Abstract
Purpose
To review the current literature on robotic assistance for ophthalmic surgery, especially vitreoretinal procedures.
Methods
MEDLINE, Embase and Web of Science databases were searched from inception to August, 2016 for articles relevant to the review topic. Queries included combinations of the terms: robotic eye surgery, ophthalmology, and vitreoretinal.
Results
In ophthalmology, proof-of-concept papers have shown the feasibility of performing many delicate anterior segment and vitreoretinal surgical procedures accurately with robotic assistance. Multiple surgical platforms have been designed and tested in animal eyes and phantom models. These platforms have the capability to measure forces generated and velocities of different surgical movements. “Smart” instruments have been designed to improve certain tasks such as membrane peeling and retinal vessel cannulations.
Conclusion
Ophthalmic surgery and particularly vitreoretinal surgery, might have reached the limits of human physiologic performance. Robotic assistance can help overcome biologic limitations and improve our surgical performance. Clinical studies of robotic assisted surgeries are needed to determine safety and feasibility of using this technology in patients.
Keywords: robotic eye surgery, retina, vitreoretinal surgery, robotic assistance
Introduction
Ophthalmic surgery has made huge leaps in the last few decades. Phacoemulsification with insertion of intra-ocular lens has revolutionized cataract surgery. The surgical time and patient recovery have been dramatically shortened, and near perfect visual outcomes are experienced almost immediately after surgery. Femto-second laser assisted cataract surgery gives the promise of even better outcomes(1).
In vitreoretinal surgery, there are still many challenges that must be solved in order to achieve near perfect outcomes. Vitreoretinal surgery is one of the most technically difficult microsurgeries. Fine, precise motion is required to manipulate extremely delicate tissue within the small, constrained space of the eye, often with forces that are below human tactile perception(2). Some of the main technical limitations are inadequate spatial resolution and depth perception of microstructures to identify tissue planes, imprecise movements during micromanipulation of tissue due to physiological tremor, and lack of force sensing since the movements required for dissection are below the surgeon’s sensory threshold.
While vitreoretinal surgery is obviously sophisticated and successful, surgical technique can be improved for all procedures, ranging from routine maneuvers such as laser photocoagulation or membrane peeling to those that are rarely performed because they are at the physiologic limitations of most surgeons. For example, robotic surgery for all surgeons offers the possibility of placing an array of standard laser burns only to ischemic retina in a single application. Likewise, robotic surgery combined with using “smart” instruments that measure physiological parameters and systems that provide auditory feedback to the surgeon, can inform the surgeon of the distance of the instrument tip from the retina or the force generated during epiretinal membrane peeling, which can be used to optimize each surgical movement(3, 4).
Robotic surgery could improve the surgical technique of the accomplished vitreoretinal surgeon who may be resistant to this innovative technology. Vitreoretinal surgeons have a physiological hand tremor with an average amplitude of 156 micrometers (5, 6). Robotic surgical techniques aim to detect and actively compensate for the tremor(7). Noda et al tested the impact of robotic assistance on performance by non-ophthalmologists and skilled ophthalmologic surgeons during vitreoretinal procedures, such as accurately approaching a target on the fundus, stabilizing the instrument tip just above the fundus, and perceiving contact of the instrument tip with the fundus. By using robotic assistance and providing objective feedback of surgical movements, technical performance was improved by both non-ophthalmologists and accomplished surgeons. This result indicates that robotic assistance can enhance even the simplest surgical movements by skilled surgeons who are given objective feedback or by overcoming physiologic limitations(8).
The benefit of robotic surgery may be especially true for technically challenging procedures such as retinal vessel microcannulation and retinal vessel sheathotomy (9, 10). Due to the small size of retinal vessels, these procedures are at the physiological limitations of most vitreoretinal surgeons due to physiological tremor. Robotic surgery will not only cancel physiological tremor, but can also help guide surgical movement to appropriately place a microcannula tip into a retinal blood vessel and maintain it in the blood vessel in order to deliver a drug. If effectively performed, vitreoretinal surgeons might be able to provide innovative surgical treatments to our patients with retinal arterial and venous occlusive diseases. Likewise, we might be able to offer to our patients, the option of either occluding or instilling chemotherapeutics into feeder vessels of intraocular tumors such as hemangioblastomas, retinoblastomas, and choroidal melanomas at optimal, effective doses only within the tumor, with the hope of improving efficacy and limiting toxicity beyond that achieved with intra-arterial chemotherapy(11).
Thus, in order to improve patient outcomes and broaden the range of treatments we can offer, alternative surgical approaches are needed. Robotic assistance provides the theoretical advantage of improved dexterity and accuracy, as well as incorporating novel technology that could translate into improved patient outcomes. In this review, we will discuss some of the latest technologies that could potentially enhance our vitreoretinal surgical practice such as automated laser application, smart instruments that improve accuracy of membrane peeling, retinal vessel cannulation and the novel area of biomicrorobotics.
The different robotic systems
The Da Vinci Robot
The Da Vinci system has been widely used in many surgical fields, and has led to a large increase in the number of robotic surgeries being performed. The number of robotic surgeries rose from 1500 in the year 2000 to more than 20,000 in 2004(12). The use of the Da Vinci robot to repair a corneal laceration and perform penetrating keratoplasty, on cadaveric human and porcine eyes, demonstrated the technical feasibility of using this system for microsurgery in the eye(13, 14). Bourla et al used the Da Vinci system to perform 25-gauge pars plana vitrectomy, intra-ocular foreign body removal, and capsulorrhexis in porcine eyes. They identified limitations of this system in ophthalmic surgery, such as moving robotic arms that were not as intuitive as moving one’s own wrist; the view through the endoscope was inferior when compared to the direct view through the operating microscope; and high stress was exerted on ocular structures at the sclerotomy site where instruments were inserted(15). This unnecessary stress was due to the remote center of motion (RCM) that was located away from the sclerotomy site, which generated tension at the entrance site. As a result, the Hexapod Surgical System (HSS) was developed for use with the Da Vinci system to provide an RCM at the site of ocular penetration, leading to improved dexterity for ocular surgery(16).
