Abstract
In addition to motor function, the cerebellum has been implicated in cognitive and social behaviors. Various structural and functional abnormalities of Purkinje cells (PCs) have been observed in schizophrenia and autism. As PCs express the gene Disrupted-In-Schizophrenia-1 (DISC1), and DISC1 variants have been associated with neurodevelopmental disorders, we evaluated the role of DISC1 in cerebellar physiology and associated behaviors using a mouse model of inducible and selective expression of a dominant-negative, C-terminus truncated human DISC1 (mutant DISC1) in PCs. Mutant DISC1 male mice demonstrated impaired social and novel placement recognition. No group differences were found in novelty-induced hyperactivity, elevated plus maze test, spontaneous alternation, spatial recognition in Y maze, sociability or accelerated rotarod. Expression of mutant DISC1 was associated with a decreased number of large somata PCs (volume: 3000–5000 μm3) and an increased number of smaller somata PCs (volume: 750–1000 μm3) without affecting the total number of PCs or the volume of the cerebellum. Compared to control mice, attached loose patch recordings of PCs in mutant DISC1 mice revealed increased spontaneous firing of PCs; and whole cell recordings showed increased amplitude and frequency of mEPSCs without significant changes in either Rinput or parallel fiber EPSC paired-pulse ratio. Our findings indicate that mutant DISC1 alters the physiology of PCs, possibly leading to abnormal recognition memory in mice.
Keywords: cerebellum, Purkinje cells, schizophrenia, autism, DISC1
Introduction
It is widely believed that the cerebellum is involved in motor activity and coordination (Evarts and Thach, 1969). However, the cerebellum also has extensive connections with the brain regions (e.g., prefrontal and posterior parietal cortex) implicated in cognitive and social aspects of human behavior (Clower et al., 2005). Consistently, decreased gyrification, smaller granular and molecular layers of the vermis and loss of Purkinje cells (PCs) have been associated with social, emotional, and cognitive dysfunction in schizophrenia (Andreasen and Pierson, 2008; Martin and Albers, 1995; Schmahmann, 1991; Schmitt et al., 2010; Snider, 1982; Supprian et al., 2000; Yeganeh-Doost et al., 2011). A decreased number of PCs (Kemper and Bauman, 1998; Kern, 2003) and abnormal sizes and shapes of neurons of the deep cerebellar nuclei were observed in autism spectrum disorders (ASD) (Amaral et al., 2008; Palmen and van Engeland, 2004). High incidence of cerebellar movement disorders such as limb dysmetria were observed in children diagnosed with ASD (Papadopoulos et al., 2012).
Disrupted In Schizophrenia 1 (DISC1) is a psychiatric gene disrupted by the balanced (1:11) (q42.1; q14.3) translocation, segregating in the Scottish family with several major psychiatric disorders, including schizophrenia, depression, and bipolar disorder (Blackwood et al., 2001; Millar et al., 2000; St Clair et al., 1990). Recent studies have reported association of DISC1 polymorphisms with ASD as well (Chakirova et al., 2011; Kanduri et al., 2016; Kilpinen et al., 2008; Zheng et al., 2011). Schurov et al (2004) and Gaudarzi et al (2013) found that PCs were predominantly positive for DISC1. However, other small cells in the molecular and the granular layer also demonstrated some DISC1-positive immunoreactivity. Ma et al (2002) and Austin et al (2003) were unable to detect DISC1 expression in PCs but found it in glial cells of the molecular layer and Bergmann glia cells only (Austin et al., 2003; Bord et al., 2006; Goudarzi et al., 2013; Ma et al., 2002; Schurov et al., 2004). However, the role of DISC1 in cerebellar physiology and associated behaviors has not been evaluated. To this end, we generated a mouse model of inducible and selective expression of C-terminus truncated human DISC1 (mutant DISC1) in PCs and assessed the morphological and electrophysiological properties of PCs as well as behavioral and cognitive phenotypes in mutant DISC1 mice.
Materials and Methods
Animals
Our mouse model of inducible expression of human mutant DISC1 in the cerebellum is based on the Tet-off system (SFig.1) (Ovanesov et al., 2008). In order to express mutant DISC1 in PCs, heterozygous Parv2A-tTA2 single transgenic male or female mice (generated and kindly provided by Dr. Hongkui Zeng at the Allen Institute for Brain Science, Seattle, WA) were crossed with homozygous single transgenic TRE-mutant DISC1 mice (line 1001) (Ovanesov et al., 2008). This breeding protocol produces litters with ~50% single transgenic mice (TRE-mutant DISC1) that do not express mutant protein but have the transgenic insertion (control mice) and ~50% double transgenic mice (Parv2A-tTA2; TRE-mutant DISC1) that express mutant protein (mutant DISC1 mice). Thus, a balanced combination of paternal or maternal backgrounds was used; and each litter had pups of both genotypes mitigating possible effects of unequal treatment from nursing dams.
All mice were backcrossed to the C57BL/6j background for at least 12 generations. Both male and female mice were used in all experiments. Mouse pups were weaned on postnatal day (P) 21 and housed in sex-matched groups of five in standard mouse cages on a 12-h light/dark cycle at a room temperature of 23°C with free access to food and water.
Mouse tails were used for genotyping as previously described (Ovanesov et al., 2008). The sequences of the primers are presented in Supplemental Table 1. The animal protocol was approved by the Johns Hopkins University Animal Care and Use Committee.
Western Blotting
In order to evaluate protein levels of mutant DISC1 across postnatal development, control and mutant male and female mice were euthanized at postnatal days (P) 0, 10, 21, 60 or 150. Brains were quickly removed and frontal cortex, hippocampus and cerebellum were isolated in ice-cold phosphate buffered saline (PBS), frozen on dry ice and were kept at 80°C until used. Expression of mutant DISC1 was measured using our published protocol (Ovanesov et al., 2008). Briefly, membranes were incubated overnight at 4°C with mouse anti-c-myc antibody (Roche Applied Science, Madison, WI, Cat#11667149001, 1:1000) to assess expression of myc-tagged mutant DISC1 followed by peroxidase-conjugated goat anti-mouse (Sigma-Aldrich, St. Louis, MO; Cat # NA931-1ML, 1:1000) secondary antibody. Optical density (O.D.) of protein bands on each digitized image was normalized to that of loading control (β-tubulin or β-actin, Cell Signaling, Danvers, MA; 1:3000). Densitometry was done using the ImageJ software (https://imagej.nih.gov/ij/). Normalized values of 3–4 mice per group of both sexes were used for analysis.
Behavioral tests
Behavioral tests were performed on 2–5 month old mice. The interval between each behavioral test was at least one week. The tests were performed in the following order: elevated plus maze, open field test, spontaneous alteration, spatial recognition memory test, novel place recognition, sociability and social novelty test, fear conditioning, and accelerating rotarod test. The behavioral protocols are described in detail in the Supplemental Materials.
Histopathological analyses
For the histopathological evaluation, we used a separate cohort of control and mutant DISC1 male and female mice (P21) and mice employed in the behavioral tests (P150). Mice were deeply anesthetized with Forane (isoflurane USP, NDC 10019-360-60, Baxter Healthcare Corporation, Deerfield, IL, USA) and transcardially perfused with 0.1 M phosphate buffer (PBS; pH 7.4) with heparin (10000 U/L), and then perfused with 4.0% paraformaldehyde in 0.1 M PBS. The brains were dissected out and post-fixed in 4.0% paraformaldehyde in 0.1 M PBS for 24 h at 4°C. After cryoprotection with 30% sucrose in 0.1 M PBS for 48 h, the brains were cut into 40 μm thick parasagittal sections. Sections were stained with cresyl violet or H&E for stereology and histopathological assessments.
Stereology assessments
We evaluated the effect of mutant DISC1 on volume of the cerebellum, total number of Purkinje cells and Purkinje cell size. Measurements and analyses were performed by an investigator blind to the groups’ identities using the Cavalieri and the Optical Disector/Fractionator and Nucleator methods (Stereo-Investigator; MBF Bioscience, Williston, VT) as previously described (Manaye et al., 2007; Subbiah et al., 1996; West, 1993). The detailed protocols are described in the Supplemental Materials.
In order to assess possible neuroinflammation in the brain of mutant mice, adjacent sections were stained with avidin–biotin immunohistochemistry (Vector, Burlingame, CA, USA) with rabbit anti-Iba1 (Wako Chemicals USA, Inc., Richmond, VA, Cat # 019-19741, 1:1000) or mouse anti-GFAP (Abcam PLC, Cambridge, MA, Cat # ab10062, 1:1000) antibodies followed by biotinylated anti-rabbit or anti-mouse Immunoglobulin G (IgG, Vector, Burlingame, CA, USA) antibodies.
Fluorescent immunostaining
Separate cohorts of male and female mice were used for immunostaining at P21. In order to visualize PCs that express mutant DISC1 for single cell immunostaining analyses and electrophysiological recording (described below), control or mutant DISC1 mice were crossed with TIGRE-Ins-TRE-tdT-D-554 (Ai63; generated and kindly provided by Dr. Hongkui Zeng at the Allen Institute for Brain Science, Seattle, WA) mice that express tdTomato under the same promoter. This breeding protocol produced double transgenic mice (Parv-2AtTA2; TIGRE-Ins-TRE-tdT-D-554) that express tdTomato only in PCs (control-tdTom mice) or triple transgenic mice (Parv2A-tTA2; TIGRE-Ins-TRE-tdT-D-554; TRE-mutant DISC1) that express both mutant DISC1 and tdTomato in the same PCs (mutant DISC1-tdTom mice). Briefly, after incubating brain sections in the blocking solution for 1 h at room temperature (RT), the sections were incubated overnight at 4°C with the primary antibodies: mouse monoclonal anti-SC35 splicing factor, (Thermo Fisher Scientific, Waltham, MA; 1:200); goat anti-mCherry, (SicGEN, Cantanhede, Portugal, Cat # AB0040-200; 1:200) or rabbit anti-Calbindin D28K (EMD Millipore, Billerica, MA, Cat # AB1778; 1:400). Afterwards, the sections were incubated for 1 h at RT with the corresponding Alexa 488-, 568-, 633-labeled species-specific secondary antibodies (Thermo Fisher Scientific, Waltham, MA former Life Technologies, Carlsbad, CA, 1:500). Images were taken with a Zeiss LSM 510 confocal laser scanning microscope with 40x/1.3 oil DIC objective at the Johns Hopkins University Neuroscience Multiphoton/Electrophysiology Core Facility.
