Highlight
Low mesophyll conductance was the most important limiter of photosynthesis in the majority of evolutionarily old species and resulted from thick cell walls and low exposed chloroplast area.
Keywords: Conifer, gm, gymnosperm, LES, LMA, nitrogen, photosynthesis.
Abstract
Mesophyll conductance is thought to be an important photosynthetic limitation in gymnosperms, but they currently constitute the most understudied plant group in regard to the extent to which photosynthesis and intrinsic water use efficiency are limited by mesophyll conductance. A comprehensive analysis of leaf gas exchange, photosynthetic limitations, mesophyll conductance (calculated by three methods previously used for across-species comparisons), and the underlying ultra-anatomical, morphological and chemical traits in 11 gymnosperm species varying in evolutionary history was performed to gain insight into the evolution of structural and physiological controls on photosynthesis at the lower return end of the leaf economics spectrum. Two primitive herbaceous species were included in order to provide greater evolutionary context. Low mesophyll conductance was the main limiting factor of photosynthesis in the majority of species. The strongest sources of limitation were extremely thick mesophyll cell walls, high chloroplast thickness and variation in chloroplast shape and size, and the low exposed surface area of chloroplasts per unit leaf area. In gymnosperms, the negative relationship between net assimilation per mass and leaf mass per area reflected an increased mesophyll cell wall thickness, whereas the easy-to-measure integrative trait of leaf mass per area failed to predict the underlying ultrastructural traits limiting mesophyll conductance.
Introduction
The 36-fold variation of net photosynthetic rate across C3 species (Wright et al., 2004) cannot be explained only by stomatal restrictions and biochemical potentials, because the CO2 diffusion efficiency from substomatal cavities to chloroplasts (mesophyll diffusion conductance; gm) plays an important role in shaping photosynthetic capacities and leaf resource-use efficiency across Earth’s ecosystems (Warren et al., 2003; Niinemets et al., 2009b, 2011; Terashima et al., 2011; Tosens et al., 2012b, 2016). The general consensus is that ignoring gm in carbon gain calculations prevents the correct simulation of photosynthesis due to biased maximum velocity of carboxylation especially in stressful field environments (Manter and Kerrigan, 2004; Niinemets et al., 2011). Still, gm has been ignored in carbon gain calculations because of our lack of knowledge about its various causes and the main drivers of its variation across species (Niinemets et al., 2009a).
Mesophyll conductance has now been estimated for more than 100 species from all major plant groups. Available data show that considerable variation of gm between plant groups exists and it is larger than that of stomatal conductance (gs) (Flexas et al., 2012; Tosens et al., 2016). gm depends on the length and characteristics of the CO2 diffusion path from substomatal cavities to chloroplasts (Evans et al., 2009; Hassiotou et al., 2009; Terashima et al., 2011; Tosens et al., 2012b). The main limitations to CO2 diffusion are in the mesophyll liquid phase. Two traits, mesophyll cell wall thickness (Tcwm) and chloroplast surface area exposed to intercellular airspace (Sc/S), have been highlighted as the strongest limiting factors of gm, but these anatomical traits are highly variable among species (Evans et al., 2009; Terashima et al., 2011; Tosens et al., 2012b). In addition, recent studies suggest that chloroplast thickness is also an important barrier limiting diffusion of CO2 to Rubisco (Tosens et al., 2012a,b, 2016; Peguero-Pina et al., 2012; Tomás et al., 2013). The reduction of photosynthesis due to gm can be about 25% for mesophytic species and up to 75% in evergreen species (Niinemets et al., 2011). The latter have more robust leaves, e.g. thicker cell walls due to their adaptations to their specific environmental stressors (Niinemets, 2016). Contrarily, ferns and allies have seemingly soft leaves and low leaf mass per area (LMA), but the extent of the photosynthetic limitation by gm and net assimilation are in a similar range to that reported for sclerophyllous angiosperms due to their very thick mesophyll cell walls and very low Sc/S (Niinemets et al., 2009,b,c; Tosens et al., 2016). That is, gm and its underlying traits cannot be simply extended across plant groups, and additional factors such as evolutionary age can play an important role in its determination.
Across spermatophytes gm is lowest in gymnosperms (Flexas et al., 2012). Six-fold variation in gm has been shown among the 13 conifer species studied so far (Warren et al., 2003; De Lucia et al., 2003; Manter and Kerrigan, 2004; Black et al., 2005; Mullin et al., 2009; Peguero-Pina et al., 2012, 2016). Collectively, the available data suggest that gm is an important limiting factor of photosynthesis across and within conifer species. Despite its undoubted importance, only Peguero-Pina et al. (2012, 2016) have investigated gm together with the underlying structural correlates. Therefore, integrative approaches are needed to characterize the nature of gm and its importance in controlling realized photosynthesis rates in gymnosperms.
The leaf economics spectrum (LES) is a dimension of ecological variation reflecting differences across species in the cost of the investment in a unit of leaf area and the return on that investment (Wright et al., 2004). Despite the broad use of LES relationships in generalizing global leaf structure–function relationships, the morpho-physiological basis underlying given trade-offs are poorly understood, especially for species placed at the low-return end (Shipley et al., 2006; Niinemets et al., 2009a,b). These species are characterized by high LMA and robust structure, and therefore the realized photosynthetic rates are modified in an important way by leaf morphology (Flexas et al., 2008; Hassiotou et al., 2009; Niinemets et al., 2011; Tosens et al., 2012b). For example, high LMA could be associated with high net assimilation rate (Aarea) if its main driver is great thickness of mesophyll and high Sc/S, or with low Aarea if it is connected with its underlying anatomical variations such as thick mesophyll cell walls (Hassiotou et al., 2009; Tomás et al., 2013). However, the integrative trait of LMA may fail for species like Australian Proteaceae, which have mesophytic mesophyll tissue embedded in highly sclerophytic tissue, or in ferns and allies, which have low LMA but high Tcwm resulting in low photosynthesis (Niinemets et al., 2009c; Tosens et al., 2012b, 2016). Understanding the underlying ultrastructural traits controlling the global LMA–photosynthesis–nitrogen relationships is inescapable for understanding photosynthetic patterns across Earth’s ecosystems.
The reasons for lower photosynthesis rates and intrinsic water use efficiency (WUEi) in gymnosperms are currently poorly known due to lack of studies separating mesophyll and stomatal diffusional and mesophyll biochemical photosynthetic limitations. Information about gm with its underlying structural traits is especially limited. Here we set out to characterize the spectrum of photosynthetic strategies and related physiological and morphological traits in gymnosperms positioned at the lower return end of the leaf economics spectrum based on 11 gymnosperm and two evolutionarily older species, Psilotum nudum and Selaginella uncinata, in order to enhance the evolutionary context. Overall, the included species are evolutionarily old with a phylogenetic age extending from 306 million years for Psilotaceae to 75 million years for the genus Podocarpus (Pryer et al., 2004; Biffin et al., 2012). Metasequoia glyptostroboides, Cycas revoluta, Macrozamia riedlei and Araucaria heterophylla can even be regarded as living fossils as their morphology and habitat have a high resemblance to fossils dated to the Mesozoic (>110 My ago) and even to the late Paleozoic (300 My ago) (e.g. Norstog and Nicholls, 1997; Rydin et al., 2004; Zhang et al., 2015). The specific aims of the study were (i) to analyse the photosynthetic limitations to understand if diffusional limitations, especially gm, are the most substantial constraints in evolutionarily old plants; (ii) to assess which structural traits are responsible for low CO2 diffusion conductance in evolutionarily old species; and (iii) to understand the morpho-physiological basis underlying LES traits in evolutionarily old species.
Materials and methods
Plant material and growth conditions
Thirteen evolutionarily old species (Fig. 1 and Supplementary Table S1 at JXB online) with widely varying shape, size, longevity and structure of photosynthetic organs covering a broad range of evolutionary ages were included in the analysis. Out of the selected species, 11 (Araucaria heterophylla, Cupressus sempervirens, Cycas revoluta, Ephedra minuta, Macrozamia riedlei, Metasequoia glyptostroboides, Picea abies, Pinus sylvestris, Podocarpus alpinus, Podocarpus nivalis and Taxus baccata) were gymnosperms from three of the four gymnosperm divisions. Two primitive herbaceous plant species (a whisk fern, Psilotum nudum, and a clubmoss, Selaginella uncinata) were also included.
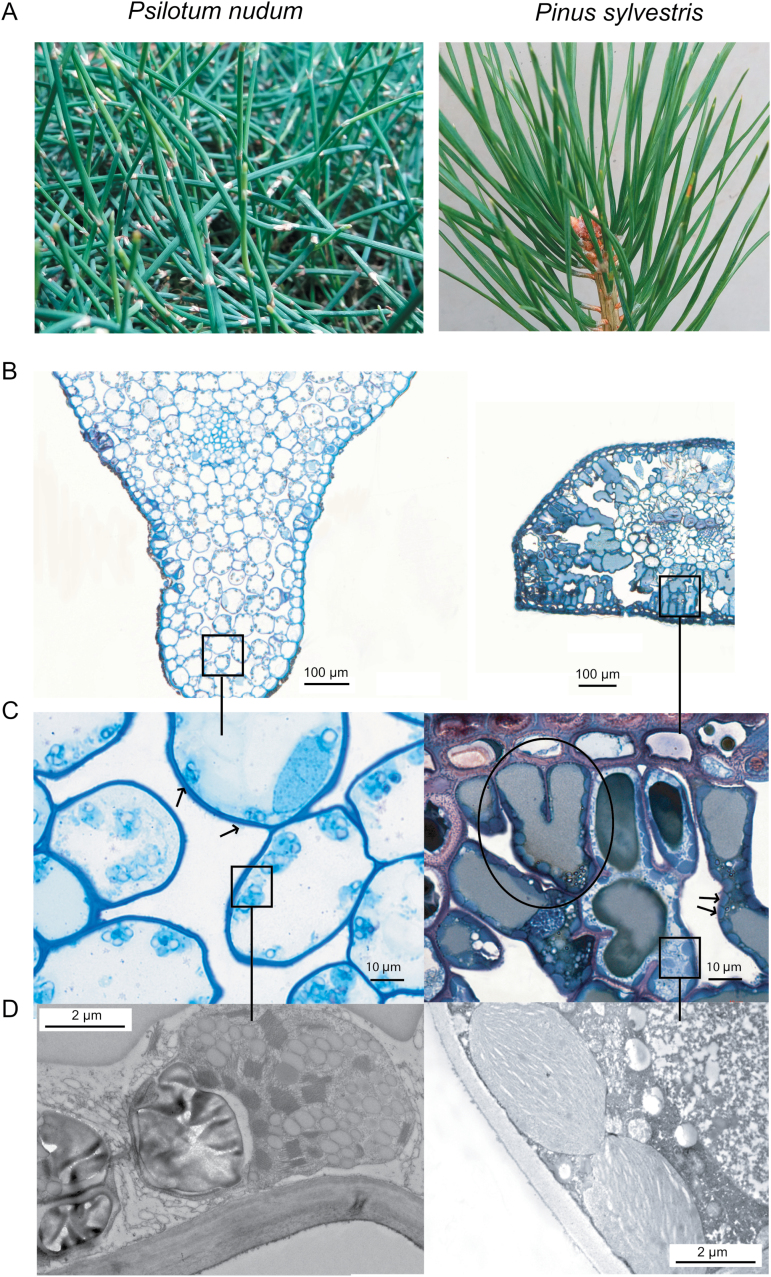
Fig. 1.
(A, B) Photographs (A) and cross-sections (B) of Psilotum nudum (left) and Pinus sylvestris (right) to illustrate different leaf internal anatomies. (C,D) Low (P. nudum, left) and high (P. sylvestris, right) chloroplast area exposed to intercellular airspace (Sc/S). Arrows in (C) indicate sparsely (left) and tightly packed chloroplasts (right). The P. sylvestris cross-section exhibits lobed cells (circled in C).
