Abstract
Study Objectives:
In young adults, napping is hypothesized to benefit episodic memory retention (eg, via consolidation). Whether this relationship is present in older adults has not been adequately tested but is an important question because older adults display marked changes in sleep and memory.
Design:
Between-subjects design.
Setting:
Sleep laboratory at Emory University School of Medicine.
Participants:
Fifty healthy young adults (18–29) and 45 community-dwelling older adults (58–83).
Intervention:
Participants were randomly assigned to a 90-minute nap opportunity or an equal interval of quiet wakefulness.
Measurements and Results:
Participants underwent an item-wise directed forgetting learning procedure in which they studied words that were individually followed by the instruction to “remember” or “forget.” Following a 90-minute retention interval filled with quiet wakefulness or a nap opportunity, they were asked to free recall and recognize those words. Young adults retained significantly more words following a nap interval than a quiet wakefulness interval on both free recall and recognition tests. There was modest evidence for greater nap-related retention of “remember” items relative to “forget” items for free recall but not recognition. Older adults’ memory retention did not differ across nap and quiet wakefulness conditions, although they demonstrated greater fragmentation, lower N3, and lower rapid eye movement duration than the young adults.
Conclusions:
In young adults, an afternoon nap benefits episodic memory retention, but such benefits decrease with advancing age.
Keywords: aging, cognition, directed forgetting, polysomnography, selective memory consolidation.
Statement of Significance
Declining cognitive function is well documented with aging, even in older adults who have not received a diagnosis of dementia. One potential contributing factor to age-related cognitive decline is an age-related change in the ability to consolidate memories during sleep. The current work compared episodic memory consolidation across polysomnography-recorded nap and quiet wakefulness intervals in young and healthy older adults. To isolate consolidation-specific effects, the memory task procedure was designed to minimize age differences at encoding and retrieval stages. The nap interval promoted episodic memory consolidation in young adults, but not in older adults. Large-scale interventional trials that manipulate various aspects of sleep in older adults are needed to determine whether increasing sleep-dependent memory consolidation slows the cognitive aging process.
INTRODUCTION
In healthy young adults, sleep benefits retention of memories.1 Enhanced post-sleep retention—hereafter, memory consolidation—is thought to be due to memory reactivation during slow-wave sleep2 (or rapid eye movement [REM] sleep3), the opportunity to stabilize memories from interference,4–6 or possibly a combination of these processes.7,8 Though there is a large literature on the relationship between sleep and memory in healthy young adults, it remains unclear whether sleep-dependent memory consolidation is preserved in older adults, whom are known to show fragmented sleep as well as less slow-wave sleep and REM sleep.8–11
Several limitations of existing studies on sleep, memory, and aging are worth consideration. First, these studies have been limited by lacking concurrently tested young adult comparison groups and wake-only control groups (for review, see Scullin and Bliwise8). Second, many existing studies on sleep, memory, and aging rely on self-report or actigraphy rather than polysomnography (PSG).9 Third, most memory consolidation studies compared memory retention across overnight sleep and daytime wakefulness conditions, which confounds time of initial learning (encoding) and time of recall (retrieval). Such time-of-day confounds are problematic within the context of studying aging, because the magnitude of age deficits in memory function is increased in the evening.12 Alternatively, the advantage of an early afternoon nap paradigm is that it matches time-of-encoding and time-of-retrieval across all conditions, it includes primarily non-REM sleep, and it has potent effects on memory retention, at least in young adults.13–17 In the present PSG study, we investigated whether an afternoon nap, relative to quiet wakefulness, would benefit memory consolidation equally in young and older adults.
We assessed memory consolidation by using the directed forgetting procedure, a technique founded in contemporary cognitive psychology.18–20 In the item-wise directed forgetting procedure, participants are presented with a series of words and instructed to selectively remember some studied words and forget other studied words.21–23 Our rationale for using the directed forgetting procedure was to assess whether memory consolidation is driven by the selective reactivation of future-relevant memories. If so, then sleep would only promote retention of words that are instructed to be remembered, and not words instructed to be forgotten.24 In contrast, if sleep stabilizes all memories against interference, then napping should promote retention of both the remember and forget words.
We hypothesized that an afternoon nap would benefit memory consolidation in young adults. For the older adults, the existing evidence conflicts and yields contrasting perspectives.8 One view is that older adults are chronically sleep deprived (eg, due to fragmented sleep) but have preserved memory/cognitive processing during intervals in which they do sleep, and therefore should evidence cognitive benefits even from naps.25 According to this “preserved-function” view, increasing older adults’ total sleep time via an afternoon nap should benefit their memory consolidation as much as young adults.26 An alternative view is that older adults’ sleep is fragmented and deficient in slow-wave sleep,27 and there are numerous age-related declines in the metabolic, neuroanatomical, and neurochemical mechanisms that may support sleep-dependent cognitive processing (eg, prefrontal atrophy).28–32 According to this latter view, an afternoon nap will not benefit memory consolidation in older adults.
METHODS
Overview
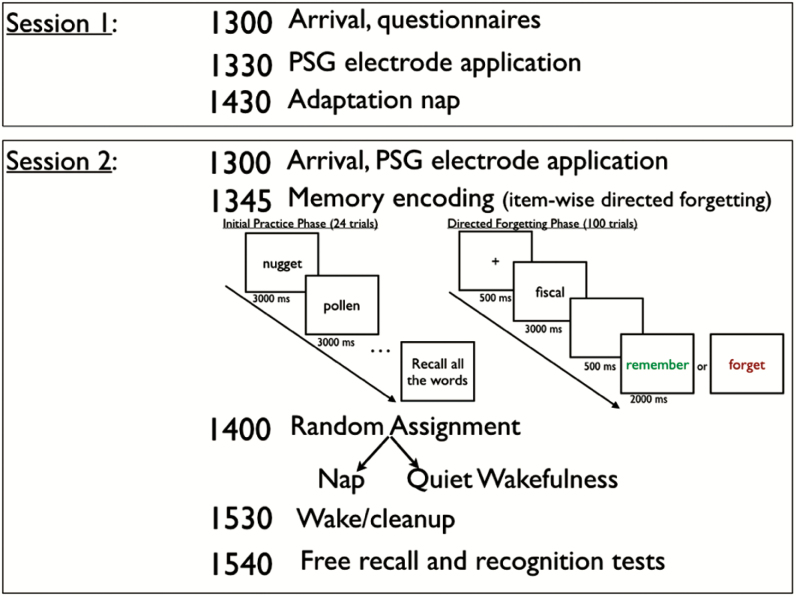
The two-session procedure is depicted in Figure 1. A general strategy was to have all participants come to the sleep laboratory for a qualifying session (Session 1) which served to screen for sleep apnea and dementia and to determine whether the person could nap during the daytime in a sleep laboratory. In Session 2, after participants encoded episodic memories (word learning with directed forgetting), they were randomly assigned to either a 90-minute nap opportunity versus 90 minutes of quiet wakefulness. The participant was then asked to recall and recognize the words from the encoding phase.
