ABSTRACT
Clostridium perfringens encodes at least two different quorum sensing (QS) systems, the Agr-like and LuxS, and recent studies have highlighted their importance in the regulation of toxin production and virulence. The role of QS in the pathogenesis of necrotic enteritis (NE) in poultry and the regulation of NetB, the key toxin involved, has not yet been investigated. We have generated isogenic agrB-null and complemented strains from parent strain CP1 and demonstrated that the virulence of the agrB-null mutant was strongly attenuated in a chicken NE model system and restored by complementation. The production of NetB, a key NE-associated toxin, was dramatically reduced in the agrB mutant at both the transcriptional and protein levels, though not in a luxS mutant. Transwell assays confirmed that the Agr-like QS system controls NetB production through a diffusible signal. Global gene expression analysis of the agrB mutant identified additional genes modulated by Agr-like QS, including operons related to phospholipid metabolism and adherence, which may also play a role in NE pathogenesis. This study provides the first evidence that the Agr-like QS system is critical for NE pathogenesis and identifies a number of Agr-regulated genes, most notably netB, that are potentially involved in mediating its effects. The Agr-like QS system thus may serve as a target for developing novel interventions to prevent NE in chickens.
KEYWORDS: NetB, Clostridium perfringens, Agr-like quorum sensing, LuxS, VirS/VirR, necrotic enteritis, poultry, quorum sensing
INTRODUCTION
Clostridium perfringens is a Gram-positive, spore-forming, anaerobic bacterium that is widely distributed in soil, feces, and foods as well as the normal intestinal microbiota of both humans and animals (1). C. perfringens is responsible for a number of human and animal diseases owing to an arsenal of at least 16 different extracellular toxins, including food poisoning, gas gangrene in humans, enterotoxemia of sheep and goats, lamb dysentery, and necrotic enteritis (NE) in poultry (2). Strains are classified into five toxinotypes (A to E) based on the production of four major typing toxins (alpha-, beta-, epsilon-, and iota-toxin) (3, 4).
Certain type A strains can produce NE, an enteric disease of poultry that, in 2015, was estimated to cost the worldwide poultry industry nearly $6 billion in losses (5). The disease occurs when C. perfringens proliferates to high numbers in the intestinal tract and produces extracellular toxins, resulting in characteristic necrotic lesions and often high rates of mortality (6, 7). In addition to producing alpha-toxin (CPA), which is carried by all type A strains, most C. perfringens strains isolated from NE infections also produce NetB, a pore-forming toxin that is essential for NE pathogenesis (8). The gene encoding NetB resides on a 42-kb pathogenicity locus (NELoc-1) that is specifically associated with NE-causing strains and, like many C. perfringens toxins, is plasmid borne (9).
The regulation of virulence in C. perfringens is complex and can differ among strains; however, some of the key regulators are now known. The production of several C. perfringens toxins is positively regulated by the VirR/VirS two-component system (10), which includes NetB (11), CPA, perfringolysin-O (PFO) (12, 13), and beta-toxin (CPB) (14). More recently, quorum sensing (QS) has also been shown to play a key role in regulating the expression of C. perfringens virulence-related proteins (15–17). QS systems coordinate collective behaviors in response to cell population density through the production and detection of small extracellular signaling molecules called autoinducers (AIs) (18–20). C. perfringens encodes at least two QS systems: the LuxS and the Agr-like systems. The LuxS QS system, common to both Gram-negative and Gram-positive species, uses a furanosyl borate diester, called autoinducer 2 (AI-2), as a signaling molecule, which is synthesized from S-adenosylmethionine (SAM) via the LuxS enzyme (21). The Agr-like system, on the other hand, is found only in Gram-positive species and involves the production of small autoinducing peptides (AIPs) as signaling molecules, which are subsequently recognized by a two-component system to ultimately modulate gene expression. This system is represented by the accessory gene regulator (Agr) locus in Staphylococcus aureus, which is comprised of four cotranscribed genes: agrB, agrD, agrC, and agrA (22). The autoinducer propeptide, encoded by agrD, is first processed to the active AIP by the AgrB transporter and then released into the extracellular environment. Once it has accumulated to a sufficient concentration, it activates the agrC-agrA two-component system (TCS) to subsequently modify gene expression. Orthologues of agrB and agrD were recently identified in the C. perfringens genome (15, 16) and found to positively regulate production of several toxins, including CPA, PFO, CPB, epsilon-toxin (ETX), beta2-toxin (CPB2), and enterotoxin (CPE) (23–26). Unlike S. aureus, however, the C. perfringens Agr-like operon does not contain a TCS, and it has been suggested that VirS/VirR serves this function instead (15). This is based on observations that several C. perfringens toxins controlled by the Agr-like QS system are also regulated by VirS/VirR, including CPA, CPB, and PFO (13, 15, 27). In contrast, ETX is regulated by the Agr-like QS system but not by VirS/VirR, indicating that this relationship is not absolute (23). While previous studies have shown that NetB is regulated by VirS/VirR (11), it is not currently known if the Agr-like QS system is also involved.
The LuxS QS system has also been shown to positively regulate CPA and PFO production in a type A gas gangrene-causing strain (17) but not CPB production in a type C strain (26). Regulation of NetB by the LuxS QS system has also not yet been investigated. The objective of the present study was therefore to investigate the roles of these two QS systems in regulating the virulence of the NE-causing C. perfringens strain CP1. Using the wild-type strain, its isogenic luxS- and agrB-null mutants, and complemented strains, we demonstrate that the Agr-like QS system positively regulates NetB production and associated cell cytotoxicity, whereas the LuxS QS system has no apparent effect. Most notably, the virulence of a CP1 agrB-null mutant was severely attenuated in a chicken NE model system and restored by complementation, demonstrating that Agr-like QS is essential for NE pathogenesis. Finally, global gene expression analysis of the agrB-null mutant revealed the Agr-mediated regulation of genes involved in phospholipid metabolism and adherence, elucidating additional possible mechanisms for its effects.
RESULTS
Sequence analysis of the agr locus in CP1.
Sequence analysis of a PCR product targeting an ∼2.9-kb region of the agr operon confirmed that CP1 possesses this locus, with predicted genes encoding hypothetical proteins CPE1562 and CPE1563 as well as AgrB and AgrD. When compared with the AgrB and AgrD amino acid sequences available for nine other C. perfringens strains, the proteins encoded by CP1 were found to share >98% identity, except for C. perfringens strain SM101, with which they share 95% and 93% identity, respectively.
Construction and genotypic characterization of CP1 mutants and complement strains.
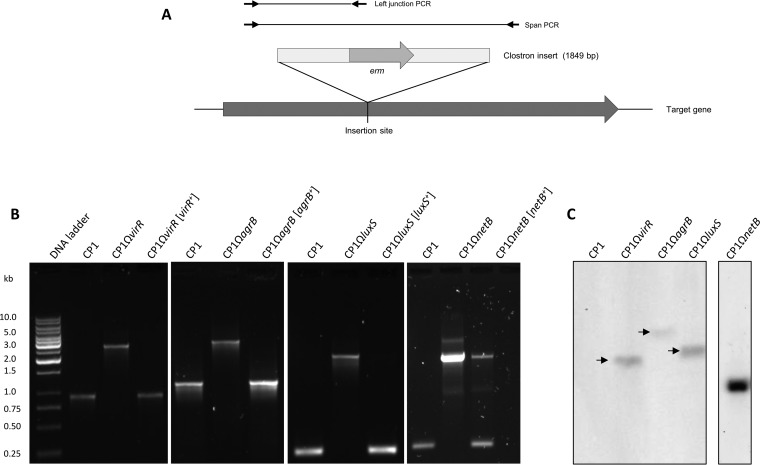
The genes encoding the putative AgrB transporter, VirR response regulator, NetB toxin, and LuxS enzyme were each insertionally inactivated in CP1 using the ClosTron system (28), and complemented strains were constructed by cloning each wild-type gene, including the upstream region, into shuttle vector pJIR750 and transforming into the mutant strain. To confirm the correct insertion of the ClosTron cassette, primers flanking the predicted insertion site in each disrupted gene were used to amplify genomic DNA (gDNA) from wild-type CP1, the isogenic mutants, and their corresponding complemented strains (Fig. 1A). In each case, a PCR product corresponding to the wild-type gene was amplified in the wild-type and complemented strains, while a larger product, corresponding to the gene containing the 1,849-bp ClosTron insert, was detected in the mutant strains (Fig. 1B). Additionally, primers were designed to amplify the region spanning the 5′ junction between the wild-type gene and the ClosTron insert, which amplified a PCR product of the correct size from the mutant and complemented strains (data not shown). Sequencing of this amplicon from each mutant confirmed that it was in fact the gene-insert junction region. Southern blot analysis was also performed, which confirmed that only one insertion event had taken place for each of the mutants (Fig. 1C).
FIG 1.
PCR and Southern blot confirmation of the isogenic CP1 mutants and complemented strains. (A) Diagram of primers used to verify ClosTron gene insertions. (B) Amplification of virR, agrB, luxS, and netB regions spanning the ClosTron insert in the wild-type CP1, mutant, and complemented strains. (C) Southern blot analysis of virR, agrB, luxS, and netB mutants. Genomic DNA was digested with DraI and hybridized with a Dig-labeled probe specific for the ClosTron insert.
