ABSTRACT
Malassezia is a genus of lipid-dependent yeasts. It is associated with common skin diseases such as pityriasis versicolor and atopic dermatitis and can cause systemic infections in immunocompromised individuals. Owing to the slow growth and lipid requirements of these fastidious yeasts, convenient and reliable antifungal drug susceptibility testing assays for Malassezia spp. are not widely available. Therefore, we optimized a broth microdilution assay for the testing of Malassezia that is based on the CLSI and EUCAST assays for Candida and other yeasts. The addition of ingredients such as lipids and esculin provided a broth medium formulation that enabled the growth of all Malassezia spp. and could be read, with the colorimetric indicator resazurin, by visual and fluorescence readings. We tested the susceptibility of 52 strains of 13 Malassezia species to 11 commonly used antifungals. MIC values determined by visual readings were in good agreement with MIC values determined by fluorescence readings. The lowest MICs were found for the azoles itraconazole, posaconazole, and voriconazole, with MIC90 values of 0.03 to 1.0 μg/ml, 0.06 to 0.5 μg/ml, and 0.03 to 2.0 μg/ml, respectively. All Malassezia spp. were resistant to echinocandins and griseofulvin. Some Malassezia spp. also showed high MIC values for ketoconazole, which is the most widely recommended topical antifungal to treat Malassezia skin infections. In summary, our assay enables the fast and reliable susceptibility testing of Malassezia spp. with a large panel of different antifungals.
KEYWORDS: antifungal agents, antifungal susceptibility testing, fungi, Malassezia, yeasts
INTRODUCTION
Malassezia is a genus of lipid-dependent yeasts that currently includes 14 species. With the exception of Malassezia pachydermatis, Malassezia species are lipid dependent. Malassezia sympodialis, Malassezia slooffiae, Malassezia furfur, Malassezia globosa, Malassezia restricta, Malassezia obtusa, Malassezia japonica, Malassezia dermatis, and Malassezia yamatoensis are considered anthropophilic, as they have been isolated from human skin (1). Malassezia is the most common fungal genus of the healthy human skin microbiome (2). Additionally, a pathogenic role is attributed to these yeasts in common skin diseases such as pityriasis versicolor, atopic dermatitis, and seborrheic dermatitis, and they can cause severe systemic infections in neonates and immunocompromised individuals (3, 4).
In infectious diseases, antimicrobial susceptibility testing is a useful tool to determine the appropriate antimicrobial treatment, particularly if antimicrobial susceptibility cannot be predicted based on the identity of the infectious agent (5). Treatment of Malassezia-related infections has thus far relied on the predicted susceptibility to common antifungals such as topical azoles. However, the antifungal treatment of Malassezia-associated skin diseases is not clinically efficacious in up to one-third of patients (6). This may be attributable to malcompliance as well as the antifungal resistance of Malassezia spp. Moreover, knowledge regarding the susceptibility of different Malassezia species and strains to particular antifungals is scarce (7–10), which supports the need for antifungal susceptibility testing for Malassezia spp. Existing assays for determination of the MIC values for antifungals, as recommended by the CLSI (11) and EUCAST (http://www.eucast.org) guidelines, are applicable only for fast-growing fungi such as Candida spp. These assays are not suitable for Malassezia spp., which are slow growing and fastidious. Therefore, some studies that tested the antifungal susceptibility of Malassezia spp. used assays modified from the CLSI and EUCAST guidelines. The modifications included variations in growth medium composition and inoculum size (Table 1) (7–9). Turbidity was the preferred readout method for most of the assays (7, 8). However, drawbacks of turbidity readings include limited reproducibility and the possibility of underestimating MIC values (12). To overcome these limitations, we have developed a new antifungal susceptibility assay, based on a broth medium, that allows growth and reliable and convenient antifungal testing of all Malassezia species. It is compatible with the fluorometric indicator resazurin, enabling the rapid and effective determination of MIC values by visual and fluorescence readings.
TABLE 1.
Synopsis of studies investigating broth microdilution assays for the antifungal susceptibility testing of Malassezia spp.
