Abstract
This short communication accompanies my presentation at the International Congress on Sudden Cardiac Death held in Prague, March 30 – April 1, 2017. It summarizes briefly studies of the cardiac electrophysiological substrate in patients with hereditary arrhythmogenic syndromes – the Long QT and Brugada syndromes – conducted noninvasively, in situ, using Electrocardiographic Imaging (ECGI). The same noninvasive approach was used to map the electrophysiological substrate of a post-infarction myocardial scar and to relate this substrate to the pattern of activation during reentrant ventricular tachycardia. My thoughts about a potential role for ECGI in cardiac research and clinical care are also expressed briefly, with examples from on-going work in my laboratory.
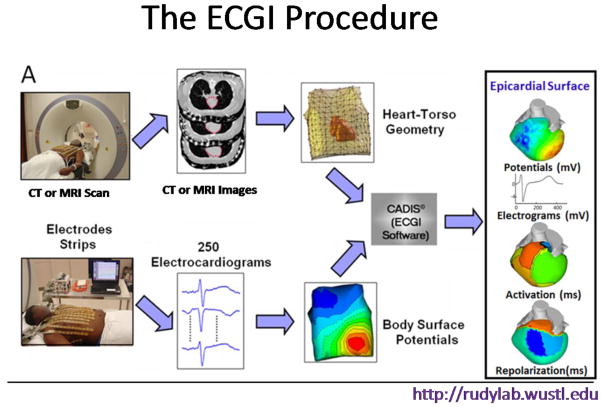
Electrocardiographic Imaging (ECGI) is a novel imaging modality, developed in my laboratory1,2, that maps noninvasively cardiac electrical activity on the heart epicardial surface. In ECGI, a multi-electrode vest (or strips) records 250 body-surface electrocardiograms; then, using these electrocardiograms together with geometrical information from a CT or MRI scan, a mathematical algorithm reconstructs electrical potentials, electrograms, activation sequences (isochrones) and repolarization patterns on the heart surface (Figure 1). ECGI has been applied to study the arrhythmic substrate in hereditary arrhythmogenic syndromes – Long QT syndrome (LQTS) and Brugada syndrome (BrS). In addition, ECGI was used to map the electrical substrate of post myocardial infarction (MI) scars in relation to the activation pattern of ventricular tachycardia (VT).
Figure 1.
The ECGI procedure. Body surface potentials are recorded from 256 electrodes. Patient-specific heart-torso geometry is obtained from thoracic CT or MRI scan. The data are combined using mathematical algorithms to reconstruct epicardial potentials and unipolar electrograms on the heart surface. Maps of epicardial activation and recovery can be further derived from the electrograms.
In LQTS3, maps of epicardial activation, recovery times (RT) and activation-recovery intervals (ARI; surrogate for local action potential duration (APD)) were reconstructed and compared with those of healthy volunteers. Activation was normal in all patients. However, RT and ARI were prolonged relative to control, indicating delayed repolarization and abnormally long APD. ARI prolongation was spatially heterogeneous, with repolarization gradients much steeper than control. This defines a substrate for reentrant arrhythmias, not detectable by surface ECG. Steeper dispersion of repolarization in symptomatic patients suggests a possible role for ECGI in risk stratification.
ECGI in BrS patients4 revealed the coexistence of structurally-based abnormal conduction and abnormal repolarization in the electrophysiological (EP) substrate. Different from LQTS, the BrS abnormal substrate was confined to the right ventricular outflow tract (RVOT). ECGI could differentiate between BrS patients (malignant and highly arrhythmogenic) and non-BrS right bundle branch block (RBBB) patients (generally considered benign) who have similar body surface ECGs, based on major differences in the cardiac EP substrate.
In ischemic cardiomyopathy patients, abnormal EP scar substrate was determined based on low electrogram voltage, and scar heterogeneity was characterized by presence of electrogram fractionation. Late potentials were also reconstructed. These parameters, reconstructed in sinus rhythm, differentiated between patients with VT and without VT. ECGI mapping during VT demonstrated close relationship between the reentry circuit and scar EP substrate5–8.
I was asked by Professor Peter Schwartz to provide an “eye to the future” in regards to ECGI applications. While one cannot predict the future with any degree of certainty, I believe that ECGI can play a central role in cardiology on two accounts: basic research – providing much needed understanding of the mechanisms of electrophysiological disorders and arrhythmias in the human heart in situ, and clinical care – as a noninvasive tool for patient-specific and mechanism–based diagnosis, risk stratification for arrhythmia and sudden death, planning and guidance of therapy, follow up for evaluation of therapy over time, and providing the basis for noninvasive ablation procedures in the future.
At present, ECGI-related research in my laboratory and in collaboration with other labs is progressing towards these goals. Together with researchers from Barts Heart Center in London, UK (Pier Lambiase, principal investigator) we completed a study of the substrate in hereditary arrhythmogenic right ventricular cardiomyopathy (ARVC). Building on our previous ECGI studies of atrial fibrillation (AF)9,10, we are conducting a longitudinal study of the mechanism(s) underlying persistent AF and its recurrence post-ablation. Through noninvasive multi-modality approach (ECGI, tagged MRI and speckles echo) we extend a previous study11 by imaging the electrical and mechanical properties of failing hearts in heart failure patients undergoing cardiac resynchronization therapy.