Figure 1.
Two-session study protocol, by time of day. Young adults completed the practice phase once whereas older adults completed the practice phase twice to minimize age-related encoding deficits. PSG = polysomnography.
Participants
One hundred and eleven healthy young adults (ages 18–29) and community-dwelling older adults (ages 58–83) were enrolled. Young adults were recruited via campus flyers. Most older adults were recruited from a control population of noncognitively impaired adults who are participating in research at the Emory Alzheimer’s Disease Research Center (ADRC). Older adults recruited from the ADRC all had a Clinical Dementia Rating = 0.33 A few additional older adults were recruited using advertisements and referrals (see Supplementary Table S1); all participants scored 26 or higher on the Mini-Mental State Examination during their qualifying session.34
Screening exclusion criteria were self-reported history of psychiatric, neurological, or cognitive disorders, use of medications that affect sleep architecture, and clinically relevant cognitive impairment (Mini-Mental State Examination < 26 or Clinical Dementia Rating > 0). Screening inclusion criterion was frequency of napping of at least once per month. Six young adults and 10 older adults were lost to follow-up after Session 1, or excluded due to inability to nap in the laboratory during Session 1, being sick during the experimental session, or for later reporting taking medications that affect sleep or memory (antipsychotics, antidepressants, acetylcholinesterase inhibitors). Our analyses focused on the remaining 50 young adults and 45 older adults, and their demographic data are presented in Table 1. Table 1 indicates that individuals assigned to the nap/wake conditions did not differ on age, gender, recent total sleep time, crystallized intelligence (Mill Hill vocabulary), Pittsburgh Sleep Quality Index global score,35 Epworth Sleepiness Scale,36 Morningness–Eveningness Questionnaire global score,37 or Morningness–Eveningness Questionnaire five-group distribution (though, as expected, morning types were more common in the older adults than in the young adults, χ2(4) = 15.18, p = .004).
Table 1.
Demographic Data Across Young and Older Adults and Nap/Wake Conditions.
| Young adults | Older adults | |||
|---|---|---|---|---|
| Nap condition (n = 30) | Wake condition (n = 20) | Nap condition (n = 29) | Wake condition (n = 16) | |
| Age (y) | 21.40 (2.61) | 21.40 (3.39) | 69.69 (7.05) | 70.13 (7.78) |
| Gender (% female) | 66.7% | 60% | 51.7% | 62.5% |
| Education (y) | 14.87 (2.11) | 14.45 (2.01) | 16.16 (3.66) | 15.47 (2.31) |
| Mini-Mental State Examination | 29.20 (0.92) | 28.90 (1.17) | 28.69 (1.20) | 29.13 (0.96) |
| Mill Hill Vocabulary Test | 0.72 (0.09) | 0.72 (0.08) | 0.72 (0.11) | 0.71 (0.16) |
| Race/ethnicity (% Caucasian) | 30% | 20% | 65.5% | 75% |
| Mean total sleep time (1-wk sleep diary) | 8.10 (1.06) | 7.92 (1.36) | 7.77 (1.28) | 7.59 (0.90) |
| Pittsburgh Sleep Quality Index—Global | 4.20 (2.27) | 4.80 (1.11) | 5.03 (2.82) | 5.19 (1.52) |
| Epworth Sleepiness Scale | 8.97 (3.66) | 8.85 (3.76) | 7.83 (3.45) | 8.06 (3.84) |
| Diabetes (self-report) | 0% | 5% | 20.7% | 12.5% |
| Hypertension (self-report) | 0% | 5% | 51.7% | 37.5% |
| MEQ (overall mean score) | 44.52 (9.73) | 46.10 (8.73) | 44.72 (14.46) | 49.25 (14.84) |
| MEQ: 16–30 (definite evening type) | n = 2 | n = 2 | n = 5 | n = 4 |
| MEQ: 31–41 (moderate evening type) | n = 9 | n = 2 | n = 6 | n = 1 |
| MEQ: 42–58 (intermediate type) | n = 16 | n = 16 | n = 11 | n = 5 |
| MEQ: 59–69 (moderate morning) | n = 1 | n = 0 | n = 5 | n = 5 |
| MEQ: 70–86 (definite morning type) | n = 1 | n = 0 | n = 0 | n = 1 |
SDs are presented in parentheses. Note that there were data missing from one young adult and two older adults on the MEQ. MEQ = Morningness-Eveningness Questionnaire.
During Session 2, participants were randomly assigned to either the nap or quiet wakefulness conditions, in a 3:2 ratio. The intent of this approach was to increase statistical power for PSG correlational analyses if a significant effect of nap/wake condition was observed. The only deviation from random assignment occurred for participants who were identified during Session 1 as having at least moderate sleep apnea (Apnea-Hypopnea Index [AHI] > 15 or self-reported diagnosis via prior PSG): Those participants were assigned to the nap group to determine whether higher AHI was associated with lower memory retention across a sleep interval (see Supplementary Table S2, for results suggesting no association). The Emory Institutional Review Board approved this study and individuals received monetary compensation for their participation.
Sleep Recording
PSG measurement was conducted using the Embla N7000 system. We recorded electroencephalography (EEG) from six standard frontal, central, and occipital sites, referenced to contralateral mastoid locations (F3-M2, F4-M1, C3-M2, C4-M1, O1-M2, O2-M1), and grounded with two midline electrodes (Fpz, Cz). The montage also included mentalis electromyography, right and left electrooculography, pulse oximetry, respiratory effort, and a nasal pressure transducer. We did not record anterior tibialis electromyography. Sleep staging followed contemporary scoring criteria.38 Quantitative EEG analyses were performed on a subset of young adults in the nap condition (n = 24) and are presented in the Supplementary Table S3.
Procedure
The two-session procedure is depicted in Figure 1. Prior to each session, participants were instructed to maintain their normal sleep schedule and to keep a sleep diary for 1 week (Table 1). They were further instructed to avoid caffeine, nicotine, and alcohol the day of their study. Session 1 was a baseline, PSG-recorded adaptation nap used for screening and ensuring that participants could nap in the laboratory.
The experimental session (Session 2) was completed approximately 1 week following the first session (Figure 1). Following PSG application, participants completed an encoding phase in which they practiced recalling some words. During this practice phase, participants were first instructed that they would be shown words sequentially and to remember as many as they could for an immediate test. We then presented 24 words sequentially (presentation rate of 3 seconds) and determined participant encoding strength by having them immediately recall those words. To avoid confounding age-related deficits in consolidation with the well-documented age-related deficits at encoding,39,40 the older adults were given a second opportunity to study and recall these words.