Five-month-old saplings of M. glyptostroboides, P. alpinus, P. nivalis, S. uncinata and T. baccata were obtained in October 2014 from a local nursery and transferred to a controlled-conditions phytotron, where they grew for 2–4 months before the measurements were begun. Other species were grown in the phytotron from seeds or seedlings brought from their country of origin (see Supplementary Table S1). All measured leaves emerged and matured in the phytotron conditions. Relative humidity was maintained at 60% and air temperature at 25 °C/22 °C (day/night). Light was provided by high pressure sodium lamps (400 W, E40 tubular clear, Beijing Luxram Lighting Ltd, China) for a 13 h photoperiod. The photosynthetic photon flux density (Q) incident to the plants was maintained at 1000 µmol m–2 s–1 reflecting the natural growth light conditions of the species (Supplementary Table S1). Plants were watered every second day with tap water and fertilized with soluble fertilizer once a week (Substral Miracle for evergreens, N:P2O5:K2O:MgO 23:9:12:2 with microelements). In all cases, fully mature non-senescent foliage was used for the measurements.
Foliage gas exchange measurements and estimation of mesophyll conductance
Simultaneous gas exchange and fluorescence measurements were conducted with a GFS-3000 portable photosynthesis system (Walz GmbH, Germany). Foliage elements were arranged side by side in the leaf chamber to avoid any overlap and a digital photograph was taken for estimation of the exact projected foliage area enclosed in the chamber after its closure. In all measurements, vapor pressure deficit was kept at ca 1.5 kPa, and leaf temperature, measured with a thermocouple, at 25 ºC. Leaf net photosynthesis, the steady-state net photosynthesis rate under saturating Q of 1000 µmol m–2 s–1 (90% red and 10% blue light) and 400 µmol CO2 mol–1 (atmospheric CO2 concentration, Ca), was recorded after the stomata opened and leaf gas exchange rates reached maximum steady state, 30–60 min after the enclosure of foliage in the chamber. Net assimilation vs. ambient CO2 response curves were measured at a Q of 1000 µmol m–2 s–1 by varying Ca between 50 and 2000 µmol CO2 mol–1. After each steady-state net assimilation rate had been estimated, the steady-state fluorescence level (F) was recorded and a saturating pulse was given by the leaf chamber fluorimeter of the GFS-3000 system (PAM-fluorometer 3055-FL) to measure the maximum fluorescence yield (Fm′) and estimate the effective photosystem II quantum yield (ΦPSII) as (Fm′–F)/Fm′ (Genty et al., 1989). The area of the leaf chamber was 8 cm2. Once the measurements for a CO2 response curve were completed, the light was switched off and the mitochondrial respiration rate (Rn) was measured after a minimum of 30 min of dark adaptation. gs and intercellular CO2 concentration (Ci) were calculated according to von Caemmerer and Farquhar (1981).
Mesophyll conductance was calculated according to Harley et al. (1992):
| (1) |
where Aarea is the net assimilation rate, JETR is the rate of photosynthetic electron transport derived from chlorophyll fluorescence measurements, Ci is the CO2 concentration in sub-stomatal cavities, Rd is the rate of non-photorespiratory respiration in the light and Γ* is the hypothetical CO2 compensation point without Rd. Rd was estimated as half of Rn (Niinemets et al., 2005). This common adaptation is supported by previous experimental observations (Villar et al., 1995; Piel et al., 2002). Γ* was taken as 42.9 μmol mol–1 at 25 °C (Bernacchi et al., 2001) as it has been shown before on vastly variable plant functional groups and gm values that there is no significant difference between gm calculated with Γ* according to Galmés et al. (2005) and Bernacchi et al. (2001) (Tomás et al., 2013). JETR was estimated from non-photorespiratory conditions (2% oxygen) from an A–Ci curve, although the calculations of gm were relatively insensitive to moderate variations in JETR (<0.5%; see Niinemets et al., 2005). Therefore, the main assumption of Harley’s variable J method was not breached. The data were corrected for chamber leaks according to Flexas et al. (2007a) and Rodeghiero et al. (2007) using empty chamber measurements. Leaf absorption was measured with an integrating sphere (leaf absorption coefficient varied between 0.84–0.90). Gaskets were changed regularly, and modelling paste and paraffin film were used with thick leaves to ensure no leakage. Mesophyll conductance was calculated from measurements of net assimilation rate over the Ci range of 150–350 μmol mol–1, because the gm values are stable over this range and its estimates are relatively insensitive to minimal Γ*, Rd and A errors (Harley et al., 1992; Niinemets et al., 2006). WUEi was defined as Aarea/gs (Flexas et al., 2013). As some plants studied here have small leaves or needles growing on several sides of the branch, not all of these were perpendicular to the light source. However, this is also the case for when they are growing in natural conditions as the branches were positioned in the same direction to the light source as they had grown.
Additionally, the one-dimensional within-leaf gas diffusion model of Niinemets and Reichstein (2003) was applied to provide an anatomically based estimate of gm as in Tosens et al. (2012b) and from A–Ci curves as in Ethier and Livingston (2004).
Quantitative limitation analyses of Aarea and partial limitations of gm
The relative controls on Aarea imposed by stomatal conductance (ls), mesophyll conductance (lm), and biochemical capacity (lb) were calculated following the Grassi and Magnani (2005) approach, as has been successfully used for a large number of highly variable species from lycophytes, horsetails and ferns to Australian sclerophylls in Tomás et al. (2013) and Tosens et al. (2012b, 2016). These three values sum to 100% and characterize the extent to which any of the three limitations curbs photosynthesis at the given values of the other two. The contribution of different components of cellular resistance to total cellular resistance to CO2 diffusion was estimated from the anatomical model following Tosens et al. (2016). This share of limitation (li) by different liquid phase components was calculated as:
| (2) |
where li is the limitation by the cell wall, the plasmalemma, cytosol, chloroplast envelope and stroma, and gi refers to the diffusion conductance of each corresponding diffusion pathway. The limitation of each cellular component was scaled up with Sc/S.
Estimation of leaf dry mass per area and nitrogen content
Foliage used for gas exchange measurements was further used for anatomical, morphological and chemical measurements. Individual foliage elements (leaves, needles or scales) were removed from the twigs and scanned at 300 dpi. The foliage was thereafter oven-dried at 70 °C for 48 h, and its dry mass was determined. Leaf projected area was determined from scanned images with ImageJ 1.48v software (Wayne Rasband/NIH, Bethesda, MD, USA) or calculated from the measured length and widths of sides measured with digital precision calipers (Mitutoyo CD-15DC, Mitutoyo Ltd, Andover, UK) for needles and cladodes (Niinemets et al., 2007). From these measurements, LMA and density (Dleaf=LMA/Tleaf) were calculated (Niinemets, 1999; Poorter et al., 2009). Nitrogen content per dry mass (Nmass) was determined by the dry combustion method with a Vario MAX CNS elemental analyser (Elementar, Hanau, Germany), and leaf nitrogen content per area as Nmass/LMA.
Light and electron microscopy
A sub-sample of leaves used for gas exchange measurements was taken for leaf anatomical measurements. Light and electron microscopy sample preparation and analyses followed Tosens et al. (2012b). Six samples were taken from three specimens of each species. Foliage cuts of approximately 6 × 4 mm (or width of the needle or cladode according to species) were taken from intercostal areas. In C. sempervirens scales and in S. uncinata whole leaves were stripped from the branches with tweezers. The plant material was infiltrated in a fixation buffer (3% glutaric aldehyde and 2% paraformaldehyde in 0.1 M phosphate buffer, pH 6.9) under vacuum in a syringe. The samples were post-fixed for 1 h in an osmium tetroxide solution (2%) and dehydrated in a series of increasingly stronger ethanol solutions and embedded in LR white resin (Electron Microscopy Sciences, Hatfield, PA, USA) according to standard procedures (Tosens et al., 2012b). Subsequently, the samples were polymerized in an oven at 60 °C for 26 h. Semi-thin cross-sections of 1 µm for light microscopy, and ultra-thin cross-sections of 70 nm were prepared by an ultramicrotome (Leica EM UC7, Leica Vienna, Austria) for transmission electron microscopy (TEM). The semi-thin sections were stained with toluidine blue for light microscopy, and the sections for TEM were stained with lead citrate and then mounted on formvar–carbon covered copper meshes (Electron Microscopy Sciences). Stained semi-thin sections were viewed in bright field ensuring that all sections were ideally straight with a Nikon Eclipse E600 microscope with phase contrast at magnifications of ×100, ×200 and ×400 and photographed with a Nikon 5 MP digital microscope camera DS-Fi1 (Nikon Corp., Kyoto, Japan). A Philips Tecnai 10 TEM (FEI, Eindhoven, Netherlands) was used to view the ultra-thin sections with the accelerating voltage of 80 kV and magnification between ×1800 and ×14 000. Between five and seven fields of view per sample were measured.
From digital images the following parameters were measured: fraction of intercellular airspace (Fias), leaf thickness from adaxial to abaxial cuticle (Tleaf), mesophyll thickness excluding vascular bundles (Tmes), cytoplasm thickness (Tcyt), chloroplast thickness (Tchl), mesophyll cell wall thickness (Tcwm), chloroplast and mesophyll cell wall area from which Sc/S and mesophyll area exposed to intercellular airspace (Sm/S) were calculated according to Evans et al. (1994) (Table 2). These characteristics were measured for at least ten spongy and ten palisade parenchyma cells and tissue-volume weighted averages were calculated in the species with distinctive separation of mesophyll to spongy and palisade parenchyma. Thirty cells were analysed for every specimen. Light and electron micrographs were analysed with ImageJ 1.48v software.
Table 2.
Leaf anatomical parameters
Data are ±SE; n=3–5. Fias, average fraction of intercellular airspace; Tcwm, mesophyll cell wall thickness; Tcyt, cytoplasm thickness; Tchl, chloroplast thickness.
| Species | F ias | T cwm (μm) | T cyt (μm) | T chl (μm) |
|---|---|---|---|---|
| Selaginella uncinata | 0.30 ± 0.03 | 0.22 ± 0.03 | 0.382 ± 0.033 | 2.359 ± 0.050 |
| Psilotum nudum | 0.15 ± 0.01 | 1.074 ± 0.074 | 0.177 ± 0.043 | 4.1 ± 0.3 |
| Araucaria heterophylla | 0.37 ± 0.09 | 0.67 ± 0.08 | 0.09 ± 0.01 | 2.727 ± 0.092 |
| Cycas revoluta | 0.20 ± 0.01 | 0.34 ± 0.05 | 0.215 ± 0.093 | 5.36 ± 0.10 |
| Macrozamia riedlei | 0.20 ± 0.03 | 0.37 ± 0.02 | 0.218 ± 0.009 | 3.918 ± 0.041 |
| Ephedra minuta | 0.21 ± 0.04 | 0.731 ± 0.010 | 0.299 ± 0.065 | 3.19 ± 0.12 |
| Metasequoia glyptostroboides | 0.29 ± 0.02 | 0.26 ± 0.05 | 0.091 ± 0.020 | 1.97 ± 0.06 |
| Pinus sylvestris | 0.21 ± 0.02 | 0.32 ± 0.08 | 0.089 ± 0.005 | 2.25 ± 0.22 |
| Picea abies | 0.18 ± 0.04 | 0.58 ± 0.05 | 0.070 ± 0.024 | 2.79 ± 0.11 |
| Taxus baccata | 0.30 ± 0.02 | 0.876 ± 0.042 | 0.087 ± 0.003 | 3.1 ± 0.2 |
| Podocarpus alpinus | 0.223 ± 0.008 | 1.18 ± 0.09 | 0.088 ± 0.009 | 2.09 ± 0.11 |
| Podocarpus nivalis | 0.26 ± 0.06 | 1.22 ± 0.09 | 0.092 ± 0.002 | 2.1 ± 0.2 |
| Cupressus sempervirens | 0.063 ± 0.003 | 0.91 ± 0.08 | 0.30 ± 0.09 | 2.81 ± 0.25 |
Statistical analyses
For each species, all measurements were replicated at least with three individual plants. Linear and non-linear regression analyses, t-tests and ANCOVA were used to examine the relationships among the traits and test for trait differences among species using Statistica 10.0 (StatSoft Inc., Tulsa, OK, USA). The choice between linear and non-linear models depended on the shape of the relationships, degree of explained variance and normality of data and residuals, and models providing the greatest r2 and lowest deviations from normality of residuals were used. Due to high variability of foliage shapes and structures, individual species were occasionally outliers in statistical relationships, especially in relationships exploring the effects of individual underlying traits on composite variables. For instance, some correlations were significant when the gymnosperms were considered, but not when the spore-bearing species were included. We denote these and analogous outliers separately in bivariate statistical relationships.