Intraocular Robotic Interventional Surgical System (IRISS)
The Intraocular Robotic Interventional Surgical System (IRISS) is a combined effort between the Jules Stein Eye Institute and the UCLA Department of Mechanical and Aerospace Engineering, to provide a platform for performing complete ophthalmic procedures. The IRISS also has a master controller and a slave manipulator. The controller includes two joysticks, which can be operated by the surgeon, and the manipulator includes two independent arms that each hold surgical instruments. The arms holding the instruments each have an independent pivot point and 7 degrees of freedom to provide significant freedom of movement for surgical maneuvers. Commercially available surgical instruments can be attached to the manipulator to perform surgical tasks. This system has been used to perform capsulorrhexis, remove lens cortex, core vitrectomies, create a PVD, and perform retinal vein microcannulation in porcine eyes(17).
Johns Hopkins Steady-Hand Eye Robot
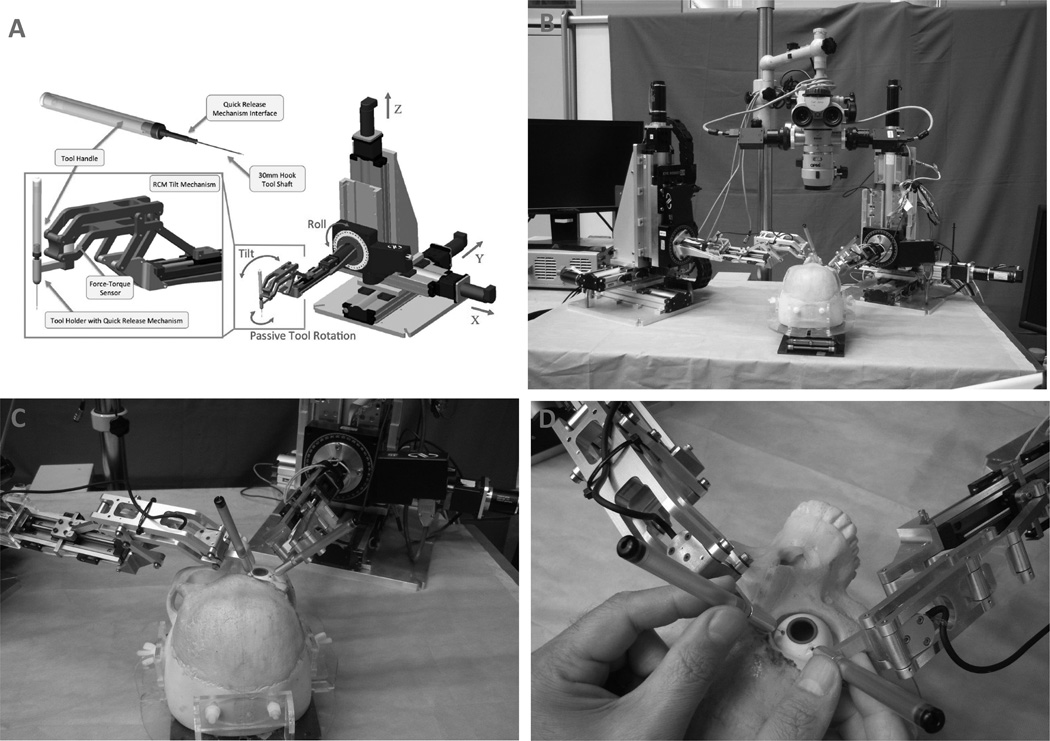
The Johns Hopkins Steady-Hand Eye Robot is a surgeon-initiated robot that is designed to share the control of surgical instruments with the retinal surgeon. The robot’s mechanical system consists of three major components: the XYZ system, the roll mechanism, and the tilt mechanism. The XYZ system allows movement of the surgical tool in all directions. The roll mechanism consists of a rotating table designed to optimize access of the surgical tool to the patient’s eye. The tilt mechanism is attached to the tool holder at one end and the roll mechanism at the other, and allows the instrument to be at any angle. The attachment to the roll mechanism is via a long tubular arm designed to separate the robot’s unsterile parts from the sterile, surgical field. Different surgical instruments, whether conventional or “smart”, can be attached to the tool holder(10), as shown in Figure 1. For example, an integrated micro-force sensing pick, which provides feedback using audio cues, can be used to guide the operator when manipulating ocular tissue (18). The robot smoothens movements to improve efficiency while the instrument’s force sensing capability guides the surgeon to apply the optimal force for each movement, thus improving the effectiveness of each movement. The group has also designed a force sensing pick instrument with 3-degrees of freedom that measures forces in all directions. This instrument can be used free-hand or incorporated into the Steady-Hand-Eye robot so the surgeon can optimally control each surgical movement (19, 20). The original Steady-Hand-Eye robot has been tested in multiple experiments, and has been recently redesigned to improve safety and ergonomics. The new design consists of a symmetric RCM tilt mechanism with a range of ±45 degrees and a stiffness of 21 N/mm, and a slim tool-holder. The tool-holder has a quick-release mechanism with two different release force thresholds for surgical instruments that allows the surgeon to quickly remove the instrument from the eye during an unexpected emergency, such as if the patient moves his/her head(21).
Figure 1.
Johns Hopkins Steady Hand Robot. (A) Scheme of the robot. (B) Photograph of two Steady Hand Robots adjacent to an operating microscope. Two instruments are held by the robots, which have been inserted through sclerotomies of a phantom eye. (C) Higher power photograph of the two Steady Hand Robots with instruments. (D) Close up view of the two instruments held by a surgeon.
