ABSTRACT
Neisseria gonorrhoeae resistance to ceftriaxone and azithromycin is increasing, which threatens the recommended dual therapy. We used molecular epidemiology to identify N. gonorrhoeae clusters and associations with azithromycin resistance in Amsterdam, the Netherlands. N. gonorrhoeae isolates (n = 143) were selected from patients visiting the Amsterdam STI Outpatient Clinic from January 2008 through September 2015. We included all 69 azithromycin-resistant isolates (MIC ≥ 2.0 mg/liter) and 74 frequency-matched susceptible controls (MIC ≤ 0.25 mg/liter). The methods used were 23S rRNA and mtrR sequencing, N. gonorrhoeae multiantigen sequence typing (NG-MAST), N. gonorrhoeae multilocus variable-number tandem-repeat analysis (NG-MLVA), and a specific PCR to detect mosaic penA genes. A hierarchical cluster analysis of NG-MLVA related to resistance and epidemiological characteristics was performed. Azithromycin-resistant isolates had C2611T mutations in 23S rRNA (n = 62, 89.9%, P < 0.001) and were NG-MAST genogroup G2992 (P < 0.001), G5108 (P < 0.001), or G359 (P = 0.02) significantly more often than susceptible isolates and were more often part of NG-MLVA clusters (P < 0.001). Two resistant isolates (2.9%) had A2059G mutations, and five (7.3%) had wild-type 23S rRNA. No association between mtrR mutations and azithromycin resistance was found. Twenty-four isolates, including 10 azithromycin-resistant isolates, showed reduced susceptibility to extended-spectrum cephalosporins. Of these, five contained a penA mosaic gene. Four of the five NG-MLVA clusters contained resistant and susceptible isolates. Two clusters consisting mainly of resistant isolates included strains from men who have sex with men and from heterosexual males and females. The co-occurrence of resistant and susceptible strains in NG-MLVA clusters and the frequent occurrence of resistant strains outside of clusters suggest that azithromycin resistance develops independently from the background genome.
KEYWORDS: NG-MAST, 23S rRNA mutation, Neisseria gonorrhoeae, antimicrobial resistance, azithromycin, sequence typing
INTRODUCTION
With an estimated 78 million infections annually, gonorrhea is the second most common bacterial sexually transmitted infection (STI) worldwide (1). Its causative agent, Neisseria gonorrhoeae, has a remarkable capacity to rapidly develop antimicrobial resistance (AMR) to many different types of antibiotic drugs when these are used widely as first-line treatment options (2). Resistance to either ceftriaxone or azithromycin (the internationally recommended combination therapy) is increasing, and the first treatment failure of dual therapy due to resistance to both drugs has been reported (3–7). Moreover, an outbreak of high-level azithromycin resistance has been observed in the United Kingdom, despite the use of dual therapy (8). In the Netherlands, we have reported a decrease in azithromycin susceptibility in recent years (9).
Genetic analyses have shown a strong association between azithromycin resistance and specific mutations in the 23S rRNA genes. These mutations prevent effective binding of azithromycin and thereby block its inhibitory effect on protein synthesis (10–12). Moderate resistance has been linked to C2611T mutations (Escherichia coli numbering), while high-level resistance has been linked to A2059G mutations in the 23S rRNA genes (11, 13, 14). In addition, out of the four 23S rRNA alleles in the N. gonorrhoeae genome, a higher cumulative number of mutated alleles is associated with a higher MIC. After introduction of a mutation in one allele, transformation of other alleles may occur, which induces high-level resistance (10, 11). However, mutations in the mtrR gene and its promoter have also been related to azithromycin resistance (15–18).
In addition to testing for specific antimicrobial resistance-related mutations, isolates can also be genetically typed according to their sequences at highly polymorphic regions and can subsequently be clustered according to their molecular sequence type (ST) (13, 19, 20). Clusters can then be linked to epidemiological data, which helps to identify possible risk groups for antimicrobial resistance.
In this study, we aimed to evaluate the molecular epidemiology of azithromycin resistance among N. gonorrhoeae isolates in patients who visited the Amsterdam STI Outpatient Clinic.
RESULTS
From January 2008 through September 2015, gonorrhea was diagnosed in 9,959 consultations. After selecting only consultations of the same patient that were at least 6 months apart and that included positive cultures and selecting only one isolate per consultation, 5,737 consultations (and, thus, 5,737 isolates) remained. Those with intermediate MICs (n = 1,212) were excluded. All 77 resistant isolates (MIC ≥ 2 mg/liter) were included as cases. From the 4,448 susceptible isolates (MIC ≤ 0.25 mg/liter), we randomly selected 77 controls frequency matched to the 77 cases on the calendar year of infection and sexual risk group. After selection, three isolates proved to be lost during storage, and four could not be repeatedly typed; these seven isolates were excluded. This resulted in a collection of 147 isolates (from 144 patients), consisting of 73 cases and 74 controls (Table 1). Three patients (all of whom were controls and all of whom were men who have sex with men [MSM]) were included twice with separate consultations.
TABLE 1.
Baseline characteristics of 147 Neisseria gonorrhoeae isolates from patients attending the Amsterdam STI Outpatient Clinic from January 2008 through September 2015 by susceptibility to azithromycina
| Characteristic | Value(s) for the following isolates: |
P | ||
|---|---|---|---|---|
| Total | Resistant | Susceptibleb | ||
| No. of isolates | 147 | 73 | 74 | |
| Azithromycin MIC (mg/liter) | ||||
| Geometric mean (range) | 0.85 (<0.016 to >256) | 6.30 (2 to >256) | 0.12 (<0.016 to 0.25) | |
| MIC50 | 0.25 | 6 | 0.125 | |
| MIC90 | 12 | 16 | 0.25 | |
| No. (%) of patients by yr of infection | —c | |||
| 2008 | 14 (9.5) | 7 (9.6) | 7 (9.5) | |
| 2009 | 20 (13.6) | 10 (13.7) | 10 (13.5) | |
| 2010 | 33 (22.5) | 16 (21.9) | 17 (23.0) | |
| 2011 | 48 (32.7) | 24 (32.9) | 24 (32.4) | |
| 2012 | 12 (8.2) | 6 (8.2) | 6 (8.1) | |
| 2013 | 6 (4.1) | 3 (4.1) | 3 (4.1) | |
| 2014 | 8 (5.4) | 4 (5.5) | 4 (5.4) | |
| 2015 | 6 (4.1) | 3 (4.1) | 3 (4.1) | |
| No. (%) of patients by sexual risk group | — | |||
| Heterosexual male | 10 (6.8) | 5 (6.9) | 5 (6.8) | |
| MSM | 119 (81.0) | 59 (80.8) | 60 (81.1) | |
| Female | 18 (12.2) | 9 (12.3) | 9 (12.2) | |
| Median (IQR) age (yr) | 33 (25–41) | 35 (27–41) | 31 (24–43) | 0.37 |
| No. (%) of patients by ethnicity | 0.006 | |||
| Dutch | 88 (69.9) | 41 (56.2) | 47 (63.5) | |
| Surinamese/Antillean | 13 (8.8) | 3 (4.1) | 10 (13.5) | |
| Other | 39 (26.5) | 22 (30.1) | 17 (23.0) | |
| Unknown | 7 (4.8) | 7 (9.6) | 0 (0.0) | |
| No. (%) of patients who were: | ||||
| Commercial sex workersd | 8 (5.4) | 5 (6.9) | 3 (4.1) | 0.49 |
| Clients of commercial sex workers | 1 (0.7) | 1 (1.4) | 0 (0.0) | 0.50 |
| Median (IQR) no. of sex partners in previous 6 mo | 6 (3–15) | 7 (3–15) | 6 (4–15) | 0.73 |
| No. (%) of patients infected at the following anatomical site: | 0.70 | |||
| Urethra | 49 (33.3) | 21 (28.8) | 28 (33.7) | |
| Rectum | 75 (51.0) | 40 (54.8) | 35 (47.3) | |
| Cervix | 11 (7.5) | 6 (8.2) | 5 (6.7) | |
| Pharynx | 12 (8.2) | 6 (8.2) | 6 (8.1) | |
| No. (%) of patients with the following HIV infection status: | 1.00 | |||
| Negative | 93 (63.3) | 46 (63.0) | 47 (63.5) | |
| Positive | 45 (30.6) | 22 (30.1) | 23 (31.1) | |
| Unknown | 9 (6.1) | 5 (6.7) | 4 (5.4) | |
| No. (%) of patients with syphilis (past or active) | 0.73 | |||
| No | 113 (76.9) | 57 (78.1) | 56 (75.7) | |
| Yes | 34 (23.1) | 16 (21.9) | 18 (24.3) | |
| No. (%) of patients with Chlamydia trachomatis infection | 0.14 | |||
| Negative | 111 (75.5) | 59 (80.8) | 52 (70.3) | |
| Positive | 36 (24.5) | 14 (19.2) | 22 (29.7) | |
| LGV positive | 3 (8.3) | 1 (7.1) | 2 (9.1) | 1.00 |
Azithromycin resistance was defined as an MIC of ≥2 mg/liter, and susceptibility was defined as an MIC of ≤0.25 mg/liter. IQR, interquartile range; LGV, lymphogranuloma venereum; MSM, men who have sex with men; STI, sexually transmitted infection.
