Abstract
Background
Arrhythmia ablation with current techniques is not universally successful. Inadequate ablation lesion formation may be responsible for some arrhythmia recurrences. Peri-procedural visualization of ablation lesions may identify inadequate lesions and gaps to guide further ablation and reduce risk of arrhythmia recurrence.
Methods
This feasibility study assessed acute post-procedure ablation lesions by MRI, and correlated these findings with clinical outcomes. Ten pediatric patients who underwent ventricular tachycardia ablation were transferred immediately post-ablation to a 1.5T MRI scanner and late gadolinium enhancement (LGE) imaging was performed to characterize ablation lesions. Immediate and mid-term arrhythmia recurrences were assessed.
Results
Patient characteristics include median age 14 years (1 – 18 years), median weight 52 kg (11 – 81kg), normal cardiac anatomy (n = 6), d-transposition of great arteries post arterial switch repair (n = 2), anomalous coronary artery origin post repair (n = 1), and cardiac rhabdomyoma (n = 1). All patients underwent radiofrequency catheter ablation of ventricular arrhythmia with acute procedural success. LGE was identified at the reported ablation site in 9/10 patients, all arrhythmia-free at median 7 months follow-up. LGE was not visible in 1 patient who had recurrence of frequent premature ventricular contractions within 2 hours, confirmed on Holter at 1 and 21 months post-procedure.
Conclusions
Ventricular ablation lesion visibility by MRI in the acute post-procedure setting is feasible. Lesions identifiable with MRI may correlate with clinical outcomes. Acute MRI identification of gaps or inadequate lesions may provide the unique temporal opportunity for additional ablation therapy to decrease arrhythmia recurrence.
Keywords: congenital heart disease, magnetic resonance imaging, arrhythmia, electrophysiology, ablation
Background
While transcatheter therapies have revolutionized management of ventricular arrhythmias, radiofrequency (RF) catheter ablation is not 100% successful and there is still a significant risk of arrhythmia recurrence. Intra-procedural assessment of adequate ablation lesion creation is currently performed via indirect measures, including termination of arrhythmia, changes in the intracardiac electrogram, measurements of impedance, power delivered and catheter tip thermal changes. Despite these outcome measures, the recurrence risk following acutely-successful ablation of ventricular arrhythmia in the pediatric population is still reported as 8–20%.(1–3) Peri-procedural visualization of the affected tissue may help to identify inadequate lesions or gaps in a planned lesion set, which could potentially be used to guide further ablation and reduce risk of arrhythmia recurrence. Preclinical studies have demonstrated that acute arrhythmia ablation lesions can be visualized by cardiac magnetic resonance imaging (CMR).(4–8) There have been limited clinical reports of acute ablation lesion imaging using CMR in adults, though these reports mainly focused on assessment of atrial lesions(9–13) or late CMR assessment of ventricular lesions.(14) There is no published data on CMR assessment of ablation lesions in children or patients with congenital heart disease.
Diagnostic CMR imaging in children generally requires higher temporal and spatial resolution compared with adults given their smaller hearts and faster heart rates.(15) Presence and conspicuity of acute ventricular tachycardia ablation lesions has thus far not been demonstrated. This pilot study therefore aimed to determine whether acute ventricular ablation lesions can be visualized by CMR in the pediatric population, and whether CMR lesion presence correlates with clinical outcomes.
Methods
With IRB approval, and informed consent and assent, where appropriate, consecutive patients referred to the Children’s National Health System electrophysiology laboratory for an electrophysiology study with potential for ablation of ventricular tachycardia were prospectively enrolled. Pre-ablation CMR, when available for clinical purposes, was reviewed for presence of any pre-existing scar.
