Abstract
Purpose of review
To date, outcomes after lung transplantation are far worse than after transplantation of other solid organs. New insights into mechanisms that contribute to graft rejection and tolerance after lung transplantation remain of great interest. This review examines the recent literature on the role of innate and adaptive immunity in shaping the fate of lung grafts.
Recent findings
Innate and adaptive immune cells orchestrate allograft rejection after transplantation. Innate immune cells such as neutrophils are recruited to the lung graft early after reperfusion and subsequently promote allograft rejection. While it is widely recognized that CD4+ T lymphocytes in concert with CD8+ T cells promote graft rejection, regulatory Foxp3+ CD4+ T, central memory CD8+ T cells and natural killer cells can facilitate tolerance.
Summary
This review highlights interactions between innate and adaptive immune pathways and how they contribute to lung allograft rejection. These findings lay a foundation for the design of new therapeutic strategies that target both innate and adaptive immune responses.
Keywords: Ischemia reperfusion injury, alloimmunity, rejection, tolerance
Introduction
Lung transplantation (LTx) is a live-saving treatment for patients with end-stage pulmonary disease. Advances in surgical technique and postoperative management have resulted in dramatic improvements in short-term outcomes 1. However, long-term survival after LTx has not significantly changed over time and outcomes remain relatively disappointing 2. According to the International Society for Heart and Lung Transplantation registry report, the median survival after primary LTx is 5.7 years with a survival rate of 31% at 10 years 1. These suboptimal outcomes are largely related to the development of chronic lung allograft dysfunction (CLAD), a condition for which no effective medical treatment exists 3.
Acute rejection (AR) represents one of the most common complications after LTx and has been recognized as a major risk factor for the development of CLAD 4,5. AR is the result of an adaptive immune process related to an MHC mismatch and T cell allorecognition 6. According to the data on long-term graft survival, currently employed immunosuppression does not protect lung allografts to the same extent as other solid organ allografts 2. Acute rejection of lungs may differ from other solid organ transplants 7. To this end, the lung is constantly exposed to the external environment and may exhibit more complex immunologic features 8,9. Moreover, the combination of recently developed small animal models and advances in intravital imaging techniques have added new insights into the cellular framework that contributes to acute and chronic lung rejection 10.
Here we will review mechanisms underlying lung allograft rejection, explore the impact of early graft injury and innate immune activation, and discuss how adaptive immune responses lead to allograft rejection.
Early graft inflammation and alloimmunity
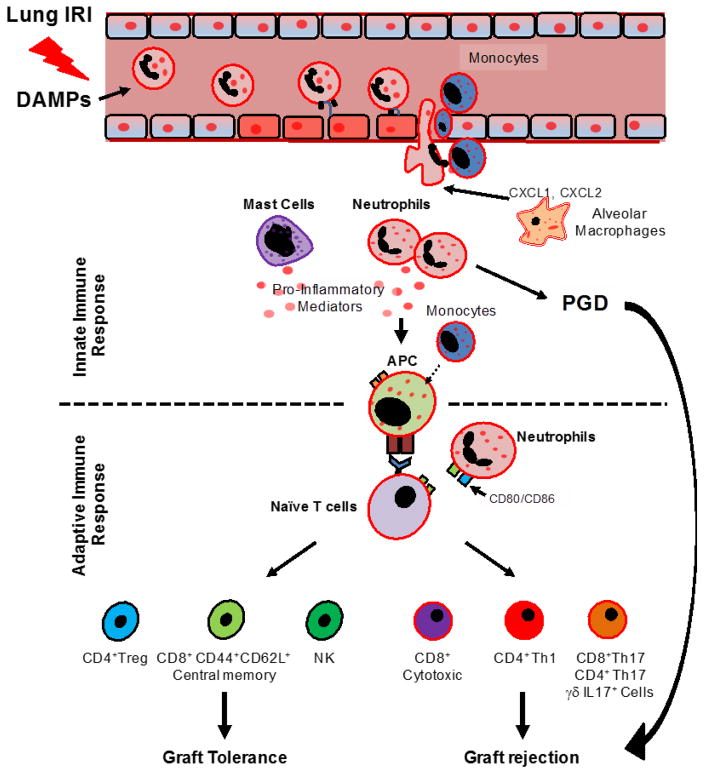
It has been recognized that early inflammatory events occurring in response to graft injury may enhance adaptive responses (Figure 1) 11–13.
Fig. 1. Diagram depicting mechanisms mediating primary graft dysfunction, rejection and the induction of tolerance.
