Abstract
Obesity is a world-wide problem, and a risk factor for cardiovascular disease, diabetes, cancer and other diseases. It is well established that sex differences influence fat storage. Males and females exhibit differences in anatomical fat distribution, utilization of fat stores, levels of adipose tissue-derived hormones, and obesity co-morbidities. The basis for these sex differences may be parsed into the effects of male vs. female gonadal hormones and the effects of XX vs. XY chromosome complement. Studies employing mouse models that allow the distinction of gonadal from chromosomal effects have revealed that X chromosome dosage influences food intake, which in turn affects adiposity and the occurrence of adverse metabolic conditions such as hyperinsulinemia, hyperlipidemia, and fatty liver. The identification of X chromosome dosage as a player in the behavior and physiology related to obesity suggests novel molecular mechanisms that may underlie sex differences in obesity and metabolism.
Keywords: body weight, sex differences, mouse models
1. Introduction to sex differences in obesity and co-morbidities
Obesity is a worldwide health issue [1]. It is a risk factor for the most prevalent diseases affecting modern populations, including cardiovascular disease, diabetes, and many forms of cancer. The United States has one of the highest incidences of obesity per capita in the entire world, with 37.7% of the population considered obese (body mass index ≥ 30), and 7.7% of the population having class 3 obesity (also known as morbid obesity, body mass index ≥ 40) [2]. Alarmingly, countries that have not traditionally had an obesity problem (including India, China, the Russian Federation, Brazil, Indonesia, Pakistan, and others) now report tens of millions of obese individuals, although the proportion of affected individuals lags significantly behind the U.S (World Obesity Federation, worldobesity.org). Despite differences in sociocultural factors such as diet, physical activity, and urbanization, there is a similarity among developed and developing countries in that the prevalence of obesity is higher in women than men [1].
Many factors likely contribute to increased obesity in women compared to men, including different cultural roles that influence the degree of physical activity, diet, and alcohol consumption. But biological factors most likely play a role as well [3–6]. The propensity for fat storage is different in males and females, which may be related in part to distinct reproductive roles, with gynoid fat serving to provide energy resources during pregnancy and a role in nurturing offspring. Key sex differences in fat storage in men and women include the distribution of fat storage (greater subcutaneous fat in women and visceral fat in men), the ability to mobilize stored fat (more efficient in men), and differential insulin sensitivity and adipokine production (which appear to be higher in women) [4,7–9]. To optimize prevention and treatment strategies for obesity, it is important to understand sex differences in fat storage and their association with the development of conditions such as hyperlipidemia, insulin resistance, and fatty liver. Studies in experimental models that allow control of environmental and genetic variables are valuable for identification of the biological differences between males and females that may influence obesity and related metabolic disease. The ability to manipulate genetic factors, including the genetic determinants of sex, makes the mouse a unique experimental model with which to investigate the causes and consequences of sex differences in fat storage and metabolism.
2. Approaches to dissect gonadal hormone and sex chromosome effects
The differences in physiological processes between males and females originate at the moment of fertilization, through the attainment of the developing embryo of either XX or XY sex chromosomes. This sets the path for the development of male or female differences, determined by a combination of genetic effects (presence of genes on the Y chromosome that influence testis development, and the presence on the X chromosome of dosage-responsive genes) and distinct gonadal secretions from testes and ovaries [10]. Thus, sex differences in metabolism and fat storage in humans and animals are likely influenced by genetic as well as hormonal differences between males and females.
There is undisputed evidence that gonadal hormones play a substantial role in regulating fat storage, affecting processes from food intake to adipocyte differentiation to energy expenditure [9,11–16]. The role of gonadal hormones in obesity in humans are most commonly evaluated by comparing obesity-related phenotypes in women at ages prior to and after the onset of menopause, or in men before and after androgen levels decline with age. These studies have provided evidence that reduced estrogen levels in women (and decreased androgen levels in men) are correlated with increased fat deposition and co-morbidities such as insulin resistance, fatty liver, hyperlipidemia and increased risk of cardiovascular disease [17,18]. However, these studies are not transparent from an experimental viewpoint; typically, the pre- and post-menopausal women in these studies are different individuals, such that genetic and lifestyle differences may also have an impact on factors that are attributed specifically to changes in gonadal hormone levels. In addition, age differs in pre- and post-menopausal women, which affects tissue function and lifestyle, and is likely to independently influence obesity and related phenotypes.
Studies in experimental models allow control of variables such as genetic background, lifestyle, and hormone levels. Comparisons of rats or mice that have been gonadectomized to remove the acute effects of gonadal hormones after adulthood have corroborated the important role of gonadal hormones in the hypothalamic-pituitary axis and effects on energy intake and expenditure [9,11,12]. In the mouse, genetic ablation of genes for the estrogen receptor have further defined the effects of estrogen on feeding behavior, muscle metabolism, glucose homeostasis and other factors that impact fat storage [19]. However, studies that utilize gonadectomy of adult animals to study the effects of gonadal hormones on obesity do not control for the effects of gonads during development, often referred to ‘organizational effects.’ These include the development of male and female secondary sex characteristics and the impact of differential gonadal hormone levels during development, which may include differences in muscle mass and establishment of fat depots. Furthermore, there is evidence that male and female mouse embryos differ in size prior to the development of the gonads, suggesting that another component of sex (i.e., the presence of XX or XY chromosomes) plays an independent role. To cleanly determine the influence of sex on fat storage, the ideal system would vary only one component of sex at a time, to allow comparisons of male and female gonad effects on the same sex chromosome background, and comparisons of XX and XY chromosome complements on the same gonadal background. This is, in fact, possible using genetic tools that are available in the mouse, such as the Four Core Genotypes mouse model.
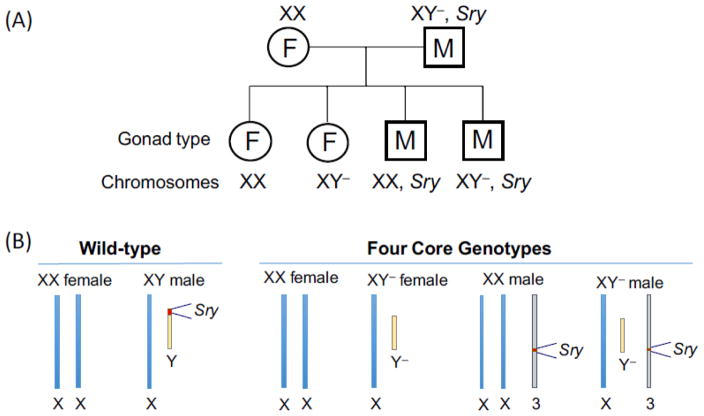
In the Four Core Genotypes (FCG) mouse model there are four genotypes related to sex: animals with an XX chromosome complement and either female (XX F) or male gonads (XX M), and animals with an XY chromosome complement and either female (XY F) or male (XY M) gonads (Fig. 1A) [20,21]. Normally, the Sry gene that is present on the Y chromosome specifies the development of male gonads, whereas the lack of an Sry gene (as in XX individuals) leads to development of female gonads (Fig. 1B, left. Because of this, male gonads are present in individuals carrying an XY chromosome complement, and absent in individuals carrying an XX chromosome complement. To investigate the independent effects of sex chromosomes and gonads requires that the process of gonad determination be separated from the sex chromosome complement. In the FCG mouse model, the Y chromosome does not contain the Sry gene, due to a spontaneous 12-kb deletion, but remains otherwise structurally normal. However, it cannot be formally ruled out that deletion of sequences within the Sry gene do not influence expression of other Y chromosome genes. Offspring that receive this Y chromosome (denoted Y−) will develop female gonads despite the fact that they carry an XY chromosome complement (Fig. 1B, right). To generate mice with male gonads in this model, the Sry gene was introduced as a transgene inserted into an autosome (chromosome 3), which therefore segregates independent of the sex chromosomes. Mice that inherit the Sry transgene will develop male gonads regardless of whether the carry an XX or XY chromosome complement. Furthermore, the presence of he Sry gene on an autosome does not alter prenatal androgen levels [22]. The The FCG model is available on a C57BL/6 inbred background, which is the most widely used strain in studies of obesity and metabolism.
