Abstract
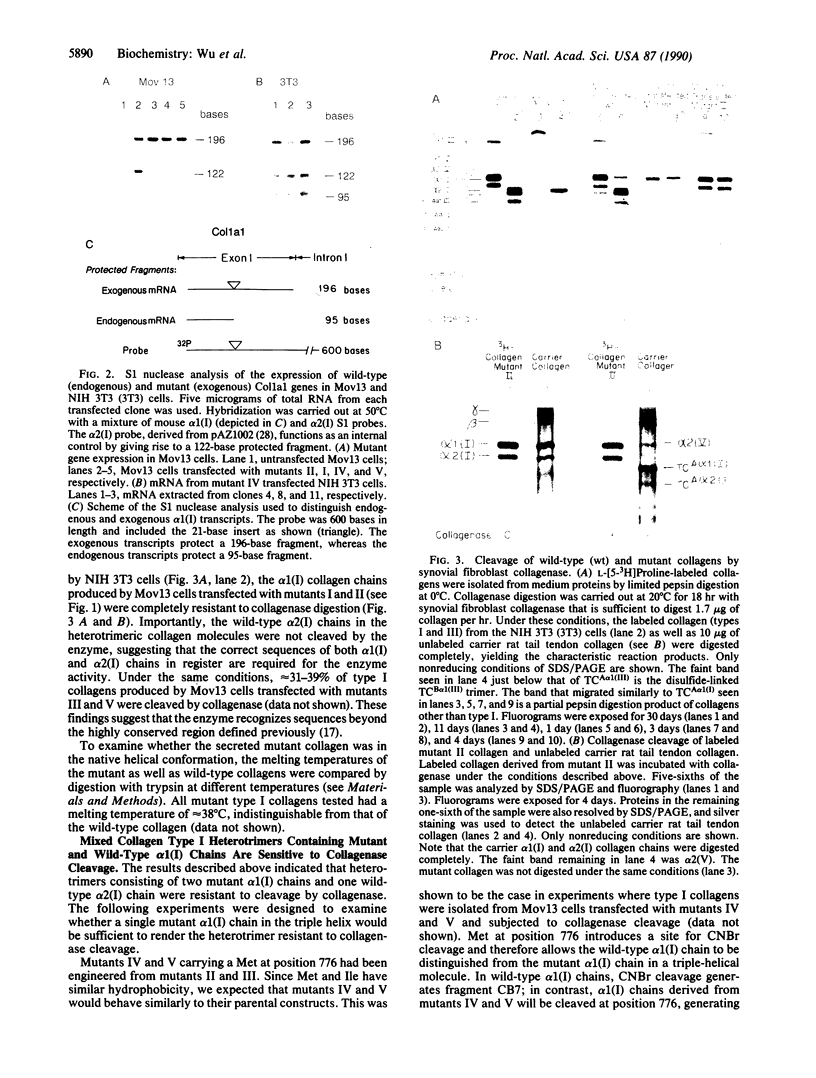
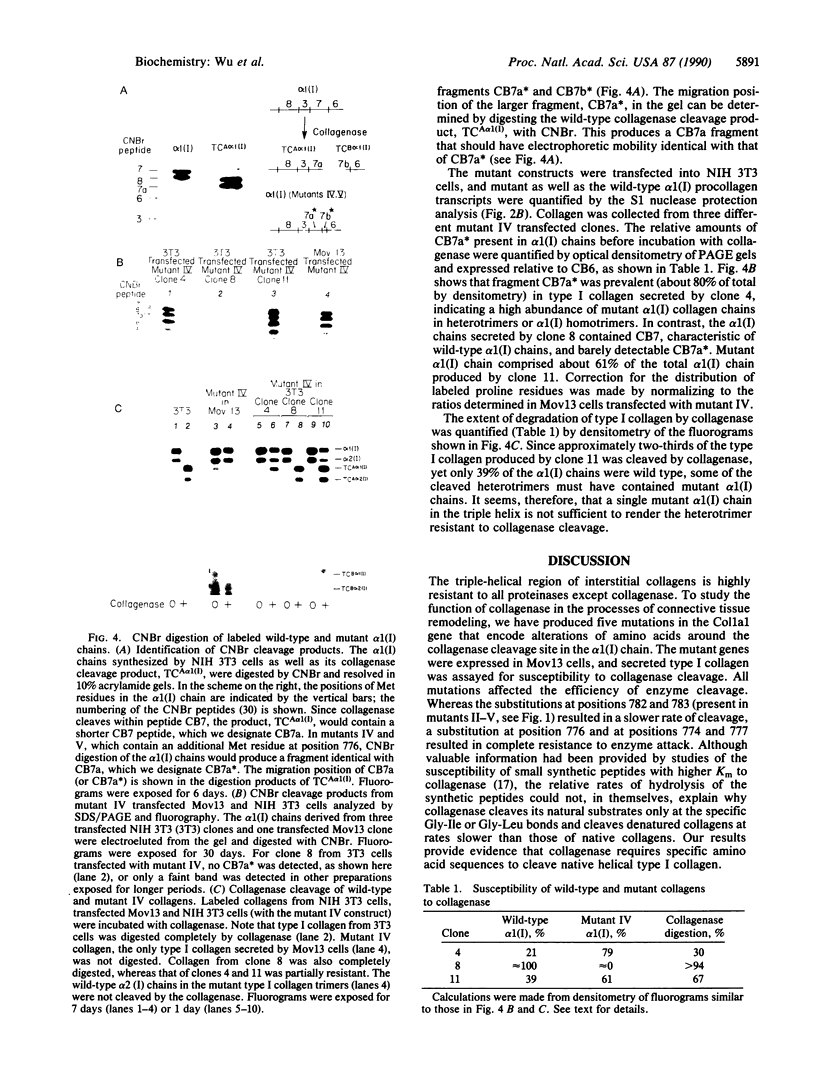
Collagenase (matrix metalloproteinase 1) cleaves type I, II, and III collagen helices at a specific site between Gly-Ile or Gly-Leu bonds (residues 775 and 776, P1-P1'). To understand the mechanism of collagen processing, mutations around the cleavage site have been introduced into the cloned murine pro alpha 1(I) collagen (Col1a1) gene. These mutant constructs have been transfected into homozygous Mov13 fibroblasts that do not express the endogenous Col1a1 gene due to a retroviral insertion. Secreted triple-helical type I collagens containing substitutions of Pro for Ile (position 776) (P1') were not cleaved by human rheumatoid synovial collagenase, whereas those containing substitutions of Met for Ile (position 776) were cleaved. Type I collagens containing double substitutions of Pro for Gln-774 (P2) and Ala-777 (P2') were not cleaved regardless of whether they contained the wild-type residue Ile at position 776 or the substitution of Met for Ile at position 776. The wild-type alpha 2(I) chains derived from the endogenous Col1a2 gene were also resistant to enzyme digestion when they were complexed with the mutant alpha 1(I) chains, indicating that the presence of normal alpha 1(I) sequences is critical for cleavage of the alpha 2(I) chains in the type I heterotrimer.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auffray C., Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Birkedal-Hansen H. Catabolism and turnover of collagens: collagenases. Methods Enzymol. 1987;144:140–171. doi: 10.1016/0076-6879(87)44177-3. [DOI] [PubMed] [Google Scholar]
- Byers P. H., Tsipouras P., Bonadio J. F., Starman B. J., Schwartz R. C. Perinatal lethal osteogenesis imperfecta (OI type II): a biochemically heterogeneous disorder usually due to new mutations in the genes for type I collagen. Am J Hum Genet. 1988 Feb;42(2):237–248. [PMC free article] [PubMed] [Google Scholar]
- Cheah K. S. Collagen genes and inherited connective tissue disease. Biochem J. 1985 Jul 15;229(2):287–303. doi: 10.1042/bj2290287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbère-Garapin F., Horodniceanu F., Kourilsky P., Garapin A. C. A new dominant hybrid selective marker for higher eukaryotic cells. J Mol Biol. 1981 Jul 25;150(1):1–14. doi: 10.1016/0022-2836(81)90321-1. [DOI] [PubMed] [Google Scholar]