In the context of ischemia-reperfusion injury (IRI), damage-associated molecular patterns (DAMPs) release contributes to the infiltration of neutrophils into the graft. Recipient-derived CCR2+ monocytes and donor alveolar macrophages facilitate neutrophil recruitment from blood vessels to the injured lung graft. Once activated, neutrophils in concert with mast cells can produce pro-inflammatory mediators such prostaglandins (PGs), interleukin (IL)-1β and IL-6, which can promote primary graft dysfunction. Allorecognition occurs locally within lung allografts through the engagement of antigen presenting cells (APCs) and T lymphocytes. Activated neutrophils can also express co-stimulatory molecules, which further promote the activation of naïve CD4+ T cells. Lymphocytes regulate outcomes after LTx. CD4+Th1, CD4+Th17, γδ IL17+, CD8+Th17 and CD8+ cytotoxic T cells promote graft loss, while regulatory CD4+Foxp3+ T cells (Tregs), CD8+CD44+CD62L+ central memory T cells and also NK cells can prevent rejection.
Ischemia-reperfusion injury and activation of TLRs
Early graft injury after organ transplantation is largely dependent on ischemia-reperfusion injury (IRI) 14. IRI induces alterations in cellular metabolism that lead to the generation of reactive oxygen species and promote cell damage and death resulting in the release of endogenous proinflammatory mediators 14. An early increase in the cell death biomarker M30 was recently demonstrated by Hashimoto in a population of LTx patients, who developed primary graft dysfunction (PGD) 15. Higher plasma levels of this biomarker were associated with worse clinical outcomes suggesting a link between early graft cell death and LTx prognosis15.
One of the most well-characterized endogenous proinflammatory mediators related to cell damage and death is the high-mobility group box1 (HMGB1). HMGB1 is a non-histone DNA binding protein actively secreted or passively released by cells undergoing necrosis 16–18. The proinflammatory effects of HMGB1 are mostly related to the stimulation of pattern recognition receptors (PRRs), such as the toll-like receptors (TLRs) and the receptor for advanced glycation end-products (RAGE) 11. Intragraft accumulation of pro-inflammatory mediators, also exacerbated by an impaired drainage function of the damaged lymphatic vessels 19, leads to leukocyte activation, which may result in graft rejection (Figure 1). An early increase in plasma levels of the soluble RAGE (sRAGE) after LTx has been associated with longer durations of mechanical ventilation, longer intensive care unit lengths of stay and development of CLAD 20. In addition, transcriptome analysis of bronchoalveolar lavage fluid and lung tissue from LTx recipients shows an overall up-regulation of TLRs 21. Using a mouse model of lung IRI, Altemeier demonstrated reduced inflammation in murine lungs deficient in the TLR signaling protein Myd88 22. Additionally, Zhang recently showed reduced signs of lung IRI in TLR3-deficient mice, which signals through the TRIF/TICAM-1 adaptor molecule in Myd88-independent fashion 23. TLR activation triggers proinflammatory effects that promote adhesion molecule expression, cytokine release from innate immune cells, and also enhance leukocyte trafficking through endothelial cell activation. In particular, TLR2 and TLR4 signaling have been shown to enhance post-ischemic vascular permeability and leukocyte migration after IRI 24. TLR2 and TLR4 activation can be driven by low molecular weight hyaluronan, which is released in response to IRI. Low molecular weight hyaluronan promotes neutrophilia and abrogates lung allograft tolerance in a TLR2/4 and MyD88-dependent manner 25.
Leukocyte trafficking into the injured graft
Despite advances in the understanding of the molecular signaling pathways that drive early graft inflammation, only recently have we gained insight into the in vivo mechanisms of intragraft leukocyte trafficking by using modern imaging techniques 26–28. Using intravital two-photon imaging and a mouse LTx model of IRI we discovered that neutrophils (PMNs) are not only rapidly recruited into the injured graft, but also tend to aggregate in dynamic clusters with intragraft monocytes. Monocytes appear to be critical in driving PMN transendothelial migration 26. Subsequent investigations found that a CCR2+ recipient-derived subset of proinflammatory monocytes promotes PGD 27. Additionally, graft-infiltrating recipient monocytes can also differentiate into dendritic cells (DC), acquire donor MHC molecules, and contribute to both indirect and direct allorecognition within the lung allograft 29. PMN recruitment into injured lungs can also be promoted by donor-derived immune cells such as alveolar macrophages 30. We have shown that the membrane-associated protein DAP12, expressed by donor alveolar macrophages, regulates the release of PMN chemoattractants such as CXCL1 and CXCL2 after IRI (Figure 1) 28.
Intragraft granulocytes and alloimmunity
Infiltrating PMNs in damaged airways are a clear hallmark of lung IRI. Thus, the role of PMNs in promoting allograft injury has been extensively investigated in the past few years 31. Besides their well-known effector functions, PMNs can also recruit activated CD8+ T cells through Fas ligand expression 32. PMNs also facilitate antigen presenting cell (APC) activity, which is critical to T cell activation and differentiation, through the expression of MHC class II and co-stimulatory molecules 33. In addition, PMNs enhance adaptive immunity after LTx 34. Using a mouse model of orthotopic LTx we showed prolonged interactions between recipient-derived PMNs and donor DCs within lung allografts; this contact-dependent mechanism promotes IL-12 production by DCs and expansion of IFN-γ+ T cells 34. In addition, our group demonstrated that graft-infiltrating neutrophils upregulate co-stimulatory molecules CD80 and CD86 during respiratory bacterial infections, which promotes the activation of T cells and triggers lung allograft rejection 35.