Fig. 1.
Four Core Genotypes mouse model. (A) Breeding scheme to generate offspring with four genotypes: mice with female (F) gonads and either XX or XY chromosome complement, and mice with male (M) gonads and either XX or XY chromosome complement. (B) Schematic view of sex chromosome and Sry gene position in wild-type vs. Four Core Genotype offspring. Y−, Y chromosome lacking Sry gene; Sry gene on chromosome 3 is a transgene.
The FCG model allows the disentanglement of gonadal from chromosomal bases for sex differences in obesity and other traits. By comparing the four genotypes for a trait of interest, it becomes apparent whether differences observed between conventional females (XX with ovaries) and males (XY with testes) are determined by the gonadal type (ovaries vs. testes), the sex chromosome type (XX vs. XY), or an interaction between the two. If a trait segregates based on the presence of female gonads (XXF and XYF mice) vs. male gonads (XXM and XYM mice) regardless of the sex chromosome complement, the difference is likely due to the gonad type (Fig. 2A). On the other hand, if the trait segregates based on the presence of XX or XY chromosomes regardless of gonad type, it is determined by sex chromosome complement (Fig. 2B). The data from studies of these four genotypes are best analyzed by two-way ANOVA using sex chromosome and gonadal type as the two variables (see graphs in Fig. 2). If differences are found to be due to effects of male vs. female gonads (as in Fig. 2A), the effects can be explored further by comparing mice that have intact gonads vs. those with gonads removed as adults. If differences between animals with ovaries and testes disappear following gonadectomy, they are likely due to acute hormonal effects; if they are not influenced by gonadectomy as adults, they may be due to the irreversible effects that gonadal hormones have during development and maturation.
Fig. 2.
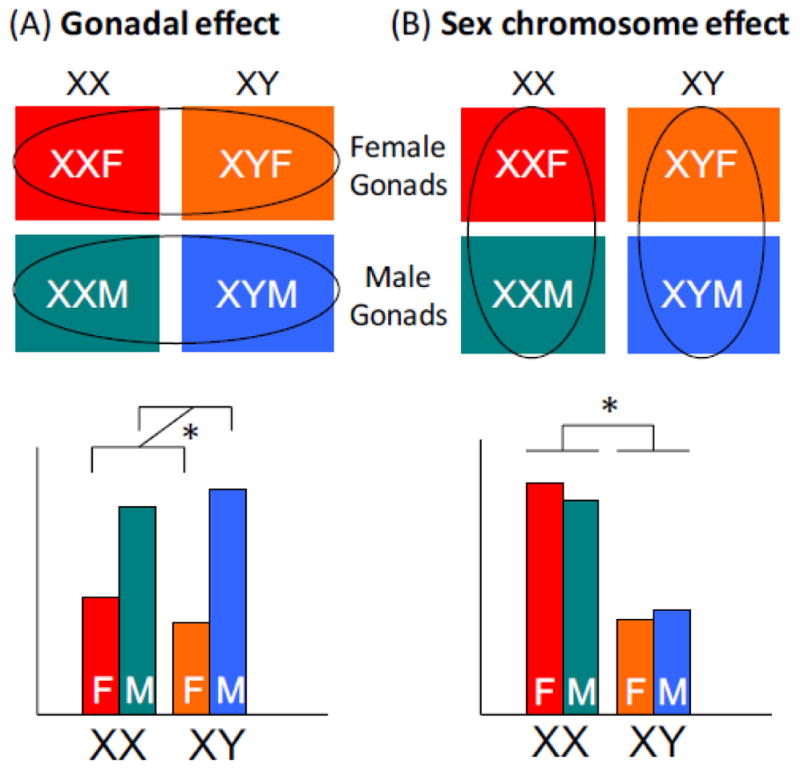
Interpretation of results from Four Core Genotypes mouse model. (A) Gonadal effects are detected as differences that are associated with male vs. female gonads. These are evident if XX and XY mice with female gonads differ from XX and XY mice with male gonads. An example of such a trait is shown in bar graph in the lower portion of the panel. (B) Sex chromosome effects are detected as differences that are association with XX vs. XY genotype. These are apparent if XX mice with female or male gonads differ from XY mice with female or male gonads. An example of such a trait is shown in bar graph in the lower portion of the panel. Gonadal and sex chromosome effects are analyzed by two-way ANOVA.
It should be noted that the FCG model (and other experimental models) do not address the potential role of gender differences in behavior that could influence obesity. The terms ‘sex’ and ‘gender’ are sometimes (incorrectly) used interchangeably in the literature, but they are not the same. ‘Sex’ refers to the genetic and physiological distinctions between males and females (i.e., presence of testes or ovaries), whereas ‘gender’ is refers to the social attributes that are associated with being masculine or feminine that are the norms in a particular society or culture [23]. Gender differences in humans that may influence obesity include differences in the accepted norms for masculine or feminine individuals in terms of food intake, physical exertion, etc. These gender-related behavioral differences may also exist in experimental models, but it has not been feasible to address them, and the studies described herein have focused on physiologically determined sex differences.
3. XX sex chromosome complement is a risk factor for obesity
3.1 Increased body fat and accelerated weight gain in XX vs. XY mice
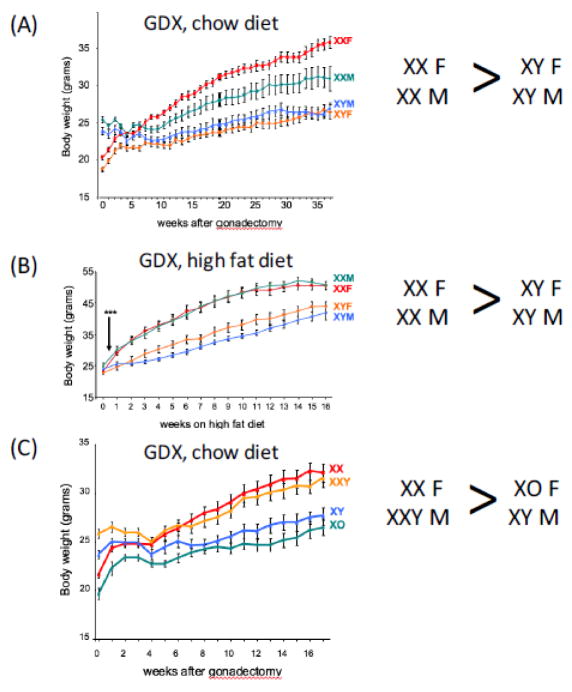
The C57BL/6 FCG mouse model was used to investigate the mechanisms underlying sex differences in adiposity, and to distinguish the contributions of gonadal hormones vs. sex chromosomes. At three weeks of age (at the time of weaning), body weight was similar in XX F, XX M, XY F and XY M mice [24]. By six weeks of age, the body weight diverged among genotypes, revealing a pattern that is familiar for many mammals, with animals having male gonads weighing ~25% more than those with female gonads. Interestingly, however, gonadal type was not the only determinant of body weight, as XX mice (with male or female gonads) weighed 3% more than XY mice (with female or male gonads). These observations suggested that gonadal hormones play a significant role in determining body weight in maturing mice, but that chromosome complement is also a determinant. To further investigate the role of acute gonadal hormones and sex chromosomes specifically on adiposity, gonads were removed from the four genotypes after mice at 8 weeks of age (after mice reached sexual maturity), and body weight was tracked for several months while mice continued to be fed a standard chow diet. Prior to gonadectomy, male mice with XX or XY chromosomes weighed more than female mice, but 4 weeks after removing gonads, the weight of all four groups of mice converged (Fig. 3A) [24]. As mice aged further, a new pattern of body weight was established, with the two groups of XX mice gaining more weight than the two groups of XY mice (Fig. 3A). Among the XX mice, those that originally had ovaries weighed more than those that originally had testes. A determination of body composition in mice at 36 weeks after gonadectomy showed that the higher body weight in XX vs. XY mice was associated with increased fat mass, with more than 25% body fat in XX mice compared to 18% body fat in XY mice [24]. The increased fat mass in XX mice was reflected in 2- to 3-fold higher levels of plasma leptin, a hormone secreted from adipose tissue [24].
