Abstract
Background: Estimated physiologic requirements (PRs) for zinc increase in late pregnancy and early lactation, but the effect on dietary zinc requirements is uncertain.
Objective: The aim of this study was to determine changes in daily fractional absorbed zinc and total absorbed zinc (TAZ) from ad libitum diets of differing phytate contents in relation to physiologic zinc requirements during pregnancy and lactation.
Methods: This was a prospective observational study of zinc absorption at 8 (phase 1) and 34 (phase 2) wk of gestation and 2 (phase 3) and 6 (phase 4) mo of lactation. Participants were indigenous Guatemalan women of childbearing age whose major food staple was maize and who had been randomly assigned in a larger study to either of 2 ad libitum feeding groups: low-phytate maize (LP; 1.6 mg/g; n = 14) or control maize (C; 7.1 mg/g; n = 8). Total dietary zinc (milligrams per day, TDZ) and phytate (milligrams per day) were determined from duplicate diets and fractional absorption (FAZ) by dual isotope ratio technique (TAZ = TDZ × FAZ). All variables were examined longitudinally and by group and compared with PRs. TAZ values at later phases were compared with phase 1. Measured TAZ was compared with predicted TAZ for nonpregnant, nonlactating (NPNL) women.
Results: TAZ was greater in the LP group than in the C group at all phases. All variables increased from phase 1 to phases 2 and 3 and declined at phase 4. TAZ increased by 1.25 mg/d (P = 0.045) in the C group and by 0.81 mg/d (P = 0.058) in the LP group at phase 2. At phase 3, the increases were 2.66 mg/d (P = 0.002) in the C group and 2.28 mg/d (P = 0.0004) in the LP group, compared with a 1.37-mg/d increase in PR. Measured TAZ was greater than predicted values in phases 2–4.
Conclusions: Upregulation of zinc absorption in late pregnancy and early lactation matches increases in PRs of pregnant and lactating women, regardless of dietary phytate, which has implications for dietary zinc requirements of pregnant and lactating women.
Keywords: zinc, absorption, diet, maize, phytate, bioavailability, pregnancy, lactation
Introduction
Adverse maternal, fetal, and offspring effects of zinc-restricted diets during the reproductive cycle have been well documented in animal models (1). Partly as a result of these findings, pregnancy and lactation have been regarded as physiologic states of special risk for adverse effects of zinc deficiency in humans (2, 3). However, consistent adverse effects have not been documented in humans whose diets are apparently deficient in bioavailable zinc (4–6). Only in special circumstances of severe zinc deficiency during pregnancy is there evidence of resulting gross fetal abnormalities (7). Nevertheless, concerns in industrialized nations about the potential for adverse effects of maternal zinc deficiency have led to the widespread prescription of prenatal maternal zinc supplements in industrialized countries. Zinc retention required during pregnancy in order to match zinc accumulation by the conceptus was carefully calculated nearly 40 y ago (8). The additional maternal zinc absorption required across lactation, assuming no change in endogenous zinc losses via other routes, has been estimated from measurements of zinc intake of exclusively breastfed offspring (9–11). These estimates have been widely accepted since then and have been given further prominence through their use in estimating dietary zinc requirements (12, 13). Furthermore, the stages of the reproductive cycle at which the changes in zinc absorption are required have been well defined. Supported by the results of single-meal studies of zinc homeostasis, the upregulation of zinc absorption has been proposed during pregnancy and lactation (14, 15). The effectiveness of upregulation is unknown in the context of matching increases in physiologic requirements for zinc in late pregnancy and especially in early lactation. Also unknown is the extent to which the inhibition of zinc absorption by high dietary phytate intake may counterbalance upregulation.
The primary objective of this research was to determine whether the estimated longitudinal changes in physiologic requirements for zinc across the reproductive cycle are matched by corresponding increases in the daily quantity of zinc absorbed [total absorbed zinc (TAZ)9; milligrams per day]. A second objective was to determine the effect of dietary phytate on the quantity of zinc absorbed, with special attention to late pregnancy and early lactation.
Methods
Study design.
This study was a double-blind, longitudinal, observational study of changes in measures of zinc homeostasis from 8 wk of gestation (phase 1) to 34 wk of gestation (phase 2) and 2 mo of lactation (phase 3) and 6 mo of lactation (phase 4). The quantity of total absorbed zinc (TAZ) was derived from measurements of zinc in duplicate diet samples [total dietary zinc (TDZ)] and the fractional absorption of zinc (FAZ) measured by extrinsic stable zinc isotope labeling of all meals for study days. The study was nested within a longitudinal trial of long-term reduction in phytate intake in indigenous Guatemalan woman of childbearing age whose habitual diet contained a high content of phytate. This allowed longitudinal comparison of TAZ from diets having widely disparate phytate content with the most recent published estimates of physiologic zinc requirements across pregnancy and lactation (13). Measures of zinc homeostasis for both dietary groups in late pregnancy and at 2 and 6 mo of lactation were compared with corresponding data for the first trimester. The total dietary phytate (TDP) content of duplicate diets was also measured, and predicted TAZ values for each phase were derived from a model on the basis of both TDZ and TDP for nonpregnant, nonlactating (NPNL) women. Differences between measured and predicted TAZs were determined for each study phase and each dietary group.
Participants.
The participants in the parent study (n = 600) were resident in the rural township of San Juan Comalapa (altitude 2100 m), Department of Chimaltenango, in the Western Highlands of Guatemala. Ninety-three percent were Kaq’chikel-speaking Native Americans, with the remainder being Ladinos. Major inclusion criteria were as follows: parous women of childbearing age, anticipating or planning a further pregnancy within the next 12 mo, no contraceptive use currently or planned, and no micronutrient supplements that contained zinc. For the parent study, including the participants reported here, control or phytate-reduced maize was provided weekly in quantities tailored to meet the entire needs of the household and commencing before conception. The participants were typically of low-middle socioeconomic status, and with a diet that continued to contain traditional nixtamalized maize as the major dietary staple. They were characteristically short in stature and overweight but otherwise apparently healthy. A convenience sample of 30 test [low-phytate (LP)] and 30 control (C) participants were enrolled in a longitudinal study of zinc homeostasis. Twenty-two of these participants (8 C and 14 LP) conceived within the time frame of the trial and successfully completed zinc homeostasis studies at 8 and 34 wk of gestation and 2 and 6 mo of lactation. These latter 22 participants are the focus of this study.