Standard electrophysiology study and radiofrequency ablation procedures were performed with a 3D electroanatomic mapping system (CARTO-3, Biosense Webster, Diamond Bar, CA, USA) in a unique interventional cardiac magnetic resonance suite, allowing patients to slide from the fluoroscopy room to the CMR room without interruption of anesthesia or need for transfer off the bed. Patients were transferred from the fluoroscopy portion of the interventional CMR suite to a 1.5 Tesla MRI scanner (Siemens Healthcare, Erlangen, Germany) for immediate post-procedural CMR imaging. Per the IRB limit on additional sedation based on available evidence(16), a maximum of 30 minutes additional sedation time was available for post-ablation CMR. Following 0.15 mmol/kg intravenous gadolinium injection, each patient underwent rapid late gadolinium enhancement (LGE) imaging during free breathing under sedation using a previously described motion-corrected, single shot sequence.(17, 18) Continuous stacks of LGE images were obtained in two planes to localize and measure the LGE in the affected ventricle. Each CMR image set was analyzed by two pediatric cardiologists expert in interpretation of CMR, blinded to location of the ablation lesion site(s). Evidence of ablation lesion by CMR was defined as per preclinical description of new late gadolinium enhancement at the predicted ablation lesion location.(4) Some patients in this study underwent additional imaging sequences for technical development of optimal acute post-ablation CMR imaging to increase likelihood of lesion identification using a novel dark blood imaging protocol using T2 prep between the IR preparation and readout allowing nulling of both myocardium and blood to increase contrast of the ablation lesion.(19)
Patients were followed at standard clinical intervals for arrhythmia recurrence including obtaining a symptom history, physical, a 12-lead electrocardiogram and 24-hour Holter monitor one month post-procedure.
Results
Between 11/2014 and 12/2015, 10 patients underwent ablation of ventricular tachycardia or frequent premature ventricular contractions with post-ablation LGE imaging, summarized in table 1 and imaging findings available in the Supplemental Figure. The 10 patients were aged 1.8 to 18 years; 6 patients had normal cardiac anatomy, 2 had D-transposition of the great arteries status post arterial switch procedure, 1 had an anomalous origin of the coronary artery status post coronary unroofing procedure, and 1 had tuberous sclerosis associated cardiac rhabdomyomas. There were no MRI or anesthesia related adverse safety events. Nine patients had clinically indicated pre-ablation CMR, and none had evidence of pre-ablation LGE. Arrhythmia was reported to have limited the technical quality of 5/9 of these studies precluding accurate assessment of cardiac function and chamber volume which rely on precise ECG-gating. No patients required additional pharmacologic therapy to obtain rate or rhythm control for adequate imaging to identify LGE using a single shot, motion-corrected SSFP technique(18) which has a high success rate for LGE imaging in the setting of ectopic beats as long as the coupling interval is adequate to allow for image acquisition, on the order of 400 ms.
Table 1.
| Case | Age (years) | Cardiac Anatomy | Pre-procedure arrhythmia | Arrhythmia during electrophysiology case | Mapping Type | Ablation site | Catheter tip (mm) | Irrigated | Number of RF ablation lesions | Post-ablation CMR LGE visible | Arrhythmia recurrence at last follow up |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1.8 | D-TGA s/p arterial switch | VT | PVCs | Activation & PaSo | Anterior RVOT | 4 | No | 10 | Yes | No |
| 2 | 2 | Normal | VT | VT | PaSo | Mid-septal LV | 4 | No | 8 | Yes | No |
| 3 | 5 | D-TGA s/p arterial switch | VT | VT | Activation & PaSo | High septal RVOT | 4 | Yes | 17 | Yes | No |
| 4 | 11 | Normal | VT | PVCs | Activation & PaSo | RV inflow | 3.5 | Yes | 42 | Yes | No |
| 5 | 13 | Normal | VT | PVCs | Activation & PaSo | Anterior septal RVOT | 3.5 | No | 11 | No | Yes |