Fig. 3.
Weight gain is influenced by X chromosome dosage. (A) Increased body weight in XX vs. XY mice fed a chow diet, regardless of male or female original gonadal type. Mice gonadectomized (GDX) as adults (time 0) were maintained on a chow diet with weekly body weight determinations. Body weights were significantly higher in XX compared to XY mice beginning at week 7 (p < 0.000005). (B) Accelerated diet-induced obesity in XX vs. XY mice. Mice were GDX as adults and started on a high fat/high carbohydrate diet 4 weeks later, when body weights of all genotypes converged (Time 0 on graph). Body weights were significantly higher in XX compared to XY mice beginning at 3 days after the start of the diet (***, p < 0.001). (C) The presence of two X chromosomes dictates higher body weight, regardless of the Y chromosome. Mice from the XY* model were GDX as adults and body weight evaluated on a chow diet. After GDX (Time 0), all body weights converged, followed by increased weight gain in mice with two X chromosomes (XX and XXY) compared to those with a single X chromosome (XO and XY); p < 0.000001). Data from [24].
Obesity in humans is typically influenced by environmental factors, most notably excess caloric intake with inadequate increases in energy expenditure. To determine whether sex chromosome effects on adiposity are still detectable when a pro-obesity environmental factor is present, the FCG mice were fed a high fat/high carbohydrate diet. The diet was administered beginning 4 weeks after gonadectomy, at the point when all genotypes had similar body weight. The diet caused accelerated weight gain in all genotypes compared to mice fed a chow diet. But XX mice gained weight more rapidly and to a greater extent than XY mice, regardless of original gonadal type; this effect was evident within 3 days of beginning the high fat diet (Fig 3B). The XX mice also had substantially higher body fat content [24]. Thus, in the absence of acute-acting gonadal hormones, XX mice have higher body weight, increased body fat, and accelerated weight and fat gain on a high fat diet. To gain a better understanding of the role of sex chromosomes in determining response to diet-induced obesity in the presence of gonadal hormones, a useful experiment for the future would be to examine the effect of a high fat diet on obesity in gonadally intact FCG mice.
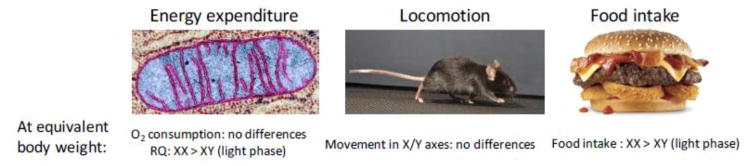
Possible contributors to increased obesity in XX mice include altered energy expenditure, physical activity, and/or food intake (Fig. 4). Assessment of energy expenditure in FCG mice using indirect calorimetry at 4 weeks after gonadectomy (when body weights were similar among all four genotypes) showed no measurable differences in energy expenditure among the four genotypes during either the dark (active) or light (inactive) phases of the circadian cycle [24]. XX mice did exhibit slightly higher respiratory quotient than XY mice during the light phase, indicating somewhat greater utilization of carbohydrates over fats for energy metabolism [24]. Physical activity, assessed both in the horizontal and vertical planes, did not differ among the four genotypes [24]. The major effect of chromosome complement on energy balance was on food intake. Continual monitoring of FCG mouse food intake for several days [24] to weeks [25] revealed that 4 weeks after gonadectomy, female mice tend to eat more than male mice during the dark phase, but that during the light phase XX mice eat more than XY mice, regardless of the original gonad type. The difference between female and male food intake at 4 weeks post- gonadectomy may be attributable to lingering effects of gonadal hormones, as this effect was not apparent at 10 months after gonadectomy [24]. Interestingly, the increased food intake in XX compared to XY mice was associated with a shift in feeding rhythm, with early onset of feeding at the end of the light phase in anticipation of normal feeding time at the beginning of the dark phase [25]. There is evidence that altered circadian cycle or increased feeding during the light phase of the circadian cycle leads to increased weight gain in mice [26–28]. These observations raise the possibility that sex chromosome complement, perhaps in conjunction with gonadal hormones, is important for regulation of the circadian clock.
Fig. 4.
Physiological mechanism for enhanced weight gain in XX compared to XY mice. Four Core Genotypes mice were analyzed for energy balance using indirect calorimetry, activity monitoring (horizontal and vertical planes), and food intake monitoring [24,25]. Results revealed that the primary difference in energy balance between XX and XY mice irrespective of original gonadal type was increased food intake in XX mice during the light phase of the circadian cycle. XX mice also had higher respiratory quotient (RQ) during the light phase, likely reflecting their food intake. Data summarized from [24,25].
3.2 Adiposity is influenced by presence of a second X chromosome, but not by the Y chromosome
The results obtained with the FCG model showed that XX mice have greater body weight than XY mice, regardless of whether mice originally had male or female gonads. The greater adiposity in XX mice could be associated with the presence of two X chromosomes, or the absence of a Y chromosome. To distinguish these possibilities, Chen et al. [24] utilized an experimental mouse model that allows manipulation of sex chromosome combinations to generate mice with genotypes nearly equivalent to XX, XY, XO and XXY. The model is known as XY*, and utilizes a genetic variant of the Y chromosome that carries the Sry gene, but contains a duplication in the pseudoautosomal region, the small region of the Y chromosome that pairs with the X chromosome during meiosis [29,30]. This duplication causes unusual pairing events with the X chromosome. Resulting offspring allow comparisons between mice with one or two X chromosomes (XX and XO vs. XY and XXY), and between mice with and without a Y chromosome (XO and XY vs. XX and XXY) [30]. A study of body weight and composition in the XY* progeny following gonadectomy to normalize the genotypes for acute hormonal affects showed that mice with two X chromosomes (XX and XXY) gained more weight and body fat than mice with a single X (XY and XO) (Fig. 3C) [24]. The presence of a Y chromosome had no effect on these traits.
The data obtained with XY* mice established that the dosage of the X chromosome is responsible for greater fat storage in XX vs. XY mice. The demonstration of enhanced adiposity in mice with two X chromosomes in the XY* model is critical for understanding the underlying mechanism. The effects of X chromosome dosage in this model are independent of the Sry transgene or the Y− chromosome that are present in FCG mice, ruling out potential unknown effects of these components. On the other hand, components that are specific to the X chromosome—particularly the dose of the X chromosome—become strong candidates for the mechanism. This is discussed further in section 5.