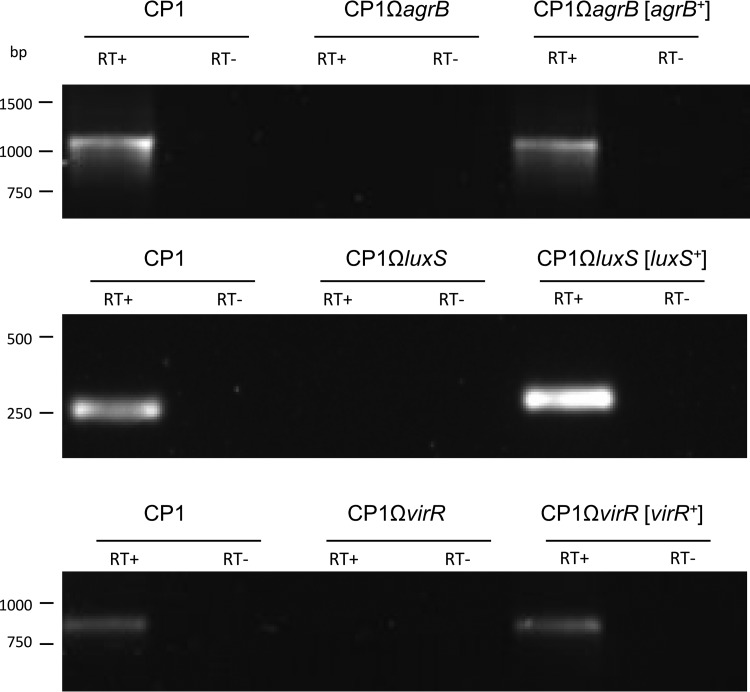
The expression of agrB, virR, and luxS in the mutant and complemented strains was also evaluated by reverse transcription (RT)-PCR, which demonstrated the production of these transcripts by wild-type CP1 and the complemented strains but not by the mutant strains (Fig. 2). The growth rates of the mutants and complemented strains were assessed and found to be comparable to that of wild-type CP1 (data not shown). The inactivation of agrB in other C. perfringens strains has been shown to exert polar effects on the downstream AgrD AIP (15, 16, 26), and it may thus be concluded that the entire agr operon is abrogated in CP1ΩagrB. Similarly, VirR and VirS are also encoded on a single operon, so the VirR-null mutant is not expected to produce VirS. Taken together, these results confirm the disruption of agrB, virR, netB, and luxS in strains CP1ΩagrB, CP1ΩvirR, CP1ΩnetB, and CP1ΩluxS and their complementation in CP1ΩagrB[agrB+], CP1ΩvirR[virR+], CP1ΩnetB[netB+], and CP1ΩluxS[luxS+].
FIG 2.
RT-PCR analyses of agrB, luxS, and virR expression in wild-type CP1, mutant, and complemented strains in TGY culture (OD600 = 0.8). As indicated, reverse transcriptase (RT) was (+) or was not (−) added to the reaction tubes.
The agr operon, but not luxS, positively regulates NetB production.
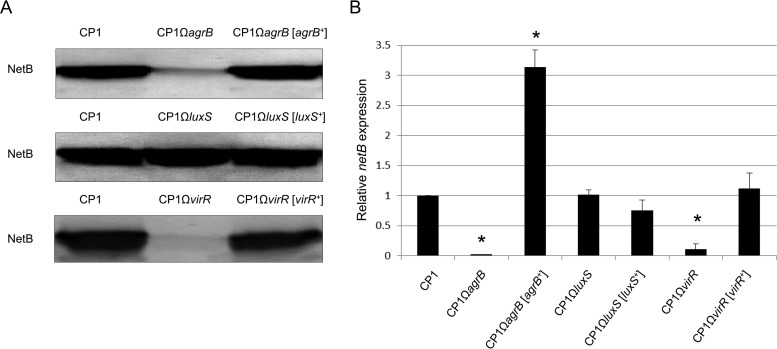
Western blot analysis using a monoclonal NetB antibody was employed to investigate whether the agr operon or luxS plays a role in regulating NetB production during in vitro growth of CP1. As shown in Fig. 3A, substantially less NetB was detected in the culture supernatant derived from CP1ΩagrB than from the wild type, while NetB production was restored to wild-type levels in the complemented strain. In contrast, no difference in NetB production was observed among wild-type, CP1ΩluxS, and CP1ΩluxS[luxS+] strains (Fig. 3A).
FIG 3.
The agr operon positively regulates in vitro NetB production in CP1 via the Agr-like QS system. (A) Western blot analyses of NetB production in wild-type CP1, agrB, luxS, and virR mutants, and complemented strains. Total proteins (100 μg) from supernatants derived from TPG cultures (OD600 = 0.8) of wild-type CP1, isogenic mutants, and complemented strains were separated by 10% SDS-PAGE, and Western blotting was performed with a mouse anti-NetB primary monoclonal antibody and goat anti-mouse AP-conjugated IgG secondary antibody. (B) qPCR analyses of netB expression in wild-type CP1, agrB, luxS, and virR mutant, and complemented strains in TPG culture (OD600 = 0.8). The data are expressed as the means ± standard deviations (SD) from three separate experiments. *, P < 0.05 versus the CP1 group.
The VirS/VirR system has been previously shown to regulate NetB production in two NE isolates, EHE-NE18 and 56 (11). To confirm this regulation in CP1, Western blot analysis was performed on CP1, the CP1ΩvirR mutant, and the complemented strain CP1ΩvirR[virR+]. As expected, only weak NetB production was detected in CP1ΩvirR, whereas wild-type CP1 and the complemented strain produced similarly high levels of NetB (Fig. 3A).
The expression of netB at the transcriptional level was additionally compared using quantitative PCR. Consistent with the Western blot results, netB expression was significantly decreased in CP1ΩagrB (−41-fold) and CP1ΩvirR (−22-fold), but not in CP1ΩluxS, compared to the wild-type control (Fig. 3B). These results clearly demonstrate that the Agr-like QS system positively regulates NetB production at the transcriptional level, whereas luxS has no effect at either the protein or the transcript level.
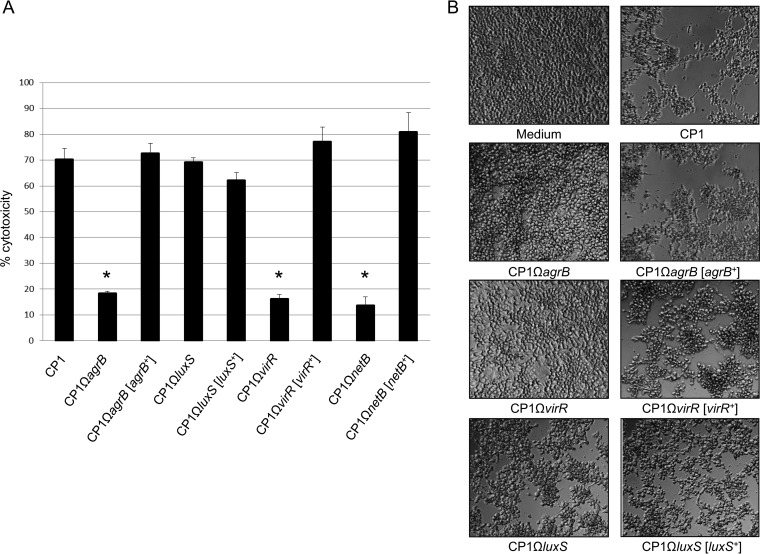
The agrB-null mutant is not cytotoxic toward chicken LMH cells.
The NetB toxin has been previously demonstrated to have specific cytotoxic effects toward the chicken Leghorn male hepatoma (LMH) cell line (ATCC CRL-2117) (8, 29). To evaluate the role of the agr operon and luxS on the cytotoxicity of CP1, we treated LMH cells with sterile supernatants from cultures of wild-type CP1 and its isogenic derivatives. After 4 h of incubation in 5% CO2, LMH cells treated with supernatant from the wild-type CP1 culture showed significantly higher LDH release than did the supernatant of the isogenic agrB mutant (Fig. 4A). Supernatant from the complemented strain CP1ΩagrB[agrB+] induced levels of LDH release similar to those of the wild-type CP1, indicating that the reduction in cytotoxicity was due to specific inactivation of the agr operon. A similar effect was observed with CP1ΩvirR and CP1ΩvirR[virR+], whereas no significant decrease in LDH release was observed in cells treated with CP1ΩluxS supernatant or the complemented strain. To verify that the cytotoxic effects toward the LMH cells were specifically due to NetB and not to the activity of other extracellular toxins produced by CP1, the CP1ΩnetB and CP1ΩnetB[netB+] strains were also examined. As expected, supernatant derived from CP1ΩnetB caused significantly less LDH release than the wild-type control, whereas the CP1ΩnetB[netB+] complemented strain caused levels similar to those of the control.
FIG 4.
In vitro LMH cytotoxicity of CP1 and its isogenic derivatives. (A) Lactate dehydrogenase (LDH) release. Wild-type CP1, agrB, luxS, and virR mutants, and complemented strains were grown in TPG culture to an OD600 of 0.8. Culture supernatant was collected and incubated with LMH cells at 37°C for 4 h. LDH release into the supernatant of LMH cells was measured as an indicator of LMH cytotoxicity. The data were expressed as the means ± SD from three separate experiments. *, P < 0.01 versus the CP1 group. (B) Morphological damage to LMH cells observed under the microscope.
As shown in Fig. 4B, the levels of LDH release were reflected in the morphological cell damage observed in the LMH cells. Obvious cytopathic effects were observed in LMH cells treated with CP1, CP1ΩluxS, and the complemented strains CP1ΩagrB[agrB+], CP1ΩvirR[virR+], and CP1ΩluxS[luxS+], whereas few to no morphological changes were observed in those treated with CP1ΩagrB or CP1ΩvirR supernatant.
The agr operon-dependent regulation of NetB production involves a secreted diffusible signal.