| Reference | Species | Antifungals | Broth medium composition | Inoculum size (CFU/ml) | Incubation time (days) | Incubation temperature (°C) | Reading method |
|---|---|---|---|---|---|---|---|
| Our study | 52 strains; M. sympodialis, M. slooffiae, M. furfur, M. globosa, M. restricta, M. obtusa, M. dermatis, M. japonica, M. yamatoensis, M. pachydermatis, Malassezia nana, M. caprae, Malassezia cuniculi | Amphotericin B, terbinafine, ketoconazole, fluconazole, posaconazole, itraconazole, voriconazole, caspofungin, micafungin, anidulafungin, griseofulvin | RPMI 1640 medium with 0.165 M MOPS, 0.2% sodium bicarbonate, 0.5% glycerol, 0.5% Tween 60, 2% oleic acid, and 1% esculin | 5.0 × 103 to 5.0 × 104 | 2 or 3 | 35 | Visual (color) and fluorescence |
| Rojas et al. (7) | M. furfur (39 strains), M. sympodialis (20 strains), M. globosa (14 strains) | Fluconazole, ketoconazole, voriconazole, itraconazole, amphotericin B, miconazole | RPMI 1640 medium with 1.8% glucose, 1% peptone, 0.5% ox bile, 0.5% malt extract, 1% glycerol, 0.5% Tween 40, 0.05% Tween 80 | 0.5 × 105 to 2.5 × 105 | 3 or 4 | 32 | Turbidity |
| Velegraki et al. (8) | 53 strains; M. furfur, M. pachydermatis, M. sympodialis, M. slooffiae, M. globosa, M. restricta, M. dermatis | Amphotericin B, itraconazole, fluconazole, voriconazole, ketoconazole, terbinafine, posaconazole | RPMI 1640 medium with 20 g glucose, 4 g ox bile, 1 ml glycerol, 0.5 g glycerol monostearate, and 0.4 ml Tween 20 | 2.0 × 103 to 3.5 × 103 or 3.0 × 103 to 4.0 × 103 | M. furfur, 2; M. pachydermatis, 2; M. sympodialis, 3; M. slooffiae, 3; M. globosa, 3; M. restricta, 3; M. dermatis, 3 | 32 | Turbidity |
| Miranda et al. (9) | M. furfur (74 strains), M. sympodialis (11 strains), M. obtusa (8 strains), M. globosa (2 strains) | Fluconazole, ketoconazole, itraconazole, voriconazole | Modified Leeming-Notman medium containing 0.1% glucose, 0.1% peptone, 0.8% bile salts, 0.2% yeast extract, 0.1%glycerol, 0.5% Tween 60, and 3% olive oil | 2.5 × 103 ± 1.0 × 103 | M. furfur, 3; M. sympodialis, 3; M. globosa, 5; M. obtusa, 5 | 32 | Turbidity |
| Gupta et al. (10)a | 55 strains; M. furfur, M. sympodialis, M. slooffiae, M. pachydermatis, M. globosa, M. obtusa, M. restricta | Ketoconazole, voriconazole, itraconazole, terbinafine | Diagnostic Sensitivity Testing agar and glycerol | 1.0 × 104 | 7 | 35 | Colony growth on agar plates |
This assay was performed using agar-based medium.
RESULTS
Growth of Malassezia spp. in OptiMAL broth medium.
Serial dilutions of each broth medium component were tested to determine the optimal concentrations for five Malassezia species, i.e., M. sympodialis, M. slooffiae, M. furfur, M. globosa, and M. pachydermatis. Optimal glycerol and Tween 60 concentrations were 0.25 to 0.5% and 0.5%, respectively. We used a concentration of 0.05 to 2% oleic acid for OptiMAL, because most Malassezia species preferred oleic acid to olive oil in assimilation assays. Glucose concentrations of more than 2% did not improve growth. The optimal pH range was pH 6.0 to 6.5.
In the absence of sodium bicarbonate, RPMI and RPMI++ tended to color bleach. Testing growth with sodium bicarbonate concentrations ranging from 0 to 32 mg/ml showed that Malassezia spp. were able to tolerate sodium bicarbonate concentrations of up to 2 mg/ml before growth inhibition occurred. A sodium bicarbonate concentration of 2 mg/ml was optimal for visual and fluorescence readings, because it boosted color intensity and improved pH buffering (see Fig. S1 in the supplemental material). The addition of 0.1 mg/ml esculin increased the fluorescence signal by 2- to 4-fold (Fig. S1). These observations support the use of sodium bicarbonate and esculin as useful broth medium additives that are compatible with resazurin for Malassezia growth and broth microdilution assays.
Optimal inoculum size for Malassezia spp.
By testing a range of inoculum sizes and varying the incubation time before the readings, we observed that the MIC values of some antifungals depended strongly on the inoculum size and the incubation time, while other antifungals were less affected. For amphotericin B, growth curves were not influenced by the inoculum size or the incubation time (Fig. S2, left column). However, growth curves were less reproducible with other antifungals, especially when an inoculum of >100,000 CFU/ml was used. To provide reproducible results for all antifungals, we decided to use a final inoculum of 5.0 × 103 to 5.0 × 104 CFU/ml for all tested Malassezia strains.
Incubation times of 18 to 48 h gave comparable growth curves, and MIC values determined at those time points did not differ by more than 1 to 2 dilution steps for inocula of 5.0 × 103 to 5.0 × 104 CFU/ml (Fig. S2, right column). Color development after 6 to 12 h of incubation was insufficient for reliable MIC readings, while the fluorescence signal faded after 60 h of incubation. Therefore, we decided to read the plates after 24 to 48 h of incubation. Ketoconazole was the only exception, for which incubation periods of 24 to 36 h resulted in a loss of growth curve sensitivity and high MIC readings.
Agreement between visual and fluorescence readings.