Prior to the studies described here, the contribution of sex chromosome complement to sex differences in adiposity was not appreciated. There are many additional questions to answer. The effects of sex chromosome complement on body weight are more obvious in mice that have been gonadectomized than in intact mice. This suggests that gonadal hormones and sex chromosomes interact, leading to a composite effect that is observed in gonadally intact animals. It is possible that some effects of hormones and sex chromosome genes are in opposite directions, such that in composite, they reduce the differences between sexes. This hypothesis has been raised in the literature previously [10] and will be important to test experimentally.
3.3 Increased adiposity in the XXY mouse—a model for Klinefelter syndrome
Sex chromosome anomalies occur in humans, with the most common being Klinefelter syndrome (XXY). XXY occurs in ~1/600 live births, although it may not be diagnosed in some individuals due to mild symptoms. Typical characteristics include small testes and low androgen levels, infertility, tall stature, and learning delays, but these vary among affected individuals [31]. Based on results from mouse studies showing that presence of two X chromosomes promotes fat storage more than a single X chromosome, the prediction would be that XXY men would have greater fat storage than XY men. Indeed, several studies have revealed increased body fat mass, particularly truncal and abdominal fat, in XXY men or boys compared to XY normal individuals or XY individuals with hypogonadism [32–37]. However, in human studies it is difficult to tease apart the contribution of the reduced androgen levels from the XXY sex chromosome complement as drivers of increased adiposity. However, studies of XXY boys before puberty revealed that there is already an increase in body fat over XY males, suggesting a genetic contribution even in the absence of major differences in androgen levels that occur following puberty [33,35].
A mouse model of Klinefelter syndrome has been used to address the influence of the XXY genotype on adiposity. The Sex Chromosome Trisomy model allows the generation of XX, XY, XXY and XYY mice with either testes or ovaries [38]. This model allowed the investigation of whether XXY chromosome complement itself causes increased adiposity over XY males, without the confounding effect of altered gonadal hormone levels. XY and XXY mice, Fat mass was assessed in XY and XXY mice under conditions of native hormone levels as well as in mice that had been gonadectomized to remove circulating gonadal hormones and then administered testosterone by implanted pellet to achieve similar levels in all genotypes. Animals analyzed with or without normalized testosterone levels showed higher body fat content in XXY than XY mice [38]. Variation in the Y chromosome copy number (XY vs. XYY) had no effect on body fat. This study corroborates the findings in multiple studies that Klinefelter men have increased adiposity, and demonstrates that this is related to X chromosome number and not dependent on the differential gonadal hormone levels that occur in Klinefelter men.
Another sex chromosome anomaly that occurs in humans is XO, Turner syndrome. Turner syndrome occurs in ~1/2500 births, but occurs even more frequently in total conceptions, many of which do not survive to term. Turner women are infertile due to impaired ovarian function and have altered levels of gonadal hormones compared to XX women, including estrogens and androgens [39]. XO mice appear not to have as many detrimental effects as XO women, most notably remaining fertile on many genetic backgrounds, with similar post-natal androgen levels to wild-type XX mice [40]. This difference between XO mice and humans precludes the use of XO mice as a model of Turner syndrome, but increases their value in studies of chromosome copy number effects since there is no confounding effect of hormone levels.
4. XX chromosome dosage and obesity co-morbidities
4.1 Increased fatty liver in XX vs. XY mice
As described above, the presence of two X chromosomes confers increased susceptibility to obesity in the mouse, most likely through effects on food intake. Obesity is of medical interest primarily because of its association with co-morbidities such as insulin resistance, hyperlipidemia and fatty liver, which in turn are linked to diseases such as type 2 diabetes mellitus and cardiovascular disease. Sex has been established as a risk factor for these conditions, but the mechanisms explored have consisted almost exclusively of gonadal hormone levels (reviewed in [41]). It was therefore of interest to determine whether XX chromosome complement is a risk factor for co-morbidities of obesity.
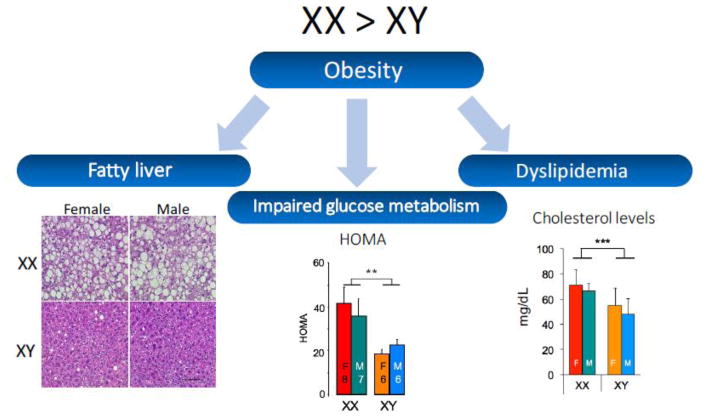
C57BL/6 mice fed a standard chow diet, which contains minimal fat and cholesterol, does not typically induce metabolic impairment. Studies were therefore performed by feeding FCG mice a high fat/high carbohydrate diet following gonadectomy [24]. All four genotypes became obese when fed this diet, gaining at least 60% of additional body weight from values on a chow diet. However, XX mice exhibited a striking difference in metabolic homeostasis, some of which is likely secondary to the greater adiposity in XX compared to XY mice (Fig. 5). XX mice had dramatically increased fat accumulation in the liver, which was comprised primarily of triglycerides [24]. This was paralleled by increased hepatic expression of lipid biosynthesis genes in XX compared to XY mice, although fatty acid oxidation genes (which would be expected to reduce fat storage) were also increased in XX compared to XY liver.
Fig. 5.
XX mice have enhanced development of obesity co-morbidities compared to XY mice. Four Core Genotypes mice that had been fed a high fat/high carbohydrate diet for 16 weeks were assessed for lipid accumulation in the liver, glucose-insulin homeostasis, and circulating lipid levels. Compared to XY mice, XX mice had: greater fatty liver as detected by staining liver sections with hematoxylin & eosin (lipids appear as colorless droplets); higher levels of insulin, leading to higher calculated HOMA value (homeostatic model assessment), and higher total cholesterol levels. Data from [24].
4.2 Increased insulin levels in XX vs. XY mice
XX chromosome complement also appeared to influence glucose-insulin homeostasis in response to a high fat/high carbohydrate diet. After 16 weeks of high fat feeding, all four genotypes maintained similar fasting glucose levels, but insulin levels were nearly double in XX compared to XY mice [24]. The HOMA index (homeostatic model assessment for insulin resistance, an evaluation of insulin resistance that is calculated from glucose and insulin levels) was also nearly twice as high in XX compared to XY mice (Fig. 5). These results suggest that after 16 weeks of a high fat diet, XX mice compensate for insulin resistance by producing more insulin in order to maintain normal glucose levels. It is possible that after additional time on a high fat diet, XX mice may be more susceptible than XY mice to impaired compensation, which could ultimately contribute to the development of type 2 diabetes. It would be interesting to perform long-term high fat feeding and hyperinsulinemic-euglycemic clamp studies in FCG mice to determine which tissues contribute to insulin resistance and to establish whether XX is a risk factor for the progression from insulin resistance to type 2 diabetes. These results have parallels in humans, as compared to XY men, XXY men have increased prevalence of insulin resistance, type 2 diabetes and metabolic syndrome [32,35,36,42].