| 6 | 14 | Cardiac rhabdomyomas | VT | PVCs | PaSo | Posterior septal LV | 3.5 | Yes | 13 | Yes | No |
| 7 | 15 | Normal | VT | PVCs | Activation & PaSo | Anterior RV | 4 | No | 15 | Yes | No |
| 8 | 16 | Normal | VT | None | PaSo with finding of abnormal diastolic potentials | LV apex | 3.5 | Yes | 27 | Yes | No |
| 9 | 17 | s/p Unroofing of RCA arising from the left sinus of Valsava | Frequent PVCs | PVCs | Activation & PaSo | RVOT and right aortic cusp | 3.5 | Yes | 16 | Yes | No |
| 10 | 18 | Normal | VT | PVCs | Activation and PaSo | Septal RVOT | 3.5 | Yes | 13 | Yes | No |
Abbreviations: CMR, cardiac magnetic resonance; LGE, late gadolinium enhancement; D-TGA, D-transposition of the great arteries; RCA, right coronary artery; VT, ventricular tachycardia; PVCs, premature ventricular contractions; PaSo (PaSo Module of CARTO system; Biosense Webster, Diamond Bar, CA, USA); RV, right ventricle; LV, left ventricle; RVOT, right ventricular outflow tract
During the electrophysiology procedure, the arrhythmogenic substrate was identified by a combination of activation and automated pace mapping using the PaSo system (Biosense Webster, Diamond Bar, CA, USA) in 9 patients with intra-procedural ventricular tachycardia (VT) and by localization of an abnormal left ventricular potential in 1 patient without intra-procedural spontaneous or inducible arrhythmia. Table 1 summarizes clinical characteristics, electrophysiology study findings and ablation parameters for the cohort. All patients underwent radiofrequency ablation; 4 patients underwent ablation with a standard radiofrequency ablation catheter, and 6 patients underwent ablation with a continuously-irrigated radiofrequency ablation catheter. The number of lesions per patient was 17.2 +/− 9.6, range 8 to 42. Acute procedural success, as defined by termination of the arrhythmia without recurrence in 30 minutes, was attained in all 10 patients.
CMR LGE was performed between 30 and 60 minutes following the application of the final ablation lesion, depending on time of overall electrophysiology procedure termination. All patients were in sinus rhythm without pharmacologic rate or rhythm control at the time of post-ablation CMR LGE. As exemplified in Figure 1, a new region of subendocardial LGE was identified on CMR LGE imaging at the reported ablation site in 9/10 patients. In patients who underwent additional research imaging, the novel dark blood imaging protocol(19) increased contrast of the ablation lesion site making the lesion easier to identify as illustrated in Figures 1 and 2. In one of the 10 patients (Supplemental Figure: case 5) there was no ventricular late enhancement seen despite successful termination of intra-procedural arrhythmia with radiofrequency ablation. CMR sternal wire artifact did not interfere with lesion identification in the three patients who had previously undergone cardiac surgery for congenital heart disease. As shown in Figure 2, one patient with a visible ablation lesion returned for repeat research CMR during the follow up period with persistence of LGE at the reported ablation site one month post-ablation.
Figure 1.
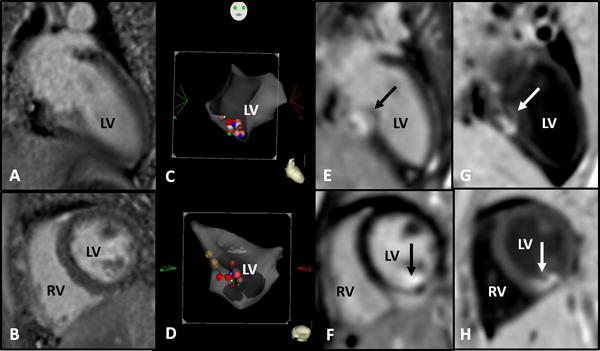
(Case 2) Two-year-old male with normal cardiac anatomy and history of ventricular tachycardia. Radiofrequency ablation performed at the mid-septal left ventricle with pre- and post- ablation cardiac MRI. Abbreviations: RV, right ventricle; LV left ventricle.
a&b. Baseline cardiac MRI with no late gadolinium enhancement in long axis (a) and short axis (b)
c&d. 3D electroanatomic map, long axis (c) and short axis (d) with red and pink dots indicating ablation lesion sites in the mid-septal left ventricle
e&f. Immediate post-ablation cardiac MRI with late gadolinium enhancement (black arrows) corresponding to the reported ablation lesion site in the long axis (e) and short axis (f)
g&h. Dark blood shows late gadolinium enhancement (white arrow) with increased contrast between the blood pool, soft tissue and the ablation lesion in the long axis (g) and short axis (h)
Figure 2.
