Abstract
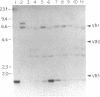
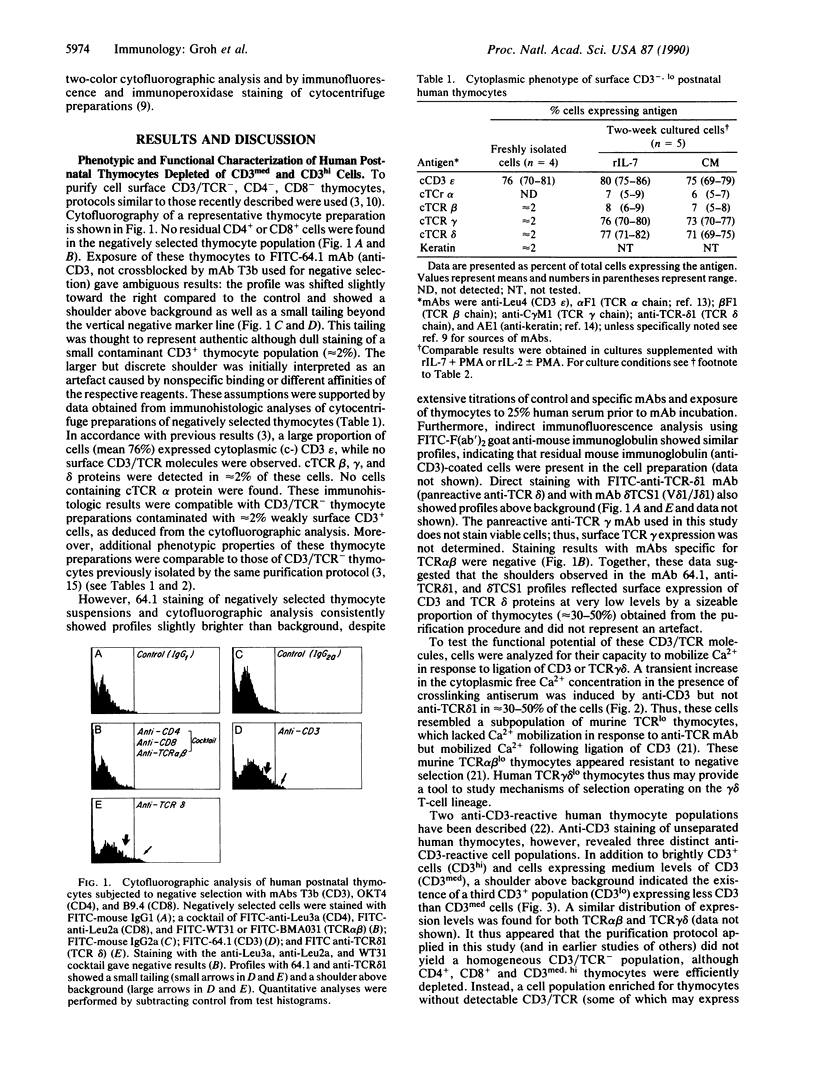
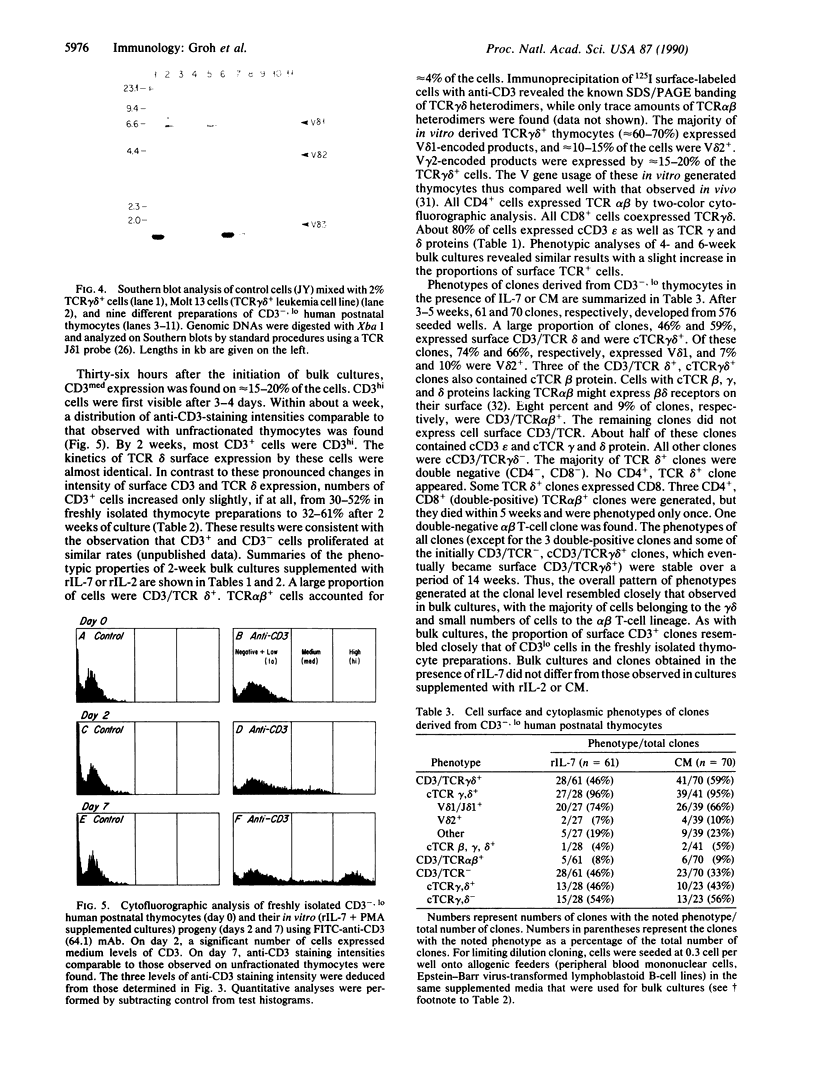
The differentiation or maturation potential of human thymocyte precursors has been studied by using a population of CD3/TCR-, CD4-, CD8- ("triple negative") thymocytes isolated by negative selection (TCR, T-cell receptor). This cell population, however, also contained 30-50% previously undescribed cells expressing very low levels of CD3/TCR gamma delta (CD3/TCR gamma delta low; approximately 60% of which expressed the variable region gene V delta 1). Correspondingly, TCR gamma and TCR delta gene rearrangements (predominantly V delta 1/joining region J delta 1) and full-length TCR gamma and TCR delta transcripts (but only immature TCR beta and no TCR alpha mRNAs) were found. These cells mobilized Ca2+ in response to ligation of CD3 but not following ligation of TCR gamma delta. When cultured in the presence of interleukin 7 or interleukin 2, these thymocytes gave rise to 30-60% CD3/TCR gamma delta medium and high cells (60-70% expressing V delta 1) seen as discrete populations. Thus, the proportion and V delta phenotype of in vitro generated CD3/TCR gamma delta cells closely resembled those of CD3/TCR gamma delta low cells in freshly isolated "thymocyte precursor" preparations. Small numbers of TCR alpha beta + cells also appeared. It is thus uncertain whether maturation, differentiation, or both account for the appearance of mature CD3/TCR+ thymocytes, although the former appears most likely.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Band H., Hochstenbach F., McLean J., Hata S., Krangel M. S., Brenner M. B. Immunochemical proof that a novel rearranging gene encodes the T cell receptor delta subunit. Science. 1987 Oct 30;238(4827):682–684. doi: 10.1126/science.3672118. [DOI] [PubMed] [Google Scholar]
- Blue M. L., Daley J. F., Levine H., Craig K. A., Schlossman S. F. Activation of immature cortical thymocytes through the T11 sheep erythrocyte binding protein. J Immunol. 1987 May 15;138(10):3108–3113. [PubMed] [Google Scholar]
- Casorati G., De Libero G., Lanzavecchia A., Migone N. Molecular analysis of human gamma/delta+ clones from thymus and peripheral blood. J Exp Med. 1989 Nov 1;170(5):1521–1535. doi: 10.1084/jem.170.5.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deans J. P., Shaw J., Pearse M. J., Pilarski L. M. CD45R as a primary signal transducer stimulating IL-2 and IL-2R mRNA synthesis by CD3-4-8- thymocytes. J Immunol. 1989 Oct 15;143(8):2425–2430. [PubMed] [Google Scholar]
- Denning S. M., Kurtzberg J., Leslie D. S., Haynes B. F. Human postnatal CD4- CD8- CD3- thymic T cell precursors differentiate in vitro into T cell receptor delta-bearing cells. J Immunol. 1989 May 1;142(9):2988–2997. [PubMed] [Google Scholar]