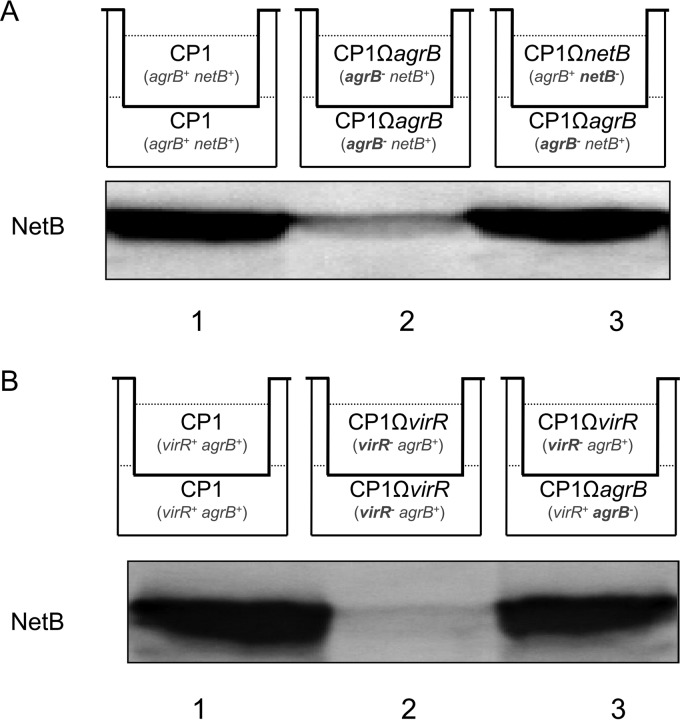
To address whether the agr operon-dependent regulation of NetB production in CP1 is mediated through a diffusible signal, a Transwell assay was performed (26). Different combinations of strains were inoculated into the top and bottom chambers of a transwell dish, which are separated by a 0.4-μm membrane filter, allowing passage of proteins and small molecules, but not bacterial cells. When wild-type CP1 was inoculated into both the top and bottom chambers, NetB was produced as expected (Fig. 5A), whereas no NetB production was detected when the netB mutant was inoculated into both chambers (data not shown). Similarly, only weak NetB production was detected when the agrB mutant was inoculated into both chambers. However, when the top chamber was inoculated with CP1ΩnetB, which carries a functional agr operon, and the bottom chamber with CP1ΩagrB, substantial NetB production was readily detectable in the bottom chamber. Given that CP1ΩnetB cannot produce NetB, it can be concluded that the NetB detected in the chamber was produced by CP1ΩagrB. A similar experiment was conducted using the CP1 VirR-null mutant, which is also deficient in NetB production (Fig. 5B). Once again, only when CP1ΩvirR and CP1ΩagrB were inoculated into separate chambers of the same Transwell did any significant production of NetB occur, indicating the physical complementation of the Agr QS system in CP1ΩagrB by the intact agr operon in CP1ΩvirR. These results demonstrate that a diffusible signal, presumably the AgrD AIP, was produced by CP1ΩnetB and CP1ΩvirR and subsequently activated the expression of NetB in CP1ΩagrB.
FIG 5.
The agr operon-mediated regulation of NetB production involves a diffusible signal. Western blot analysis of NetB production in CP1ΩagrB induced by a diffusible signal provided by CP1ΩnetB (A) or CP1ΩvirR (B) culture. The top schematic indicates the combinations of strains inoculated into each Transwell chamber and their relevant genotypes, with insertionally inactivated genes in bold. Supernatants were collected from a 5-h TPG culture in the bottom chambers for Western blot analyses of NetB production. Shown are representative results that were reproducible over three repetitions.
The agr operon is required for NE pathogenesis in an in vivo NE model system.
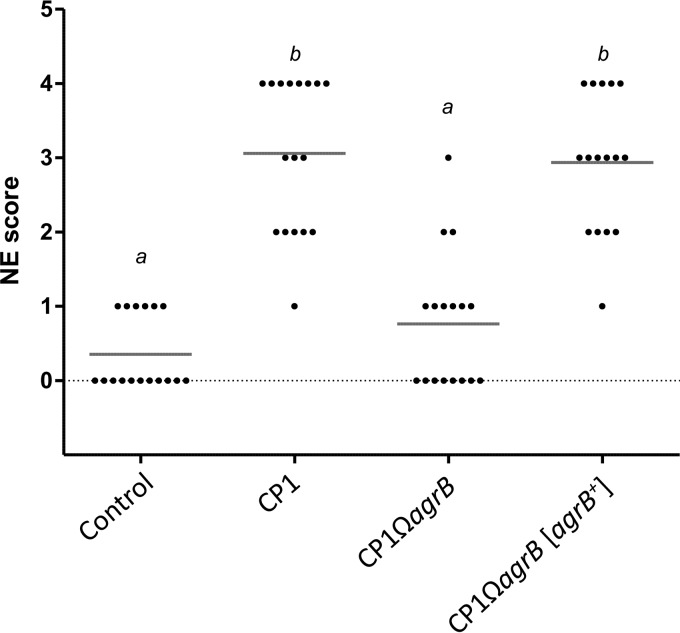
To further investigate the role of the agr operon in NE pathogenesis, the virulence of CP1 and its isogenic derivatives was evaluated in a chicken NE model system. Four groups of Ross 708 broiler chickens were challenged with either wild-type CP1, the mutant CP1ΩagrB, the complemented CP1ΩagrB[agrB+] strain, or uninoculated fluid thioglycolate (FTG) medium for 3 days and then scored for intestinal lesions characteristic of NE. The cultures used to prepare the infected inoculum were enumerated by plating at each time point and revealed comparable growths of CP1 (2.52 × 108 ± 1.01 × 108 CFU/ml), CP1ΩagrB (1.40 × 108 ± 0.49 × 108 CFU/ml), and CP1ΩagrB[agrB+] (2.42 × 108 ± 0.50 × 108 CFU/ml). A significantly higher level of disease was detected in birds infected with the wild-type CP1 than in the medium-only control, with an average lesion score of 3.06 (Fig. 6). In contrast, challenge with the mutant CP1ΩagrB resulted in significantly lower NE lesion scores (mean score, 0.76; P < 0.01 compared to the CP1 group), whereas the complemented strain CP1ΩagrB[agrB+] restored the virulence of the agrB mutant, with lesion scores similar to those of the wild-type strain (mean score, 2.94; P < 0.01 compared to the CP1ΩagrB group).
FIG 6.
In vivo virulence of CP1 and its isogenic derivatives. Virulence of C. perfringens strains in an in vivo NE challenge model. The lesion scores and means of individual 16-day-old broiler chickens challenged with different C. perfringens strains are shown. Control, CP1, and CP1ΩagrB groups consisted of 17 birds, and the CP1ΩagrB[agrB+] complemented strain group consisted of 16 birds. Groups that differ significantly (P < 0.01, Tukey's HSD) are annotated with different letters.
Global gene expression in CP1ΩagrB.
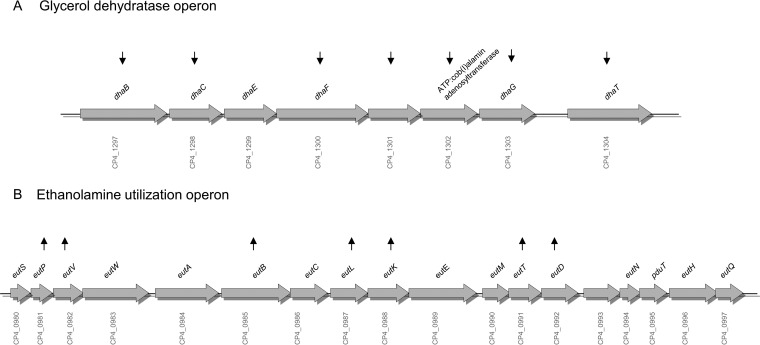
To identify additional genes regulated by the agr operon in CP1 that could potentially play a role in NE pathogenesis, microarray analysis was performed with RNA isolated from CP1 and CP1ΩagrB, using a custom C. perfringens microarray (9). Of the 3,335 probes on the array, 2,651 (79.5%) were discarded due to low signal intensity, and of the remaining 684 probes, 66 genes were found to be significantly differentially expressed (false discovery rate [FDR] < 0.1; >2-fold change) (Tables 1 and 2). Of these, 34 genes were significantly upregulated in CP1ΩagrB compared to wild-type CP1 (Table 1), indicating that their expression is normally repressed by Agr-like QS under the conditions examined, while 32 genes were downregulated in CP1ΩagrB (Table 2), indicating positive regulation by Agr-like QS in the wild-type strain. Five genes were selected for confirmation by quantitative PCR (qPCR), which exhibited fold changes comparable to those obtained by microarray analysis (Table 3). As expected, agrB exhibited the greatest downregulation (−35-fold), followed by netB and netH (−27- and −29-fold, respectively), which are cotranscribed. In addition to individual genes, several groups of closely linked genes that likely form operons were differentially expressed. These included two different systems involved in the catabolism of phosphotidylethanolamine, an abundant phospholipid found in animal cell membranes. Specifically, seven of eight genes of the dha operon, responsible for the conversion of glycerol to 1,3-propanediol, were downregulated (Fig. 7A), while seven genes within the ethanolamine utilization (eut) regulon, encoding enzymes and bacterial microcompartment proteins for catabolism of ethanolamine, were upregulated (Fig. 7B). Additionally, three closely linked genes (CP4_1816, CP4_1817, and CP4_1819) involved in the phosphotransferase (PTS) sugar uptake system were upregulated in CP1ΩagrB and two genes (CP4_0573 and CP4_0575) that are part of a larger operon recently identified as involved in adhesion (30) were downregulated in CP1ΩagrB.
TABLE 1.
Genes upregulated in CP1ΩagrB compared to wild-type CP1
| Locus tag | GenBank accession no. | Gene | Putative gene product | Fold change | q value (%) |
|---|---|---|---|---|---|
| CP4_0093 | AM596_00445 | Protein NT01CX_0824 | 2.17 | 4.64 | |
| CP4_0143 | AM596_00685 | Conserved hypothetical protein | 3.64 | 4.64 | |
| CP4_0362 | AM596_01730 | Protein of unknown function | 2.29 | 5.92 | |
| CP4_0600 | AM596_02865 | NAD-dependent 4-hydroxybutyrate dehydrogenase | 2.25 | 4.64 | |
| CP4_0623 | AM596_02980 | Alpha,alpha-phosphotrehalase | 18.14 | 0.00 | |
| CP4_0732 | AM596_03505 | Conserved hypothetical protein | 2.22 | 10.72 | |
| CP4_0860 | AM596_04125 | Flavohemoprotein | 2.04 | 10.72 | |
| CP4_0862 | AM596_04135 | Rubredoxin | 3.66 | 11.51 | |
| CP4_0981 | AM596_04675 | eutP | Ethanolamine utilization protein, EutP | 2.75 | 4.64 |
| CP4_0982 | AM596_04680 | eutV | Response regulator | 2.65 | 7.77 |
| CP4_0985 | AM596_04695 | eutB | Ethanolamine ammonia-lyase, large subunit | 7.72 | 12.41 |
| CP4_0987 | AM596_04705 | eutL | Ethanolamine utilization protein EutL | 36.33 | 7.77 |
| CP4_0988 | AM596_04710 | pduJ | Propanediol utilization protein | 11.43 | 7.77 |
| CP4_0991 | AM596_04725 | eutT | Ethanolamine utilization cobalamin | 6.68 | 11.51 |
| CP4_0992 | AM596_04730 | pduL | Propanediol utilization protein | 5.93 | 12.41 |
| CP4_1183 | AM596_05605 | Ferredoxin hydrogenase | 2.48 | 11.51 | |
| CP4_1290 | AM596_06085 | Conserved hypothetical protein | 2.16 | 5.92 | |
| CP4_1391 | AM596_06550 | Putative endoribonuclease L-PSP | 2.79 | 7.77 | |
| CP4_1492 | AM596_07040 | Threonine dehydratase | 2.97 | 5.92 | |
| CP4_1587 | AM596_07465 | Magnesium transporter | 3.01 | 7.77 | |
| CP4_1648 | AM596_07745 | Phosphopyruvate hydratase | 5.33 | 0.00 | |
| CP4_1816 | AM596_08535 | PTS system, IIC component | 4.17 | 10.72 | |
| CP4_1817 | AM596_08540 | Probable phosphotransferase enzyme IIB component | 2.96 | 4.64 | |
| CP4_1819 | AM596_08550 | 6-Phosphofructokinase | 77.67 | 0.00 | |
| CP4_1947 | AM596_09175 | Conserved hypothetical protein | 3.62 | 12.41 | |
| CP4_2614 | AM596_12415 | CTP synthase | 3.25 | 7.77 | |
| CP4_2695 | AM596_12805 | Inosine-5′-monophosphate dehydrogenase | 3.34 | 7.77 | |
| CP4_2724 | AM596_12945 | Putative cadmium efflux system accessory protein | 2.44 | 7.77 | |
| CP4_2788 | AM596_13240 | Chain B X-ray structures | 16.21 | 12.41 | |
| CP4_2799 | AM596_13295 | Thioredoxin reductase | 2.06 | 10.72 | |
| CP4_2800 | AM596_13300 | Thioredoxin | 2.79 | 7.77 | |
| CP4_2802 | AM596_13310 | Phosphoenolpyruvate-protein phosphotransferase | 3.96 | 2.92 | |
| CP4_2909 | AM596_13825 | UDP-N-acetylmuramoylalanine-d-glutamate ligase | 9.48 | 7.77 | |
| CP4_2940 | AM596_13975 | Two-component sensor histidine kinase | 2.01 | 7.77 |
TABLE 2.
