Abstract
In mid-2012 we conducted survey of immunization program managers (IPMs) for the purpose of describing relationships between immunization programs and emergency preparedness programs, IPM's perceptions of challenges encountered and changes made or planned in programmatic budgeting, vaccine allocation and pandemic plans as a result of the H1N1 vaccination campaign. Over 95% of IPMs responded (61/64) to the survey. IPMs reported that a primary budget-related challenge faced during H1N1 included staff-related restrictions that limited the ability to hire extra help or pay regular staff overtime resulting in overworked regular staff. Other budget-related challenges related to operational budget shortfalls and vaccine procurement delays. IPMs described overcoming these challenges by increasing staff where possible, using executive order or other high-level support by officials to access emergency funds and make policy changes, as well as expedite hiring and spending processes according to their pandemic influenza plan or by direction from leadership. Changes planned for response to future pandemic vaccine allocation strategies were to “tailor the strategy to the event” taking into account disease virulence, vaccine production rates and public demand, having flexible vaccine allocation strategies, clarifying priority groups for vaccine receipt to providers and the public, and having targeted clinics such as through pharmacies or schools. Changes already made to pandemic plans were improving strategies for internal and external communication, improving vaccine allocation efficiency, and planning for specific scenarios. To prepare for future pandemics, programs should ensure well-defined roles, collaborating during non-emergency situations, sustaining continuity in preparedness funding, and improved technologies.
Keywords: budget, communication, emergency preparedness, immunization programs, leadership, pandemic influenza plan, vaccine allocation, staff, vaccine procurement
Abbreviations
- ICS
incident command structures
- IIS
immunization information systems
- PIP
pandemic influenza plan
- IPM
immunization program manager
- CDC
Centers for Disease Control and Prevention
- POD
point of distribution
- OB
obstetrician
- IP
immunization program
- EP
emergency preparedness programs
- AIM
Association of Immunization Managers
- FAQ
frequently asked questions
Introduction
Previous research examining the US response to the 2009 H1N1 pandemic and vaccine campaign have focused on leadership tactics, use of paradigms like incident command structures (ICS), community partnerships, funding pathways, immunization information systems (IIS) and communication strategies. We found from our previous survey that shared leadership, flexible pandemic influenza plans (PIP), and expanded uses of IIS were associated with perceptions of successful vaccine campaigns at the state level.1 Several studies and commentaries have outlined the importance of investments in public health infrastructure to build capacity for distributing, dispensing and administering countermeasures in future emergency responses. Additionally, implementation of expanded seasonal influenza recommendations, development of relationships with providers, and enhancement of federal support of state and local activities have also been cited as key to improve the US public health response capability.2,3 Beyond the US, the World Health Organization declared the world unsuited to respond to future emergencies without advancements in global preparedness through a suite of strategies which include research, reliance on a multisectoral approach, strengthened health care delivery systems, economic development in low and middle income countries, and overall improved health status.4
In a comparison of the 2009-2010 H1N1 pandemic to the 1976 H1N1 pandemic, Sencer describes lessons from the 2 events including managing expectations and risk communication, the importance of accurate surveillance, and flexible planning and decision making5 Both research and organizations’ self-examination (via “hot wash” activities* ) have shown that having planning models in place to address continuity of operations, managing temporary staff, building community partnerships, streamlining communications, and improving vaccination strategies and logistics are important ways to augment pandemic influenza plans.6,7
Though much has been written about retrospective lessons from previous pandemic responses, we are not aware of any studies examining what actual changes have been made to policy, infrastructure, practice, and relationships that reflect lessons learned from challenges encountered. Our third survey of immunization program managers (IPMs) attempts to discern the extent to which changes to formal and informal plans, official policies and practice have been planned or implemented at the state level.1,8
Results
Response
Over 95% of IPMs responded (61/64). Non-responding programs were 3 of the 8 outlying territories; resulting in coverage representing roughly 99% of the United States population.9 Most surveys (92%) were completed online.