Phylogenetic analyses
Sequences of the plant barcode genes maturase K (matK; a gene that encodes an intron splicing protein) and RbcL (a gene that encodes the large subunit of Rubisco; both genes are in the chloroplastic DNA; Hollingsworth et al., 2009) were extracted from NCBI GenBank (www.ncbi.nlm.nih.gov, last accessed July 2015). This information was not available for Macrozamia riedlei, so the closest available relative, M. moorei, was used. Phylogenetic analyses were conducted and phylogenetic trees were created by the neighbor-joining method using standard procedures in MEGA6 (Tamura et al., 2013). The maximum composite likelihood model was used for estimates of evolutionary divergence (Tamura et al., 2004), and the maximum likelihood method based on the Tamura–Nei model (Tamura and Nei, 1993) was used to create phylogenetic topology with the superior log-likelihood value. The evolutionary age of genera was based on previously published literature (Table 1: smaller numbers indicate evolutionarily closer species). This information was correlated with the study parameters using t-tests and ANCOVA. The Akaike information criterion was used as a measure of the relative quality of the multiple linear models used here.
Table 1.
Phylogenetic ages of the genera, growth form, leaf habit and distances of studied taxa and estimates of evolutionary divergence
Species are in the order of their evolutionary age. Evolutionary divergence is defined as the number of base substitutions per site between sequences.
| Species (abbreviation) | Family | Evolutionary age of genus (My) | Growth form | Evolutionary divergence | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Selaginella uncinata (S.u.) | Selaginellaceae | 405 (Lang et al., 2010) | Herb | S.u. | Ps.n. | A.h. | C.r. | Ma.r. | E.m. | Me.g. | Pin.s. | Pic.a. | T.b. | Po.a. | Po.n. |
| Psilotum nudum (Ps.n.) | Psilotaceae | 306 (Pryer et al., 2004) | Herb | 0.87 | |||||||||||
| Araucaria heterophylla (A.h.) | Araucariaceae | 197 (Knapp et al., 2007) | Tree | 0.76 | 0.61 | ||||||||||
| Cycas revoluta (C.r.) | Cycadaceae | 175 (Magallón and Sanderson, 2005) | Tree | 0.69 | 0.59 | 0.23 | |||||||||
| Macrozamia riedlei (Ma.r.) | Zamiaceae | 175 (Magallón and Sanderson, 2005) | Tree | 0.67 | 0.61 | 0.23 | 0.094 | ||||||||
| Ephedra minuta (E.m.) | Ephedraceae | 168 (Rydin et al., 2004) | Shrub | 1.23 | 0.85 | 0.68 | 0.67 | 0.70 | |||||||
| Metasequoia glyptostroboides (Me.g.) | Cupressaceae | 146 (Hemsley and Poole, 2004) | Tree | 0.87 | 0.66 | 0.18 | 0.27 | 0.27 | 0.71 | ||||||
| Pinus sylvestris (Pin.s.) | Pinaceae | 140 (Wang et al., 2000) | Tree | 0.80 | 0.77 | 0.22 | 0.30 | 0.29 | 0.67 | 0.25 | |||||
| Picea abies (Pic.a) | Pinaceae | 130 (Wang et al., 2000) | Tree | 0.79 | 0.69 | 0.19 | 0.27 | 0.26 | 0.68 | 0.23 | 0.080 | ||||
| Taxus baccata (T.b.) | Taxaceae | 120 (Cheng et al., 2000) | Tree | 0.84 | 0.65 | 0.15 | 0.26 | 0.24 | 0.73 | 0.14 | 0.26 | 0.22 | |||
| Podocarpus alpinus (Po.a.) | Podocarpaceae | 75 (Biffin et al., 2012) | Shrub | 0.88 | 0.71 | 0.20 | 0.29 | 0.29 | 0.75 | 0.24 | 0.32 | 0.31 | 0.25 | ||
| Podocarpus nivalis (Po.n) | Podocarpaceae | 75 (Biffin et al., 2012) | Shrub | 0.87 | 0.71 | 0.20 | 0.28 | 0.29 | 0.75 | 0.24 | 0.31 | 0.30 | 0.24 | 0.004 | |
| Cupressus sempervirens (Cu.s.) | Cupressaceae | 75 (Stewart, 1993) | Tree | 0.88 | 0.70 | 0.19 | 0.25 | 0.29 | 0.70 | 0.085 | 0.28 | 0.26 | 0.16 | 0.26 | 0.26 |
Results
Variation in leaf anatomy and morphology in evolutionarily old species
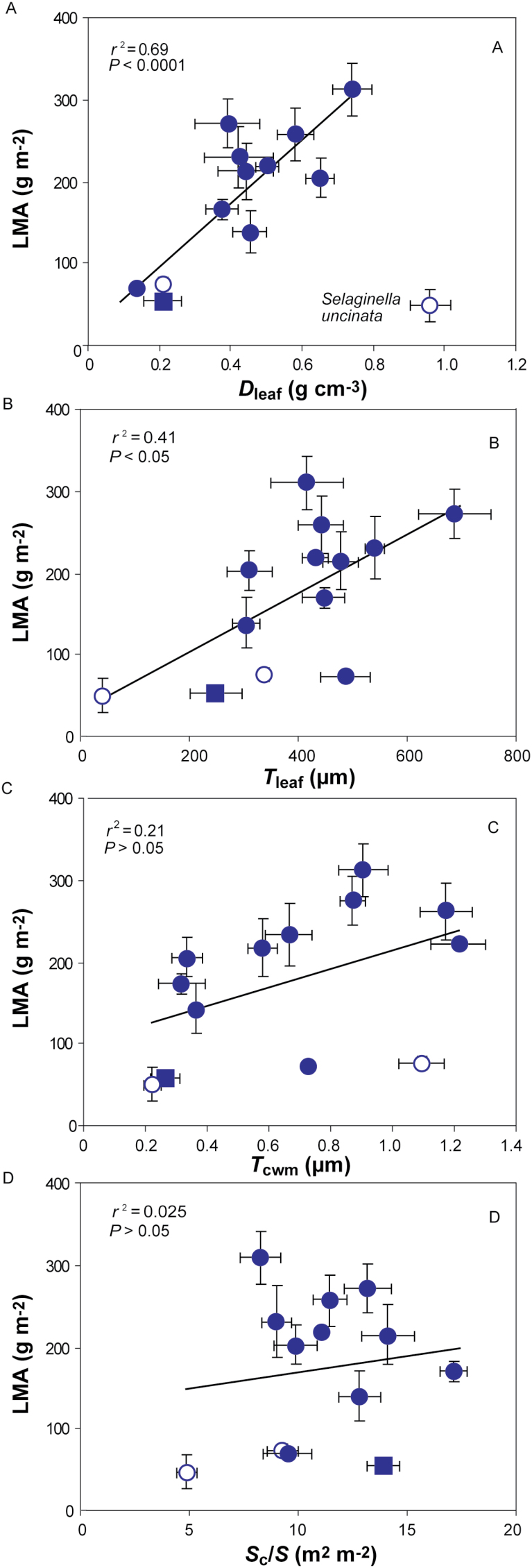
In order to understand the mesophyll diffusional limitations to photosynthesis, a complete structural and ultrastructural analysis was performed of the photosynthetic organs of all the species. A large variability was observed in their macroscopic anatomy (Fig. 1A), but also structural and ultrastructural parameters (Figs 1B–D and 2), e.g. a P. sylvestris cross-section exhibited lobed cells, increasing mesophyll surface area exposed to the intercellular airspace (Fig. 1C). LMA varied about 7-fold between species, from 45 ± 12 g m–2 in S. uncinata to 308 ± 22 g m–2 in C. sempervirens. Leaf density (Dleaf) and thickness varied 16- and 24-fold, respectively. Tcwm varied 5.5-fold (from 0.2 ± 0.05 µm in S. uncinata to 1.2 ± 0.37 µm in P. nivalis), Sc/S varied 3.5-fold (from 4.9 ± 0.47 m2 m–2 in S. uncinata to 17.1 ± 0.62 m2 m–2 in P. sylvestris), and Sm/S varied 2.9-fold (6.1 ± 0.18 to 17.5 ± 0.72 m2 m–2). The variation in LMA was attributed to variation in both of its components, but there was a stronger positive relationship with Dleaf (excluding S. uncinata due to its extremely thin leaves) than Tleaf (Fig. 3A, B). However, there was no correlation between LMA and mesophyll cell wall thickness (Fig. 3C). There was a strong positive relationship between Sc/S and Sm/S (see Supplementary Fig. S1A), but neither of them was related to LMA (Fig. 3D for Sc/S; r2=0.077, P=0.36 for the correlation with Sm/S). Similarly, Sm/S was not correlated to fraction of intercellular airspaces, Fias (r2=0.007; P=0.78). Sc/S was unrelated to Tmes (Supplementary Fig. S1B), but Sm/S had a positive correlation (Supplementary Fig. S1C). However, this correlation was not significant when only gymnosperms were included (r2=0.068; P=0.37).
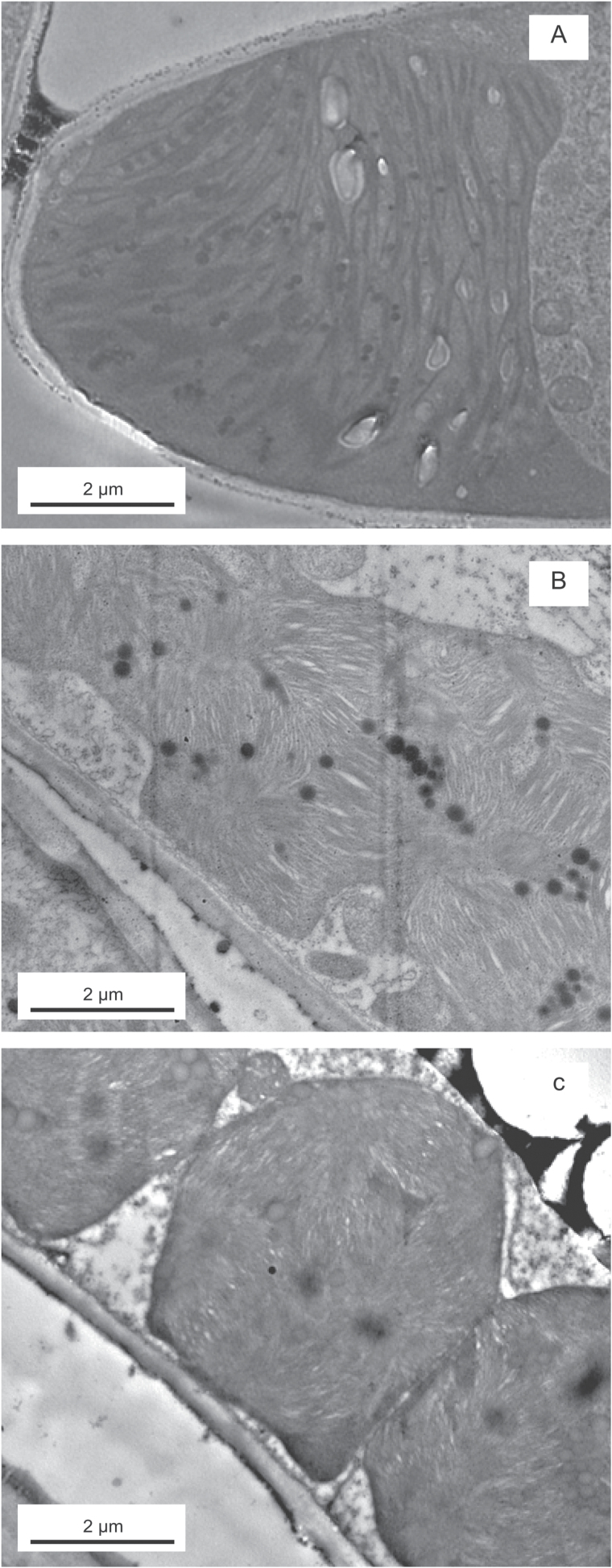
Fig. 2.