Participants then were instructed that for the next block of words, they would be instructed to “remember” (in green font) or “forget” (in red font) individual words (Figure 1). This item-wise directed forgetting procedure41 is used to assess selective encoding and intentional forgetting and has previously been used in sleep and memory retention studies in young adults.21–23 During the test block, participants viewed 100 words sequentially (including the practice words as remember words). For each trial, we presented a fixation cross for 500 ms, the word to study for 3000 ms, a blank screen for 500 ms, and then the instruction to “remember” or “forget” for 2000 ms. There were 50 “remember” words and 50 “forget” words, with item type counterbalanced across participants. A short, self-paced break was given after every 25 trials. The word lists were generated using the English Lexicon Project Database42 and each word was 6 letters, 1–2 syllables, and medium frequency (log hyperspace analogue to language ranged from 4.97 to 8.50, with M = 6.50).
Following the encoding phase, participants were randomly assigned to either a 90-minute nap opportunity or to rest while remaining awake for 90 minutes. In the quiet wakefulness condition, participants sat upright in bed and watched television, and the research technician monitored their EEG to confirm absence of sleep. To minimize possible detrimental effects of sleep inertia, participants in the nap condition were not awakened at the end of the study from slow-wave sleep or REM (in which case, they were allowed to sleep until their first spontaneous arousal). Otherwise, at the 90-minute time mark, participants had their electrodes removed and were given a short break (~10 minutes) before the memory testing began.
We assessed memory consolidation by using both free recall and recognition test measures.43 For the free recall test, participants were given 5 minutes to write down all the words they studied. They were instructed to write all the words they remembered, regardless of the original remember/forget instruction. Next they completed a recognition test on the computer in which they viewed the 100 “old” studied words and 100 “new” lure words (order randomized, and study/lure lists were counterbalanced across participants). Participants were instructed to indicate which words were old words (ie, studied words) and which were new words (ie, nonstudied words). The rationale for including the recognition test was to avoid confounding age-related deficits in consolidation, with known age-related deficits at retrieval. Because recognition tests can rely on familiarity processes, which are automatic processes that are preserved with increasing age,44 older adults often perform as well as young adults on recognition memory tests.45
Statistical Analysis
Free recall responses were scored as hits or false alarms (ie, a word not studied; this metric corrects for guessing) by independent raters who were masked to condition. Total recognition accuracy was calculated as total correct responses (correct hits and correct lure rejections) divided by total number of trials. Recognition performance was further corrected for false alarm rate by calculating d-prime (https://memory.psych.mun.ca/models/dprime/). We used t tests, analyses of variance (ANOVA), and analyses of (co)variance to determine whether condition (nap, quiet wakefulness) differentially affected memory performance in young and older adults. When necessary, we used Levene’s correction for unequal variances. We report Cohen’s d and partial eta squared (ηp2) as measures of effect size. Though we suggest the value of effect sizes is as continuous measures (rather than as categorical measures), typical benchmarks for small, medium, and large effect sizes would be a Cohen’s d of .2, .5, and .8, respectively, or a partial eta squared of .01, .09, and .25, respectively. To protect against Type I error, we only examined correlations between memory measures and PSG variables when the nap condition produced significantly different results than the quiet wakefulness condition for that memory measure.
RESULTS
PSG Results
The PSG features across Sessions 1 and 2 by age groups are displayed in Table 2. In young adults, most PSG features were relatively stable from the first nap (Session 1) to the second nap (Session 2), except for REM sleep and N1. In the older adults, PSG features such as total sleep time, wake after sleep onset, sleep efficiency, N2, and REM were not stable across naps. However, the older adults showed very strong correlations between the first and second naps for slow-wave sleep (N3) duration, AHI, and mean SaO2. The inter-nap correlation was significantly stronger in the older adults than in the young adults for AHI, Z = 2.64, p = .008.
Table 2.
Polysomnographic Data Across Young and Older Adults Who Completed Both Sessions in the Nap Condition.
| Young adults (n = 30) | Older adults (n = 29) | Session 2 age effect (p value) | |||||
|---|---|---|---|---|---|---|---|
| Adaptation nap | Experimental nap | r | Adaptation nap | Experimental nap | r | ||
| Total sleep time (min) | 66.87 (22.95) | 73.20 (21.63) | .44* | 47.23 (21.58) | 54.12 (21.14) | .23 | .001 |
| Sleep efficiency (%) | 69.80 (20.94) | 76.02 (23.75) | .54** | 54.17 (25.77) | 59.49 (21.40) | .17 | .007 |
| Sleep onset latency (min) | 10.22 (8.12) | 7.06 (6.46) | .62*** | 9.40 (8.62) | 8.96 (9.30) | .42* | .36 |
| Wake after sleep onset (min) | 22.99 (25.45) | 12.90 (17.38) | .33† | 27.70 (21.97) | 27.04 (16.15) | .10 | .002 |
| N1 (min) | 16.45 (16.04) | 13.08 (5.44) | .25 | 19.67 (11.28) | 16.59 (10.07) | .42* | .11 |
| N2 (min) | 28.58 (10.66) | 30.52 (12.72) | .45* | 21.05 (16.37) | 30.82 (18.94) | .16 | .94 |
| N3 (min) | 14.85 (12.19) | 18.92 (17.16) | .53** | 5.42 (8.57) | 5.23 (8.48) | .75*** | <.001 |
| REM (min) | 10.00 (12.23) | 10.12 (6.95) | .12 | 1.45 (3.55) | 1.47 (2.98) | .23 | <.001 |
| Apnea-Hypopnea Index | 0.89 (1.65) | 0.98 (1.53) | .31† | 16.53 (22.17) | 16.43 (25.34) | .78*** | .003 |
| Mean SaO2 (%) | 97.01 (1.40) | 97.19 (1.02) | .71*** | 94.08 (1.99) | 93.80 (2.27) | .71*** | <.001 |
SDs in parentheses. Pulse oximeter data were missing for one young adult participant (experimental nap) and one older adult participant (adaptation nap). The correlations (Pearson r) between Session 1 and 2 polysomnography variables are provided within age groups (***p < .001, **p < .01, * p < .05, †p < .10). The p values for the main effects comparing young and older adults for experimental nap (Session 2) polysomnographic variables are provided in the far right column. REM = rapid eye movement.
During Session 2, older adults slept less than did young adults, t(57) = 3.43, p = .001, d = .91, due to greater wake after sleep onset, t(57) = 3.23, p = .002, d = .86, including more breathing events (AHI), t(28.20) = 3.28, p = .003, d = .86 (Levene’s correction). Older adults showed reduced N3 duration, t(42.69) = 3.90, p < .001, d = 1.01 (Levene’s correction), and REM sleep duration, t(39.59) = 6.26, p < .001, d = 1.62 (Levene’s correction).
Pre-Experimental Memory Performance (Encoding Phase)
For memory performance, we first determined whether there were any pre-experimental differences during the encoding phase (ie, immediate testing before PSG). There was a significant age-related increase in total items recalled during the study phase (of 24 possible), F(1, 91) = 7.27, MSE = 11.10, p = .008, ηp2 = .07 (young adults: MNap= 7.73, MWake= 6.45; older adults: MNap= 9.31, MWake= 8.69). This confirmed that our manipulation of having older adults test on the words twice during the encoding phase (and young adults once) produced the intended effect of demonstrating that older adults were capable of learning such word lists if allowed the extra time to do so. There was not a nap/wake condition main effect, F(1, 91) = 1.82, MSE = 11.10, p = .18, ηp2 = .02, or a condition by age group interaction, F < 1, p = .64, ηp2 < .01, demonstrating that assignment to the napping or quiet wakefulness conditions was not biased by pre-experimental memory (encoding) ability.