In addition to the role that PMNs play in LTx alloimmunity, some conflicting observations come from studies investigating the putative role of mast cells (MC) in this process 36–38. In various animal models of lung IRI, MCs have been shown not only to be recruited into injured lungs, but also to actively contribute to the proinflammatory microenvironment through the release of thromboxane B2, leukotriene B4, PGD2, TNF-α and IL-6 (Figure 1) 36,37. However, using adoptively transferred MCs in a stringent MC-deficient mouse model, Greenland recently showed a minor contribution for MCs in IRI 38. Moreover, evidence stemming from a skin transplant model has even suggested a beneficial role for MCs in inducing peripheral tolerance through a mechanism involving an IL-9-dependent interplay with CD4+CD25+Foxp3+ regulatory T cells (Treg) 39. Conflicting evidence regarding the role of MCs following LTx may be reconciled by recent reports suggesting that MCs can exhibit a “phenotypic switch” after solid organ transplantation 40,41. Banga recently showed a time-dependent transition from a tryptase+ to a tryptase+, chymase+ MC phenotype; this phenotypic switch was associated with a progressive decline in allograft function 40. These observations suggest that MCs are recruited into lung allografts and may play an important, albeit uncertain, role in modulating the early inflammatory events and adaptive immune responses that bring about allograft dysfunction.
Mechanisms of allorecognition
Allorecognition is a process where donor antigens are presented to recipient immune cells, resulting in the activation of an adaptive alloimmune response. Recent investigations suggest that T cell allorecognition is accomplished by at least two different mechanisms, termed “direct” and “indirect” pathways 42. The direct pathway involves recognition of allogeneic MHC molecules on donor APCs by recipient T cells. Meanwhile, T cells recognize processed alloantigens on recipient-derived APCs in the indirect pathway. For many years, it was believed that allorecognition was initiated only in recipient secondary lymphoid organs; this hypothesis was supported by a landmark study demonstrating that rejection of skin grafts could be prevented when the afferent lymphatic drainage was surgically disrupted 43. Similarly, Lakkis demonstrated that the survival of skin and heart grafts was extended in recipient mice lacking secondary lymphoid tissues44. However, this notion has been challenged by recent findings suggesting that the lung can be a site for the priming of adaptive responses. In a mouse model of viral infection, Moyron-Quiroz demonstrated that mice lacking secondary lymphoid organs are still able to mount a strong adaptive immune response and clear viral lung infections 9. Constant suggested that activated APCs could locally promote T cell differentiation towards a Th2 subtype in lung tissue, and that T cell priming in secondary lymphoid organs is not required 45. Using a mouse model of orthotopic LTx, we demonstrated that graft-infiltrating naïve T cells are activated locally in allogeneic lung grafts early after transplantation 46. Moreover, pulmonary allografts are rejected in non-immunosuppressed recipients that lack secondary lymphoid organs, supporting the notion that allogeneic immune responses are generated within lung grafts (Figure 1).
Role of T cells in acute cellular rejection
Recently, research has focused on the role of Th17 cells, characterized by the secretion of interleukin (IL)-17A and the expression of a transcription factor RORγT, in lung allograft rejection. In mice and humans, Th17 cells may play an important role in the development of CLAD 47–49. Mechanistically, Th17 cells downregulate complement-regulatory proteins on airway epithelial cells, which results in robust complement activation that damages the donor graft 48. Lendermon demonstrated that a robust induction of IL-17 production in CD8+ T cells triggers obliterative airway inflammation in t-bet-deficient hosts that are treated with CD154 blockade 50. This finding extends previous reports which suggested that CD8+ T cells are sufficient to trigger allograft rejection in lungs, challenging the notion that allograft rejection is dependent on CD4+ T cells 51. In addition to Th17 cells and IL17- producing CD8+ T cells, IL-17-producing γδT lymphocytes may also play a role in triggering alloimmune responses that lead to graft rejection (Figure 1) 50. Finally, development of acute rejection and inflammation can be blunted by neutralization of IL-17 49,52. These findings suggest that IL-17 is a key player of allograft rejection, and may be a suitable therapeutic target.
Regulatory Foxp3+ Tregs and Allograft Tolerance
Regulatory CD4+ Foxp3+ T cells (Tregs) have emerged as key regulators in allograft tolerance (Figure 1) 53. Tregs modulate immune responses through: 1) production of immunosuppressive cytokines (TGF-β and IL-10), 2) suppression of effector cells (i.e. Th1 and Th17 cells), and 3) regulation of DC maturation and function 54, all of which were shown to mediate transplant tolerance 53,55.
