Abstract
Even though elderly populations lack visible or other clinical signs of inflammation, their serum cytokine and C reactive protein levels typically are elevated. However, the origin of age-associated systemic inflammation is unknown. Our previous studies showed that abnormalities in epidermal function provoke cutaneous inflammation, and because intrinsically aged skin displays compromised permeability barrier homeostasis, as well as reduced stratum corneum hydration, we hypothesized here that epidermal dysfunction could contribute to the elevations in serum cytokines in the elderly. Our results show first that acute disruption of the epidermal permeability barrier in young mice led not only to a rapid increase in cutaneous cytokine mRNA expression, but also an increase in serum cytokine levels. Second, cytokine levels in both the skin and serum increased in otherwise normal, aged mice (>12 months). Third, expression of TNFα and amyloid A mRNA levels increased in the epidermis, but not in the liver, in parallel with significant elevations in serum levels of cytokines. Fourth, disruption of the permeability barrier induced similar elevations in epidermal and serum cytokine levels in normal and athymic mice, suggesting the T cells play a negligible role in the elevations in cutaneous and serum inflammatory cytokines induced by epidermal dysfunction. Fifth, correction of epidermal function significantly reduced cytokine levels not only in the skin, but also in the serum of aged mice. Together, these results indicate that the sustained abnormalities in epidermal function in chronologically aged skin contribute to the elevated serum levels of inflammatory cytokines, potentially predisposing the elderly to the subsequent development or exacerbation of chronic inflammatory disorders.
Keywords: Aging, Inflammation, Permeability Barrier, Cytokines, Hydration, T cells
Introduction
Although the majority of aged humans display no clinical indications of inflammation, they typically demonstrate elevated serum cytokine levels (Kim et al., 2011; Mariani et al., 2006). While an increase in adipose tissue that commonly occurs with aging is well known to increase serum cytokine levels, whether other organs, such as the skin, might also contribute to these elevations are unknown. Yet, in order for a single organ with inapparent inflammation to cause systemic inflammation, it should account for a substantial portion of body size. In this respect, the skin qualifies, as it accounts for ≥15% of total body weight (Kanitakis, 2002). Previous studies from our group and others have demonstrated that either acute or chronic abrogation of epidermal permeability barrier function stimulates epidermal cytokine and chemokine production, inflammatory cell infiltration, and Langerhans’ cell maturation and proliferation (Katoh et al., 1997; Lin et al., 2013; Nishijima et al., 1997; Onoue et al., 2009; Proksch et al., 1996; Tsai et al., 1994; Wood et al., 1992, 1994 & 1997), while also predisposing to Staphylococcus aureus colonization of aged skin (Wanke et al., 2013). Finally, it should be noted that prolonged reductions in stratum corneum hydration also induce or aggravate cutaneous inflammation, independent of barrier disruption (Ashida et al., 2001 & 2003; Denda et al., 1998 & 2003).
Because previous studies from our group and others demonstrated that chronologically aged skin displays both compromised permeability homeostasis and reduced stratum corneum hydration, both of which increase cutaneous cytokine production (Choi et al., 2007; Ghadially et al., 1995; Kikuchi et al., 2003; Man et al., 2015; Tsai et al., 1994; Wood et al., 1992 & 1997), and increased susceptibility to infections (Laube, 2004), we hypothesized that epidermal dysfunction-induced cutaneous inflammation could lead to elevations in serum cytokines. In support of our hypothesis, both psoriasis and atopic dermatitis display prominent abnormalities in epidermal function, which has been proposed to ‘drive’ the inflammation of these disorders (Elias and Steinhoff, 2008; Man et al., 2015, Sano, 2015), and elevated serum cytokine levels correlate with severity of these disorders (Arican et al., 2005; Jacob et al., 2003; Yamamoto et al., 2013; Yoshizawa et al., 2002). However, the origin of serum cytokines in these two inflammatory dermatoses is controversial since these disorders are considered to be T cell-mediated, immune diseases. In the present study, we assessed whether the epidermal dysfunction, which inevitably accompanies intrinsically aged skin, could account for or contribute to the elevated levels of serum cytokines in the elderly. We present evidence here that age-related epidermal dysfunction leads to elevations in serum inflammatory cytokines in aged mice; and conversely, that correction of epidermal functional abnormalities reduces both cutaneous and serum cytokine levels. Together, these studies raise the intriguing possibility that epidermal functional abnormalities could contribute to the development of certain chronic age-associated systemic disorders.
Results
Acute Disruption of the Epidermal Permeability Barrier Increases Cutaneous and Serum Inflammatory Cytokines in Young Mice
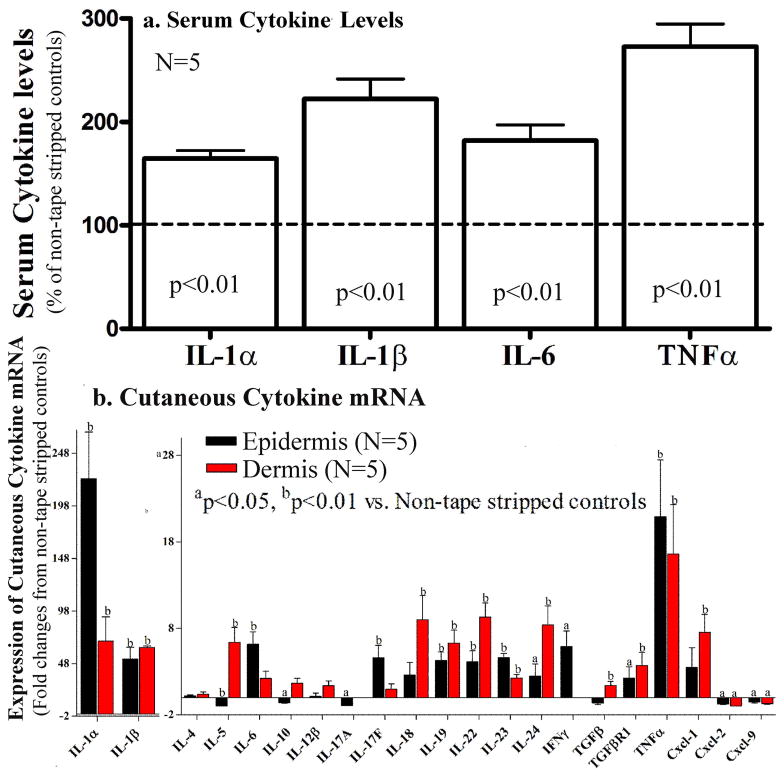
Prior studies from our group and others have demonstrated that disruption of the epidermal permeability barrier increases epidermal cytokine production, eventually inducing dermal inflammation in murine models (Lin et al., 2013; Proksch et al., 1996; Tsai et al., 1994; Wood et al., 1992, 1994 & 1997). Yet, whether acute barrier disruption elevates serum levels of inflammatory cytokines is unknown. To test our hypothesis that epidermal dysfunction can induce an increase in serum inflammatory cytokine levels, we first determined whether acute abrogation of epidermal permeability barrier function increases serum levels of cytokines in young mice. As seen in Figure 1a, serum levels of IL-1α, IL-1β, IL-6, and TNFα increased significantly three hours after acute barrier abrogation induced by repeated tape-stripping. In a separate experiment, we assessed whether the levels of serum amyloid A, a well-accepted marker of acute systemic inflammation, changes following barrier disruption. Our results show that the serum levels of amyloid A dramatically increased after acute barrier disruption (by over 200% and 100% at two and six hours, respectively; P<0.001 for both time points).