“Smart” Instruments
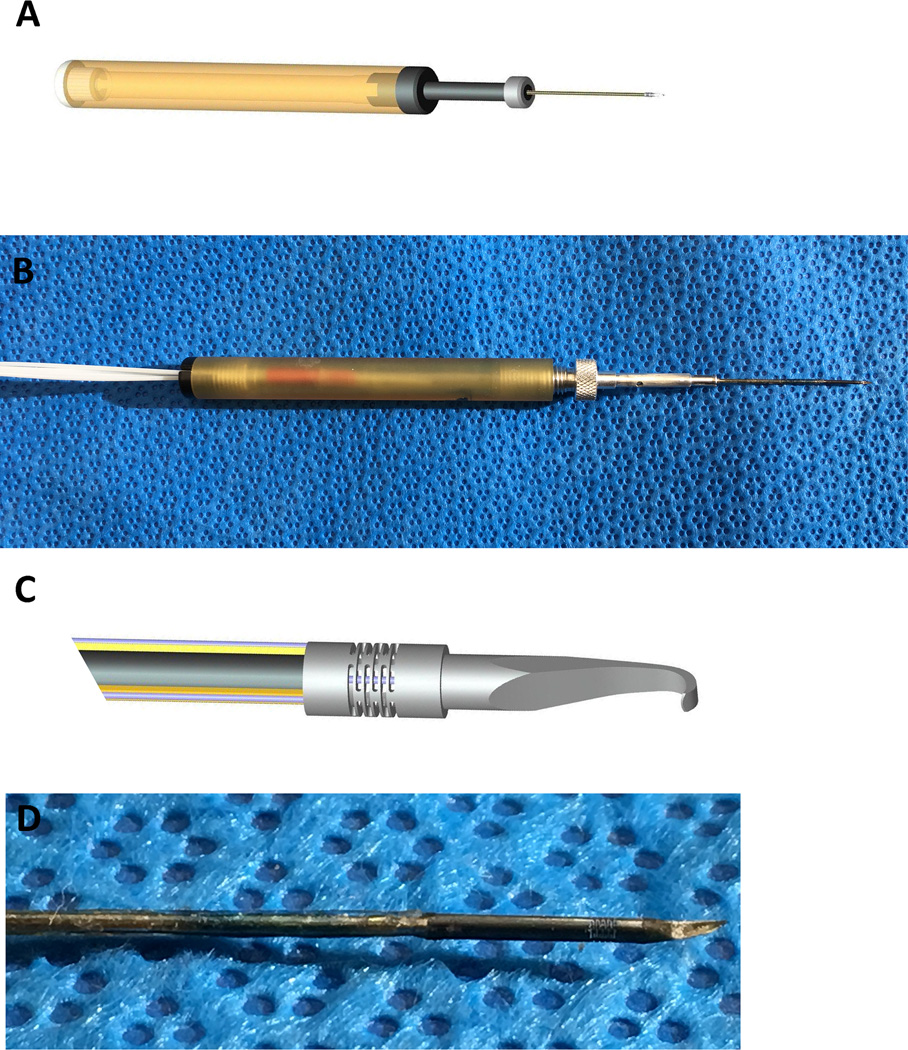
To compliment the robotic system, additional systems and “smart” instruments have been developed for the robotic surgery platform to improve technical performance. Some of these innovations will transform current ophthalmic instruments into “smart” tools that will provide the surgeon with real time physiological information during each surgical maneuver. For example, a “smart” forceps has been developed from a commercially available forceps where sensors have been incorporated to measure the forces being applied to ocular tissues, which can be communicated to the surgeon in real time using an auditory feedback system(22). This instrument can detect micro-forces that are lower than human tactile sensation yet can distinguish between forces that are needed for a normal maneuver from those that may exceed tissue tensile strength, which leads to a surgical complication (Figure 2) (3).
Figure 2.
Force sensing micro-pick. (A) Scheme of the 3 degree-of-freedom (DOF) force sensing micro-pick. (B) Photograph of the 3DOF micro-pick. (C) Magnified scheme showing the shaft which contains 3 grooves containing FBG (Fiber Bragg Grating) sensors (Red arrow) and the flexure (red arrowhead) connected to an inner FBG sensor for measuring axial forces. With any force generated, the instrument shaft “bends” or develops strain, which induces a waveform change that is proportional to the force. The FBG sensor detects the waveform change. (D) Magnified photograph of the distal shaft of the micro-pick where the force sensing takes place.
OCT has become an invaluable tool for managing retinal diseases in the clinic. Likewise, obtaining live intraocular OCT images in the operating room can also help surgeons make decisions based on real-time information. Tao et al demonstrated this concept in the operating room when they obtained in vivo images of human retinal structures using an intra-operative microscope mounted OCT (23). Instead of mounting the OCT on the operating microscope, Yang et al further developed this technology by designing an intraocular OCT. Thus, ocular structures using intraocular optical coherence tomography (OCT) instruments can be imaged in real-time before, during, or after a surgical maneuver to facilitate removing an epiretinal or internal limiting membrane, or to determine the completeness of the delamination. The OCT has been incorporated into a 25 gauge force sensing microsurgical pick to provide a dual function, “smart” instrument for membrane peeling (24)
The Micron is a handheld micromanipulator designed to improve tremor and increase positional accuracy. It operates using piezoelectric actuators, each having 400-micron range of motion, which are activated by sensing motion of the handle. Positional information is detected by optical sensors that track light emitting diodes (LEDs) mounted on the micron. The Micron detects movements, identifies hand tremors, and then moves its tip to counteract the involuntary motion. Since it is a hand piece, it occupies minimal space compared to the robot systems previously described. Importantly, commercially available instruments can be attached to the Micron handpiece. For example, a force-sensing pick can be mounted on the tip of the Micron, to measure forces applied to the tissue in real-time while at the same time minimizing physiologic tremor (25, 26). The intraocular OCT instrument has also been attached to the Micron, which, by decreasing tremor, improves the quality of OCT images acquired in real time (27).