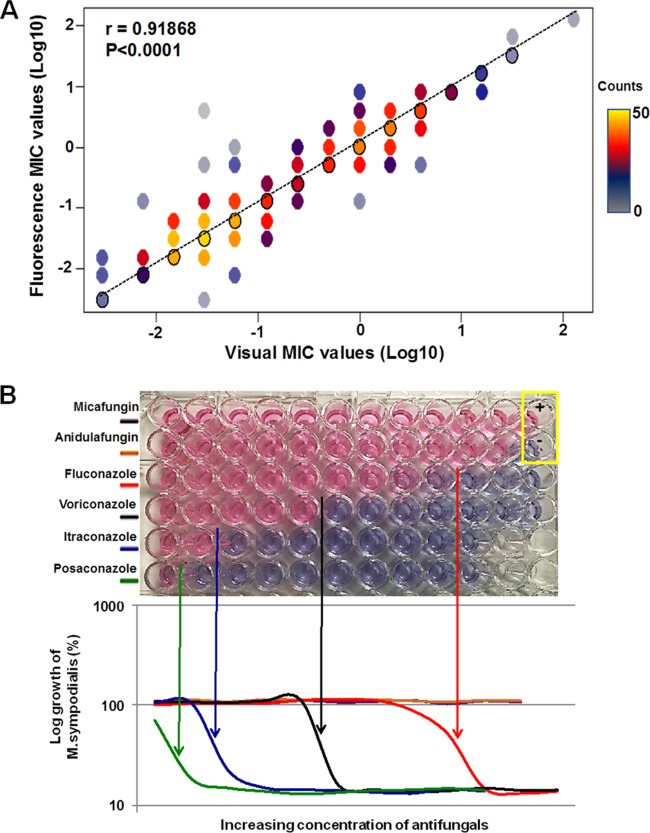
We were interested in assessing whether MIC values determined by visual readings differed from MIC values determined by fluorescence readings. MIC values determined by fluorescence readings appeared slightly higher than those determined by visual readings, but the difference was not significant (P = 0.25). Overall, the MIC values determined by the two readout methods were strongly correlated with each other for all antifungals (Pearson's correlation coefficients of >0.857 for all drugs) (Fig. 1). For most antifungals, MIC values determined by the two methods were within 2 dilution steps in 96 to 100% of cases (Table 2). The agreement of MIC values for ketoconazole was slightly lower, with MIC values within 2 dilution steps in 88% of cases (Table 2).
FIG 1.
(A) MIC values of antifungals (except griseofulvin and echinocandins) for 52 Malassezia strains, determined by visual readings (x axis) and fluorescence readings (y axis). Black circles represent points at which the two data readings were in 100% agreement. The number of MIC values per data point in the graph is indicated by the color bar. Agreement between visual and fluorescence readings was determined by Pearson correlation analysis. (B) Agreement between MIC values determined by visual readings and fluorescence readings from a representative antifungal resistance assay plate testing M. sympodialis. Each row represents a particular antifungal, with concentrations increasing from left to right. In the yellow box, the plus sign indicates the positive control without antifungal and the minus sign indicates the negative control containing broth medium without M. sympodialis. Pink wells indicate the growth of M. sympodialis, and blue wells indicate no growth. The graph represents the fluorescence readings of the color changes.
TABLE 2.
Agreement of MIC values from visual and fluorescence readings
| Agreement level | Agreement (%) |
||||||
|---|---|---|---|---|---|---|---|
| Amphotericin B | Terbinafine | Ketoconazole | Fluconazole | Itraconazole | Posaconazole | Voriconazole | |
| MICs identical | 75 | 73 | 63 | 67 | 67 | 73 | 75 |
| MICs within 1 dilution | 96 | 94 | 84 | 88 | 88 | 94 | 96 |
| MICs within 2 dilutions | 98 | 96 | 88 | 96 | 98 | 100 | 98 |
Susceptibility of Malassezia spp. to antifungals.
We assessed the antifungal susceptibility profiles of 52 Malassezia strains, including 13 reference strains and 39 clinical strains (Table 3), with 11 antifungals commonly used in clinical practice. The lowest MIC values were found for itraconazole, posaconazole, and voriconazole for most strains tested (Fig. 2 and Table 4). Among the azoles, the highest MIC values and the widest MIC ranges were observed for fluconazole (Table 4; also see Table S1). Ketoconazole showed low MICs for most strains but very high MICs for a few strains (Fig. 2 and Table 4). MICs for terbinafine and amphotericin B showed wide ranges; some strains showed low MICs while others had very high MIC values. All strains had very high MIC values for the echinocandins anidulafungin, caspofungin, and micafungin, as well as griseofulvin, suggesting an intrinsic resistance of Malassezia spp. to these antifungals (Fig. 2); therefore, MIC values for these antifungals are not shown in Tables 2 and 4. Two species, namely, the anthropophilic M. obtusa and the zoophilic Malassezia caprae, appeared to be resistant to most antifungals (Fig. 2 and Table 4). Terbinafine was the only antifungal that was effective against M. caprae.
TABLE 3.
Malassezia species and proposed inocula for antifungal susceptibility testing
| Strain no. | Species | Host | No. of strains tested | Reference strain | OD600 for inoculuma |
|---|---|---|---|---|---|
| 1 | M. sympodialis | Human | 24 | CBS 7222 | 0.1 |
| 2 | M. slooffiae | Human | 10 | CBS 7956 | 0.05 |
| 3 | M. furfur | Human | 5 | CBS 1878 | 0.005 |
| 4 | M. globosa | Human | 3 | CBS 7966 | 0.2–1 |
| 5 | M. restricta | Human | 2 | CBS 7877 | 0.2–1 |
| 6 | M. obtusa | Human | 2 | CBS 7876 | 0.2–1 |
| 7 | M. dermatis | Human | 1 | CBS 9169 | 0.1 |
| 8 | M. japonica | Human | 1 | CBS 9432 | 0.2 |
| 9 | M. yamatoensis | Human | 1 | CBS 9725 | 0.02 |
| 10 | M. nana | Animal | 1 | CBS 9557 | 0.1 |
| 11 | M. pachydermatis | Animal | 1 | CBS 1879 | 0.01 |
| 12 | M. cuniculi | Animal | 1 | CBS 11721 | 0.1 |
| 13 | M. caprae | Animal | 1 | CBS 10434 | 0.1 |
OD600 values for broth microdilution testing, as determined by plating 10 μl of a 1:10 dilution to achieve inocula of 104 to 105 CFU/ml. For the susceptibility assay, 50 μl of inoculum was added to 50 μl of 2× concentrations of antifungals, to achieve final cell densities of 5.0 × 103 to 5.0 × 104 CFU/ml.