Figure 1. Acute Barrier Disruption Induces Not only Cutaneous, but also Systemic Inflammation in Normal Mice.
Both flanks of 8-week old C57BL/6J mice were repeatedly tape-stripped until in transepidermal water loss rates increased 4-fold. One group of non-tape stripped mice served as normal controls. Three hours after tape-stripping, blood was collected for cytokine analysis (see Methods). Skin samples were collected for determination of cytokine mRNA levels in the dermis and epidermis, separated by brief heating (Feingold et al., 1991). Fig 1a: Serum cytokine levels, measured by ELISA assay; 1b: Changes in mRNA levels, measured by Q-PCR in the dermis and epidermis 3 hrs after acute barrier disruption. Data were normalized to non-tape stripped normal controls, setting normal controls as 100% (dotted line in Fig 1b). A Mann Whitney two-tailed test was used to determine the significances between tape-stripped vs. non-tape stripped mice.
Since our previous studies focused on the influence of epidermal permeability disruption on epidermal cytokine production (Tsai et al., 1994; Wood et al., 1992, 1994 & 1997), we next determined whether disruption of epidermal permeability barrier upregulates cytokine expression not only in the epidermis, but also in the dermis. In agreement with our previous findings, barrier disruption induced a significant increase in the mRNA levels of IL-1α, β, IL-17F, IL-19, 22, 23, and TNFα in the epidermis, and further that it also upregulated expression of these cytokine mRNAs in the dermis (Red blocks in Fig 1b). In contrast, epidermal mRNA levels of certain Th2 cytokines, i,e., IL-4 and IL-5, as well as IL-10, either did not change or decreased (Fig 1b), and mRNA level of T-cell chemoattractant, CXCL-9, decreased in both the dermis and epidermis following barrier disruption (Fig 1b). Taken together, these results demonstrate that acute abrogation of the epidermal permeability barrier function induces an increase in inflammatory cytokines in both the skin and serum.
Aged Mice Display Increased Levels of Cutaneous and Serum Inflammatory Cytokines
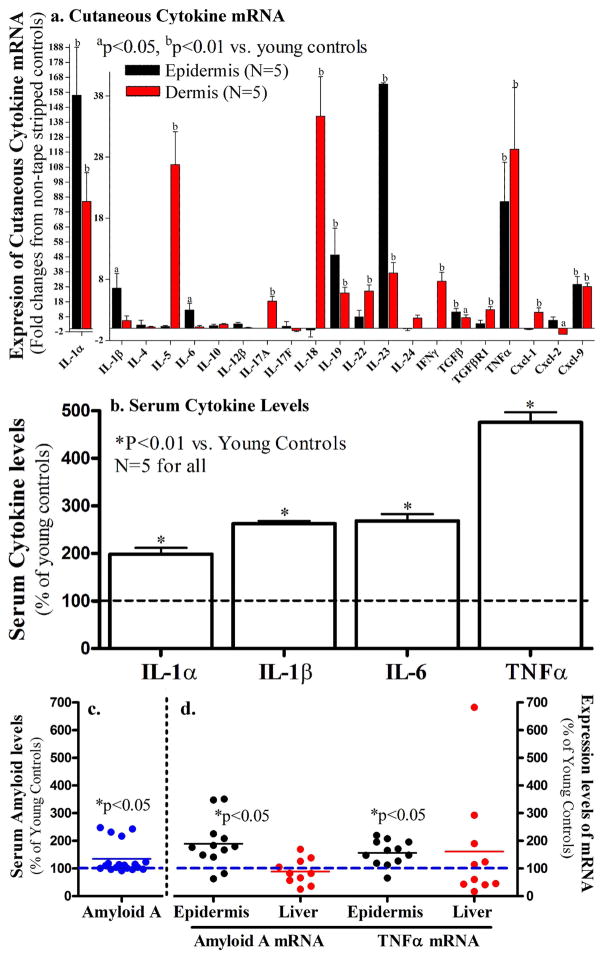
Both aged human and mouse epidermis exhibit functional abnormalities, including reduced stratum corneum hydration, and compromised epidermal permeability barrier homeostasis, which first appears ≥50 years in humans (12–15 months in mice) (Choi et al., 2007; Ghadially et al., 1995). To examine whether age-associated epidermal dysfunction is accompanied by elevations in cutaneous and serum inflammatory cytokines, we next assessed changes in pro-inflammatory cytokine levels in both the skin and serum of otherwise normal-appearing, aged mice under basal condition. As shown in Figure 2a, the expression levels of mRNA for IL-1α, IL-19, 23, TGFβ, TNFα and CXCL-9 increased significantly in both the dermis and epidermis of the 12-month old vs. 8-week old mice (red blocks in Fig 2a). Consistent with these increases in mRNA levels, epidermal TNFα protein levels also increased 5-fold in aged mice vs. young mice (0.87 ± 0.16 in young vs. 5.33 ± 0.56 in aged; p=0.0016). In contrast, the mRNA levels of epidermal IFNγ, a cytokine primarily produced by natural killer T cells, diminished in the aged mice (Fig 2a). Similarly, the levels of dermal CXCL-2, a chemokine predominantly secreted by monocytes and macrophages, also decreased in aged mice. To determine whether aged skin displays increased infiltration of inflammatory cells, immunostaining of various inflammatory cells were performed. As seen in Supplemental Fig 1, there were no differences in cutaneous inflammatory infiltrates between young and aged mice. Taken together, these results indicate that otherwise normal-appearing aged skin displays evidence of increased cytokine expression, but no inflammatory cell infiltration.
Figure 2. Cutaneous and Serum Cytokines Increase in Aged Mice.
Both flanks of 12-month old C57BL/6J mice were used in this study. The dermis and epidermis were separated by heat (Feingold et al., 1991). Fig 2a: Expression levels of cytokine mRNA in the skin; Fig 2b: Serum cytokine levels; Fig 2c: Serum amyloid A levels in 7-week old vs. 12-month old mice. Fig 2d: mRNA levels for amyloid A and TNFα in the liver (red dots) and the epidermis (black dots) of 12-month old mice. Data were normalized to normal young controls, setting normal young controls as 100% (dotted lines). A Mann Whitney two-tailed test was used to determine the significances between aged and young mice. P values were vs. the non-tape stripped normal controls.
We next determined whether the increased cutaneous cytokine expression in aged skin is accompanied by increased levels of serum cytokines. The serum levels of several cytokines were compared in 12-month old vs. 8-week old C57BL/6J mice. In comparison with young mice, the serum levels of IL-1α, IL-1β, IL-6 and TNFα were elevated significantly (Fig 2b). Moreover, the levels of serum amyloid A also increased by >30% in 12-month old vs. 8-week old mice (14.5 ± 0.5 vs. 19.5 ± 1.9; p=0.031, Fig 2c).
To determine whether the serum cytokines and amyloid A could originate from the skin, rather than distal organs, such as the liver, a known source of serum amyloid A (Upragarin et al., 2005), we next compared mRNA levels of amyloid A and TNFα in the epidermis and liver of aged mice. While mRNA levels for both amyloid A and TNFα increased in aged epidermis, neither of them increased in the liver (Fid 2d), suggesting that the skin could be a major source of serum inflammatory markers, possibly in association with epidermal dysfunction in aged mice. Together, these results indicate that normal-appearing, aged mice exhibit an increase in both cutaneous and serum cytokines, possibly linked to epidermal dysfunction.