Post-Nap/Wake Free Recall Performance
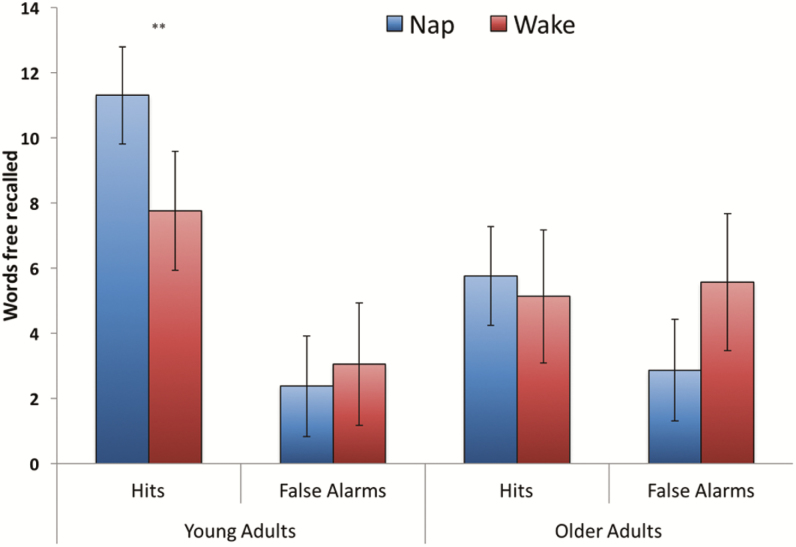
The results of the free recall test (of a possible 100 words) are displayed in Figure 2. We conducted a 2 (hit, false alarm) × 2 (young, older) × 2 (nap, wake) mixed ANOVA. There was a significant age group by condition interaction, F(1, 91) = 5.33, MSE = 12.66, p = .03, ηp2 = .06, indicating that hit rate was greater following the nap rather than following quiet wakefulness in the young adults, t(48) = 2.74, p = .009, d = .79, but not in the older adults, t < 1, p = .58, d = .17. False alarm rates did not differ significantly across conditions in the young adults, t < 1, p = .41, d = .12, or older adults, t(16.71) = 1.61, p = .23, d = .44 (Levene’s correction). The age group by condition interaction remained significant even after controlling for number of words recalled during the encoding phase, F(1, 90) = 5.05, MSE = 12.22, p = .03, ηp2 = .05, mean total sleep time during the previous week (sleep diary), F(1, 90) = 5.22, MSE = 12.66, p = .03, ηp2 = .06, Pittsburgh Sleep Quality Index global score, F(1, 90) = 5.31, MSE = 12.80, p = .02, ηp2 = .06, Epworth Sleepiness Score, F(1, 90) = 5.23, MSE = 12.54, p = .03, ηp2 = .06, and when removing participants with abnormal morningness–eveningness questionnaire scores (>2 SDs from mean), F(1, 86) = 6.72, MSE = 12.88, p = .01, ηp2 = .07. Presence versus absence of sleep apnea did not moderate whether older adults showed evidence in favor of sleep-dependent memory consolidation (Supplementary Table S2). Additional, secondary outcomes from the omnibus ANOVA were a greater number of hits than false alarms, F(1, 91) = 32.28, MSE = 22.25, p < .001, ηp2 = .26, particularly following sleep (MHit = 8.53, MFalse-Alarm = 2.61) relative to wake (MHit = 6.44, MFalse-Alarm = 4.31), F(1, 91) = 7.14, MSE = 22.25, p = .009, ηp2 = .07. The age-related increase in false alarm rate (and decrease in hit rate), F(1, 91) = 15.56, MSE = 22.25, p < .001, ηp2 = .15 (Figure 2), may reflect an underlying age-related deficit in associative memory processing.46 The three-way interaction between hits/false alarms, age group, and nap/wake condition was not significant (F < 1, p = .75, ηp2 < .01).
Figure 2.
Free recall performance across age groups and nap/wake conditions. Error bars reflect 95% confidence intervals. Hits refer to words correctly recalled (max possible: 100) and false alarms refer to words generated on the free recall test that were not previously studied. **p < .01 (all other nap/wake condition comparisons were nonsignificant).
Planned comparisons in the young adult conditions showed that, as predicted by the directed forgetting literature,41 retention was better for “remember” items (M = 7.58) than for “forget” items (M = 0.52), t(49) = 11.94, p < .001, d = 2.34. For “remember” items (out of 50 possible), memory retention was better following a nap (M = 8.57) than quiet wakefulness (M = 6.10), t(48) = 2.11, p = .04, d = .61. For “forget” items (out of 50 possible), recall was at floor levels suggesting caution in interpreting the trend difference between nap (M = 0.67) and wake (M = 0.30) conditions (t(48) = 1.77, p = .08, d = .51). When subtracting “forget” item recall from “remember” item recall, there was a marginally significant nap/wake condition difference, t(48) = 1.78, p = .08, d = .51. When selecting only those words that were practiced and tested during the encoding phase (out of 24 possible), young adults showed significantly better retention following the nap (M = 6.10) than quiet wakefulness (M = 4.0), t(48) = 2.50, p = .02, d = .72.
In the older adults, retention was better for “remember” items (M = 3.47) than for “forget” items (M = 0.24), t(44) = 8.57, p < .001, d = 1.78, but memory retention did not significantly differ across nap and quiet wakefulness conditions for “remember” items (MNap= 3.48, MWake= 3.44; t < 1, p = .95, d = .02), “forget” items (MNap= 0.21, MWake= 0.31; t < 1, p = .53, d = .19), or when limited to words that were practiced and tested during the encoding phase (MNap= 4.93, MWake= 3.63; t(43) = 1.33, p = .19, d = .41). The directed forgetting effect was larger in the young adults than in the older adults, as indicated by an age group difference in “remember” minus “forget” item score, t(81.74) = 5.48, p < .001, d = 1.11 (Levene’s correction). Similar results obtained when limiting analyses to individuals with minimal-to-no sleep apnea or when comparing healthy adults recruited from the ADRC versus non-ADRC general community (Supplementary Tables S1 and S2).
Post-Nap/Wake Recognition Performance
The recognition test data were congruent with the free recall results. There was significantly better recognition performance for “remember” items than “forget” items (young adults: t(49) = 9.63, p < .001, d = 1.37; older adults: t(44) = 6.99, p < .001, d = .65). Furthermore, the directed forgetting effect (“remember” minus “forget” performance) was larger for the young adults than for the older adults, t(87.26) = 3.69, p < .001, d = .75 (Levene’s correction).