Huschman et al described the use of “the microhand” for robotically assisted vitreoretinal surgery. This pneumatically operated micro-forceps consists of balloon-based joints and attached silicon phalanges. It has four 4mm long and 800-micron wide fingers with 6-micron thick balloons. The authors showed that the microhand could be successfully used to remove retinal tissue from retinal pigment epithelium in porcine eyes. Iatrogenic injuries to the retina could be avoided by using an instrument such as the microhand to control delicate movements during surgical maneuvers(28).
There is continued development and optimization of tools that can help enhance a surgeon’s motor skills for microsurgery. A microsurgical platform, called smart micromanipulation aided robotic-surgical tool (SMART), uses swept source OCT that helps the surgeon minimize tremor and undesirable movements during surgery. SMART tool assistance showed improved surgical movements compared to freehand performance (29).
Biomicrorobotics
Biomicrorobotics combines the principles of robotic surgery with the technological advancements of microelectromechanical systems (MEMS) to allow minimally invasive or noninvasive means of diagnosis and treatment. Ergeneman et al have developed a minimally invasive wireless optical sensor that measures oxygen levels inside the eye. This device can be inserted into the vitreous cavity via a small scleral incision. It is controlled by magnetic fields and visual tracking through the pupil, and can be used to generate oxygen maps inside the eye, such as the preretinal area. This concept can be further extended to measure other physiological variables such as temperature, carbon dioxide, lactate, or glucose levels. Future models of this sensor will focus on reducing the size of the sensor while maintaining its functionality to enable its use during vitreoretinal surgery (30). Micro-robotic devices have the potential to provide localized delivery of drugs into the vitreous cavity. Bergeles et al describe an electromagnetically controlled micro-robotic device that can be inserted and removed in a minimally invasive fashion without vitrectomy. The device was tested in vitro in synthetic vitreous humor and ex vivo in cadaveric porcine eyes. The authors noted that the forces generated from these devices were very low and unlikely to lead to complications such as retinal tears or retinal penetration. However, further optimization and safety studies would be needed before this technology is ready for use in human eyes(31).
Applications of robotic assistance in vitreoretinal surgery
Retinal vessel cannulation
Laboratory experiments have shown that the success rate of small vessel cannulation is enhanced with robotic aid as it minimizes tremor, allowing reliable cannulation of 80-micrometer diameter veins (9, 10). Ueta et al developed a surgeon assisted robot prototype that consists of a master console and a slave manipulator, which communicate with each other via a local area network. The performance of this system was assessed using a pointing accuracy test on graph paper and during several tasks including creating a posterior vitreous detachment (PVD), retinal vessel sheathotomy, and retinal vessel microcannulation and drug injection in harvested porcine eyes. Two engineering graduate students and two ophthalmologists, one with 6 and another with 20 years of clinical experience, performed the surgical tasks manually and using robotic assistance. When asked to place the instrument tip at an aiming point on graph paper, the mean distance between aiming point and instrument tip was 327±121 microns when tasks were performed manually and 32.3±4.5 microns when performed with robotic assistance. The results in porcine eyes similarly showed better performance when tasks were performed with robotic assistance as compared to without assistance (32). The role of robotic assistance was more apparent during drug injection, which is a slow process due to the small gauge of the injection needle. In manually performed procedures, the micropipette was inadvertently removed prematurely from the vessel because the surgeon’s innate tremor prevented stabilization of the micropipette during the injection. The higher motion stability of the robotic system also led to fewer unnecessary forces being applied to the model eye (33, 34). A recently published paper on retinal vein cannulation in porcine eyes showed that a junior retinal surgeon was able to successfully cannulate and inject fluid in retinal vessels in 20 of 20 attempts when using robotic assistance with a force-sensitive needle(35).
Membrane Peeling
Membrane peeling is one of the most essential and commonly performed tasks in vitreoretinal surgery. An imperceptible increase in force or unintentional movement can lead to retinal hemorrhages or tears that can lead to sub-optimal outcomes or prolonged surgery times. Experiments show that this task can be performed more accurately with robotic assistance. Sunshine et al tested the micro-force sensor incorporated into a micro-pick to measure forces generated during vitrectomy in rabbit eyes and membrane peeling in the “raw egg” and chick chorioallantoic membrane models. They showed that there were subtle, sub-threshold differences that separated the forces required for normal maneuvers from those that caused complications during surgery (3). Providing auditory feedback of the forces generated during a maneuver can enable the surgeon to utilize this information to influence surgical performance. Cutler et al used a 25 gauge force-sensing micro-forceps that was linked to an auditory force feedback system, and instructed participants with varying surgical experience to peel strips off of a platform as quickly as possible without exceeding a preset threshold of 9mN of force. When surgeons used audio feedback, the magnitude and variability of the forces generated during membrane peeling were decreased compared to manual peeling (4). Thus, the combination of the force-sensing instrument with auditory feedback provides the potential to make membrane peeling standardized and safer (14), either in eyes with typical macular pucker or in highly myopic eyes with unusual anatomy and delicate retinal tissue.
OCT based instruments can help surgeons identify the largest space between an epiretinal membrane and retina to decide where to initiate membrane peeling. The surgeon can use the intraocular OCT to visualize the completeness of membrane peeling by reimaging the recently peeled surgical area, and comparing it with pre-operative images. This technology may obviate the need for using dyes such as indocyanine green, which is currently used to highlight internal limiting membranes for peeling, and may be toxic to the retina (36). Ultimately, the dual function OCT and force sensing 25 gauge microsurgical pick can combined the benefits of both technology during membrane peeling(24). During membrane peeling, the OCT measures the distance of the tool tip from the membrane or retina, while the force sensor will quantify the forces generated. The OCT integrated device can be especially helpful in situations where the normal retinal anatomy is distorted.