- Cole W. G., Jaenisch R., Bateman J. F. New insights into the molecular pathology of osteogenesis imperfecta. Q J Med. 1989 Jan;70(261):1–4. [PubMed] [Google Scholar]
- Constantinou C. D., Vogel B. E., Jeffrey J. J., Prockop D. J. The A and B fragments of normal type I procollagen have a similar thermal stability to proteinase digestion but are selectively destabilized by structural mutations. Eur J Biochem. 1987 Mar 2;163(2):247–251. doi: 10.1111/j.1432-1033.1987.tb10794.x. [DOI] [PubMed] [Google Scholar]
- Dayer J. M., Stephenson M. L., Schmidt E., Karge W., Krane S. M. Purification of a factor from human blood monocyte-macrophages which stimulates the production of collagenase and prostaglandin E2 by cells cultured from rheumatoid synovial tissues. FEBS Lett. 1981 Feb 23;124(2):253–253. doi: 10.1016/0014-5793(81)80149-4. [DOI] [PubMed] [Google Scholar]
- Dziadek M., Timpl R., Jaenisch R. Collagen synthesis by cell lines derived from Mov-13 mouse embryos which have a lethal mutation in the collagen alpha 1(I) gene. Biochem J. 1987 Jun 1;244(2):375–379. doi: 10.1042/bj2440375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields G. B., Van Wart H. E., Birkedal-Hansen H. Sequence specificity of human skin fibroblast collagenase. Evidence for the role of collagen structure in determining the collagenase cleavage site. J Biol Chem. 1987 May 5;262(13):6221–6226. [PubMed] [Google Scholar]
- Goldring M. B., Krane S. M. Modulation by recombinant interleukin 1 of synthesis of types I and III collagens and associated procollagen mRNA levels in cultured human cells. J Biol Chem. 1987 Dec 5;262(34):16724–16729. [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Harris E. D., Jr, Vater C. A. Vertebrate collagenases. Methods Enzymol. 1982;82(Pt A):423–452. doi: 10.1016/0076-6879(82)82077-6. [DOI] [PubMed] [Google Scholar]
- Harris E. D., Jr, Welgus H. G., Krane S. M. Regulation of the mammalian collagenases. Coll Relat Res. 1984 Dec;4(6):493–512. doi: 10.1016/s0174-173x(84)80015-1. [DOI] [PubMed] [Google Scholar]