This project received ethical approval from both the Interinstitutional Ethics Board of Guatemala and the Colorado Multiple Institutional Review Board. All of the participants gave their written, informed consent before enrollment into this substudy, which took place between 2003 and 2006.
Diet.
Participants in the parent study were randomly assigned to receive either maize with an 80% reduction in phytate or a near-isohybrid control maize (16, 17). The phytate content of the control maize averaged 710 mg/100 g dry weight, a concentration typical for maize. The phytate content of the phytate-reduced maize was 160 mg/100 g dry weight. The zinc content of both the test and control maize was relatively high, with a mean of 2.6 mg/100 g dry weight. Details of the source of the maize have been described previously (17). Participants received a weekly supply of maize sufficient to meet the requirements of the entire household, typically an 18-kg sack. The test or control maize was the only source of this major food staple for a minimum of 3 mo before conception through pregnancy and the first 6 mo of lactation.
Metabolic studies.
Dietary intake of zinc was measured in duplicate diet samples collected for all meals on the metabolic study day. These diets were derived from additional food prepared for each meal on the isotope-administration day with the same weights for each food item as those actually consumed.
FAZ for all meals on the test days was measured by using our dual isotope tracer technique (18, 19), which compares the ratio of urine enrichment of orally (70Zn) and intravenously (67Zn) administered stable isotopes of zinc. TAZ was determined from the product of FAZ and the zinc content of duplicate composite diets. 67Zn and 70Zn stable isotope dose solutions were prepared from enriched zinc oxide (Trace Sciences International) at the University of Colorado and hand-carried to the project site in Guatemala. 70Zn was administered orally as described previously (20). Sterile precautions and longitudinal monitoring for sterility and pyrogenicity were tightly controlled for the intravenous solution of 67Zn. Precise concentrations and volumes of 70Zn (∼300 μg total) were administered orally commencing at approximately the midpoint of each of the 3 meals on study day 1. The isotope container was rinsed twice with water and the rinses were also administered orally. Any losses were collected on ashless filter paper, which was subsequently analyzed for tracer and subtracted from the administered dose.
During the afternoon at a recorded hour, an accurately measured quantity (∼800 μg) of sterile 67Zn was administered intravenously into a superficial arm vein over a 5-min period, followed by 2 rinses with normal saline. Clean, timed spot-urine samples were collected before isotope administration and twice daily on study days 4–8. Participants were trained to collect these samples at home, and they were subsequently picked up daily by a member of the research staff. Samples were stored at −20°C in the research facility in Guatemala before transfer to the Pediatric Nutrition Laboratory, University of Colorado Denver (UCD).
On the day of zinc stable isotope administration, participants cooked and consumed all of their meals in their home in the presence of a field research assistant. Intakes of each food were accurately measured at each of 3 meals and a duplicate diet sample prepared accordingly. The duplicate diet samples, adjusted for plate waste, were combined in a plastic container and homogenized into a single pool, and four 50-g aliquots stored at −20°C until transfer of 3 aliquots to the Pediatric Nutrition Laboratory, UCD.
Sample analyses.
Two replicates of the duplicate diet samples were dried in an electric oven at UCD. Samples for zinc analyses were then dry ashed at 450°C, wet ashed with HNO3, and then dry ashed again. Samples were subsequently reconstituted in 6 N HCl. Total zinc concentration was measured by atomic absorption spectrophotometry fitted with a deuterium arc background lamp (day-to-day variation <2%; model AAnalyst 400; Perkin-Elmer Corporation) (21). The zinc intake from tortillas was also measured.
Urine samples were digested by using a MARS microwave sample preparation system (CEM Corporation) as previously described (21). Isotope enrichment was determined by the measurement of the isotope ratios 67Zn:66Zn and 70Zn:66Zn by inductively coupled plasma MS (ICP-MS) (<0.5% CV for inter- and intra-assay results; Agilent 7700X ICP-MS; Agilent Technologies), and conversion of ratios to enrichment was determined with a mathematical matrix. Tracer enrichment was defined as all of the zinc in the sample derived from the isotopically enriched tracer preparation divided by the total zinc in the sample (20). Dietary sample aliquots were transferred to one of the author’s (VR’s) USDA laboratories in Aberdeen, Idaho, where they were freeze-dried and analyzed for phytic acid phosphorus by a modification of the ferric precipitation method (22).
Reference data.
The reference data were derived from the most recent estimates of zinc physiologic requirements for NPNL women (2.9 mg Zn/d) (13) and the estimated additional physiologic requirement in late pregnancy (0.73 mg Zn/d) (8) and 2 mo of lactation (1.37 mg Zn/d) (23). On the basis of a mean prepregnancy weight for the women in this study of 54 kg, a baseline requirement of 2.7 mg Zn/d was calculated by using a model described in the European Food Safety Authority document (13). Thus, the adjusted estimates of average requirements for the study population are 3.43 and 4.07 mg Zn/d for late pregnancy and early lactation, respectively.
Data analyses.
Summary statistics for TDZ, TDP, the phytate-to-zinc molar ratio, FAZ, and TAZ were calculated separately by study phase and group (C compared with LP). Data distributions were examined and normality assessed. Mean TDZ and FAZ data were plotted separately. Measured mean values for TAZ at each phase were plotted to compare with longitudinal changes in estimated physiologic requirements for zinc across pregnancy and lactation. A model of zinc absorption as a function of dietary zinc and phytate was derived from our published model (24, 25) by limiting data included in that model to those derived from NPNL women of childbearing age. There were 171 women in 10 publications (26–35). This model was used to predict TAZ for NPNL women ingesting the mean measured TDZ and TDP values from duplicate diet samples for each phase (1 phase 2 phytate value was interpolated for the LP group). The TAZ predictions from this model were slightly lower (0.14 mg/d, on average) than those of the published model (24, 25). Paired 2-tailed t tests were applied to derive P values for differences in measured FAZ and TAZ between study phases and between measured and predicted TAZ.