Budgetary challenges encountered during and lessons learned after H1N1 vaccination campaigns
Themes that emerged among IPM open-ended responses to a question asking “what budget-related challenges limited their program's ability to increase staff and operational activities under a disaster scenario?” were difficulties hiring temporary staff or increasing workloads on existing staff to handle extra work/increased paperwork (n = 31), operational budget shortfalls (n = 7), and vaccine procurement delays (n = 6). Specific challenges cited were delays in contract approvals, hiring freezes, restricted overtime, and difficulties training new staff quickly enough. When asked “how did you overcome these challenges?” IPMs reported using volunteers or temporary employees (n = 19) with specific mention of allowing overtime, or borrowing staff from another agency (e.g. National Guard, CDC). Other themes were expedited processes such as hiring, emergency funding or vaccine procurement (n = 10), and executive order or support of other high-level officials that facilitated the release of funds (n = 10).
When asked “who or what helped you overcome challenges?” themes included leadership from executive administration within the department (n = 8), collaboration with other public health programs such as emergency preparedness (n = 8), temporary staff (n = 6), increased funding (n = 6) (e.g., public health emergency preparedness released funds), and assistance from an outside organization (n = 5) (e.g. collaboration with National Guard, CDC, schools, etc.).
Vaccine allocation challenges encountered during and lessons learned after H1N1 vaccination campaigns
When asked “during a future hypothetical pandemic event similar to H1N1 would you change your vaccine allocation strategy?” 37% of IPMs said they would not, 28% said they “don't know,” and 35% said they would. When these 35% were asked to describe “how would you change your strategy,” response themes were tailoring their plan to the specific event (i.e. factoring in event-specific disease virulence, vaccine production rates and public demand), using population-based allocation (e.g., allocating to local health authorities) and alternate strategies such as tier order frequency (where high volume users continue to order as needed, others can only order during certain timeframes) or reduced volume shipping. [Fig. 1] One program said, “Vaccines were only available in 100-dose minimums [packaging], which did not work when vaccine was very limited at the beginning. In a rural state, often providers' equitable share of vaccine was less than 100. This meant the state had to have vaccine sent to a central location and broken down into smaller increment [packaging]. This disrupted the cold chain and actually caused some vaccine wastage.” Another allocation change described was to clarify priority groups (e.g. specify whether the priority group “healthcare personnel” includes fire and police personnel, school nurses, or even teachers).10 Other less common but noteworthy responses included initiating a faster response (e.g., opening to non-priority patient groups sooner), expanding vaccine registry use (i.e., using IIS for data entry), opening more targeted clinics (e.g. school located vaccine clinics, hospital systems, mass public clinics, etc.), increasing staff dedicated to the emergency response, establishing new management inventory tools, and distributing vaccine through pharmacies to maximize availability through extended locations and hours.
Figure 1.
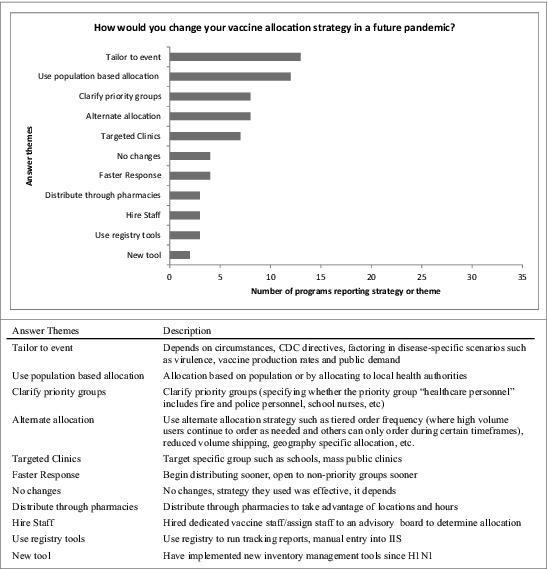
Program changes planned for future pandemic event vaccine allocation strategy. US immunization programs’ qualitative responses regarding planned changes to vaccine allocation strategies in a future pandemic.
Planning and programmatic changes since H1N1 vaccination campaigns [note these questions were asked in 2012 about the 2009-2010 pandemic]
We asked “how has your pandemic influenza plan been updated due to your experiences in the H1N1 influenza vaccination campaign?,” and many IPMs cited improved communication between agencies and external providers, including better defined roles of agencies and staff. IPMs also reported specific updates to sections describing vaccine allocation strategies (e.g., timing, high risk groups, improved vaccine storage, ordering or distribution) and improved plans for specific scenarios (e.g. H5N1, POD plans). [Fig. 2] When asked to “describe how you plan to increase the number of providers offering influenza vaccine” most who responded described outreach to specific provider types such as obstetricians and pharmacists. [Fig. 3]
Figure 2.