4.3 X chromosome complement influences plasma lipid profile
Plasma lipoprotein levels are a common clinical predictor for cardiovascular disease risk, with high levels of cholesterol and triglycerides each considered to be potential risk factors. In very broad terms, pre-menopausal women are likely to have a lipoprotein profile that is associated with protection against cardiovascular disease (higher concentrations of large high density lipoprotein (HDL) particles), whereas men are more likely to have a profile that is associated with cardiovascular disease risk (including small HDL particles, higher concentrations of small low density lipoprotein (LDL) particles, and increased levels of very low density lipoproteins) [43]. After menopause, lipid levels in women tend to change to resemble those in men suggesting a key effect of gonadal hormones on lipid profiles [44,45]. However, careful analyses of the effects of exogenous estrogen or androgen administration indicates that changes occurring in response to these hormones account for only part of the differences observed in lipid levels between men and women [46]. This raises the possibility that sex chromosome complement may be an additional contributor to sex differences in lipid levels.
Some insight into the roles of gonadal hormones and sex chromosome complement on determining circulating lipid levels has been provided by studies of FCG mice in both gonad-intact and gonadectomized states. In studies of intact FCG mice fed a chow diet, effects of both gonadal sex and chromosomal sex were observed. Total plasma cholesterol levels and triglyceride levels were higher in male than female mice, and cholesterol levels were also higher in XX compared to XY mice [47]. After gonadectomy, the difference between mice with male and female gonads was abolished, and total cholesterol levels remained higher in XX compared to XY mice (Fig. 5). Stressing gonadally intact mice with a cholesterol-enriched diet raised total cholesterol levels in all mice and there was no difference among the four genotypes, whereas triglyceride levels were higher in XY compared to XX mice [47]. The cholesterol-enriched diet in gonadectomized mice led to the emergence of higher total cholesterol levels in mice with male vs. female gonads and higher triglyceride levels in male mice that also carried an XY chromosome complement. XX chromosome complement was associated with elevated HDL cholesterol levels in all diet treatment groups and gonadal states. Levels of proteins carried on HDL also showed higher levels in XX compared to XY mice.
Using offspring from XY* mouse crosses, it was further demonstrated that the presence of two X chromosomes was the determinant of differences in lipid levels between XX and XY mice [47]. This was analogous to findings regarding sex chromosome effect on adiposity, and neither case showed an effect of the Y chromosome. An analysis of hepatic gene expression for processes such as cholesterol synthesis, cholesterol transport, and bile acid synthesis identified several differences related to gonadal sex and sex chromosome complement, which could contribute to, but not fully explain, some of the observed sex differences in lipid levels. Thus, XX genotype is one key factor in determining sex differences in plasma lipid levels.
5. Potential mechanisms for the effects of X chromosome dosage on obesity and related traits
Data presented in the preceding sections and summarized in Table 1 provide evidence for the X chromosome dosage as a determinant of body weight, fat storage and food intake. Several cardio-metabolic processes have also been shown to be influenced by sex chromosome complement, including stroke sensitivity with aging, cardiac ischemia/reperfusion injury, aortic aneurisms, hypertension, body fluid homeostasis and blood pressure regulation [48–53]. Multiple mechanisms could potentially contribute. The X chromosome is special in that the copy number normally differs between males and females and some genes on the X chromosome are dosage-sensitive, such that phenotype may differ with one vs. two doses [20,54]. The difference between XX and XY cells is suppressed by the transcriptional inactivation of one copy of the X chromosome in every cell of an XX individual. This normalizes the expression level between XX and XY cells for the majority of X chromosome genes. However, higher expression levels of selected X chromosome genes may nevertheless occur in XX cells due to the escape of specific genes from X chromosome inactivation [55–58]. In mice, about 3–6% of genes escape X chromosome inactivation, and in humans ~15% may escape [55,56,59]. Those genes that escape in mice are also known escapees in humans.
Table 1.
Summary of X chromosome effects on adiposity in mouse and human models.
| Model | This model tests: | Comparisons | Relative adiposity |
|---|---|---|---|
| FCG | Is XX different from XY? | XXF vs. XYF XXM vs. XYM |
XXF > XYF XXM > XYM |
| XY* | Is difference caused by number of X or Y? | XX vs. XO XY vs. XXY XX vs. XXY |
2 X (XX, XXY) > 1 X (XO, XY) |
| Sex Chrom Trisomy | Is difference caused by number of X or Y? | XY vs. XXY XY vs. XYY |
2 X (XXY) > 1 X (XY) no effect of Y chrom number |
| Human Klinefelter syndrome (XXY) | Does X chrom dosage affect adiposity in humans? | XXY vs. XY | XXY > XY |
A second mechanism by which two vs. one X chromosome could affect phenotype is by parental imprinting on the X chromosome, such that some genes are expressed only when inherited from the mother or the father [54]. In XY cells, all X chromosomes are of maternal origin and are not subject to paternal imprinting that occurs in XX cells, which could lead to differences in gene expression levels between XX and XY cells. As a third potential mechanism, the presence of an inactive, heterochromatic X chromosome exclusively in XX cells due to X inactivation could conceivably produce an ‘epigenetic sink,’ reducing the concentration of epigenetic regulators for action on autosomes of XX compared to XY cells [60].
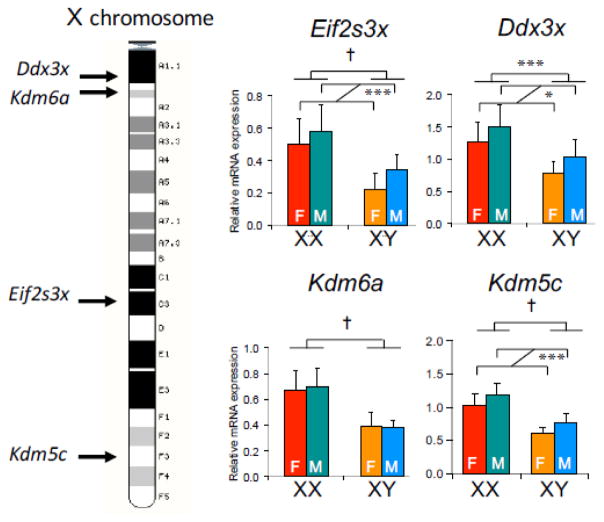
Future studies must focus on evaluating the hypotheses outlined above to determine the mechanism(s) by which X chromosome dosage influences feeding behavior, adiposity, and related disease development. The possibility that specific genes that escape X chromosome inactivation and therefore have expression levels that increase with the number of X chromosomes is attractive. As an initial study, ten genes that were shown to escape X inactivation in mouse fibroblasts and testes were examined for expression levels in FCG mouse metabolic tissues that could influence fat storage, including adipose tissues, liver, and hypothalamus. Six of these genes showed significantly higher expression levels in XX compared to XY mice in the presence of both male and female gonads [24,47,61]. In particular, four of these genes showed at least 40% higher gene expression in XX vs. XY tissues (Fig. 6), and these differences were robust on different diets [47]. These genes represent interesting candidates, as they encode proteins that function in gene expression, histone methylation at sites that determine promoter/enhancer activity, and protein translation. As such, the levels of these X escapee gene products could have wide-ranging impact on cellular function. The involvement of these candidates in determination of adiposity can be tested by generating XX mice that have ablation of one copy of these genes (individually or in combination) and comparing them with wild-type XX mice that have both gene copies intact. The identification of specific X chromosome gene(s) that influence adiposity could reveal downstream genes that bring about effects on obesity and other phenotypes and provide novel mechanistic insight into sex differences in obesity and other traits.