- Finkel T. H., Cambier J. C., Kubo R. T., Born W. K., Marrack P., Kappler J. W. The thymus has two functionally distinct populations of immature alpha beta + T cells: one population is deleted by ligation of alpha beta TCR. Cell. 1989 Sep 22;58(6):1047–1054. doi: 10.1016/0092-8674(89)90503-5. [DOI] [PubMed] [Google Scholar]
- Fuller T. C., Trevithick J. E., Fuller A. A., Colvin R. B., Cosimi A. B., Kung P. C. Antigenic polymorphism of the T4 differentiation antigen expressed on human T helper/inducer lymphocytes. Hum Immunol. 1984 Feb;9(2):89–102. doi: 10.1016/0198-8859(84)90031-4. [DOI] [PubMed] [Google Scholar]
- Groh V., Porcelli S., Fabbi M., Lanier L. L., Picker L. J., Anderson T., Warnke R. A., Bhan A. K., Strominger J. L., Brenner M. B. Human lymphocytes bearing T cell receptor gamma/delta are phenotypically diverse and evenly distributed throughout the lymphoid system. J Exp Med. 1989 Apr 1;169(4):1277–1294. doi: 10.1084/jem.169.4.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata S., Clabby M., Devlin P., Spits H., De Vries J. E., Krangel M. S. Diversity and organization of human T cell receptor delta variable gene segments. J Exp Med. 1989 Jan 1;169(1):41–57. doi: 10.1084/jem.169.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata S., Satyanarayana K., Devlin P., Band H., McLean J., Strominger J. L., Brenner M. B., Krangel M. S. Extensive junctional diversity of rearranged human T cell receptor delta genes. Science. 1988 Jun 10;240(4858):1541–1544. doi: 10.1126/science.3259726. [DOI] [PubMed] [Google Scholar]
- Haynes B. F., Denning S. M., Singer K. H., Kurtzberg J. Ontogeny of T-cell precursors: a model for the initial stages of human T-cell development. Immunol Today. 1989 Mar;10(3):87–91. doi: 10.1016/0167-5699(89)90232-6. [DOI] [PubMed] [Google Scholar]
- Haynes B. F., Martin M. E., Kay H. H., Kurtzberg J. Early events in human T cell ontogeny. Phenotypic characterization and immunohistologic localization of T cell precursors in early human fetal tissues. J Exp Med. 1988 Sep 1;168(3):1061–1080. doi: 10.1084/jem.168.3.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry L., Tian W. T., Rittershaus C., Ko J. L., Marsh H. C., Jr, Ip S. H. Two distinct immunogenic epitopes on the alpha chain of human T cell antigen receptor. Hybridoma. 1989 Dec;8(6):577–588. doi: 10.1089/hyb.1989.8.577. [DOI] [PubMed] [Google Scholar]
- Hochstenbach F., Brenner M. B. T-cell receptor delta-chain can substitute for alpha to form a beta delta heterodimer. Nature. 1989 Aug 17;340(6234):562–565. doi: 10.1038/340562a0. [DOI] [PubMed] [Google Scholar]