- Herskowitz I. Functional inactivation of genes by dominant negative mutations. Nature. 1987 Sep 17;329(6136):219–222. doi: 10.1038/329219a0. [DOI] [PubMed] [Google Scholar]
- Jaenisch R., Harbers K., Schnieke A., Löhler J., Chumakov I., Jähner D., Grotkopp D., Hoffmann E. Germline integration of moloney murine leukemia virus at the Mov13 locus leads to recessive lethal mutation and early embryonic death. Cell. 1983 Jan;32(1):209–216. doi: 10.1016/0092-8674(83)90511-1. [DOI] [PubMed] [Google Scholar]
- Jaenisch R. Transgenic animals. Science. 1988 Jun 10;240(4858):1468–1474. doi: 10.1126/science.3287623. [DOI] [PubMed] [Google Scholar]
- Kivirikko K. I., Myllylä R. Posttranslational enzymes in the biosynthesis of collagen: intracellular enzymes. Methods Enzymol. 1982;82(Pt A):245–304. doi: 10.1016/0076-6879(82)82067-3. [DOI] [PubMed] [Google Scholar]
- Liau G., Yamada Y., de Crombrugghe B. Coordinate regulation of the levels of type III and type I collagen mRNA in most but not all mouse fibroblasts. J Biol Chem. 1985 Jan 10;260(1):531–536. [PubMed] [Google Scholar]
- Löhler J., Timpl R., Jaenisch R. Embryonic lethal mutation in mouse collagen I gene causes rupture of blood vessels and is associated with erythropoietic and mesenchymal cell death. Cell. 1984 Sep;38(2):597–607. doi: 10.1016/0092-8674(84)90514-2. [DOI] [PubMed] [Google Scholar]
- Mann K. G., Fish W. W. Protein polypeptide chain molecular weights by gel chromatography in guanidinium chloride. Methods Enzymol. 1972;26:28–42. doi: 10.1016/s0076-6879(72)26004-9. [DOI] [PubMed] [Google Scholar]
- Miller E. J., Finch J. E., Jr, Chung E., Butler W. T., Robertson P. B. Specific cleavage of the native type III collagen molecule with trypsin. Similarity of the cleavage products to collagenase-produced fragments and primary structure at the cleavage site. Arch Biochem Biophys. 1976 Apr;173(2):631–637. doi: 10.1016/0003-9861(76)90300-3. [DOI] [PubMed] [Google Scholar]
- Monson J. M., Friedman J., McCarthy B. J. DNA sequence analysis of a mouse pro alpha 1 (I) procollagen gene: evidence for a mouse B1 element within the gene. Mol Cell Biol. 1982 Nov;2(11):1362–1371. doi: 10.1128/mcb.2.11.1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prockop D. J., Kivirikko K. I. Heritable diseases of collagen. N Engl J Med. 1984 Aug 9;311(6):376–386. doi: 10.1056/NEJM198408093110606. [DOI] [PubMed] [Google Scholar]