Tregs have been shown to inhibit Th17 responses. An imbalance of the Th17:Treg ratio has been linked to graft rejection after LTx 56. Additionally, administration of inducible Tregs can induce lung allograft tolerance through the suppression Th17-mediated responses 56. In human lung grafts the number of Foxp3+ Tregs correlates with a better prognosis, fewer episodes of AR, and better pulmonary function after transplantation 57–59. The quantity of Tregs in transbronchial biopsies positively correlates with the degree of AR after lung transplantation, suggesting that Tregs are actively recruited to sites of AR and may play a role in downregulating inflammation 57,58. Interestingly, the recruitment of Tregs occurs in concert with the upregulation of the chemokine CCL22 in the graft, suggesting that naïve Tregs may be primed in secondary lymphoid organs prior to their entry into the lung graft in response to CCL22 60.
Our group has reported that Tregs are critical in maintaining lung allograft acceptance 61. Dodd-o further demonstrated that CD154/CD40 signaling blockade blunts lung allograft rejection, in part by enhancing CD4+ Treg expansion 62. It is widely believed that alloantigen-specific Treg expansion predominantly takes place in secondary lymphoid organs 63. Treg trafficking to donor graft tissue from recipients appears to be a dynamic, yet crucial process for immune tolerance. Furthermore, migration of Tregs from allograft tissue to draining lymph nodes is required for optimal immune suppression and allograft tolerance 64. However, evidence has emerged suggesting that Tregs can be activated locally within the allograft itself. The discovery of Foxp3-rich ectopic lymphoid aggregates within renal and pulmonary allografts, also called tertiary lymphoid organs (TLOs), supports this notion 61,65.
Central Memory CD8+ T cells and Allograft Tolerance
The activation of alloreactive CD8+ T cells has been linked to allograft rejection in both experimental and clinical settings. Alloreactive memory CD8+ T cells infiltrate heart and kidney grafts early after transplantation and can facilitate rejection 66–70. Memory CD8+ T cells are rather resilient to immunosuppression and represent a barrier to tolerance induction 71. Depleting CD8+ T cells in mouse heart and skin transplant models prolongs long-term graft survival 72,73. By contrast, our group demonstrated that central memory CD8+ T cell, characterized by high surface expression of CD62 ligand (CD62L) and CD44, promote lung allograft tolerance induction (Figure 1) 74. This finding may represent a challenge for currently employed immunosuppressive regimens, which target T cells nonselectively potentially depleting beneficial T cell populations. In addition to central memory CD8+ T cells, natural killer (NK) cells may also play a crucial role in tolerance induction 75. Jungraithmayr demonstrated that lung allograft infiltration with NK cells is associated with tolerance. These NK cells recognize and destroy donor-derived DCs, which would otherwise activate T-cell-dependent alloimmune responses (Figure 1).
Concluding Remarks
Due to unique immunological features, the lung represents a challenge in designing effective immunosuppressive therapies. Rejection after LTx may start with the activation of innate immune pathways in response to early graft injury. The resulting proinflammatory microenvironment is capable of shaping the adaptive immune response. Targeting innate immune pathways in the peri-operative period may blunt graft rejection. Furthermore, indiscriminately targeting T cells at the time of LTx should be reconsidered since beneficial cell populations may be depleted. These findings present a more complex framework for lung allograft injury, allorecognition, and tolerance induction than previously recognized. Additional translational research is needed to examine the impact of the above mentioned findings on human lung transplant patients.
Key points.
Ischemia reperfusion injury drives innate immune activation, which plays a critical role in enhancing adaptive immune responses.
Lungs provide a suitable environment for either promoting allograft rejection or tolerance.
IL-17+ T lymphocytes drive rejection while CD4+Foxp3+ Tregs, CD8+ central memory T cells and NK cells may promote tolerance after lung transplantation.
Acknowledgments
Financial support and sponsorship
This work was supported by National Institutes of Health grants 1P01AI116501, R01 HL113931, R01 HL094601, T32-HL-007776 (HMH) and a research fellowship award from The International Society for Heart and Lung Transplantation (HMH).
Footnotes
Conflicts of interest
The authors have no conflicts of interest to declare.