We follow changes in these properties over time in an attempt to characterize reverse-remodeling processes in these hearts. Together with collaborators from cardiology (Phillip Cuculich) and radiation oncology (Clifford Robinson) at Washington University, we have started to test a noninvasive stereotactic ablation radiotherapy for VT with very promising results as evaluated for longer than one-year post-procedure.12 In short, these are exciting times with new electrophysiological data obtained from the in situ human heart using a novel imaging modality for noninvasive mapping. My hope is that better understanding of the electrophysiologic cardiac substrate and the mechanisms of human cardiac arrhythmias will lead to improved prevention and treatment of electrophysiological disturbances of the heart.
Highlights.
Electrocardiographic Imaging (ECGI) can map noninvasively the cardiac electrophysiologic and arrhythmic substrate in patients.
In the long QT syndrome, epicardial repolarization is delayed and action potential duration is prolonged nonuniformly, creating steep dispersion of repolarization.
In Brugada syndrome, abnormal substrate is confined to the right ventricular outflow tract; where both repolarization and conduction abnormalities are observed.
ECGI can localize noninvasively the origin and circuits of ventricular tachycardia (VT) in myocardial scars, paving the way for noninvasive ablation of VT.
Acknowledgments
Funding Sources: The studies summarized in this communication were supported by NIH – National Heart, Lung and Blood Institute grants R01-HL-033343 and R01-HL-049054 to Yoram Rudy.
Footnotes
Conflict of Interest: Yoram Rudy receives royalties from CardioInsight Technologies (CIT). CIT does not support any research conducted in Dr. Rudy’s laboratory.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ramanathan C, Ghanem RN, Jia P, Ryu K, Rudy Y. Electrocardiographic Imaging (ECGI): A Noninvasive Imaging Modality for Cardiac Electrophysiology and Arrhythmia. Nature Medicine. 2004;10:422–428. doi: 10.1038/nm1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ramanathan C, Jia P, Ghanem RN, Ryu K, Rudy Y. Activation and repolarization of the normal human heart under complete physiological conditions. Proc Natl Acad Sci USA (PNAS) 2006;103:6309–14. doi: 10.1073/pnas.0601533103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vijayakumar Ramya, Silva Jennifer NA, Desouza Kavit A, Abraham Robert L, Strom Maria, Sacher Frederic, Van Hare George F, Haïssaguerre Michel, Roden Dan M, Rudy Yoram. Electrophysiologic Substrate in Congenital Long QT Syndrome: Noninvasive Mapping with Electrocardiographic Imaging (ECGI) Circulation. 2014;130:1936–1943. doi: 10.1161/CIRCULATIONAHA.114.011359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang J, Sacher F, Hoffmayer K, O’Hara T, Strom M, Cuculich P, Silva J, Cooper D, Faddis M, Hocini M, Haissaguerre M, Scheinman M, Rudy Y. The Cardiac Electrophysiologic Substrate Underlying the ECG Phenotype and Electrogram Abnormalities in Brugada Syndrome Patients. Circulation. 2015;131:1950–1959. doi: 10.1161/CIRCULATIONAHA.114.013698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cuculich PS, Zhang J, Wang Y, Desouza KA, Vijayakumar R, Woodard PK, Rudy Y. The Electrophysiologic Cardiac Ventricular Substrate in Patients after Myocardial Infarction: Noninvasive Characterization with ECG Imaging (ECGI) J Am Col Cardiol (JACC) 2011;58:1893–1902. doi: 10.1016/j.jacc.2011.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Y, Cuculich PS, Zhang J, Desouza KA, Vijayakumar R, Chen J, Faddis MN, Lindsay BD, Smith TW, Rudy Y. Noninvasive Electroanatomic Mapping of Human Ventricular Arrhythmias Using ECG Imaging (ECGI) Science Translational Medicine. 2011 Aug 31;3(98):191–200. 98ra84. doi: 10.1126/scitranslmed.3002152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rudy Y. Noninvasive Electrocardiographic Imaging of Arrhythmogenic Substrate in Humans. Circulation Research. 2013;112:863–874. doi: 10.1161/CIRCRESAHA.112.279315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang J, Cooper DH, Desouza KA, Cuculich PS, Woodard PK, Smith TW, Rudy Y. Electrophysiologic Scar Substrate in Relation to VT: Noninvasive High-Resolution Mapping and Risk Assessment with ECGI. Pacing Clin Electrophysiol (PACE) 2016 Aug;39(8):781–91. doi: 10.1111/pace.12882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cuculich PS, Wang Y, Lindsay BD, Faddis MN, Schuessler RB, Damiano RD, Li L, Rudy Y. Noninvasive Characterization of Epicardial Activation in Humans with Diverse Atrial Fibrillation Patterns. Circulation. 2010;122:1364–1372. doi: 10.1161/CIRCULATIONAHA.110.945709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vijayakumar, Vasireddi SK, Cuculich PS, Faddis MN, Rudy Y. Methodology Considerations in Phase Mapping of Human Cardiac Arrhythmias. Circulation Arrhythmia and Electrophysiology. 2016;9:e004409. doi: 10.1161/CIRCEP.116.004409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jia P, Ramanathan C, Ghanem RN, Ryu K, Varma N, Rudy Y. Electrocardiographic Imaging of Cardiac Resynchronization Therapy in Heart Failure: Observations of Variable Electrophysiological Responses. Heart Rhythm Journal. 2006;3:296–310. doi: 10.1016/j.hrthm.2005.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cuculich P, Schill M, Kashani R, Cooper D, Faddis M, Gleva M, Noheria A, Smith T, Rudy Y, Robinson C. First report of entirely noninvasive stereotactic cardiac ablation radiotherapy (NO-SCAR) for VT in humans. Heart Rhythm; Heart Rhythm Society 37th Annual Scientific Sessions; May 2016; 2016. p. S138. [Google Scholar]