Image analysis
To measure levels of SC35-immunioreactivity (SC35-ir) of individual PCs in confocal images of the cerebellum in mutant DISC1-tdTom and control-tdTom mice, the Imaris software (Bitplane AG, Zurich, Switzerland) was used. Using mCherry (tdTomato) channel, we selected the entire surface of the soma of a PC to generate a region of interest (ROI) defined by the program as “3D soma surface” for the selected PC. We then generated a new “SC35in” channel that included SC35-ir signal within the 3D surface of the PC. During image processing the images were compared with the original raw data. The thresholds were determined to contain PC soma and SC35 volumes inside the PC soma, and were applied to all images. Subsequently, SC35-ir intensity within the 3D surface of individual PCs was calculated as volumetric ratio of SC35-ir (Volume of SC35in/PC Soma Volume) and compared between randomly selected PCs of the cerebellar vermis of control-tdTom and mutant DISC1-tdTom mice.
In addition, we determined a correlation between calculated volume of PC soma and total volume of SC35-ir within the 3-D surface of that PC for each group of mice (17 PCs from 3 control tdTom mice and 11 PCs from 3 mutant DISC1-tdTom mice were used for analysis).
Slice preparation, electrophysiology and visualization of dendritic tree in Purkinje cells
The method used for slice preparation and recording of mEPSCs was adapted from Shin and Linden (2005)(Shin and Linden, 2005). Briefly, parasagittal slices of cerebellar vermis (300 μm thick) were prepared from control-tdTom or mutant DISC1-tdTom mice at P28-35. A vibrating tissue slicer (Leica VT1000S) was used to cut slices in ice-cold standard artificial cerebrospinal fluid (ACSF) containing (in mM): 124 NaCl, 2.5 KCl, 1.3 MgCl2, 2.5 CaCl2, 1 NaH2PO4, 26.2 NaHCO3 and 20 glucose bubbled with 95% O2 /5% CO2 (pH 7.4). Slices were allowed to recover in a submerged chamber for 30 min at 32°C, and then at RT until they were used. The slices were placed in a submerged recording chamber (SD Instruments) that was perfused at a flow rate of 2 mL/min with room temperature ACSF and 5 μM gabazine to block GABA-A receptors. Visualized whole cell patch-clamp recording was performed with a Zeiss Axioskop FS with tdTomato fluorescence to identify Purkinje cells with mutant DISC1 expression and a Multiclamp 700A amplifier (Axon Instruments, Union City, CA). Glass electrodes (PG 10165-4, World Precision Instruments) for stable recording (2–4 MΩ) of Purkinje cell miniature excitatory postsynaptic currents (mEPSCs) were filled with a solution containing (in mM) 88 Cs2SO4, 10 EGTA, 4 MgSO4, 4 CaCl2,1.5 MgCl2, 4 Na2-ATP, 0.3 Na3-GTP, and 0.1 D600 (pH 7.2) (Dittman and Regehr, 1996). Cells were voltage-clamped at −70 mV unless otherwise indicated. The currents were filtered at 2 kHz and digitized at 10 kHz. For mEPSC analysis, a template was made to detect events in pClamp10 software (Axon Instruments), by averaging 30 hand-picked mEPSC events. When detecting events, the template match threshold was set to 4. For extracellular stimulation, standard patch pipettes filled with ACSF were used. To estimate paired-pulse ratio, parallel fibers were stimulated in the distal part of the molecular layer. Test stimulation was given using paired-pulses (80 ms or 120 ms intervals) at a frequency of 0.33 Hz using 10–20 μA pulses (100-μs duration). Stimulus strength was adjusted so that the first EPSC did not exceed 200 pA. After 15–20 single EPSCs with stable amplitude a total of 20 trains (alternating 10 trains with 80 ms and 10 trains with 120 ms intervals) were applied at 20 s intervals one by one.
The method of extracellular recordings was adapted from Dizon and Khodakhah (2011) (Dizon and Khodakhah, 2011). Briefly, the slice temperature was maintained at 35+1 °C by adjusting the temperature of the bathing solution. Single-unit extracellular recordings were made from cerebellar Purkinje cells of anterior lobules with glass pipettes filled with ACSF. Purkinje cells with mutant DISC1 expression were identified by their tdTomato fluorescence. The pipette tip was placed near the axon hillock where the largest current changes were recorded (Womack and Khodakhah, 2004), resulting in spike heights of ~ 50–400 pA. A total of 20 epochs with a 5-s duration were recorded with minimal pauses between epochs.
To select healthy Purkinje cells for analysis and to quantify PC dendritic tree morphology, a fluorescent dye, Alexa 488 (Thermo Fisher Scientific, Waltham, MA former Life Technologies, Carlsbad, CA) was added in the recording pipette solution. Alexa 488 was excited by an Argon ion laser (488 nm), and emission was collected using a 505-nm dichroic mirror and a 505–530 nm bandpass filter. Images of PCs were taken 30 min after patching the cell in z-stack confocal mode with 1024 x 1024 pixel resolution and z-spacing 1 μm. For image analysis we used a method adapted from Kaneko et al. (2011) (Kaneko et al., 2011). Briefly, the dendrites of stained PCs were reconstructed in 3-D and automatically measured using Imaris software (Bitplane AG, Zurich, Switzerland). The area occupied by the dendritic tree (a polygon of dendritic tips), length of a dendrite branch, total length of all dendrites and total number of dendrite branches for each PC were calculated and used for analysis.
SR 95531 hydrobromide (gabazine) was purchased from Tocris Cookson (Ellisville, MO), and TTX from Abcam (Cambridge, MA). All other chemicals were purchased from Sigma-Aldrich (St. Louis, MO).
Statistical analysis
Results are expressed as mean ± standard error of the mean (±SEM) throughout. The behavioral data was analyzed with ANOVA, with the genetic background, sex and time of testing (if applicable) as independent variables. Significant main effects were explored further with lower level ANOVAs and/or post hoc comparisons. The morphometric results and electrophysiological data were analyzed with two-tailed Student t-test. p<0.05 was used for the significance level.
Results
Selective expression of mutant DISC1 in Purkinje cells
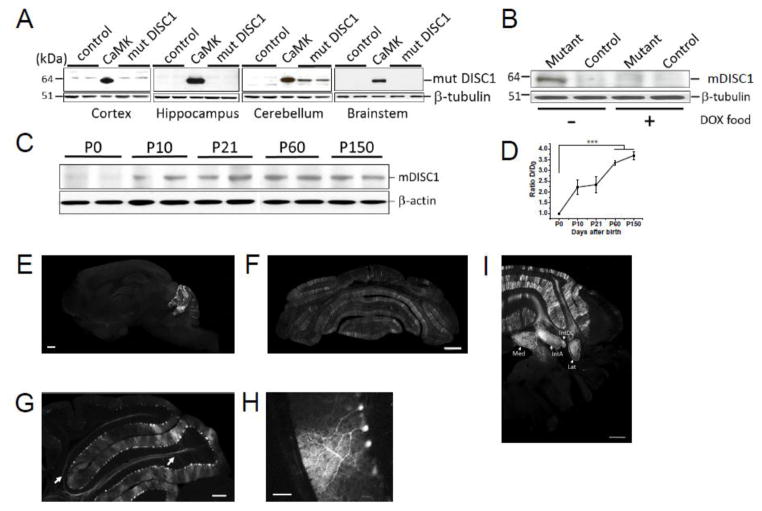
We detected expression of mutant DISC1 in the cerebellum but not in the cortex or hippocampus (Fig. 1A). Consistent with the features of a Tet-off system, cerebellum-restricted expression can be regulated by doxycycline (Fig. 1B). Expression of mutant DISC1 in the cerebellum starts at approximately the first postnatal week and rises to the maximal levels by adulthood (Fig. 1C–D). In order to identify the cerebellar cells that express mutant DISC1, Parv-2AtTA2 mice were crossed with the reporter line, TIGRE-Ins-TRE-tdTomato-D-554. tdTomato fluorescence was observed in Purkinje cells (PCs) of the cerebellar vermis and hemispheres (Fig. 1E–H). In the cerebellar vermis, tdTomato-positive PCs were predominantly found in lobuli II - VI of the anterior cerebellum. Fewer tdTomato-positive PCs were observed in lobuli VI (the external side) – X of posterior cerebellum (Larsell, 1970) (Fig.1E, SFig. 2). We also observed axons of tdTomato-positive PCs in the white matter and cerebellar nuclei (Fig. 1E, 1I). No other types of tdTomato-positive cells were seen in the cerebellum or other brain regions.
Figure 1. Selective expression of mutant DISC1 in the cerebellum.