References
- 1.Yusen RD, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: Thirty-second Official Adult Lung and Heart-Lung Transplantation Report--2015; Focus Theme: Early Graft Failure. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2015;34(10):1264–1277. doi: 10.1016/j.healun.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 2.Lodhi SA, Lamb KE, Meier-Kriesche HU. Solid organ allograft survival improvement in the United States: the long-term does not mirror the dramatic short-term success. Am J Transplant. 2011;11(6):1226–1235. doi: 10.1111/j.1600-6143.2011.03539.x. [DOI] [PubMed] [Google Scholar]
- 3.Gauthier JM, Hachem RR, Kreisel D. Update on Chronic Lung Allograft Dysfunction. Current Transplantation Reports. 2016;3(3):185–191. doi: 10.1007/s40472-016-0112-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burton CM, Iversen M, Carlsen J, et al. Acute cellular rejection is a risk factor for bronchiolitis obliterans syndrome independent of post-transplant baseline FEV1. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2009;28(9):888–893. doi: 10.1016/j.healun.2009.04.022. [DOI] [PubMed] [Google Scholar]
- 5.Hachem RR, Khalifah AP, Chakinala MM, et al. The significance of a single episode of minimal acute rejection after lung transplantation. Transplantation. 2005;80(10):1406–1413. doi: 10.1097/01.tp.0000181161.60638.fa. [DOI] [PubMed] [Google Scholar]
- 6.Taylor AL, Watson CJ, Bradley JA. Immunosuppressive agents in solid organ transplantation: Mechanisms of action and therapeutic efficacy. Critical reviews in oncology/hematology. 2005;56(1):23–46. doi: 10.1016/j.critrevonc.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 7.Prop J, Wildevuur CR, Nieuwenhuis P. Lung allograft rejection in the rat. II. Specific immunological properties of lung grafts. Transplantation. 1985;40(2):126–131. doi: 10.1097/00007890-198508000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Thaunat O, Patey N, Morelon E, Michel JB, Nicoletti A. Lymphoid neogenesis in chronic rejection: the murderer is in the house. Curr Opin Immunol. 2006;18(5):576–579. doi: 10.1016/j.coi.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 9.Moyron-Quiroz JE, Rangel-Moreno J, Kusser K, et al. Role of inducible bronchus associated lymphoid tissue (iBALT) in respiratory immunity. Nat Med. 2004;10(9):927–934. doi: 10.1038/nm1091. [DOI] [PubMed] [Google Scholar]
- 10.Lin X, Li W, Lai J, et al. Five-year update on the mouse model of orthotopic lung transplantation: Scientific uses, tricks of the trade, and tips for success. Journal of thoracic disease. 2012;4(3):247–258. doi: 10.3978/j.issn.2072-1439.2012.06.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Braza F, Brouard S, Chadban S, Goldstein DR. Role of TLRs and DAMPs in allograft inflammation and transplant outcomes. Nat Rev Nephrol. 2016;12(5):281–290. doi: 10.1038/nrneph.2016.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Whitson BA, Prekker ME, Herrington CS, et al. Primary graft dysfunction and long-term pulmonary function after lung transplantation. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2007;26(10):1004–1011. doi: 10.1016/j.healun.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 13.Shen H, Song Y, Colangelo CM, et al. Haptoglobin activates innate immunity to enhance acute transplant rejection in mice. The Journal of clinical investigation. 2012;122(1):383–387. doi: 10.1172/JCI58344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laubach VE, Sharma AK. Mechanisms of lung ischemia-reperfusion injury. Current opinion in organ transplantation. 2016;21(3):246–252. doi: 10.1097/MOT.0000000000000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *15.Hashimoto K, Besla R, Zamel R, et al. Circulating Cell Death Biomarkers May Predict Survival in Human Lung Transplantation. American journal of respiratory and critical care medicine. 2016;194(1):97–105. doi: 10.1164/rccm.201510-2115OC. This manuscript provides insights into the level of circulating cell death molecules and the clinical outcomes of lung transplantation in human. [DOI] [PubMed] [Google Scholar]
- 16.Lin Y, Chen L, Li W, Fang J. Role of high-mobility group box-1 in myocardial ischemia/reperfusion injury and the effect of ethyl pyruvate. Experimental and therapeutic medicine. 2015;9(4):1537–1541. doi: 10.3892/etm.2015.2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kashiwadate T, Miyagi S, Hara Y, et al. Soluble Thrombomodulin Ameliorates Ischemia-Reperfusion Injury of Liver Grafts by Modulating the Proinflammatory Role of High-Mobility Group Box 1. The Tohoku journal of experimental medicine. 2016;239(4):315–323. doi: 10.1620/tjem.239.315. [DOI] [PubMed] [Google Scholar]