Automated laser application
Robotic assistance in laser photocoagulation can increase the efficiency and decrease error (37). Yang et al tested the feasibility of automated laser photocoagulation in artificial eye models. Two experienced retinal surgeons performed the procedure manually and with a manipulator attached to the endolaser probe. The manipulator corrected any errors between the aiming laser beam and the target. In the automated trials, the depth of the laser tip was maintained within 18±2 µm root-mean-square (RMS) of its original position, while it varied in manual trials with an error of 296±30µm RMS. Errors were particularly apparent at high firing rates of more than 2.5Hz, when manual photocoagulation was associated with 30% failed burns as opposed to no failed burns when using the automated laser photocoagulation technique(38). Yang et al described using a handheld robotic device, the Micron, for automated delivery of retinal laser photocoagulation. They found their system to perform better than manual or semi-automated delivery of laser photocoagulation in phantom eyes, and they aim to develop techniques that would optimize operation of their system in a real eye, where the optics are different from those in a phantom eye(39).
Telesurgery
Robotic assisted surgery has the potential to be used for telesurgery, where a surgeon is physically located many miles away from the area where his/her expertise may be needed to provide training or patient care. Belyea and colleagues showed that the use of a remotely controlled diode laser was as effective as direct manual diode laser for ciliary ablation of fresh enucleated human eyes (40). Marescaux et al showed the feasibility of performing successful laparoscopic cholecystectomy across the Atlantic Ocean using robotic telesurgery. The surgeons were located in New York, while the subjects were located in France. The robotic system consisted of two sub-systems: a surgeon system and a patient system connected via a high-speed optical fiber network which transports data using asynchronous transfer mode technology. Surgeons evaluated the procedure on a 0–10 scale (0 being worst possible and 10 being best possible). Image quality was graded at 9.1, time lag at 8.5 and safety at 8.7 (41). This study serves as an important proof-of-concept in favor of telesurgery with robotic assistance. This has tremendous potential to be further developed to provide surgical expertise for patient care and surgical training around the world, particularly in regions with poor access to trained surgeons. It can also help further develop the surgical field as it would allow surgeons to share their expertise with colleagues across the world, not just at conferences, but continuously in real time. Finally, telesurgery can be used for surgical training where the learning surgeon can feel the proper movements provided by the master surgeon just as a child learns to ride a bike with “training wheels”.
Developing a language of surgery and its role in surgical training
Currently, there is no standardized method of quantifying surgical performance that will allow comparison of surgical outcomes or for surgical trainees to objectively assess their progress. Surgeons are typically credentialed to perform procedures based on the number of surgical cases performed, and by their competence after being subjectively assessed by senior surgeons. Further evaluation of surgical competence is based on an assessment of knowledge of the surgical procedure, which provides insight into cognitive competence, but minimal evidence of a surgeon’s technical ability. There is no standardized means of measuring surgical movements to quantify surgical precision or dexterity. In a workshop held on the assessment of surgical skills, the need for a skill called “tissue handling” was identified. Currently there are no validated method to objectively measure this or other surgical skills (42). Robotic surgery has introduced devices with the potential to quantify surgical movements such as velocity, distance traveled, force generated, and impact on tissue. Uemura et al described a computerized system that measures the laparoscopic suturing skill of surgeons performing intestinal anastomosis. Suture tension, air pressure leak across the anastomosis, deformity of intestinal wall after suturing, number of sutures, and time taken to complete the suturing task differed significantly between novice and experienced surgeons. Quantitative assessment of performance, such as the one obtained from this study, can provide a useful guide for junior surgeons as they work to improve their skills and aim to improve their performance to the level of skilled surgeons(43).
Similarly, in ophthalmology, electromagnetic tracking systems and force-sensing tools can quantify surgical movements by providing the amplitude and rate of forces being generated during tissue manipulation, the distance of the instrument tip and shaft from ocular structures, or the velocity movement of instruments by the surgeon (44, 45). This information can be used to develop a library of quantified movements for any surgical procedure, thus creating a “language of surgery”. This library would include both successful movements and those that induce tissue injury. The educational value is enormous. Quantitative data can be used to provide objective feedback based on which surgical trainees can improve their surgical technique, or to enhance the performance of skilled surgeons.
Being able to measure surgeon skill can be useful when determining outcomes from clinical trials involving surgical interventions or in the testing of a new surgical device. Any surgical outcome depends upon the success of the surgery and the inherent ability of the tissue to heal. Theoretically, by performing every surgical maneuver within an optimized, standardized range, it is possible for the first time, to determine the extent that surgical intervention impacts the outcome of a disease by eliminating variability due to inadequately performed surgical technique. Likewise, by quantifying surgical movements, surgical performance can be objectively assessed when obtaining hospital privileges or when evaluating negligence in malpractice cases.
Future directions
Future innovations in robotic surgery will increase the utility of robotic surgery in ophthalmologic surgery. With continued improvement of robotic assistance technology, we can perhaps more efficiently, effectively, and precisely perform procedures. Since current surgery is at human physiological performance limitations, inclusion of robotic technology will enable us to perform other procedures that are not currently feasible, such as delivering genes to specific cells in the retina, cannulating feeder vessels of choroidal neovascularization or choroidal tumors, or precise debridement individual layers of the retina, RPE, Bruch’s membrane, and choroid.