These predictions were compared with the means of the measured TAZ data for both groups at all study phases, and the differences between measured and predicted values were calculated. Mean measured TAZ, predicted TAZ, and reference estimates of the zinc physiologic requirements were plotted longitudinally to examine changes in TAZ for both C and LP groups from phase 1 to phases 2, 3, and 4 and their relation to the predictions and estimated requirements across the reproductive cycle. Mean increases in TAZ relative to the first trimester of pregnancy and to the predicted values for late pregnancy and early lactation were calculated and P values were determined.
Results
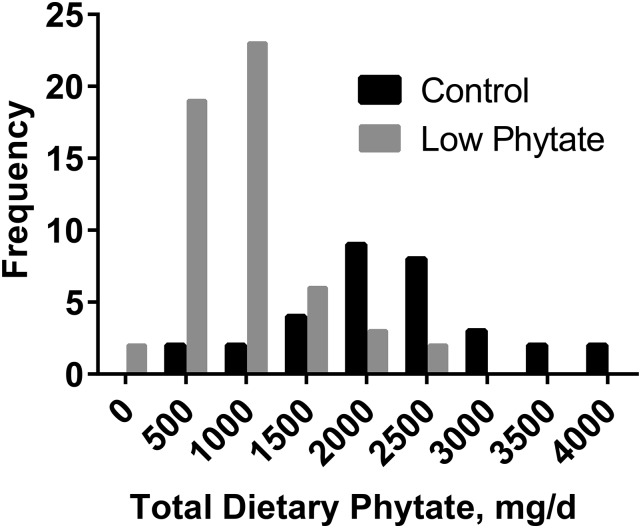
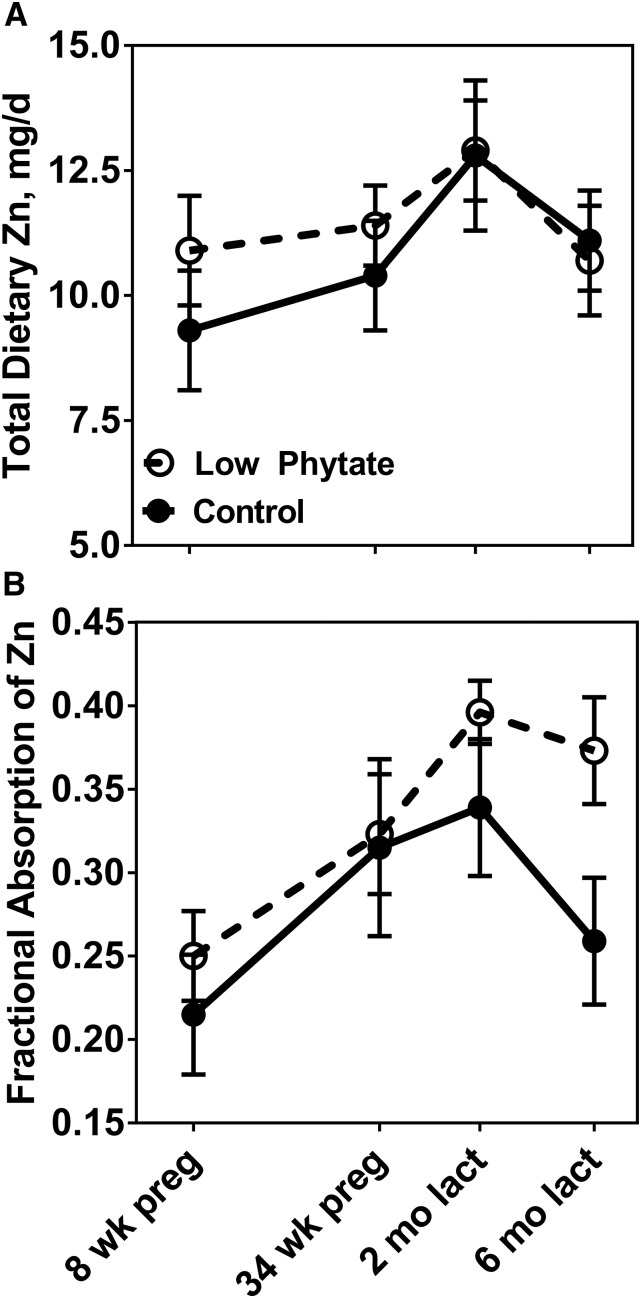
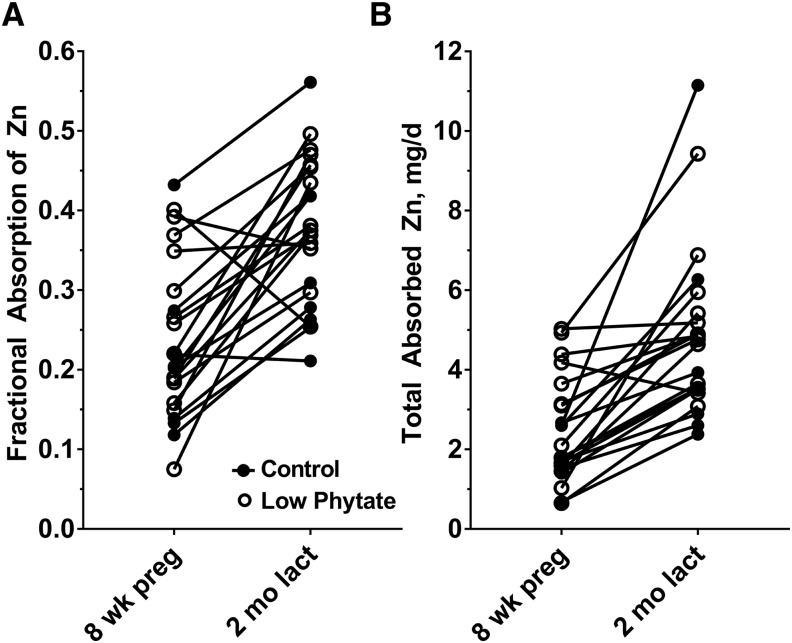
Anthropometric, demographic, and socioeconomic status data were similar for the 2 dietary groups (Table 1). Dietary zinc measured in duplicate diet samples was similar for both groups, averaging 10.9 and 11.5 mg Zn/d for the C and LP groups, respectively, for all phases combined. Dietary phytate differed between the 2 groups, with averages for all 4 phases of 2220 mg/d for the C group and 950 mg/d for the LP group. Corresponding phytate-to-zinc molar ratios were 22.4 and 8.8, respectively. A comparison of the frequency distributions of phytate intakes further shows the difference between the 2 groups (Figure 1). Dietary zinc and phytate intakes did vary by study phase, increasing across pregnancy and peaking in early lactation before declining in later lactation (Table 2). This pattern was similar for both groups. Both the mean measured quantities of zinc ingested during ad libitum feeding day of duplicate collection of all meals (TDZ) and the mean FAZs at each study phase increased across pregnancy and peaked in early lactation for both the C and LP groups (Figure 2). The percentage increases for FAZ between phases 1 (first trimester) and 3 (2 mo of lactation) were 58% for both the C (P = 0.0008) and LP (P = 0.002) groups, with the increases evident in almost all individuals (Figure 3). FAZ was higher for the LP than for the C group at each phase (Figure 2B).