Ultrathin transmission electron microscopy cross-sections showing species with atypical chloroplast shapes and sizes: (A) Selaginella uncinata, (B) Cycas revoluta and (C) Macrozamia riedlei.
Fig. 3.
Leaf dry mass per area (LMA) in relation to (A) leaf density (Dleaf), (B) leaf thickness (Tleaf), (C) mesophyll cell wall thickness (Tcwm), and (D) chloroplast surface area exposed to intercellular airspaces (Sc/S). Each data point corresponds to one species (n=3–5). The non-gymnosperms are marked as open circles, while the only deciduous conifer, Metasequoia glyptostroboides, is marked as a filled square. Excluded species are marked with the species’ name. Error bars show average±SE of all presented species. Note that some standard errors are so small that they are not visible under the data points. Data were fitted by linear regression (all are significant at P<0.05).
Interspecific variation in photosynthetic capacity and its relationships with diffusional and structural determinants
Area- and mass-based net assimilation (Amass) varied about 5- and 8-fold, respectively (from 2.1 ± 0.34 µmol m–2 s–1 in P. nudum to 11.0 ± 0.78 µmol m–2 s–1 in P. sylvestris, and from 21 ± 3.9 nmol m–2 s–1 in C. revoluta to 165 ± 9 nmol m–2 s–1 in M. glyptostroboides). Mesophyll conductance per unit leaf area (gm/area) varied over 12-fold (from 10 ± 2.1 mmol m–2 s–1 in Ephedra minuta to 124 ± 9 mmol m–2 s–1 in P. sylvestris) and mass-based gm (gm/mass) over 15-fold (from 0.08 ± 0.005 mmol g–1 s–1 in P. nivalis to 1.20 ± 0.06 mmol g–1 s–1 in M. glyptostroboides).
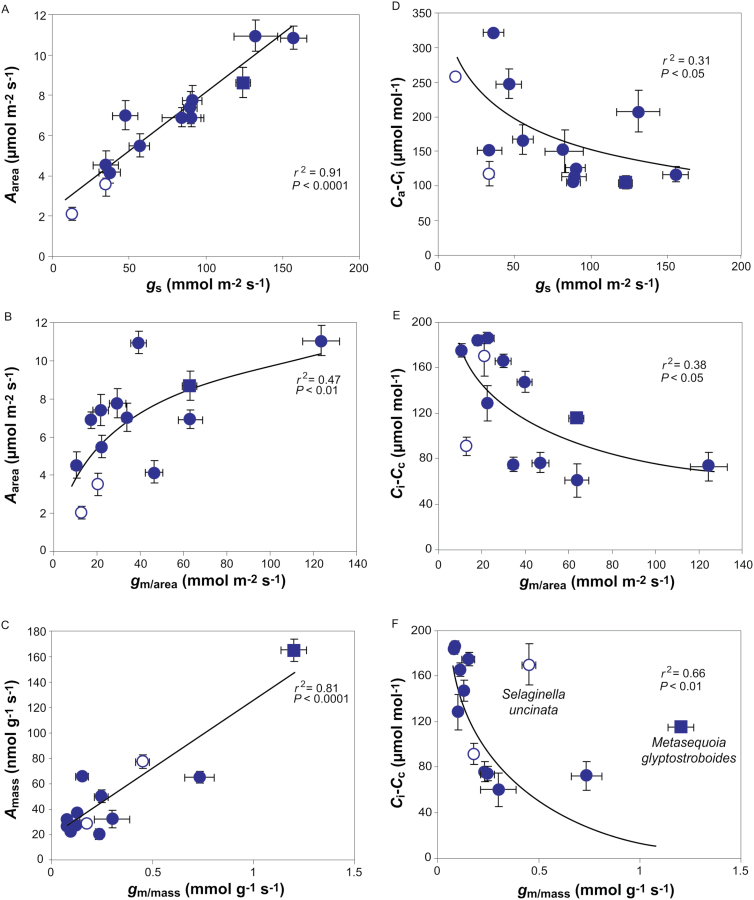
A area depended strongly on gs (Fig. 4A). Importantly, Aarea scaled positively with gm/area (Fig. 4B), but there was stronger positive correlation between Amass and gm/mass (Fig. 4C), which was significant even with the exclusion of M. glyptostroboides (r2=0.42; P=0.016). A similar strength negative curvilinear relationship was found between Ca–Civs gs (Fig. 4D) and the CO2 drawdown from the intercellular airspace to chloroplasts (Ci–Cc) vs gm/area (Fig. 4E). However, a stronger relationship was found between Ci–Ccvs gm/mass when S. uncinata and M. glyptostroboides were excluded (Fig. 4F).
Fig. 4.
(A, B) Area-based net assimilation (Aarea) in relation to (A) stomatal conductance (gs) and (B) mesophyll conductance per area (gm/area). (C) Mass-based photosynthesis (Amass) in relation to mass-based mesophyll conductance (gm/mass). (D) The draw-down from the atmosphere to the intercellular airspaces (Ca–Ci) in relation to gs. (E, F) The draw-down from intercellular airspaces to chloroplasts (Ci–Cc) in relation to (E) gm/area and (F) gm/mass. Data in (B) were fitted by non-linear regression of the form y=2.73ln(x)–2.81; data in (D) of the form y=–53ln(x)+389; data in (E) of the form y=–36ln(x)+252; and data in (F) of the form y=–30ln(x)+80. Data are presented as in Fig. 3.
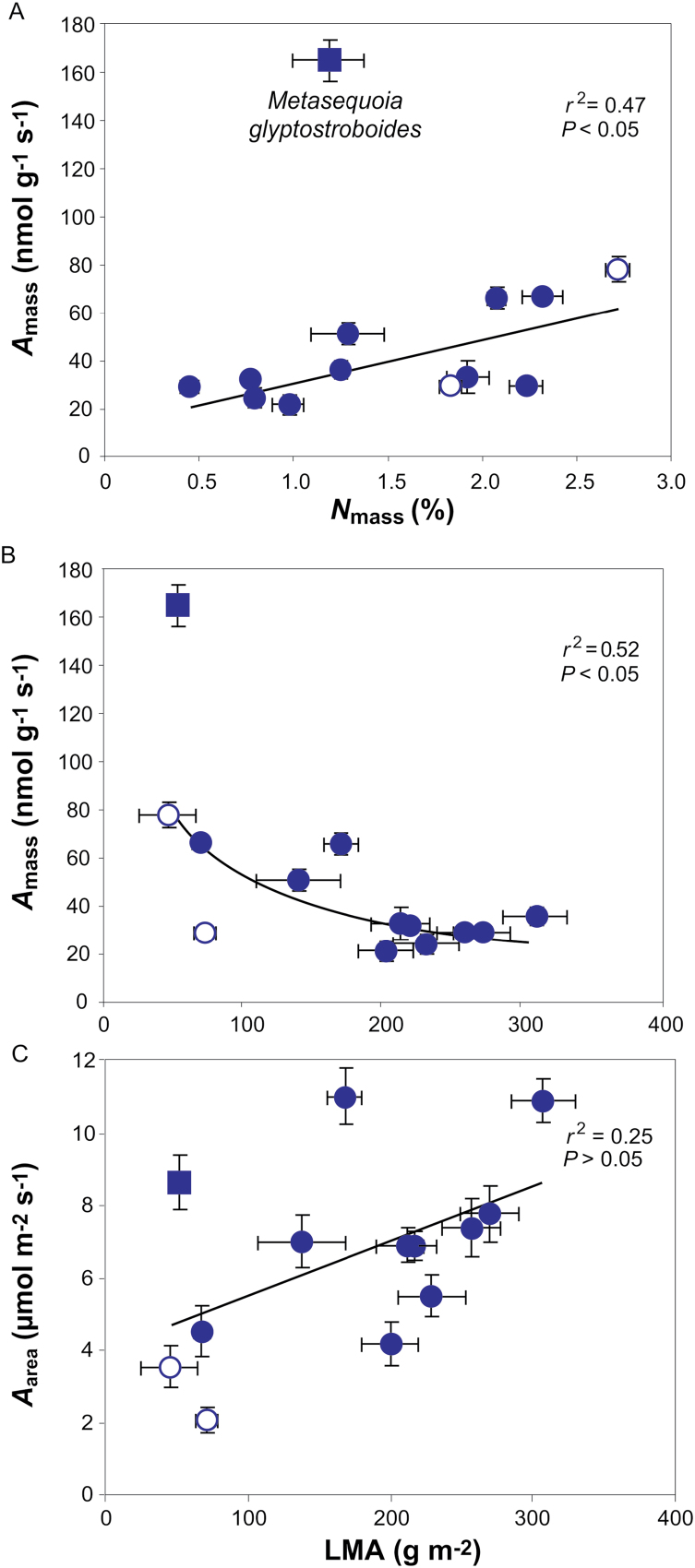
In addition to large variations in LMA and its components, nitrogen content per dry mass (Nmass) varied 6-fold (from 0.45 ± 0.02% to 2.72 ± 0.07%) and area-based nitrogen (Narea) varied 12-fold (0.6 ± 0.01 to 6.1 ± 0.09 g m–2). Amass scaled positively with Nmass, but the only deciduous conifer in this study, M. glyptostroboides, diverged from this relationship by having higher Amass (Fig. 5A). There was no relationship between Narea and Aarea (r2=0.20; P=0.12). Similarly, Amass was negatively correlated with LMA, while there was no relationship between Aarea and LMA (Fig. 5B, C).
Fig. 5.
Mass- and area-based net assimilation (respectively Amass and Aarea) in relation to (A) mass-based nitrogen content (Nmas) and (B, C) LMA. The data in (B) were fitted with a curvilinear regression of the form y=896x–0.63. Data presentation and fitting in (A, C) are as in Fig. 3.