The ideal system might be a computer assisted robot rather than a fully automated system. The robot or computer would receive input from the surgeon and the eye. From the surgeon, it would receive details regarding the surgical maneuver. The computer could assist by damping tremor, optimizing the velocity of the movement, the pace of force generation of a movement, and guide the direction and amplitude of a movement. From the patient’s eye, the computer could receive real time physiological information, such as information on oxygen or carbon dioxide levels, lactic acid levels, changes in tissue thickness, and light exposure. With fluorescent tags, it might be possible to inject into an eye a marker for a particular cell or problem, e.g. a fibroblastic marker so the surgeon can distinguish scar tissue from normal ocular tissue. From both the surgeon and the patient’s eye, the computer can build “virtual fixtures” which can prevent an instrument tip from moving into a forbidden zone, such as the shaft of a vitrectomy tip from accidently hitting the lens, or a microforceps or instrument tip from unintentionally entering the retinal surface. The surgeon could use these data to make more informed decisions when performing a given procedure.
Limitations
The added value of managing surgical cases with robotic assistance in human eyes still needs to be established. There are no randomized controlled trials on this topic and even small studies on the use of robotic assistance for surgery in human eyes are lacking. If we are to advance our field, we need to have an open mind toward this radical paradigm shift in the approach toward surgery with this new technology. The safety of this technology in human eyes will need to be unequivocally established. As with any new device, there will be a learning curve for the entire surgical team, which will add to the cost as well as the time involved in performing a given procedure. The financial cost of robotic surgery is likely to be high (46), at least initially, and this is an important consideration, especially in today’s era of escalating health care costs. However, it is possible that robotic surgery may yield better or more consistent outcomes than conventional surgery, ultimately making it overall, more cost-effective. Study from a nation-wide database showed that for certain general surgical procedures including colorectal procedures, adrenalectomy and lysis of adhesions, when overall cost including post-operative care was considered, robotic surgery was as cost-effective as conventional surgery(47). Data are needed to make this cost determination for ophthalmic procedures. There is room for improvement in our surgical outcomes and in the measurement of our surgical skill. Developing new technology can help improve outcomes for our patients and expand the range of treatments we can successfully offer.
Summary Statement.
Robotic assistance in vitreoretinal surgery has many potential applications: enhancing surgical performance, developing a “language of surgery”, surgical training, and telesurgery. Multiple platforms have shown promise in phantom models and animals. Clinical studies of this technology are warranted to determine their benefit and safety in patients, and their cost-effectiveness.
Acknowledgments
Funding: Unrestricted grant from Research to Prevent Blindness, Robert Bond Welch Professorship, gifts from the Merlau family and Aleda Wright, National Institute of Health grant R01EB000526
Footnotes
The authors have no proprietary interest in any of the content discussed in the manuscript.
References
- 1.Nagy Z, Takacs A, Filkorn T, Sarayba M. Initial clinical evaluation of an intraocular femtosecond laser in cataract surgery. Journal of Refractive Surgery. 2009;25(12):1053. doi: 10.3928/1081597X-20091117-04. [DOI] [PubMed] [Google Scholar]
- 2.Gupta PK, Jensen PS, de Juan E Jr, editors. Medical Image Computing and Computer-Assisted Intervention–MICCAI’99. Springer; 1999. Surgical forces and tactile perception during retinal microsurgery. [Google Scholar]
- 3.Sunshine S, Balicki M, He X, Olds K, Kang JU, Gehlbach P, et al. A force-sensing microsurgical instrument that detects forces below human tactile sensation. Retina. 2013;33(1):200–206. doi: 10.1097/IAE.0b013e3182625d2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balicki M, Uneri A, Iordachita I, Handa J, Gehlbach P, Taylor R. Micro-force sensing in robot assisted membrane peeling for vitreoretinal surgery; Medical Image Computing and Computer-Assisted Intervention–MICCAI 2010; 2010. pp. 303–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Riviere CN, Gangloff J, De Mathelin M. Robotic compensation of biological motion to enhance surgical accuracy. Proceedings-ieee. 2006;94(9):1705. [Google Scholar]
- 6.Peral-Gutierrez F, Liao AL, Riviere CN, editors. Engineering in Medicine and Biology Society, 2004 IEMBS'04 26th Annual International Conference of the IEEE. IEEE; 2004. Static and dynamic accuracy of vitreoretinal surgeons. [DOI] [PubMed] [Google Scholar]
- 7.Tatinati S, Ang WT, Veluvolu K. Multi-Dimensional Modeling of Physiological Tremor for Active Compensation in Hand-Held Surgical Robotics. IEEE Transactions on Industrial Electronics. 2016;PP(99) [Google Scholar]