Three patients were included twice with a consultation and an isolate.
—, frequency matched between resistant (cases) and susceptible (controls).
Of the eight commercial sex workers, five were female and three were male.
Baseline characteristics and azithromycin susceptibility.
The geometric mean MIC was 6.30 mg/liter (range, 2 to >256 mg/liter) for resistant isolates and 0.12 mg/liter (range, <0.016 to 0.25 mg/liter) for susceptible isolates (Table 1). The MIC50 was 0.25 mg/liter for all isolates, 0.125 mg/liter for susceptible isolates, and 6 mg/liter for resistant isolates. The MIC90 results for these three groups of isolates were 12, 0.25, and 16 mg/liter, respectively. Reduced susceptibility to extended-spectrum cephalosporins (ESC) was defined as an MIC of >0.016 mg/liter for cefotaxime (for isolates collected before 2010) or ceftriaxone (for isolates collected since 2010). This reduced susceptibility was noted among 24 isolates. Of these, 14 isolates were susceptible to azithromycin and 10 were resistant to azithromycin. Of the 147 strains, most were collected in 2011 (n = 48, 32.7%) and in 2010 (n = 33, 22.5%). Patients were mainly MSM (n = 119, 81.0%), but 18 females (12.2%) and 10 heterosexual males (6.8%) were also included. The median age was 33 years (interquartile range [IOR], 25 to 41 years), and 88 (69.9%) patients were of Dutch origin. Commercial sex work was reported by eight patients (5.4%), of whom five were female and three were male. The median number of sex partners in the previous 6 months was 6 (interquartile range [IQR], 3 to 15). Most isolates were rectal (n = 75, 51.0%) or urethral (n = 49, 33.3%), followed by 12 pharyngeal (8.2%) and 11 cervical (7.5%) isolates. Forty-five patients (30.6%) were human immunodeficiency virus (HIV) positive, and the HIV infection status was unknown for nine (6.1%). Coinfections were common: 34 (23.1%) had past or active syphilis, and 36 (24.5%) had a concurrent infection with Chlamydia trachomatis. Of the patients with concurrent infection with Chlamydia trachomatis, 3 (8.3%) had lymphogranuloma venereum (LGV).
23S rRNA typing.
We determined the presence of 23S rRNA mutations for all 147 included isolates. Nine resistant isolates had wild-type 23S rRNA. Because of the strong link between azithromycin resistance and 23S rRNA mutations, we retested the MICs for these nine isolates. For five isolates the new MIC values were within 1 dilution of the reported MIC, and wild-type 23S rRNA was confirmed by repeated testing. One isolate could not be confirmed to be N. gonorrhoeae and was excluded post hoc. For the remaining three of nine retested isolates, the new MIC value was more than 1 dilution lower than the reported MIC, and these were also excluded post hoc. After excluding the 4 isolates, we included 143 isolates for typing and cluster analysis.
Of the 143 included isolates (69 resistant and 74 susceptible), 78 (54.6%) had wild-type 23S rRNA genes, 62 (43.4%) had a C2611T mutation, 2 (1.4%) had a A2059G mutation (both were resistant), and the result for 1 was undetermined (Table 2). Among the 69 resistant isolates, 62 (89.9%) had a C2611T mutation, 2 (2.9%) had an A2059G mutation, and 5 (7.2%) were wild type (geometric mean MIC, 2.6 mg/liter; range, 2 to 8 mg/liter). The association between resistance and C2611T mutations was highly significant (P < 0.001). Among the 62 isolates with C2611T mutations, 1 isolate (1.6%) had 2 mutated alleles, 4 had 3 mutated alleles (6.5%), and 57 had the mutation in all 4 alleles (91.9%) (Fig. 1A).
TABLE 2.
Typing results for 143 Neisseria gonorrhoeae isolates at the Amsterdam STI Outpatient Clinic from January 2008 through September 2015 by susceptibility to azithromycina
| Characteristic | Value(s) for the following isolates: |
P | ||
|---|---|---|---|---|
| Total | Resistant | Susceptible | ||
| No. (%) of isolates | 143 | 69 (48.3) | 74 (51.8) | |
| No. (%) of isolates with the following 23S rRNAb: | ||||
| Wild type | 78 (54.6) | 5 (7.2) | 73 (98.7) | <0.001 |
| C2611T mutation | 62 (43.4) | 62 (89.9) | 0 (0.0) | <0.001 |
| A2059G mutation | 2 (1.4) | 2 (2.9) | 0 (0.0) | 0.2 |
| No. (%) of isolates with the following NG-MLVA result: | ||||
| Isolate in cluster | 65 (45.5) | 45 (65.2) | 20 (27.0) | <0.001 |
| Isolates not in clusters | 78 (54.6) | 24 (34.8) | 54 (73.0) | |
| No. (%) of isolates in the following NG-MAST genogroupc: | ||||
| G2992 | 23 (16.1) | 19 (27.5) | 4 (5.4) | <0.001 |
| G5108 | 17 (11.9) | 17 (24.6) | 0 (0.0) | <0.001 |
| G2400 | 14 (9.8) | 3 (4.4) | 11 (14.9) | 0.03 |
| G1407 | 11 (7.7) | 5 (7.3) | 6 (8.1) | 0.8 |
| G359 | 9 (6.3) | 8 (11.6) | 1 (1.4) | 0.02 |
| Not in the main genogroups | 65 (46.8) | 17 (24.6) | 48 (68.6) | <0.001 |
NG-MLVA, N. gonorrhoeae multilocus variable-number tandem-repeat analysis; NG-MAST, N. gonorrhoeae multiantigen sequence typing.