Genes downregulated in CP1ΩagrB compared to wild-type CP1
| Locus tag | GenBank accession no. | Gene | Putative gene product | Fold change | q value (%) |
|---|---|---|---|---|---|
| CP4_0126 | AM596_00605 | Glucose kinase | −8.53 | 0.00 | |
| CP4_0211 | AM596_01000 | colA | Microbial collagenase | −3.07 | 7.77 |
| CP4_0349 | AM596_01670 | Transcriptional regulator | −2.05 | 10.72 | |
| CP4_0398 | AM596_01900 | Pyruvate kinase | −2.22 | 7.77 | |
| CP4_0473 | AM596_02265 | Phosphopentomutase | −12.79 | 0.00 | |
| CP4_0573 | AM596_02740 | Signal peptidase I | −4.17 | 7.77 | |
| CP4_0575 | AM596_02750 | Sortase, Srtb family | −4.05 | 1.72 | |
| CP4_1164 | AM596_05510 | UBA/TS-N domain-containing protein | −2.07 | 10.72 | |
| CP4_1297 | AM596_06115 | dhaB | Glycerol dehydratase, large subunit | −6.25 | 10.72 |
| CP4_1298 | AM596_06120 | dhaC | Glycerol dehydratase, medium subunit | −6.11 | 0.00 |
| CP4_1300 | AM596_06130 | dhaF | Glycerol dehydratase reactivation factor, large | −25.47 | 0.00 |
| CP4_1301 | AM596_06135 | Glycerol dehydratase reactivation factor, small | −10.38 | 1.72 | |
| CP4_1302 | AM596_06140 | ATP:cob(I)alamin adenosyltransferase | −10.13 | 0.00 | |
| CP4_1303 | AM596_06145 | dhaG | DhaG protein | −7.33 | 0.00 |
| CP4_1304 | AM596_06150 | dhaT | 1,3-Propanediol dehydrogenase | −6.04 | 4.08 |
| CP4_1328 | AM596_06270 | Conserved hypothetical protein | −4.32 | 11.51 | |
| CP4_1396 | AM596_06575 | Aminodeoxychorismate synthase, component I | −2.26 | 7.77 | |
| CP4_1422 | AM596_06695 | Iron chelate ABC transporter solute-binding | −2.26 | 2.92 | |
| CP4_1497 | AM596_07060 | Conserved hypothetical protein | −4.24 | 4.08 | |
| CP4_1531 | AM596_07215 | Coenzyme A substrate-specific enzyme activase | −2.19 | 10.72 | |
| CP4_1720 | AM596_08080 | Sodium:neurotransmitter symporter family | −3.01 | 2.92 | |
| CP4_1915 | AM596_09015 | Lipoprotein, putative | −13.79 | 0.00 | |
| CP4_1916 | AM596_09020 | agrB | Accessory gene regulator protein B | −35.45 | 0.00 |
| CP4_2070 | AM596_09775 | Ribosomal protein S16 | −2.55 | 7.77 | |
| CP4_2113 | AM596_09990 | Fe-S oxidoreductase | −2.84 | 4.64 | |
| CP4_2294 | AM596_10880 | Adenine phosphoribosyltransferase | −2.00 | 1.72 | |
| CP4_2457 | AM596_11635 | Deoxyribose-phosphate aldolase | −3.80 | 0.00 | |
| CP4_2753 | AM596_13080 | Na+/H+ antiporter family protein | −4.07 | 10.72 | |
| CP4_2949 | AM596_14020 | Nucleoside permease Nupx | −6.95 | 4.64 | |
| CP4_3445 | AM596_16310 | Putative radical SAM domain-containing protein | −15.75 | 0.00 | |
| CP4_3449 | AM596_16320 | netB | Necrotic enteritis toxin B | −27.03 | 0.00 |
| CP4_3450 | AM596_16325 | netH | Ricin-type beta-trefoil domain protein | −29.24 | 0.00 |
TABLE 3.
qPCR verification of microarray results
| Locus tag | Putative gene product | Fold change by: |
qPCR SD | |
|---|---|---|---|---|
| Microarray | qPCR | |||
| CP4_0573 | Signal peptidase I | −4.17 | −5.42 | 0.35 |
| CP4_0575 | Sortase, Srtb family | −4.05 | −3.67 | 0.21 |
| CP4_3445 | Putative radical SAM domain-containing protein | −15.75 | −14.17 | 7.29 |
| CP4_3449 | Necrotic enteritis toxin B | −27.03 | −41.18 | 1.86 |
| CP4_3450 | Ricin-type beta-trefoil domain protein | −29.24 | −22.97 | 4.19 |
FIG 7.
Glycerol dehydratase and ethanolamine utilization operon regulation by the Agr system. Diagram of the dha (A) and eut (B) operons, indicating which genes were significantly upregulated (up arrows) and downregulated (down arrows) in CP1ΩagrB compared to CP1.
DISCUSSION
The current study establishes a key role for the Agr-like QS system in the pathogenesis of NE in chickens and elucidates several possible mechanisms by which the system may influence in vivo virulence. QS systems related to the S. aureus Agr system are widespread among Firmicutes (31) and typically act to regulate concerted, population-wide behaviors such as sporulation, biofilm formation, and virulence (32–34). The development of NE in poultry is generally attributed to the rapid overgrowth of C. perfringens in the gut and subsequent elaboration of virulence factors that induce damage to the host (35, 36), a progression that is consistent with a QS-based regulatory mechanism. It follows that, by coordinating the production of extracellular toxins and enzymes to occur only once a sufficient population density is reached, a pathogen may inflict the greatest damage to the host while at the same time minimizing its expenditure of metabolic resources.
In the current study, the virulence of a CP1 agrB-null mutant was found to be severely attenuated in an in vivo NE model system compared to the wild-type control and restored in the complemented strain. One other study to date has demonstrated the requirement for Agr-like QS in in vivo pathogenesis, which examined CPB production in a type C strain (26); however, this has remained an open question for type A-associated disease (37). This previous study demonstrated that an agrB null mutant was avirulent in a rabbit intestinal loop model of enteropathogenicity and had reduced lethality in a mouse model of enterotoxemia. This attenuated virulence was attributed to the reduced production of CPB by the agrB mutant as detected in the intestinal loop, after ruling out differences in in vivo growth. Similarly, the current study found that the expression of NetB was dramatically reduced in the CP1 agrB-null mutant in vitro; however, NetB production was not quantified in the chicken intestinal lumen during challenge, so it is not known if a similar reduction also occurred in vivo, though the previous findings regarding CPB regulation tend to suggest that this may be the case. While the reduced production of NetB by CP1ΩagrB offers the most plausible explanation for its attenuated in vivo virulence, given its essential role in NE pathogenesis, other possibilities exist, which are further explored below.
The Agr-like QS system in C. perfringens has been shown to regulate a number of extracellular toxins in vitro, including CPA, CPB, CPB2, CPE, PFO, and ETX in type A to D strains (15, 16, 23–26). This is the first study, however, to demonstrate the involvement of Agr-like QS in the regulation of NetB, the key toxin responsible for NE in chickens. We demonstrate that inactivation of agrB in the virulent CP1 strain leads to a dramatic in vitro decrease in NetB expression at both the transcriptional and protein levels. Similarly, as a result of this reduced expression, the cytotoxic activity of CP1ΩagrB culture supernatant was strongly attenuated, as shown by the decreased cytotoxicity toward the chicken LMH cell line. These effects can be specifically attributed to the inactivation of the agr operon, since wild-type levels of cytotoxicity were restored following complementation with an agrB-carrying plasmid. While C. perfringens can produce a number of extracellular toxins capable of causing cellular damage, the LMH cell line has been shown to be specifically susceptible to the pore-forming activity of the NetB toxin (29). This is also supported by the observation that CP1ΩnetB is significantly less cytotoxic than the wild-type strain, even though it still produces the full complement of other extracellular toxins produced by the parent strain.
