γH2AX refers to absolute FITC intensity. The gate for high γH2AX was determined by applying a threshold cutoff at the mean top 2.5% of cells across triplicates in the vitamin B12- and folate-replete (control) condition stained for total γH2AX (Fig. 3, 1, B). This gate for high γH2AX was applied to replicates in all treatment conditions and cell cycle phases. Significance between the control and other treatment conditions in all cells, G1, S, and G2/M was assessed using a two-way ANOVA. In this model, the independent variables included folate, vitamin B12, and the interaction term (folate × vitamin B12); the dependent variable was the percent high γH2AX for individual triplicates in each treatment and cell cycle. The percent high γH2AX values for triplicates were log transformed to better satisfy the assumptions of the model. Specific pairwise post hoc comparisons were made between the percent high γH2AX in the control condition for all cells, G1, S, or G2/M to that observed in the corresponding cell cycle phase for each treatment column. A Bonferroni correction was applied to account for the multiple comparisons between conditions (n = 4).
Microbiological Lactobacillus casei Assay.
The concentration of total folate in HeLa cells was determined using the microbiological L. casei assay as described elsewhere (50).
Formate Suppression Assay.
Flux through the de novo and salvage purine biosynthetic pathways was measured in human fibroblasts by quantifying the amount of 14C-formate (de novo) and 3H-hypoxanthine (salvage) incorporated into nuclear DNA as previously described (48). Cells (2 × 104) were seeded in triplicate in six-well plates in folate-replete or folate-depleted culture medium with cyanocobalamin and containing 20 μM [14C]-formate (Moravek) and 200 nM [3H]-hypoxanthine (Moravek). Cells were cultured for two doublings. Cell pellets, DNA extraction, and radioisotope abundance in DNA were performed as described elsewhere (see dU Suppression Assay above). Statistical analyses were performed using a two-way ANOVA to assess the impact of folate, fibroblast genotype, as well as the interaction term on the mean ratio of 14C-formate/3H-hypoxanthine. The mean 14C/3H values were rank transformed to improve the normal distribution assumption of the model.
γH2AX Immunostaining and Quantification.
Cells (2 × 104) were seeded in duplicate on no. 1.5 microscope cover glass slides (Fisher Scientific) in six-well plates, and maintained in modified folate-replete or -depletes culture medium with cyanocobalamin until ∼80% confluent. Cell fixation and immunostaining was performed as previously described (48) with the modification that 0.5% Triton in PBS was added for permeabilizing cells and diluting the primary antibody. Representative images (n = 10 per slide) were recorded with a Leica TCS SP2 confocal microscope. Quantification of the percent γH2AX positive area for each cell was performed as previously described (48). The percent thresholded area of γH2AX staining for each cell within an image was averaged to improve the normal distribution assumption, and statistical analyses were performed using the averaged values across all treatments (n = 160). A linear mixed-effects model assessed the impact of folate and vitamin B12 status on γH2AX response (mean percent threshold area). Fixed effects included: folate, vitamin B12, and the interaction term. Random effects included: subject and slide. Slide was nested within subject.
Stable Isotope Tracer Studies.
The flux of isotopically labeled one-carbon units into the de novo dTMP biosynthesis pathway was quantified in thymidine in DNA using l-[2,3,3-2H3]-serine as described previously (19). Briefly, human fibroblasts (8 × 105) were plated in duplicate in folate-replete modified culture medium containing cyanocobalamin (see Cell Culture above) supplemented with 250 μM l-[2,3,3-2H3]-serine (Cambridge Isotope Laboratories) and allowed to undergo two doublings. HeLa cells (4 × 105) were plated in duplicate for three media conditions: (i) vitamin B12 and folate replete, (ii) vitamin B12 depleted and folate replete, and (iii) vitamin B12 and folate depleted. Vitamin B12-depleted conditions were treated with nitrous oxide for 48 h following 24 h in culture. All groups were then seeded at a 1:4 density in duplicate into appropriate media containing 250 μM l-[2,3,3-2H3]-serine, treated with nitrous oxide for an additional 48 h to allow for two population doublings. The cell pellets were washed twice with PBS, and genomic DNA was isolated as previously described (see dU Suppression Assay above).
Supplementary Material
Acknowledgments
The authors thank Dr. Lynn Johnson for helpful advice regarding statistical analyses. Proband and control fibroblast cell lines were a generous gift from Dr. David S. Rosenblatt (McGill University). Funding for this study was provided by National Institutes of Health Grants R37DK58144 (to P.J.S.) and F31HD081858 (to A.M.P.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1619582114/-/DCSupplemental.