The results for one sample were undetermined.
Only the five most common genogroups are mentioned separately; the results for 4 isolates were undetermined. Genogroups were assigned to sequence types with at least one identical allele and a difference of ≤4 bp (tbpB) or ≤5 bp (por) for the other allele.
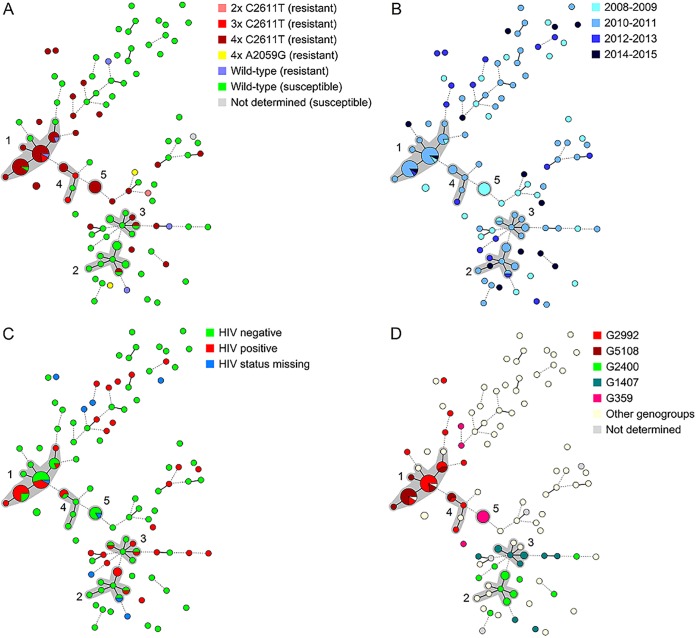
FIG 1.
Minimum spanning tree of 143 N. gonorrhoeae isolates, based on NG-MLVA, by 23S rRNA type and resistance to azithromycin (A), calendar year (B), HIV status (C), and NG-MAST genogroup (D). Each circle represents a different NG-MLVA type, the size of the circle represents the number of isolates, a solid line represents a difference of one locus, and a dashed line represents a difference of two loci. A cluster (gray area) was assigned to groups of NG-MLVA types that differed at a maximum of one locus and contained at least five isolates. Azithromycin susceptibility was defined as an MIC of ≤0.25 mg/liter, and resistance was defined as an MIC of ≥2 mg/liter. NG-MAST genogroups were assigned to sequence types with at least one identical allele and a difference of ≤4 bp (tbpB) or ≤5 bp (por) for the other allele. HIV, human immunodeficiency virus; NG-MAST, Neisseria gonorrhoeae multiantigen sequence type; NG-MLVA, Neisseria gonorrhoeae multilocus variable-number tandem-repeat analysis.
NG-MAST.
When testing the 143 isolates by N. gonorrhoeae multiantigen sequence typing (NG-MAST), we noted 65 different STs, and most of these were represented by 1 or 2 isolates (see Table S1 in the supplemental material). Four isolates could not be assigned an NG-MAST ST. When assigning STs into genogroups, we noted five genogroups that consisted of more than five isolates: G2992 (n = 23, 16.1%), G5108 (n = 17, 11.9%), G2400 (n = 14, 9.8%), G1407 (n = 11, 7.7%), and G359 (n = 9, 6.3%) (Table 2). Resistant isolates were assigned to genogroups G2992 (P < 0.001), G5108 (P < 0.001), and G359 (P = 0.02) significantly more often than susceptible isolates. Genogroup G2400 was significantly more common among susceptible than among resistant isolates (P = 0.03). Genogroup G1407 was equally common among resistant and susceptible isolates (P = 0.8). The remaining 65 strains belonged to uncommon genogroups (≤5 isolates each) and mainly included susceptible isolates (n = 48, 73.9%) but also included 17 resistant isolates (26.2%).
NG-MLVA typing and cluster analysis.
The hierarchical cluster analysis using N. gonorrhoeae multilocus variable-number tandem-repeat analysis (NG-MLVA) types showed five clusters containing 65 of the 143 isolates (45.5%) (Table 2; Fig. 1). Seventy-eight isolates (54.6%) were not part of a cluster. Azithromycin-resistant isolates were present in a cluster (n = 45, 65.2%) significantly more often than susceptible isolates (n = 20, 27.0%) (P < 0.001). Clusters 1, 4, and 5 mainly consisted of resistant isolates with 23S rRNA with the C2611T mutation, whereas clusters 2 and 3 mainly consisted of susceptible isolates (Fig. 1A). All clusters except cluster 5 contained isolates from 2010 and 2011, whereas clusters 1 and 2 also contained more recent isolates from 2014 and 2015 (Fig. 1B). HIV positivity varied within and outside of clusters (P = 0.1; Fig. 1C). Age and anatomical site of infection were also not associated with clusters (data not shown). In addition, although the majority of patients were Dutch, most clusters contained a mixture of isolates from patients with diverse ethnic origins. Interestingly, of the 12 Surinamese or Antillean patients, only the isolate from 1 patient was in a cluster (cluster 3) (Fig. S1).
Azithromycin resistance, 23S rRNA mutation, and genotype.
Although the 62 isolates carrying a C2611T mutation were found throughout the genetic tree, 40 (64.5%) were found in the predominantly resistant NG-MLVA clusters 1, 4, and 5 (Fig. 1A). Three isolates (4.8%) were included in the predominantly susceptible clusters 2 and 3, of which two belonged to common NG-MAST genogroups (one was G2400 and one was G1407). Nineteen (30.7%) of the isolates with the C2611T mutation were not included in any cluster, and 11 (57.9%) of these also did not belong to the five most common NG-MAST genogroups (Fig. 1D; Table 3). The two isolates with A2059G mutations were unrelated to any other isolate (Fig. 1A). Of the five resistant isolates with wild-type 23S rRNA, two were included in NG-MLVA cluster 1 and were NG-MAST G2992. The other three were not in a cluster; two were G1407, and one was G6327.
TABLE 3.
Results by NG-MLVA cluster of 65 Neisseria gonorrhoeae isolates at the Amsterdam STI Outpatient Clinic from January 2008 through September 2015a
| Cluster | No. (%) of isolates |
Yr | No. (%) of patients HIV positive | NG-MAST genogroupb (no. of isolates) | 23S rRNA mutation | ||
|---|---|---|---|---|---|---|---|
| Total | Resistant | Susceptible | |||||
| 1 | 34 | 31 (91.2) | 3 (8.8) | 2009–2012, 2015 | 17 (50.0) | G2992 (15), G5108 (15), G4544 (2), G14375 (2) | C2611T (4 alleles; n = 27), C2611T (3 alleles; n = 2), wild type (n = 5) |
| 2 | 11 | 1 (9.1) | 10 (90.9) | 2010-2011, 2013-2014 | 4 (36.4) | G2400 (8), G5031 (1), G21 (1), G11072 (1) | C2611T (4 alleles; n = 1), wild type (n = 10) |
| 3 | 8 | 2 (25.0) | 6 (75.0) | 2009–2011 | 3 (37.5) | G1407 (6), G3806 (1), G14345 (1) | C2611T (4 alleles; n = 2), wild type (n = 6) |
| 4 | 6 | 5 (83.3) | 1 (16.7) | 2010–2012 | 2 (33.3) | G2992 (3), G5108 (2), G14347 (1) | C2611T (4 alleles; n = 3), C2611T (3 alleles; n = 2), wild type (n = 1) |
| 5 | 6 | 6 (100.0) | 0 (0.0) | 2008 | 0 (0.0) | G359 (6) | C2611T (4 alleles; n = 6) |
HIV, human immunodeficiency virus; NG-MLVA, N. gonorrhoeae multilocus variable-number tandem-repeat analysis; NG-MAST, N. gonorrhoeae multiantigen sequence typing; STI, sexually transmitted infection.