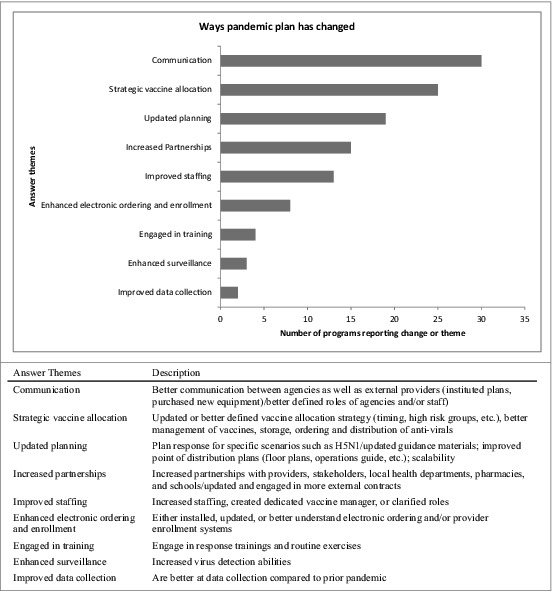
Changes made to pandemic influenza plans since H1N1. US immunization programs’ qualitative responses regarding actual changes to pandemic influenza plan since H1N1.
Figure 3.
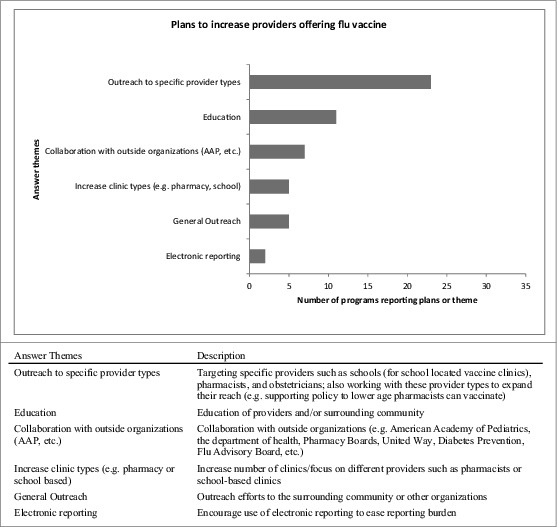
US immunization programs’ plans to increase providers offering influenza vaccine. US immunization programs qualitative responses describing which type of providers each program plans to recruit for influenza vaccination and how they plan to recruit them.
When asked to “describe other relevant policy changes you have made since the H1N1influenza pandemic that you feel are important,” IPMs described changes to IIS (n = 10) (e.g., tracking vaccine, electronic enrollment, vaccine management, new system, etc.) and increased collaborations with emergency preparedness (n = 10). Six mentioned supporting specific provider types (e.g. OBs and pharmacists).
Changes in collaborations between Immunization and Emergency Preparedness Programs as a result of the H1N1 vaccination campaign
Sixty 4 percent (39/61) of IPMs thought the relationship between immunization programs (IPs) and emergency preparedness programs (EPs) in their jurisdiction was strengthened as a result of the H1N1 vaccination campaign; 30% (18/61) reported the relationship remained the same, only 1 of the 61 (<2%) thought the relationship had weakened and 5% (3/61) said that they didn't know. We asked “after the H1N1 Vaccination campaign, what steps were taken to establish/maintain routine communication and relationships between immunization programs and emergency preparedness program?,” and provided pre-defined answers for respondents to check; 71% (41/58) of responding IPMs indicated participation in scheduled preparedness activities and 62% (36/58) indicated participation in regular meetings. Almost half indicated maintaining contact lists and staff directories (48% or 28/58) and sharing reports between programs (48% or 28/58). Fewer indicated participation in scheduled immunization activities (41% or 24/58) and social events (10% or 6/58). Ten percent (6/58) responded that no steps had been taken to improve inter-program communication. From the open-ended “other” response field 8 IPMs mentioned specific plans for collaboration and ongoing regular communication (e.g., medical surge shelters, Flu Advisory Board), resulting in strong relationships.
When asked “what factors contributed to a change in relationship between programs after the H1N1 campaign?” the most prominent theme was the importance of collaboration (n = 17), which included currently working together, having well-defined roles and having previous experience working together. One respondent said, “We dealt with them on a near daily basis and established relationships…we also educated them on vaccines and the vaccine infrastructure in our state…they gave us insight into how hospitals and regional threat preparations work.” Other themes were education and communication (n = 7) and working together during a crisis (n = 6).