TABLE 1.
Summary of anthropometric, demographic, and SES data at enrollment for Guatemalan women randomly assigned to receive high phytate (control) or phytate-reduced diets1
| Control (n = 8) | Low phytate (n = 14) | |
| Age, y | 31 ± 3 | 31 ± 6 |
| Height, cm | 144.6 ± 3.9 | 145.3 ± 4.4 |
| Weight, kg | 52 ± 9 | 54 ± 9 |
| BMI, kg/m2 | 25.1 ± 4.9 | 25.4 ± 3.6 |
| Birth weight of offspring, g | 3239 ± 388 | 3220 ± 356 |
| Education, y | 3.4 ± 2.5 | 2.5 ± 1.7 |
| Parity, n | 5.0 ± 2.1 | 5.0 ± 2.8 |
| SES score2 | 0.38 ± 0.31 | 0.24 ± 0.29 |
Values are means ± SDs. SES, socioeconomic status.
SES score was calculated as the sum of 5 components: persons per bedroom, flooring, principal source of potable water, main type of toilet facility, and type of land farmed by the family.
FIGURE 1.
Frequency distribution of phytate intake by pregnant and lactating Guatemalan women in control high-phytate (n = 8) and low-phytate (n = 14) diet groups in all phases of pregnancy and lactation combined.
TABLE 2.
Longitudinal measurements of TDZ, FAZ, TAZ, and TDP together with corresponding model-predicted absorbed zinc, measured-to-predicted absorbed zinc ratio, and phytate-to-zinc MRs in pregnant and lactating Guatemalan women1
| Phase 1 (first trimester of pregnancy) |
Phase 2 (third trimester of pregnancy) |
Phase 3 (2 mo of lactation) |
Phase 4 (6 mo of lactation) |
|||||
| Control | LP | Control | LP | Control | LP | Control | LP | |
| TDZ, mg/d | 9.3 ± 3.52 | 10.9 ± 4.1 | 10.4 ± 3.2 | 11.5 ± 3.0 | 12.8 ± 4.1 | 12.9 ± 3.6 | 11.1 ± 2.7 | 10.8 ± 4.1 |
| FAZ | 0.22 ± 0.10 | 0.25 ± 0.10 | 0.32 ± 0.15 | 0.32 ± 0.13 | 0.34 ± 0.12 | 0.40 ± 0.07 | 0.26 ± 0.11 | 0.37 ± 0.12 |
| TAZ, mg/d | 1.87 ± 0.70 | 2.76 ± 1.49 | 3.12 ± 1.37 | 3.57 ± 1.38 | 4.53 ± 2.94 | 5.04 ± 1.63 | 3.01 ± 1.72 | 3.84 ± 1.66 |
| TAZ, predicted, mg/d | 2.12 | 3.25 | 2.20 | 3.13 | 2.28 | 3.28 | 2.20 | 2.90 |
| TAZ, measured:predicted | 0.88 | 0.85 | 1.42 | 1.14 | 1.99 | 1.54 | 1.37 | 1.32 |
| TDP, mg/d | 1880 ± 776 | 728 ± 353 | 2071 ± 791 | 954 ± 468 | 2636 ± 773 | 1040 ± 610 | 2290 ± 1016 | 1071 ± 560 |
| Phytate:zinc MR | 20.1 ± 6.4 | 6.6 ± 2.7 | 19.9 ± 4.8 | 8.6 ± 4.4 | 20.7 ± 3.2 | 8.9 ± 8.2 | 20.7 ± 7.8 | 11.1 ± 6.9 |
Control (habitual high-phytate diet), n = 8, and LP, n = 14. FAZ, fractional absorption of zinc; LP, low phytate; MR, molar ratio; TAZ, total absorbed zinc; TDP, total dietary phytate; TDZ, total dietary zinc.
Mean ± SD (all such values).
FIGURE 2.
Longitudinal changes in total dietary zinc (A) and fractional absorption of zinc (B) in pregnant and lactating Guatemalan women in control high-phytate (n = 8) and low-phytate (n = 14) diet groups during pregnancy and lactation. Values are means ± SEMs. lact, lactation; preg, pregnancy.
FIGURE 3.
Individual changes in fractional absorption of zinc (A) and total absorbed zinc (B) in Guatemalan women in the control high-phytate (n = 8) and low-phytate (n = 14) diet groups from 8 wk of pregnancy to 2 mo of lactation. lact, lactation; preg, pregnancy.