FIG 2.
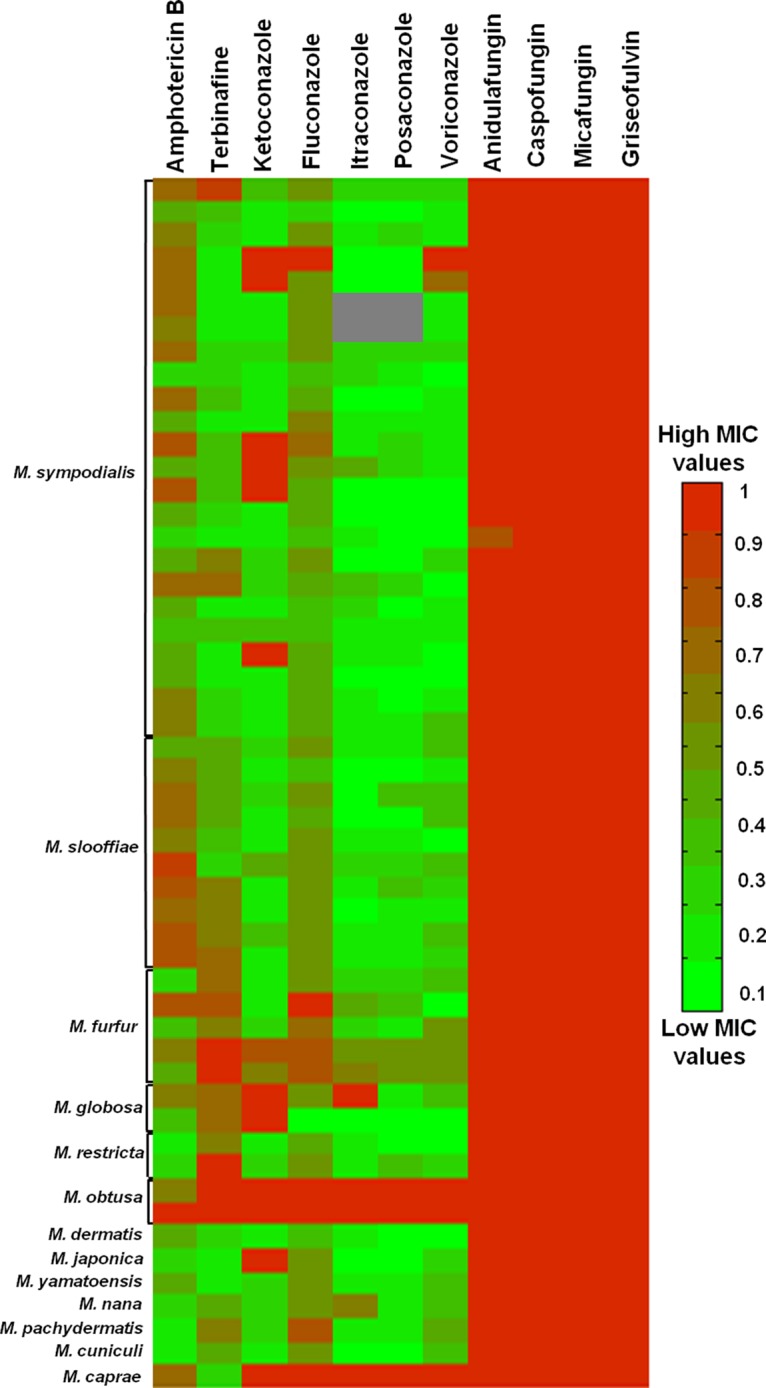
MIC values of 11 antifungals for 52 Malassezia strains, as determined by visual readings. MIC values were normalized from 0 to 1, with 1 being the highest antifungal concentration tested. The first row for each Malassezia species represents the susceptibility profile of the reference strain. Gray sections indicate inconclusive MIC results.
TABLE 4.