(a) Western blotting detection of mutant DISC1 revealed selective expression of mutant DISC1 in the cerebellum but not in the cortex, hippocampus or brainstem; tissue brain samples were collected from cortex, hippocampus, cerebellum and brainstem of mutant DISC1 (mDISC1; double transgenic mice with expression of mDISC1) and control (single transgenic mDISC1 mice without expression of this mutant protein) mice; CaMKII denotes a forebrain sample from a mouse with expression of mDISC1 driven by the CaMKII promoter; this sample was used as a positive control (Pletnikov et al., 2008); mDISC1 was visualized with anti-c-myc antibody (1:1000);
(b) Consistent with the properties of the Tet-off system, adding doxycycline (DOX) to mouse food shuts down expression of mDISC1. Note the absence of expression of mutant DISC1 (mDISC1) in the sample from a mouse maintained for at least 7 days on DOX-containing food (DOX food); mDISC1 (64 kDa) was visualized with anti-c-myc antibody (1:1000), β– tubulin was visualized with anti-tubulin antibody (1:3000);
(c) Expression of mDISC1 was minimal in newborn mice and was gradually rising by adulthood. Whole cerebellum tissue samples were collected from mutant DISC1 mice at P0, P10, P21, P60, and P150; mDISC1 (64 kDa) was visualized with anti-c-myc antibody (1:1000), β– actin was visualized with anti-actin antibody (1:3000).
(d) Quantitative analysis of expression of mDISC1 across postnatal period confirmed the WB results, with expression of mDISC1 being minimal at P0 and significantly rising by adulthood, n=3–4 in each sample; data are mean ± SEM; one-way ANOVA with Bonferroni correction of the expression data showed a significant effect of time, F(4, 15)= 23.39, p<0.001 ; post-hoc comparisons showed significantly greater expression at P60 and 150 compared to P0, all ps<0.05
(e–i) Crossing TRE-tdTomato reporter mice and Parv2A-tTA mice allowed us to evaluate the regional and cell type activity of the Parv2A-promoter used to drive expression of mDISC1; we found selective activity of the Parv2A promoter in the cerebellum but not in other brain regions consistent with our WB data (e–f, scale bar –500 μm, f – Bregma -5.64mm); and selective activity of the Parv2A promoter in PCs but not in other type of cerebellar cells (g–h; scale bar –200 μm for g and 50 μm for h). Note dtTomato+ fibers that are likely axons of PCs (g, arrows) that innervate the cerebellar nuclei: Med – medial nucleus; IntA – interposed nucleus, anterior part; IntDL interposed nucleus, dorsolateral protuberance; Lat lateral nucleus (i; scale bar – 400 μm, Bregma -6.0 mm).
Impaired social and spatial recognition
There are multiple reports on behavioral effects of mutant (a.k.a. dominant-negative or C-terminus truncated) DISC1 (Ayhan et al., 2011; Kaminitz et al., 2014; Kuroda et al., 2011; Ma et al., 2013; Ovanesov et al., 2008) . Thus, we performed a series of behavioral tests to assess whether selective expression of mutant DISC1 in PCs would affect different domains of mouse behavioral repertoire.
Novel place recognition test
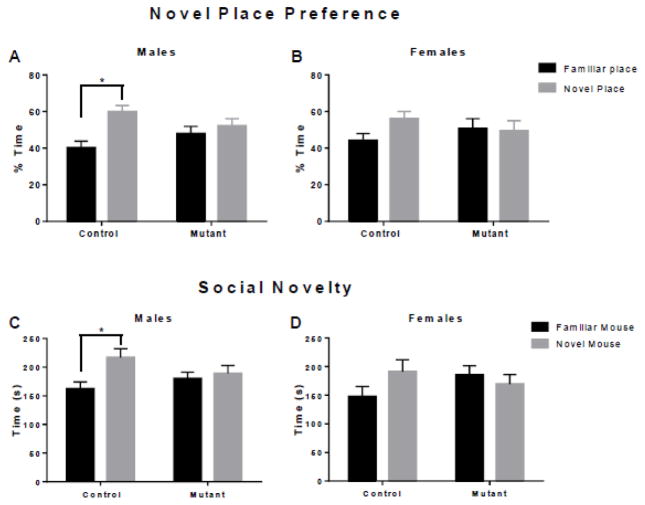
To evaluate the effects of mutant DISC1 on novel place recognition, mice were tested in the novel place test (Antunes and Biala, 2012; Leger et al., 2013). Male control mice showed a preference for an object in a new location (t=3.22, p=0.004 (Fig. 2A) and female control mice displayed a trend for a preference of an object in a new location (t=2.01, p=0.10) (Fig. 2B). Both male and female mutant DISC1 mice, however, showed no preference for an object in a new location (t=0.80 p=0.67, t=0.16, p=0.98) (Fig. 2A–B).
Figure 2. Impaired recognition memory in mutant DISC1 mice.
(a) Novel place recognition test; control but not mutant DISC1 male mice spent significantly more time exploring an object moved to a novel place compared to the identical object remaining in the familiar place; the Y axis shows the time of exploration of the object in the familiar (black bar) or the novel (grey bar) place as the percentage of the total time spent exploring two objects in both places; n=12–16 in each group; * denotes p< 0.05;
(b) Control but not mutant DISC1 female mice displayed a strong trend towards preference for the object moved to the novel place, n=9–13 in each group; all labels are same as in (a);
(c) Social novelty preference test; control but not mutant DISC1 male mice spent significantly more time exploring an unfamiliar mouse compared to the familiar one; the Y axis shows the time of exploration of familiar (black bar) or novel (grey bar) mouse as the percentage of the total time spent exploring two mice; n=12–16, * denotes p<0.05;
(d) Control but not mutant DISC1 female mice showed a strong trend towards preference for an unfamiliar mouse, n=9–13; all labels are same as in (c).
Social behaviors
Mice were tested in a 3-chamber test box (Moy et al., 2004). All groups of mice preferred a mouse over an inanimate object, F(1, 46)=14.59, p=0.02 (SFig. 3A–B). In the preference for social novelty test, planned comparisons indicate that control male mice prefer a novel mouse (t=2.76, p=0.01) and control female mice show a trend toward a preference for a novel mouse (t=1.60, p=0.12), but neither mutant male nor female mice do (t=0.504, p=0.62 and t=0.70, p=0.50) (Fig. 2C–D).
Anxiety related behavior
To assess anxiety related behavior, mice were tested in the elevated plus maze, a standard test for anxiety in rodents (Pellow et al., 1985; Walf and Frye, 2007). There was no effect of sex, F(1, 46)=0.27, p=0.61, or genotype, F(1, 46)=3.28, p=0.08, on the percent time spent in the open arms (SFig. 4). Furthermore, expression of mutant DISC1 in PCs did not alter locomotor behavior in the open field test, F(3, 46)=0.17, p=0.92 (SFig. 5).
Spatial working and recognition memory
To assess spatial working memory and spatial recognition memory, mice were tested in the Y-maze (Deacon and Rawlins, 2006) . There was no effect of genotype on spontaneous alternations in the Y-maze, F(1, 46)=0.42, p=0.52 (SFig. 6A). There was no effect of genotype on spatial recognition measured as the time spent in the previously blocked arm of the Y maze, F(1, 46)=0.91, p=0.35 (SFig. 6B).
Fear conditioning
To determine the effect of mutant DISC1 on associative learning, fear conditioning was conducted where mice learned to associate a context and a tone with a foot shock. Freezing behavior increased significantly over time for all groups as the cue and shock were presented together during training, F(4, 184)=35.41, p<0.0001 (SFig. 7A). There were no genotype-dependent differences in the context-dependent freezing behavior, F(1, 46)=0.37, p=0.55, or to the cue, F(1, 41)=0.02, p=0.89 (SFig. 7B–C). There was, however a significant difference in the cue-dependent freezing behavior between male and female mice, F(1, 41)=17.87, p=0.0001 (SFig. 7C).
Effect of mutant DISC1 expression in PC on motor coordination
To determine the effect of mutant DISC1 expression in PC on motor coordination, mice were assessed in the accelerating rotarod test. There were no differences between genotypes in the amount of time mice stayed on the rotarod, F(1, 26)=0.004, p=0.95 (SFig. 8).
Collectively, our behavioral results indicated impaired recognition memory in the novel place and novel social partner recognition tests in mutant DISC1 mice.
Decreased number of PCs with bigger soma size
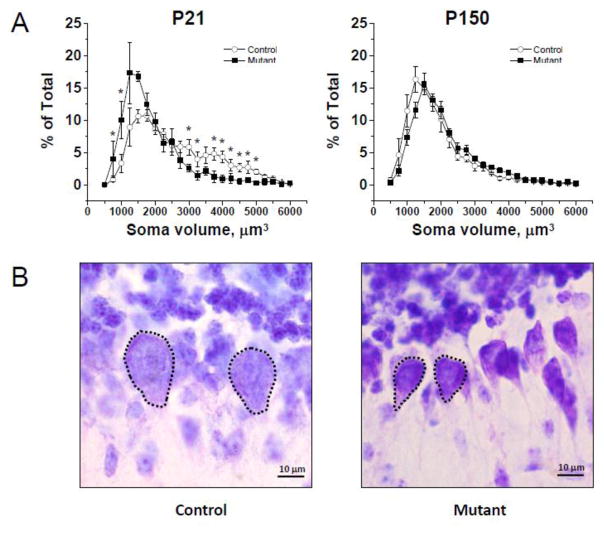
Various brain abnormalities (e.g., lateral ventricle enlargement) have been reported in several DISC1 mouse models (Ayhan et al., 2011; Doyle et al., 2015; Hikida et al., 2007). Thus, we assessed the brain effects of mutant DISC1 at postnatal day 21 (P21) and P150. We found no genotype-dependent effects on total volume of the cerebellum or total number of PCs (SFig. 9A–B). DISC1 has been also implicated in the regulation of cell soma size and neurite outgrowth (Duan et al., 2007; Kang et al., 2011; Kim et al., 2009). Analysis of PC soma size across of a range of the values revealed that compared to control mice, mutant DISC1 mice had significantly more PCs with smaller soma sizes (750–1000 μm3) and significantly fewer PCs with larger soma sizes (4000–5000 μm3) at P21 but not P150. Two-way repeated measures ANOVA of the PC size results found a significant effect of cell size, F(22,137)=28.81, p<0.001, as well as the genotype by PC size interaction, F(22,137)=3.71, p<0.001 (Fig. 3A–B). These results indicate that mutant DISC1 mice affected PC growth, leading to decreased PC soma size.