NG-MAST genogroups were assigned to sequence types with at least one identical allele and a difference of ≤4 bp (tbpB) or ≤5 bp (por) for the other allele.
Mosaic penA gene determination.
The previously noted 24 isolates with reduced susceptibility to cefotaxime (n = 12) or ceftriaxone (n = 12) were analyzed for the presence of a penA mosaic gene. This was present in seven isolates. Of these seven isolates, five were azithromycin resistant, and four of these isolates were NG-MAST G1407 and one was G3807.
Characteristics of each NG-MLVA cluster.
Cluster 1 included 34 strains, of which 31 (91.2%) were resistant and 29 had C2611T mutations (Table 3). Two resistant isolates had wild-type 23S rRNA. Isolates from almost the entire study period (2009 to 2012 and 2015) were part of this cluster. Isolates were predominantly from MSM, but they were also from two heterosexual males and four females. Half of the isolates were from HIV-positive patients.
Cluster 2 included 11 isolates, of which 10 (90.9%) were susceptible and had wild-type 23S rRNA; one isolate was resistant and had a C2611T mutation (Table 3). Isolates were from 2010 and 2011 and from 2013 and 2014. All patients in this cluster were MSM, and 36.4% were HIV positive.
Cluster 3 included eight isolates, of which six (75.0%) were susceptible and had wild-type 23S rRNA; two isolates were resistant and had C2611T mutations (Table 3). Isolates were from 2009 to 2011. All patients were MSM, and 37.5% were HIV positive.
Cluster 4 included six isolates, of which five were resistant and had C2611T mutations. One was susceptible with wild-type 23S rRNA (Table 3). Isolates were from 2010 to 2012. The patients included four MSM and one female, and 33.3% were HIV positive.
Cluster 5 included six identical isolates, all resistant, and all had C2611T mutations on 4 alleles (Table 3). All the strains were obtained in 2008 from the rectums of Dutch MSM. Five patients were HIV negative, and the HIV infection status of one was unknown (Fig. 1C).
Correlation of NG-MAST and NG-MLVA typing.
Figure 1D shows a clear overlapping of NG-MLVA clustering and NG-MAST genogroups. Isolates in the most common NG-MAST genogroups, genogroups G2992 and G5108, were significantly more often included in an NG-MLVA cluster (G2992, P = 0.001; G5108, P < 0.001). NG-MLVA clusters 1 and 4 included 18 of the 23 isolates in G2992 (78.3%) and all 17 isolates in G5108 (Table 3). The tbpB sequences of all isolates in clusters 1 and 4 were identical, regardless of NG-MAST genogroup. However, the por sequences of both genogroups differed by more than 20 bp. The remaining five isolates (21.7%) in genogroup G2992 were not included in NG-MLVA cluster 1 or 4. However, three of these differed from isolates in cluster 1 at only two NG-MLVA loci instead of one.
NG-MAST genogroups G2400 (P = 0.4), G359 (P = 0.3), and G1407 (P = 0.5) were not significantly more often included in an NG-MLVA cluster, possibly due to the small sample size. However, in cluster 2, 8 of the 11 isolates (72.7%) were G2400, which was 57.1% of all G2400 strains. In cluster 3, six of the eight isolates (75.0%) were G1407, which was 54.6% of all G1407 strains. Cluster 5 consisted only of G359 strains, and three additional G359 isolates were not in this (or any other) cluster.
mtrR sequence analysis.
We selected 45 isolates for mtrR promoter and gene sequence analysis (Table 4). These 45 isolates included all five azithromycin-resistant isolates with a wild-type 23S rRNA. Two of the five resistant isolates with wild-type 23S rRNA could not be successfully sequenced for mtrR mutations; both were part of NG-MLVA cluster 1 and were NG-MAST genogroup G2992. Of the remaining three isolates, two (both G1407) had a −35A deletion and one (G6327) had both an A39T mutation and a Y105H mutation. None of these three were included in an NG-MLVA cluster. The mtrR promoter or gene mutations relative to the sequence of strain FA1090 (GenBank accession number YP_208426) were seen in all other resistant isolates and all susceptible isolates. Almost all of the isolates in NG-MLVA clusters 2 and 3 had a −35A deletion, while none of those in clusters 1, 4, and 5 did. Those in clusters 1, 2, 4, and 5 had other mtrR mutations, while all in cluster 3 were wild type. Likewise, all isolates with NG-MAST genogroups G1407 and G2400 had a −35A deletion, while this did not occur in any of the other genogroups. In addition, isolates in G1407 and G2992 had no other mtrR mutations, while all isolates in the other genogroups did have mutations.
TABLE 4.
Mutations of the mtrR promoter and the mtrR gene in 43 selected strains, categorized by azithromycin susceptibility, NG-MAST genogroup, and NG-MLVA clustera
| Characteristic | No. of isolates |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | Azithromycin resistant | With mtrR promoter −35A deletion | With mtrR gene mutation(s) |
||||||
| A39T, R49H, Y105H | D79N, T86A | G45D, Y105H | A39T, Y105H | Y105H | WT | ||||
| Azithromycin | |||||||||
| Susceptible | 20 | 0 | 10 | 5 | 6 | 1 | 2 | 2 | 4 |
| Resistant | |||||||||
| WT 23S rRNAb | 3 | 3 | 2 | 0 | 0 | 0 | 1 | 0 | 2 |
| Mutated 23S rRNA | 20 | 20 | 7 | 10 | 1 | 3 | 0 | 0 | 6 |
| NG-MAST genogroupc | |||||||||
| G1407 | 7 | 4 | 7 | 0 | 0 | 0 | 0 | 0 | 7 |
| G2400 | 6 | 1 | 6 | 0 | 6 | 0 | 0 | 0 | 0 |
| G2992b | 8 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 8 |
| G359 | 4 | 3 | 0 | 0 | 0 | 4 | 0 | 0 | 0 |
| G4544 | 2 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 |
| G5108 | 4 | 4 | 0 | 0 | 0 | 0 | 4 | 0 | 0 |
| G6327 | 2 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 0 |
| NG-MLVA cluster | |||||||||
| 1b | 9 | 3 | 0 | 9 | 0 | 0 | 0 | 0 | 0 |
| 2 | 6 | 1 | 5 | 0 | 5 | 0 | 0 | 1 | 0 |
| 3 | 5 | 2 | 5 | 0 | 0 | 0 | 0 | 0 | 5 |
| 4 | 4 | 3 | 0 | 4 | 0 | 0 | 0 | 0 | 0 |
| 5 | 2 | 2 | 0 | 0 | 0 | 2 | 0 | 0 | 0 |
NG-MLVA, N. gonorrhoeae multilocus variable-number tandem-repeat analysis; NG-MAST, N. gonorrhoeae multiantigen sequence typing; WT, wild type.