- 18.Wu H, Ma J, Wang P, et al. HMGB1 contributes to kidney ischemia reperfusion injury. J Am Soc Nephrol. 2010;21(11):1878–1890. doi: 10.1681/ASN.2009101048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **19.Cui Y, Liu K, Monzon-Medina ME, et al. Therapeutic lymphangiogenesis ameliorates established acute lung allograft rejection. J Clin Invest. 2015;125(11):4255–4268. doi: 10.1172/JCI79693. This study describes a novel mechanism by which newly formed lymphatic vesesels facilitate the clearance of hyaluronan, a potent inflammatory molecule, from lung allografts thereby ameoliating rejection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shah RJ, Bellamy SL, Lee JC, et al. Early plasma soluble receptor for advanced glycation end-product levels are associated with bronchiolitis obliterans syndrome. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2013;13(3):754–759. doi: 10.1111/ajt.12062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cantu E, Lederer DJ, Meyer K, et al. Gene set enrichment analysis identifies key innate immune pathways in primary graft dysfunction after lung transplantation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2013;13(7):1898–1904. doi: 10.1111/ajt.12283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Altemeier WA, Liles WC, Villagra-Garcia A, Matute-Bello G, Glenny RW. Ischemia-reperfusion lung injury is attenuated in MyD88-deficient mice. PLoS One. 2013;8(10):e77123. doi: 10.1371/journal.pone.0077123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang XY, Chen C, Zhang YB, et al. Role of Toll-Like Receptor 3 in Lung Ischemia-Reperfusion Injury. Shock (Augusta, Ga) 2016 doi: 10.1097/SHK.0000000000000600. [DOI] [PubMed] [Google Scholar]
- 24.Khandoga AG, Khandoga A, Anders HJ, Krombach F. Postischemic vascular permeability requires both TLR-2 and TLR-4, but only TLR-2 mediates the transendothelial migration of leukocytes. Shock (Augusta, Ga) 2009;31(6):592–598. doi: 10.1097/SHK.0b013e318193c859. [DOI] [PubMed] [Google Scholar]
- 25.Todd JL, Wang X, Sugimoto S, et al. Hyaluronan contributes to bronchiolitis obliterans syndrome and stimulates lung allograft rejection through activation of innate immunity. Am J Respir Crit Care Med. 2014;189(5):556–566. doi: 10.1164/rccm.201308-1481OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kreisel D, Nava RG, Li W, et al. In vivo two-photon imaging reveals monocyte-dependent neutrophil extravasation during pulmonary inflammation. Proc Natl Acad Sci U S A. 2010;107(42):18073–18078. doi: 10.1073/pnas.1008737107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu Y, Li W, Luehmann HP, et al. Noninvasive Imaging of CCR2+ Cells in Ischemia-Reperfusion Injury After Lung Transplantation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2016 doi: 10.1111/ajt.13907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *28.Spahn JH, Li W, Bribriesco AC, et al. DAP12 expression in lung macrophages mediates ischemia/reperfusion injury by promoting neutrophil extravasation. J Immunol. 2015;194(8):4039–4048. doi: 10.4049/jimmunol.1401415. This study demonstrates a role for DAP12-expressing donor macrophages in promoting lung ischemia reperfusion injury by facilitating neutrophil recruitment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gelman AE, Okazaki M, Sugimoto S, et al. CCR2 regulates monocyte recruitment as well as CD4 T1 allorecognition after lung transplantation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2010;10(5):1189–1199. doi: 10.1111/j.1600-6143.2010.03101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsushima Y, Jang JH, Yamada Y, et al. The depletion of donor macrophages reduces ischaemia-reperfusion injury after mouse lung transplantation. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2014;45(4):703–709. doi: 10.1093/ejcts/ezt489. [DOI] [PubMed] [Google Scholar]
- 31.Scozzi D, Ibrahim M, Menna C, Krupnick AS, Kreisel D, Gelman AE. The Role of Neutrophils in Transplanted Organs. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2016 doi: 10.1111/ajt.13940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kish DD, Gorbachev AV, Parameswaran N, Gupta N, Fairchild RL. Neutrophil expression of Fas ligand and perforin directs effector CD8 T cell infiltration into antigen-challenged skin. J Immunol. 2012;189(5):2191–2202. doi: 10.4049/jimmunol.1102729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abi Abdallah DS, Egan CE, Butcher BA, Denkers EY. Mouse neutrophils are professional antigen-presenting cells programmed to instruct Th1 and Th17 T-cell differentiation. Int Immunol. 2011;23(5):317–326. doi: 10.1093/intimm/dxr007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kreisel D, Sugimoto S, Zhu J, et al. Emergency granulopoiesis promotes neutrophil-dendritic cell encounters that prevent mouse lung allograft acceptance. Blood. 2011;118(23):6172–6182. doi: 10.1182/blood-2011-04-347823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamamoto S, Nava RG, Zhu J, et al. Cutting edge: Pseudomonas aeruginosa abolishes established lung transplant tolerance by stimulating B7 expression on neutrophils. J Immunol. 2012;189(9):4221–4225. doi: 10.4049/jimmunol.1201683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wingard CJ, Walters DM, Cathey BL, et al. Mast cells contribute to altered vascular reactivity and ischemia-reperfusion injury following cerium oxide nanoparticle instillation. Nanotoxicology. 2011;5(4):531–545. doi: 10.3109/17435390.2010.530004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Su M, Chi EY, Bishop MJ, Henderson WR., Jr Lung mast cells increase in number and degranulate during pulmonary artery occlusion/reperfusion injury in dogs. The American review of respiratory disease. 1993;147(2):448–456. doi: 10.1164/ajrccm/147.2.448. [DOI] [PubMed] [Google Scholar]