Figure 3. Fewer PCs with large soma in mutant DISC1 mice.
(a) Quantitative analyses of volumes of PCs across the range of different values found the significantly greater number of PCs with small soma in mutant DISC1 mice and the significantly greater number of PCs with large soma in control mice at P21 but not P150; * denotes p<0.05 vs. the opposite group; the Y axes show the percentage of PCs of different soma size ranges that are depicted on the X axis;
(b) Representative images of PCs with the large soma, 4000–5000 μm3, (the left panel, Control) and smaller soma, 1000–2000 μm3, (the right panel, Mutant) in the cerebellum of mice at P21, scale bar –10 μm. PC somas are outlined with dotted lines.
We next evaluated whether mutant DISC1 could also influence the dendritic arborization of PCs. As strong overlapping of dendritic trees of neighboring PCs complicates estimation of dendritic morphology of PCs in situ, we performed a single cell analysis by sparingly filling PCs of the bank region with the fluorescent dye Alexa 488 through the recording pipette using ex vivo cerebellar slices (as described below). We found no significant genotype-dependent alterations in dendritic arborization of PCs in any of the measures taken, including area occupied by the dendritic tree (a polygon of dendritic tips), average length of dendrites, total length of all dendrites, or average number of dendritic branches (SFig. 10A–E).
As there is a possibility that expression of a heterologous mutant protein might trigger the innate immune response in the brain, we assessed the cellular markers of immune activation in the brain in mutant DISC1 mice. No up-regulation of the cellular markers of immune activation or inflammation (i.e., Iba1 and GFAP) was observed in the entire cerebellum of mutant DISC1 mice (SFig. 11A–B), suggesting that decreased soma size in mutant DISC1 PCs was unlikely related to the activation of the local innate immune response in the brain.
Reduced PC expression of the splicing factor, SC35
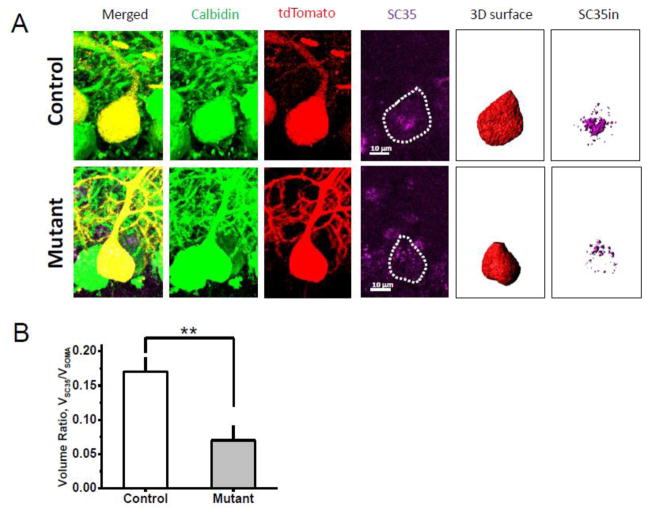
Reduction of total RNA and rRNA expression, as well as protein translation, has been found in the hippocampus and primary neurons that express mutant DISC1(Ji et al., 2014), suggesting that abnormalities in PC soma size distribution in mutant mice might be, at least in part, associated with the effects of mutant DISC1 on splicing and translational processes. We measured expression of the splicing factor, SC35, an indicator of RNA/protein synthesis (Sahebi et al., 2016; Zhou and Fu, 2013). We first measured levels of SC35-immunoreactivity (SC35-ir) in single PCs of control and mutant mice and then calculated a correlation between SC35-ir and the volume of PCs (Fig. 4A). We found a significant reduction of SC35-ir volumetric density (defined as a ratio of SC35-ir/Volume of PC soma) in mutant DISC1 mice compared to control mice (Fig. 4B). There was also a significant correlation between volume of SC35in inside PC soma and volume of the soma in control PCs (r=0.91, p<0.05; n=17 cells analyzed from 3–4 mice), and the absence of correlation in mutant DISC1 mice (r= −0.14, n=11 cells analyzed from 3–4 mice). These findings suggest mutant DISC1 could affect expression of the SC35 splicing factor, possibly accelerating maturation and growth of PCs.
Figure 4. Decreased expression of the splicing factor, SC35, in mutant PCs.
a) Representative images of immunostaining of PCs of control-tdTom mice (Control; the upper row) and mutant DISC1-tdTom mice (Mutant; the bottom row); anti-MCherry staining (red) anti-Calbindin staining (green); and anti-splicing factor SC35 (purple, PC somas are outlined with dotted lines); scale bar – 10 μm; using MCherry (tdTomato) channel, we created the entire surface of the soma of a PC (3D surface) and generated a new “SC35in” channel that included SC35-ir (purple) signal within the 3D surface of the PC soma and calculated SC35in volume;
(b) Quantitative analysis of SC35-ir in PC presented as the ratio of volume of the SC35-ir within the PC (i.e., 3D volume) to total volume of the soma of the selected tdTom+ PC; decreased SC35 volume ratio was detected in mutant DISC1 PCs compared to control PCs control – control-tdTom mice (n=17 PCs from 3 mice); mutant–mutant DISC1-tdTom mice (n=11 PCs from 3 mice); * denotes p=0.0018 vs. control mice.
Increased amplitude and frequency of mEPSCs and spontaneous firing in PCs
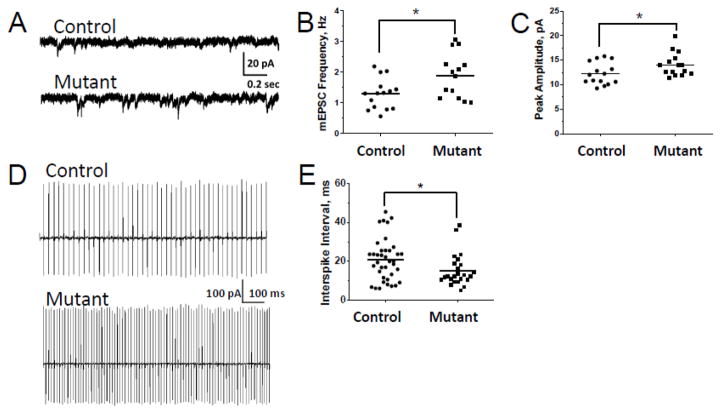
Altered electrophysiological and synaptic characteristics have been reported in several mutant DISC1 models. For example, mutant DISC1 reduced excitability of pyramidal neurons of the layer II/III of the medial prefrontal cortex and increased a transmitter release (Juan et al., 2014), enhanced mEPSCs frequency and altered kinetics of the evoked glutamate transient in cortical pyramidal neurons of layer 2/3 (Maher and LoTurco, 2012) and increased spontaneous EPSCs in forebrain neurons (Holley et al., 2013). Thus, we evaluated the effects of mutant DISC1 on the physiology of PCs. To assess the pre- and/or postsynaptic effects of mutant DISC1, miniature excitatory synaptic currents (mEPSCs) were recorded from PCs in the presence of 500 nM tetrodotoxin (TTX). We found that the frequency and amplitude of mEPSCs mediated by glutamatergic synaptic activity were significantly increased in mutant DISC1-tdTom PCs compared to controls (Fig. 5A–C). We did not find any group differences in mEPSC kinetics as indexed by the time to peak, half-width or 90–10% decay time (SFig.12A–C). We observed no significant differences in the input resistance between control and mutant PCs (233±28 and 244±19 MΩ, respectively, p=0.77; n=15).
Figure 5. Increased frequency and amplitude of mEPSCs and firing in mutant PCs.
(a) Parasagittal slices of cerebellar vermis (300 μm thick) were prepared from control-tdTom or mutant DISC1-tdTom mice at P28-35 for whole cell patch-clamp recording of PCs mEPSCs; representative traces from PCs of a control-tdTom (control) and a mutant DISC1-tdTom (mutant DISC1) mouse are presented; TTX (500 nM) was bath-applied to the ACSF solution at least for 10 min before the recording;
(b) Quantitative analysis of frequency of mEPSCs in PCs revealed a significantly greater frequency of mEPSCs in mutant DISC1-tdTom PCs (Mutant, n=14 cells from 5 mice) compared to control-tdTom PCs (Control, n=15 cells from 8 mice); * denotes p=0.014 vs. control mice;
(c) Quantitative analysis of the PC peak amplitude revealed a significantly greater amplitude of mEPSCs in mutant DISC1-tdTom PCs (Mutant, n=14 cells from 5 mice) compared to control tdTom PCs (Control, n=15 cells from 8 mice);* denotes p=0.039 vs. control mice;
(d) Attached loose patch recording was performed to assess spontaneous firing activity of PCs; representative traces of spontaneous firing of PCs of control-tdTom (control) and mutant DISC1-tdTom (mutant DISC1) mice;
(e) Quantitative analysis showed a significant decrease of the inter-spike interval in PCs of control-tdTom (Control, n=29 cells from 4 mice) compared to that in PCs of mutant DISC1-tdTom (Mutant, n=15 cells from 4 mice); * denotes p=0.018 vs. control mice.
Greater frequency and amplitude of mEPSCs in mutant PCs may be a result of increased probability of presynaptic release and/or altered sensitivity of the postsynaptic membrane. Thus, we assessed the effects of mutant DISC1 on the probability of glutamate release by measuring paired-pulse ratios (PPR) of evoked parallel fiber EPSCs in PCs of control-tdTom and mutant DISC1-tdTom mice. PCs were patch-clamped and held at 70 mV. A stimulation electrode was placed in the distal part of the molecular layer, and a paired-pulse test stimulus (80- or 120-ms interval) was delivered every 20 s to activate parallel fibers. No genotype-related differences in PPR were observed (SFig. 13A-B), arguing against a difference in probability of glutamate release underlying the observed increase in mEPSC frequency.