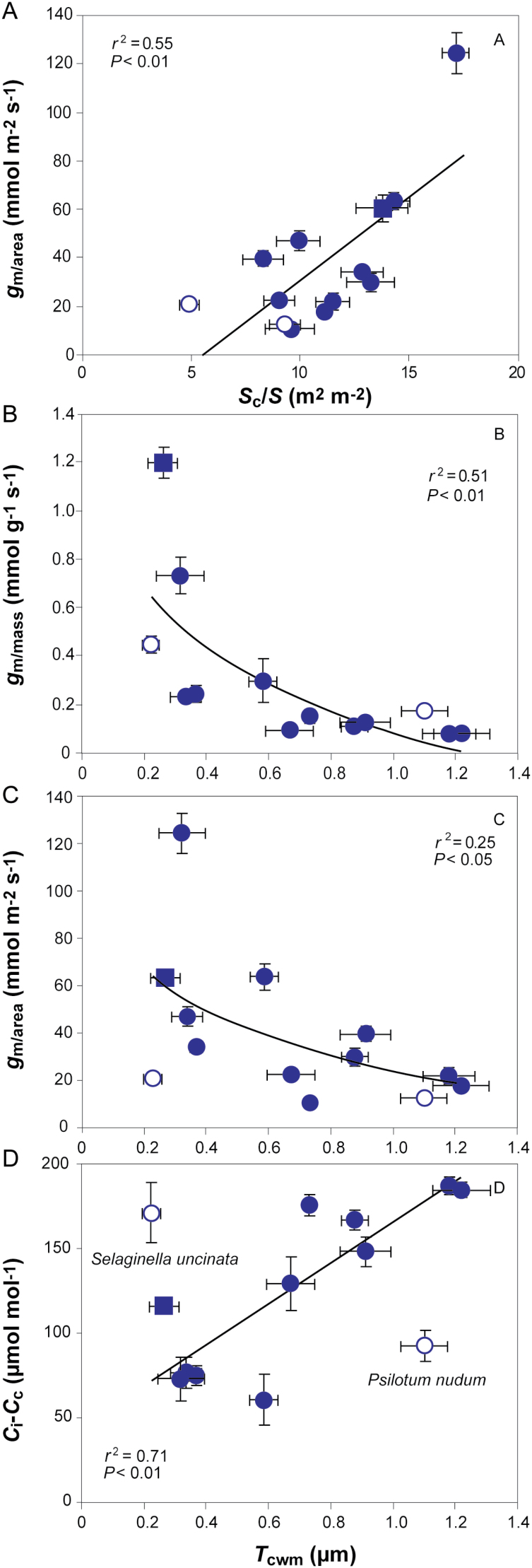
Analysis of the ultra-anatomical controls on gm demonstrated that gm/area was positively correlated with Sc/S. On the other hand, gm/mass and gm/area were negatively associated with Tcwm. Ci–Cc scaled positively with Tcwm across gymnosperms, but not across the whole sample (Fig. 6). Tcwm did not correlate with density (r2=0.030; P=0.55).
Fig. 6.
(A) The influence of chloroplast surface area exposed to intercellular airspaces (Sc/S) on mesophyll conductance (gm/area). (B–D) The influence of cell wall thickness (Tcwm) on (B) mesophyll conductance per mass (gm/mass), (C) mesophyll conductance per area (gm/area), and (D) the draw-down from intercellular airspaces to chloroplasts (Ci–Cc). The data in (B) were fitted with a non-linear regression of the form y=–0.37ln(x)+0.09 and data in (C) of the form y=–26ln(x)+25. Data presentation and fitting are as in Fig. 3.
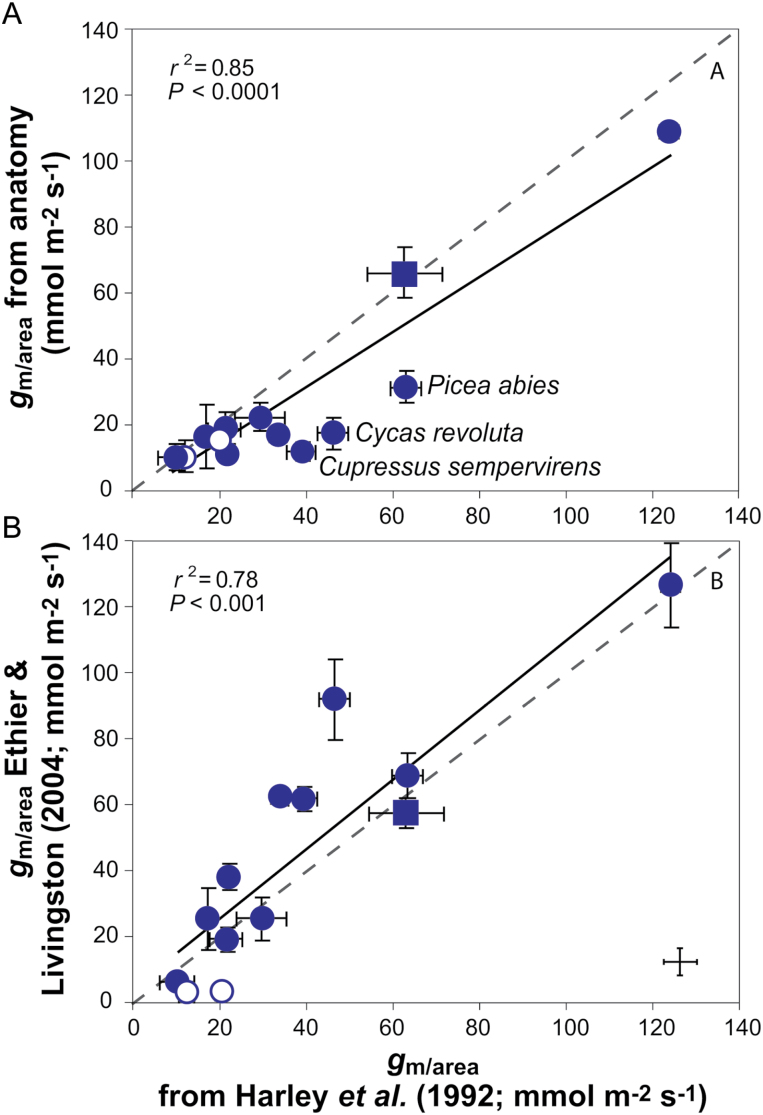
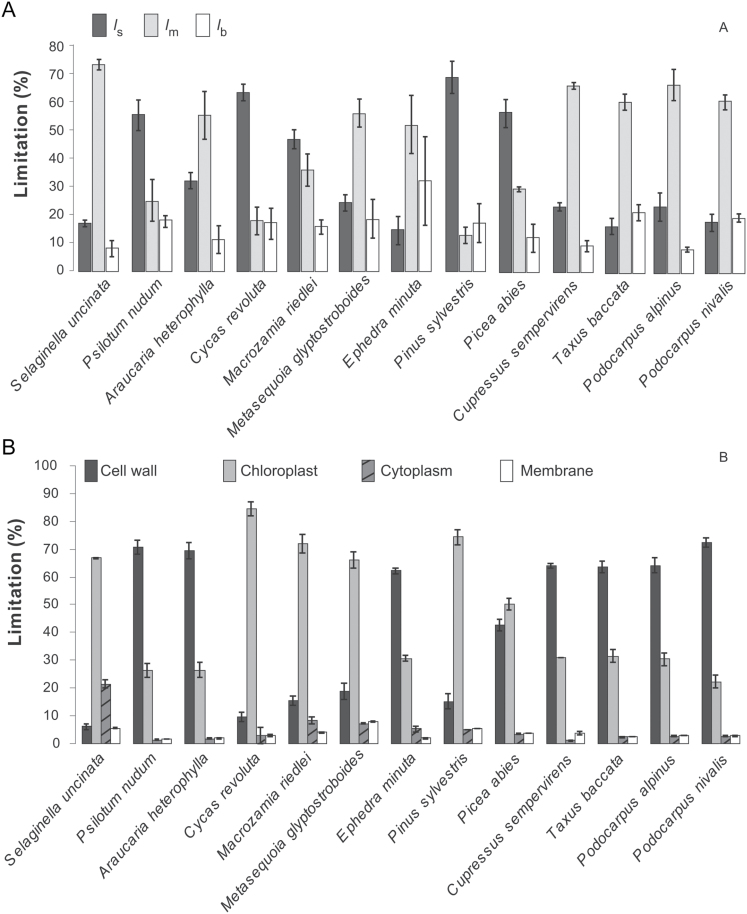
In addition to gas exchange–fluorescence methodology, gm was estimated by two alternative methods: it was modeled from mesophyll anatomical traits by using the anatomical model of Niinemets and Reichstein (2003) and from A–Ci curves (Ethier and Livingston, 2004, Fig. 7). gm calculated from anatomical measurements correlated better with the estimates obtained from gas exchange–fluorescence measurements than gm obtained from A–Ci curves (average discrepancy between gm from gas exchange–fluorescence was 27% with gm from anatomy and 33% with gm from Ethier and Livingston (2004)). Based on the quantitative limitation analysis, the photosynthetic capacity was strongly limited by both gs (range 15–70%) and gm/area (range 12–74%), while limited biochemical capacity (range 8–33%) restricted Aarea less than stomata and mesophyll (Fig. 8A). With respect to the structural limitations of gm, the estimated gas phase limitation inside the leaf was <1% of the total limitations for all the species (data not shown). Among all the components of liquid phase limitations, cytoplasm and membrane limitations played a minor role, whereas the predominant limitation was exerted by cell walls and chloroplast stroma (Fig. 8B and Table 2).
Fig. 7.
Comparison of gm/area calculated based on Harley et al. (1992) with (A) gm/area calculated from anatomical measurements according to Niinemets and Reichstein (2003) and (B) gm/area calculated based on Ethier and Livingston (2004). Dashed lines represent 1:1 correlation. Data presentation and fitting are as in Fig. 3, but all species are included in the analyses. C. revoluta, C. sempervirens and P. abies were underestimated by the anatomical model.
Fig. 8.
(A) The percentage of assimilation limited by stomatal conductance (ls), mesophyll conductance (lm) and biochemistry (lb). (B) The percentage of mesophyll conductance per area limited by liquid phase components: cell wall, chloroplast, cytoplasm and membrane. Error bars denominate standard errors (n=3–5). The species are in the order of evolutionary age.
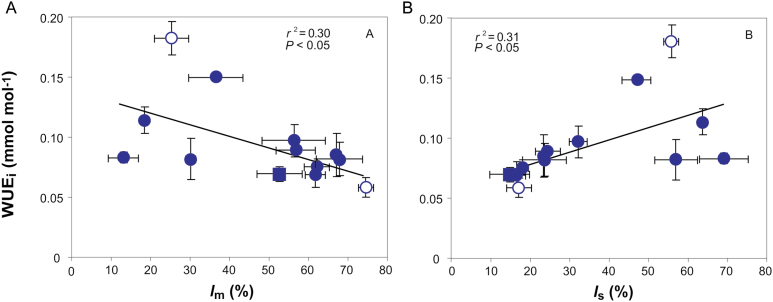
The intrinsic water use efficiency (WUEi) varied ca 3-fold across species. It was negatively correlated with the share of photosynthesis limited by gm/area (Fig. 9A). WUEi also scaled positively with the photosynthesis limitation by stomata (Fig. 9B). When considered together, the interspecific variation in WUEi was driven by both ls and lm (see Supplementary Table S2).
Fig. 9.
Correlations of intrinsic water use efficiency (WUEi) with (A) the share of photosynthesis limited by mesophyll conductance (lm) and (B) share of photosynthesis limited by stomatal conductance (ls). Data presentation and fitting are as in Fig. 3.
The effect of divergence time on structure and physiology
In the given dataset, LMA and Aarea were negatively correlated with the estimated evolutionary age of the genus (Supplementary Fig. S2 and Supplementary Table S3a; r2=0.45; p=0.012). However, gm/area itself was not related to plant evolutionary age (r2=0.055; p=0.44) and gm/area influenced Aarea regardless of evolutionary age (Supplementary Table S3b). Nevertheless, the lowest mesophyll conductances were observed for the oldest genera (Ephedra: 10.3 mmol m–2 s–1; Psilotum: 12.5 mmol m–2 s–1; Selaginella: 20.5 mmol m–2 s–1). However, the highest average gm/area was recorded for Pinus (124 ± 9 mmol m–2 s–1), although this genus is older than four others (Picea, Podocarpus, Taxus, Cupressus) in this study. In addition, gs correlated negatively with the age of the genus (r2=0.41; P=0.017), but there was no significant correlation of WUEi with the evolutionary age of the genus (r2=0.083; P=0.34).