The attenuated virulence of CP1ΩagrB in the NE challenge model could also be explained by a reduced ability of the mutant to survive and reproduce in vivo. In an attempt to evaluate this possibility, the intestinal contents of 6 or 7 birds from each challenge group were enumerated for C. perfringens at the time of euthanization; however, due to the large variability in counts, it was not possible to conclude whether the populations differed significantly between groups. Despite this fact, it could be qualitatively determined that all of the strains, including CP1ΩagrB, were able to survive and proliferate to considerable numbers in the chicken intestinal tract, with similar average numbers of C. perfringens recovered from birds challenged with CP1 (6.03 × 107 ± 5.69 × 107 CFU/g), CP1ΩagrB (1.31 × 107 ± 0.63 × 107 CFU/g), and CP1ΩagrB[agrB+] (1.89 × 107 ± 2.3 × 107 CFU/g). Furthermore, the in vitro growth rates of the wild-type, mutant, and complemented strains were also examined and found to be comparable, and enumeration of the inoculum added to the chicken feed at each challenge time point confirmed similar numbers for each strain. Together these results suggest that the attenuated in vivo virulence of CP1ΩagrB was not likely due to differences in its ability to survive and reproduce in vivo, though this possibility cannot be ruled out entirely.
A third alternative explanation for the CP1ΩagrB attenuated in vivo virulence that does not involve NetB is the requirement of other agr-regulated genes for NE pathogenesis, although a more likely scenario is that additional agr-regulated genes contribute to virulence alongside NetB. Recent advances have made it clear that the onset of NE is a complex process involving multiple C. perfringens virulence factors related to colonization, adhesion, and acquisition of nutrients (35). Global gene expression analysis of CP1ΩagrB revealed 66 genes that were significantly differentially expressed compared to the wild-type CP1 control. Among these, agrB and netB exhibited the greatest amount of downregulation, providing strong validation of the microarray results. Due to the relatively low sensitivity of the microarray (∼80% of probes were not detected), these results cannot be considered a comprehensive survey of the agr regulon; however, they do provide some valuable insights into key pathways regulated by the Agr-like QS system. Interestingly, two genes (CP4_0573 and CP4_0575) identified in a previous comparative genomic screen for NE-associated loci (38) were found to be positively regulated by Agr-like QS, further implicating a role for this locus in NE pathogenesis. These two genes are part of a larger cluster of seven genes, designated VR-10B, that encode a collagen adhesin, sortases, and structural proteins predicted to produce an adhesive pilus (30, 38).
Two operons involved in the phosphotidylethanolamine (PE) catabolic pathway were also differentially expressed in CP1ΩagrB; specifically, the dha and eut operons, responsible for the breakdown of the PE metabolites glycerol and ethanolamine, respectively. PE is the most abundant phospholipid present in intestinal epithelial cells (39) and is therefore a probable nutrient source during lesion formation at the intestinal mucosa, where C. perfringens cells are present in high numbers. PE can also be hydrolyzed by CPA (40), a zinc metallophospholipase C that, although no longer considered to be the key toxin involved (41), still appears to play a role in NE pathogenesis (42–44). Ethanolamine has been specifically implicated in virulence, due to its ability to serve as a sole carbon and nitrogen source for some bacterial pathogens, including C. perfringens (45). Ethanolamine utilization (EU) has been most extensively studied in Salmonella enterica, where it has been shown to provide S. enterica with a competitive advantage during infection, allowing it to outgrow resident members of the microbiota (46, 47). A systematic survey of bacterial genomes revealed that, while eut operons are absent from most prokaryotes, those carried by S. enterica, Listeria monocytogenes, and C. perfringens are highly similar, suggesting that EU may be a key fitness adaptation of intestinal pathogens (48). Notably, a recent transcriptomic analysis of C. perfringens in ligated chicken intestinal loops also detected upregulation of the eut operon (49). Further studies into the role of EU in the pathogenesis of NE, and perhaps other diseases caused by C. perfringens, are therefore warranted.
A hallmark of the Agr-like QS system is the mediation of its regulatory effects through a diffusible AIP signal. To confirm this phenomenon in the regulation of NetB by the agr operon, a Transwell assay in which a semipermeable membrane was used to separate two bacterial cultures but allow passage of small molecules such as the Agr-like QS AIP was performed. NetB- and AgrB-deficient strains were used to demonstrate that substantial NetB was produced only when the two strains were inoculated together into separate chambers of the same Transwell. Since CP1ΩnetB is unable to produce NetB, it can therefore be concluded that the observed toxin production must have arisen from CP1ΩagrB and in all likelihood was induced by the AIP produced by CP1ΩnetB, which still carries an intact agr locus. The physical complementation of a QS-deficient strain (CP1ΩagrB) by a strain with an intact QS system (CP1ΩnetB) provides strong evidence for the involvement of a diffusible-signaling molecule and in previous studies has confirmed the AIP-mediated regulation of both ETX and CPB (23, 26).
As mentioned previously, the C. perfringens agr operon lacks a TCS, and it is speculated that the VirS/VirR TCS serves this role (15). Construction and examination of a VirR mutant (CP1ΩvirR) in this study demonstrated the requirement of VirS/VirR in netB transcription and protein production in CP1, confirming previous findings in two other type A strains (11). The fact that the CP1ΩvirR and CP1ΩagrB mutants share the same NetB-negative phenotype suggests that the VirS/VirR system plays the role of the downstream TCS to detect the Agr-like QS system AIP in CP1. Future experiments demonstrating that the Agr AIP can stimulate autophosphorylation of the VirS sensor histine kinase would confirm this hypothesis.
Although the LuxS QS system has also been previously implicated in the regulation of C. perfringens virulence (17), in the current study a LuxS-null mutant did not exhibit any reduction in NetB expression or cytotoxicity toward LMH cells compared to wild-type controls. Ohtani et al. (17) initially reported that LuxS QS regulates CPA and PFO expression in a type A strain; however, subsequent studies examining the production of CPA and PFO in LuxS-null type C strains failed to identify a LuxS QS effect (14, 26). The regulation of CPB was also found to be independent of LuxS in a type C strain (26). The current findings further support a more limited role for the LuxS QS system in the regulation of C. perfringens toxin production than for the Agr-like QS system.
In summary, the present study provides the first evidence that the Agr-like QS system is critical for NE pathogenesis of C. perfringens in a disease model. Furthermore, our in vitro results suggest several mechanisms by which Agr-like QS may influence virulence. Currently, efforts are under way to develop therapies against S. aureus infections using AIP analogues to interfere with the Agr QS system (50, 51), which have the added benefit of not promoting the development of antibiotic resistance, and some progress has also been made to target the C. perfringens AIP (52). Considering that Agr-like QS is essential for the pathogenesis of NE in vivo, this system may serve as a target for developing novel interventions to prevent NE disease in chickens, as an alternative to the antibiotic therapies currently in use.
MATERIALS AND METHODS
Bacterial strains and media.
C. perfringens strain CP1 used in this study was a field isolate from an NE case in Ontario, Canada (53). The bacterial culture media used throughout this study included tryptone glucose yeast (TGY), tryptone proteose peptone glucose (TPG), cooked meat medium (CMM), and Perfringens oleandomycin-polymyxin-sulfadiazin-perfringens-selective agar (OPSP). Escherichia coli Top 10 (Invitrogen, Burlington, ON, Canada) was used for cloning in Luria-Bertani (LB) broth or agar (Difco, Sparks, MD, USA), supplemented with 34 μg ml−1 chloramphenicol (Sigma-Aldrich, St. Louis, MO, USA) as required.
Sequencing of the agr locus in C. perfringens strain CP1.
Genomic DNA (gDNA) was extracted from CP1 with the Gentra Puregene Yeast/Bact kit (Qiagen, Mississauga, ON, Canada). The CP1 agr locus was amplified by PCR with Platinum Taq High Fidelity (Invitrogen) using the primers agr-F1 and agr-R1 (16). The ∼2.9-kb PCR product was cloned into pCR4-TOPO (Invitrogen), and the insert was sequenced at the Laboratory Services Division, University of Guelph.
Construction of mutant strains and complement strains in C. perfringens strain CP1.
The ClosTron system was used to insertionally inactivate agrB, luxS, netB, and virR in CP1 as described previously (28, 54). ClosTron intron-targeting regions were designed to insert at the following gene positions using the Perutka algorithm implemented at www.clostron.com: bp 344 of the agrB sense strand, bp 161 of the luxS antisense strand, bp 178 of the netB sense strand, and bp 97 of the virR sense strand. The intron-targeting regions were synthesized and cloned into ClosTron plasmid pMTL007C-E2 by DNA 2.0 (Menlo Park, CA, USA) and named pMTL007C-E2::agrB344s, pMTL007C-E2::luxS161a, pMTL007C-E2::netB178s, and pMTL007C-E2::virR97s, respectively. The resultant plasmids were separately electroporated into CP1 as described previously with minor modifications (55). Briefly, after growing at 37°C anaerobically overnight in 5 ml TGY broth, CP1 was subcultured into 50 ml TGY and grown to exponential phase (optical density at 600 nm [OD600], 0.8). The cells were harvested by centrifugation at 6,000 × g for 10 min at 20°C and washed once in 10 ml sucrose electroporation buffer (SEB) (272 mM sucrose, 1 mM MgCl2, 5 mM Na2HPO4, pH 7.4) and then resuspended in 5 ml SEB. Aliquots (0.2 ml) were mixed with 2 μg concentrated plasmid DNA in prechilled cuvettes (0.2-cm gap), and plasmid DNA was introduced into the cells by electroporation (1,000 V, 25 μF) using a Bio-Rad Gene Pulser Xcell apparatus (Bio-Rad, Hercules, CA, USA). Immediately after transformation, the mixture was transferred into 1 ml of TGY broth and incubated anaerobically at 37°C for 3 h, followed by plating onto TGY agar containing 15 μg ml−1 thiamphenicol anaerobically at 37°C overnight for selecting transformants. The resulting colonies were subcultured onto TGY agar containing 10 μg ml−1 erythromycin for selecting integrants and then passaged for 10 consecutive days to cure the shuttle vector. Those clones resistant to erythromycin but sensitive to thiamphenicol were chosen for further analysis.
To verify that the intron had inserted into the expected location, PCR was carried out to amplify the region spanning the insertion site with the primer pairs agrBF/agrR1 for agrB, luxS-1F/luxsS-1R for luxS, virRP-L-KpnI/virR-R-XbaI for virR, and CT_netB-F2/qnetB-R for netB (Table 4). To amplify the gene insert junction region, PCR was performed with primer pairs agrBF/ErmF for agrB, luxS-1F/ErmR for luxS, virRP-L-KpnI/ErmF for virR, and CT_netB-F2/Erm-F for netB (Fig. 1A).
TABLE 4.