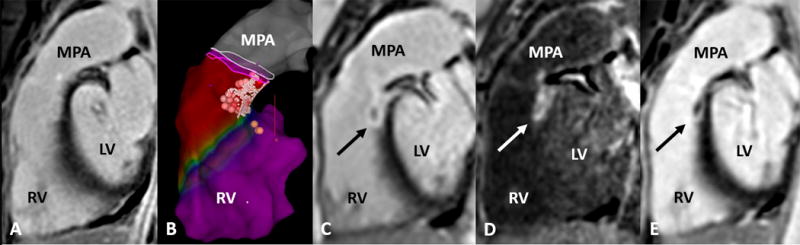
(Case 10) Eighteen-year-old female with normal cardiac anatomy and history of ventricular tachycardia. Radiofrequency ablation performed at the post-septal right ventricular outflow tract with pre- and post-ablation cardiac MRI. Abbreviations: MPA, main pulmonary artery; RV, right ventricle; LV left ventricle.
a. Baseline cardiac MRI, sagittal right ventricular outflow tract view, with no late gadolinium enhancement
b. 3D electroanatomic map with red and pink dots indicating ablation lesion sites and yellow dots indicated the bundle of His location
c. Immediate post-ablation late gadolinium enhancement (black arrow) corresponding to the reported ablation lesion site
d. Dark blood shows late gadolinium enhancement (white arrow) with increased contrast between the blood pool, soft tissue and the ablation lesion
e. Late gadolinium enhancement (black arrow) still present on follow up imaging one month post ablation
The 9 patients with CMR identifiable lesions were arrhythmia- and symptom-free at median 7 months post ablation (range 1–20 months). The patient in whom a lesion could not be identified by CMR had early recurrence of frequent premature ventricular contractions within 2 hours post-procedure, confirmed on Holter monitoring one month later and at last follow up 21 months post-procedure.
Discussion
We chose to study ventricular arrhythmia ablation lesions in the pediatric population given the relatively high risk of life-threatening ventricular arrhythmia recurrence following ablation(1–3), the hypothesis that acute confirmation of a visible CMR LGE lesion at the ablation site may correlate with arrhythmia recurrence risk and the lack of other studies of this type in the pediatric age range. This pilot feasibility study is the first to demonstrate that acute ventricular radiofrequency ablation lesions can be visualized by CMR with LGE imaging in children immediately following ablation with various types of ablation methods (standard and irrigated tip RF ablation catheters). Our imaging findings are keeping with the expected time course of CMR ablation lesion development and appearance described in preclinical studies(4–6). Given that there was no LGE identified on pre-procedure CMR in the 9 patients who had studies performed, we believe the LGE identified post-procedure in the region of the reported ablation site was appropriately attributed to the ablation lesion. Despite the challenges of higher temporal and spatial resolution required for pediatric CMR, LGE was identifiable at the reported ablation site in the 9 patients who had successful ablation without arrhythmia recurrence. Transfer to MRI immediately post ablation and the additional sedation time required for imaging (on the range of 20–25 minutes) were not associated with any adverse safety outcomes.