To assess whether increased mEPSCs in mutant DISC1 PCs could give rise to alterations in spontaneous spiking activity PCs, we used loose-patch attached extracellular recordings on single PCs in cerebellar slices of mutant DISC1-tdTom and control-tdTom mice without any receptor antagonists in ACSF. Compared to control PCs, we found a significant decrease in the inter-spike interval in mutant DISC1 PCs (Fig. 5D–E). Thus, mutant DISC1 increased amplitude and frequency of mEPSCs and spiking activity of PCs, indicating that expression of mutant DISC1 could affect these neurophysiological functions of PCs.
Discussion
We generated a mouse model of selective and inducible expression of mutant DISC1 in Purkinje cells (PCs), with its predominant expression in the anterior cerebellum. Although mutant DISC1 mice did not show gross cerebellar abnormalities, expression of mutant DISC1 in PCs led to impaired social and novel placement recognition in male but not female mice. These behavioral abnormalities were associated with a decreased number of large soma PCs, increased amplitude and frequency of mEPSCs and increased spontaneous firing of PCs as assessed at P21.
Possible outcomes of the chromosomal translocation in the DISC1 Scottish pedigree include haploinsufficiency or the putative production of a mutant truncated DISC1 protein. The truncated human DISC1 may lose its normal localization and association with interacting proteins and affect the organization of protein interacting complexes via dominant-negative mechanisms (Kamiya et al., 2005; Ovanesov et al., 2008; Pletnikov et al., 2007). In the context of the present findings, it appears interesting to compare our data with those reported for several mouse models that over-express dominant-negative (a.k.a. mutant) DISC1 in forebrain neurons. Hikida et al. generated the model of constitutive expression of mutant DISC1 driven by the Ca2+/calmodulin-dependent protein kinase II (CAMKII) promoter (Hikida et al., 2007). In this model, mutant DISC1 was found in the olfactory bulb, frontal cortex, hippocampus, and basal ganglia starting from the neonatal period. Mutant DISC1 mice displayed increased activity, deficits in prepulse inhibition of the acoustic startle and increased immobility in the Porsolt forced swim test. These mice were also less sociable and exhibited decreased exploration of a novel an inanimate object. In addition, decreased reversal learning and mental flexibility in cognitive and reward-related paradigms were observed (Johnson et al., 2013). Another model of inducible expression of mutant human DISC1 in forebrain neurons was generated using the Tet-off system (Ovanesov et al., 2008). In this model, expression of mutant DISC1 is also regulated by the CAMKII promoter and can be turned off by adding tetracycline or a related compound, doxycycline, to food or water. Similar to other mutant DISC1 mouse models, inducible expression of mutant DISC1 driven by the CAMKII promoter produced no gross developmental defects but significantly increased spontaneous locomotor activity in male but not female mice, decreased social interaction in male mice, enhanced their aggressive behavior and was associated with poorer spatial memory in the Morris water maze task in female mice (Ayhan et al., 2011; Ovanesov et al., 2008). Expression of mutant DISC1 driven by the hamster Prion protein promoter led to increased spontaneous and methamphetamine-induced locomotor activity, deficits in PPI and increased immobility in FST only after adolescent isolation stress (Niwa et al., 2013)
Collectively, the above data indicate that behavioral outcomes of over-expression of mutant DISC1 are dependent on where and when this dominant-negative factor is expressed.
Our behavioral data on autistic-like behaviors in mice with predominant expression of mutant DISC1 in PCs are consistent with those reported for various genetic and environmental models focused on cerebellar pathology and dysfunction, including the maternal immune activation mouse model (Shi et al., 2009; Xu et al., 2013), an early postnatal hypothyroidism rat model (Akaike et al., 1991; Sadamatsu et al., 2006), Staggerer mice (Doulazmi et al., 2001; Goldowitz and Koch, 1986; Herrup and Mullen, 1979; Herrup et al., 1996; Lalonde et al., 1996; Misslin et al., 1986), Shank2 and 3 knockout (KO) mice (Beri et al., 2007; Jiang and Ehlers, 2013; Peca et al., 2011; Peter et al., 2016), Enlarged2 KO mice (Brielmaier et al., 2012; Cheh et al., 2006; Kuemerle et al., 2007; Kuemerle et al., 1997; Millen et al., 1994), Fmr1 KO mice (Ellegood et al., 2010; Koekkoek et al., 2005; Olmos-Serrano et al., 2011; Yuskaitis et al., 2010), and PTEN conditional KO mice (Cupolillo et al., 2016).
Similar to the mild brain abnormalities in most DISC1 mouse models (Ayhan et al., 2011; Ovanesov et al., 2008), expression of mutant DISC1 did not produce gross pathology of the cerebellum. Still, it is tempting to speculate that the altered social behavior and deficient recognition memory may at least in part result from the cellular pathology observed in the cerebellum of mutant DISC1 mice. There were significant effects of mutant DISC1 on PC soma sizes at P21, with the genotype-related effect being insignificant at P150. We found a significantly greater number of smaller PCs in mutant mice and significantly more PCs with larger sizes were observed in control mice. Given the role of DISC1 in cell growth (Duan et al., 2007; Kang et al., 2011; Lee et al., 2015; Ren et al., 2016) and postnatal increase in PC soma size (Takacs and Hamori, 1994), one could speculate that expression of mutant DISC1 might affect PC growth, leading to an increased number of smaller PCs in mutant mice. This suggestion appears consistent with the findings of smaller PC soma size in the cerebellar vermis of patients with autism (Fatemi et al., 2002), although we see this difference only in early life in the present mutant mice.
One potential underlying mechanism of stunted growth of individual PCs in mutant mice could include decreased transcriptional and/or translational activity produced by mutant DISC1. Thus, we evaluated levels of expression of Serine/arginine-rich splicing factor 2 (SRSF2, a.k.a. SC35) that is a member of the SR family of proteins involved in RNA splicing (Sahebi et al., 2016; Zhou and Fu, 2013). In support of our hypothesis, we found decreased expression of SC35 in PCs that also express mutant DISC1. Moreover, there was a significant positive correlation between PC soma size and level of SC35, with bigger PC somas having greater expression of SC35. Our results are in line with previous reports on the effects of mutant DISC1 on rRNA expression and protein translation in the hippocampus and primary neurons from DISC1 humanized mice (Ji et al., 2014). In contrast to soma size effects, no significant effects of mutant DISC1 were found on dendritic arborization of PCs in mutant DISC1 mice. Our findings appear inconsistent with several studies that demonstrate that manipulation of expression of DISC1 in neurons leads to abnormal formation of axons or dendrites (Duan et al., 2007; Kaneko et al., 2011; Kang et al., 2011; Lee et al., 2015; Shinoda et al., 2007; Steinecke et al., 2012). It is possible that postnatal expression of mutant DISC1 might have more subtle effects on PC physiology that might underlie the behavioral phenotypes observed in mutant mice.
In order to address this possibility, we recorded miniature excitatory postsynaptic currents (mEPSC) in PCs and found significantly increased frequency and peak amplitude in mutant DISC1 PCs compared to control ones. These synaptic abnormalities can be a result of changes in postsynaptic functions and/or presynaptic probability of the glutamate release. However, no alterations in paired-pulse ratio were observed in mutant PCs, suggesting that the increased frequency and amplitude of mEPSCs in mutant PCs is more likely due to some postsynaptic dysfunction.
PCs spontaneously fire action potentials (Llinas and Sugimori, 1980a; Llinas and Sugimori, 1980b; Nam and Hockberger, 1997; Womack and Khodakhah, 2002). This tonic pacemaking activity of PCs is assumed to be crucial for the correct encoding of cortical cerebellar information (De Zeeuw et al., 2011; Hoebeek et al., 2005; Kasumu and Bezprozvanny, 2012). Thus, we sought to evaluate how increased frequency of mEPSCs influences spontaneous spiking in PCs. We found a shorter inter-spike intervals in mutant DISC1 PCs. Our results are congruent with synaptic changes observed in other genetic mouse models of PCs abnormalities. Conditional deletion of the protein rictor (rapamycin-insensitive companion of mTOR) in PCs reduced frequency and amplitude of mEPSCs (Thomanetz et al., 2013). Although no synaptic alterations were found in mice that express mutant Tuberous Sclerosis Complex 1 (TSC1) protein, there was a significantly lower graded spontaneous spiking rate and reduced excitability in mutant TSC1 PCs (Tsai et al., 2012). Homozygous Staggerer mice (sg/sg) with functional loss of the transcription factor retinoid-related orphan receptor α (RORα) have anomalous passive electrical properties of PCs, parallel fibers-evoked EPSCs with faster kinetics and a slightly decreased paired pulse facilitation (Mitsumura et al., 2011). Expression of human mutant ataxin-2 under the PC-specific L7/pcp2 promoter increased the percentage of PCs with bursting and an irregular pattern of spontaneous activity (Egorova et al., 2016). PC pacemaker firing becomes disrupted and is accompanied by abnormal depolarization after the onset of motor dysfunction in SCA1[82Q] mice (Dell'Orco et al., 2015). Thus, similar to other genetic mutations expressed in PCs, mutant DISC1 affects synaptic and spontaneous firing activities of PCs.