References
- 1.Finkelstein JL, Layden AJ, Stover PJ. Vitamin B-12 and perinatal health. Adv Nutr. 2015;6:552–563. doi: 10.3945/an.115.008201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stabler SP. Clinical practice. Vitamin B12 deficiency. N Engl J Med. 2013;368:149–160. doi: 10.1056/NEJMcp1113996. [DOI] [PubMed] [Google Scholar]
- 3.Carmel R. Nutritional anemias and the elderly. Semin Hematol. 2008;45:225–234. doi: 10.1053/j.seminhematol.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 4.Nielsen MJ, Rasmussen MR, Andersen CBF, Nexø E, Moestrup SK. Vitamin B12 transport from food to the body’s cells: A sophisticated, multistep pathway. Nat Rev Gastroenterol Hepatol. 2012;9:345–354. doi: 10.1038/nrgastro.2012.76. [DOI] [PubMed] [Google Scholar]
- 5.Marshall RA, Jandl JH. Responses to “physiologic” doses of folic acid in the megaloblastic anemias. Arch Intern Med. 1960;105:352–360. doi: 10.1001/archinte.1960.00270150006002. [DOI] [PubMed] [Google Scholar]
- 6.Morris MS, Jacques PF, Rosenberg IH, Selhub J. Folate and vitamin B-12 status in relation to anemia, macrocytosis, and cognitive impairment in older Americans in the age of folic acid fortification. Am J Clin Nutr. 2007;85:193–200. doi: 10.1093/ajcn/85.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Selhub J, Morris MS, Jacques PF, Rosenberg IH. Folate-vitamin B-12 interaction in relation to cognitive impairment, anemia, and biochemical indicators of vitamin B-12 deficiency. Am J Clin Nutr. 2009;89:702S–706S. doi: 10.3945/ajcn.2008.26947C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller JW, Garrod MG, Allen LH, Haan MN, Green R. Metabolic evidence of vitamin B-12 deficiency, including high homocysteine and methylmalonic acid and low holotranscobalamin, is more pronounced in older adults with elevated plasma folate. Am J Clin Nutr. 2009;90:1586–1592. doi: 10.3945/ajcn.2009.27514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mills JL, et al. Homocysteine and neural tube defects. J Nutr. 1996;126:756S–760S. doi: 10.1093/jn/126.suppl_3.756S. [DOI] [PubMed] [Google Scholar]
- 10.Bailey LB, Gregory JF., 3rd Folate metabolism and requirements. J Nutr. 1999;129:779–782. doi: 10.1093/jn/129.4.779. [DOI] [PubMed] [Google Scholar]
- 11.Kutzbach C, Stokstad EL. Mammalian methylenetetrahydrofolate reductase. Partial purification, properties, and inhibition by S-adenosylmethionine. Biochim Biophys Acta. 1971;250:459–477. doi: 10.1016/0005-2744(71)90247-6. [DOI] [PubMed] [Google Scholar]
- 12.Metz J, Kelly A, Swett VC, Waxman S, Herbert V. Deranged DNA synthesis by bone marrow from vitamin B-12-deficient humans. Br J Haematol. 1968;14:575–592. doi: 10.1111/j.1365-2141.1968.tb00364.x. [DOI] [PubMed] [Google Scholar]
- 13.Herbert V, Zalusky R. Interrelations of vitamin B12 and folic acid metabolism: Folic acid clearance studies. J Clin Invest. 1962;41:1263–1276. doi: 10.1172/JCI104589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scott JM, Weir DG. The methyl folate trap. A physiological response in man to prevent methyl group deficiency in kwashiorkor (methionine deficiency) and an explanation for folic-acid induced exacerbation of subacute combined degeneration in pernicious anaemia. Lancet. 1981;2:337–340. doi: 10.1016/s0140-6736(81)90650-4. [DOI] [PubMed] [Google Scholar]
- 15.Anderson DD, Woeller CF, Chiang EP, Shane B, Stover PJ. Serine hydroxymethyltransferase anchors de novo thymidylate synthesis pathway to nuclear lamina for DNA synthesis. J Biol Chem. 2012;287:7051–7062. doi: 10.1074/jbc.M111.333120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anderson DD, Stover PJ. SHMT1 and SHMT2 are functionally redundant in nuclear de novo thymidylate biosynthesis. PLoS One. 2009;4:e5839. doi: 10.1371/journal.pone.0005839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anderson DD, Woeller CF, Stover PJ. Small ubiquitin-like modifier-1 (SUMO-1) modification of thymidylate synthase and dihydrofolate reductase. Clin Chem Lab Med. 2007;45:1760–1763. doi: 10.1515/CCLM.2007.355. [DOI] [PubMed] [Google Scholar]