Though recognition tests often yield lower effect sizes than free recall tests for measuring memory consolidation, at least in young adults,43 recognition tests avoid floor effects in older adults because recognition can be supported by familiarity retrieval processes, which are preserved with increasing age.45 As demonstrated in Table 3, d-prime analyses showed that the older adults performed statistically as well as the young adults, F(1, 91) = 2.41, MSE = 0.289, p = .12, ηp2 = .03. Furthermore, d-prime analyses showed a significant age group by nap/wake condition interaction, F(1, 91) = 5.85, MSE = 0.289, p = .02, ηp2 = .06. A nap-related benefit to memory retention was observed in the young adults, t(48) = 2.17, p = .03, d = .63, but not in the older adults (t(43) = 1.23, p = .23, d = .38). The age group by condition interaction for d-prime scores remained significant even after controlling for number of words recalled during the encoding phase, F(1, 90) = 5.88, MSE = 0.24, p = .02, ηp2 = .06, mean total sleep time during the previous week (sleep diary), F(1, 90) = 4.65, MSE = 0.31, p = .03, ηp2 = .06, Pittsburgh Sleep Quality Index global score, F(1, 90) = 5.97, MSE = 0.29, p = .02, ηp2 = .06, Epworth Sleepiness Score, F(1, 90) = 6.10, MSE = 0.29, p = .02, ηp2 = .06, and when removing participants with abnormal morningness–eveningness questionnaire scores (>2 SDs from mean), F(1, 86) = 7.64, MSE = 0.28, p = .007, ηp2 = .08. Of particular importance, when minimizing age-related encoding differences by selecting the words that were tested during the encoding phase (twice in older adults, once in younger adults), and minimizing age-related retrieval differences by using a recognition test, there was still no evidence for napping benefiting episodic memory consolidation in the older adult group (t < 1, p = .41, d = .24; Table 3). In other words, even when controlling for other known memory deficits, sleep still benefits memory less in older adults than in young adults.
Table 3.
Mean Proportion of Words Correctly Recognized (or, for Lures, Correctly Rejected) Across Word Type, Age Group, and Nap/Wake Condition.
| Young adults | Older adults | |||
|---|---|---|---|---|
| Nap Condition (n = 30) | Wake Condition (n = 20) | Nap Condition (n = 29) | Wake Condition (n = 16) | |
| Remember words (proportion out of 50) | 0.69 (0.12) | 0.64 (0.16) | 0.69 (0.14) | 0.67 (0.16) |
| Forget words (proportion out of 50) | 0.49 (0.12) | 0.45 (0.16) | 0.59 (0.15) | 0.57 (0.19) |
| Lure words (proportion out of 100) | 0.78 (0.21) | 0.70 (0.18) | 0.73 (0.12) | 0.76 (0.18) |
| Words tested during encoding phase (proportion out of 24) | 0.77 (0.15) | 0.70 (0.14) | 0.83 (0.17) | 0.86 (0.10) |
| Total for all words (proportion out of 200) | 0.69 (0.09) | 0.62 (0.09)a | 0.68 (0.06) | 0.69 (0.07) |
| D-prime for all words | 1.12 (0.67) | 0.73 (0.55)a | 1.02 (0.40) | 1.18 (0.44) |
SDs are presented in parentheses.
aSignificant nap/wake condition main effect within that age group.
PSG—Memory Correlations
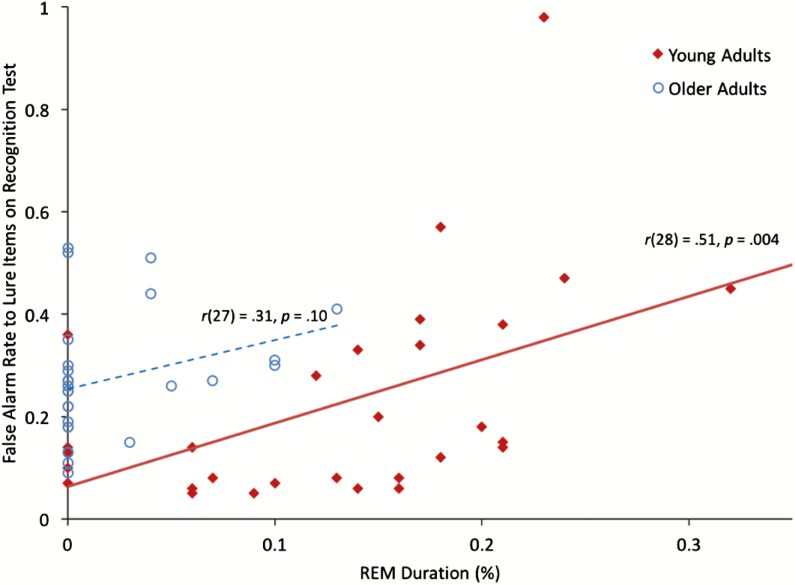
To protect against Type I error rate, we limited our correlational analyses to the primary dependent measures in young adults that showed a significant nap/wake condition effect8: total free recall hits and d-prime recognition accuracy. We found no significant correlations with free recall accuracy and total sleep time, sleep efficiency, wake after sleep onset, sleep stage duration, or AHI (all ps > .05; also see Supplementary Table S4). Though these PSG measures have been implicated in episodic memory consolidation, afternoon naps show restricted range and limited variability in each of these measures (eg, almost all young adult participants had one cycle of N343), thereby decreasing the likelihood of observing a significant correlation.13,14,47 However, d-prime recognition accuracy was negatively correlated with REM sleep duration, r(28) = −.48, p = .008 (REM %: r(28) = −.52, p = .003), even after controlling for number of words recalled during the encoding phase, rp(27) = −.50, p = .006 (REM %: rp(27) = −.53, p = .003), mean total sleep time during the previous week (sleep diary), rp(27) = −.47, p = .009 (REM %: rp(27) = −.52, p = .004), Pittsburgh Sleep Quality Index global score, rp(27) = −.47, p = .01 (REM %: rp(27) = −.53, p = .003), Epworth Sleepiness Score, rp(27) = −.49, p = .007 (REM %: rp(27) = −.53, p = .003), and when removing participants with abnormal morningness–eveningness questionnaire scores (2 SDs from the mean), rp(25) = −.47, p = .01 (REM %: rp(25) = −.51, p = .006). Interestingly, the correlation with REM duration was not observed for remember words, r(28) = .15, p = .44 (REM %: r(28) = .04, p = .83) or forget words, r(28) = .20, p = .29 (REM %: r(28) = .16, p = .39), but as shown in Figure 3, was driven by false alarm rates on lure items, r(28) = .51, p = .004 (REM %: r(28) = .51, p = .004).
Figure 3.
Scatterplot illustrating relationship between REM percent and false alarm rate to lure items (proportion out of 100 possible) on the recognition test in young adults. The older adult data are provided for archival purposes. Note that after removing the outlier young adult data point (at top of graph), the correlation between REM percent and false alarm rate was still significant, r(27) = .51, p = .005. REM = rapid eye movement.