Fig. 6.
Elevated expression in XX mouse tissues of genes that escape X chromosome inactivation. Left, X chromosome diagram showing position of some genes that escape X chromosome inactivation. Right, Hepatic gene expression levels for X escapee genes in Four Core Genotypes mice that had been gonadectomized and fed a chow diet. All genes exhibit significantly higher expression levels in tissue from XX compared to XY mice; some genes also exhibit effects based on long-lasting effects from the original male or female gonads. Data were analyzed by two-way ANOVA. **, p < 0.01; †, p <0.0001.
6. Summary and future perspectives
As outlined here, sex chromosome complement influences food intake, fat accumulation and obesity-related conditions such as fatty liver, hyperinsulinemia and hyperlipidemia. These findings expand the more prevalent view that differences in fat accumulation and metabolism in males and females are determined by differences in gonadal hormones. It has been difficult to distinguish effects of gonads and sex chromosome complement in traditional experimental models or in human studies, but models such as the Four Core Genotypes, XY*, and Sex Chromosome Trisomy models allow a more complete picture to emerge. Most likely, there are interactions between gonadal and genetic components that ultimately lead to the key differences between males and females, and both components should be taken into account.
Recent recommendations by the National Institutes of Heath call for greater inclusion of both sexes in both pre-clinical and clinical research [62]. Given that studies of both sexes may incur greater cost and time, there has been some backlash to this policy. But it has also been pointed out that the cost of failing to study both sexes may be greater—leading to incomplete understanding of fundamental biology and diseases processes, and potential misdiagnosis and suboptimal disease treatments (particularly for females, due to inadequate representation in pre-clinical and clinical studies) [63–66]. To take this one step further, the study of both sexes—and consideration of novel mechanisms such as the role of X chromosome gene dosage—may lead to new discoveries about the regulation of basic processes such as regulation food intake and fat accumulation.
Highlights.
Mouse models allow dissection of sex into gonadal and sex chromosomal components
Sex differences in obesity are influenced by gonadal hormones and sex chromosomes
The dosage of the X chromosome is correlated with increased body weight and adiposity
0X chromosome genes that escape inactivation may contribute to sex differences
Acknowledgments
This work was supported by the Public Health Service (R01 DK083561 and R56 DK083561). I acknowledge the contributions of colleagues who have collaborated in the area of sex differences in obesity for the past several years: Arthur P. Arnold, Jenny C. Link, and Xuqi Chen.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, Mullany EC, Biryukov S, Abbafati C, Abera SF, Abraham JP, Abu-Rmeileh NME, Achoki T, AlBuhairan FS, Alemu ZA, Alfonso R, Ali MK, Ali R, Guzman NA, Ammar W, Anwari P, Banerjee A, Barquera S, Basu S, Bennett DA, Bhutta Z, Blore J, Cabral N, Nonato IC, Chang J-C, Chowdhury R, Courville KJ, Criqui MH, Cundiff DK, Dabhadkar KC, Dandona L, Davis A, Dayama A, Dharmaratne SD, Ding EL, Durrani AM, Esteghamati A, Farzadfar F, Fay DFJ, Feigin VL, Flaxman A, Forouzanfar MH, Goto A, Green MA, Gupta R, Hafezi-Nejad N, Hankey GJ, Harewood HC, Havmoeller R, Hay S, Hernandez L, Husseini A, Idrisov BT, Ikeda N, Islami F, Jahangir E, Jassal SK, Jee SH, Jeffreys M, Jonas JB, Kabagambe EK, Khalifa SEAH, Kengne AP, Khader YS, Khang Y-H, Kim D, Kimokoti RW, Kinge JM, Kokubo Y, Kosen S, Kwan G, Lai T, Leinsalu M, Li Y, Liang X, Liu S, Logroscino G, Lotufo PA, Lu Y, Ma J, Mainoo NK, Mensah GA, Merriman TR, Mokdad AH, Moschandreas J, Naghavi M, Naheed A, Nand D, Narayan KMV, Nelson EL, Neuhouser ML, Nisar MI, Ohkubo T, Oti SO, Pedroza A, Prabhakaran D, Roy N, Sampson U, Seo H, Sepanlou SG, Shibuya K, Shiri R, Shiue I, Singh GM, Singh JA, Skirbekk V, Stapelberg NJC, Sturua L, Sykes BL, Tobias M, Tran BX, Trasande L, Toyoshima H, van de Vijver S, Vasankari TJ, Veerman JL, Velasquez-Melendez G, Vlassov VV, Vollset SE, Vos T, Wang C, Wang X, Weiderpass E, Werdecker A, Wright JL, Yang YC, Yatsuya H, Yoon J, Yoon S-J, Zhao Y, Zhou M, Zhu S, Lopez AD, Murray CJL, Gakidou E. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet (London, England) 2014;384:766–81. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in Obesity Among Adults in the United States, 2005 to 2014. JAMA. 2016;315:2284–91. doi: 10.1001/jama.2016.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fried SK, Lee M-J, Karastergiou K. Shaping fat distribution: New insights into the molecular determinants of depot- and sex-dependent adipose biology. Obesity (Silver Spring) 2015;23:1345–52. doi: 10.1002/oby.21133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mauvais-Jarvis F. Sex differences in metabolic homeostasis, diabetes, and obesity. Biol Sex Differ. 2015;6:14. doi: 10.1186/s13293-015-0033-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Link JC, Chen X, Arnold AP, Reue K. Metabolic impact of sex chromosomes. Adipocyte. 2013;2:74–9. doi: 10.4161/adip.23320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peterfy M, Phan J, Reue K. Alternatively Spliced Lipin Isoforms Exhibit Distinct Expression Pattern, Subcellular Localization, and Role in Adipogenesis. J Biol Chem. 2005;280:32883–32889. doi: 10.1074/jbc.M503885200. [DOI] [PubMed] [Google Scholar]
- 7.Karastergiou K, Smith SR, Greenberg AS, Fried SK. Sex differences in human adipose tissues - the biology of pear shape. Biol Sex Differ. 2012;3:13. doi: 10.1186/2042-6410-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palmer BF, Clegg DJ. The sexual dimorphism of obesity. Mol Cell Endocrinol. 2015;402:113–119. doi: 10.1016/j.mce.2014.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mauvais-Jarvis F, Clegg DJ, Hevener AL. The Role of Estrogens in Control of Energy Balance and Glucose Homeostasis. Endocr Rev. 2013;34:309–338. doi: 10.1210/er.2012-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arnold AP. A general theory of sexual differentiation. J Neurosci Res. 2017;95:291–300. doi: 10.1002/jnr.23884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Asarian L, Geary N. Sex differences in the physiology of eating. AJP Regul Integr Comp Physiol. 2013;305:R1215–R1267. doi: 10.1152/ajpregu.00446.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown LM, Clegg DJ. Central effects of estradiol in the regulation of food intake, body weight, and adiposity. J Steroid Biochem Mol Biol. 2010;122:65–73. doi: 10.1016/j.jsbmb.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lovejoy JC, Sainsbury A Stock Conference 2008 Working Group. Sex differences in obesity and the regulation of energy homeostasis. Obes Rev. 2009;10:154–167. doi: 10.1111/j.1467-789X.2008.00529.x. [DOI] [PubMed] [Google Scholar]