There are multiple reports on the role of DISC1 in functioning of different types of neurons. Duan et al. (2007) (Duan et al., 2007) found lower membrane resistances and higher excitability in hippocampal newborn granular cells at 14 dpi after DISC1 knockdown. DISC1 knockdown was also shown to produce longer bursting activity of hippocampal primary cultured neurons at DIV11-12 (MacLaren et al., 2011). Patch-clamp analysis revealed no genotype dependence of the intrinsic properties of CA1 pyramidal neurons, but enhanced theta burst-induced long-term potentiation in the Schaffer collateral commissural pathway in mice expressing mutant DISC1 in pyramidal neurons (Booth et al., 2014). DISC1Tm1Kara mice demonstrated a significant decrease in input resistance, a decrease in excitability and reduced profound short-term synaptic plasticity (Kvajo et al., 2011). Expression of mutant DISC1 in forebrain neurons produced greater spontaneous EPSCs, the increased ratio of excitatory to inhibitory events and diminished cortical GABAergic neurotransmission in male mutant mice, with female mutant DISC1 mice showing increased frequency of small-amplitude sIPSCs (Holley et al., 2013). Mutant DISC1 mice also demonstrated a reduced excitability of pyramidal neurons of the layer II/III of the medial prefrontal cortex and increased a transmitter release (Juan et al., 2014). In utero electroporation of truncated (mutant) DISC1 enhanced mEPSCs frequency and altered kinetics of the evoked glutamate transient in cortical pyramidal neurons of layer 2/3 (Maher and LoTurco, 2012). Taken together, the available data indicate that perturbation of expression of DISC1 in neurons either with a RNAi approach or with expression of DN-DISC1 can be associated with changes in both intrinsic and synaptic properties of targeted neurons. The nature of the outcome is dependent on the method by which levels of endogenous DISC1 were affected, time of changes and the brain region being evaluated. The future studies will utilize the inducible feature of this model to determine timing and reversibility of the effects of mutant DISC1 on PC physiology and associated behaviors to evaluate whether the actions of DISC1 could be a target for pharmacological manipulation.
Our findings demonstrate expression of mutant DISC1+ PCs and their axonal projections in the medial, anterior interposed and lateral and posterior interposed nuclei. Functional roles of the cerebellar nuclei have been extensively studied. The consensus is that PCs of the vermis are mostly involved in regulation of locomotion, while PCs of the cerebellar hemispheres are important for cognitive function (Buckner, 2013; Klein et al., 2016; Šveljo and Ćulić , 2015). Similarly, a gradient of functional specialization has been reported for the cerebellar nuclei. The medial and interposed nuclei have been implicated in motion and assessing the body’s position, while the lateral nuclei may be involved in cognition and emotions (Brooks and Cullen, 2009; Strick et al., 2009). Given the wide distribution of various cerebellar projections in the cortex via the thalamic relay nuclei, one could speculate that expression of mutant DISC1 in the vermis and hemispheres could at least in part contribute to deficient recognition memory in mutant DISC1 mice. Additional studies are necessary to identify the circuits that mediate the cognitive effects of mutant DISC1+ PCs.
In conclusion, the present data demonstrate that altered functioning of DISC1 in PCs of the cerebellum could lead to abnormal cell growth and synaptic properties that might contribute to impaired object and social recognition memory in mice, consistent with aspects of schizophrenia and ASD.
Supplementary Material
Highlights.
Expression of mutant DISC1 in Purkinje cells (PCs) of the cerebellum impaired novel place and social recognition memory
Expression of mutant DISC1 decreased the number of PCs with large body size without affecting the total number or dendritic arborization of PCs at P21 but not P150
Expression of mutant DISC1 increased spontaneous spiking activity as well as the amplitude and frequency of mEPSCs in PCs at P21
DISC1 may be involved in physiology of PCs to contribute to cognitive and social behaviors
Acknowledgments
The authors are grateful to Dr. Hongkui Zeng (Allen Institute for Brain Science, Seattle, WA) for providing the Parv-2AtTA2 and TIGRE-Ins-TRE-tdT-D-554 mice, Mrs. Olga A. Mychko for help with immunostaining, Dr. Stanislav A. Gordiienko for help with analysis of PC size, Dr. Sofya Abazyan for help with Western blotting; Mrs. Chunxia Yang for maintaining mouse cohorts; Drs. Matthew Gastinger (Bitplane USA), Michele Pucak, Amit Agarwal, and Shu-Ling Chiu for their help with confocal microscopy, immunostaining, individual cell analysis of SC35-immunoreactivity and analysis of PC dendritic arborization; and Dr. Hirofumi Fujita for his help with analysis of tdTomato positive Purkinje cell projections in cerebellar nuclei.
Finding: The study was supported by 1F05MH097457-01 (AVS) and 5R01MH083728-04 (MVP and AVS); the work at the JHU Neuroscience Multiphoton Imaging Core was supported by the NINDS grant P30NS050274.
Footnotes
Disclosure
The authors declare no competing financial interest and have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akaike M, et al. Hyperactivity and spatial maze learning impairment of adult rats with temporary neonatal hypothyroidism. Neurotoxicol Teratol. 1991;13:317–22. doi: 10.1016/0892-0362(91)90077-a. [DOI] [PubMed] [Google Scholar]
- Amaral DG, et al. Neuroanatomy of autism. Trends Neurosci. 2008;31:137–45. doi: 10.1016/j.tins.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Pierson R. The role of the cerebellum in schizophrenia. Biol Psychiatry. 2008;64:81–8. doi: 10.1016/j.biopsych.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antunes M, Biala G. The novel object recognition memory: neurobiology, test procedure, and its modifications. Cogn Process. 2012;13:93–110. doi: 10.1007/s10339-011-0430-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin CP, et al. DISC1 (Disrupted in Schizophrenia-1) is expressed in limbic regions of the primate brain. Neuroreport. 2003;14:951–4. doi: 10.1097/01.wnr.0000074342.81633.63. [DOI] [PubMed] [Google Scholar]
- Ayhan Y, et al. Differential effects of prenatal and postnatal expressions of mutant human DISC1 on neurobehavioral phenotypes in transgenic mice: evidence for neurodevelopmental origin of major psychiatric disorders. Mol Psychiatry. 2011;16:293–306. doi: 10.1038/mp.2009.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beri S, et al. DNA methylation regulates tissue-specific expression of Shank3. J Neurochem. 2007;101:1380–91. doi: 10.1111/j.1471-4159.2007.04539.x. [DOI] [PubMed] [Google Scholar]
- Blackwood DH, et al. Schizophrenia and affective disorders--cosegregation with a translocation at chromosome 1q42 that directly disrupts brain-expressed genes: clinical and P300 findings in a family. Am J Hum Genet. 2001;69:428–33. doi: 10.1086/321969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth CA, et al. Neurophysiological modification of CA1 pyramidal neurons in a transgenic mouse expressing a truncated form of disrupted-in-schizophrenia 1. Eur J Neurosci. 2014;39:1074–90. doi: 10.1111/ejn.12549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bord L, et al. Primate disrupted-in-schizophrenia-1 (DISC1): high divergence of a gene for major mental illnesses in recent evolutionary history. Neurosci Res. 2006;56:286–93. doi: 10.1016/j.neures.2006.07.010. [DOI] [PubMed] [Google Scholar]
- Brielmaier J, et al. Autism-relevant social abnormalities and cognitive deficits in engrailed-2 knockout mice. PLoS One. 2012;7:e40914. doi: 10.1371/journal.pone.0040914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks JX, Cullen KE. Multimodal integration in rostral fastigial nucleus provides an estimate of body movement. J Neurosci. 2009;29:10499–511. doi: 10.1523/JNEUROSCI.1937-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL. The cerebellum and cognitive function: 25 years of insight from anatomy and neuroimaging. Neuron. 2013;80:807–15. doi: 10.1016/j.neuron.2013.10.044. [DOI] [PubMed] [Google Scholar]
- Chakirova G, et al. The effects of DISC1 risk variants on brain activation in controls, patients with bipolar disorder and patients with schizophrenia. Psychiatry Res. 2011;192:20–8. doi: 10.1016/j.pscychresns.2011.01.015. [DOI] [PubMed] [Google Scholar]
- Cheh MA, et al. En2 knockout mice display neurobehavioral and neurochemical alterations relevant to autism spectrum disorder. Brain Res. 2006;1116:166–76. doi: 10.1016/j.brainres.2006.07.086. [DOI] [PubMed] [Google Scholar]
- Clower DM, et al. Basal ganglia and cerebellar inputs to 'AIP'. Cereb Cortex. 2005;15:913–20. doi: 10.1093/cercor/bhh190. [DOI] [PubMed] [Google Scholar]
- Cupolillo D, et al. Autistic-Like Traits and Cerebellar Dysfunction in Purkinje Cell PTEN Knock-Out Mice. Neuropsychopharmacology. 2016;41:1457–66. doi: 10.1038/npp.2015.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Zeeuw CI, et al. Spatiotemporal firing patterns in the cerebellum. Nat Rev Neurosci. 2011;12:327–44. doi: 10.1038/nrn3011. [DOI] [PubMed] [Google Scholar]
- Deacon RMJ, Rawlins JNP. T-maze alternation in the rodent. Nat Protocols. 2006;1:7–12. doi: 10.1038/nprot.2006.2. [DOI] [PubMed] [Google Scholar]