- 18.Fox JT, Shin WK, Caudill MA, Stover PJ. A UV-responsive internal ribosome entry site enhances serine hydroxymethyltransferase 1 expression for DNA damage repair. J Biol Chem. 2009;284:31097–31108. doi: 10.1074/jbc.M109.015800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herbig K, et al. Cytoplasmic serine hydroxymethyltransferase mediates competition between folate-dependent deoxyribonucleotide and S-adenosylmethionine biosyntheses. J Biol Chem. 2002;277:38381–38389. doi: 10.1074/jbc.M205000200. [DOI] [PubMed] [Google Scholar]
- 20.MacFarlane AJ, et al. Nuclear localization of de novo thymidylate biosynthesis pathway is required to prevent uracil accumulation in DNA. J Biol Chem. 2011;286:44015–44022. doi: 10.1074/jbc.M111.307629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blount BC, et al. Folate deficiency causes uracil misincorporation into human DNA and chromosome breakage: Implications for cancer and neuronal damage. Proc Natl Acad Sci USA. 1997;94:3290–3295. doi: 10.1073/pnas.94.7.3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jensen MK. Cytogenetic findings in pernicious anaemia. Comparison between results obtained with chromosome studies and the micronucleus test. Mutat Res. 1977;45:249–252. doi: 10.1016/0027-5107(77)90024-0. [DOI] [PubMed] [Google Scholar]
- 23.Rana SR, Colman N, Goh KO, Herbert V, Klemperer MR. Transcobalamin II deficiency associated with unusual bone marrow findings and chromosomal abnormalities. Am J Hematol. 1983;14:89–96. doi: 10.1002/ajh.2830140111. [DOI] [PubMed] [Google Scholar]
- 24.Minnet C, Koc A, Aycicek A, Kocyigit A. Vitamin B12 treatment reduces mononuclear DNA damage. Pediatr Int. 2011;53:1023–1027. doi: 10.1111/j.1442-200X.2011.03448.x. [DOI] [PubMed] [Google Scholar]
- 25.Wickramasinghe SN, Fida S. Bone marrow cells from vitamin B12- and folate-deficient patients misincorporate uracil into DNA. Blood. 1994;83:1656–1661. [PubMed] [Google Scholar]
- 26.Kapiszewska M, Kalemba M, Wojciech U, Milewicz T. Uracil misincorporation into DNA of leukocytes of young women with positive folate balance depends on plasma vitamin B12 concentrations and methylenetetrahydrofolate reductase polymorphisms. A pilot study. J Nutr Biochem. 2005;16:467–478. doi: 10.1016/j.jnutbio.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 27.Field MS, et al. Nuclear enrichment of folate cofactors and methylenetetrahydrofolate dehydrogenase 1 (MTHFD1) protect de novo thymidylate biosynthesis during folate deficiency. J Biol Chem. 2014;289:29642–29650. doi: 10.1074/jbc.M114.599589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilson A, et al. Functionally null mutations in patients with the cblG-variant form of methionine synthase deficiency. Am J Hum Genet. 1998;63:409–414. doi: 10.1086/301976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stover P, Schirch V. 5-Formyltetrahydrofolate polyglutamates are slow tight binding inhibitors of serine hydroxymethyltransferase. J Biol Chem. 1991;266:1543–1550. [PubMed] [Google Scholar]
- 30.Woeller CF, Anderson DD, Szebenyi DME, Stover PJ. Evidence for small ubiquitin-like modifier-dependent nuclear import of the thymidylate biosynthesis pathway. J Biol Chem. 2007;282:17623–17631. doi: 10.1074/jbc.M702526200. [DOI] [PubMed] [Google Scholar]
- 31.MacFarlane AJ, et al. Cytoplasmic serine hydroxymethyltransferase regulates the metabolic partitioning of methylenetetrahydrofolate but is not essential in mice. J Biol Chem. 2008;283:25846–25853. doi: 10.1074/jbc.M802671200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lamm N, et al. Folate levels modulate oncogene-induced replication stress and tumorigenicity. EMBO Mol Med. 2015;7:1138–1152. doi: 10.15252/emmm.201404824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hagen L, et al. Cell cycle-specific UNG2 phosphorylations regulate protein turnover, activity and association with RPA. EMBO J. 2008;27:51–61. doi: 10.1038/sj.emboj.7601958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wickremasinghe RG, Hoffbrand AV. Reduced rate of DNA replication fork movement in megaloblastic anemia. J Clin Invest. 1980;65:26–36. doi: 10.1172/JCI109656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stover P, Schirch V. The metabolic role of leucovorin. Trends Biochem Sci. 1993;18:102–106. doi: 10.1016/0968-0004(93)90162-g. [DOI] [PubMed] [Google Scholar]