Primers used in this study
| Primer name | Purpose | Sequence (5′–3′)a |
|---|---|---|
| agr-F1 | PCR | TTAGCTCTTTATATTGGATATACAG |
| agr-R1 | PCR/mutant verification/RT-PCR | CCGGTTTAAAACCGACCTTTAG |
| agrBF | Mutant verification/RT-PCR | GATGTTAGCCATGTATGCTTTCG |
| agrF1-SacI | agrB complementation | TAGAGAGCTCTTAGCTCTTTATATTGGATATACAG |
| agrR1-BamHI | agrB complementation | TAGAGGATCCCCGGTTTAAAACCGACCTTTAG |
| CT_netB-F2 | Mutant verification | AGTGTAATTAGTACAAGCC |
| qNetB-R | Mutant verification | GGCCATTTCATTTTTCCGTAA |
| CTC-netF | netB complementation | CGGGATCCGTACCATTTAAATTAAGCAC |
| CTC-netR | netB complementation | TCGAGCTCCCCTCTATATACTATTGATTG |
| mNetB-F | NetB protein expression | CGGCGAATTCAGTGAATTAAATGACATAAACA |
| mNetB-R | NetB protein expression | CGGCAAGCTTCAGATAATATTCTATTTTATGA |
| virRP-L KpnI | Mutant verification/virR complementation/RT-PCR | TTGGTACCTTATGTTCATAAAATAGAAAGTGG |
| virR-R XbaI | Mutant verification/virR complementation/RT-PCR | TTTCTAGATTAACATATTAAATCCCCTAAAAGGC |
| luxS-1F | Mutant verification/RT-PCR | GTGGTTAAAGTTGAAAGTTTG |
| luxS-1R | Mutant verification/RT-PCR | ATATCTTAACATCAATATCTCC |
| luxS-F3-KpnI | luxS complementation | TTGGTACCATTAGCTGATCCAGTAGGTTC |
| luxS-R3-XbaI | luxS complementation | TTTCTAGACTTTAGATTATAAATAGTAATTAC |
| ErmF | Mutant verification | GATATTCACCGAACACTAGG |
| ErmR | Mutant verification | TTACCTGTTCCAATTTCGTA |
| CT_probe-F1 | Southern blotting | ACAACTTAATTATACCCACT |
| CT_probe-R1 | Southern blotting | CTTGTGTTTATGAATCACG |
| QnetBF | qPCR | AGTGTAATTAGTACAAGCC |
| QnetBR | qPCR | GGCCATTTCATTTTTCCGTAA |
| rpoA-F1 | qPCR | TTACCTGGAGTGGCTCCAAC |
| rpoA-R1 | qPCR | ACACCTGGTCCTTGAGCATC |
| CP4_0573-F1 | qPCR | AAGCCATCAGATACGGTGGT |
| CP4_0573-R1 | qPCR | AGATTCGGCTATCCTTTGCAT |
| CP4_0575-F1 | qPCR | TCATCATGCAGATGCTTCCT |
| CP4_0575-R1 | qPCR | CACCTTTTGCATCAGTATTGACA |
| rSAM-F | qPCR | GAGTTTTTCCAGATGGTTC |
| rSAM-R | qPCR | GATGTAAATTTATGGCTATAC |
Restriction enzyme sites are underlined.
To construct the complementation plasmids, the respective genes, including 5′ upstream regions, were amplified using the primers given in Table 4, digested with the appropriate restriction enzymes, and cloned into the E. coli-C. perfringens shuttle vector pJIR750 (16), which confers resistance to thiamphenicol. Specifically, agrB and netB were cloned into the SacI/BamHI sites of pJIR750, while luxS and virR were cloned into the KpnI/XbaI sites. The resulting complementation plasmids were confirmed by sequencing and introduced into the corresponding mutant strains by electroporation as described above. The transformation mixture was selected on TGY agar supplemented with 15 μg ml−1 thiamphenicol and 10 μg ml−1 erythromycin.
RNA isolation and RT-PCR.
Total RNA was extracted from CP1 or its isogenic derivatives using the RiboPure-Bacteria kit (Applied Biosystems, Austin, TX, USA) according to the manufacturer's instructions. Total RNA (10 μg) was DNase treated with the Turbo DNase kit (Bio-Rad), and the purified RNA was quantified by absorbance at 260 nm using a NanoDrop 2000 spectrophotometer (Thermo Scientific, Wilmington, DE, USA).
Reverse transcription was performed using the High-Capacity cDNA reverse transcription kit (Invitrogen). Briefly, 1 μg of total purified RNA was incubated with 1 μl 20× RT enzyme mix at 37°C for 1 h and then at 95°C for 5 min to stop the reaction. The resulting cDNA was then used as the template in PCRs with primers agrBF/agrR1 (targeting agrB), primers luxS-1F/luxS-1R (targeting luxS), and primers virRP-L-KpnI/virR-R-XbaI (targeting virR).
Southern blot analysis.
Genomic DNA (1 μg) isolated from CP1, CP1ΩagrB, CP1ΩvirR, CP1ΩluxS, or CP1ΩnetB was digested with DraI for 4 h at 37°C and separated on a 1% agarose gel. Southern blotting was performed with the DIG Luminescent Detection kit (Roche, Laval, QC, Canada) according to the manufacturer's instructions. A digoxigenin (DIG)-labeled probe was generated by PCR using the PCR DIG Probe synthesis kit (Roche) with primers CT_probe-F1 and CT_probe-R1 and 50 ng CP1ΩagrB genomic DNA as the template.
Production of rNetB MAb.
The netB open reading frame was amplified by PCR using primers mNetB-F and mNetB-R and cloned into the EcoRI/HindIII restriction sites of pET28 His tag expression vector (Novagen, Gibbstown, NJ). The resulting construct was transformed into E. coli BL21-Star (DE3) pLysS and subsequently grown in LB broth supplemented with appropriate antibiotics (chloramphenicol [34 μg/ml] and ampicillin [100 μg/ml]) at 37°C on an orbital shaker (200 rpm) until the absorbance at 600 nm reached 0.5. Protein expression was induced by adding 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside) at 37°C for 4 h. Purification of rNetB protein from E. coli BL21 was performed using Ni-nitrilotriacetic acid (Ni-NTA) resin under denaturing conditions according to the manufacturer's instructions (Qiagen). The rNetB protein was further purified by electroelution to remove residual urea and then sent to ImmunoPrecise Antibodies Ltd. (Victoria, BC, Canada) for monoclonal antibody (MAb) development in mice.
Western blot analysis.
A 0.1-ml aliquot of an overnight TPG culture of CP1 or its isogenic derivatives was inoculated into 10 ml of TPG broth and grown to an OD600 of 0.8. Culture supernatants were obtained by centrifugation at 18,000 × g for 15 min and concentrated by Amicon Ultra Centrifugal Filter Devices 10K (Millipore, Billerica, MA, USA). Equal amounts (100 μg) of total protein were mixed with SDS loading buffer and boiled for 5 min. The proteins were separated in a 10% SDS polyacrylamide gel and transferred to a nitrocellulose membrane (Invitrogen) by electroblotting. Detection was performed with the WesternBreeze chromogenic Western blot immunodetection kit (Invitrogen), according to the manufacturer's protocol. Briefly, the membrane was incubated in blocking solution overnight at 4°C and then incubated with mouse anti-NetB MAb (1:2,500) at room temperature for 1 h. After washing three times, the membrane was incubated with goat anti-mouse IgG secondary antibody conjugated to alkaline phosphatase at room temperature for 30 min. Alkaline phosphatase activity was detected using a chromogenic substrate containing 5-bromo-4-chloro-3-indolyl-1-phosphate (BCIP) and nitroblue tetrazolium (NBT). To assess the specificity of the rNetB mAB, Western blotting of culture supernatant from CP1 and CP1ΩnetB was performed, which revealed a single ∼33-kDa band in the CP1 supernatant that was absent in CP1ΩnetB (data not shown).
Quantitative real-time PCR.
CP1 and its isogenic derivatives were grown in TPG medium to an OD600 of 0.8. One microgram of purified total RNA was reverse transcribed to cDNA as described above. The cDNA was diluted 100 times and used as the template for quantitative real-time PCR in a reaction mixture consisting of 5 μl cDNA, 5 μl of 1.5 μM each primer, and 10 μl iTaq Universal SYBR green Supermix (Bio-Rad). The amplifications were done in a Viia7 real-time PCR instrument (Applied Biosystems) using cycling conditions of 1 cycle of 95°C for 10 min and 45 cycles of 95°C for 15 s, 55°C for 1 min, and 68°C for 1 min, followed by a melting curve analysis using the default parameters. The rpoA gene was used as the housekeeping gene for normalization of target gene expression. qPCR data were analyzed using the 2−ΔΔCT method (where CT is threshold cycle) (56). The values were represented as fold changes relative to wild-type CP1, while the value of wild-type CP1 was set as 1. Groups that differed significantly (P < 0.05) from the control were identified by performing Student's t test on the 2−ΔΔCT values.
Transwell assay.
CP1 and its isogenic derivatives were grown in TPG broth at 37°C overnight. Different combinations of strains were inoculated (30 μl) into the top or bottom chamber of the Transwell plates (0.4-μm pore size; Corning, Corning, NY, USA) containing 3 ml sterile TPG. The physical barrier present in the Transwell plates is impermeable to bacteria but allows passage of small molecules. After 5 h of incubation, the supernatants were collected by centrifugation and subjected to Western blot analysis using NetB MAb.
LMH cell cytotoxicity assay.
The Leghorn male hepatoma cell line (ATCC CRL-2117) was maintained in Earl's minimum essential medium (EMEM) (Invitrogen) supplemented with l-glutamine, MEM nonessential amino acids solution, 100 U ml−1 penicillin, 100 U ml−1 streptomycin, and 10% fetal bovine serum in a humidified environment of 5% CO2 at 37°C. Flasks and 96-well plates were precoated with 0.1% gelatin (Millipore) prior to use.