Acute Disruption of the Epidermal Permeability Barrier Increases both Cutaneous and Serum Inflammatory Cytokines in Athymic Mice
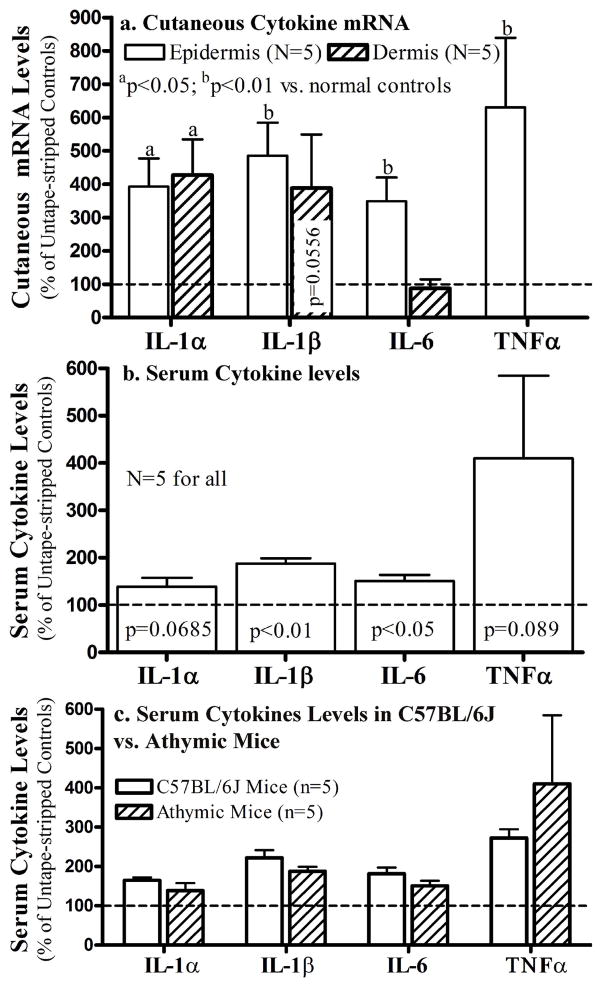
Although our results clearly show that epidermal dysfunction is accompanied with increased serum inflammatory cytokines, the cellular origin of these cytokines is still obscure. To determine whether T cells, one of the major non-epithelial sources of cytokines, contribute to the epidermal dysfunction-induced elevations in cutaneous and serum inflammatory cytokines, we next compared cutaneous cytokine mRNA and serum cytokine levels in normal C57BL/6J vs. athymic mice following barrier disruption. As shown in Fig 3a, epidermal mRNA levels of all four cytokines dramatically increased 3 hours after barrier disruption, while TNFα mRNA was undetectable in the dermis of athymic mice, suggesting the importance of T cells in the origin of dermal TNFα production. Moreover, serum levels of cytokines increased significantly, and to an extent comparable to normal mice, in spite of T cell deficiency (Fig 3b,c). These results strongly suggest that T cells are not a major contributor to epidermal dysfunction-induced increases in either cutaneous or serum inflammatory cytokines.
Figure 3. Acute Barrier Disruption Induces Increases in Cutaneous and Systemic Cytokines in Athymic Mice.
Both flanks of 8-week old athymic mice were repeatedly tape-stripped until transepidermal water loss rates ≥4-fold increases. Groups of mice and experimental methods were the same as that in C57BL/6J mice. Fig 3a: Changes in mRNA levels in the dermis and epidermis; Fig 3b: Serum cytokine levels 3 hrs after barrier disruption. Fig 3c: Changes in serum cytokine levels in athymic vs. C57BL/6J mice following acute barrier disruption by sequential tape stripping. Data were normalized to non-tape stripped normal controls, setting normal controls as 100% (dotted line). A Mann Whitney two-tailed test was used to determine the significances between tape-stripped vs. non-tape stripped mice. P values were vs. the non-tape stripped normal controls.
Improvements in Epidermal Function Reduce Cutaneous and Serum Cytokines in Aged Mice
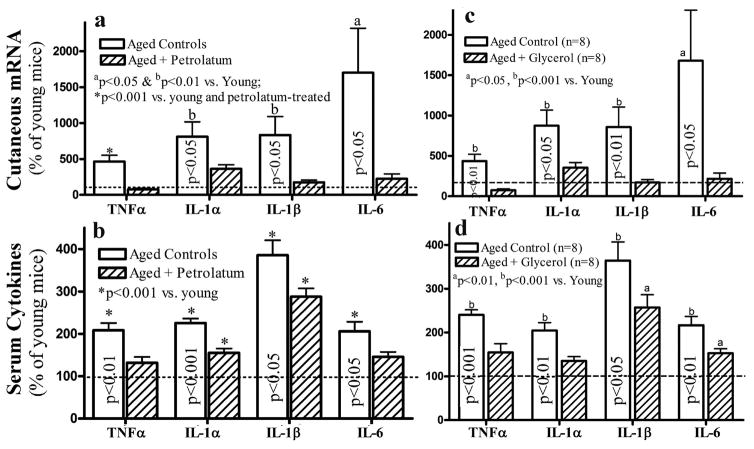
Our prior studies showed that correction of chronic abnormalities in epidermal permeability barrier function lowers epidermal cytokine levels in murine skin (Wood et al., 1994), suggesting a regulatory role of the epidermal permeability barrier in cutaneous inflammation. Because aged skin displays compromised epidermal permeability barrier and reduced stratum corneum hydration (Choi et al., 2007; Ghadially et al., 1995; Man et al., 2009 & 2015), should the increased inflammation in serum and the epidermis of aged mice be primarily due to epidermal dysfunction, correction of the epidermal function should reduce levels of inflammatory markers not only in the epidermis, both also in the serum of aged mice. Therefore, we next determined whether correction of the epidermal functional abnormalities with topical petrolatum lowers cutaneous and serum cytokine levels in aged mice. We initially treated 12-month old C57BL/6J mice, which exhibit epidermal permeability barrier dysfunction (Ghadially et al., 1995; Man et al., 2015), with topical petrolatum, an agent previously shown to improve epidermal permeability barrier function in aged murine skin (Ghadially et al., 1992; Mao-Qiang et al., 1995), three times daily for 10 days. As seen in Figure 4a, topical petrolatum treatment lowered mRNA levels of cutaneous cytokines to levels comparable to those that occur in the skin of young mice (dotted line, setting levels in young mice as 100%). Consistent with these changes in cytokine mRNA, topical petrolatum also induced a 42% reduction in cutaneous TNFα protein level in aged skin (p<0.05). Finally, serum levels of the same cytokines also significantly declined following topical petrolatum treatments (Fig 4b).
Figure 4. Improvements in Epidermal Function Alleviate Not only Cutaneous, but also Systemic Inflammation.