MIC ranges and mean MICs of antifungals determined by visual readings
| Species | MIC range (mean MIC) (μg/ml) |
||||||
|---|---|---|---|---|---|---|---|
| Amphotericin B | Terbinafine | Ketoconazole | Fluconazole | Itraconazole | Posaconazole | Voriconazole | |
| M. sympodialis (24 strains) | 0.125–4 (1.27) | ≤0.125 to 16 (1.15) | ≤0.03 to >4 (1.03) | 0.25 to >128 (7.09) | ≤0.015 to >8 (0.37) | ≤0.015 to >8 (0.36) | ≤0.003 to >8 (0.38) |
| M. slooffiae (10 strains) | 0.5 to >8 (2.85) | 0.25–4 (1.48) | ≤0.03 to 0.125 (0.07) | 0.5–4 (1.95) | ≤0.015 to 0.06 (0.04) | ≤0.015 to 0.125 (0.03) | ≤0.003 to 0.03 (0.02) |
| M. furfur (5 strains) | 0.125–4 (1.18) | 2 to >16 (9.2) | ≤0.03 to 2 (0.52) | 4 to >128 (42.4) | 0.06–1 (0.37) | 0.03–0.5 (0.24) | 0.03–2 (0.51) |
| M. globosa (3 strains) | 0.25–1 | 4 | >4 | 4 | ≤0.015 to >8 | ≤0.015 to 0.03 | ≤0.003 to 0.03 |
| M. restricta (2 strains) | ≤0.06 to >0.125 | 2 to >16 | ≤0.03 to 0.06 | 1–4 | 0.03 | ≤0.015 to 0.125 | ≤0.003 to 0.015 |
| M. obtusa (2 strains) | 1 to >8 | >16 | >4 | >128 | >8 | >8 | >8 |
| M. dermatis (1 strain)a | 0.5 | 0.25 | ≤0.03 | 0.5 | 0.03 | ≤0.015 | ≤0.003 |
| M. japonica (1 strain)a | 0.125 | ≤0.125 | >4 | 2 | ≤0.015 | ≤0.015 | 0.015 |
| M. yamatoensis (1 strain)a | 0.5 | ≤0.125 | 0.06 | 4 | 0.06 | 0.03 | 0.03 |
| M. pachydermatis (1 strain)a | ≤0.06 | 2 | 0.06 | 32 | 0.03 | 0.03 | 0.06 |
| M. caprae (1 strain)a | 2 | 0.25 | >4 | >128 | >8 | >8 | >8 |
| M. nana (1 strain)a | 0.125 | 1 | 0.06 | 4 | 0.06 | 1 | 0.03 |
| M. cuniculi (1 strain)a | ≤0.06 | 1 | ≤0.03 | 4 | ≤0.015 | ≤0.015 | 0.03 |
No range; only the reference strain was tested for these species.
DISCUSSION
RPMI 1640 medium containing 0.165 M 3-(N-morpholino)propanesulfonic acid (MOPS) buffer is the basis for the broth microdilution methods recommended by the CLSI and EUCAST (6, 7). This broth is not optimal for the growth of fastidious fungi such as Malassezia spp., which require lipid supplementation. By adding multiple components, including lipids (such as oils and Tween compounds) and esculin, to our broth medium, we facilitated the growth of Malassezia spp. We have named this new broth medium OptiMAL. OptiMAL may also be suitable for other yeast species, such as Candida, that utilize additives such as Tween compounds (13).
Resazurin (also known as alamarBlue) is used as an indicator in the colorimetric YeastOne Sensititre assay (Thermo Scientific, Zug, Switzerland) for routine antifungal susceptibility testing of yeasts and molds such as Candida spp. and Aspergillus spp. (14, 15). It changes from blue to pink as a result of growth (16) and allows for the visual and spectrophotometric determination of MIC values. Until now, it has not been used in broth media for Malassezia spp. because it is not always color stable during prolonged incubation and therefore needs to be buffered (16, 17). The careful buffering of OptiMAL with sodium bicarbonate and esculin allowed for the concurrent use of resazurin as a color indicator. The use of esculin in OptiMAL improved the growth of all Malassezia species by up to 4-fold (Fig. S1), and it was a key ingredient to improve the accuracy and reproducibility of our assay. Studies on fungal growth usually omit sodium bicarbonate because it causes growth inhibition of many fungal species (18). We observed that Malassezia spp. grew well in OptiMAL containing 2 mg/ml sodium bicarbonate. The bicarbonate served as a complementary buffer to MOPS; it boosted the intensity of the resazurin color change and yielded higher sensitivity of fluorescence detection.
The lack of standardized guidelines for drug susceptibility testing in Malassezia spp. has led some studies (8, 9) to use small inocula, in accordance with the guidelines specified by the CLSI for yeasts (11), because overtly large inocula are associated with inaccurate MIC values (19). However, the EUCAST guidelines for yeasts use a 100-fold larger inoculum than do the CLSI guidelines. To test the relevance of these differences between the CLSI and EUCAST guidelines for Malassezia spp., we performed inoculum titration studies. We decided on 5.0 × 103 to 5.0 × 104 CFU/ml as the optimal inoculum size for Malassezia broth microdilution assays, because this size gave reproducible MIC values within 2 dilution steps for repeats with the same Malassezia strain. Ketoconazole was particularly sensitive to variations in inoculum size and incubation time.
Visual readings for changes in color and turbidity are the most common form of MIC determinations. Quantitative readings can be performed by measuring absorbance or fluorescence, with the latter being more sensitive (20). In general, visual determinations of color changes may be preferable to absorbance or fluorescence readings in laboratory practice, because they do not require additional technical equipment, are faster, and therefore are cheaper (12). Few studies have investigated the agreement of MIC values determined by visual and fluorescence readings. For example, a comparison of MIC values of azoles for 88 Aspergillus fumigatus isolates determined by absorbance and visual turbidity reading were in good agreement (21). Neither absorbance nor turbidity readings were an option in our assay, however, because (i) some very slow-growing species such as M. furfur would have required much larger inocula than recommended to visualize turbidity; (ii) the clumpiness of some species, such as M. globosa and M. restricta, hampered reliable accurate absorbance or turbidity readings; and (iii) our assay is based on resazurin, and turbidity readings are not recommended when resazurin is used (22). Therefore, we decided to use visual color changes and fluorescence readings to determine MIC values. Overall, our MIC values determined by visual and fluorescence readings were in good agreement. Slightly higher MIC values were obtained with fluorescence readings, presumably because of the higher sensitivity of fluorescence readings. These differences were generally within 1 to 2 dilution steps, which is acceptable by CLSI and EUCAST standards. Our findings substantiate the concordance of visual and fluorescence readings for MIC values in resazurin-based assays.