- Dell'Orco JM, et al. Neuronal Atrophy Early in Degenerative Ataxia Is a Compensatory Mechanism to Regulate Membrane Excitability. J Neurosci. 2015;35:11292–307. doi: 10.1523/JNEUROSCI.1357-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittman JS, Regehr WG. Contributions of calcium-dependent and calcium-independent mechanisms to presynaptic inhibition at a cerebellar synapse. J Neurosci. 1996;16:1623–33. doi: 10.1523/JNEUROSCI.16-05-01623.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dizon MJ, Khodakhah K. The role of interneurons in shaping Purkinje cell responses in the cerebellar cortex. J Neurosci. 2011;31:10463–73. doi: 10.1523/JNEUROSCI.1350-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doulazmi M, et al. A comparative study of Purkinje cells in two RORalpha gene mutant mice: staggerer and RORalpha(−/−) Brain Res Dev Brain Res. 2001;127:165–74. doi: 10.1016/s0165-3806(01)00131-6. [DOI] [PubMed] [Google Scholar]
- Doyle OM, et al. The cortical thickness phenotype of individuals with DISC1 translocation resembles schizophrenia. J Clin Invest. 2015;125:3714–22. doi: 10.1172/JCI82636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan X, et al. Disrupted-In-Schizophrenia 1 regulates integration of newly generated neurons in the adult brain. Cell. 2007;130:1146–58. doi: 10.1016/j.cell.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egorova PA, et al. In vivo analysis of cerebellar Purkinje cell activity in SCA2 transgenic mouse model. J Neurophysiol. 2016;115:2840–51. doi: 10.1152/jn.00913.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellegood J, et al. Anatomical phenotyping in a mouse model of fragile X syndrome with magnetic resonance imaging. Neuroimage. 2010;53:1023–9. doi: 10.1016/j.neuroimage.2010.03.038. [DOI] [PubMed] [Google Scholar]
- Evarts EV, Thach WT. Motor mechanisms of the CNS: cerebrocerebellar interrelations. Annu Rev Physiol. 1969;31:451–98. doi: 10.1146/annurev.ph.31.030169.002315. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, et al. Purkinje cell size is reduced in cerebellum of patients with autism. Cell Mol Neurobiol. 2002;22:171–5. doi: 10.1023/A:1019861721160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldowitz D, Koch J. Performance of normal and neurological mutant mice on radial arm maze and active avoidance tasks. Behav Neural Biol. 1986;46:216–26. doi: 10.1016/s0163-1047(86)90696-5. [DOI] [PubMed] [Google Scholar]
- Goudarzi S, et al. Interaction of DISC1 with the PTB domain of Tensin2. Cell Mol Life Sci. 2013;70:1663–72. doi: 10.1007/s00018-012-1228-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrup K, Mullen RJ. Staggerer chimeras: intrinsic nature of Purkinje cell defects and implications for normal cerebellar development. Brain Res. 1979;178:443–57. doi: 10.1016/0006-8993(79)90705-4. [DOI] [PubMed] [Google Scholar]
- Herrup K, et al. The numerical matching of source and target populations in the CNS: the inferior olive to Purkinje cell projection. Brain Res Dev Brain Res. 1996;96:28–35. doi: 10.1016/0165-3806(96)00069-7. [DOI] [PubMed] [Google Scholar]
- Hikida T, et al. Dominant-negative DISC1 transgenic mice display schizophrenia-associated phenotypes detected by measures translatable to humans. Proc Natl Acad Sci U S A. 2007;104:14501–6. doi: 10.1073/pnas.0704774104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoebeek FE, et al. Increased noise level of purkinje cell activities minimizes impact of their modulation during sensorimotor control. Neuron. 2005;45:953–65. doi: 10.1016/j.neuron.2005.02.012. [DOI] [PubMed] [Google Scholar]
- Holley SM, et al. Frontal cortical synaptic communication is abnormal in Disc1 genetic mouse models of schizophrenia. Schizophr Res. 2013;146:264–72. doi: 10.1016/j.schres.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji B, et al. Inhibition of protein translation by the DISC1-Boymaw fusion gene from a Scottish family with major psychiatric disorders. Hum Mol Genet. 2014;23:5683–705. doi: 10.1093/hmg/ddu285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang YH, Ehlers MD. Modeling autism by SHANK gene mutations in mice. Neuron. 2013;78:8–27. doi: 10.1016/j.neuron.2013.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AW, et al. Cognitive and motivational deficits together with prefrontal oxidative stress in a mouse model for neuropsychiatric illness. Proc Natl Acad Sci U S A. 2013;110:12462–7. doi: 10.1073/pnas.1307925110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juan LW, et al. Phenotypic characterization of C57BL/6J mice carrying the Disc1 gene from the 129S6/SvEv strain. Brain Struct Funct. 2014;219:1417–31. doi: 10.1007/s00429-013-0577-8. [DOI] [PubMed] [Google Scholar]
- Kaminitz A, et al. Dominant negative DISC1 mutant mice display specific social behaviour deficits and aberration in BDNF and cannabinoid receptor expression. World J Biol Psychiatry. 2014;15:76–82. doi: 10.3109/15622975.2013.841993. [DOI] [PubMed] [Google Scholar]
- Kamiya A, et al. A schizophrenia-associated mutation of DISC1 perturbs cerebral cortex development. Nat Cell Biol. 2005;7:1167–78. doi: 10.1038/ncb1328. [DOI] [PubMed] [Google Scholar]
- Kanduri C, et al. The landscape of copy number variations in Finnish families with autism spectrum disorders. Autism Res. 2016;9:9–16. doi: 10.1002/aur.1502. [DOI] [PubMed] [Google Scholar]
- Kaneko M, et al. Remodeling of monoplanar Purkinje cell dendrites during cerebellar circuit formation. PLoS One. 2011;6:e20108. doi: 10.1371/journal.pone.0020108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang E, et al. Interaction between FEZ1 and DISC1 in regulation of neuronal development and risk for schizophrenia. Neuron. 2011;72:559–71. doi: 10.1016/j.neuron.2011.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasumu A, Bezprozvanny I. Deranged calcium signaling in Purkinje cells and pathogenesis in spinocerebellar ataxia 2 (SCA2) and other ataxias. Cerebellum. 2012;11:630–9. doi: 10.1007/s12311-010-0182-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemper TL, Bauman M. Neuropathology of infantile autism. J Neuropathol Exp Neurol. 1998;57:645–52. doi: 10.1097/00005072-199807000-00001. [DOI] [PubMed] [Google Scholar]
- Kern JK. Purkinje cell vulnerability and autism: a possible etiological connection. Brain Dev. 2003;25:377–82. doi: 10.1016/s0387-7604(03)00056-1. [DOI] [PubMed] [Google Scholar]
- Kilpinen H, et al. Association of DISC1 with autism and Asperger syndrome. Mol Psychiatry. 2008;13:187–96. doi: 10.1038/sj.mp.4002031. [DOI] [PubMed] [Google Scholar]
- Kim JY, et al. DISC1 regulates new neuron development in the adult brain via modulation of AKT-mTOR signaling through KIAA1212. Neuron. 2009;63:761–73. doi: 10.1016/j.neuron.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein AP, et al. Nonmotor Functions of the Cerebellum: An Introduction. American Journal of Neuroradiology. 2016;37:1005–1009. doi: 10.3174/ajnr.A4720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koekkoek SK, et al. Deletion of FMR1 in Purkinje cells enhances parallel fiber LTD, enlarges spines, and attenuates cerebellar eyelid conditioning in Fragile X syndrome. Neuron. 2005;47:339–52. doi: 10.1016/j.neuron.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Kuemerle B, et al. The mouse Engrailed genes: a window into autism. Behav Brain Res. 2007;176:121–32. doi: 10.1016/j.bbr.2006.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuemerle B, et al. Pattern deformities and cell loss in Engrailed-2 mutant mice suggest two separate patterning events during cerebellar development. J Neurosci. 1997;17:7881–9. doi: 10.1523/JNEUROSCI.17-20-07881.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda K, et al. Behavioral alterations associated with targeted disruption of exons 2 and 3 of the Disc1 gene in the mouse. Hum Mol Genet. 2011;20:4666–83. doi: 10.1093/hmg/ddr400. [DOI] [PubMed] [Google Scholar]
- Kvajo M, et al. Altered axonal targeting and short-term plasticity in the hippocampus of Disc1 mutant mice. Proc Natl Acad Sci U S A. 2011;108:E1349–58. doi: 10.1073/pnas.1114113108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalonde R, et al. Sensorimotor learning in three cerebellar mutant mice. Neurobiol Learn Mem. 1996;65:113–20. doi: 10.1006/nlme.1996.0013. [DOI] [PubMed] [Google Scholar]
- Lee H, et al. DISC1-mediated dysregulation of adult hippocampal neurogenesis in rats. Front Syst Neurosci. 2015;9:93. doi: 10.3389/fnsys.2015.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leger M, et al. Object recognition test in mice. Nat Protocols. 2013;8:2531–2537. doi: 10.1038/nprot.2013.155. [DOI] [PubMed] [Google Scholar]
- Llinas R, Sugimori M. Electrophysiological properties of in vitro Purkinje cell dendrites in mammalian cerebellar slices. J Physiol. 1980a;305:197–213. doi: 10.1113/jphysiol.1980.sp013358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinas R, Sugimori M. Electrophysiological properties of in vitro Purkinje cell somata in mammalian cerebellar slices. J Physiol. 1980b;305:171–95. doi: 10.1113/jphysiol.1980.sp013357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, et al. Cloning and characterization of Disc1, the mouse ortholog of DISC1 (Disrupted-in-Schizophrenia 1) Genomics. 2002;80:662–72. doi: 10.1006/geno.2002.7012. [DOI] [PubMed] [Google Scholar]
- Ma TM, et al. Pathogenic disruption of DISC1-serine racemase binding elicits schizophrenia-like behavior via D-serine depletion. Mol Psychiatry. 2013;18:557–67. doi: 10.1038/mp.2012.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLaren EJ, et al. Knockdown of mental disorder susceptibility genes disrupts neuronal network physiology in vitro. Mol Cell Neurosci. 2011;47:93–9. doi: 10.1016/j.mcn.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher BJ, LoTurco JJ. Disrupted-in-schizophrenia (DISC1) functions presynaptically at glutamatergic synapses. PLoS One. 2012;7:e34053. doi: 10.1371/journal.pone.0034053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manaye KF, et al. Neuropathological quantification of dtg APP/PS1: neuroimaging, stereology, and biochemistry. Age (Dordr) 2007;29:87–96. doi: 10.1007/s11357-007-9035-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P, Albers M. Cerebellum and schizophrenia: a selective review. Schizophr Bull. 1995;21:241–50. doi: 10.1093/schbul/21.2.241. [DOI] [PubMed] [Google Scholar]