12-month old C57BL/6J mice were treated topically with either petrolatum or glycerol twice-daily for 10 days, followed by collection of blood and skin for analyses of serum cytokines and cutaneous cytokine mRNA. Fig 4a and c: Expression levels of cutaneous pro-inflammatory cytokine mRNA levels in aged mice following treatment with petrolatum or glycerol; Fig 4b and d: Changes in serum cytokine levels in aged mice treated with petrolatum and glycerol, respectively. The data are expressed as % of untreated young mice, setting the level of young mice as 100% (dotted line). One way ANOVA was used to determine the statistical significances. The significances are indicated in the figures. N=8 for all groups. Significances indicated in column bars represent the differences between treated vs. untreated aged mice.
To further confirm the regulatory role of epidermal dysfunction in serum inflammatory cytokines, we next treated the 12-month old mice topically with glycerol, another agent that improves epidermal permeability barrier function and stratum corneum hydration (Atrux-Tallau et al., 2010; Fluhr et al., 1999 & 2003). As shown in Figure 4c, topical glycerol treatments significantly reduced the mRNA levels of several cytokines in aged mouse skin. In parallel, serum levels of the same cytokines also declined significantly (Figure 4d). Collectively, these results show first that the increase in pro-inflammatory cytokines in aged skin and serum can be ascribed to co-existent abnormalities in epidermal function; and second, that strategies that improve epidermal function in aged skin can attenuate the increases in both serum and cutaneous cytokines.
Discussion
In the present study, we found higher level of pro-inflammatory cytokines in both the intact skin and serum of aged mice in comparison with young mice. The increased production of cytokines by aged epidermis likely reflects homeostatic signaling responses to the co-existent permeability barrier abnormality, because: 1) an increase in IL-1α levels improves epidermal permeability barrier function, by stimulating lipid synthesis not only in aged skin, but also in barrier-compromised young skin (Barland et al., 2004; Jung et al., 2011); 2) both exogenous IL-1α and TNFα accelerate permeability barrier formation during fetal development in vitro (Jiang et al., 2009); and 3) IL-6 deficiency delays epidermal permeability barrier recovery in mice, while exogenous IL-6 improves permeability barrier function in vivo and in vitro (Jiang et al., 2010; Wang et al., 2001). While permeability barrier disruption increases epidermal cytokine production and release (Tsai et al., 1994; Wood et al., 1992 & 1997), correction of the barrier abnormalities by occlusion conversely decreases epidermal cytokine levels (Tsai et al., 1994; Wood et al., 1994 & 1997). Thus, increased cytokine production in aged mouse skin, which begins to demonstrate functional abnormalities by 12-month of age, likely reflects a compensatory homeostatic response to compromised permeability barrier function. In addition to a permeability barrier defect, aged skin displays low stratum corneum hydration, which likely also contributes to cutaneous inflammation, because reductions in stratum corneum hydration alone increase cutaneous cytokine expression (Denda et al., 1998), while conversely improvements in stratum corneum hydration relieve cutaneous inflammation (Kikuchi et al., 2003; Xu et al., 2014). Therefore, increased levels of epidermal cytokines likely result from both the compromised permeability barrier function and reduced stratum corneum hydration levels in aged skin.
While previous studies demonstrate that both keratinocytes and fibroblasts can produce proinflammatory cytokines upon stimulation (Ablett et al., 2003; Huleihel et al., 1993; Köck et al., 1990; Mustafa et al., 2000; Urbanski et al., 1990; Wolf et al., 2013), our results further suggest that the skin is likely an important contributor to the age-associated increase in serum inflammatory cytokines. While aged mice display elevated serum amyloid A and TNFα levels, mRNA levels of both amyloid A and TNFα increase only in the aged skin, but not in the liver, a putative source of serum amyloid A (Upragarin et al., 2005). It is also worth noting that T cells negligibly influence serum inflammatory cytokines following permeability disruption. Because aged skin exhibits reductions in the densities of Langerhans cells, Thy-1+ dendritic cells and eosinophils (Gunin, et al., 2011; Sprecher et al., 1990; Xu et al., 2012), as well as decreased production of TNFα by macrophages (Agius et al., 2009), the age-associated increase in serum inflammatory cytokines likely instead originate from aged keratinocytes and fibroblasts, both of which are known cytokine producers (Doles et al., 2012; Wolf et al., 2012). However, the increased cytokines could also be ascribed in part to cutaneous mast cells, because aged skin exhibits a greater than normal density of mast cells (Gunin, et al., 2011). Nevertheless, further studies will be required to delineate the extent to which different cutaneous cell types contribute to the age-associated increases in serum and cutaneous cytokines.
The prevention and treatment of inflammation associated systemic disorders in the elderly have been a challenge in part due to its uncertain origins. We demonstrate here that aged mice, as in otherwise normal, aged humans (Banerjee et al., 2011; Kim et al., 2011; Mariani et al., 2006), exhibit elevated levels of serum cytokines. Systemic inflammation is a well-known complication of inflammatory skin disorders, such as atopic dermatitis (Yoshizawa et al., 2002) and psoriasis (Jacob et al., 2003; Yamamoto et al., 2013). Pertinently, serum cytokine levels also increase during cold seasons (Valmadrid et al., 2000; Wannamethee et al., 2011), when the epidermal permeability barrier is further stressed by both reduced environmental humidities and low temperatures, which compromise epidermal permeability barrier homeostasis (Denda et al., 1999; Halkier-Sørensen et al., 1995; Lin et al., 2009; Muizzuddin et al., 2013). Finally, the link between epidermal dysfunction and serum inflammatory cytokines is most convincingly demonstrated by our observation that correction of epidermal functional abnormality lowers the levels of serum inflammatory cytokines in aged mice. Indeed, we demonstrated here that improvements in epidermal permeability barrier function and hydration in aged skin reduce levels of pro-inflammatory cytokines in both the epidermis and serum. Collectively, the bulk of evidence suggests that permeability barrier dysfunction could contribute to the development of systemic inflammation in aged humans.
Inflammatory skin disorders (atopic dermatitis and psoriasis) are closely associated with the co-morbidity for certain systemic disorders, including cardiovascular disease and diabetes (Dregan et al., 2014; Donath, 2014; Horreau et al., 2013; Marques-Vidal et al., 2013; Silverberg et al., 2015). Acute disruption in epidermal barrier function regulates homeostatic metabolic responses in the underlying epidermis, in part through cytokine and growth factor signaling (Elias et al., 1999). Low stratum corneum hydration, another feature of aged skin, also induces cutaneous inflammation (Denda et al., 1998). However, when these abnormalities persist, epidermal cytokine production eventually produces cutaneous inflammation, which we now show could lead to increase in serum inflammatory cytokines. It is likely that epidermal dysfunction could play a role, at least in part, in the pathogenesis of chronic systemic diseases, because 1) both cardiovascular disease and diabetes, which impact each other (Muizzuddin et al., 2013; Valmadrid et al., 2000) , occur primarily in aged populations, who inevitably display a defective epidermal permeability barrier (Choi et al., 2007; Ghadially et al., 1995) , with higher cytokine levels (Kim et al., 2011; Mariani et al., 2006); 2) defective skin barrier function is associated with type 2 diabetes (http://www.oncologypractice.com/single-view/mutations-in-the-skin-barrier-correlate-with-increased-type-2-diabetes-risk.html). However, further clinical studies are required to determine whether enhancement of epidermal functions (permeability barrier and/or stratum corneum hydration) could alleviate/prevent chronic inflammation and certain inflammation-associated disorders in elderly humans.