DISCUSSION
One objective of this study was to confirm whether afternoon naps benefit memory functioning in healthy, young adults (for review, see Ficca et al.17). Certainly, napping has previously been demonstrated to promote episodic memory consolidation, and we affirmed those findings using item-wise directed forgetting.13–17,23,48 Whereas much sleep and memory research has attempted to isolate the effects of memory reactivation49 versus stabilization of memories from interference,4–6 both of these interpretations have some limitations when their influences are considered separately. For example, if stabilization against interference was the only factor promoting sleep-related benefits, then one would predict both young and older adults to benefit from the nap. If selective memory reactivation were the only important factor, then one would expect more pronounced evidence for retention of “remember” items over “forget” items. Recent models suggest that a combination of reduced interference and increased memory reactivation (and perhaps other factors) underlie the benefit of sleep on memory,7,8 and we interpret the current results as consistent with this framework.
The broader goal of this work was to determine the extent to which the memory benefits of an afternoon nap observed in young adults also extend to older adults. We found that afternoon naps did not benefit episodic memory in older adults. An afternoon nap showed no significant differences relative to a nearly equal duration of quiet wakefulness for retention of strongly encoded (or weakly encoded) memories or for recognition of studied words. Thus, simply gaining more total sleep time is insufficient to produce memory benefits in older adults.50 By contrast, our findings are compatible with the view that age-related changes in sleep physiology, cognitive processing, or other relevant underlying neurophysiology preclude an afternoon nap from being as strongly restorative for episodic memory in older adults as in healthy young adults. Regarding sleep-specific mechanisms, if a specific quantity/quality of slow-wave sleep is necessary for any memory consolidation to occur, then no nap duration will benefit older adults’ memory if the slow-wave sleep requirement is not met. Regarding cognitive processing mechanisms, if memory consolidation requires that new learning becomes associated with a “tag” of future relevance during encoding,51 and if older adults demonstrate associative encoding deficits,46 then it is possible that older adults will not consolidate memories without successful associative “tagging” at encoding. Regarding neurophysiological mechanisms, there are several brain regions (eg, prefrontal cortex, hippocampus) and neuromodulators (eg, dopamine, acetylcholine) that are hypothesized to be critical to successful memory consolidation, but it is also known that these neurophysiological mechanisms decline with aging.52–54 Therefore, age-related neurophysiological deficits may block memories from being consolidated even during high-quality naps. Sleep, cognitive, and neurophysiological mechanisms deserve further research to inform why memory consolidation declines with increasing age.
The current findings fit within the developing field of studies on memory consolidation, sleep, and aging. Many such studies have compared memory retention across overnight sleep versus daytime wakefulness intervals,8 which assumes that young adults and older adults have similar chronotypes (cf. Table 1). By contrast, the magnitude of age differences in memory functioning depends on the time of learning and testing, such that young adults tend to encode memories better in the evening and older adults tend to encode memories better in the morning.12,55 An afternoon nap paradigm in which all participants encode materials at the same time helps to neutralize these time-of-day confounds. There are a few existing memory consolidation, sleep, and aging studies that have used napping paradigms, but these studies focused on procedural, or motor, memory consolidation.56–59 The important point here is that whereas most research has indicated an age-related change in sleep-dependent procedural memory consolidation,60 some researchers have reported that episodic memory consolidation is preserved in older adults.61 The current evidence compels the conclusion that sleep intervals will not always benefit episodic memory consolidation in older adults, even when using methods that aim to minimize age deficits at encoding and retrieval. Large-scale interventional trials (eg, pharmacologic, behavioral, deep brain stimulation62–65) that manipulate various aspects of sleep in older adults, and include a diversity of cognitive outcomes, will be required to conclude with certainty whether sleep does (or does not) benefit specific cognitive functions.
One limitation of the current study was low free recall performance, which might be expected given the 90-minute retention interval and that our methodology did not require participants to learn words to a ≥80% criterion. The low recall for the “forget” items, however, limits our ability to draw conclusions regarding whether memory consolidation is specific for future-relevant information (for supportive evidence not limited by floor performance, see Rauchs et al.22, Saletin et al.23, and Scullin and McDaniel66). The primary goal of the current research, however, was to examine whether aging affects retention following sleep intervals, and therefore, it is reassuring that we observed the same age by nap/wake condition interactions for both recall and recognition performance. One consideration for future research on cognitive processing mechanisms of age-related changes in memory consolidation should be the specific parameters of the memory task. Whereas one does not normally modify a neuropsychological test (eg, Wechsler Memory Scale), in cognitive psychology experiments researchers customize many procedural details (number of test items, total study repetitions, duration of study, arousal, and valence, etc.), which results in many variants of a task (eg, directed forgetting) in the research literature. Each customization comes with advantages and disadvantages, and researchers should consider and set each design parameter based upon the primary goals of their project. In the current study, we aimed to measure directed forgetting (traditionally, all items studied once), but did not want to miss evidence of memory consolidation if such evidence were to only emerge with multiple study repetitions; thus, we included a subset of words that were studied multiple times.
A second “fly in the ointment” was the lack of significant positive correlations between PSG measures and memory retention. The absence of positive correlations might be due to minimal variability during afternoon naps, the specific types of analyses we conducted, or some unexpected nuisance variable (these explanations, of course, assume a true association between PSG and memory retention67). Despite the lack of significant positive correlations, we observed a negative REM—recognition performance correlation (Figure 3), which would seem to support Crick and Mitchison’s68 idea that REM sleep promotes forgetting: Higher amounts of REM sleep during the nap were related to poorer recognition memory. Although consistent with Crick and Mitchison’s putative function for REM, when analyzed in greater detail, we noted that the association in Figure 3 was due entirely to accuracy on the “lure” items. In other words, individuals with greater REM sleep were more likely to falsely believe that lure words were studied words (even after controlling for encoding strength and recent sleep duration). This implies that REM sleep might promote integration of associations among learned information and result in false memories,24 a phenomenon partially subsumed by cognitive psychologists under the rubric of “gist memory.” Such a finding could conceivably bear relevance for REM-related phenomenology across a broad range of psychiatric conditions (eg, posttraumatic stress disorder).69
SUPPLEMENTARY MATERIAL
Supplementary data are available at Sleep online.
FUNDING
This work was supported by National Institute on Aging (F32-AG041543, 2P50AG025688-06), Sleep Research Society Foundation Early Career Development Award, and the Cottrell Foundation.
DISCLOSURE STATEMENT
MKS, JF, and MJD have no conflicts of interest to disclose. DLB is a Consultant for Merck and Ferring. This was not an industry-supported study.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Derelle Kirksey, Kaleb Swanson, Chandini Virenjan, Bjorn Anderson, Mericyn Daunis, and Natalya Pruett for their assistance with data collection and analysis.