The mtrR sequences of two additional azithromycin-resistant strains with WT 23S rRNA, NG-MAST genogroup G2992, and NG-MLVA cluster 1 could not be sequenced.
Only genogroups in which at least two strains were sequenced are shown. Genogroups were assigned to sequence types with at least one identical allele and a difference of ≤4 bp (tbpB) or ≤5 bp (por) for the other allele.
DISCUSSION
We describe the molecular epidemiology and 23S rRNA mutations of 69 azithromycin-resistant N. gonorrhoeae isolates and 74 susceptible isolates from Amsterdam, the Netherlands, collected from January 2008 through September 2015. Our data show that 92.8% of resistant strains had a 23S rRNA mutation, which consisted of a C2611T mutation in almost all cases; A2059G mutations occurred infrequently. These results are similar to those of three recent studies analyzing azithromycin-resistant strains using whole-genome sequencing (WGS) (13, 14, 20). While the study by Jacobsson et al. reported 23S rRNA mutations in all azithromycin-resistant strains, we found five resistant strains (7.2%) with a wild-type 23S rRNA (13). This is in marked contrast to the rate of 43.5% reported among strains with reduced susceptibility by Grad et al. (14) and to the rate of 31.8% reported among resistant strains by Demczuk et al. (20). Remarkably, most of these strains, including those from our study, had MICs just above the breakpoint. Given the slight differences in MIC depending on the method used (agar dilution or Etest), it is possible that these low-level-resistant strains were not equally frequently included in the different studies (21, 22). Another explanation could be resistance due to mutations in the mtrR gene (encoding the repressor of the MtrCDE efflux pump) or its promoter region (10, 11, 14–18, 20, 23). When testing the five resistant strains with wild-type 23S rRNA and an additional selection of resistant strains (with 23S rRNA mutations) and susceptible strains, we found no association of azithromycin resistance with specific mtrR mutations. In contrast, the mtrR mutations from our study were in complete agreement with the genetic background of the strains characterized either by NG-MLVA or by NG-MAST. We cannot exclude the possibility that mtrR mutations play a role in the development of low-level resistance, as these stains were not included in this study. It is possible that other, currently unknown, mutations are involved in azithromycin resistance.
Although resistance to ESC was not present among the isolates in our study, reduced susceptibility was noted in 24/143 (17%) of the included isolates, with resistance to ESC being detected at similar frequencies in azithromycin-susceptible and -resistant isolates. It is of concern that resistance to azithromycin due to mutations in the 23S rRNA gene combined with reduced susceptibility to ESC due to the presence of a penA mosaic gene was present in a number of isolates, including those belonging to widely spread NG-MAST genogroup G1407.
A strength of our study was a direct comparison between resistant strains and susceptible control strains. Five NG-MLVA clusters were identified, and three of these consisted predominantly of azithromycin-resistant isolates and two consisted predominantly of susceptible isolates (24, 25). Resistant isolates were included in a cluster significantly more often than susceptible isolates. Despite this clear distribution, four of the five clusters included both resistant and susceptible isolates. Moreover, some of the resistant strains in clusters dominated by susceptible strains were assigned to the same NG-MAST genotype as the susceptible strains, and vice versa. Both the distribution of resistant strains within clusters and susceptible strains outside of clusters and the combination of susceptible and resistant strains within genetic clusters, as determined by WGS-based methods, have previously been reported (13, 14, 20).
We noted a considerable overlap between NG-MLVA clusters and NG-MAST genogroups: the larger clusters (1 and 4) were significantly associated with G2992 and G5108 (P < 0.001 for both). Combined with the C2611T mutations of resistant isolates and the inclusion of both susceptible and resistant strains in NG-MLVA clusters, this implies that susceptible N. gonorrhoeae isolates can accumulate resistance (by C2611T mutations) without significant changes to the background genome. Furthermore, the finding that 34.8% of resistant isolates did not belong to an NG-MLVA cluster suggests that 23S rRNA mutations frequently occur de novo.
The association between azithromycin resistance and NG-MAST genogroups G2992, G5108, and G359 in our study was significant. This was also reported for G2992 in a recent European study (13) but not in other studies (5, 11, 12, 26–28). The association between G5108 or G359 and resistance was not reported before (5, 12, 13, 20, 26–28). Whereas in our study strains in genogroup G2400 were significantly more often susceptible, this was associated with resistance in the European study by Jacobsson et al. (13). However, in the latter study only resistant strains were characterized (13). Genogroup G1407 has been associated with resistance to third-generation cephalosporins (29, 30). The association with azithromycin resistance was reported in some studies (5, 13, 26, 28, 29, 31), but a recent study from France reported an association with susceptibility (12). Although genogroup G1407 was common among our isolates, we found no significant association with either resistance or susceptibility to azithromycin. These differences indicate not only that NG-MAST genotypes differ between geographic regions but also that azithromycin resistance evolves separately from the background genome (13, 32).
The NG-MLVA clusters not only represent genetically related N. gonorrhoeae isolates but also could reflect sexual networks of patients. This is important because four of the five clusters included strains from both HIV-negative and HIV-positive patients, and HIV infection status was not associated with azithromycin resistance. This adds further evidence that both azithromycin resistance and sexual networks do not occur in strict association with HIV infection status (33). As was previously described in Europe and Canada, as determined using whole-genome sequencing (13, 20), most of our clusters included isolates from different calendar years. The largest cluster contained isolates from 2009 through 2015, indicating that azithromycin resistance evolves through time and that the sexual network that was the source of these isolates could still be active. Patients belonging to this sexual network may be at continued risk of acquiring an N. gonorrhoeae infection which is resistant to azithromycin.
There are some limitations of this study. We included only isolates from Amsterdam, the Netherlands, which could limit the generalizability of the findings of this study to other parts of the world, where different types of sexual networks exist. Also, because we selected all available resistant isolates and only a selection of susceptible isolates from a larger population, resistant isolates could be derived from the same sexual network and, thus, could be genetically more related than susceptible isolates. Finally, a policy change at the Amsterdam STI Outpatient Clinic in May 2014 to no longer use culture as the routine method for the diagnosis of gonorrhea could have influenced the selection of isolates in our study before and after May 2014.
In conclusion, the azithromycin resistance of N. gonorrhoeae isolates from Amsterdam was associated with C2611T mutations of 23S rRNA, NG-MAST genogroups G2992, G5108, and G359, and three NG-MLVA clusters, but it was not associated with HIV infection status. Azithromycin resistance was also observed in isolates with wild-type 23S rRNA. Moreover, the NG-MLVA clusters included both resistant and susceptible strains. This suggests that azithromycin resistance develops independently from the background genome. Because azithromycin is the preferred option for the treatment of C. trachomatis infections and urethritis, exposure in patients potentially coinfected with N. gonorrhoeae is high (4, 34, 35). This could induce the accumulation of resistance mutations in susceptible strains and increase the spread of azithromycin resistance within sexual networks. A further increase in the rate of azithromycin resistance will threaten the use of azithromycin as part of dual therapy for gonorrhea.
MATERIALS AND METHODS
Selection of isolates.