We asked “what are the challenges your program faces in participating in future preparedness activities such as tabletop, functional or full scale exercises with both programs?” with pre-defined answer choices; 45% (27/60) indicated not enough staffing, 45% (27/60) indicated competing priorities and 20% (12/60) indicated not enough funding. Eight percent (5/60) said this was the role of local health departments and 13% (8/60) said that the preparedness program did not ask them to participate. Thirty 7 percent (22/60) said that they currently have no known challenges.
Forty-one percent (25/61) of IPMs indicated that they continue to receive emergency funding, staffing support or other resources from the emergency preparedness program. IPMs largely reported that the funds came from pandemic influenza specific funding, and were being used for increased staff (n = 10), emergency supplies (n = 8), and IIS improvements (n = 8).
Thirty-7 percent (22/60) said that they do have plans to share funding with the preparedness program, 57% (34/60) said they do not and 7% (4/60) said they didn't know. Major themes regarding a description of these plans included pre-planned discussions (n = 5) (e.g. “…we conduct quarterly meetings to improve storage and handling best practices”) ad hoc solutions (n = 4), and pre-arranged financial agreements (n = 4).
Improving budget readiness
We asked “does the immunization program have any plans to investigate how to improve budget-readiness in preparation of a future pandemic event?” and gave the following examples: use of contract workers, use of coalitions, agreements with preparedness programs. Twenty-3 percent (14/60) said yes, 60% (36/60) said no, and 17% (10/60) said they didn't know. When we asked them to “please briefly describe your plans” IPMs indicated that they planned to improve their ability to pay for contract staff and other contractors, (n = 9) and support coalitions with internal programs (n = 3).
When asked “how does the current organizational structure of your health department promote or hinder collaboration between the 2 programs?,” predominant themes included leadership (programs governed by the same leadership led to improved collaboration, programs having different leadership said this hindered collaboration), routine interactions (i.e. regular meetings, briefings, and regular pandemic plan review), geographically shared or divided programs (programs having shared locations said this helped collaboration, programs being in different buildings or even locations said this hindered collaborations), existence of a liaison or shared staff and funding allocation. [Fig. 4]
Figure 4.
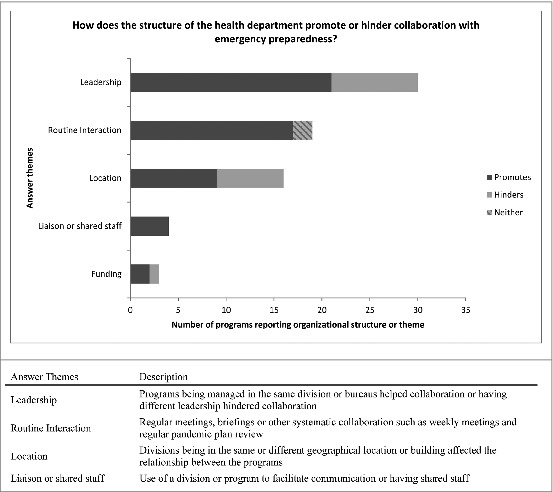
Health departments structure and its effect on collaborations between emergency preparedness and immunization staff. US immunization programs qualitative responses describing whether and how the structure of their health department hinders or promotes collaboration between emergency preparedness and immunization staff.
Discussion
In our survey, we found changes that have been planned or incorporated into to pandemic plans since H1N1 included: improving strategies for internal and external communication including better defined roles of staff and agencies, improving ability to expand staffing resources during emergencies, improving vaccine procurement and allocation efficiency through scenario-specific planning. Changes to vaccine allocation that may be on the horizon were allowing further flexibility for disease-specific responses as well as expediting hiring and procurement processes. These kinds of vaccine allocation changes would be important, for instance, in the case of event-specific disease virulence, high virulence would likely be associated with greater public demand for vaccination and more vaccine may need to be allocated to the local level than in a milder pandemic when public demand is less, whereas population-based allocation may be an important tactic in the case of accommodating evacuees fleeing a natural disaster which can often overwhelm nearby local health departments.