- 8.Noda Y, Ida Y, Tanaka S, Toyama T, Roggia MF, Tamaki Y, et al. Impact of robotic assistance on precision of vitreoretinal surgical procedures. PLoS One. 2013;8(1):e54116. doi: 10.1371/journal.pone.0054116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gonenc B, Tran N, Riviere CN, Gehlbach P, Taylor RH, Iordachita I, editors. Multisensor Fusion and Integration for Intelligent Systems (MFI), 2015 IEEE International Conference on. IEEE; 2015. Force-based puncture detection and active position holding for assisted retinal vein cannulation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fleming I, Balicki M, Koo J, Iordachita I, Mitchell B, Handa J, et al. Cooperative robot assistant for retinal microsurgery. Medical image computing and computer-assisted intervention : MICCAI International Conference on Medical Image Computing and Computer-Assisted Intervention. 2008;11(Pt 2):543–550. doi: 10.1007/978-3-540-85990-1_65. [DOI] [PubMed] [Google Scholar]
- 11.Wyse E, Handa JT, Friedman AD, Pearl MS. A review of the literature for intra-arterial chemotherapy used to treat retinoblastoma. Pediatric radiology. 2016;46(9):1223–1233. doi: 10.1007/s00247-016-3554-6. [DOI] [PubMed] [Google Scholar]
- 12.Kumar R, Hemal AK. Emerging role of robotics in urology. Journal of minimal access surgery. 2005;1(4):202. doi: 10.4103/0972-9941.19268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsirbas AMC, Dutson E. Robotic ocular surgery. The British journal of ophthalmology. 2007;91:18–21. doi: 10.1136/bjo.2006.096040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bourges JL, Hubschman JP, Burt B, Culjat M, Schwartz SD. Robotic microsurgery: Corneal transplantation. British Journal of Ophthalmology. 2009;93(12):1672–1675. doi: 10.1136/bjo.2009.157594. [DOI] [PubMed] [Google Scholar]
- 15.Bourla M, * Dan H, Hubschman Jean Pierre, MD,*, Culjat P, † Martin, Tsirbas Angelo, MD,*, Gupta Anurag, MD,*, Schwartz Steven D., MD FEASIBILITY STUDY OF INTRAOCULAR ROBOTIC SURGERY WITH THE da VINCI SURGICAL SYSTEM. Retina. 2008;28:154–158. doi: 10.1097/IAE.0b013e318068de46. [DOI] [PubMed] [Google Scholar]
- 16.Bourges JL, Hubschman JP, Wilson J, Prince S, Tsao TC, Schwartz S. Assessment of a hexapod surgical system for robotic micro-macro manipulations in ocular surgery. Ophthalmic Research. 2011;46(1):25–30. doi: 10.1159/000314719. [DOI] [PubMed] [Google Scholar]
- 17.Rahimy E, Wilson J, Tsao TC, Schwartz S, Hubschman JP. Robot-assisted intraocular surgery: Development of the IRISS and feasibility studies in an animal model. Eye (Basingstoke) 2013;27(8):972–978. doi: 10.1038/eye.2013.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uneri A, Balicki MA, Handa J, Gehlbach P, Taylor RH, Iordachita I. New Steady-Hand Eye Robot with Micro-Force Sensing for Vitreoretinal Surgery. Proceedings of the IEEE/RAS-EMBS International Conference on Biomedical Robotics and Biomechatronics IEEE/RAS-EMBS International Conference on Biomedical Robotics and Biomechatronics. 2010;2010(26–29):814–819. doi: 10.1109/BIOROB.2010.5625991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He X, Handa J, Gehlbach P, Taylor R, Iordachita I. A submillimetric 3-DOF force sensing instrument with integrated fiber Bragg grating for retinal microsurgery. IEEE transactions on bio-medical engineering. 2014;61(2):522–534. doi: 10.1109/TBME.2013.2283501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mitchell B, Koo J, Iordachita I, Kazanzides P, Kapoor A, Handa J, et al., editors. Robotics and Automation, 2007 IEEE International Conference on. IEEE; 2007. Development and application of a new steady-hand manipulator for retinal surgery. [Google Scholar]
- 21.He X, Roppenecker D, Gierlach D, Balicki M, Olds K, Gehlbach P, et al., editors. ASME 2012 International Mechanical Engineering Congress and Exposition. American Society of Mechanical Engineers; 2012. Toward clinically applicable Steady-Hand Eye Robot for vitreoretinal surgery. [Google Scholar]
- 22.Cutler N, Balicki M, Finkelstein M, Wang J, Gehlbach P, McGready J, et al. Auditory force feedback substitution improves surgical precision during simulated ophthalmic surgery. Investigative ophthalmology & visual science. 2013;54(2):1316–1324. doi: 10.1167/iovs.12-11136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tao YK, Ehlers JP, Toth CA, Izatt JA. Intraoperative spectral domain optical coherence tomography for vitreoretinal surgery. Optics letters. 2010;35(20):3315–3317. doi: 10.1364/OL.35.003315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Balicki MHJ-H, Iordachita I, Gehlbach P, Handa J, Taylor R, et al. Single fiber optical coherence tomography microsurgical instruments for computer and robot-assisted retinal surgery. Med Image Comput Comput-Assist Interv MICCAI Int Conf Med Image Comput Comput-Assist Interv. 2009;12:108–115. doi: 10.1007/978-3-642-04268-3_14. [DOI] [PubMed] [Google Scholar]
- 25.Gonenc B, Balicki MA, Handa J, Gehlbach P, Riviere CN, Taylor RH, et al. Evaluation of a Micro-Force Sensing Handheld Robot for Vitreoretinal Surgery. Proceedings of the IEEE/RSJ International Conference on Intelligent Robots and Systems IEEE/RSJ International Conference on Intelligent Robots and Systems. 2012;2012:4125–4130. doi: 10.1109/IROS.2012.6385715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maclachlan RA, Becker BC, Tabares JC, Podnar GW, Lobes LA, Jr, Riviere CN. Micron: an Actively Stabilized Handheld Tool for Microsurgery. IEEE transactions on robotics : a publication of the IEEE Robotics and Automation Society. 2012;28(1):195–212. doi: 10.1109/TRO.2011.2169634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang S, Balicki M, Wells TS, Maclachlan RA, Liu X, Kang JU, et al. Improvement of optical coherence tomography using active handheld micromanipulator in vitreoretinal surgery. Conference proceedings : Annual International Conference of the IEEE Engineering in Medicine and Biology Society IEEE Engineering in Medicine and Biology Society Annual Conference. 2013;2013:5674–5677. doi: 10.1109/EMBC.2013.6610838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hubschman JP, Bourges JL, Choi W, Mozayan A, Tsirbas A, Kim CJ, et al. The Microhand: A new concept of micro-forceps for ocular robotic surgery. Eye. 2010;24(2):364–367. doi: 10.1038/eye.2009.47. [DOI] [PubMed] [Google Scholar]