N. gonorrhoeae strains for which an azithromycin MIC was available were selected from patients who visited the STI Outpatient Clinic of Amsterdam, the Netherlands, between January 2008 and September 2015. Depending on sexual techniques, patients could be infected at up to four anatomical sites per consultation. To prevent inclusion of the same isolate twice, we included only one isolate (the one with the highest azithromycin MIC) per consultation. If the MICs for isolates from different anatomical sites were equal, we aimed to include isolates from a balanced distribution of anatomical sites and selected isolates from one site using the following order of priority, based on increasing prevalence: (i) pharynx, (ii) cervix or vagina, (iii) rectum, and (iv) urethra. We allowed for multiple inclusions per patient, as long as consultations were more than 6 months apart.
Selection of cases and controls.
We categorized isolates into those that were azithromycin susceptible (MICs, ≤0.25 mg/liter), intermediate (MICs, >0.25 and ≤1.5 mg/liter), or resistant (MICs, ≥2 mg/liter) on the basis of the clinical breakpoints of EUCAST (36) and the Clinical and Laboratory Standards Institute (37). Resistant isolates were included as cases, and susceptible isolates were considered controls. Intermediate isolates were excluded, because azithromycin MICs of about 1 mg/liter can fluctuate and are sometimes difficult to reproduce (13). As both risk behavior and year of infection are associated with resistance, we selected controls using a 1:1 random frequency matching on calendar year of infection and sexual risk group (heterosexual males, men who have sex with men [MSM], or females) (9, 38–40). Clinical and epidemiological data were collected from the electronic patient files. Due to the use of routinely collected samples and data and an anonymous analysis of this retrospective study, ethical clearance or informed consent was not required according to Dutch law.
Antimicrobial susceptibility testing.
Until May 2014, samples for direct culture of N. gonorrhoeae were routinely obtained if patients reported symptoms suggestive of an STI or reported any of the following: being an MSM, being a commercial sex worker, or being notified of an STI by a sex partner. For other patients, a nucleic acid amplification test (NAAT) was performed, and samples for culture were obtained only if NAAT results were positive (9, 19). From May 2014 onwards, NAAT was the routine diagnostic method for all patients. Samples for culture were obtained from symptomatic patients with a positive Gram-stained smear and from patients with a positive NAAT result (9). MICs were routinely determined using Etest according to the manufacturer's instructions (bioMérieux SA, Marcy l'Etoile, France) (41). Azithromycin and cefotaxime MICs were routinely determined during the entire study period, whereas ceftriaxone MIC testing started in 2010.
Preparation of isolates for typing.
Included isolates were collected from −80°C storage, samples were added to 100 μl phosphate-buffered saline, the mixture was heated at 95°C for 15 min to release the DNA, and the DNA was stored at −20°C. These samples were used for all PCRs and sequence typing methods.
23S rRNA sequencing.
23S rRNA was amplified using PCR and sequenced using an ABI 3130 automated sequencer. An allele-specific PCR was performed as described by Ng et al. (10). In a number of strains, we directly sequenced the internal 712-bp fragment using the PCR primers reported by Ng et al., followed by sequencing (10). In cases of a double peak at position 2058, 2059, or 2611, allele-specific PCR amplification was performed to determine the number of alleles with the 23S rRNA mutation. This approach was validated in our laboratory (the Public Health Laboratory in Amsterdam), using strains with a known number of mutated genes determined by the allele-specific PCR (10). Sequence data were analyzed using BioNumerics software (version 7.5; Applied Maths, Sint-Martens-Latem, Belgium).
NG-MAST.
N. gonorrhoeae multiantigen sequence typing (NG-MAST) uses variations in two genes: porB (490 bp) and tbpB (390 bp). These genes were amplified using PCR and sequenced at the sequencing facility of the Academic Medical Center, Amsterdam, the Netherlands (42, 43). Sequence data were analyzed using BioNumerics software and entered into the NG-MAST website (www.ng-mast.net) to assign allele numbers and sequence types.
NG-MLVA.
The N. gonorrhoeae multilocus variable-number tandem-repeat analysis (NG-MLVA) typing technique has been previously described in detail (24, 25). In short, the variable-number tandem repeats (VNTR) of five different loci on the N. gonorrhoeae genome were amplified using two multiplex PCRs. Fragment sizes were measured using an ABI 3130 automated sequence analyzer. The number of repeats was analyzed using GeneMarker software (version 1.8; SoftGenetics, State College, PA). The combination of the number of repeats for all five loci determined the NG-MLVA sequence type.
mtrR sequencing.
The full-length mtrR gene and an additional 120 bp of the promoter region were amplified as described by Liao et al. (44). The mtrR gene was sequenced from both ends using additional primers: Ng-mtrR F (5′-ACC TCG CTC AAC GAA ATC G-3′) and Ng mtrR-R (5′-GTT GGA ACA ACG CGT CAA AC-3′).
Mosaic penA gene detection.
For rapid detection of the mosaic penA gene in N. gonorrhoeae strains, a PCR was developed using forward primer 5′-GGCGGTCGAATACCATCAG-3′, recognizing a conserved region of the penA gene, and reverse primers 5′-TTCTGAACAAACCTGCAGTTTCC-3′ and 5′-GTAAACGGCTTCATGGCA-3′, recognizing specific sequences in the wild-type and mosaic penA genes, respectively. Separate PCRs were performed with the forward and each reverse primer in the presence of Sybr green for 45 cycles at an annealing temperature of 58°C. The presence of the wild-type or mosaic penA gene was assessed by melting curve analysis. The assay has been validated in our laboratory by testing strains with a known sequence of the penA gene.
Statistical analysis.
Baseline characteristics were compared between cases and controls using the χ2 test, Fisher's exact test, or the Kruskal-Wallis test. Mean MICs were calculated as geometric means. NG-MLVA hierarchical cluster analysis was performed to create a minimum spanning tree (MST) using BioNumerics software (24, 25). Isolates were assigned to a cluster if they differed in no more than one VNTR locus and if at least five isolates were included in the cluster. Using NG-MAST sequence types (STs), we assigned genogroups if one allele (por or tbpB) was identical and the other allele differed by ≤4 bp (tbpB) or ≤5 bp (por), as previously described (12, 26, 29). Genogroups were named after the ST with the highest frequency within that genogroup. All analyses were performed using Stata software (version 13; StataCorp, College Station, TX, USA).
Supplementary Material
ACKNOWLEDGMENTS
We thank Priscilla van Doorn, Kawtar Mouajib, and Michelle Himschoot (Public Health Laboratory, Amsterdam, the Netherlands) for their help in performing the molecular typing; Martijn van Rooijen (STI Outpatient Clinic, Amsterdam, the Netherlands) for his help with the patient data; and Irene Martin (Public Health Agency of Canada) for her help in assigning new NG-MAST allele numbers and sequence types.
We have no conflicts of interest to declare.
This study was funded by the Public Health Service of Amsterdam, the Netherlands.
C.M.W., S.M.B., M.F.S.V.D.L., H.J.C.D.V., and A.P.V.D. designed the study. C.M.W. coordinated the study, collected and analyzed the data, and wrote the manuscript. C.M.W. and M.D. performed the laboratory testing and analyzed the results. All authors interpreted the data, critically reviewed the manuscript, and approved the final version.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.02374-16.