- Prockop D. J. Mutations in collagen genes. Consequences for rare and common diseases. J Clin Invest. 1985 Mar;75(3):783–787. doi: 10.1172/JCI111773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose G. D., Geselowitz A. R., Lesser G. J., Lee R. H., Zehfus M. H. Hydrophobicity of amino acid residues in globular proteins. Science. 1985 Aug 30;229(4716):834–838. doi: 10.1126/science.4023714. [DOI] [PubMed] [Google Scholar]
- Schnieke A., Dziadek M., Bateman J., Mascara T., Harbers K., Gelinas R., Jaenisch R. Introduction of the human pro alpha 1(I) collagen gene into pro alpha 1(I)-deficient Mov-13 mouse cells leads to formation of functional mouse-human hybrid type I collagen. Proc Natl Acad Sci U S A. 1987 Feb;84(3):764–768. doi: 10.1073/pnas.84.3.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnieke A., Harbers K., Jaenisch R. Embryonic lethal mutation in mice induced by retrovirus insertion into the alpha 1(I) collagen gene. 1983 Jul 28-Aug 3Nature. 304(5924):315–320. doi: 10.1038/304315a0. [DOI] [PubMed] [Google Scholar]
- Sillence D. O. Osteogenesis imperfecta nosology and genetics. Ann N Y Acad Sci. 1988;543:1–15. doi: 10.1111/j.1749-6632.1988.tb55311.x. [DOI] [PubMed] [Google Scholar]
- Stacey A., Bateman J., Choi T., Mascara T., Cole W., Jaenisch R. Perinatal lethal osteogenesis imperfecta in transgenic mice bearing an engineered mutant pro-alpha 1(I) collagen gene. Nature. 1988 Mar 10;332(6160):131–136. doi: 10.1038/332131a0. [DOI] [PubMed] [Google Scholar]
- Traub W., Piez K. A. The chemistry and structure of collagen. Adv Protein Chem. 1971;25:243–352. doi: 10.1016/s0065-3233(08)60281-8. [DOI] [PubMed] [Google Scholar]
- Welgus H. G., Jeffrey J. J., Eisen A. Z. The collagen substrate specificity of human skin fibroblast collagenase. J Biol Chem. 1981 Sep 25;256(18):9511–9515. [PubMed] [Google Scholar]
- Wu H., Bateman J. F., Schnieke A., Sharpe A., Barker D., Mascara T., Eyre D., Bruns R., Krimpenfort P., Berns A. Human-mouse interspecies collagen I heterotrimer is functional during embryonic development of Mov13 mutant mouse embryos. Mol Cell Biol. 1990 Apr;10(4):1452–1460. doi: 10.1128/mcb.10.4.1452. [DOI] [PMC free article] [PubMed] [Google Scholar]