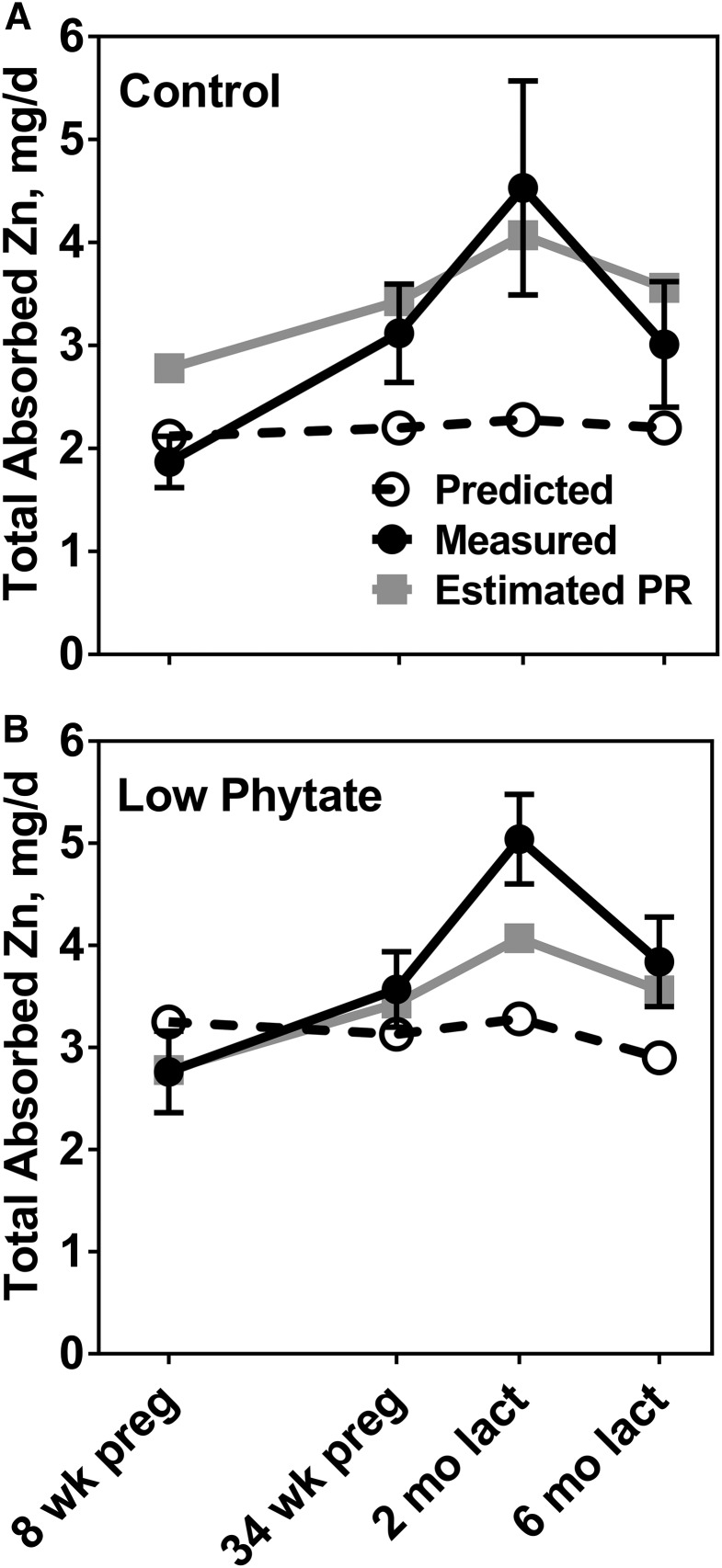
TAZ also increased across pregnancy and especially in early lactation (Figure 4). Mean TAZ for the LP group was greater than for the C group at all phases (Figure 4). The pattern of zinc absorption observed across the reproductive cycle was similar to that for estimated physiologic zinc requirements (13) (Figure 4). The mean TAZ for the LP group was similar to or slightly exceeded the physiologic requirement across all study phases. The mean TAZ for the C group approached the estimated physiologic zinc requirement in late pregnancy and exceeded the estimated physiologic requirement in early lactation. Increases in TAZ from the first to third trimester were 1.25 mg/d (P = 0.045) and 0.80 mg/d (P = 0.058) for the C and LP groups, respectively. Corresponding increases from the first trimester to 2 mo of lactation were 2.66 mg Zn/d (142%; P = 0.02) and 2.28 mg Zn/d (83%; P = 0.0004).
FIGURE 4.
Comparison of measured zinc absorption in Guatemalan women in the control high-phytate (n = 8) (A) and low-phytate (n = 14) (B) diet groups with the estimated longitudinal changes in PRs for zinc at the same phases of pregnancy and lactation (13) and with predicted zinc absorption from the same diets in NPNL women. Measured values are means ± SEMs. lact, lactation; NPNL, nonpregnant, nonlactating; PR, physiologic requirement; preg, pregnancy.
The predictions of TAZ for adults not in the reproductive cycle (NPNL), produced by a model of TAZ as a function of dietary zinc and phytate (24), provided an additional set of references values to compare with the observed TAZ data (Table 2, Figure 4). The predicted TAZ, based on measured quantities of phytate and zinc at each phase, varied little across pregnancy and lactation. In addition, as would be expected given the inhibitory effect of phytate on zinc absorption, TAZ was predicted to be greater for NPNL women ingesting the LP diet at each phase. With the exception of the measurements in the first trimester of pregnancy, all mean TAZ values exceeded the NPNL predictions for both LP and C groups. The greatest difference was in early lactation, when the mean increment in TAZ for C participants was 2.3 mg/d greater, and for the LP participants 1.8 mg/d greater, than that predicted for NPNL women consuming the same diet. The percentage increases were 99% (P = 0.0007) and 54% (P = 0.053) for the C and LP groups, respectively. Corresponding increases in the third trimester of pregnancy were 42% (P = 0.09) and 14% (P = 0.28) for the C and LP groups, respectively.