- 38.Greenland JR, Xu X, Sayah DM, et al. Mast cells in a murine lung ischemia-reperfusion model of primary graft dysfunction. Respir Res. 2014;15:95. doi: 10.1186/s12931-014-0095-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lu LF, Lind EF, Gondek DC, et al. Mast cells are essential intermediaries in regulatory T-cell tolerance. Nature. 2006;442(7106):997–1002. doi: 10.1038/nature05010. [DOI] [PubMed] [Google Scholar]
- 40.Banga A, Han Y, Wang X, Hsieh FH. Mast cell phenotypes in the allograft after lung transplantation. Clinical transplantation. 2016;30(7):845–851. doi: 10.1111/ctr.12758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamada M, Ueda M, Naruko T, et al. Mast cell chymase expression and mast cell phenotypes in human rejected kidneys. Kidney international. 2001;59(4):1374–1381. doi: 10.1046/j.1523-1755.2001.0590041374.x. [DOI] [PubMed] [Google Scholar]
- 42.Herrera OB, Golshayan D, Tibbott R, et al. A novel pathway of alloantigen presentation by dendritic cells. J Immunol. 2004;173(8):4828–4837. doi: 10.4049/jimmunol.173.8.4828. [DOI] [PubMed] [Google Scholar]
- 43.Barker CF, Billingham RE. The role of afferent lymphatics in the rejection of skin homografts. J Exp Med. 1968;128(1):197–221. doi: 10.1084/jem.128.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lakkis FG, Arakelov A, Konieczny BT, Inoue Y. Immunologic ‘ignorance’ of vascularized organ transplants in the absence of secondary lymphoid tissue. Nat Med. 2000;6(6):686–688. doi: 10.1038/76267. [DOI] [PubMed] [Google Scholar]
- 45.Constant SL, Brogdon JL, Piggott DA, et al. Resident lung antigen-presenting cells have the capacity to promote Th2 T cell differentiation in situ. J Clin Invest. 2002;110(10):1441–1448. doi: 10.1172/JCI16109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gelman AE, Li W, Richardson SB, et al. Cutting edge: Acute lung allograft rejection is independent of secondary lymphoid organs. J Immunol. 2009;182(7):3969–3973. doi: 10.4049/jimmunol.0803514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Burlingham WJ, Love RB, Jankowska-Gan E, et al. IL-17-dependent cellular immunity to collagen type V predisposes to obliterative bronchiolitis in human lung transplants. J Clin Invest. 2007;117(11):3498–3506. doi: 10.1172/JCI28031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Suzuki H, Lasbury ME, Fan L, et al. Role of complement activation in obliterative bronchiolitis post-lung transplantation. J Immunol. 2013;191(8):4431–4439. doi: 10.4049/jimmunol.1202242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fan L, Benson HL, Vittal R, et al. Neutralizing IL-17 prevents obliterative bronchiolitis in murine orthotopic lung transplantation. Am J Transplant. 2011;11(5):911–922. doi: 10.1111/j.1600-6143.2011.03482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *50.Wu Q, Gupta PK, Suzuki H, et al. CD4 T Cells but Not Th17 Cells Are Required for Mouse Lung Transplant Obliterative Bronchiolitis. Am J Transplant. 2015;15(7):1793–1804. doi: 10.1111/ajt.13215. This paper identifies subtypes of IL17-producing cells that mediate lung allograft rejection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gelman AE, Okazaki M, Lai J, et al. CD4+ T lymphocytes are not necessary for the acute rejection of vascularized mouse lung transplants. J Immunol. 2008;180(7):4754–4762. doi: 10.4049/jimmunol.180.7.4754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lendermon EA, Dodd-o JM, Coon TA, et al. CD8(+)IL-17(+) T Cells Mediate Neutrophilic Airway Obliteration in T-bet-Deficient Mouse Lung Allograft Recipients. Am J Respir Cell Mol Biol. 2015;52(5):622–633. doi: 10.1165/rcmb.2014-0059OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6(4):345–352. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 54.Misra N, Bayry J, Lacroix-Desmazes S, Kazatchkine MD, Kaveri SV. Cutting edge: human CD4+CD25+ T cells restrain the maturation and antigen-presenting function of dendritic cells. J Immunol. 2004;172(8):4676–4680. doi: 10.4049/jimmunol.172.8.4676. [DOI] [PubMed] [Google Scholar]
- 55.Li W, Carper K, Zheng XX, et al. The role of Foxp3+ regulatory T cells in liver transplant tolerance. Transplant Proc. 2006;38(10):3205–3206. doi: 10.1016/j.transproceed.2006.10.093. [DOI] [PubMed] [Google Scholar]
- *56.Zhou W, Zhou X, Gaowa S, et al. The Critical Role of Induced CD4+ FoxP3+ Regulatory Cells in Suppression of Interleukin-17 Production and Attenuation of Mouse Orthotopic Lung Allograft Rejection. Transplantation. 2015;99(7):1356–1364. doi: 10.1097/TP.0000000000000526. This paper provides evidence that Foxp3+ regulatory T cells attenuate lung rejection by suppressing Th17-mediated pathways. [DOI] [PubMed] [Google Scholar]