REFERENCES
- 1. Rasch B, Born J. About sleep’s role in memory. Physiol Rev. 2013; 93(2): 681–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wilson MA, McNaughton BL. Reactivation of hippocampal ensemble memories during sleep. Science. 1994; 265(5172): 676–679. [DOI] [PubMed] [Google Scholar]
- 3. Louie K, Wilson MA. Temporally structured replay of awake hippocampal ensemble activity during rapid eye movement sleep. Neuron. 2001; 29(1): 145–156. [DOI] [PubMed] [Google Scholar]
- 4. Wixted JT. The psychology and neuroscience of forgetting. Annu Rev Psychol. 2004; 55(1): 235–269. [DOI] [PubMed] [Google Scholar]
- 5. Mednick SC, Cai DJ, Shuman T, Anagnostaras S, Wixted JT. An opportunistic theory of cellular and systems consolidation. Trends Neurosci. 2011; 34(10): 504–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rickard TC, Cai DJ, Rieth CA, Jones J, Ard MC. Sleep does not enhance motor sequence learning. J Exp Psychol Learn Mem Cogn. 2008; 34(4): 834–842. [DOI] [PubMed] [Google Scholar]
- 7. Berry JA, Cervantes-Sandoval I, Chakraborty M, Davis RL. Sleep facilitates memory by blocking dopamine neuron-mediated forgetting. Cell. 2015; 161(7): 1656–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Scullin MK, Bliwise DL. Sleep, cognition, and normal aging: integrating a half century of multidisciplinary research. Perspect Psychol Sci. 2015; 10(1): 97–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lo JC, Groeger JA, Cheng GH, Dijk DJ, Chee MW. Self-reported sleep duration and cognitive performance in older adults: a systematic review and meta-analysis. Sleep Med. 2016; 17: 87–98. [DOI] [PubMed] [Google Scholar]
- 10. Yaffe K, Falvey CM, Hoang T. Connections between sleep and cognition in older adults. Lancet Neurol. 2014; 13(10): 1017–1028. [DOI] [PubMed] [Google Scholar]
- 11. Ju YE, Lucey BP, Holtzman DM. Sleep and Alzheimer disease pathology—a bidirectional relationship. Nat Rev Neurol. 2014; 10(2): 115–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schmidt C, Collette F, Cajochen C, Peigneux P. A time to think: circadian rhythms in human cognition. Cogn Neuropsychol. 2007; 24(7): 755–789. [DOI] [PubMed] [Google Scholar]
- 13. Lo JC, Dijk DJ, Groeger JA. Comparing the effects of nocturnal sleep and daytime napping on declarative memory consolidation. PLoS ONE. 2014; 9(9): e108100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tucker MA, Fishbein W. Enhancement of declarative memory performance following a daytime nap is contingent on strength of initial task acquisition. Sleep. 2008; 31(2): 197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rudoy JD, Voss JL, Westerberg CE, Paller KA. Strengthening individual memories by reactivating them during sleep. Science. 2009; 326(5956): 1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schabus M, Hödlmoser K, Pecherstorfer T, Klösch G. Influence of midday naps on declarative memory performance and motivation. Somnologie. 2005; 9(3): 148–153. [Google Scholar]
- 17. Ficca G, Axelsson J, Mollicone DJ, Muto V, Vitiello MV. Naps, cognition and performance. Sleep Med Rev. 2010; 14(4): 249–258. [DOI] [PubMed] [Google Scholar]
- 18. Zacks RT, Radvansky G, Hasher L. Studies of directed forgetting in older adults. J Exp Psychol Learn Mem Cogn. 1996; 22(1): 143–156. [DOI] [PubMed] [Google Scholar]
- 19. Titz C, Verhaeghen P. Aging and directed forgetting in episodic memory: a meta-analysis. Psychol Aging. 2010; 25(2): 405–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sahakyan L, Delaney PF, Goodmon LB. Oh, honey, I already forgot that: strategic control of directed forgetting in older and younger adults. Psychol Aging. 2008; 23(3): 621–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Abel M, Bäuml KH. Sleep can eliminate list-method directed forgetting. J Exp Psychol Learn Mem Cogn. 2013; 39(3): 946–952. [DOI] [PubMed] [Google Scholar]
- 22. Rauchs G, Feyers D, Landeau B, et al. Sleep contributes to the strengthening of some memories over others, depending on hippocampal activity at learning. J Neurosci. 2011; 31(7): 2563–2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Saletin JM, Goldstein AN, Walker MP. The role of sleep in directed forgetting and remembering of human memories. Cereb Cortex. 2011; 21(11): 2534–2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stickgold R, Walker MP. Sleep-dependent memory triage: evolving generalization through selective processing. Nat Neurosci. 2013; 16(2): 139–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Campbell SS, Murphy PJ, Stauble TN. Effects of a nap on nighttime sleep and waking function in older subjects. J Am Geriatr Soc. 2005; 53(1): 48–53. [DOI] [PubMed] [Google Scholar]
- 26. Milner CE, Cote KA. A dose-response investigation of the benefits of napping in healthy young, middle-aged and older adults. Sleep Biol Rhythms 2008; 6(1): 2–15. [Google Scholar]
- 27. Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004; 27(7): 1255–1273. [DOI] [PubMed] [Google Scholar]
- 28. Raz N, Lindenberger U, Rodrigue KM, et al. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb Cortex. 2005; 15(11): 1676–1689. [DOI] [PubMed] [Google Scholar]
- 29. Bäckman L, Nyberg L, Lindenberger U, Li SC, Farde L. The correlative triad among aging, dopamine, and cognition: current status and future prospects. Neurosci Biobehav Rev. 2006; 30(6): 791–807. [DOI] [PubMed] [Google Scholar]
- 30. Grady CL. Age-related changes in the functional neuroanatomy of memory. In: Naveh-Benjamin M, Moscovitch M, Roediger HL, III, eds. Perspectives on Human Memory and Cognitive Aging: Essays in Honor of Fergus Craik. New York, NY: Psychology Press; 2013: 325–333. [Google Scholar]
- 31. Scullin MK. Sleep, memory, and aging: the link between slow-wave sleep and episodic memory changes from younger to older adults. Psychol Aging. 2013; 28(1): 105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chan MY, Park DC, Savalia NK, Petersen SE, Wig GS. Decreased segregation of brain systems across the healthy adult lifespan. Proc Natl Acad Sci USA. 2014; 111(46): E4997–E5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Morris JC. Clinical Dementia Rating: a reliable and valid diagnostic and staging measure for dementia of the Alzheimer type. Int Psychogeriatr. 1997; 9(Suppl 1): 173–176; discussion 177. [DOI] [PubMed] [Google Scholar]
- 34. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975; 12(3): 189–198. [DOI] [PubMed] [Google Scholar]