REFERENCES
- 1.Newman L, Rowley J, Vander Hoorn S, Wijesooriya NS, Unemo M, Low N, Stevens G, Gottlieb S, Kiarie J, Temmerman M. 2015. Global estimates of the prevalence and incidence of four curable sexually transmitted infections in 2012 based on systematic review and global reporting. PLoS One 10:e0143304. doi: 10.1371/journal.pone.0143304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Unemo M, Shafer WM. 2014. Antimicrobial resistance in Neisseria gonorrhoeae in the 21st century: past, evolution, and future. Clin Microbiol Rev 27:587–613. doi: 10.1128/CMR.00010-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bignell C, Unemo M. 2013. 2012 European guideline on the diagnosis and treatment of gonorrhoea in adults. Int J STD AIDS 24:85–92. doi: 10.1177/0956462412472837. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. 2015. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recommend Rep 64(RR03):1–137. doi: 10.15585/mmwr.rr6404a1. [DOI] [Google Scholar]
- 5.Brunner A, Nemes-Nikodem E, Jeney C, Szabo D, Marschalko M, Karpati S, Ostorhazi E. 2016. Emerging azithromycin-resistance among the Neisseria gonorrhoeae strains isolated in Hungary. Ann Clin Microbiol Antimicrob 15:53. doi: 10.1186/s12941-016-0166-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olsen B, Pham TL, Golparian D, Johansson E, Tran HK, Unemo M. 2013. Antimicrobial susceptibility and genetic characteristics of Neisseria gonorrhoeae isolates from Vietnam, 2011. BMC Infect Dis 13:40. doi: 10.1186/1471-2334-13-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fifer H, Natarajan U, Jones L, Alexander S, Hughes G, Golparian D, Unemo M. 2016. Failure of dual antimicrobial therapy in treatment of gonorrhea. N Engl J Med 374:2504–2506. doi: 10.1056/NEJMc1512757. [DOI] [PubMed] [Google Scholar]
- 8.Chisholm SA, Wilson J, Alexander S, Tripodo F, Al-Shahib A, Schaefer U, Lythgow K, Fifer H. 2016. An outbreak of high-level azithromycin resistant Neisseria gonorrhoeae in England. Sex Transm Infect 92:365–367. doi: 10.1136/sextrans-2015-052312. [DOI] [PubMed] [Google Scholar]
- 9.Wind CM, Schim van der Loeff MF, van Dam AP, de Vries HJC, van der Helm JJ. 2017. Trends in antimicrobial susceptibility for azithromycin and ceftriaxone in Neisseria gonorrhoeae isolates in Amsterdam, The Netherlands, between 2012 and 2015. Euro Surveill 22(1):pii=30431. doi: 10.2807/1560-7917.ES.2017.22.1.30431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ng LK, Martin I, Liu G, Bryden L. 2002. Mutation in 23S rRNA associated with macrolide resistance in Neisseria gonorrhoeae. Antimicrob Agents Chemother 46:3020–3025. doi: 10.1128/AAC.46.9.3020-3025.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chisholm SA, Dave J, Ison CA. 2010. High-level azithromycin resistance occurs in Neisseria gonorrhoeae as a result of a single point mutation in the 23S rRNA genes. Antimicrob Agents Chemother 54:3812–3816. doi: 10.1128/AAC.00309-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Belkacem A, Jacquier H, Goubard A, Mougari F, La Ruche G, Patey O, Micaelo M, Semaille C, Cambau E, Bercot B. 2016. Molecular epidemiology and mechanisms of resistance of azithromycin-resistant Neisseria gonorrhoeae isolated in France during 2013-14. J Antimicrob Chemother 71:2471–2478. doi: 10.1093/jac/dkw182. [DOI] [PubMed] [Google Scholar]
- 13.Jacobsson S, Golparian D, Cole M, Spiteri G, Martin I, Bergheim T, Borrego MJ, Crowley B, Crucitti T, Van Dam AP, Hoffmann S, Jeverica S, Kohl P, Mlynarczyk-Bonikowska B, Pakarna G, Stary A, Stefanelli P, Pavlik P, Tzelepi E, Abad R, Harris SR, Unemo M. 2016. WGS analysis and molecular resistance mechanisms of azithromycin-resistant (MIC >2 mg/liter) Neisseria gonorrhoeae isolates in Europe from 2009 to 2014. J Antimicrob Chemother 71:3109–3116. doi: 10.1093/jac/dkw279. [DOI] [PubMed] [Google Scholar]
- 14.Grad YH, Harris SR, Kirkcaldy RD, Green AG, Marks DS, Bentley SD, Trees D, Lipsitch M. 2016. Genomic epidemiology of gonococcal resistance to extended spectrum cephalosporins, macrolides, and fluoroquinolones in the US, 2000-2013. J Infect Dis 214:1579–1587. doi: 10.1093/infdis/jiw420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pan W, Spratt BG. 1994. Regulation of the permeability of the gonococcal cell envelope by the mtr system. Mol Microbiol 11:769–775. doi: 10.1111/j.1365-2958.1994.tb00354.x. [DOI] [PubMed] [Google Scholar]
- 16.Hagman KE, Pan W, Spratt BG, Balthazar JT, Judd RC, Shafer WM. 1995. Resistance of Neisseria gonorrhoeae to antimicrobial hydrophobic agents is modulated by the mtrRCDE efflux system. Microbiology 141(Pt 3):611–622. [DOI] [PubMed] [Google Scholar]
- 17.Zarantonelli L, Borthagaray G, Lee EH, Shafer WM. 1999. Decreased azithromycin susceptibility of Neisseria gonorrhoeae due to mtrR mutations. Antimicrob Agents Chemother 43:2468–2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zarantonelli L, Borthagaray G, Lee EH, Veal W, Shafer WM. 2001. Decreased susceptibility to azithromycin and erythromycin mediated by a novel mtr(R) promoter mutation in Neisseria gonorrhoeae. J Antimicrob Chemother 47:651–654. doi: 10.1093/jac/47.5.651. [DOI] [PubMed] [Google Scholar]
- 19.Heymans R, Bruisten SM, Golparian D, Unemo M, de Vries HJC, van Dam AP. 2012. Clonally related Neisseria gonorrhoeae isolates with decreased susceptibility to the extended-spectrum cephalosporin cefotaxime in Amsterdam, the Netherlands. Antimicrob Agents Chemother 56:1516–1522. doi: 10.1128/AAC.05481-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Demczuk W, Martin I, Peterson S, Bharat A, Van Domselaar G, Graham M, Lefebvre B, Allen V, Hoang L, Tyrrell G, Horsman G, Wylie J, Haldane D, Archibald C, Wong T, Unemo M, Mulvey MR. 2016. Genomic epidemiology and molecular resistance mechanisms of azithromycin-resistant Neisseria gonorrhoeae in Canada from 1997 to 2014. J Clin Microbiol 54:1304–1313. doi: 10.1128/JCM.03195-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wind CM, de Vries HJ, van Dam AP. 2015. Determination of in vitro synergy for dual antimicrobial therapy against resistant Neisseria gonorrhoeae using Etest and agar dilution. Int J Antimicrob Agents 45:305–308. doi: 10.1016/j.ijantimicag.2014.10.020. [DOI] [PubMed] [Google Scholar]
- 22.Enriquez RP, Goire N, Kundu R, Gatus BJ, Lahra MM. 2016. A comparison of agar dilution with the calibrated dichotomous sensitivity (CDS) and Etest methods for determining the minimum inhibitory concentration of ceftriaxone against Neisseria gonorrhoeae. Diagn Microbiol Infect Dis 86:40–43. doi: 10.1016/j.diagmicrobio.2016.04.016. [DOI] [PubMed] [Google Scholar]