There was no identifiable ablation lesion on CMR LGE imaging in the only patient who had early recurrence of arrhythmia, leading us to speculate that there is a possible correlation between lesion visibility with CMR, and arrhythmia recurrence risk. As detailed in table 1, there were no specific alterations to the standard electrophysiology procedure that would explain absence of CMR LGE for the patient with arrhythmia recurrence (case 5). Our study is obviously limited by the number of patients and further studies are necessary to verify clinical correlation of acute ablation lesion CMR appearance before considering applying post-ablation CMR to routine clinical practice. If the findings are confirmed through larger studies, acute post-ablation CMR could be used to ensure adequate ablation lesion creation by guiding targeted ablation of lesion gaps or extension of lesion depth. Similar studies have been performed in adults post atrial fibrillation ablation with CMR scar quantification results suggesting that poor scar formation correlates with risk of atrial fibrillation arrhythmia recurrence.(20,21)
During the imaging protocol, CMR lesion identification was partially guided by knowledge of the ablation site, allowing for specific site imaging in orthogonal planes. In cases with discrepancy between reported ablation site and actual ablation site, lesions may be difficult to identify by CMR. Even with knowledge of the likely ablation lesion location, the selected CMR orthogonal planes may not capture the lesion in all planes due to size discrepancy between CMR slice thickness (usually about 10 mm) and ablation lesion (usually about 4–6 mm). In-plane image resolution for the single shot motion-corrected LGE technique is adequate to characterize lesions within the imaging plane, but lesions may be missed between imaging planes. As lesions may only be partially captured within the constraints of selected imaging planes and slice thickness, they cannot be reliably quantified in terms of a percentage or even depth of the substrate tissue. Lesion conspicuity may also be dependent on tissue type and thickness. CMR is used in clinical practice to identify ventricular lesions including myocarditis and myocardial infarction, though is less commonly employed for study of the atria or other thin walled cardiac structures, which have a different tissue profile with increased challenges in differentiating tissue and lesion boundaries from the surrounding blood pool(19). Therefore, our findings in ventricular ablation lesions may not necessarily be applicable to atrial ablation lesions. With further optimization of imaging protocols we hope to continue this study in pediatric patients undergoing atrial arrhythmia ablation. The novel dark blood imaging protocol increased conspicuity of the ablation lesions in this study as illustrated in Figure 2. One could hypothesize that this type of dark blood imaging protocol could potentially add to the sensitivity of CMR ablation lesion detection compared to more traditional LGE bright blood techniques. Of course, a major limitation of using CMR or any other imaging modality determining lesion visibility to confirm adequate lesion creation is that ablation in any location that is not at the origin of arrhythmia may result in a visible lesion but will not correlate with successful arrhythmia treatment.
The 30-minute limit for additional sedation time including patient transfer to MRI and research imaging necessitated prioritization of LGE in the imaging protocol and limited time for additional sequences to identify and quantify edema and fibrosis, such as T2-weighted imaging and T1 native contrast. (9, 12, 22) The results of follow-up imaging for 1 patient (Figure 3) with a visible acute ablation lesion persisting at 1 month follow-up imaging in our study suggest that the acute LGE CMR may correlate with permanent tissue injury rather than simply acute edema. While CMR imaging at a time point beyond the acute post-ablation period may more accurately identify ablation scar as opposed to temporary edematous changes, the need for an additional sedation in young children is avoided by acute post-procedure imaging. In addition, the temporal nature of immediate post-ablation imaging may provide the opportunity for identification of inadequate lesions allowing for lesion modification to reinforce ablation lines of block or inadequate ablated tissue during the same sedation procedure. This may reduce the chance of needing repeat ablation attempts for arrhythmia recurrence. Ultimately with the advent of technology for real-time MRI-guided atrial arrhythmia ablation currently being trialed in adults(23–25), the finding that acute ventricular ablation lesions are visible by CMR in the pediatric population may be of tremendous importance if real-time MRI guided technology proves to be feasible and efficacious.
Other imaging modalities have been studied for identification of acute arrhythmia ablation lesions including intracardiac echocardiography(26) and optical coherence tomography,(27) which has higher axial resolution than MRI and are not adversely affected by arrhythmia, though these techniques usually rely on fluoroscopic guidance for catheter manipulation especially in the pediatric population. Despite the aforementioned resolution challenges associated with CMR in the pediatric and arrhythmia populations we believe that it is still may be the imaging modality of choice for identification of acute ablation lesions. Children are particularly susceptible to effects of ionizing radiation and patients with congenital heart disease who have increased lifetime cumulative radiation exposure risk;(28) therefore, any adjunctive imaging technology for electrophysiology procedures should ideally be radiation sparing.