Materials and Methods
Materials
For the studies to determine whether acute barrier disruption influences serum amyloid A and TNFα levels, and their mRNA in the epidermis and liver, C57BL/6J mice were purchased from Charles River Laboratories (Wilmington, MA, USA) (results in Figures 2c and d). For the rest of studies in this manuscript, animals were from Laboratory Animal Center, Academy of Military Medical Science (Beijing, China) and housed for two weeks in the same room at the animal facility of Tianjin Medical University, China. Cytokine ELISA kits were purchased from R & D Systems, Minneapolis, MN, USA. Mouse amyloid A ELISA kit was from Life Technologies (Grand Island, NY, USA). All primers for cytokines were from Elim Biopharmaceuticals (Hayward, CA, USA). Anti-mouse TNFα monoclonal antibody was from Santa Cruz Biotechnology (Dallas, TX, USA). Petrolatum jelly (made by Shanghai Hualing Health Machinery Plant, Xuhui, Shanghai, China) and glycerol (made by Shanghai Huayin Daily Article Co., Ltd, Minhang, Shanghai, China) were purchased from local drug store in Tianjin, China.
Experimental protocols
All animal procedures were approved by the Animal Studies Subcommittee of the San Francisco Veterans Affairs Medical Center and Tianjin Medical University, and performed in accordance with their guidelines. For studies in aged mice, both flanks of 12-month old C57BL/6J mice were treated topically with 60 μl of glycerol or 200 mg of petrolatum twice daily for 10 days. Because the purpose of using glycerol and petrolatum was to explore the concept that improvement of epidermal function can alleviate cutaneous and systemic inflammation, controls should comprise treatment with agents that affect neither stratum corneum biophysical properties nor epidermal function, including innate immunity. But all topical agents impact either epidermal function or stratum corneum biophysical property. For example, repeatedly topical applications of water could change stratum corneum hydration, which can affect cytokine expression. Ethanol can alter skin microflora, which could also affect cutaneous immune system. Therefore, untreated 12-month old mice, as well as untreated 8 week old mice served as controls in this study. 18 hours after the last topical treatment, both skin and blood samples were collected. The epidermis was separated from dermis by heat separation method as we previously described (Mao-Qiang, et al., 1995). The expression levels of mRNA for proinflammatory cytokine in the epidermis were determined by qPCR. The primer inflammation is detailed in supplemental Table 1. The levels of serum cytokines were measured with ELISA from (R & D Systems, Minneapolis, MN). In acute barrier abrogation model, permeability barrier in both 8-week old C57BL/6J and athymic mice was disrupted by repeated applications of cellophane tape until 10-fold increase in TEWL levels (Tsai et al., 1994; Wood et al., 1992 & 1997). Skin and blood were collected 3 hours after barrier disruption.
Q-PCR for mRNA expression
Because we have shown that the peak increase in epidermal mRNA occurs 3 hr after a single insult to the barrier, and returns to basal levels by 24 hr (Wood et al., 1996). We anticipated that the barrier abrogation induced changes in serum cytokine levels would require longer time, we chose both 3 and 6 hr time points in pilot studies. Our results showed that serum cytokine levels already increased significantly at 3 hours after barrier disruption. Therefore, 3 hr time point was used in all studies in the present paper except otherwise stated. Total cutaneous RNA was isolated from mice as described above using TRI Reagent (Sigma). First strand cDNA was synthesized from 1μg of total RNA with the iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA). The real-time PCR contained 20 ng of reversed transcribed total RNA, 450 nM forward and reverse primers, and 10 μl of 2× LightCycler 480 SYBR Green I Master in a final volume of 20 μl in 96-well plates using Mx3000P7™ Real-time PCR System (Stratagene, La Jolla, CA). Quantification was performed by the comparative CT method with mouse 36B4 used for normalization. Primers sequences are listed in supplemental Table. Relative expression of the mRNAs compared to mRNA in normal young mice was calculated. Data are expressed as percentage of control (setting normal young controls as 100%) (Man et al., 2015).
Measurements of Serum Cytokines and Amyloid A
The levels of serum cytokines and amyloid A were measured using respective ELISA kit according to manufactures’ instruction. Relative expression of the cytokines and amyloid A compared to that in normal young control mice was calculated. Data are expressed as percentage of normal young controls (setting normal young controls as 100%).
Immunohistochemical Staining
Cutaneous infiltration of various inflammatory cells was assessed with immunohistoehcmical staining (antibodies are listed in Table S2).
Statistics
Data are expressed as the mean ± SEM. GraphPad Prism 4 software (San Diego, CA, USA) was used for all statistical analyses. Unpaired two-tailed student’s t-test with Mann Whitney test was used to determine the statistical significances when two groups were compared. One-Way ANOVA with Tukey’s multiple comparison was used when three groups were compared.
Supplementary Material
Acknowledgments
This work was supported in part by National Institutes of Health grant (PEM, AR19089; TMM, AR051930), China National Natural Science Foundation (LH, NSFC 81301360 and 81573075; GW, 81220108016) and the Science Foundation of Tianjin Medical University (LH, 2013KY06), and the resources and uses of facilities at the Veterans Affairs Medical Center, San Francisco, California, USA.
Footnotes
Conflicts of Interest
All authors declare no conflicts of interest except that invention disclosure has been filed for the concept of preventing/treating systemic disorders using strategies to improve epidermal functions.