- Huck S., Lefranc M. P. Rearrangements to the JP1, JP and JP2 segments in the human T-cell rearranging gamma gene (TRG gamma) locus. FEBS Lett. 1987 Nov 30;224(2):291–296. doi: 10.1016/0014-5793(87)80472-6. [DOI] [PubMed] [Google Scholar]
- Krangel M. S., Band H., Hata S., McLean J., Brenner M. B. Structurally divergent human T cell receptor gamma proteins encoded by distinct C gamma genes. Science. 1987 Jul 3;237(4810):64–67. doi: 10.1126/science.2955517. [DOI] [PubMed] [Google Scholar]
- Kurtzberg J., Denning S. M., Nycum L. M., Singer K. H., Haynes B. F. Immature human thymocytes can be driven to differentiate into nonlymphoid lineages by cytokines from thymic epithelial cells. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7575–7579. doi: 10.1073/pnas.86.19.7575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiden J. M., Dialynas D. P., Duby A. D., Murre C., Seidman J., Strominger J. L. Rearrangement and expression of T-cell antigen receptor genes in human T-lymphocyte tumor lines and normal human T-cell clones: evidence for allelic exclusion of Ti beta gene expression and preferential use of a J beta 2 gene segment. Mol Cell Biol. 1986 Sep;6(9):3207–3214. doi: 10.1128/mcb.6.9.3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissey P. J., Goodwin R. G., Nordan R. P., Anderson D., Grabstein K. H., Cosman D., Sims J., Lupton S., Acres B., Reed S. G. Recombinant interleukin 7, pre-B cell growth factor, has costimulatory activity on purified mature T cells. J Exp Med. 1989 Mar 1;169(3):707–716. doi: 10.1084/jem.169.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namen A. E., Lupton S., Hjerrild K., Wignall J., Mochizuki D. Y., Schmierer A., Mosley B., March C. J., Urdal D., Gillis S. Stimulation of B-cell progenitors by cloned murine interleukin-7. Nature. 1988 Jun 9;333(6173):571–573. doi: 10.1038/333571a0. [DOI] [PubMed] [Google Scholar]
- Namen A. E., Schmierer A. E., March C. J., Overell R. W., Park L. S., Urdal D. L., Mochizuki D. Y. B cell precursor growth-promoting activity. Purification and characterization of a growth factor active on lymphocyte precursors. J Exp Med. 1988 Mar 1;167(3):988–1002. doi: 10.1084/jem.167.3.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker C. M., Groh V., Band H., Porcelli S. A., Morita C., Fabbi M., Glass D., Strominger J. L., Brenner M. B. Evidence for extrathymic changes in the T cell receptor gamma/delta repertoire. J Exp Med. 1990 May 1;171(5):1597–1612. doi: 10.1084/jem.171.5.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picker L. J., De los Toyos J., Telen M. J., Haynes B. F., Butcher E. C. Monoclonal antibodies against the CD44 [In(Lu)-related p80], and Pgp-1 antigens in man recognize the Hermes class of lymphocyte homing receptors. J Immunol. 1989 Mar 15;142(6):2046–2051. [PubMed] [Google Scholar]