Conclusion
Acute ventricular radiofrequency ablation lesions can be visualized by CMR in the pediatric population with LGE imaging techniques. CMR lesion appearance may correlate with clinical outcomes and therefore may be useful to predict arrhythmia recurrence risk. Ablation lesion LGE could guide further fluoroscopic and 3D electroanatomic mapping ablation procedures, and in the future real-time MRI guided ablation. Further study will determine reproducibility of these results and optimize imaging protocols to increase resolution, decrease scan time and definitively distinguish edema from long-term scar.
Supplementary Material
Acknowledgments
This work is funded by the National Heart Lung and Blood Institute (HHSN268201500001C).
Footnotes
Disclosures: None
Clinical Trial Registration: NCT02761343 https://clinicaltrials.gov/ct2/show/NCT02761343?term=mri+ablation&rank=1
References
- 1.Morwood JG, Triedman JK, Berul CI, Cecchin F, Alexander ME, Khairy P, Walsh EP. Catheter ablation for ventricular tachycardia in young patients. Heart Rhythm. 2004;1:301–308. doi: 10.1016/j.hrthm.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 2.Li X, Jiang H, Li Y, Zhang Y, Liu H, Ge H, Zhang Y, Li M. Effectiveness of Radiofrequency Catheter Ablation of Outflow Tract Ventricular Arrhythmias in Children and Adolescents. Pediatric Cardiology. 2016;37(8):1475–1481. doi: 10.1007/s00246-016-1460-1. [DOI] [PubMed] [Google Scholar]
- 3.Akdeniz C, Elvin E, Celik N, Karacan M, Tuzcu V. Catheter ablation of idiopathic right ventricular arrhythmias in children with limited fluoroscopy. J Interv Card Electrophysiol. 2016;46:355–360. doi: 10.1007/s10840-016-0133-6. [DOI] [PubMed] [Google Scholar]
- 4.Dickfield T, Kato R, Zviman M, Lai S, Meininger G, Lardo AC, Roquin A, Blumke D, Berger R, Calkins H, Halperin H. Characterization of radiofrequency ablation lesions with gadolinium enhanced cardiovascular magnetic resonance imaging. Journal of the American College of Cardiology. 2006;47:370–378. doi: 10.1016/j.jacc.2005.07.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lardo AC, McVeigh ER, Jumrussirikul P, Berger RD, Calkins H, Lima J, Halperin HR. Visualization and temporal/spatial characterization of cardiac radiofrequency ablation lesions using magnetic resonance imaging. Circulation. 2000;102:698–705. doi: 10.1161/01.cir.102.6.698. [DOI] [PubMed] [Google Scholar]
- 6.Ranjan R, Kato R, Zviman MM, Dickfeld TM, Roquin A, Berger RD, Tomaselli GF, Halperin HR. Gaps in the ablation line as a potential cause of recovery from electrical isolation and their visualization using MRI. Circulation Arrhythmia and Electrophysiology. 2011;4:279–86. doi: 10.1161/CIRCEP.110.960567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ranjan R, Khomovski EG, Blauer J, Vijayakumar S, Volland NA, Salama ME, Parker DL, MacLeod R, Marrouche NF. Identification and acute targeting of gaps in atrial ablation lesion sets using a real-time magnetic resonance imaging system. Circulation Arrhythmia and Electrophysiology. 2012;5:1130–1135. doi: 10.1161/CIRCEP.112.973164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vergara GR, Vijayakumar S, Kholmovski EG, Blauer JJ, Guttman MA, Gloschat C, Payne G, Vij K, Akoum NW, Daccarett M, McGann CJ, Macleod RS, Marrouche NF. Real-time magnetic resonance imaging-guided radiofrequency atrial ablation and visualization of lesion formation at 3 Tesla. Heart rhythm: the official journal of the Heart Rhythm Society. 