Despite the use of an optimized growth medium and a standardized inoculum size, repeated testing (at least duplicates) showed that the azoles, and to a lesser extent terbinafine, might be prone to considerable test-to-test variations. In particular, ketoconazole testing required more repeats. This may be attributable to (i) the antagonization of medium components (10) and/or (ii) the susceptibility of ketoconazole to variations in inoculum size and incubation time, as described above.
We have applied this optimized broth microdilution method to test the susceptibility of 52 Malassezia strains to 11 antifungals. Our assay included the echinocandin class as well as griseofulvin. The data showed that Malassezia is resistant to these antifungals, which is in line with literature reports (3, 23). The azoles itraconazole, posaconazole, and voriconazole appeared to be the most effective antifungals against Malassezia spp. High MIC values for these azoles were rare, which is in line with previous studies (10, 24). Most of our Malassezia strains were susceptible to concentrations of fluconazole and ketoconazole described previously (7–9, 25). However, some strains were resistant to ketoconazole. This could be of clinical significance, because ketoconazole is the first-line topical antifungal in the treatment of Malassezia-associated skin diseases such as pityriasis versicolor or seborrheic dermatitis (26). Terbinafine, a widely used allylamine, is active against dermatophytes and molds and can be applied topically or systemically (27). It is not recommended as a first-line treatment for Malassezia-associated diseases (26). The relatively low MIC values of terbinafine against many of our Malassezia strains suggest that terbinafine may be a treatment alternative for Malassezia infections that do not respond to azoles.
In summary, we have developed an optimized broth microdilution assay, compatible with the colorimetric indicator resazurin, for the fast and efficient profiling of antifungal susceptibility in Malassezia spp. Determinations of MIC values by visual readings of color changes versus fluorescence readings were comparably reliable. Among the 52 Malassezia strains tested, azoles such as voriconazole, itraconazole, and posaconazole were the most effective antifungals. Terbinafine might be a treatment alternative for Malassezia infections.
MATERIALS AND METHODS
Malassezia strains.
Malassezia reference strains were obtained from the Centraalbureau voor Schimmelcultures (CBS) (Utrecht, The Netherlands) (Table 3). Clinical strains were obtained from patients who were seen at the Department of Dermatology, University Hospital of Zurich (Zurich, Switzerland). All strains were maintained on modified Leeming-Notman (mLN) agar and were identified as described previously (28, 29).
Optimization of broth medium formulation.
The basis of the broth medium was filtered RPMI 1640 medium with 0.165 M MOPS without sodium bicarbonate (Sigma-Aldrich, Zurich, Switzerland). Sodium bicarbonate, glucose, Tween 60, oleic acid, glycerin, and esculin (Sigma-Aldrich) were selectively titrated and added to the broth medium. Reference strains of M. sympodialis, M. slooffiae, M. furfur, M. globosa, and M. pachydermatis were inoculated in triplicate at the stipulated optical density (OD) (Table 3) and incubated for 48 h at 35°C. Growth was assessed by scoring the turbidity of the broth medium on an arbitrary scale ranging from 0 (no growth) to 4 (highest growth). This led to the formulation of three candidate media (Table 5). RPMI++ with esculin provided the best conditions for growth and colorimetric readings for all tested Malassezia strains. We have named this medium OptiMAL and used it for antifungal susceptibility testing.
TABLE 5.
Broth media for Malassezia spp.
| Component | RPMI+a | RPMI ++ | RPMI++ with esculin (OptiMAL) |
|---|---|---|---|
| MOPS (M) | 0.165 | 0.165 | 0.165 |
| Sodium bicarbonate (mg/ml) | 0.2 | 0.2 | 0.2 |
| Glucose (% of volume) | 2 | 2 | 2 |
| Glycerol (% of volume) | 0.1 | 1 | 0.5 |
| Glycerol monostearate (% of volume) | 0.05 | ||
| Tween 20 (% of volume) | 0.04 | ||
| Tween 60 (% of volume) | 0.5 | 0.5 | |
| Olive oil | |||
| Oleic acid (% of volume) | 2 | 2 | |
| Esculin (% of volume) | 0.1 | ||
| Resazurin (μg/ml) | 12.5 | 12.5 | 12.5 |
| pH | 6.32 | 6.25 | 6.26 |
Adapted from Velegraki et al. (8).
Malassezia inoculum quantification and optimization.
Malassezia inocula were quantified in suspension by measurements of the OD at 600 nm (OD600) (Cytation 3 plate reader; BioTek, Winooski, VT, USA). To correlate OD600 readings with the numbers of yeast cells, serial dilutions of the suspensions were plated on mLN agar at 35°C and the CFU were counted after 4 to 7 days.
To determine the optimal inoculum range for Malassezia spp., five different inocula (approximately 2,500, 5,000, 50,000, 100,000, and 200,000 CFU/ml) of M. furfur CBS 1878 were tested together with seven antifungals (amphotericin B, terbinafine, ketoconazole, fluconazole, itraconazole, posaconazole, and voriconazole). Plates were read every 6 h for the first 24 h and subsequently at 12-h intervals for up to 60 h, as described below.
Broth microdilution assay.