Discussion
The risk of zinc deficiency is considered to be greater in human populations consuming plant-based diets, which is attributed in substantial part to high intakes of phytate. There has been a strong incentive, therefore, to implement a study that not only achieves comparability of the quantities of zinc absorbed with longitudinal changes in physiologic zinc requirements across pregnancy and lactation, but simultaneously assesses the inhibitory effects of a range of dietary phytate on zinc absorption during these special physiologic circumstances. One essential facet of the study design was the selection of a population with a traditional diet containing maize as the major food staple and, therefore, a very high habitual phytate intake. A second important feature was the link with a larger randomized controlled trial that involved the long-term distribution of a very-low-phytate maize to half of the study population. This provided a control group with a high phytate intake and a phytate-to-zinc molar ratio of >20:1 and a second group with a low phytate intake and a phytate-to-zinc molar ratio of <10:1. Furthermore, interpretation of the data was facilitated by application of our trivariate model of the quantity of zinc absorbed as a function of dietary zinc and phytate (24). This allowed comparison with predictions based on NPNL reference data not only for the effect of dietary phytate but also for the effect of the quantity of zinc ingested.
The principal outcome of this study was the demonstration of the similarity in the longitudinal pattern of change in TAZ across pregnancy and lactation between the measured values and the estimated physiologic requirements (Figure 4). This pattern is so robust that it would not be compromised by any differences in estimated physiologic requirements, which were, for example, slightly higher for the DRIs of the Institute of Medicine (12) than for those used in this report (13). FAZ increased from phase 1 to phases 2 and 3 despite concurrent increases in TDZ, in contrast to the inverse relation of these 2 variables in NPNL participants (Figure 2). The determination of TAZ allowed quantitative longitudinal comparison of daily zinc absorption with physiologic requirements, which has the potential to provide new insights into the roles of both host and dietary factors in affecting zinc requirements during these demanding physiologic periods.
The 2 options for obtaining quantitative measures of the increases in TAZ in late pregnancy and early lactation were to subtract the corresponding measures for the first trimester and to subtract the corresponding model-based predictions for NPNL participants consuming the same quantities of zinc and phytate. The increases were greater with the first-trimester approach, because predicted values were greater than measured values in the first trimester. However, the predicted values were more informative because they depended on precisely the same dietary zinc and phytate intakes as those actually consumed at each phase. The differences between measured TAZ in late pregnancy, and especially early lactation, and those predicted in NPNL participants ingesting the same quantities of zinc and phytate were positive and large for both the LP and C groups. We did not anticipate the magnitude of the positive differences for the C group between the measured and predicted TAZ in late pregnancy and early lactation. These differences compared favorably with those for the LP group and with the estimated increases in physiologic zinc requirements for the corresponding phases. This narrowing of the gap between the longitudinal plot for C compared with LP groups was sufficient to ensure that the high phytate intake of the C group did not prevent the TAZ from matching the estimated physiologic requirement in early lactation. It is worth emphasizing that the high phytate intake of the C group had no negative effect on the magnitude of the increase in measured zinc absorption in late pregnancy and early lactation beyond any possible inhibitory effect of the more modest phytate intake of the LP group.
With a dietary phytate of 900 mg/d, an intake close to the average dietary intake of phytate by the LP group, the model-predicted quantities of dietary zinc necessary to achieve TAZs to match the physiologic zinc requirement are 12 and 18 mg Zn/d in late pregnancy and early lactation, respectively. With intakes of 2200 mg phytate/d, an intake similar to that of the C group, the dietary zinc estimated as necessary to meet NPNL model–predicted physiologic requirements for zinc absorption are in excess of 20 mg Zn/d for both late pregnancy and early lactation. These estimations highlight the extent to which NPNL model–estimated phytate effects are modified as a result of the upregulation of zinc absorption in late pregnancy and early lactation. The implication is that physiologic requirements for zinc can be met by pregnant and lactating women from some diets alone even when these contain substantial quantities of phytate.
With the very high phytate intake of the C group, an intake of 10.5 mg Zn is not quite adequate to meet the estimated physiologic zinc requirement in late pregnancy and 13 mg Zn/d is required to meet the physiologic requirement in early lactation. With the more modest phytate intakes of the LP group, 11 mg Zn/d in late pregnancy and 12.5 mg Zn/d in early lactation were at least adequate. Dietary zinc intakes were unexpectedly high in this study, which is attributable to the high zinc concentrations in the particular maize, which provided the major food staple. Maize tortillas provided ∼75% of the zinc ingested. The extent to which the mean zinc intakes for the LP group could be reduced and still meet physiologic requirements remains uncertain. Results of a cross-sectional study of zinc homeostasis in rural China (36) were supportive of the potential for meeting physiologic zinc requirements during lactation with lower intakes of dietary zinc than those ingested by participants in the current study. On the basis of the relation of endogenous fecal zinc to TAZ (25), the results of this study were also interpreted to be consistent with relative downregulation of endogenous intestinal zinc excretion, an aspect of zinc homeostasis that may affect future estimates of physiologic zinc requirements during lactation. However, cross-sectional measurements of TAZ in impoverished rural Ethiopian women during the third trimester of pregnancy whose zinc intake was ∼6 mg/d indicate that there is a limit to upregulation (37).
This study provides convincing evidence of major upregulation of zinc absorption in late pregnancy and early lactation, which appears to be of a magnitude that essentially matches the increase in physiologic requirements for zinc. Although there is substantial information on the regulation of zinc homeostasis (38–40), including in the enterocyte, nothing is yet known about the trigger or triggers responsible for these notable changes in zinc homeostasis during the reproductive cycle. The upregulation is not related to dietary zinc intake (41, 42). However, although not defined at a molecular level, zinc provides a clear example of a prominent nutrient for which host homeostatic mechanisms at least partially mitigate the need for increases in intake to meet physiologic increases in requirements during pregnancy and lactation.
A limitation of the study is the inevitable restriction in the range of dietary intake of zinc that could be included in a single study. The number of women who completed all phases of the study was smaller than the goal of 20 participants in each of the 2 groups. However, the study was strengthened by the longitudinal design. Other strengths included the quantitative determination of total daily absorbed zinc and the access to diets with both high and modest phytate concentration.