Immunization programs have made substantial progress since H1N1 to prepare for the next pandemic through improvements to response times, continuity of operations, effective communication and activation of emergency operations centers and medical staff. 3, 6 Despite this substantial progress, the CDC reported that only about 24% of Americans and 35% of healthcare workers were vaccinated against the H1N1 virus leaving considerable room for improvement.11,12 Economic investments in public health, the stability of which is important for continuity of planning, have been volatile in the past few years, especially in the area of preparedness.3
Though less than half of immunization programs reported continuing to receive funding directly from emergency preparedness programs, this may have changed since our survey with recent updates to routine pandemic influenza planning. Having an established revenue stream dedicated to emergency response not only enhances emergency response directly through the use of funding, but can also enhance emergency response by providing an avenue for quick hiring of additional staff or contractors. Our study showed that budget challenges pertained largely to increased workloads and the resulting burden on staff, especially when supplemental staff could not be accessed. Identifying normal duties that can be deferred or scaled back during an emergency could be worked into future plans. Also important to integrate into emergency and pandemic plans are strategies to keep volunteers trained, engaged and ready to respond, as well as policies to expedite hiring staff with various levels of skills. A strong well-trained executive administration can facilitate problem solving and retention of support staff is important for maintaining institutional memory. Key areas for improvement in our study were budget-readiness and expedited hiring ability during emergencies. For many at the state level, maintaining staff for every day duties is becoming increasingly difficult due to state hiring freezes and budget cuts.13 A pandemic scenario – planning for and responding to – compounds this issue. Maintaining core resources and staff are fundamental to being able to prepare for a pandemic scenario. States should continue to improve budget readiness and make plans with preparedness programs to share resources and plan scenarios to increase staff capacity rapidly.
We found that clearly and explicitly defining response-time roles and responsibilities of staff and programs appears to contribute to better communication and may be important pandemic plan changes to consider– the predominant theme in 2 areas of our survey. Increasing partnerships, another frequent theme from both this survey and our previous survey in 2010, can help leverage tight budgets by distributing the workload across a broader spectrum. Many have changed IIS structures and functions, some even using emergency funds to implement these changes. IISs may be able to play an important role in streamlining and improving preparedness and response activities around vaccine-related emergencies. While changes related specifically to IIS are important to consider, we have addressed those more in-depth in a separate manuscript.14
Strengths and limitations
Our response rate of 95% along with a mixed-methods approach lends credibility to our results. However, IPMs had to rely on self-recall which may be limited given the time lapsed and turnover of staff since the H1N1 campaign. Moreover, no verification of reported changes or other results was conducted.
Conclusion
Vaccine allocation emerged as a key area for improvement in our findings and many IPMs suggested or are implementing strategies such as the use of population based allocation (e.g., allocation based on the proportion of the population served) or tiered order frequency1 to improve readiness for future mass allocation events. Assuring flexibility into federal vaccine allocation strategies and investments in research supporting mapping tools and other technology based tools may be well timed and fill important, emerging gaps.
Our study indicates that some problems with staffing and collaboration can be mitigated to some extent by ensuring that everyone on both immunization and emergency preparedness teams has well-defined roles during response efforts as well as plenty of experience collaborating during non-emergency times. The key to successfully preparing for future pandemics is a sustainable, but flexible approach to funding public health, ensuring improved technologies, and maintaining collaborations between immunizations and preparedness programs and staff.
Materials and Methods
Survey development
In preparation for developing our survey, we collaborated with the Association of Immunization Managers (AIM), to conduct a focus group among 9 IPMs attending the AIM-CDC Program Managers annual meeting in March 2012. Results from this focus group were used along with input from the AIM research subcommittee to develop our survey instrument which was deployed in July 2012 to IPMs associated with all 64 Centers for Disease Control and Prevention (CDC) grantee IPs.15
The final survey consisted of 39 questions in total, including 12 questions on post H1N1 policy changes, 10 questions on post H1N1 relationships, and 17 questions on IIS (results of IIS questions published elsewhere).14,16 Questions were structured in 3 ways:
quantitative questions: we asked questions that had pre-defined multiple-choice or yes/no answer choices
qualitative questions: we asked respondents to describe answers in their own words
combined approach questions: we asked questions with pre-defined answer choices but also gave respondents a chance to describe “other” options or expand on why they selected a particular choice
IPMs could complete the survey by mail, fax, or online. The survey instrument can be found at http://web1.sph.emory.edu/PHSR/Emory_PERRC/documents/Emory%20PERRC%202012%20IPM%20Survey.pdf
Survey implementation
We followed a pre-established survey protocol that has been previously used in other surveys and thus previously described.14,17 Briefly, we sent pre-survey notices by fax and email, followed by FedEx delivery of a “survey kit” containing a paper copy of the survey, a Frequently Asked Questions (FAQ) sheet which provided the information they needed to make an informed decision to participate in the survey, a cover letter, an addressed, stamped envelope, a pen, and a signed copy of Dr. William Foege's 2012 book, House on Fire as a thank you. Follow-up emails and phone calls were conducted to verify receipt of the survey kit, address concerns and questions and later as a reminder to non-respondents.