Antifungals tested in the assay were amphotericin B (concentration range, 0.06 to 8 μg/ml), caspofungin (0.06 to 8 μg/ml), griseofulvin (0.06 to 8 μg/ml), terbinafine (0.125 to 16 μg/ml), fluconazole (0.06 to 128 μg/ml), ketoconazole (0.03 to 4 μg/ml), itraconazole (0.015 to 8 μg/ml), posaconazole (0.015 to 8 μg/ml), voriconazole (0.003 to 8 μg/ml) (all from Sigma-Aldrich), micafungin, and anidulafungin (all from MedChemExpress Europe, Sollentuna, Sweden). Serial 2-fold dilutions of 200× stocks of antifungals were prepared in dimethyl sulfoxide (DMSO) (Sigma-Aldrich) and stored at −80°C. Inocula (50 μl) were added to 50 μl of 2× concentrated antifungals to achieve a final cell density of 5.0 × 103 to 5.0 × 104 CFU/ml. For inoculum verification, 10 μl of a 1:10 diluted inoculum was plated onto mLN agar and incubated for 4 to 7 days at 35°C. MIC values from each well plate were deemed reliable only if counts on agar plates were within 10 to 100 CFU.
Candida parapsilosis ATCC 22019 was used as a quality control strain to assess the accuracy of our antifungal dilutions and the reproducibility of results for fluorescence and visual readings, according to CLSI (11) and EUCAST (http://www.eucast.org) guidelines.
Analysis of antifungal susceptibility assay data.
Plates were analyzed when the indicator resazurin (Sigma-Aldrich) changed from blue to pink in the positive control. The color change was assessed by (i) visual reading and (ii) fluorescence analysis at 530 nm and 590 nm (Cytation 3 plate reader). Growth was measured relative to the positive control. MIC values for azoles and echinocandins were defined as 50% growth inhibition and those for amphotericin B as complete growth inhibition, in accordance with CLSI standards (11). For terbinafine, the MIC was defined as the lowest drug concentration that completely inhibited growth.
For each strain, at least duplicate testing was performed. If the MIC values differed by more than 2 dilution steps, then experiments were repeated. In cases in which repeat testing gave MIC values on extreme ends of the dilution scale, an inconclusive MIC result was recorded.
Heat maps of the final MIC values for each strain were plotted using the minimum and maximum drug ranges tested, normalized on a scale of 0 to 1. Analysis of variance (ANOVA) and correlation analysis were applied by calculating Pearson's r values. Inferential statistics were calculated with StatPlus:mac 2016 (AnalystSoft Inc., Walnut, CA, USA), and P values of <0.05 were considered significant.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Nada Juricevic, Department of Dermatology at the University Hospital of Zurich, for technical support.
We declare no conflicts of interest.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JCM.00338-17.
REFERENCES
- 1.Cabanes FJ. 2014. Malassezia yeasts: how many species infect humans and animals? PLoS Pathog 10:e1003892. doi: 10.1371/journal.ppat.1003892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Findley K, Oh J, Yang J, Conlan S, Deming C, Meyer JA, Schoenfeld D, Nomicos E, Park M, NIH Intramural Sequencing Center Comparative Sequencing Program, Kong HH, Segre JA. 2013. Topographic diversity of fungal and bacterial communities in human skin. Nature 498:367–370. doi: 10.1038/nature12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Sweih N, Ahmad S, Joseph L, Khan S, Khan Z. 2014. Malassezia pachydermatis fungemia in a preterm neonate resistant to fluconazole and flucytosine. Med Mycol Case Rep 5:9–11. doi: 10.1016/j.mmcr.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tragiannidis A, Bisping G, Koehler G, Groll AH. 2010. Minireview: Malassezia infections in immunocompromised patients. Mycoses 53:187–195. doi: 10.1111/j.1439-0507.2009.01814.x. [DOI] [PubMed] [Google Scholar]
- 5.Jenkins SG, Schuetz AN. 2012. Current concepts in laboratory testing to guide antimicrobial therapy. Mayo Clin Proc 87:290–308. doi: 10.1016/j.mayocp.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Samarei R, Gharebaghi N, Zayer S. 2017. Evaluation of 30 cases of mucormycosis at a university hospital in Iran. Mycoses doi: 10.1111/myc.12614. [DOI] [PubMed] [Google Scholar]
- 7.Rojas FD, Sosa MDLA, Fernandez MS, Cattana ME, Cordoba SB, Giusiano GE. 2014. Antifungal susceptibility of Malassezia furfur, Malassezia sympodialis, and Malassezia globosa to azole drugs and amphotericin B evaluated using a broth microdilution method. Med Mycol 52:641–646. doi: 10.1093/mmy/myu010. [DOI] [PubMed] [Google Scholar]
- 8.Velegraki A, Alexopoulos EC, Kritikou S, Gaitanis G. 2004. Use of fatty acid RPMI 1640 media for testing susceptibilities of eight Malassezia species to the new triazole posaconazole and to six established antifungal agents by a modified NCCLS M27-A2 microdilution method and Etest. J Clin Microbiol 42:3589–3593. doi: 10.1128/JCM.42.8.3589-3593.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miranda KC, de Araujo CR, Costa CR, Passos XS, de Fatima Lisboa Fernandes O, do Rosario Rodrigues Silva M. 2007. Antifungal activities of azole agents against the Malassezia species. Int J Antimicrob Agents 29:281–284. doi: 10.1016/j.ijantimicag.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 10.Gupta AK, Kohli Y, Li A, Faergemann J, Summerbell RC. 2000. In vitro susceptibility of the seven Malassezia species to ketoconazole, voriconazole, itraconazole and terbinafine. Br J Dermatol 142:758–765. doi: 10.1046/j.1365-2133.2000.03294.x. [DOI] [PubMed] [Google Scholar]