- 14.Law J, Bloor I, Budge H, Symonds ME. The influence of sex steroids on adipose tissue growth and function. Horm Mol Biol Clin Investig. 2014;19:13–24. doi: 10.1515/hmbci-2014-0015. [DOI] [PubMed] [Google Scholar]
- 15.Michalakis K, Mintziori G, Kaprara A, Tarlatzis BC, Goulis DG. The complex interaction between obesity, metabolic syndrome and reproductive axis: A narrative review. Metabolism. 2013;62:457–478. doi: 10.1016/j.metabol.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 16.Quarta C, Mazza R, Pasquali R, Pagotto U. Role of sex hormones in modulation of brown adipose tissue activity. J Mol Endocrinol. 2012;49:R1–R7. doi: 10.1530/JME-12-0043. [DOI] [PubMed] [Google Scholar]
- 17.Escobar-Morreale HF, Alvarez-Blasco F, Botella-Carretero JI, Luque-Ramirez M. The striking similarities in the metabolic associations of female androgen excess and male androgen deficiency. Hum Reprod. 2014;29:2083–2091. doi: 10.1093/humrep/deu198. [DOI] [PubMed] [Google Scholar]
- 18.Lizcano F, Guzmán G. Estrogen Deficiency and the Origin of Obesity during Menopause. Biomed Res Int. 2014;2014:1–11. doi: 10.1155/2014/757461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hevener AL, Clegg DJ, Mauvais-Jarvis F. Impaired estrogen receptor action in the pathogenesis of the metabolic syndrome. Mol Cell Endocrinol. 2015;418:306–321. doi: 10.1016/j.mce.2015.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arnold AP, Reue K, Eghbali M, Vilain E, Chen X, Ghahramani N, Itoh Y, Li J, Link JC, Ngun T, Williams-Burris SM. The importance of having two X chromosomes. Philos Trans R Soc B Biol Sci. 2016;371:20150113. doi: 10.1098/rstb.2015.0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arnold AP. Mouse models for evaluating sex chromosome effects that cause sex differences in non-gonadal tissues. J Neuroendocrinol. 2009;21:377–86. doi: 10.1111/j.1365-2826.2009.01831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Itoh Y, Mackie R, Kampf K, Domadia S, Brown JD, O’Neill R, Arnold AP. Four core genotypes mouse model: localization of the Sry transgene and bioassay for testicular hormone levels. BMC Res Notes. 2015;8:69. doi: 10.1186/s13104-015-0986-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ritz SA, Antle DM, Côté J, Deroy K, Fraleigh N, Messing K, Parent L, St-Pierre J, Vaillancourt C, Mergler D. First steps for integrating sex and gender considerations into basic experimental biomedical research. FASEB J. 2014;28:4–13. doi: 10.1096/fj.13-233395. [DOI] [PubMed] [Google Scholar]
- 24.Chen X, McClusky R, Chen J, Beaven SW, Tontonoz P, Arnold AP, Reue K. The number of x chromosomes causes sex differences in adiposity in mice. PLoS Genet. 2012;8:e1002709. doi: 10.1371/journal.pgen.1002709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen X, Wang L, Loh DH, Colwell CS, Taché Y, Reue K, Arnold AP. Sex differences in diurnal rhythms of food intake in mice caused by gonadal hormones and complement of sex chromosomes. Horm Behav. 2015;75:55–63. doi: 10.1016/j.yhbeh.2015.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bass J, Takahashi JS. Circadian Integration of Metabolism and Energetics. Science (80- ) 2010;330:1349–1354. doi: 10.1126/science.1195027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arble DM, Bass J, Laposky AD, Vitaterna MH, Turek FW. Circadian Timing of Food Intake Contributes to Weight Gain. Obesity. 2009;17:2100–2102. doi: 10.1038/oby.2009.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang W, Ramsey KM, Marcheva B, Bass J. Circadian rhythms, sleep, and metabolism. J Clin Invest. 2011;121:2133–2141. doi: 10.1172/JCI46043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burgoyne PS, Mahadevaiah SK, Perry J, Palmer SJ, Ashworth A. The Y* rearrangement in mice: new insights into a perplexing PAR. [accessed February 25, 2016];Cytogenet Cell Genet. 1998 80:37–40. doi: 10.1159/000014954. http://www.ncbi.nlm.nih.gov/pubmed/9678332. [DOI] [PubMed] [Google Scholar]
- 30.Burgoyne PS, Arnold AP. A primer on the use of mouse models for identifying direct sex chromosome effects that cause sex differences in non-gonadal tissues. Biol Sex Differ. 2016;7:68. doi: 10.1186/s13293-016-0115-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bonomi M, Rochira V, Pasquali D, Balercia G, Jannini EA, Ferlin A. Klinefelter ItaliaN Group (KING), Klinefelter syndrome (KS): genetics, clinical phenotype and hypogonadism. J Endocrinol Invest. 2016 doi: 10.1007/s40618-016-0541-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiang-Feng M, Hong-Li X, Xue-Yan W, Min N, Shuang-Yu L, Hong-Ding X, Liang-Ming L. Prevalence and risk factors of diabetes in patients with Klinefelter syndrome: a longitudinal observational study. Fertil Steril. 2012;98:1331–1335. doi: 10.1016/j.fertnstert.2012.07.1122. [DOI] [PubMed] [Google Scholar]
- 33.Aksglaede L, Molgaard C, Skakkebaek NE, Juul A. Normal bone mineral content but unfavourable muscle/fat ratio in Klinefelter syndrome. Arch Dis Child. 2008;93:30–34. doi: 10.1136/adc.2007.120675. [DOI] [PubMed] [Google Scholar]
- 34.Ishikawa T, Yamaguchi K, Kondo Y, Takenaka A, Fujisawa M. Metabolic Syndrome in Men with Klinefelter’s Syndrome. Urology. 2008;71:1109–1113. doi: 10.1016/j.urology.2008.01.051. [DOI] [PubMed] [Google Scholar]
- 35.Bardsley MZ, Falkner B, Kowal K, Ross JL. Insulin resistance and metabolic syndrome in prepubertal boys with Klinefelter syndrome. Acta Paediatr. 2011;100:866–70. doi: 10.1111/j.1651-2227.2011.02161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gravholt CH, Jensen AS, Høst C, Bojesen A. Body composition, metabolic syndrome and type 2 diabetes in Klinefelter syndrome. Acta Paediatr. 2011;100:871–7. doi: 10.1111/j.1651-2227.2011.02233.x. [DOI] [PubMed] [Google Scholar]
- 37.Bojesen A, Kristensen K, Birkebaek NH, Fedder J, Mosekilde L, Bennett P, Laurberg P, Frystyk J, Flyvbjerg A, Christiansen JS, Gravholt CH. The Metabolic Syndrome Is Frequent in Klinefelter’s Syndrome and Is Associated With Abdominal Obesity and Hypogonadism. Diabetes Care. 2006;29:1591–1598. doi: 10.2337/dc06-0145. [DOI] [PubMed] [Google Scholar]
- 38.Chen X, Williams-Burris SM, McClusky R, Ngun TC, Ghahramani N, Barseghyan H, Reue K, Vilain E, Arnold AP. The Sex Chromosome Trisomy mouse model of XXY and XYY: metabolism and motor performance. Biol Sex Differ. 2013;4:15. doi: 10.1186/2042-6410-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bondy CA. Turner Syndrome 2008. Horm Res Paediatr. 2009;71:52–56. doi: 10.1159/000178039. [DOI] [PubMed] [Google Scholar]