- 57.Krustrup D, Madsen CB, Iversen M, Engelholm L, Ryder LP, Andersen CB. The number of regulatory T cells in transbronchial lung allograft biopsies is related to FoxP3 mRNA levels in bronchoalveolar lavage fluid and to the degree of acute cellular rejection. Transpl Immunol. 2013;29(1–4):71–75. doi: 10.1016/j.trim.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 58.Krustrup D, Iversen M, Martinussen T, Andersen CB. Time elapsed after transplantation influences the relationship between the number of regulatory T cells in lung allograft biopsies and subsequent acute rejection episodes. Transpl Immunol. 2014;31(1):42–47. doi: 10.1016/j.trim.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 59.Nakagiri T, Warnecke G, Avsar M, et al. Lung function early after lung transplantation is correlated with the frequency of regulatory T cells. Surg Today. 2012;42(3):250–258. doi: 10.1007/s00595-011-0087-3. [DOI] [PubMed] [Google Scholar]
- 60.Bhorade SM, Chen H, Molinero L, et al. Decreased percentage of CD4+FoxP3+ cells in bronchoalveolar lavage from lung transplant recipients correlates with development of bronchiolitis obliterans syndrome. Transplantation. 2010;90(5):540–546. doi: 10.1097/TP.0b013e3181e8dabe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li W, Bribriesco AC, Nava RG, et al. Lung transplant acceptance is facilitated by early events in the graft and is associated with lymphoid neogenesis. Mucosal Immunol. 2012;5(5):544–554. doi: 10.1038/mi.2012.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dodd-o JM, Lendermon EA, Miller HL, et al. CD154 blockade abrogates allospecific responses and enhances CD4(+) regulatory T-cells in mouse orthotopic lung transplant. Am J Transplant. 2011;11(9):1815–1824. doi: 10.1111/j.1600-6143.2011.03623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ochando JC, Yopp AC, Yang Y, et al. Lymph node occupancy is required for the peripheral development of alloantigen-specific Foxp3+ regulatory T cells. J Immunol. 2005;174(11):6993–7005. doi: 10.4049/jimmunol.174.11.6993. [DOI] [PubMed] [Google Scholar]
- 64.Zhang N, Schroppel B, Lal G, et al. Regulatory T cells sequentially migrate from inflamed tissues to draining lymph nodes to suppress the alloimmune response. Immunity. 2009;30(3):458–469. doi: 10.1016/j.immuni.2008.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brown K, Sacks SH, Wong W. Tertiary lymphoid organs in renal allografts can be associated with donor-specific tolerance rather than rejection. Eur J Immunol. 2011;41(1):89–96. doi: 10.1002/eji.201040759. [DOI] [PubMed] [Google Scholar]
- 66.Ascon M, Ascon DB, Liu M, et al. Renal ischemia-reperfusion leads to long term infiltration of activated and effector-memory T lymphocytes. Kidney Int. 2009;75(5):526–535. doi: 10.1038/ki.2008.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Donckier V, Craciun L, Miqueu P, et al. Expansion of memory-type CD8+ T cells correlates with the failure of early immunosuppression withdrawal after cadaver liver transplantation using high-dose ATG induction and rapamycin. Transplantation. 2013;96(3):306–315. doi: 10.1097/TP.0b013e3182985414. [DOI] [PubMed] [Google Scholar]
- 68.Koyama I, Nadazdin O, Boskovic S, et al. Depletion of CD8 memory T cells for induction of tolerance of a previously transplanted kidney allograft. Am J Transplant. 2007;7(5):1055–1061. doi: 10.1111/j.1600-6143.2006.01703.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schenk AD, Nozaki T, Rabant M, Valujskikh A, Fairchild RL. Donor-reactive CD8 memory T cells infiltrate cardiac allografts within 24-h posttransplant in naive recipients. Am J Transplant. 2008;8(8):1652–1661. doi: 10.1111/j.1600-6143.2008.02302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Adams AB, Williams MA, Jones TR, et al. Heterologous immunity provides a potent barrier to transplantation tolerance. J Clin Invest. 2003;111(12):1887–1895. doi: 10.1172/JCI17477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Trambley J, Bingaman AW, Lin A, et al. Asialo GM1(+) CD8(+) T cells play a critical role in costimulation blockade-resistant allograft rejection. J Clin Invest. 1999;104(12):1715–1722. doi: 10.1172/JCI8082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Benichou G, Yamada Y, Yun SH, Lin C, Fray M, Tocco G. Immune recognition and rejection of allogeneic skin grafts. Immunotherapy. 2011;3(6):757–770. doi: 10.2217/imt.11.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Halamay KE, Kirkman RL, Sun L, et al. CD8 T cells are sufficient to mediate allorecognition and allograft rejection. Cell Immunol. 2002;216(1–2):6–14. doi: 10.1016/s0008-8749(02)00530-0. [DOI] [PubMed] [Google Scholar]
- 74.Krupnick AS, Lin X, Li W, et al. Central memory CD8+ T lymphocytes mediate lung allograft acceptance. J Clin Invest. 2014;124(3):1130–1143. doi: 10.1172/JCI71359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jungraithmayr W, Codarri L, Bouchaud G, et al. Cytokine complex-expanded natural killer cells improve allogeneic lung transplant function via depletion of donor dendritic cells. Am J Respir Crit Care Med. 2013;187(12):1349–1359. doi: 10.1164/rccm.201209-1749OC. [DOI] [PMC free article] [PubMed] [Google Scholar]