In conclusion, the patterns of change in TAZ across pregnancy and lactation conformed to the estimated physiologic zinc requirements across the reproductive cycle with diets both high and relatively low in phytate. With the modest phytate intakes of the LP group, the increases in TAZ during late pregnancy and at 2 mo of lactation ensured that physiologic requirements were met with the zinc intakes from the ad libitum study diets. Moreover, the inhibitory effect of high dietary phytate on zinc absorption is greatly diminished in late pregnancy and early lactation. The results of this study provide strong evidence of upregulation of zinc absorption in late pregnancy and early lactation, which may explain the limited evidence for severe manifestations of maternal zinc deficiency globally.
Acknowledgments
We thank the following individuals for their valuable contributions to this project: Amanda Harvey (USDA); Mark Kindem (RTI International); and Brenda Barahona, Raquel Campos, and Pilar Mux (Guatemalan field staff). The authors’ responsibilities were as follows—KMH and NFK: designed the study and had overall responsibility for the research with input from LVM, MM, JW, NWS, VR, AD, TH, and LW; MM: directed the field component of the parent trial and for this study; VR: directed the production and supply of maize for the parent study and provided analyses of dietary phytate; JW: provided major support for the conduct of all aspects of this study; JFK: responsible for the laboratory analyses; AD, NG, TH, and LW: responsible for data management and external oversight of the parent trial; LVM: processed, analyzed, and interpreted the data; KMH and LVM: wrote the manuscript; and all authors: had responsibility for the final content, and read and approved the final manuscript.
Footnotes
Abbreviations used: C, control (group); FAZ, fractional absorption of zinc; LP, low-phytate (group); NPNL, nonpregnant, nonlactating; TAZ, total absorbed zinc; TDP, total dietary phytate; TDZ, total dietary zinc; UCD, University of Colorado, Denver.
References
- 1.Hambidge KM, Casey CE, Krebs NF. Zinc Mertz W, editor. Trace elements in human and animal nutrition Orlando (FL): Academic Press; 1986. p. 1–137. [Google Scholar]
- 2.Shah PS, Ohlsson A. Effects of prenatal multimicronutrient supplementation on pregnancy outcomes: a meta-analysis. CMAJ 2009;180:E99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Donangelo CM, King JC. Maternal zinc intakes and homeostatic adjustments during pregnancy and lactation. Nutrients 2012;4:782–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Osendarp SJM, West CE, Black RE. The need for maternal zinc supplementation in developing countries: an unresolved issue. J Nutr 2003;133:817S–27S. [DOI] [PubMed] [Google Scholar]
- 5.Mahomed K, Bhutta Z, Middleton P. Zinc supplementation for improving pregnancy and infant outcome. Cochrane Database Syst Rev 2007;5:CD000230. [DOI] [PubMed] [Google Scholar]
- 6.Hess SY, King JC. Effects of maternal zinc supplementation on pregnancy and lactation outcomes. Food Nutr Bull 2009;30:S60–78. [DOI] [PubMed] [Google Scholar]
- 7.Kasana S, Din J, Maret W. Genetic causes and gene-nutrient interactions in mammalian zinc deficiencies: acrodermatitis enteropathica and transient neonatal zinc deficiency as examples. J Trace Elem Med Biol 2015;29:47–62. [DOI] [PubMed] [Google Scholar]
- 8.Swanson CA, King JC. Zinc and pregnancy outcome. Am J Clin Nutr 1987;46:763–71. [DOI] [PubMed] [Google Scholar]
- 9.Brown KH, Engle-Stone R, Krebs NF, Peerson JM. Dietary intervention strategies to enhance zinc nutrition: promotion and support of breastfeeding for infants and young children. Food Nutr Bull 2009;30:S144–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krebs NF, Reidinger CJ, Robertson AD, Hambidge KM. Growth and intakes of energy and zinc in infants fed human milk. J Pediatr 1994;124:32–9. [DOI] [PubMed] [Google Scholar]
- 11.Moser-Veillon PB, Reynolds RD. A longitudinal study of pyridoxine and zinc supplementation of lactating women. Am J Clin Nutr 1990;52:135–41. [DOI] [PubMed] [Google Scholar]
- 12.Food and Nutrition Board, Institute of Medicine. Dietary Reference Intakes for vitamin A, vitamin K, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium and zinc. Washington (DC): National Academies Press; 2001. [PubMed] [Google Scholar]
- 13.EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition, and Allergies). Scientific opinion on dietary reference values for zinc. EFSA J 2014;12:3844. [Google Scholar]
- 14.Fung EB, Ritchie LD, Woodhouse LR, Roehl R, King JC. Zinc absorption in women during pregnancy and lactation: a longitudinal study. Am J Clin Nutr 1997;66:80–8. [DOI] [PubMed] [Google Scholar]
- 15.Donangelo CM, Zapata CL, Woodhouse LR, Shames DM, Mukherjea R, King JC. Zinc absorption and kinetics during pregnancy and lactation in Brazilian women. Am J Clin Nutr 2005;82:118–24. [DOI] [PubMed] [Google Scholar]
- 16.Hambidge KM, Huffer JW, Raboy V, Grunwald GK, Westcott JL, Sian L, Miller LV, Dorsch JA, Krebs NF. Zinc absorption from low-phytate hybrids of maize and their wild-type isohybrids. Am J Clin Nutr 2004;79:1053–9. [DOI] [PubMed] [Google Scholar]
- 17.Mazariegos M, Hambidge KM, Westcott JE, Solomons NW, Raboy V, Das A, Goco N, Kindem M, Wright LL, Krebs NF. Neither a zinc supplement nor phytate-reduced maize nor their combination enhance growth of 6- to 12-month-old Guatemalan infants. J Nutr 2010;140:1041–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krebs NF, Miller LV, Naake VL, Lei S, Westcott JE, Fennessey PV, Hambidge KM. The use of stable isotope techniques to assess zinc metabolism. J Nutr Biochem 1995;6:292–301. [Google Scholar]