Authors’ Contribution: LH, ED, JZ, GM and DL performed experiments; TMM, GW, KRF and PME interpreted data and critically reviewed the manuscript; MQM originated concept, designed experiment, analyzed and interpreted data, and wrote manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ablett E, Whiteman DC, Boyle GM, Green AC, Parsons PG. Induction of metallothionein in human skin by routine exposure to sunlight: evidence for a systemic response and enhanced induction at certain body sites. J Invest Dermatol. 2003;120:318–24. doi: 10.1046/j.1523-1747.2003.12025.x. [DOI] [PubMed] [Google Scholar]
- Agius E, Lacy KE, Vukmanovic-Stejic M, Jagger AL, Papageorgiou AP, Hall S, et al. Decreased TNF-alpha synthesis by macrophages restricts cutaneous immunosurveillance by memory CD4+ T cells during aging. J Exp Med. 2009;206:1929–40. doi: 10.1084/jem.20090896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arican O, Aral M, Sasmaz S, Ciragil P. Serum levels of TNF-alpha, IFN-gamma, IL-6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severity. Mediators Inflamm. 2005;2005(5):273–9. doi: 10.1155/MI.2005.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashida Y, Denda M. Dry environment increases mast cell number and histamine content in dermis in hairless mice. Br J Dermatol. 2003;149:240–7. doi: 10.1046/j.1365-2133.2003.05408.x. [DOI] [PubMed] [Google Scholar]
- Ashida Y, Ogo M, Denda M. Epidermal interleukin-1 alpha generation is amplified at low humidity: implications for the pathogenesis of inflammatory dermatoses. Br J Dermatol. 2001;144:238–43. doi: 10.1046/j.1365-2133.2001.04007.x. [DOI] [PubMed] [Google Scholar]
- Atrux-Tallau N, Romagny C, Padois K, Denis A, Haftek M, Falson F, et al. Effects of glycerol on human skin damaged by acute sodium lauryl sulphate treatment. Arch Dermatol Res. 2010;302:435–41. doi: 10.1007/s00403-009-1021-z. [DOI] [PubMed] [Google Scholar]
- Banerjee C, Ulloor J, Dillon EL, Dahodwala Q, Franklin B, Storer T, et al. Identification of serum biomarkers for aging and anabolic response. Immun Ageing. 2011;8:5. doi: 10.1186/1742-4933-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barland CO, Zettersten E, Brown BS, Ye J, Elias PM, Ghadially R. Imiquimod-induced interleukin-1 alpha stimulation improves barrier homeostasis in aged murine epidermis. J Invest Dermatol. 2004;122:330–6. doi: 10.1046/j.0022-202X.2004.22203.x. [DOI] [PubMed] [Google Scholar]
- Choi EH, Man MQ, Xu P, Xin S, Liu Z, Crumrine DA, et al. Stratum corneum acidification is impaired in moderately aged human and murine skin. J Invest Dermatol. 2007;127:2847–56. doi: 10.1038/sj.jid.5700913. [DOI] [PubMed] [Google Scholar]
- Denda M, Sato J, Tsuchiya T, Elias PM, Feingold KR. Low humidity stimulates epidermal DNA synthesis and amplifies the hyperproliferative response to barrier disruption: implication for seasonal exacerbations of inflammatory dermatoses. J Invest Dermatol. 1998;111:873–8. doi: 10.1046/j.1523-1747.1998.00364.x. [DOI] [PubMed] [Google Scholar]
- Denda M, Sokabe T, Fukumi-Tominaga T, Tominaga M. Effects of skin surface temperature on epidermal permeability barrier homeostasis. J Invest Dermatol. 2007;127:654–9. doi: 10.1038/sj.jid.5700590. [DOI] [PubMed] [Google Scholar]
- Doles J, Storer M, Cozzuto L, Roma G, Keyes WM. Age-associated inflammation inhibits epidermal stem cell function. Genes Dev. 2012;26:2144–53. doi: 10.1101/gad.192294.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donath MY. Targeting inflammation in the treatment of type 2 diabetes: time to start. Nat Rev Drug Discov. 2014;13:465–76. doi: 10.1038/nrd4275. [DOI] [PubMed] [Google Scholar]
- Dregan A, Charlton J, Chowienczyk P, Gulliford MC. Chronic inflammatory disorders and risk of type 2 diabetes mellitus, coronary heart disease, and stroke: a population-based cohort study. Circulation. 2014;130:837–44. doi: 10.1161/CIRCULATIONAHA.114.009990. [DOI] [PubMed] [Google Scholar]
- Elias PM, Steinhoff M. “Outside-to-inside” (and now back to “outside”) pathogenic mechanisms in atopic dermatitis. J Invest Dermatol. 2008;128:1067–70. doi: 10.1038/jid.2008.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias PM, Wood LC, Feingold KR. Epidermal pathogenesis of inflammatory dermatoses. Am J Contact Dermat. 1999;10:119–26. [PubMed] [Google Scholar]
- Feingold KR, Man MQ, Proksch E, Menon GK, Brown BE, Elias PM. The lovastatin-treated rodent: a new model of barrier disruption and epidermal hyperplasia. J Invest Dermatol. 1991;96:201–9. doi: 10.1111/1523-1747.ep12461153. [DOI] [PubMed] [Google Scholar]
- Fernandes R, Fink GD, Galligan JJ, Watts SW, Pereira-Hicks C, Watson RE, et al. Hypertension and Type II diabetes are not associated with visceral inflammation or vascular remodeling/fibrosis in obese women. FASEB J. 2016 Apr;30:738.6. [Google Scholar]
- Fluhr JW, Gloor M, Lehmann L, Lazzerini S, Distante F, Berardesca E. Glycerol accelerates recovery of barrier function in vivo. Acta Derm Venereol. 1999;79:418–21. doi: 10.1080/000155599750009825. [DOI] [PubMed] [Google Scholar]
- Fluhr JW, Mao-Qiang M, Brown BE, Wertz PW, Crumrine D, Sundberg JP, et al. Glycerol regulates stratum corneum hydration in sebaceous gland deficient (asebia) mice. J Invest Dermatol. 2003;120:728–37. doi: 10.1046/j.1523-1747.2003.12134.x. [DOI] [PubMed] [Google Scholar]
- Ghadially R, Brown BE, Sequeira-Martin SM, Feingold KR, Elias PM. The aged epidermal permeability barrier. Structural, functional, and lipid biochemical abnormalities in humans and a senescent murine model. J Clin Invest. 1995;95:2281–90. doi: 10.1172/JCI117919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghadially R, Halkier-Sorensen L, Elias PM. Effects of petrolatum on stratum corneum structure and function. J Am Acad Dermatol. 1992;26:387–96. doi: 10.1016/0190-9622(92)70060-s. [DOI] [PubMed] [Google Scholar]
- Ginaldi L, Di Benedetto MC, De Martinis M. Osteoporosis, inflammation and ageing. Immun Ageing. 2005;2:14. doi: 10.1186/1742-4933-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunin AG, Kornilova NK, Vasilieva OV, Petrov VV. Age-related changes in proliferation, the numbers of mast cells, eosinophils, and cd45-positive cells in human dermis. J Gerontol A Biol Sci Med Sci. 2011;66:385–92. doi: 10.1093/gerona/glq205. [DOI] [PubMed] [Google Scholar]
- Halkier-Sørensen L, Menon GK, Elias PM, Thestrup-Pedersen K, Feingold KR. Cutaneous barrier function after cold exposure in hairless mice: a model to demonstrate how cold interferes with barrier homeostasis among workers in the fish-processing industry. Br J Dermatol. 1995;132:391–401. doi: 10.1111/j.1365-2133.1995.tb08672.x. [DOI] [PubMed] [Google Scholar]
- Horreau C, Pouplard C, Brenaut E, Barnetche T, Misery L, Cribier B, et al. Cardiovascular morbidity and mortality in psoriasis and psoriatic arthritis: a systematic literature review. J Eur Acad Dermatol Venereol. 2013;27(Suppl 3):12–29. doi: 10.1111/jdv.12163. [DOI] [PubMed] [Google Scholar]