Discussion
Structural and ultrastructural traits determining LES relationships in evolutionarily old species
LMA in our study depended on leaf density while its relationship with Tleaf was weaker (Fig. 3A, B) as shown by Tomás et al. (2013) across a variety of genera. Contrarily, LMA was more strongly related to Tleaf than to Dleaf in Australian sclerophylls (Niinemets et al., 2009c). The median value of LMA for evergreen gymnosperms is higher (230 g m–2) than what was found here (214 g m–2), but the minimum and maximum values here fall into the gymnosperm range (Poorter et al., 2009) placing them at the low end of LES characterized by overall robust foliage and low photosynthetic capacity (Wright et al., 2004).
Prior this study the highest Tcwm was recorded in ferns and allies: 0.81 µm (Tosens et al., 2016). The species here had even thicker mesophyll cell walls: up to 1.22 µm in P. nivalis. Both the average across the sample and across the gymnosperms (0.68 µm) were also very high compared with what has been previously found for non-stressed fully expanded leaves: from approximately 0.1–0.2 µm in annual to 0.4–0.5 µm in sclerophyllous species (Hanba et al., 2001; Terashima et al., 2006; Hassiotou et al., 2009; Tosens et al., 2012b, 2016; Tomás et al., 2013). On the other hand, values of Sc/S in our study were average in the majority of species as it varies from 1.6 to 32 m2 m–2 worldwide (Terashima et al., 2006; Peguero-Pina et al., 2012; Tosens et al., 2016) and the lowest values have been recorded in ferns (in the majority of species Sc/S<8) (Tosens et al., 2016), but across evergreen gymnosperms Sc/S had only been measured in Abies alba and A. pinsapo (17 and 32 m2 m–2, respectively) (Peguero-Pina et al., 2012). However, CO2 diffusion to Rubisco is greatly enhanced if the cell walls are fully lined with chloroplasts, with Sc/Sm=1, a condition that in practice is rare (Terashima et al., 2011). The ratio was remarkably high in the gymnosperms studied here, being over 0.96 for five species (see Supplementary Fig. S1A).
The LMA components thickness and density alter leaf photosynthetic capacity in opposite directions (Niinemets, 1999). Leaf thickness often reflects a greater number of chloroplasts, higher Sc/S and Sm/S for CO2 diffusion, and greater concentration of photosynthetic machinery, while greater density is associated with lower net assimilation due to increased cell wall resistance (Hanba et al., 1999; Niinemets, 1999; Terashima et al., 2006). Contrarily, Slaton and Smith (2002) studied a wide range of genera, but did not find a correlation between Sm/S and Tmes. Similarly, no relationship was found between Sc/S or Sm/S and Tmes across Australian sclerophylls (Tosens et al., 2012b). However, across the evolutionarily old species studied in this work, Tmes correlated positively with Sm/S, but regardless of the positive trend was not correlated with Sc/S (Supplementary Fig. S1B, C). However, the species with high Sm/S also had higher Sc/S (Supplementary Fig. S1A). This somewhat counterintuitive outcome results from the variability of Sc/S due to the spore-bearing plants in the sample as their Sc/Sm ratio was lower than average. These relationships were not significant, when these two species were excluded from the analyses. Sm/S and Sc/S depended on mesophyll architecture rather than Tmes or Fias due to variability in density, size and shape of mesophyll cells (e.g. lobes; Fig. 1C) at a given thickness as was previously suggested by Tosens et al. (2012a). Likewise, this resulted in LMA not correlating with Sc/S (Fig. 3D).
Additionally, Tcwm was not related to LMA (Fig. 3C) or density as is often assumed (e.g. Syvertsen et al., 1995) showing that Tcwm may not vary with total leaf tissue density or other leaf tissue’s cell wall thicknesses (Tosens et al., 2012b). Collectively, these results suggest that widely varying combinations of leaf anatomical traits occur at given values of LMA, and detailed anatomical studies are needed to relate gm to mesophyll structure.
Structural modifications of photosynthesis and mesophyll diffusion conductance
A area and gs were correlated and comparable to those found in spore-bearing plants and gymnosperms (James et al., 1994; Equiza et al., 2006; Franks, 2006; La Porta et al., 2006; Pardo et al., 2009; Zhang et al., 2015; Tosens et al., 2016). Flexas et al. (2012) set an average gm slightly above 100 mmol m–2 s–1 for conifers, and studies have shown a wide variation from as low as 18 up to 110 mmol m–2 s–1 in evergreen gymnosperms (Warren et al., 2003; De Lucia et al., 2003; Manter and Kerrigan, 2004; Black et al., 2005; Mullin et al., 2009; Peguero-Pina et al., 2012, 2016). Accordingly, gm varied from 10 to 124 mmol m–2 s–1 in this study. The values of Ci–Cc here fell in the higher part of the range found for angiosperms and were higher than previously found for conifers (Niinemets et al., 2005, 2009b; Warren, 2008; Hassiotou et al., 2009; Tosens et al., 2012b; Peguero-Pina et al., 2012). Importantly, gm influenced both Ci–Cc and photosynthesis significantly with stronger mass-based relations (Fig. 4).
Nitrogen content was similar to that previously found in conifers and positively related to Amass, as was also shown by Reich et al. (1995). M. glyptostroboides diverged in the analysis, which can be explained by its deciduous nature (Wright et al., 2002). A negative relationship between LMA and Amass has been shown previously for evergreens while area-based photosynthesis seems not to correlate with LMA, which is contrary to deciduous species, where higher LMA has been shown to correlate with higher Aarea (Reich et al., 1995; Wright et al., 2004). Accordingly, the same was found here (Fig. 5). Altogether, this illustrates strong investment in supportive structure (Niinemets et al., 2009c; Hassiotou et al., 2009).
Similarly to recent studies, the most important anatomical correlations with gm were Sc/S and Tcwm (Terashima et al., 2011; Tosens et al., 2012b, 2016; Peguero-Pina et al., 2012; Tomás et al., 2013) (Fig. 6). The significance of each depends on foliage structure, e.g. in sclerophylls higher Tcwm overshadows the influence of Sc/S on gm (Tomás et al., 2013). The gm/mass–Tcwm correlation was stronger than the area-based one (Fig. 6B, C) as gm/mass indicates the investment in cell walls and gm/area in the mesophyll area (Niinemets et al., 2009b).
The importance of structure as a limiting factor of photosynthesis
In order to overcome the various uncertainties related to gm estimations, it was additionally modelled from anatomy and estimated from A–Ci curves (Ethier and Livingston, 2004). All three methods show similarly wide variation in gm across evolutionarily old species (Fig. 7). However, gm from anatomy tended to underestimate, whereas the A–Ci curve method overestimated, gm for some species. When both methods were considered, gm from the variable J method was more strongly correlated with gm obtained from anatomy than from A–Ci curves. Likewise, Tomás et al. (2013) found this across species exhibiting a wide variation of leaf morphologies. Yet, the model tends to over- or underestimate at the lower or higher end of gm (Tosens et al., 2012a,b; Peguero-Pina et al., 2012; Tomás et al., 2013; Fini et al., 2016). In this sample, gm from anatomy mostly underestimated for C. revoluta, C. sempervirens and P. abies (Fig. 7), while the model estimations where in strong agreement with gm from gas exchange for species with thick mesophyll cell walls and low gm. This is different from Tosens et al. (2016), where the model overestimated for evolutionarily old Ophioglossum and Lycopodium species exhibiting very high Tcwm (0.7–0.81 µm). The discrepancy between measured and modelled gm may arise from various uncertainties associated with both estimates. As for combined gas exchange–fluorescence estimation, gm is not the only component to affect Cc: the amount of respiratory and photorespiratory CO2 should be considered as an independent source of CO2 (Tholen et al., 2012). Concerning the predictive power of the anatomical model for species exhibiting a wide variation in Tcwm, heterogeneous chloroplast thickness and morphology (Figs 1 and 2), the strongest uncertainties are related to the unknown variation of mesophyll cell wall porosity and the actual determinants of stroma resistance including the role of carbonic anhydrase (CA). Nobel (1991) proposed an upper value of cell wall porosity of 0.3 and that it varies linearly with Tcwm. Indeed, 0.3 was used to calculate gm for Populus tremula with low Tcwm and it gave good results (Tosens et al., 2012a). In a multispecies context the relationship between gm from anatomy and gas exchange was improved when porosity was modelled varying between 0.04 and 0.095 and being negatively correlated with Tcwm (Tosens et al., 2012b; Peguero-Pina et al., 2012; Tomás et al., 2013). Some species here and in Tosens et al. (2016) exhibited very high Tcwm compared with prior research, and therefore lower porosity values were used for species where Tcwm>0.5 μm. However, as was emphasized by Tosens et al. (2012b), the linear decline of porosity with Tcwm is hypothetical and the discrepancy between modelled and measured gm could be resolved by some other trait.
Quantitative limitation analysis further confirmed that the mesophyll gas phase limitation to CO2 diffusion is negligible as it accounted for less than 1% of the total limitation. This is even less than previously observed in a wide range of sclerophyllous angiosperm genera or in spore-bearing plants (Tosens et al., 2012a,b; Tomás et al., 2013). Using limitation analysis to further separate the contribution of the components of the liquid phase revealed that the limitation of Tcwm and Tchl had the greatest span, ranging from 6 to 72% and from 22 to 84%, respectively (Fig. 8B). This is in agreement with the negative correlation between gm/mass and Tcwm (Fig. 6B). CO2 faces the longest diffusion distance in the liquid phase after entering the chloroplasts (Evans et al., 2009). In this set of species, Tchl varied between 1.97 and 5.36 μm (Fig. 2), which is thicker than previously reported explaining the high limitation by Tchl. It is debated whether the CO2 diffusion efficiency in the chloroplast stroma is mainly controlled by CA activity in the chloroplast (Flexas et al. 2012) and if it varies across species (at normal cytosolic pH), being higher in evergreen sclerophylls in order to compensate for the low CO2 diffusion efficiency through thick mesophyll cell walls (Gillon and Yakir, 2000), or whether CA is always sufficient and CO2 interconversion is not a limiting factor of gm. Equally, this analysis adds to the evidence that CO2 diffusion is importantly controlled by the physical diffusion distance within the chloroplasts (Tosens et al., 2012a,b; Peguero-Pina et al., 2012; Tomás et al., 2013).
Overall, the good fit between gm calculated from anatomy and gas exchange confirms that gm is explained to a large extent by inherent variations in mesophyll anatomy, while mesophyll cell walls and chloroplasts are the most important structural determinants of liquid phase conductance in species exhibiting high Tcwm, Tchl and heterogeneous chloroplast morphology. However, the limitation amplitude was large implicating versatility in its importance among different species (Fig. 8A). The smallest limitation on Aarea on average was biochemistry, which is similar to previous interspecies studies by Tomás et al. (2013) and Tosens et al. (2016). As the mesophyll and stomatal conductances were low, especially compared with maximal values measured so far (Wright et al., 2004; Flexas et al., 2012), it is expected that biochemistry plays a smaller role in limitations, because the photosynthetic enzymes are not saturated with CO2 due to constraints on the previous parts of the pathway (Terashima et al., 2011; Niinemets et al., 2011). The limitations by stomata and mesophyll were similarly significant in the control of WUEi showing again that leaf mesophyll structure plays an important role in realized WUEi (Fig. 9).
The effect of divergence time on structure and physiology
The rate of photosynthesis depended on the age of the genera. This, however, was mostly due to the inclusion of spore-bearing plants reflecting the evolutionary increase in photosynthetic capacity from early plants to angiosperms (Brodribb et al., 2009; Carriquí et al., 2015; McAdam and Brodribb, 2015). However, when broad taxonomic groups are considered, species’ evolutionary adaptation to light and water conditions can actually drive photosynthetic capacity more strongly than their evolutionary age (Tosens et al., 2016). In this regard, it is important that Aarea depended on gm/area positively regardless of evolutionary age, showing that this correlation remained significant including the effect of divergence time in the model (see Supplementary Table S3a, b).