- 11.Clinical and Laboratory Standards Institute. 2008. Reference methods for broth dilution antifungal susceptibility testing of yeasts; approved standard—3rd ed. CLSI document M27-A3 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 12.Rahman M, Kuhn I, Rahman M, Olsson-Liljequist B, Mollby R. 2004. Evaluation of a scanner-assisted colorimetric MIC method for susceptibility testing of Gram-negative fermentative bacteria. Appl Environ Microbiol 70:2398–2403. doi: 10.1128/AEM.70.4.2398-2403.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rudek W. 1978. Esterase activity in Candida species. J Clin Microbiol 8:756–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pfaller MA, Chaturvedi V, Diekema DJ, Ghannoum MA, Holliday NM, Killian SB, Knapp CC, Messer SA, Miskov A, Ramani R. 2008. Clinical evaluation of the Sensititre YeastOne colorimetric antifungal panel for antifungal susceptibility testing of the echinocandins anidulafungin, caspofungin, and micafungin. J Clin Microbiol 46:2155–2159. doi: 10.1128/JCM.00493-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meletiadis J, Mouton JW, Meis JF, Bouman BA, Verweij PE. 2002. Comparison of the Etest and the Sensititre colorimetric methods with the NCCLS proposed standard for antifungal susceptibility testing of Aspergillus species. J Clin Microbiol 40:2876–2885. doi: 10.1128/JCM.40.8.2876-2885.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rampersad SN. 2012. Multiple applications of Alamar Blue as an indicator of metabolic function and cellular health in cell viability bioassays. Sensors (Basel) 12:12347–12360. doi: 10.3390/s120912347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Natto MJ, Savioli F, Quashie NB, Dardonville C, Rodenko B, de Koning HP. 2012. Validation of novel fluorescence assays for the routine screening of drug susceptibilities of Trichomonas vaginalis. J Antimicrob Chemother 67:933–943. doi: 10.1093/jac/dkr572. [DOI] [PubMed] [Google Scholar]
- 18.Letscher-Bru V, Obszynski CM, Samsoen M, Sabou M, Waller J, Candolfi E. 2013. Antifungal activity of sodium bicarbonate against fungal agents causing superficial infections. Mycopathologia 175:153–158. doi: 10.1007/s11046-012-9583-2. [DOI] [PubMed] [Google Scholar]
- 19.Egervarn M, Lindmark H, Roos S, Huys G, Lindgren S. 2007. Effects of inoculum size and incubation time on broth microdilution susceptibility testing of lactic acid bacteria. Antimicrob Agents Chemother 51:394–396. doi: 10.1128/AAC.00637-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tiballi RN, He X, Zarins LT, Revankar SG, Kauffman CA. 1995. Use of a colorimetric system for yeast susceptibility testing. J Clin Microbiol 33:915–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meletiadis J, Leth Mortensen K, Verweij PE, Mouton JW, Arendrup MC. 2017. Spectrophotometric reading of EUCAST antifungal susceptibility testing of Aspergillus fumigatus. Clin Microbiol Infect 23:98–103. doi: 10.1016/j.cmi.2016.10.017. [DOI] [PubMed] [Google Scholar]
- 22.Trek Diagnostic Systems. 2012. SENSITITRE® YEASTONE® for in vitro diagnostics. Trek Diagnostic Systems, Oakwood Village, OH. [Google Scholar]
- 23.Bennassar A, Grimalt R. 2010. Management of tinea capitis in childhood. Clin Cosmet Investig Dermatol 3:89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Warrilow AG, Price CL, Parker JE, Rolley NJ, Smyrniotis CJ, Hughes DD, Thoss V, Nes WD, Kelly DE, Holman TR, Kelly SL. 2016. Azole antifungal sensitivity of sterol 14α-demethylase (CYP51) and CYP5218 from Malassezia globosa. Sci Rep 6:27690. doi: 10.1038/srep27690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ashbee HR. 2007. Update on the genus Malassezia. Med Mycol 45:287–303. doi: 10.1080/13693780701191373. [DOI] [PubMed] [Google Scholar]
- 26.Hald M, Arendrup MC, Svejgaard EL, Lindskov R, Foged EK, Saunte DM. 2015. Evidence-based Danish guidelines for the treatment of Malassezia-related skin diseases. Acta Derm Venereol 95:12–19. doi: 10.2340/00015555-1825. [DOI] [PubMed] [Google Scholar]
- 27.Gupta AK, Kohli Y. 2003. In vitro susceptibility testing of ciclopirox, terbinafine, ketoconazole and itraconazole against dermatophytes and nondermatophytes, and in vitro evaluation of combination antifungal activity. Br J Dermatol 149:296–305. doi: 10.1046/j.1365-2133.2003.05418.x. [DOI] [PubMed] [Google Scholar]
- 28.Kaneko T, Makimura K, Sugita T, Yamaguchi H. 2006. Tween 40-based precipitate production observed on modified chromogenic agar and development of biological identification kit for Malassezia species. Med Mycol 44:227–231. doi: 10.1080/13693780500354313. [DOI] [PubMed] [Google Scholar]
- 29.Ciardo DE, Schar G, Bottger EC, Altwegg M, Bosshard PP. 2006. Internal transcribed spacer sequencing versus biochemical profiling for identification of medically important yeasts. J Clin Microbiol 44:77–84. doi: 10.1128/JCM.44.1.77-84.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.