- 19.Friel JK, Naake VL Jr., Miller LV, Fennessey PV, Hambidge KM. The analysis of stable isotopes in urine to determine the fractional absorption of zinc. Am J Clin Nutr 1992;55:473–7. [DOI] [PubMed] [Google Scholar]
- 20.Huffer JW, Hambidge KM, Raboy VE, Drosch JA, Krebs NF. Comparison of zinc absorption from two low phytic acid corns. J Investig Med 2001;49:42A. [Google Scholar]
- 21.Krebs NF, Westcott JE, Culbertson DL, Sian L, Miller LV, Hambidge KM. Comparison of complementary feeding strategies to meet zinc requirements of older breastfed infants. Am J Clin Nutr 2012;96:30–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raboy V, Dickinson DB, Below FE. Variation in seed total phosphorus, phytic acid, zinc, calcium, magnesium, and protein among lines of Glycine max and G. Soja. Crop Sci 1984;24:431–4. [Google Scholar]
- 23.International Zinc Nutrition Consultative Group (IiNCG); Brown KH, Rivera JA, Bhutta Z, Gibson RS, King JC, Lonnerdal B, Ruel MT, Sandtrom B, Wasantwisut E, Hotz C. Assessment of the risk of zinc deficiency in populations and options for its control. Food Nutr Bull 2004;25:S99–203. [International Zinc Nutrition Consultative Group (IZiNCG) technical document 1]. [PubMed] [Google Scholar]
- 24.Miller LV, Krebs NF, Hambidge KM. A mathematical model of zinc absorption in humans as a function of dietary zinc and phytate. J Nutr 2007;137:135–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hambidge KM, Miller LV, Westcott JE, Sheng X, Krebs NF. Zinc bioavailability and homeostasis. Am J Clin Nutr 2010;91(Suppl):1478S–83S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hunt JR, Mullen LK, Lykken GI. Zinc retention from an experimental diet based on the US FDA Total Diet Study. Nutr Res 1992;126:2345S–53S. [Google Scholar]
- 27.Sian L, Mingyan X, Miller LV, Tong L, Krebs NF, Hambidge KM. Zinc absorption and intestinal losses of endogenous zinc in young Chinese women with marginal zinc intakes. Am J Clin Nutr 1996;63:348–53. [DOI] [PubMed] [Google Scholar]
- 28.Lowe NM, Shames DM, Woodhouse LR, Matel JS, Roehl R, Saccomani MP, Toffolo G, Cobelli C, King JC. A compartmental model of zinc metabolism in healthy women using oral and intravenous stable isotope tracers. Am J Clin Nutr 1997;65:1810–9. [DOI] [PubMed] [Google Scholar]
- 29.Hunt JR, Matthys LA, Johnson LK. Zinc absorption, mineral balance, and blood lipids in women consuming controlled lactoovovegetarian and omnivorous diets for 8 wk. Am J Clin Nutr 1998;67:421–30. [DOI] [PubMed] [Google Scholar]
- 30.Kristensen MB, Hels O, Morberg CM, Marving J, Bugel S, Tetens I. Total zinc absorption in young women, but not fractional zinc absorption, differs between vegetarian and meat-based diets with equal phytic acid content. Br J Nutr 2006;95:963–7.16611387 [Google Scholar]
- 31.Kim J, Paik HY, Joung H, Woodhouse LR, Li S, King JC. Effect of dietary phytate on zinc homeostasis in young and elderly Korean women. J Am Coll Nutr 2007;26:1–9. [DOI] [PubMed] [Google Scholar]
- 32.Rosado JL, Hambidge KM, Miller LV, Garcia OP, Westcott J, Gonzalez K, Conde J, Hotz C, Pfeiffer W, Ortiz-Monesterio I, et al. . The quantity of zinc absorbed from wheat in adult women is enhanced by biofortification. J Nutr 2009;139:1920–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sheng XY, Hambidge KM, Miller LV, Westcott JE, Lei S, Krebs NF. Measurement of zinc absorption from meals: comparison of extrinsic zinc labeling and independent measurements of dietary zinc absorption. Int J Vitam Nutr Res 2009;79:230–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hunt JR, Beiseigel JM, Johnson LK. Adaptation in human zinc absorption as influenced by dietary zinc and bioavailability. Am J Clin Nutr 2008;87:1336–45. [DOI] [PubMed] [Google Scholar]
- 35.Hunt JR, Beiseigel JM. Dietary calcium does not exacerbate phytate inhibition of zinc absorption by women from conventional diets. Am J Clin Nutr 2009;89:839–43. [DOI] [PubMed] [Google Scholar]
- 36.Sian L, Krebs NF, Westcott JE, Fengliang L, Tong L, Miller LV, Sonko B, Hambidge M. Zinc homeostasis during lactation in a population with a low zinc intake. Am J Clin Nutr 2002;75:99–103. [DOI] [PubMed] [Google Scholar]
- 37.Hambidge KM, Abebe Y, Gibson RS, Westcott JE, Miller LV, Lei S, Stoecker BJ, Arbide I, Teshome A, Bailey KB, et al. . Zinc absorption during late pregnancy in rural southern Ethiopia. Am J Clin Nutr 2006;84:1102–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cousins RJ. Gastrointestinal factors influencing zinc absorption and homeostasis. Int J Vitam Nutr Res 2010;80:243–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hojyo S, Fukada T, Shimoda S, Ohashi W, Bin BH, Koseki H, Hirano T. The zinc transporter SLC39A14/ZIP14 controls G-protein coupled receptor-mediated signaling required for systemic growth. PLoS One 2011;6: e18059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lichten LA, Cousins RJ. Mammalian zinc transporters: nutritional and physiologic regulation. Annu Rev Nutr 2009;29:153–76. [DOI] [PubMed] [Google Scholar]
- 41.Chung CS, Stookey J, Dare D, Welch R, Nguyen TQ, Roehl R, Peerson JM, King JC, Brown KH. Current dietary zinc intake has a greater effect on fractional zinc absorption than does longer term zinc consumption in healthy adult men. Am J Clin Nutr 2008;87:1224–9. [DOI] [PubMed] [Google Scholar]
- 42.Krebs NE, Hambidge KM. Zinc metabolism and homeostasis: the application of tracer techniques to human zinc physiology. Biometals 2001;14:397–412. [DOI] [PubMed] [Google Scholar]