Analogously to Aarea, it has been suggested that there is an increase in gm/area through the evolution and diversification of embryophytes (Carriquí et al., 2015). While indeed gymnosperms studied here had a very low average gm/area, much lower than angiosperms with comparably tough foliage structure (e.g. Flexas et al., 2012), gm/area did not significantly depend on evolutionary age. As with within-group variability in ferns (Tosens et al., 2016) and angiosperms (Flexas et al., 2007b), this likely reflects individual species adaptation to specific habitat conditions.
Notwithstanding the lack of within-group evolutionary signal, low average gm/area and Aarea and the corresponding thick mesophyll cell walls for species with widely contrasting ecological strategies do support the preservation of old traits through evolution, suggesting apparent constraints on evolution. Different CO2/O2 selection pressures at the time of divergence for ferns and angiosperms have been hypothesized to be the reason for lower average Aarea values for the former (Carriquí et al., 2015) as ferns evolved in several-fold higher CO2 and slightly lower O2 concentrations than angiosperms (Brodribb et al., 2009; Carriquí et al., 2015). In fact, in the high-CO2 atmosphere where several of the thick-cell-walled species evolved (about 65–200 My ago), diffusional limitations exercised a lower control on the rate of photosynthesis. However, while this ancient trait has been preserved through evolution, gm has started to exercise an increasingly stronger control on foliage assimilation rates. Yet, this might be inevitable given the inefficient dynamic stomatal control under water-limited conditions potentially leading to excess plant water loss until hydraulic constraints force stomatal closure (Brodribb et al., 2005; Carnicer et al., 2013).
Conclusions
This study demonstrates important relationships between mesophyll structure and physiology in evolutionarily old plants. A large variability was found in ultrastructural traits determining LES relationships. Although LMA depended on leaf density and thickness, they were not correlated with Tcwm or Sc/S illustrating that LMA cannot be used as a universal explanation for photosynthetic structural constraints.
Mesophyll anatomy exerted major control on net assimilation rates. The three methods used in previous studies for interspecies comparisons were used to confirm gm values from gas exchange–fluorescence measurements and their anatomical nature. Although high variation was uncovered between species in gm, it was on average very low. This resulted mainly from the exceptionally thick cell walls, large chloroplasts, and low Sc/S. The role of stroma deserves further attention.
These characteristics in evolutionarily older taxa support the preservation of ancient traits in evolution, although the role of functional adaptation and evolutionary constraint in leaf anatomy continues to be debated.
Supplementary Material
Acknowledgements
This work was supported by the Estonian Ministry of Science and Education (grants IUT-8-3, PUT1409 and and PUT1473) and the European Commission through the European Regional Fund (the Center of Excellence EcolChange).
Glossary
Abbreviations:
- Aarea
net assimilation rate per area
- Amass
net assimilation rate per dry mass
- Ca
atmospheric CO2 concentration
- CA
carbonic anhydrase
- Ca–Ci
CO2 drawdown from atmosphere to intercellular airspace
- Ci
intercellular CO2 concentration
- Ci–Cc
CO2 drawdown from intercellular airspace to chloroplasts
- Dleaf
leaf density
- Fias
fraction of intercellular airspace
- LMA
leaf dry mass per area
- lb, lm and ls
photosynthetic limitation by biochemistry, mesophyll and stomatal conductance, respectively
- Narea
nitrogen content per area
- Nmass
nitrogen content per mass
- gm
mesophyll diffusion conductance
- gm/area and gm/mass
mesophyll conductance per area and per mass
- gs
stomatal conductance
- Sc/S and Sm/S
chloroplast and mesophyll area exposed to intercellular airspace
- Tchl
chloroplast thickness
- Tcwm
mesophyll cell wall thickness
- Tleaf
leaf thickness
- Tmes
mesophyll thickness
- WUEi
intrinsic water use efficiency.
References
- Bernacchi CJ, Singsaas EL, Pimentel C, Portis AR, Jr, Long SP. 2001. Improved temperature response functions for models of Rubisco-limited photosynthesis. Plant, Cell and Environment 24, 253–259. [Google Scholar]
- Biffin E, Brodribb TJ, Hill RS, Thomas P, Lowe AJ. 2012. Leaf evolution in Southern Hemisphere conifers tracks the angiosperm ecological radiation. Proceedings of the Royal Society B. Biological Sciences 279, 341–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black K, Davis P, McGrath J, Doherty P, Osborne B. 2005. Interactive effects of irradiance and water availability on the photosynthetic performance of Picea sitchensis seedlings: implications for seedling establishment under different management practices. Annals of Forest Science 62, 413–422. [Google Scholar]
- Brodribb TJ, Holbrook NM, Zwieniecki MA, Palma B. 2005. Leaf hydraulic capacity in ferns, conifers and angiosperms: impacts on photosynthetic maxima. The New Phytologist 165, 839–846. [DOI] [PubMed] [Google Scholar]
- Brodribb TJ, McAdam SA, Jordan GJ, Feild TS. 2009. Evolution of stomatal responsiveness to CO2 and optimization of water-use efficiency among land plants. The New Phytologist 183, 839–847. [DOI] [PubMed] [Google Scholar]
- Carnicer J, Barbeta A, Sperlich D, Coll M, Peñuelas J. 2013. Contrasting trait syndromes in angiosperms and conifers are associated with different responses of tree growth to temperature on a large scale. Frontiers in Plant Science 4, 409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carriquí M, Cabrera HM, Conesa MÀ, et al. 2015. Diffusional limitations explain the lower photosynthetic capacity of ferns as compared with angiosperms in a common garden study. Plant, Cell and Environment 38, 448–460. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Nicolson RG, Tripp K, Chaw SM. 2000. Phylogeny of Taxaceae and Cephalotaxaceae genera inferred from chloroplast matK gene and nuclear rDNA ITS region. Molecular Phylogenetics and Evolution 14, 353–365. [DOI] [PubMed] [Google Scholar]
- De Lucia EH, Whitehead D, Clearwater MJ. 2003. The relative limitation of photosynthesis by mesophyll conductance in co-occurring species in a temperate rainforest dominated by the conifer Dacrydium cupressinum. Functional Plant Biology 30, 1197–1204. [DOI] [PubMed] [Google Scholar]
- Equiza MA, Day ME, Jagels R. 2006. Physiological responses of three deciduous conifers (Metasequoia glyptostroboides, Taxodium distichum and Larix laricina) to continuous light: adaptive implications for the early Tertiary polar summer. Tree Physiology 26, 353–364. [DOI] [PubMed] [Google Scholar]
- Ethier GJ, Livingston NJ. 2004. On the need to incorporate sensitivity to CO2 transfer conductance into the Farquhar–von Caemmerer–Berry leaf photosynthesis model. Plant, Cell and Environment 27, 137–153. [Google Scholar]
- Evans JR, Kaldenhoff R, Genty B, Terashima I. 2009. Resistances along the CO2 diffusion pathway inside leaves. Journal of Experimental Botany 60, 2235–2248. [DOI] [PubMed] [Google Scholar]
- Evans JR, Setchell BA, von, Caemmerer S, Hudson GS. 1994. The relationship between CO2 transfer conductance and leaf anatomy in transgenic tobacco with a reduced content of Rubisco. Plant Environmental Biology 21, 475–495. [Google Scholar]
- Fini A, Loreto F, Tattini M, Giordano C, Ferrini F, Brunetti C, Centritto M. 2016. Mesophyll conductance plays a central role in leaf functioning of Oleaceae species exposed to contrasting sunlight irradiance. Physiologia Plantarum 157, 54–68. [DOI] [PubMed] [Google Scholar]
- Flexas J, Barbour MM, Brendel O, et al. 2012. Mesophyll diffusion conductance to CO2: an unappreciated central player in photosynthesis. Plant Science 193–194, 70–84. [DOI] [PubMed] [Google Scholar]
- Flexas J, Díaz-Espejo A, Berry JA, Cifre J, Galmés J, Kaldenhoff R, Medrano H, Ribas-Carbó M. 2007a Analysis of leakage in IRGA’s leaf chambers of open gas exchange systems: quantification and its effects in photosynthesis parameterization. Journal of Experimental Botany 58, 1533–1543. [DOI] [PubMed] [Google Scholar]
- Flexas J, Diaz-Espejo A, Galmés J, Kaldenhoff R, Medrano H, Ribas-Carbo M. 2007b Rapid variations of mesophyll conductance in response to changes in CO2 concentration around leaves. Plant, Cell and Environment 30, 1284–1298. [DOI] [PubMed] [Google Scholar]
- Flexas J, Niinemets U, Gallé A, et al. 2013. Diffusional conductances to CO2 as a target for increasing photosynthesis and photosynthetic water-use efficiency. Photosynthesis Research 117, 45–59. [DOI] [PubMed] [Google Scholar]
- Flexas J, Ribas-Carbó M, Diaz-Espejo A, Galmés J, Medrano H. 2008. Mesophyll conductance to CO2: current knowledge and future prospects. Plant, Cell and Environment 31, 602–621. [DOI] [PubMed] [Google Scholar]
- Franks PJ. 2006. Higher rates of leaf gas exchange are associated with higher leaf hydrodynamic pressure gradients. Plant, Cell and Environment 29, 584–592. [DOI] [PubMed] [Google Scholar]
- Galmés J, Flexas J, Keys AJ, Cifre J, Mitchell RAC, Madgwick PJ, Haslam RP, Medrano H, Parry MAJ. 2005. Rubisco specificity factor tends to be larger in plant species from drier habitats and in species with persistent leaves. Plant, Cell and Environment 28, 571–579. [Google Scholar]
- Genty B, Briantais J-M, Baker NR. 1989. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochimica et Biophysica Acta 990, 87–92. [Google Scholar]
- Gillon JS, Yakir D. 2000. Internal conductance to CO2 diffusion and C18OO discrimination in C3 leaves. Plant Physiology 123, 201–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassi G, Magnani F. 2005. Stomatal, mesophyll conductance and biochemical limitations to photosynthesis as affected by drought and leaf ontogeny in ash and oak trees. Plant, Cell and Environment 28, 834–849. [Google Scholar]
- Hanba YT, Miyazawa SI, Kogami H, Terashima I. 2001. Effects of leaf age on internal CO2 transfer conductance and photosynthesis in tree species having different types of shoot phenology. Australian Journal of Plant Physiology 28, 1075–1084. [Google Scholar]
- Hanba YT, Miyazawa S-I, Terashima I. 1999. The influence of leaf thickness on the CO2 transfer conductance and leaf stable carbon isotope ratio for some evergreen tree species in Japanese warm-temperate forests. Functional Ecology 13, 632–639. [Google Scholar]
- Harley PC, Loreto F, Di Marco G, Sharkey TD. 1992. Theoretical considerations when estimating the mesophyll conductance to CO2 flux by analysis of the response of photosynthesis to CO2. Plant Physiology 98, 1429–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassiotou F, Ludwig M, Renton M, Veneklaas EJ, Evans JR. 2009. Influence of leaf dry mass per area, CO2, and irradiance on mesophyll conductance in sclerophylls. Journal of Experimental Botany 60, 2303–2314. [DOI] [PubMed] [Google Scholar]
- Hemsley AR, Poole I. 2004. The evolution of plant physiology. London: Academic Press. [Google Scholar]
- Hollingsworth PM, Forrest LL, Spouge JL, et al. 2009. A DNA barcode for land plants. Proceedings of the National Academy of Sciences of the United States of America 106, 12794–12797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James JC, Grace J, Hoad SP. 1994. Growth and photosynthesis of Pinus sylvestris at its altitudinal limit in Scotland. Journal of Ecology 82, 297–306. [Google Scholar]