2011;8:295–303. doi: 10.1016/j.hrthm.2010.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arujuna A, Karim R, Caulfield D, Knowles B, Rhode K, Schaeffter T, Kato B, Rinaldi CA, Cooklin M, Razavi R, O’Neill MD, Gill J. Acute pulmonary vein isolation is achieved by a combination of reversible and irreversible atrial injury after catheter ablation. Circulation Arrhythmia and Electrophysiology. 2012;5:691–700. doi: 10.1161/CIRCEP.111.966523. [DOI] [PubMed] [Google Scholar]
- 10.Ozgun M, Maintz D, Bunck AC, Monnig G, Eckardt L, Wasmer K, Heindel W, Botnar RM, Kirchhof P. Right atrial scar detection after catheter ablation: Comparison of 2D and high spatial resolution 3D-late enhancement magnetic resonance imaging. Academic radiology. 2011;18:488–94. doi: 10.1016/j.acra.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 11.Khurram IM, Catanzaro JN, Zimmerman S, Zipunnikov V, Berger RD, Cheng A, Sinha S, Dewire J, Marine J, Spragg D, Ashikaga H, Halperin H, Calkins H, Nazarian S. MRI evaluation of radiofrequency, cryothermal, and laser left atrial lesion formation in patients with atrial fibrillation. Pacing Clin Electrophysiol. 2015;38(11):1317–1324. doi: 10.1111/pace.12696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yokokawa M, Tada H, Koyama K, Ino T, Naito S, Oshima S, Taniguchi K. The change in the tissue characterization detected by magnetic resonance imaging after radiofrequency ablation of isthmus-dependent atrial flutter. Int J Cardiol. 2011;148(1):30–5. doi: 10.1016/j.ijcard.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 13.Parmar BR, Jarrett TR, Burgon NS, Kholmovski EG, Akoum NW, Hu N, Macleod RS, Marrouche NF, Ranjan R. Comparison of left atrial area marked ablated in electroanatomical maps with scar in MRI. J Cardiovasc Electrophysiol. 2014;25:457–63. doi: 10.1111/jce.12357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ilg K, Baman TS, Gupta SK, Swanson S, Good E, Chugh A, Jongnarangsin K, Pelosi F, Jr, Crawford T, Oral H, Morady F, Bogun F. Assessment of radiofrequency ablation lesions by CMR imaging after ablation of idiopathic ventriculararrhythmias. JACC Cardiovasc Imaging. 2010 Mar;3(3):278–85. doi: 10.1016/j.jcmg.2009.09.028. [DOI] [PubMed] [Google Scholar]
- 15.Bonnemains L, Raimondi F, Odille F. Specifics of cardiac magnetic resonance imaging in children. Arch Cardiovasc Dis. 2016;109(2):143–9. doi: 10.1016/j.acvd.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 16.Kiringoda R, Thurm AE, Hirschtritt ME, Koziol D, Wesley R, Swedo SE, O’Grady NP, Quezado ZM. Risks of propofol sedation/anesthesia for imaging studies in pediatric research: Eight years of experience in a clinical research center. Arch Pediatr Adolesc Med. 2010;164:554–560. doi: 10.1001/archpediatrics.2010.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ledesma-Carbayo MJ, Kellman P, Arai AE, McVeigh ER. Motion Corrected Free-Breathing Delayed Enhancement Imaging of Myocardial Infarction Using Nonrigid Registration. J Magn Reson Imaging. 2007;26:184–190. doi: 10.1002/jmri.20957. [DOI] [PubMed] [Google Scholar]
- 18.Olivieri L, Cross R, O’Brien KJ, Xue H, Kellman P, Hansen MS. Free-breathing motion-corrected late-gadolinium enhancement imaging improves image quality in children. Pediatr Radiol. 2016;46(7):983–990. doi: 10.1007/s00247-016-3553-7. [DOI] [PubMed] [Google Scholar]