To test for cytotoxicity, wells of a 96-well plate (BD Biosciences, Bedford, MA, USA) were seeded with 4 × 104 LMH cells and incubated at 37°C with 5% CO2 for 2 to 3 days until 100% confluent. A 0.1-ml aliquot of an overnight TPG culture of CP1 or its isogenic derivatives was inoculated into 10 ml of TPG broth. When the OD600 reached 0.8, the culture supernatants were collected by centrifugation at 18,000 × g for 15 min and filter sterilized by a 0.22-μm filter (Millipore). Those sterile supernatants were added to the LMH cell medium in triplicate and incubated for 4 h at 37°C with 5% CO2. Both visual cytopathic effects and lactate dehydrogenase (LDH) release were used as indicators of cytolysis.
The cytopathic effects caused by supernatants from different strains were analyzed and photographed using a Canon Powershot G5 fitted to a Zeiss Axiovert 25 microscope.
The Cyto-Tox Non-Radioactive kit (Promega, Madison, WI, USA) was used to measure LDH release into the supernatant of LMH cells. A maximum lysis control well was included, to which lysis buffer was added. The results of this LDH cytotoxicity assay were expressed as the average percentage cytotoxicity relative to the maximum lysis control from three replicate experiments. Significant differences between the CP1 group and the various mutants were determined by Tukey's honestly significant difference (HSD) test.
Microarray analysis.
Total RNA was extracted from wild-type CP1 and CP1ΩagrB as described above in three separate experiments and hybridized to a custom C. perfringens microarray consisting of probes representing two NE-causing isolates, CP4 and JGS4143 (9). Cyanine dye-labeled cDNA was prepared from 15 μg of total bacterial RNA by indirect labeling using cDNA containing aminoallyl-dUTP and Cy3 or Cy5 monoreactive dyes (Amersham Biosciences). Hybridizations were incubated for 18 h at 42°C, washed once for 5 min in 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)–0.01% SDS, twice for 5 min in 0.1× SSC–0.01% SDS, and 5 times for 1 min in 0.1× SSC. Slide scanning and quantitation of fluorescence intensities were performed using Scanarray Express software (PerkinElmer, Waltham, MA). Statistical analyses were performed using BRB ArrayTools v4.3.0 (http://brb.nci.nih.gov/BRB-ArrayTools/index.html) and samr. Data were first filtered to exclude spots with raw intensities of <1,000 relative fluorescence units (RFU) for both channels or with >1 missing value. Log-transformed data were normalized using the lowess function, and differentially expressed genes between the treatment and control samples were identified with the significance analysis for microarrays method using the R package samr v2.0 (57). Genes with a false-discovery rate (FDR) of <0.1 and displaying >2-fold altered expression were considered significantly affected. Five differentially expressed genes were validated by quantitative real-time PCR as described above, using the primers listed in Table 4.
Animal trials.
Experiments with chickens and conditions for their use were approved by the University of Guelph Animal Care Committee in accordance with the Canadian Council on Animal Care's Guidelines. Commercial 1-day-old male Ross 708 broiler chickens (Bonnie's Chick Hatchery, Elmira, ON, Canada) were randomly divided into 4 experimental groups (n = 16 or 17 per group). The chickens were fed an antibiotic-free broiler starter ration containing 20% protein for 15 days and then changed to a turkey starter ration containing 28% protein (Arkell Research Station, University of Guelph). For the experimental infection (challenge) of birds, wild-type CP1, CP1ΩagrB, and CP1ΩagrB[agrB+] were grown in CMM for 24 h at 37°C under anaerobic conditions and then inoculated (3%, vol/vol) into fresh CMM. On days 16 to 18, each bird was orally challenged twice daily with 0.2 ml of C. perfringens culture of each strain (108 cells/chick). The cultures were grown in fresh CMM and incubated at 37°C for 16 h and 24 h, respectively, before gavage. On day 20, chickens were euthanized with carbon dioxide, and at necropsy the small intestine was examined for grossly visible lesions. NE lesions were scored blindly using the system described by Keyburn et al. (8). Intestinal damage was graded as follows: 0 (no gross lesions), 1 (degeneration of superficial mucosa, loss of villous tips, no fibrin present), 2 (superficial villous degeneration and mild fibrin exudation), 3 (superficial mucosal necrosis with loss of villous tips, some exudation of fibrin, bacteria present), and 4 (extensive ulceration, erosions, and/or mucosal necrosis, extensive fibrin, fibrinous casts, and/or fibrinous pseudomembrane, and bacteria present). Significant pairwise differences in NE scores between groups were determined by Tukey's HSD test.
Dilutions of the intestinal digesta were plated on Perfringens OPSP and blood agar plates, and colonies were counted after anaerobic incubation overnight at 37°C.
Accession number(s).
The newly determined sequence of the agr locus in C. perfringens strain CP1 was deposited in GenBank under accession no. KY202922. The microarray data have been deposited into the GEO archives under accession no. GSE97874.
ACKNOWLEDGMENTS
This work was supported by Agriculture and Agri-Food Canada and the Canadian Poultry Research Council.
Plasmid pJIR750 was a gift from J. I. Rood at Monash University, Australia.
Q.Y. and H.Z. were visiting PhD students financially supported by the China Scholarship Council through the MOE-AAFC Ph.D. Research Program. T.W. was a visiting scientist financially supported by the China Scholarship Council.
We have no conflict of interest to declare.
REFERENCES
- 1.McClane BA, Uzal FA, Miyakawa MEF, Lyerly D, Wilkins T. 2006. The enterotoxic clostridia, p 698–752. In Dworkin M, Falkow S, Rosenberg E, Schleifer K-H, Stackenbrandt E (ed), The prokaryotes, 3rd ed Springer, Berlin, Germany. [Google Scholar]
- 2.Uzal FA, Freedman JC, Shrestha A, Theoret JR, Garcia J, Awad MM, Adams V, Moore RJ, Rood JI, McClane BA. 2014. Towards an understanding of the role of Clostridium perfringens toxins in human and animal disease. Future Microbiol 9:361–377. doi: 10.2217/fmb.13.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McClane BA, Rood JI. 2001. Clostridial toxins involved in human enteric and histotoxic infections, p 169–209. In Bahl H, D̈urre P (ed), Clostridia: biotechnology and medical applications. Wiley, Hoboken, NJ. [Google Scholar]
- 4.Smedley JG III, Fisher D, Sayeed S, Chakrabarti G, McClane B. 2004. The enteric toxins of Clostridium perfringens. Rev Physiol Biochem Pharmacol 152:183–204. [DOI] [PubMed] [Google Scholar]
- 5.Wade B, Keyburn AL. 2015. The true cost of necrotic enteritis. World Poultry 9:2. [Google Scholar]
- 6.Van Immerseel F, Rood JI, Moore RJ, Titball RW. 2009. Rethinking our understanding of the pathogenesis of necrotic enteritis in chickens. Trends Microbiol 17:32–36. doi: 10.1016/j.tim.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 7.Si W, Gong J, Han Y, Yu H, Brennan J, Zhou H, Chen S. 2007. Quantification of cell proliferation and alpha-toxin gene expression of Clostridium perfringens in the development of necrotic enteritis in broiler chickens. Appl Environ Microbiol 73:7110–7113. doi: 10.1128/AEM.01108-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keyburn AL, Boyce JD, Vaz P, Bannam TL, Ford ME, Parker D, Di Rubbo A, Rood JI, Moore RJ. 2008. NetB, a new toxin that is associated with avian necrotic enteritis caused by Clostridium perfringens. PLoS Pathog 4:e26. doi: 10.1371/journal.ppat.0040026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lepp D, Roxas B, Parreira VR, Marri PR, Rosey EL, Gong J, Songer JG, Vedantam G, Prescott JF. 2010. Identification of novel pathogenicity loci in Clostridium perfringens strains that cause avian necrotic enteritis. PLoS One 5:e10795. doi: 10.1371/journal.pone.0010795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ohtani K, Hirakawa H, Tashiro K, Yoshizawa S, Kuhara S, Shimizu T. 2010. Identification of a two-component VirR/VirS regulon in Clostridium perfringens. Anaerobe 16:258–264. doi: 10.1016/j.anaerobe.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 11.Cheung JK, Keyburn AL, Carter GP, Lanckriet AL, Van Immerseel F, Moore RJ, Rood JI. 2010. The VirSR two-component signal transduction system regulates NetB toxin production in Clostridium perfringens. Infect Immun 78:3064–3072. doi: 10.1128/IAI.00123-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shimizu T, Ba-Thein W, Tamaki M, Hayashi H. 1994. The virR gene, a member of a class of two-component response regulators, regulates the production of perfringolysin O, collagenase, and hemagglutinin in Clostridium perfringens. J Bacteriol 176:1616–1623. doi: 10.1128/jb.176.6.1616-1623.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ba-Thein W, Lyristis M, Ohtani K, Nisbet IT, Hayashi H, Rood JI, Shimizu T. 1996. The virR/virS locus regulates the transcription of genes encoding extracellular toxin production in Clostridium perfringens. J Bacteriol 178:2514–2520. doi: 10.1128/jb.178.9.2514-2520.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vidal JE, Ohtani K, Shimizu T, McClane BA. 2009. Contact with enterocyte-like Caco-2 cells induces rapid upregulation of toxin production by Clostridium perfringens type C isolates. Cell Microbiol 11:1306–1328. doi: 10.1111/j.1462-5822.2009.01332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ohtani K, Yuan Y, Hassan S, Wang R, Wang Y, Shimizu T. 2009. Virulence gene regulation by the agr system in Clostridium perfringens. J Bacteriol 191:3919–3927. doi: 10.1128/JB.01455-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vidal JE, Chen J, Li J, McClane BA. 2009. Use of an EZ-Tn5-based random mutagenesis system to identify a novel toxin regulatory locus in Clostridium perfringens strain 13. PLoS One 4:e6232. doi: 10.1371/journal.pone.0006232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ohtani K, Hayashi H, Shimizu T. 2002. The luxS gene is involved in cell-cell signalling for toxin production in Clostridium perfringens. Mol Microbiol 44:171–179. doi: 10.1046/j.1365-2958.2002.02863.x. [DOI] [PubMed] [Google Scholar]