- Knapp M, Mudaliar R, Havell D, Wagstaff SJ, Lockhart PJ. 2007. The drowning of New Zealand and the problem of Agathis. Systematic Biology 56, 862–870. [DOI] [PubMed] [Google Scholar]
- Lang D, Weiche B, Timmerhaus G, Richardt S, Riaño-Pachón DM, Corrêa LG, Reski R, Mueller-Roeber B, Rensing SA. 2010. Genome-wide phylogenetic comparative analysis of plant transcriptional regulation: a timeline of loss, gain, expansion, and correlation with complexity. Genome Biology and Evolution 2, 488–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Porta N, Bertamini M, Nedunchezhian N, Muthuchelian K. 2006. Photosynthetic changes that occur during aging of cypress (Cupressus sempervirens L.) needles. Photosynthetica 44, 555–560. [Google Scholar]
- McAdam SA, Brodribb TJ. 2015. The evolution of mechanisms driving the stomatal response to vapor pressure deficit. Plant Physiology 167, 833–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magallón SA, Sanderson MJ. 2005. Angiosperm divergence times: the effect of genes, codon positions, and time constraints. Evolution 59, 1653–1670. [DOI] [PubMed] [Google Scholar]
- Manter DK, Kerrigan J. 2004. A/Ci curve analysis across a range of woody plant species: influence of regression analysis parameters and mesophyll conductance. Journal of Experimental Botany 55, 2581–2588. [DOI] [PubMed] [Google Scholar]
- Mullin LP, Sillett SC, Koch GW, Tu KP, Antoine ME. 2009. Physiological consequences of height-related morphological variation in Sequoia sempervirens foliage. Tree Physiology 29, 999–1010. [DOI] [PubMed] [Google Scholar]
- Niinemets Ü. 1999. Research review. Components of leaf dry mass per area—thickness and density —alter leaf photosynthetic capacity in reverse directions in woody plants. The New Phytologist 144, 35–47. [Google Scholar]
- Niinemets Ü. 2016. Does the touch of cold make evergreen leaves tougher? Tree Physiology 36, 267–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niinemets Ü, Cescatti A, Rodehiero M, Tosens T. 2005. Leaf internal diffusion conductance limits photosynthesis more strongly in older leaves of Mediterranean evergreen broad-leaved species. Plant, Cell and Environment 28, 1552–1566. [Google Scholar]
- Niinemets Ü, Cescatti A, Rodeghiero M, Tosens T. 2006. Complex adjustments of photosynthetic potentials and internal diffusion conductance to current and previous light availabilities and leaf age in Mediterranean evergreen species Quercus ilex. Plant, Cell and Environment 29, 1159–1178. [DOI] [PubMed] [Google Scholar]
- Niinemets Ü, Díaz-Espejo A, Flexas J, Galmés J, Warren CR. 2009a Importance of mesophyll diffusion conductance in estimation of plant photosynthesis in the field. Journal of Experimental Botany 60, 2271–2282. [DOI] [PubMed] [Google Scholar]
- Niinemets Ü, Díaz-Espejo A, Flexas J, Galmés J, Warren CR. 2009b Role of mesophyll diffusion conductance in constraining potential photosynthetic productivity in the field. Journal of Experimental Botany 60, 2249–2270. [DOI] [PubMed] [Google Scholar]
- Niinemets Ü, Flexas J, Peñuelas J. 2011. Evergreens favored by higher responsiveness to increased CO2. Trends in Ecology and Evolution 26, 136–142. [DOI] [PubMed] [Google Scholar]
- Niinemets Ü, Lukjanova A, Turnbull MH, Sparrow AD. 2007. Plasticity in mesophyll volume fraction modulates light-acclimation in needle photosynthesis in two pines. Tree Physiology 27, 1137–1151. [DOI] [PubMed] [Google Scholar]
- Niinemets Ü, Reichstein M. 2003. Controls on the emission of plant volatiles through stomata: Differential sensitivity of emission rates to stomatal closure explained. Journal of Geophysical Research 108, 4208. [Google Scholar]
- Niinemets Ü, Wright IJ, Evans JR. 2009c Leaf mesophyll diffusion conductance in 35 Australian sclerophylls covering a broad range of foliage structural and physiological variation. Journal of Experimental Botany 60, 2433–2449. [DOI] [PubMed] [Google Scholar]
- Nobel PS. 1991. Physicochemical and environmental plant physiology. San Diego, CA, USA: Academic Press. [Google Scholar]
- Norstog K, Nicholls T. 1997. The biology of the cycads. Ithaca, NY, USA: Cornell University Press. [Google Scholar]
- Pardo A, Aranjuelo I, Biel C, Savé R, Azcón-Bieto J, Nogués S. 2009. Effects of long-term exposure to elevated CO2 conditions in slow-growing plants using a 12C-enriched CO2labelling technique. Rapid Communications in Mass Spectrometry 23, 282–290. [DOI] [PubMed] [Google Scholar]
- Peguero-Pina JJ, Flexas J, Galmés J, Niinemets U, Sancho-Knapik D, Barredo G, Villarroya D, Gil-Pelegrín E. 2012. Leaf anatomical properties in relation to differences in mesophyll conductance to CO2 and photosynthesis in two related Mediterranean Abies species. Plant, Cell and Environment 35, 2121–2129. [DOI] [PubMed] [Google Scholar]
- Peguero-Pina JJ, Sancho-Knapik D, Flexas J, Galmés J, Niinemets Ü, Gil-Pelegrín E. 2016. Light acclimation of photosynthesis in two closely related firs (Abies pinsapo Boiss. and Abies alba Mill.): the role of leaf anatomy and mesophyll conductance to CO2. Tree Physiology 36, 300–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piel C, Frak E, Le Roux X, Genty B. 2002. Effect of local irradiance on CO2 transfer conductance of mesophyll in walnut. Journal of Experimental Botany 53, 2423–2430. [DOI] [PubMed] [Google Scholar]
- Poorter H, Niinemets U, Poorter L, Wright IJ, Villar R. 2009. Causes and consequences of variation in leaf mass per area (LMA): a meta-analysis. The New Phytologist 182, 565–588. [DOI] [PubMed] [Google Scholar]
- Pryer KM, Schuettpelz E, Wolf PG, Schneider H, Smith AR, Cranfill R. 2004. Phylogeny and evolution of ferns (monilophytes) with a focus on the early leptosporangiate divergences. American Journal of Botany 91, 1582–1598. [DOI] [PubMed] [Google Scholar]
- Reich P, Kloeppel B, Ellsworth D, Walters M. 1995. Different photosynthesis-nitrogen relations in deciduous hardwood and evergreen coniferous tree species. Oecologia 104, 24–30. [DOI] [PubMed] [Google Scholar]
- Rodeghiero M, Niinemets U, Cescatti A. 2007. Major diffusion leaks of clamp-on leaf cuvettes still unaccounted: how erroneous are the estimates of Farquhar et al. model parameters? Plant, Cell and Environment 30, 1006–1022. [DOI] [PubMed] [Google Scholar]
- Rydin C, Pedersen KR, Friis EM. 2004. On the evolutionary history of Ephedra: Cretaceous fossils and extant molecules. Proceedings of the National Academy of Sciences of the United States of America 101, 16571–16576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipley B, Lechowicz MJ, Wright I, Reich PB. 2006. Fundamental trade-offs generating the worldwide leaf economics spectrum. Ecology 87, 535–541. [DOI] [PubMed] [Google Scholar]
- Slaton MR, Smith WK. 2002. Mesophyll architecture and cell exposure to intercellular air space in alpine, desert, and forest species. International Journal of Plant Sciences 163, 937–948. [Google Scholar]
- Stewart WN. 1993. Paleobotany and the evolution of plants. New York: Cambridge University Press. [Google Scholar]
- Syvertsen JP, Lloyd J, McConchie C, Kriedemann PE, Farquhar GD. 1995. On the relationship between leaf anatomy and CO2 diffusion through the mesophyll of hypostomatous leaves. Plant, Cell and Environment 18, 149–157. [Google Scholar]
- Tamura K, Nei M. 1993. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Molecular Biology and Evolution 10, 512–526. [DOI] [PubMed] [Google Scholar]
- Tamura K, Nei M, Kumar S. 2004. Prospects for inferring very large phylogenies by using the neighbor-joining method. Proceedings of the National Academy of Sciences of the United States of America 101, 11030–11035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. 2013. MEGA6: molecular evolutionary genetics analysis version 6.0. Molecular Biology and Evolution 30, 2725–2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terashima I, Hanba YT, Tazoe Y, Vyas P, Yano S. 2006. Irradiance and phenotype: comparative eco-development of sun and shade leaves in relation to photosynthetic CO2 diffusion. Journal of Experimental Botany 57, 343–354. [DOI] [PubMed] [Google Scholar]
- Terashima I, Hanba YT, Tholen D, Niinemets Ü. 2011. Leaf functional anatomy in relation to photosynthesis. Plant Physiology 155, 108–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tholen D, Ethier G, Genty B, Pepin S, Zhu XG. 2012. Variable mesophyll conductance revisited: theoretical background and experimental implications. Plant, Cell and Environment 35, 2087–2103. [DOI] [PubMed] [Google Scholar]
- Tomás M, Flexas J, Copolovici L, et al. 2013. Importance of leaf anatomy in determining mesophyll diffusion conductance to CO2 across species: quantitative limitations and scaling up by models. Journal of Experimental Botany 64, 2269–2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosens T, Niinemets U, Vislap V, Eichelmann H, Castro Díez P. 2012a Developmental changes in mesophyll diffusion conductance and photosynthetic capacity under different light and water availabilities in Populus tremula: how structure constrains function. Plant, Cell and Environment 35, 839–856. [DOI] [PubMed] [Google Scholar]
- Tosens T, Niinemets Ü, Westoby M, Wright IJ. 2012b Anatomical basis of variation in mesophyll resistance in eastern Australian sclerophylls: news of a long and winding path. Journal of Experimental Botany 63, 5105–5119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosens T, Nishida K, Gago J, et al. 2016. The photosynthetic capacity in 35 ferns and fern allies: mesophyll CO2 diffusion as a key trait. The New Phytologist 209, 1576–1590. [DOI] [PubMed] [Google Scholar]
- Villar R, Held AA, Merino J. 1995. Dark leaf respiration in light and darkness of an evergreen and a deciduous plant species. Plant Physiology 107, 421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Caemmerer S, Farquhar GD. 1981. Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 153, 376–387. [DOI] [PubMed] [Google Scholar]
- Wang XQ, Tank DC, Sang T. 2000. Phylogeny and divergence times in Pinaceae: evidence from three genomes. Molecular Biology and Evolution 17, 773–781. [DOI] [PubMed] [Google Scholar]
- Warren CR. 2008. Stand aside stomata, another actor deserves centre stage: the forgotten role of the internal conductance to CO2 transfer. Journal of Experimental Botany 59, 1475–1487. [DOI] [PubMed] [Google Scholar]
- Warren CR, Ethier GJ, Livingston NJ, Grant NJ, Turpin DH, Harrison DL, Black TA. 2003. Transfer conductance in second growth Douglas-fir (Pseudotsuga menziesii (Mirb.) Franco) canopies. Plant, Cell and Environment 26, 1215–1227. [Google Scholar]
- Wright IJ, Reich PB, Westoby M, et al. 2004. The worldwide leaf economics spectrum. Nature 428, 821–827. [DOI] [PubMed] [Google Scholar]
- Wright IJ, Westoby M, Reich PB. 2002. Convergence towards higher leaf mass per area in dry and nutrient-poor habitats has different consequences for leaf life span. Journal of Ecology 90, 534–543. [Google Scholar]
- Zhang YJ, Cao KF, Sack L, Li N, Wei XM, Goldstein G. 2015. Extending the generality of leaf economic design principles in the cycads, an ancient lineage. The New Phytologist 206, 817–829. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.