- 23.Allen VG, Seah C, Martin I, Melano RG. 2014. Azithromycin resistance is coevolving with reduced susceptibility to cephalosporins in Neisseria gonorrhoeae in Ontario, Canada. Antimicrob Agents Chemother 58:2528–2534. doi: 10.1128/AAC.02608-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heymans R, Golparian D, Bruisten SM, Schouls LM, Unemo M. 2012. Evaluation of Neisseria gonorrhoeae multiple-locus variable-number tandem-repeat analysis, N. gonorrhoeae multiantigen sequence typing, and full-length porB gene sequence analysis for molecular epidemiological typing. J Clin Microbiol 50:180–183. doi: 10.1128/JCM.05386-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heymans R, Schouls LM, van der Heide HG, van der Loeff MF, Bruisten SM. 2011. Multiple-locus variable-number tandem repeat analysis of Neisseria gonorrhoeae. J Clin Microbiol 49:354–363. doi: 10.1128/JCM.01059-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carannante A, Renna G, Dal Conte I, Ghisetti V, Matteelli A, Prignano G, Impara G, Cusini M, D'Antuono A, Vocale C, Antonetti R, Gaino M, Busetti M, Latino MA, Mencacci A, Bonanno C, Cava MC, Giraldi C, Stefanelli P. 2014. Changing antimicrobial resistance profiles among Neisseria gonorrhoeae isolates in Italy, 2003 to 2012. Antimicrob Agents Chemother 58:5871–5876. doi: 10.1128/AAC.00103-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liang JY, Cao WL, Li XD, Bi C, Yang RD, Liang YH, Li P, Ye XD, Chen XX, Zhang XB. 2016. Azithromycin-resistant Neisseria gonorrhoeae isolates in Guangzhou, China (2009-2013): coevolution with decreased susceptibilities to ceftriaxone and genetic characteristics. BMC Infect Dis 16:152. doi: 10.1186/s12879-016-1469-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shigemura K, Osawa K, Miura M, Tanaka K, Arakawa S, Shirakawa T, Fujisawa M. 2015. Azithromycin resistance and its mechanism in Neisseria gonorrhoeae strains in Hyogo, Japan. Antimicrob Agents Chemother 59:2695–2699. doi: 10.1128/AAC.04320-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chisholm SA, Unemo M, Quaye N, Johansson E, Cole MJ, Ison CA, Van de Laar MJ. 2013. Molecular epidemiological typing within the European Gonococcal Antimicrobial Resistance Surveillance Programme reveals predominance of a multidrug-resistant clone. Euro Surveill 18(3):pii=20358 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20358. [PubMed] [Google Scholar]
- 30.Unemo M, Golparian D, Nicholas R, Ohnishi M, Gallay A, Sednaoui P. 2012. High-level cefixime- and ceftriaxone-resistant Neisseria gonorrhoeae in France: novel penA mosaic allele in a successful international clone causes treatment failure. Antimicrob Agents Chemother 56:1273–1280. doi: 10.1128/AAC.05760-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tanaka M, Furuya R, Irie S, Kanayama A, Kobayashi I. 2015. High prevalence of azithromycin-resistant Neisseria gonorrhoeae isolates with a multidrug resistance phenotype in Fukuoka, Japan. Sex Transm Dis 42:337–341. doi: 10.1097/OLQ.0000000000000279. [DOI] [PubMed] [Google Scholar]
- 32.Mahajan N, Sood S, Singh R, Kapil A, Das BK, Sreenivas V, Kar HK, Sharma VK. 2016. Antimicrobial resistance and Neisseria gonorrhoeae multiantigen sequence typing profile of Neisseria gonorrhoeae in New Delhi, India. Sex Transm Dis 43:506–516. doi: 10.1097/OLQ.0000000000000471. [DOI] [PubMed] [Google Scholar]
- 33.Heymans R, Matser A, Bruisten SM, Heijman T, Geskus RB, Speksnijder AG, Davidovich U, de Vries HJ, Coutinho RA, Schim van der Loeff MF. 2012. Distinct Neisseria gonorrhoeae transmission networks among men who have sex with men in Amsterdam, The Netherlands. J Infect Dis 206:596–605. doi: 10.1093/infdis/jis399. [DOI] [PubMed] [Google Scholar]
- 34.World Health Organization. 2016. WHO guidelines for the treatment of Chlamydia trachomatis. World Health Organization, Geneva, Switzerland. [PubMed] [Google Scholar]
- 35.Horner P, Blee K, O'Mahony C, Muir P, Evans C, Radcliffe K, Clinical Effectiveness Group of the British Association for Sexual Health and HIV. 2016. 2015 UK national guideline on the management of non-gonococcal urethritis. Int J STD AIDS 27:85–96. doi: 10.1177/0956462415586675. [DOI] [PubMed] [Google Scholar]
- 36.European Committee on Antimicrobial Susceptibility Testing. 2016. Breakpoint table for interpretation of MICs and zone diameters, version 6.0. European Committee on Antimicrobial Susceptibility Testing, Stockholm, Sweden. [Google Scholar]
- 37.Clinical and Laboratory Standards Institute. 2012. Performance standards for antimicrobial susceptibility testing; twenty-second informational supplement. Document M100-S22. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 38.European Centre for Disease Prevention and Control. 2015. Gonococcal antimicrobial susceptibility surveillance in Europe, 2013. European Centre for Disease Prevention and Control, Stockholm, Sweden. [Google Scholar]
- 39.Cole MJ, Spiteri G, Jacobsson S, Pitt R, Grigorjev V, Unemo M, Euro GN. 2015. Is the tide turning again for cephalosporin resistance in Neisseria gonorrhoeae in Europe? Results from the 2013 European surveillance. BMC Infect Dis 15:321. doi: 10.1186/s12879-015-1013-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trecker MA, Waldner C, Jolly A, Liao M, Gu W, Dillon JA. 2014. Behavioral and socioeconomic risk factors associated with probable resistance to ceftriaxone and resistance to penicillin and tetracycline in Neisseria gonorrhoeae in Shanghai. PLoS One 9:e89458. doi: 10.1371/journal.pone.0089458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wind CM, de Vries HJC, Schim van der Loeff MF, Unemo M, van Dam AP. 2015. Successful combination of nucleic acid amplification test diagnostics and targeted deferred Neisseria gonorrhoeae culture. J Clin Microbiol 53:1884–1890. doi: 10.1128/JCM.00369-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martin IM, Ison CA, Aanensen DM, Fenton KA, Spratt BG. 2004. Rapid sequence-based identification of gonococcal transmission clusters in a large metropolitan area. J Infect Dis 189:1497–1505. doi: 10.1086/383047. [DOI] [PubMed] [Google Scholar]
- 43.Unemo M, Dillon JA. 2011. Review and international recommendation of methods for typing Neisseria gonorrhoeae isolates and their implications for improved knowledge of gonococcal epidemiology, treatment, and biology. Clin Microbiol Rev 24:447–458. doi: 10.1128/CMR.00040-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liao M, Gu WM, Yang Y, Dillon JA. 2011. Analysis of mutations in multiple loci of Neisseria gonorrhoeae isolates reveals effects of PIB, PBP2 and MtrR on reduced susceptibility to ceftriaxone. J Antimicrob Chemother 66:1016–1023. doi: 10.1093/jac/dkr021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.