- 18.Henke JM, Bassler BL. 2004. Bacterial social engagements. Trends Cell Biol 14:648–656. doi: 10.1016/j.tcb.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 19.Bassler BL, Losick R. 2006. Bacterially speaking. Cell 125:237–246. doi: 10.1016/j.cell.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 20.Parker CT, Sperandio V. 2009. Cell-to-cell signalling during pathogenesis. Cell Microbiol 11:363–369. doi: 10.1111/j.1462-5822.2008.01272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen X, Schauder S, Potier N, Van Dorsselaer A, Pelczer I, Bassler BL, Hughson FM. 2002. Structural identification of a bacterial quorum-sensing signal containing boron. Nature 415:545–549. doi: 10.1038/415545a. [DOI] [PubMed] [Google Scholar]
- 22.Novick RP, Geisinger E. 2008. Quorum sensing in staphylococci. Annu Rev Genet 42:541–564. doi: 10.1146/annurev.genet.42.110807.091640. [DOI] [PubMed] [Google Scholar]
- 23.Chen J, Rood JI, McClane BA. 2011. Epsilon-toxin production by Clostridium perfringens type D strain CN3718 is dependent upon the agr operon but not the VirS/VirR two-component regulatory system. mBio 2(6):e00275-11. doi: 10.1128/mBio.00275-11. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Li J, Chen J, Vidal JE, McClane BA. 2011. The Agr-like quorum-sensing system regulates sporulation and production of enterotoxin and beta2 toxin by Clostridium perfringens type A non-food-borne human gastrointestinal disease strain F5603. Infect Immun 79:2451–2459. doi: 10.1128/IAI.00169-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen J, McClane BA. 2012. Role of the Agr-like quorum-sensing system in regulating toxin production by Clostridium perfringens type B strains CN1793 and CN1795. Infect Immun 80:3008–3017. doi: 10.1128/IAI.00438-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vidal JE, Ma M, Saputo J, Garcia J, Uzal FA, McClane BA. 2012. Evidence that the Agr-like quorum sensing system regulates the toxin production, cytotoxicity and pathogenicity of Clostridium perfringens type C isolate CN3685. Mol Microbiol 83:179–194. doi: 10.1111/j.1365-2958.2011.07925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ohtani K, Kawsar HI, Okumura K, Hayashi H, Shimizu T. 2003. The VirR/VirS regulatory cascade affects transcription of plasmid-encoded putative virulence genes in Clostridium perfringens strain 13. FEMS Microbiol Lett 222:137–141. doi: 10.1016/S0378-1097(03)00255-6. [DOI] [PubMed] [Google Scholar]
- 28.Heap JT, Cartman ST, Kuehne SA, Cooksley C, Minton NP. 2010. ClosTron-targeted mutagenesis. Methods Mol Biol 646:165–182. doi: 10.1007/978-1-60327-365-7_11. [DOI] [PubMed] [Google Scholar]
- 29.Keyburn AL, Yan XX, Bannam TL, Van Immerseel F, Rood JI, Moore RJ. 2010. Association between avian necrotic enteritis and Clostridium perfringens strains expressing NetB toxin. Vet Res 41:21. doi: 10.1051/vetres/2009069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wade B, Keyburn AL, Seemann T, Rood JI, Moore RJ. 2015. Binding of Clostridium perfringens to collagen correlates with the ability to cause necrotic enteritis in chickens. Vet Microbiol 180:299–303. doi: 10.1016/j.vetmic.2015.09.019. [DOI] [PubMed] [Google Scholar]
- 31.Wuster A, Babu MM. 2008. Conservation and evolutionary dynamics of the agr cell-to-cell communication system across firmicutes. J Bacteriol 190:743–746. doi: 10.1128/JB.01135-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cooksley CM, Davis IJ, Winzer K, Chan WC, Peck MW, Minton NP. 2010. Regulation of neurotoxin production and sporulation by a putative agrBD signaling system in proteolytic Clostridium botulinum. Appl Environ Microbiol 76:4448–4460. doi: 10.1128/AEM.03038-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qin X, Singh KV, Weinstock GM, Murray BE. 2000. Effects of Enterococcus faecalis fsr genes on production of gelatinase and a serine protease and virulence. Infect Immun 68:2579–2586. doi: 10.1128/IAI.68.5.2579-2586.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sturme MH, Nakayama J, Molenaar D, Murakami Y, Kunugi R, Fujii T, Vaughan EE, Kleerebezem M, de Vos WM. 2005. An agr-like two-component regulatory system in Lactobacillus plantarum is involved in production of a novel cyclic peptide and regulation of adherence. J Bacteriol 187:5224–5235. doi: 10.1128/JB.187.15.5224-5235.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prescott JF, Parreira VR, Mehdizadeh Gohari I, Lepp D, Gong J. 2016. The pathogenesis of necrotic enteritis in chickens: what we know and what we need to know: a review. Avian Pathol 45:288–294. doi: 10.1080/03079457.2016.1139688. [DOI] [PubMed] [Google Scholar]
- 36.Timbermont L, Haesebrouck F, Ducatelle R, Van Immerseel F. 2011. Necrotic enteritis in broilers: an updated review on the pathogenesis. Avian Pathol 40:341–347. doi: 10.1080/03079457.2011.590967. [DOI] [PubMed] [Google Scholar]
- 37.Chen J, Ma M, Uzal FA, McClane BA. 2014. Host cell-induced signaling causes Clostridium perfringens to upregulate production of toxins important for intestinal infections. Gut Microbes 5:96–107. doi: 10.4161/gmic.26419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lepp D, Gong J, Songer JG, Boerlin P, Parreira VR, Prescott JF. 2013. Identification of accessory genome regions in poultry Clostridium perfringens isolates carrying the netB plasmid. J Bacteriol 195:1152–1166. doi: 10.1128/JB.01032-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kawai K, Fujita M, Nakao M. 1974. Lipid components of two different regions of an intestinal epithelial cell membrane of mouse. Biochim Biophys Acta 369:222–233. doi: 10.1016/0005-2760(74)90253-7. [DOI] [PubMed] [Google Scholar]
- 40.Urbina P, Collado MI, Alonso A, Goñi FM, Flores-Díaz M, Alape-Girón A, Ruysschaert J-M, Lensink MF. 2011. Unexpected wide substrate specificity of C. perfringens α-toxin phospholipase C. Biochim Biophys Acta 1808:2618–2627. doi: 10.1016/j.bbamem.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 41.Keyburn AL, Sheedy SA, Ford ME, Williamson MM, Awad MM, Rood JI, Moore RJ. 2006. Alpha-toxin of Clostridium perfringens is not an essential virulence factor in necrotic enteritis in chickens. Infect Immun 74:6496–6500. doi: 10.1128/IAI.00806-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fernandes da Costa SP, Mot D, Geeraerts S, Bokori-Brown M, Immerseel FV, Titball RW. 2016. Variable protection against experimental broiler necrotic enteritis after immunization with the C-terminal fragment of Clostridium perfringens alpha-toxin and a non-toxic NetB variant. Avian Pathol 45:381–388. doi: 10.1080/03079457.2015.1129663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Coursodon CF, Trinh HT, Mallozzi M, Vedantam G, Glock RD, Songer JG. 2010. Clostridium perfringens alpha toxin is produced in the intestines of broiler chicks inoculated with an alpha toxin mutant. Anaerobe 16:614–617. doi: 10.1016/j.anaerobe.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 44.Cooper KK, Trinh HT, Songer JG. 2009. Immunization with recombinant alpha toxin partially protects broiler chicks against experimental challenge with Clostridium perfringens. Vet Microbiol 133:92–97. doi: 10.1016/j.vetmic.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 45.Garsin DA. 2010. Ethanolamine utilization in bacterial pathogens: roles and regulation. Nat Rev Microbiol 8:290–295. doi: 10.1038/nrmicro2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Winter SE, Thiennimitr P, Winter MG, Butler BP, Huseby DL, Crawford RW, Russell JM, Bevins CL, Adams LG, Tsolis RM, Roth JR, Baumler AJ. 2010. Gut inflammation provides a respiratory electron acceptor for Salmonella. Nature 467:426–429. doi: 10.1038/nature09415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thiennimitr P, Winter SE, Winter MG, Xavier MN, Tolstikov V, Huseby DL, Sterzenbach T, Tsolis RM, Roth JR, Baumler AJ. 2011. Intestinal inflammation allows Salmonella to use ethanolamine to compete with the microbiota. Proc Natl Acad Sci U S A 108:17480–17485. doi: 10.1073/pnas.1107857108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Korbel JO, Doerks T, Jensen LJ, Perez-Iratxeta C, Kaczanowski S, Hooper SD, Andrade MA, Bork P. 2005. Systematic association of genes to phenotypes by genome and literature mining. PLoS Biol 3:e134. doi: 10.1371/journal.pbio.0030134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Parreira VR, Russell K, Athanasiadou S, Prescott JF. 2016. Comparative transcriptome analysis by RNAseq of necrotic enteritis Clostridium perfringens during in vivo colonization and in vitro conditions. BMC Microbiol 16:186. doi: 10.1186/s12866-016-0792-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gray B, Hall P, Gresham H. 2013. Targeting agr- and agr-like quorum sensing systems for development of common therapeutics to treat multiple gram-positive bacterial infections. Sensors (Basel) 13:5130–5166. doi: 10.3390/s130405130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Broderick AH, Stacy DM, Tal-Gan Y, Kratochvil MJ, Blackwell HE, Lynn DM. 2014. Surface coatings that promote rapid release of peptide-based AgrC inhibitors for attenuation of quorum sensing in Staphylococcus aureus. Adv Healthc Mater 3:97–105. doi: 10.1002/adhm.201300119. [DOI] [PubMed] [Google Scholar]
- 52.Ma M, Li J, McClane BA. 2015. Structure-function analysis of peptide signaling in the Clostridium perfringens Agr-like quorum sensing system. J Bacteriol 197:1807–1818. doi: 10.1128/JB.02614-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thompson D, Parreira V, Kulkarni R, Prescott J. 2006. Live attenuated vaccine-based control of necrotic enteritis of broiler chickens. Vet Microbiol 113:25–34. doi: 10.1016/j.vetmic.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 54.Heap JT, Kuehne SA, Ehsaan M, Cartman ST, Cooksley CM, Scott JC, Minton NP. 2010. The ClosTron: mutagenesis in Clostridium refined and streamlined. J Microbiol Methods 80:49–55. doi: 10.1016/j.mimet.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 55.Jirásková A, Vítek L, Fevery J, Ruml T, Branny P. 2005. Rapid protocol for electroporation of Clostridium perfringens. J Microbiol Methods 62:125–127. doi: 10.1016/j.mimet.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 56.Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 57.Tusher VG, Tibshirani R, Chu G. 2001. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci U S A 98:5116–5121. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]