Quantitative analysis
All quantitative analyses were conducted with SAS v9.3 (The SAS Institute, Cary, N. C.). Proportions were only calculated for quantitative analyses. No statistical comparisons were conducted for the proportions given.
Qualitative analysis
Answers to qualitative questions from the survey were analyzed by 2 investigators, and codebooks for each question and answer set were created through coder consensus. Investigators assigned themes for the answer choices after agreeing on a codebook with descriptions for each theme. No proportions were calculated for these theme counts due to lack of a consistent denominator. Both investigators coded 100% of each question and in the 2 instances where coder agreement was less than 80% for the total number of responses to a given question, a third investigator resolved the differences by assigning the appropriate code. Some responses warranted more than one code, therefore, in some cases the number of coded responses is greater than the number of responding IPMs. Likewise, if a response did not fit into a broader theme, no code was assigned (rare). Moreover, not all IPMs responded to each question. Survey question answers were analyzed using Microsoft Excel 2007 (Microsoft, Redmond, WA). Qualitative analyses were conducted among the responses to the following questions:
What budget-related challenges limited their program's ability to increase staff and operational activities under a disaster scenario? (EC, CO, KS)
How did you overcome these (budget-related) challenges? (EC, CO, KS)
Who or what helped you overcome these (budget-related) challenges? (EC, CO, KS)
How would you change your (vaccine allocation) strategy? (EC, CO, KS)
How has your pandemic influenza plan been updated due to your experiences in the H1N1 influenza vaccination campaign? (EC, CO, KS)
Describe how you plan to increase the number of providers offering influenza vaccine. (EC, CO, KS)
Describe other relevant policy changes have you made since the H1N1influenza pandemic that you feel are important. (EC, CO, KS)
What factors contributed to a change in relationship between programs after the H1N1 campaign? (EC, CO, KS)
Please briefly describe your plans (to investigate how to improve budget-readiness in preparation for a future pandemic event). (EC, CO, KS)
How does the current organizational structure of your health department promote or hinder collaboration between the 2 programs? (EC, CO, KS)
The Emory University institutional review board (IRB) approved the survey as an exempt study.
Footnotes
a hot wash is a participant-led debrief conducted directly after an exercise or event.
Tiered Order Frequency, similar to Economic Order Quantity which is CDC's current standard for the federal vaccine program for routine childhood immunizations, refers to a strategy that is designed to minimize the total inventory and holding costs for the entire system.
Disclosure of Potential Conflict of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank the immunization program managers for their responses to this survey. Their insights were invaluable and we appreciate their participation during a very busy time in their grant cycle. We also thank our graduate research assistants Chikaodili Obidike, Joshua Van Otterloo and Alyssa Parr for their assistance in administering the survey and cleaning and analyzing data from this survey.
Funding
This study was supported by a grant from the CDC, grant # 5P01TP000300, to the Emory Preparedness and Emergency Response Research Center, Emory University (Atlanta, GA). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the CDC.