- Huleihel M, Douvdevani A, Segal S, Apte RN. Different regulatory levels are involved in the generation of hemopoietic cytokines (CSFs and IL-6) in fibroblasts stimulated by inflammatory products. Cytokine. 1993;5:47–56. doi: 10.1016/1043-4666(93)90023-x. [DOI] [PubMed] [Google Scholar]
- Huleihel M, Douvdevani A, Segal S, Apte RN. Regulation of interleukin 1 generation in immune-activated fibroblasts. Eur J Immunol. 1990;20:731–8. doi: 10.1002/eji.1830200404. [DOI] [PubMed] [Google Scholar]
- Indulekha K, Surendar J, Mohan V. High sensitivity C-reactive protein, tumor necrosis factor-α, interleukin-6, and vascular cell adhesion molecule-1 levels in Asian Indians with metabolic syndrome and insulin resistance (CURES-105) J Diabetes Sci Technol. 2011;5:982–8. doi: 10.1177/193229681100500421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob SE, Nassiri M, Kerdel FA, Vincek V. Simultaneous measurement of multiple Th1 and Th2 serum cytokines in psoriasis and correlation with disease severity. Mediators Inflamm. 2003;12:309–13. doi: 10.1080/09629350310001619753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang YJ, Lu B, Crumrine D, Elias PM, Feingold KR. IL-6 stimulates but is not essential for stratum corneum formation and permeability barrier development during gestation. Exp Dermatol. 2010;19:e31–6. doi: 10.1111/j.1600-0625.2009.00968.x. [DOI] [PubMed] [Google Scholar]
- Jiang YJ, Lu B, Crumrine D, Man MQ, Elias PM, Feingold KR. IL-1alpha accelerates stratum corneum formation and improves permeability barrier homeostasis during murine fetal development. J Dermatol Sci. 2009;54:88–98. doi: 10.1016/j.jdermsci.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Jung YJ, Jung M, Kim M, Hong SP, Choi EH. IL-1α stimulation restores epidermal permeability and antimicrobial barriers compromised by topical tacrolimus. J Invest Dermatol. 2011;131:698–705. doi: 10.1038/jid.2010.344. [DOI] [PubMed] [Google Scholar]
- Kanitakis J. Anatomy, histology and immunohistochemistry of normal human skin. Eur J Dermatol. 2002;12:390–9. [PubMed] [Google Scholar]
- Katoh N, Hirano S, Kishimoto S, Yasuno H. Acute cutaneous barrier perturbation induces maturation of Langerhans’ cells in hairless mice. Acta Derm Venereol. 1997;77:365–9. doi: 10.2340/0001555577365369. [DOI] [PubMed] [Google Scholar]
- Kikuchi K, Kobayashi H, Hirao T, Ito A, Takahashi H, Tagami H. Improvement of mild inflammatory changes of the facial skin induced by winter environment with daily applications of a moisturizing cream. A half-side test of biophysical skin parameters, cytokine expression pattern and the formation of cornified envelope. Dermatology. 2003;207:269–75. doi: 10.1159/000073089. [DOI] [PubMed] [Google Scholar]
- Kim HO, Kim HS, Youn JC, Shin EC, Park S. Serum cytokine profiles in healthy young and elderly population assessed using multiplexed bead-based immunoassays. J Transl Med. 2011;9:113. doi: 10.1186/1479-5876-9-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köck A, Schwarz T, Kirnbauer R, Urbanski A, Perry P, Ansel JC, et al. Human keratinocytes are a source for tumor necrosis factor alpha: evidence for synthesis and release upon stimulation with endotoxin or ultraviolet light. J Exp Med. 1990;172:1609–14. doi: 10.1084/jem.172.6.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laube S. Skin infections and ageing. Ageing Res Rev. 2004;3:69–89. doi: 10.1016/j.arr.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Lin TK, Man MQ, Santiago JL, Park K, Roelandt T, Oda Y, et al. Topical antihistamines display potent anti-inflammatory activity linked in part to enhanced permeability barrier function. J Invest Dermatol. 2013;133:469–78. doi: 10.1038/jid.2012.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin ZX. Study on skin barrier function and its associated factors in 160 normal Chinese. Fudan University; http://cdmd.cnki.com.cn/Article/CDMD-10246-2009184487.htm. [Google Scholar]
- Lucas R, Parikh SJ, Sridhar S, Guo DH, Bhagatwala J, Dong Y, et al. Cytokine profiling of young overweight and obese female African American adults with prediabetes. Cytokine. 2013;64:310–5. doi: 10.1016/j.cyto.2013.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest. 2011;121:2111–7. doi: 10.1172/JCI57132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man G, Mauro TM, Zhai Y, Kim PL, Cheung C, Hupe M, et al. Topical hesperidin enhances epidermal function in an aged murine model. J Invest Dermatol. 2015;135:1184–7. doi: 10.1038/jid.2014.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man MQ, Man G, Elias PM. Could psoriasis be preventable. Dermatol Sinica. 2015;33:243–244. [Google Scholar]
- Man MQ, Xin SJ, Song SP, Cho SY, Zhang XJ, Tu CX, et al. Variation of skin surface pH, sebum content and stratum corneum hydration with age and gender in a large Chinese population. Skin Pharmacol Physiol. 2009;22:190–9. doi: 10.1159/000231524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao-Qiang M, Brown BE, Wu-Pong S, Feingold KR, Elias PM. Exogenous nonphysiologic vs physiologic lipids. Divergent mechanisms for correction of permeability barrier dysfunction. Arch Dermatol. 1995;131:809–16. doi: 10.1001/archderm.131.7.809. [DOI] [PubMed] [Google Scholar]
- Mariani E, Cattini L, Neri S, Malavolta M, Mocchegiani E, Ravaglia G, et al. Simultaneous evaluation of circulating chemokine and cytokine profiles in elderly subjects by multiplex technology: relationship with zinc status. Biogerontology. 2006;7:449–59. doi: 10.1007/s10522-006-9060-8. [DOI] [PubMed] [Google Scholar]
- Marques-Vidal P, Bastardot F, von Känel R, Paccaud F, Preisig M, Waeber G, et al. Association between circulating cytokine levels, diabetes and insulin resistance in a population-based sample (CoLaus study) Clin Endocrinol (Oxf) 2013;78:232–41. doi: 10.1111/j.1365-2265.2012.04384.x. [DOI] [PubMed] [Google Scholar]
- Mirza S, Hossain M, Mathews C, Martinez P, Pino P, Gay JL, et al. Type 2-diabetes is associated with elevated levels of TNF-alpha, IL-6 and adiponectin and low levels of leptin in a population of Mexican Americans: a cross-sectional study. Cytokine. 2012;57:136–42. doi: 10.1016/j.cyto.2011.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muizzuddin N, Ingrassia M, Marenus KD, Maes DH, Mammone T. Effect of seasonal and geographical differences on skin and effect of treatment with an osmoprotectant: Sorbitol. J Cosmet Sci. 2013;64:165–74. [PubMed] [Google Scholar]
- Mustafa M, Wondimu B, Bakhiet M, Modéer T. Induction of interferon gamma in human gingival fibroblasts challenged with phytohaemagglutinin. Cytokine. 2000;12:368–73. doi: 10.1006/cyto.1999.0565. [DOI] [PubMed] [Google Scholar]
- Mutations in the Skin Barrier Correlate with Increased Type 2 Diabetes Risk. http://www.oncologypractice.com/single-view/mutations-in-the-skin-barrier-correlate-with-increased-type-2-diabetes-risk.html (obtained on November 2, 2015)