- Millar JK, et al. Chromosomal location and genomic structure of the human translin-associated factor X gene (TRAX; TSNAX) revealed by intergenic splicing to DISC1, a gene disrupted by a translocation segregating with schizophrenia. Genomics. 2000;67:69–77. doi: 10.1006/geno.2000.6239. [DOI] [PubMed] [Google Scholar]
- Millen KJ, et al. Abnormal embryonic cerebellar development and patterning of postnatal foliation in two mouse Engrailed-2 mutants. Development. 1994;120:695–706. doi: 10.1242/dev.120.3.695. [DOI] [PubMed] [Google Scholar]
- Misslin R, et al. Responses to novelty in staggerer mutant mice. Behav Processes. 1986;12:51–6. doi: 10.1016/0376-6357(86)90070-7. [DOI] [PubMed] [Google Scholar]
- Mitsumura K, et al. Disruption of metabotropic glutamate receptor signalling is a major defect at cerebellar parallel fibre-Purkinje cell synapses in staggerer mutant mice. J Physiol. 2011;589:3191–209. doi: 10.1113/jphysiol.2011.207563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy SS, et al. Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes, Brain and Behavior. 2004;3:287–302. doi: 10.1111/j.1601-1848.2004.00076.x. [DOI] [PubMed] [Google Scholar]
- Nam SC, Hockberger PE. Analysis of spontaneous electrical activity in cerebellar Purkinje cells acutely isolated from postnatal rats. J Neurobiol. 1997;33:18–32. doi: 10.1002/(sici)1097-4695(199707)33:1<18::aid-neu3>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Niwa M, et al. Adolescent stress-induced epigenetic control of dopaminergic neurons via glucocorticoids. Science. 2013;339:335–9. doi: 10.1126/science.1226931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmos-Serrano JL, et al. The GABA(A) receptor agonist THIP ameliorates specific behavioral deficits in the mouse model of fragile X syndrome. Dev Neurosci. 2011;33:395–403. doi: 10.1159/000332884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovanesov MV, et al. Astrocytes play a key role in activation of microglia by persistent Borna disease virus infection. J Neuroinflammation. 2008;5:50. doi: 10.1186/1742-2094-5-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmen SJ, van Engeland H. Review on structural neuroimaging findings in autism. J Neural Transm (Vienna) 2004;111:903–29. doi: 10.1007/s00702-003-0068-9. [DOI] [PubMed] [Google Scholar]
- Papadopoulos N, et al. An investigation of upper limb motor function in high functioning autism and Asperger's disorder using a repetitive Fitts’ aiming task. Research in Autism Spectrum Disorders. 2012;6:286–292. [Google Scholar]
- Peca J, et al. Shank3 mutant mice display autistic-like behaviours and striatal dysfunction. Nature. 2011;472:437–42. doi: 10.1038/nature09965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellow S, et al. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–67. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- Peter S, et al. Dysfunctional cerebellar Purkinje cells contribute to autism-like behaviour in Shank2-deficient mice. Nat Commun. 2016;7:12627. doi: 10.1038/ncomms12627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pletnikov MV, et al. PC12 cell model of inducible expression of mutant DISC1: new evidence for a dominant-negative mechanism of abnormal neuronal differentiation. Neurosci Res. 2007;58:234–44. doi: 10.1016/j.neures.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Ren J, et al. Interaction between DISC1 and CHL1 in regulation of neurite outgrowth. Brain Res. 2016;1648:290–7. doi: 10.1016/j.brainres.2016.06.033. [DOI] [PubMed] [Google Scholar]
- Sadamatsu M, et al. Review of animal models for autism: implication of thyroid hormone. Congenit Anom (Kyoto) 2006;46:1–9. doi: 10.1111/j.1741-4520.2006.00094.x. [DOI] [PubMed] [Google Scholar]
- Sahebi M, et al. Towards understanding pre-mRNA splicing mechanisms and the role of SR proteins. Gene. 2016;587:107–19. doi: 10.1016/j.gene.2016.04.057. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD. An emerging concept. The cerebellar contribution to higher function. Arch Neurol. 1991;48:1178–87. doi: 10.1001/archneur.1991.00530230086029. [DOI] [PubMed] [Google Scholar]
- Schmitt A, et al. Gene expression of NMDA receptor subunits in the cerebellum of elderly patients with schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2010;260:101–11. doi: 10.1007/s00406-009-0017-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurov IL, et al. Expression of disrupted in schizophrenia 1 (DISC1) protein in the adult and developing mouse brain indicates its role in neurodevelopment. Mol Psychiatry. 2004;9:1100–10. doi: 10.1038/sj.mp.4001574. [DOI] [PubMed] [Google Scholar]
- Shi L, et al. Activation of the maternal immune system alters cerebellar development in the offspring. Brain Behav Immun. 2009;23:116–23. doi: 10.1016/j.bbi.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin JH, Linden DJ. An NMDA receptor/nitric oxide cascade is involved in cerebellar LTD but is not localized to the parallel fiber terminal. J Neurophysiol. 2005;94:4281–9. doi: 10.1152/jn.00661.2005. [DOI] [PubMed] [Google Scholar]
- Shinoda T, et al. DISC1 regulates neurotrophin-induced axon elongation via interaction with Grb2. J Neurosci. 2007;27:4–14. doi: 10.1523/JNEUROSCI.3825-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider SR. Cerebellar pathology in schizophrenia--cause or consequence? Neurosci Biobehav Rev. 1982;6:47–53. doi: 10.1016/0149-7634(82)90006-9. [DOI] [PubMed] [Google Scholar]
- St Clair D, et al. Association within a family of a balanced autosomal translocation with major mental illness. Lancet. 1990;336:13–6. doi: 10.1016/0140-6736(90)91520-k. [DOI] [PubMed] [Google Scholar]
- Steinecke A, et al. Disrupted-in-Schizophrenia 1 (DISC1) is necessary for the correct migration of cortical interneurons. J Neurosci. 2012;32:738–45. doi: 10.1523/JNEUROSCI.5036-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strick PL, et al. Cerebellum and nonmotor function. Annu Rev Neurosci. 2009;32:413–34. doi: 10.1146/annurev.neuro.31.060407.125606. [DOI] [PubMed] [Google Scholar]
- Subbiah P, et al. Stereological analysis of cerebral atrophy in human immunodeficiency virus-associated dementia. J Neuropathol Exp Neurol. 1996;55:1032–7. [PubMed] [Google Scholar]
- Supprian T, et al. Cerebellar vermis area in schizophrenic patients - a post-mortem study. Schizophr Res. 2000;42:19–28. doi: 10.1016/s0920-9964(99)00103-6. [DOI] [PubMed] [Google Scholar]
- Šveljo O, Ćulić M. Cerebellar Nonmotor Functions–Approaches and Significance. Neurophysiology. 2015;47:337–347. [Google Scholar]
- Takacs J, Hamori J. Developmental dynamics of Purkinje cells and dendritic spines in rat cerebellar cortex. J Neurosci Res. 1994;38:515–30. doi: 10.1002/jnr.490380505. [DOI] [PubMed] [Google Scholar]
- Thomanetz V, et al. Ablation of the mTORC2 component rictor in brain or Purkinje cells affects size and neuron morphology. J Cell Biol. 2013;201:293–308. doi: 10.1083/jcb.201205030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai PT, et al. Autistic-like behaviour and cerebellar dysfunction in Purkinje cell Tsc1 mutant mice. Nature. 2012;488:647–51. doi: 10.1038/nature11310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Frye CA. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protocols. 2007;2:322–328. doi: 10.1038/nprot.2007.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West MJ. Regionally specific loss of neurons in the aging human hippocampus. Neurobiol Aging. 1993;14:287–93. doi: 10.1016/0197-4580(93)90113-p. [DOI] [PubMed] [Google Scholar]
- Womack M, Khodakhah K. Active contribution of dendrites to the tonic and trimodal patterns of activity in cerebellar Purkinje neurons. J Neurosci. 2002;22:10603–12. doi: 10.1523/JNEUROSCI.22-24-10603.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womack MD, Khodakhah K. Dendritic control of spontaneous bursting in cerebellar Purkinje cells. J Neurosci. 2004;24:3511–21. doi: 10.1523/JNEUROSCI.0290-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, et al. Aberrant cerebellar neurotrophin-3 expression induced by lipopolysaccharide exposure during brain development. Cerebellum. 2013;12:316–8. doi: 10.1007/s12311-012-0446-7. [DOI] [PubMed] [Google Scholar]
- Yeganeh-Doost P, et al. The role of the cerebellum in schizophrenia: from cognition to molecular pathways. Clinics (Sao Paulo) 2011;66(Suppl 1):71–7. doi: 10.1590/S1807-59322011001300009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuskaitis CJ, et al. Evidence of reactive astrocytes but not peripheral immune system activation in a mouse model of Fragile X syndrome. Biochim Biophys Acta. 2010;1802:1006–12. doi: 10.1016/j.bbadis.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng F, et al. Evidence for association between Disrupted-in-Schizophrenia 1 (DISC1) gene polymorphisms and autism in Chinese Han population: a family-based association study. Behav Brain Funct. 2011;7:14. doi: 10.1186/1744-9081-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Fu XD. Regulation of splicing by SR proteins and SR protein-specific kinases. Chromosoma. 2013;122:191–207. doi: 10.1007/s00412-013-0407-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.