References
- 1.Chamberlain AT, Seib K, Wells K, Hannan C, Orenstein WA, Whitney EA, et al. Perspectives of immunization program managers on 2009-10 H1N1 vaccination in the United States: a national survey. Biosecurity and bioterrorism : biodefense strategy, practice, and science 2012; 10:142-50; PMID:22360580; http://dx.doi.org/ 10.1089/bsp.2011.0077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rambhia KJ, Watson M, Sell TK, Waldhorn R, Toner E. Mass vaccination for the 2009 H1N1 pandemic: approaches, challenges, and recommendations. Biosecurity and bioterrorism : biodefense strategy, practice, and science 2010; 8:321-30; PMID:21043791; http://dx.doi.org/ 10.1089/bsp.2010.0043 [DOI] [PubMed] [Google Scholar]
- 3.Khan AS. Public health preparedness and response in the USA since 9/11: a national health security imperative. Lancet 2011; 378:953-6; PMID:21890060; http://dx.doi.org/ 10.1016/S0140-6736(11)61263-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization Implementation of the international health regulations (2005). Report of the review committee on the functioning of the international health regulations (2005) in relation to pandemic (H1N1) 2009 http://apps.who.int/gb/ebwha/pdf_files/WHA64/A64_10-en.pdf, May 5, 2011 [Google Scholar]
- 5.Sencer DJ. Perspective: Swine-origin influenza: 1976 and 2009. Clin Infect Dis 2011; 52(Suppl 1):S4-7; PMID:21342898; http://dx.doi.org/ 10.1093/cid/ciq006 [DOI] [PubMed] [Google Scholar]
- 6.National Association of County & City Health Officials Stories from the field- search. http://www.naccho.org/topics/H1N1/stories_search.cfm, 2014 [Google Scholar]
- 7.DiBiase LM, Davis SE, Rosselli R, Horney J. Evaluation of the implementation of the H1N1 pandemic influenza vaccine in local health departments (LHDs) in North Carolina. Vaccine 2011; 29:3969-76; PMID:21477677; http://dx.doi.org/ 10.1016/j.vaccine.2011.03.085 [DOI] [PubMed] [Google Scholar]
- 8.Chamberlain AT, Wells K, Seib K, Kudis A, Hannan C, Orenstein WA, et al. Lessons learned from the 2007 to 2009 Haemophilus influenzae type B vaccine shortage: implications for future vaccine shortages and public health preparedness. Journal of public health management and practice : JPHMP 2012; 18:E9-E16; PMID:22473128; http://dx.doi.org/ 10.1097/PHH.0b013e31821dce27 [DOI] [PubMed] [Google Scholar]
- 9. United States Census Bureau. Table 1. Annual Estimates of the Population for the United States, Regions, States, and Puerto Rico: April 1, 2010 to July 1, 2012. http://www.census.gov/popest/data/state/totals/2012/tables/NST-EST2012-01.xls, 2012 [Google Scholar]
- 10. Pennsylvania Vaccines for Children Handbook. Section 3-Vaccine ordering. http://www.portal.state.pa.us/portal/server.pt?open=18andobjID=1342884andmode=2, 2012 [Google Scholar]
- 11.Centers for Disease Control and Prevention Interim results: state-specific influenza A (H1N1) 2009 monovalent vaccination coverage - United States, October 2009-January 2010. MMWR Morbidity and mortality weekly report 2010; 59:363-8; PMID:20360670 [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention Interim results: influenza A (H1N1) 2009 monovalent and seasonal influenza vaccination coverage among health-care personnel - United States, August 2009-January 2010. MMWR Morbidity and mortality weekly report 2010; 59:357-62; PMID:20360669 [PubMed] [Google Scholar]
- 13.National Vaccine Advisory Committee Protecting the Public's Health: Critical Functions of the Section 317 Immunization Program — A Report of the National Vaccine Advisory Committee. http://www.hhs.gov/nvpo/nvac/meetings/pastmeetings/2012/protecting_the_public_health.pdf, 2012:1-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Curran EA, Seib KG, Wells K, Hannan C, Bednarczyk RA, Hinman AR, et al. A National Survey of Immunization Programs Regarding Immunization Information Systems Data Sharing and Use. Journal of public health management and practice : JPHMP 2013 [DOI] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention Grantee immunization websites. 2012 [Google Scholar]
- 16. Emory Preparedness and Emergency Response Center. 2012 Immunization Program Manager Survey. http://web1.sph.emory.edu/PHSR/Emory_PERRC/documents/Emory%20PERRC%202012%20IPM%20Survey.pdf, 2012 [Google Scholar]
- 17.Van Otterloo J, Richards JL, Seib K, Weiss P, Omer SB. Gift card incentives and non-response bias in a survey of vaccine providers: the role of geographic and demographic factors. PloS one 2011; 6:e28108; PMID:22132224; http://dx.doi.org/ 10.1371/journal.pone.0028108 [DOI] [PMC free article] [PubMed] [Google Scholar]