- 40.Bonthuis PJ, Cox KH, Rissman EF. X-chromosome dosage affects male sexual behavior. Horm Behav. 2012;61:565–572. doi: 10.1016/j.yhbeh.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Link JC, Reue K. The genetic basis for sex differences in obesity and lipid metabolism. Annu Rev Nutr. 2017 doi: 10.1146/annurev-nutr-071816-064827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Han SJ, Kim KS, Kim W, Kim JH, Lee YH, Nam JS, Seo JA, Kim BK, Lee J, Chung JO, Kim MH, Sohn TS, Choi HS, Bin Hong S, Chung YS. Obesity and Hyperglycemia in Korean Men with Klinefelter Syndrome: The Korean Endocrine Society Registry. Endocrinol Metab (Seoul, Korea) 2016;31:598–603. doi: 10.3803/EnM.2016.31.4.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johnson JL, Slentz CA, Duscha BD, Samsa GP, McCartney JS, Houmard JA, Kraus WE. Gender and racial differences in lipoprotein subclass distributions: the STRRIDE study. Atherosclerosis. 2004;176:371–377. doi: 10.1016/j.atherosclerosis.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 44.Matthews KA, Crawford SL, Chae CU, Everson-Rose SA, Sowers MF, Sternfeld B, Sutton-Tyrrell K. Are Changes in Cardiovascular Disease Risk Factors in Midlife Women Due to Chronological Aging or to the Menopausal Transition? J Am Coll Cardiol. 2009;54:2366–2373. doi: 10.1016/j.jacc.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cífková R, Krajčoviechová A. Dyslipidemia and Cardiovascular Disease in Women. Curr Cardiol Rep. 2015;17:52. doi: 10.1007/s11886-015-0609-5. [DOI] [PubMed] [Google Scholar]
- 46.Wang X, Magkos F, Mittendorfer B. Sex Differences in Lipid and Lipoprotein Metabolism: It’s Not Just about Sex Hormones. J Clin Endocrinol Metab. 2011;96:885–893. doi: 10.1210/jc.2010-2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Link JC, Chen X, Prien C, Borja MS, Hammerson B, Oda MN, Arnold AP, Reue K. Increased high-density lipoprotein cholesterol levels in mice with XX versus XY sex chromosomes. Arterioscler Thromb Vasc Biol. 2015;35:1778–86. doi: 10.1161/ATVBAHA.115.305460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McCullough LD, Mirza MA, Xu Y, Bentivegna K, Steffens EB, Ritzel R, Liu F. Stroke sensitivity in the aged: sex chromosome complement vs. gonadal hormones. Aging (Albany NY) 2016;8:1–10. doi: 10.18632/aging.100997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li J, Chen X, McClusky R, Ruiz-Sundstrom M, Itoh Y, Umar S, Arnold AP, Eghbali M. The number of X chromosomes influences protection from cardiac ischaemia/reperfusion injury in mice: one X is better than two. Cardiovasc Res. 2014;102:375–384. doi: 10.1093/cvr/cvu064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alsiraj Y, Thatcher SE, Charnigo R, Kuey C, Blalock E, Daugherty A, Cassis LA. Female Mice with an XY Sex Chromosome Complement Develop Severe Angiotensin II-Induced Abdominal Aortic Aneurysms. Circulation. 2016 doi: 10.1161/CIRCULATIONAHA.116.023789. CIRCULATIONAHA.116.023789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ji H, Zheng W, Wu X, Liu J, Ecelbarger CM, Watkins R, Arnold AP, Sandberg K. Sex Chromosome Effects Unmasked in Angiotensin II-Induced Hypertension. Hypertension. 2010;55:1275–1282. doi: 10.1161/HYPERTENSIONAHA.109.144949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vivas L, Dadam FM, Caeiro XE. Sex differences in body fluid homeostasis: Sex chromosome complement influences on bradycardic baroreflex response and sodium depletion induced neural activity. Physiol Behav. 2015;152:416–421. doi: 10.1016/j.physbeh.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 53.Caeiro XE, Mir FR, Vivas LM, Carrer HF, Cambiasso MJ. Sex chromosome complement contributes to sex differences in bradycardic baroreflex response. Hypertens (Dallas, Tex 1979) 2011;58:505–11. doi: 10.1161/HYPERTENSIONAHA.111.175661. [DOI] [PubMed] [Google Scholar]
- 54.Abramowitz LK, Stichelen SO-V, Hanover JA. Chromosome Imbalance as a Driver of Sex Disparity in Disease. J Genomics. 2014;2:77–88. doi: 10.7150/jgen.8123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Carrel L, Cottle AA, Goglin KC, Willard HF. A first-generation X-inactivation profile of the human X chromosome. [accessed February 25, 2016];Proc Natl Acad Sci U S A. 1999 96:14440–4. doi: 10.1073/pnas.96.25.14440. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=24455&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lopes AM, Arnold-Croop SE, Amorim A, Carrel L. Clustered transcripts that escape X inactivation at mouse XqD. Mamm Genome. 2011;22:572–82. doi: 10.1007/s00335-011-9350-6. [DOI] [PubMed] [Google Scholar]
- 57.Disteche CM. Dosage compensation of the sex chromosomes. Annu Rev Genet. 2012;46:537–60. doi: 10.1146/annurev-genet-110711-155454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Berletch JB, Ma W, Yang F, Shendure J, Noble WS, Disteche CM, Deng X. Escape from X inactivation varies in mouse tissues. PLoS Genet. 2015;11:e1005079. doi: 10.1371/journal.pgen.1005079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang F, Babak T, Shendure J, Disteche CM. Global survey of escape from X inactivation by RNA-sequencing in mouse. Genome Res. 2010;20:614–22. doi: 10.1101/gr.103200.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wijchers PJ, Festenstein RJ. Epigenetic regulation of autosomal gene expression by sex chromosomes. Trends Genet. 2011;27:132–140. doi: 10.1016/j.tig.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 61.Bonthuis PJ, Rissman EF. Neural growth hormone implicated in body weight sex differences. Endocrinology. 2013;154:3826–35. doi: 10.1210/en.2013-1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Clayton JA, Collins FS. Policy: NIH to balance sex in cell and animal studies. [accessed February 25, 2016];Nature. 2014 509:282–3. doi: 10.1038/509282a. http://www.ncbi.nlm.nih.gov/pubmed/24834516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McCullough LD, de Vries GJ, Miller VM, Becker JB, Sandberg K, McCarthy MM. NIH initiative to balance sex of animals in preclinical studies: generative questions to guide policy, implementation, and metrics. Biol Sex Differ. 2014;5:15. doi: 10.1186/s13293-014-0015-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Danska JS. Sex Matters for Mechanism. Sci Transl Med. 2014;6:258fs40-258fs40. doi: 10.1126/scitranslmed.3009859. [DOI] [PubMed] [Google Scholar]
- 65.Miller LR, Marks C, Becker JB, Hurn PD, Chen W-J, Woodruff T, McCarthy MM, Sohrabji F, Schiebinger L, Wetherington CL, Makris S, Arnold AP, Einstein G, Miller VM, Sandberg K, Maier S, Cornelison TL, Clayton JA. Considering sex as a biological variable in preclinical research. FASEB J. 2016 doi: 10.1096/fj.201600781R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sandberg K, Umans JG Georgetown Consensus Conference Work Group. Recommendations concerning the new U.S. National Institutes of Health initiative to balance the sex of cells and animals in preclinical research. FASEB J. 2015;29:1646–1652. doi: 10.1096/fj.14-269548. [DOI] [PMC free article] [PubMed] [Google Scholar]