- 36.Field MS, Szebenyi DME, Stover PJ. Regulation of de novo purine biosynthesis by methenyltetrahydrofolate synthetase in neuroblastoma. J Biol Chem. 2006;281:4215–4221. doi: 10.1074/jbc.M510624200. [DOI] [PubMed] [Google Scholar]
- 37.Kirke PN, et al. Maternal plasma folate and vitamin B12 are independent risk factors for neural tube defects. Q J Med. 1993;86:703–708. [PubMed] [Google Scholar]
- 38.Molloy AM, et al. Maternal vitamin B12 status and risk of neural tube defects in a population with high neural tube defect prevalence and no folic acid fortification. Pediatrics. 2009;123:917–923. doi: 10.1542/peds.2008-1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ray JG, et al. Vitamin B12 and the risk of neural tube defects in a folic-acid-fortified population. Epidemiology. 2007;18:362–366. doi: 10.1097/01.ede.0000257063.77411.e9. [DOI] [PubMed] [Google Scholar]
- 40.Czeizel AE, Dudás I. Prevention of the first occurrence of neural-tube defects by periconceptional vitamin supplementation. N Engl J Med. 1992;327:1832–1835. doi: 10.1056/NEJM199212243272602. [DOI] [PubMed] [Google Scholar]
- 41.Group MVSR. MRC Vitamin Study Research Group Prevention of neural tube defects: Results of the Medical Research Council Vitamin Study. Lancet. 1991;338:131–137. [PubMed] [Google Scholar]
- 42.Martiniova L, Field MS, Finkelstein JL, Perry CA, Stover PJ. Maternal dietary uridine causes, and deoxyuridine prevents, neural tube closure defects in a mouse model of folate-responsive neural tube defects. Am J Clin Nutr. 2015;101:860–869. doi: 10.3945/ajcn.114.097279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dunlevy LPE, et al. Abnormal folate metabolism in foetuses affected by neural tube defects. Brain. 2007;130:1043–1049. doi: 10.1093/brain/awm028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Beaudin AE, et al. Shmt1 and de novo thymidylate biosynthesis underlie folate-responsive neural tube defects in mice. Am J Clin Nutr. 2011;93:789–798. doi: 10.3945/ajcn.110.002766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Girgis S, Suh JR, Jolivet J, Stover PJ. 5-Formyltetrahydrofolate regulates homocysteine remethylation in human neuroblastoma. J Biol Chem. 1997;272:4729–4734. doi: 10.1074/jbc.272.8.4729. [DOI] [PubMed] [Google Scholar]
- 46.Anguera MC, et al. Methenyltetrahydrofolate synthetase regulates folate turnover and accumulation. J Biol Chem. 2003;278:29856–29862. doi: 10.1074/jbc.M302883200. [DOI] [PubMed] [Google Scholar]
- 47.Suzuki K, Bose P, Leong-Quong RY, Fujita DJ, Riabowol K. REAP: A two minute cell fractionation method. BMC Res Notes. 2010;3:294. doi: 10.1186/1756-0500-3-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Field MS, Kamynina E, Watkins D, Rosenblatt DS, Stover PJ. Human mutations in methylenetetrahydrofolate dehydrogenase 1 impair nuclear de novo thymidylate biosynthesis. Proc Natl Acad Sci USA. 2015;112:400–405. doi: 10.1073/pnas.1414555112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Muslimovic A, Ismail IH, Gao Y, Hammarsten O. An optimized method for measurement of gamma-H2AX in blood mononuclear and cultured cells. Nat Protoc. 2008;3:1187–1193. doi: 10.1038/nprot.2008.93. [DOI] [PubMed] [Google Scholar]
- 50.Molloy AM, Scott JM. Microbiological assay for serum, plasma, and red cell folate using cryopreserved, microtiter plate method. Methods Enzymol. 1997;281:43–53. doi: 10.1016/s0076-6879(97)81007-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.