- 29.Song C, Gehlbach PL, Kang JU. Active tremor cancellation by a "smart" handheld vitreoretinal microsurgical tool using swept source optical coherence tomography. Opt Express. 2012;20(21):23414–23421. doi: 10.1364/OE.20.023414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ergeneman O, Dogangil G, Kummer MP, Abbott JJ, Nazeeruddin MK, Nelson BJ. A magnetically controlled wireless optical oxygen sensor for intraocular measurements. Sensors Journal, IEEE. 2008;8(1):29–37. [Google Scholar]
- 31.Bergeles C, Kummer MP, Kratochvil BE, Framme C, Nelson BJ. Medical Image Computing and Computer-Assisted Intervention–MICCAI 2011. Springer; 2011. Steerable intravitreal inserts for drug delivery: in vitro and ex vivo mobility experiments; pp. 33–40. [DOI] [PubMed] [Google Scholar]
- 32.Ueta T, Yamaguchi Y, Shirakawa Y, Nakano T, Ideta R, Noda Y, et al. Robot-Assisted Vitreoretinal Surgery. Development of a Prototype and Feasibility Studies in an Animal Model. Ophthalmology. 2009;116(8):1538.e2–1543.e2. doi: 10.1016/j.ophtha.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 33.Tanaka S, Harada K, Ida Y, Tomita K, Kato I, Arai F, et al. Quantitative assessment of manual and robotic microcannulation for eye surgery using new eye model. The International Journal of Medical Robotics and Computer Assisted Surgery. 2014 doi: 10.1002/rcs.1586. n/a-n/a. [DOI] [PubMed] [Google Scholar]
- 34.Ueta T, Nakano T, Ida Y, Sugita N, Mitsuishi M, Tamaki Y. Comparison of robot-assisted and manual retinal vessel microcannulation in an animal model. British Journal of Ophthalmology. 2011;95(5):731–734. doi: 10.1136/bjo.2010.193391. [DOI] [PubMed] [Google Scholar]
- 35.Gijbels A, Willekens K, Esteveny L, Stalmans P, Reynaerts D, Vander Poorten EB, editors. 2016 6th IEEE International Conference on Biomedical Robotics and Biomechatronics (BioRob) IEEE; 2016. Towards a clinically applicable robotic assistance system for retinal vein cannulation. [Google Scholar]
- 36.Ando F, Sasano K, Ohba N, Hirose H, Yasui O. Anatomic and visual outcomes after indocyanine green-assisted peeling of the retinal internal limiting membrane in idiopathic macular hole surgery. American journal of ophthalmology. 2004;137(4):609–614. doi: 10.1016/j.ajo.2003.08.038. [DOI] [PubMed] [Google Scholar]
- 37.Becker BC, MacLachlan RA, Lobes LA, Riviere CN. Semiautomated intraocular laser surgery using handheld instruments. Lasers Surg Med. 2010;42(3):264–273. doi: 10.1002/lsm.20897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang S, Lobes LA, Jr, Martel JN, Riviere CN. Handheld-automated microsurgical instrumentation for intraocular laser surgery. Lasers Surg Med. 2015;47(8):658–668. doi: 10.1002/lsm.22383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang S, MacLachlan RA, Martel JN, Lobes LA, Jr, Riviere CN. Comparative Evaluation of Handheld Robot-Aided Intraocular Laser Surgery. IEEE transactions on robotics : a publication of the IEEE Robotics and Automation Society. 2016;32(1):246–251. doi: 10.1109/TRO.2015.2504929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Belyea DA, Mines MJ, Yao WJ, Dan JA, Bower KS. Telerobotic contact transscleral cyclophotocoagulation of the ciliary body with the diode laser. Journal of Robotic Surgery. 2014;8(1):49–55. doi: 10.1007/s11701-013-0424-1. [DOI] [PubMed] [Google Scholar]
- 41.Marescaux J, Leroy J, Gagner M, Rubino F, Mutter D, Vix M, et al. Transatlantic robot-assisted telesurgery. Nature. 2001;413(6854):379–380. doi: 10.1038/35096636. [DOI] [PubMed] [Google Scholar]
- 42.Satava RM, Cuschieri A, Hamdorf J. Metrics for objective assessment. Surgical endoscopy. 2003;17(2):220–226. doi: 10.1007/s00464-002-8869-8. [DOI] [PubMed] [Google Scholar]
- 43.Uemura M, Yamashita M, Tomikawa M, Obata S, Souzaki R, Ieiri S, et al. Objective assessment of the suture ligature method for the laparoscopic intestinal anastomosis model using a new computerized system. Surg Endosc. 2015;29(2):444–452. doi: 10.1007/s00464-014-3681-9. [DOI] [PubMed] [Google Scholar]
- 44.He X, Balicki M, Gehlbach P, Handa J, Taylor R, Iordachita I. A Multi-Function Force Sensing Instrument for Variable Admittance Robot Control in Retinal Microsurgery. IEEE International Conference on Robotics and Automation : ICRA : [proceedings] IEEE International Conference on Robotics and Automation. 2014;2014:1411–1418. doi: 10.1109/ICRA.2014.6907037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hubschman JP, Son J, Allen B, Schwartz SD, Bourges JL. Evaluation of the motion of surgical instruments during intraocular surgery. Eye. 2011;25(7):947–953. doi: 10.1038/eye.2011.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lanfranco AR, Castellanos AE, Desai JP, Meyers WC. Robotic surgery: a current perspective. Annals of surgery. 2004;239(1):14. doi: 10.1097/01.sla.0000103020.19595.7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Salman M, Bell T, Martin J, Bhuva K, Grim R, Ahuja V. Use, cost, complications, and mortality of robotic versus nonrobotic general surgery procedures based on a nationwide database. The American Surgeon. 2013;79(6):553–560. [PubMed] [Google Scholar]