- Nanus DE, Filer AD, Yeo L, Scheel-Toellner D, Hardy R, Lavery GG, et al. Differential glucocorticoid metabolism in patients with persistent versus resolving inflammatory arthritis. Arthritis Res Ther. 2015;17:121. doi: 10.1186/s13075-015-0633-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishijima T, Tokura Y, Imokawa G, Seo N, Furukawa F, Takigawa M. Altered permeability and disordered cutaneous immunoregulatory function in mice with acute barrier disruption. J Invest Dermatol. 1997;109:175–82. doi: 10.1111/1523-1747.ep12319282. [DOI] [PubMed] [Google Scholar]
- Onoue A, Kabashima K, Kobayashi M, Mori T, Tokura Y. Induction of eosinophil- and Th2-attracting epidermal chemokines and cutaneous late-phase reaction in tape-stripped skin. Exp Dermatol. 2009;18:1036–43. doi: 10.1111/j.1600-0625.2009.00899.x. [DOI] [PubMed] [Google Scholar]
- Osiecki H. The role of chronic inflammation in cardiovascular disease and its regulation by nutrients. Altern Med Rev. 2004;9:32–53. [PubMed] [Google Scholar]
- Patrick L, Uzick M. Cardiovascular disease: C-reactive protein and the inflammatory disease paradigm: HMG-CoA reductase inhibitors, alpha-tocopherol, red yeast rice, and olive oil polyphenols. A review of the literature. Altern Med Rev. 2001;6:248–71. [PubMed] [Google Scholar]
- Proksch E, Brasch J, Sterry W. Integrity of the permeability barrier regulates epidermal Langerhans cell density. Br J Dermatol. 1996;134:630–8. [PubMed] [Google Scholar]
- Sakura M, Chiba Y, Kamiya E, Kurukawa A, Kawamura N, Niwa M, et al. Differences in the Histopathology and Cytokine Expression Pattern between Chronological Aging and Photoaging of Hairless Mice Skin. Modern Research in Inflammation. 2014;3:82–89. [Google Scholar]
- Sano S. Psoriasis as a barrier disease. Dermatol Sinica. 2015;33:64–69. [Google Scholar]
- Silverberg JI, Greenland P. Eczema and cardiovascular risk factors in 2 US adult population studies. J Allergy Clin Immunol. 2015;135:721–8. e6. doi: 10.1016/j.jaci.2014.11.023. [DOI] [PubMed] [Google Scholar]
- Spranger J, Kroke A, Möhlig M, Hoffmann K, Bergmann MM, Ristow M, et al. Inflammatory cytokines and the risk to develop type 2 diabetes: results of the prospective population-based European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Diabetes. 2003;52:812–7. doi: 10.2337/diabetes.52.3.812. [DOI] [PubMed] [Google Scholar]
- Sprecher E, Becker Y, Kraal G, Hall E, Harrison D, Shultz LD. Effect of aging on epidermal dendritic cell populations in C57BL/6J mice. J Invest Dermatol. 1990;94:247–53. doi: 10.1111/1523-1747.ep12874586. [DOI] [PubMed] [Google Scholar]
- Surmi BK, Hasty AH. Macrophage infiltration into adipose tissue: initiation, propagation and remodeling. Future Lipidol. 2008;3:545–556. doi: 10.2217/17460875.3.5.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai JC, Feingold KR, Crumrine D, Wood LC, Grunfeld C, Elias PM. Permeability barrier disruption alters the localization and expression of TNF alpha/protein in the epidermis. Arch Dermatol Res. 1994;286:242–8. doi: 10.1007/BF00387595. [DOI] [PubMed] [Google Scholar]
- Upragarin N, Landman WJ, Gaastra W, Gruys E. Extrahepatic production of acute phase serum amyloid A. Histol Histopathol. 2005;20:1295–307. doi: 10.14670/HH-20.1295. [DOI] [PubMed] [Google Scholar]
- Urbanski A, Schwarz T, Neuner P, Krutmann J, Kirnbauer R, Köck A, et al. Ultraviolet light induces increased circulating interleukin-6 in humans. J Invest Dermatol. 1990;94:808–11. doi: 10.1111/1523-1747.ep12874666. [DOI] [PubMed] [Google Scholar]
- Valmadrid CT, Klein R, Moss SE, Klein BE. The risk of cardiovascular disease mortality associated with microalbuminuria and gross proteinuria in persons with older-onset diabetes mellitus. Arch Intern Med. 2000;160:1093–100. doi: 10.1001/archinte.160.8.1093. [DOI] [PubMed] [Google Scholar]
- Wang XP, Schunck M, Kallen KJ, Neumann C, Trautwein C, Rose-John S, et al. The interleukin-6 cytokine system regulates epidermal permeability barrier homeostasis. J Invest Dermatol. 2004;123:124–31. doi: 10.1111/j.0022-202X.2004.22736.x. [DOI] [PubMed] [Google Scholar]
- Wanke I, Skabytska Y, Kraft B, Peschel A, Biedermann T, Schittek B. Staphylococcus aureus skin colonization is promoted by barrier disruption and leads to local inflammation. Exp Dermatol. 2013;22:153–5. doi: 10.1111/exd.12083. [DOI] [PubMed] [Google Scholar]
- Wannamethee SG, Shaper AG, Whincup PH, Lennon L, Sattar N. Impact of diabetes on cardiovascular disease risk and all-cause mortality in older men: influence of age at onset, diabetes duration, and established and novel risk factors. Arch Intern Med. 2011;171:404–10. doi: 10.1001/archinternmed.2011.2. [DOI] [PubMed] [Google Scholar]
- Wolf J, Weinberger B, Arnold CR, Maier AB, Westendorp RG, Grubeck-Loebenstein B. The effect of chronological age on the inflammatory response of human fibroblasts. Exp Gerontol. 2012;47:749–53. doi: 10.1016/j.exger.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf P, Gruber-Wackernagel A, Rinner B, Griesbacher A, Eberhard K, Groselj-Strele A, et al. Phototherapeutic hardening modulates systemic cytokine levels in patients with polymorphic light eruption. Photochem Photobiol Sci. 2013;12:166–73. doi: 10.1039/c2pp25187f. [DOI] [PubMed] [Google Scholar]
- Wood LC, Elias PM, Sequeira-Martin SM, Grunfeld C, Feingold KR. Occlusion lowers cytokine mRNA levels in essential fatty acid-deficient and normal mouse epidermis, but not after acute barrier disruption. J Invest Dermatol. 1994;103:834–8. doi: 10.1111/1523-1747.ep12413597. [DOI] [PubMed] [Google Scholar]
- Wood LC, Jackson SM, Elias PM, Grunfeld C, Feingold KR. Cutaneous barrier perturbation stimulates cytokine production in the epidermis of mice. J Clin Invest. 1992;90:482–7. doi: 10.1172/JCI115884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood LC, Stalder AK, Liou A, Campbell IL, Grunfeld C, Elias PM, et al. Barrier disruption increases gene expression of cytokines and the 55 kD TNF receptor in murine skin. Exp Dermatol. 1997;6:98–104. doi: 10.1111/j.1600-0625.1997.tb00154.x. [DOI] [PubMed] [Google Scholar]
- Xu W, Jia S, Xie P, Zhong A, Galiano RD, Mustoe TA, et al. The expression of proinflammatory genes in epidermal keratinocytes is regulated by hydration status. J Invest Dermatol. 2014;134:1044–55. doi: 10.1038/jid.2013.425. [DOI] [PubMed] [Google Scholar]
- Xu YP, Qi RQ, Chen W, Shi Y, Cui ZZ, Gao XH, et al. Aging affects epidermal Langerhans cell development and function and alters their miRNA gene expression profile. Aging (Albany NY) 2012;4:742–54. doi: 10.18632/aging.100501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, Imai Y, Sakaguchi Y, Haneda T, Yamanishi K. Serum cytokines correlated with the disease severity of generalized pustular psoriasis. Dis Markers. 2013;34:153–61. doi: 10.3233/DMA-120958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, Nakajima K, Takaishi M, Kitaba S, Magata Y, Kataoka S, et al. Psoriatic inflammation facilitates the onset of arthritis in a mouse model. J Invest Dermatol. 2015;135:445–53. doi: 10.1038/jid.2014.426. [DOI] [PubMed] [Google Scholar]
- Yoshizawa Y, Nomaguchi H, Izaki S, Kitamura K. Serum cytokine levels in atopic dermatitis. Clin Exp Dermatol. 2002;27:225–9. doi: 10.1046/j.1365-2230.2002.00987.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.