- 19.Kellman P, Xui H, Olivieri LJ, Cross RR, Grant EK, Fontana M, Ugander M, Moon JC, Hansen MS. Dark Blood Late Enhancement Imaging. Journal of Cardiovascular Magnetic Resonance. 2016;18(1):17. doi: 10.1186/s12968-016-0297-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parmar B, Jarrett TR, Kholmovski EG, Hu N, Parker D, MacLeod RS, Marrouche NF, Ranjan R. Poor scar formation after ablation is associated with atrial fibrillation recurrence. J Interv Card Electrophysiol. 2015;44(3):247–256. doi: 10.1007/s10840-015-0060-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peters DC, Wylie JV, Hauser TH, Nezafat R, Han Y, Woo JJ, Taclas J, Kissinger KV, Goddu B, Josephson ME, Manning WJ. Recurrence of atrial fibrillation correlates with the extent of post-procedural late gadolinium enhancement: a pilot study. JACC Cardiovasc Imaging. 2009;2(3):308–316. doi: 10.1016/j.jcmg.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Celik H, Ramanan V, Barry J, Ghate S, Leber V, Oduneye S, Gu Y, Jamali M, Ghugre N, Stainsby JA, Shurrab M, Crystal E, Wright GA. Intrinsic contrast for characterization of acute radiofrequency ablation lesions. Circ Arrhythm Electrophysiol. 2014;7(4):718–727. doi: 10.1161/CIRCEP.113.001163. [DOI] [PubMed] [Google Scholar]
- 23.Grothoff M, Piorkowski C, Eitel C, Gaspar T, Lehmkuhl L, Lucke C, Hoffmann J, Hildebrand L, Wedan S, Lloyd T, Sunnarborg D, Schnackenburg B, Hindricks G, Sommer P, Gutberlet M. MR imaging-guided electrophysiological ablation studies in humans with passive catheter tracking: initial results. Radiology. 2014;271(3):695–702. doi: 10.1148/radiol.13122671. [DOI] [PubMed] [Google Scholar]
- 24.Hilbert S, Sommer P, Gutberlet M, Gaspar T, Foldyna B, Piorkowski C, Weiss S, Lloyd T, Schnackenburg B, Krueger S, Fleiter C, Paetsch I, Jahnke C, Hindricks G, Grothoff M. Real-time magnetic resonance-guided ablation of typical right atrial flutter using a combination of active catheter tracking and passive catheter visualization in man: initial results from a consecutive patient series. Europace. 2016;18(4):572–577. doi: 10.1093/europace/euv249. [DOI] [PubMed] [Google Scholar]
- 25.Nordbeck P, Beer M, Kostler H, Ladd ME, Quick HH, Bauer WR, Ritter O. Cardiac catheter ablation under real-time magnetic resonance guidance. European Heart Journal. 2012;33:1977. doi: 10.1093/eurheartj/ehs139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Filgueiras-Rama D, de Torres-Alba F, Castrejón-Castrejón S, Estrada A, Figueroa J, Salvador-Montañés Ó, López T, Moreno-Yanguela M, López Sendón JL, Merino JL. Utility of intracardiac echocardiography for catheter ablation of complex cardiac arrhythmias in a medium-volume training center. Echocardiography. 2015;32(4):660–70. doi: 10.1111/echo.12714. [DOI] [PubMed] [Google Scholar]
- 27.Herranz D, Lloret J, Jiménez-Valero S, Rubio-Guivernau JL, Margallo-Balbás E. Novel catheter enabling simultaneous radiofrequency ablation and optical coherence reflectometry. Biomed Opt Express. 2015;6(9):3268–75. doi: 10.1364/BOE.6.003268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson JN, Hornik CP, Li JS, Benjamin DK, Jr, Yoshizumi TT, Reiman RE, Frush DP, Hill KD. Cumulative radiation exposure and cancer risk estimation in children with heart disease. Circulation. 2014;130(2):161–167. doi: 10.1161/CIRCULATIONAHA.113.005425. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