- Preffer F. I., Kim C. W., Fischer K. H., Sabga E. M., Kradin R. L., Colvin R. B. Identification of pre-T cells in human peripheral blood. Extrathymic differentiation of CD7+CD3- cells into CD3+ gamma/delta+ or alpha/beta+ T cells. J Exp Med. 1989 Jul 1;170(1):177–190. doi: 10.1084/jem.170.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quertermous T., Strauss W. M., Van Dongen J. J., Seidman J. G. Human T cell gamma chain joining regions and T cell development. J Immunol. 1987 Apr 15;138(8):2687–2690. [PubMed] [Google Scholar]
- Sim G. K., Yagüe J., Nelson J., Marrack P., Palmer E., Augustin A., Kappler J. Primary structure of human T-cell receptor alpha-chain. Nature. 1984 Dec 20;312(5996):771–775. doi: 10.1038/312771a0. [DOI] [PubMed] [Google Scholar]
- Singer K. H., Harden E. A., Robertson A. L., Lobach D. F., Haynes B. F. In vitro growth and phenotypic characterization of mesodermal-derived and epithelial components of normal and abnormal human thymus. Hum Immunol. 1985 Jul;13(3):161–176. doi: 10.1016/0198-8859(85)90009-6. [DOI] [PubMed] [Google Scholar]
- Solomon K. R., Krangel M. S., McLean J., Brenner M. B., Band H. Human T cell receptor-gamma and -delta chain pairing analyzed by transfection of a T cell receptor-delta negative mutant cell line. J Immunol. 1990 Feb 1;144(3):1120–1126. [PubMed] [Google Scholar]
- Takeshita T., Goto Y., Tada K., Nagata K., Asao H., Sugamura K. Monoclonal antibody defining a molecule possibly identical to the p75 subunit of interleukin 2 receptor. J Exp Med. 1989 Apr 1;169(4):1323–1332. doi: 10.1084/jem.169.4.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toribio M. L., de la Hera A., Borst J., Marcos M. A., Márquez C., Alonso J. M., Bárcena A., Martínez C. Involvement of the interleukin 2 pathway in the rearrangement and expression of both alpha/beta and gamma/delta T cell receptor genes in human T cell precursors. J Exp Med. 1988 Dec 1;168(6):2231–2249. doi: 10.1084/jem.168.6.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triebel F., Faure F., Graziani M., Jitsukawa S., Lefranc M. P., Hercend T. A unique V-J-C-rearranged gene encodes a gamma protein expressed on the majority of CD3+ T cell receptor-alpha/beta- circulating lymphocytes. J Exp Med. 1988 Feb 1;167(2):694–699. doi: 10.1084/jem.167.2.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson J. D., Morrissey P. J., Namen A. E., Conlon P. J., Widmer M. B. Effect of IL-7 on the growth of fetal thymocytes in culture. J Immunol. 1989 Aug 15;143(4):1215–1222. [PubMed] [Google Scholar]
- de la Hera A., Marston W., Aranda C., Toribio M. L., Martinez C. Thymic stroma is required for the development of human T cell lineages in vitro. Int Immunol. 1989;1(5):471–478. doi: 10.1093/intimm/1.5.471. [DOI] [PubMed] [Google Scholar]