- 35. Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989; 28(2): 193–213. [DOI] [PubMed] [Google Scholar]
- 36. Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991; 14(6): 540–545. [DOI] [PubMed] [Google Scholar]
- 37. Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976; 4(2): 97–110. [PubMed] [Google Scholar]
- 38. Iber C, Ancoli-Israel S, Chesson A, Quan S. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 39. Craik FIM, Byrd M. Aging and cognitive deficits. In: Craik FIM, Trehub S, eds. Aging and Cognitive Processes. New York, NY: Plenum Press; 1982: 191–211. [Google Scholar]
- 40. Grady CL, McIntosh AR, Craik FI. Age-related differences in the functional connectivity of the hippocampus during memory encoding. Hippocampus. 2003; 13(5): 572–586. [DOI] [PubMed] [Google Scholar]
- 41. MacLeod CM. Long-term recognition and recall following directed forgetting. J Exp Psychol: Learn Mem Cogn 1975; 1(3): 271–279. [Google Scholar]
- 42. Balota DA, Yap MJ, Cortese MJ, et al. The English Lexicon Project. Behav Res Methods. 2007; 39(3): 445–459. [DOI] [PubMed] [Google Scholar]
- 43. Diekelmann S, Wilhelm I, Born J. The whats and whens of sleep-dependent memory consolidation. Sleep Med Rev. 2009; 13(5): 309–321. [DOI] [PubMed] [Google Scholar]
- 44. Yonelinas AP. The nature of recollection and familiarity: a review of 30 years of research. J Mem Language 2002; 46(3): 441–517. [Google Scholar]
- 45. Jennings JM, Jacoby LL. An opposition procedure for detecting age-related deficits in recollection: telling effects of repetition. Psychol Aging. 1997; 12(2): 352–361. [DOI] [PubMed] [Google Scholar]
- 46. Naveh-Benjamin M, Shing YL, Kilb A, Werkle-Bergner M, Lindenberger U, Li SC. Adult age differences in memory for name-face associations: the effects of intentional and incidental learning. Memory. 2009; 17(2): 220–232. [DOI] [PubMed] [Google Scholar]
- 47. Alger SE, Lau H, Fishbein W. Slow wave sleep during a daytime nap is necessary for protection from subsequent interference and long-term retention. Neurobiol Learn Mem. 2012; 98(2): 188–196. [DOI] [PubMed] [Google Scholar]
- 48. Tucker MA, Hirota Y, Wamsley EJ, Lau H, Chaklader A, Fishbein W. A daytime nap containing solely non-REM sleep enhances declarative but not procedural memory. Neurobiol Learn Mem. 2006; 86(2): 241–247. [DOI] [PubMed] [Google Scholar]
- 49. Rasch B, Büchel C, Gais S, Born J. Odor cues during slow-wave sleep prompt declarative memory consolidation. Science. 2007; 315(5817): 1426–1429. [DOI] [PubMed] [Google Scholar]
- 50. Monk TH, Buysse DJ, Carrier J, Billy BD, Rose LR. Effects of afternoon “siesta” naps on sleep, alertness, performance, and circadian rhythms in the elderly. Sleep. 2001; 24(6): 680–687. [DOI] [PubMed] [Google Scholar]
- 51. Bennion KA, Payne JD, Kensinger EA. Selective effects of sleep on emotional memory: what mechanisms are responsible? Transl Issues Psychol Sci 2015; 1(1): 79–88. [Google Scholar]
- 52. Mander BA, Rao V, Lu B, et al. Prefrontal atrophy, disrupted NREM slow waves and impaired hippocampal-dependent memory in aging. Nat Neurosci. 2013; 16(3): 357–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Scullin MK, Trotti LM, Wilson AG, Greer SA, Bliwise DL. Nocturnal sleep enhances working memory training in Parkinson’s disease but not Lewy body dementia. Brain. 2012; 135(Pt 9): 2789–2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hornung OP, Regen F, Danker-Hopfe H, Schredl M, Heuser I. The relationship between REM sleep and memory consolidation in old age and effects of cholinergic medication. Biol Psychiatry. 2007; 61(6): 750–757. [DOI] [PubMed] [Google Scholar]
- 55. May CP, Hasher L, Stoltzfus ER. Optimal time of day and the magnitude of age differences in memory. Psychol Sci. 1993; 4(5): 326–330. [Google Scholar]
- 56. Backhaus W, Braass H, Renné T, Gerloff C, Hummel FC. Motor performance is not enhanced by daytime naps in older adults. Front Aging Neurosci. 2016; 8: 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Gudberg C, Wulff K, Johansen-Berg H. Sleep-dependent motor memory consolidation in older adults depends on task demands. Neurobiol Aging. 2015; 36(3): 1409–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Korman M, Dagan Y, Karni A. Nap it or leave it in the elderly: a nap after practice relaxes age-related limitations in procedural memory consolidation. Neurosci Lett. 2015; 606: 173–176. [DOI] [PubMed] [Google Scholar]
- 59. Fogel SM, Albouy G, Vien C, et al. fMRI and sleep correlates of the age-related impairment in motor memory consolidation. Hum Brain Mapp. 2014; 35(8): 3625–3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Spencer RM, Gouw AM, Ivry RB. Age-related decline of sleep-dependent consolidation. Learn Mem. 2007; 14(7): 480–484. [DOI] [PubMed] [Google Scholar]
- 61. Aly M, Moscovitch M. The effects of sleep on episodic memory in older and younger adults. Memory. 2010; 18(3): 327–334. [DOI] [PubMed] [Google Scholar]
- 62. Paßmann S, Külzow N, Ladenbauer J, et al. Boosting slow oscillatory activity using tDCS during early nocturnal slow wave sleep does not improve memory consolidation in healthy older adults. Brain Stimul. 2016; 9(5): 730–739. [DOI] [PubMed] [Google Scholar]
- 63. Ladenbauer J, Külzow N, Passmann S, et al. Brain stimulation during an afternoon nap boosts slow oscillatory activity and memory consolidation in older adults. Neuroimage. 2016; 142: 311–323. [DOI] [PubMed] [Google Scholar]
- 64. Eggert T, Dorn H, Sauter C, Nitsche MA, Bajbouj M, Danker-Hopfe H. No effects of slow oscillatory transcranial direct current stimulation (tDCS) on sleep-dependent memory consolidation in healthy elderly subjects. Brain Stimul. 2013; 6(6): 938–945. [DOI] [PubMed] [Google Scholar]
- 65. Westerberg CE, Florczak SM, Weintraub S, et al. Memory improvement via slow-oscillatory stimulation during sleep in older adults. Neurobiol Aging. 2015; 36(9): 2577–2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Scullin MK, McDaniel MA. Remembering to execute a goal: sleep on it! Psychol Sci. 2010; 21(7): 1028–1035. [DOI] [PubMed] [Google Scholar]
- 67. Ackermann S, Hartmann F, Papassotiropoulos A, de Quervain DJ, Rasch B. No associations between interindividual differences in sleep parameters and episodic memory consolidation. Sleep. 2015; 38(6): 951–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Crick F, Mitchison G. The function of dream sleep. Nature. 1983; 304(5922): 111–114. [DOI] [PubMed] [Google Scholar]
- 69. Krystal AD, Stein MB, Szabo ST. Anxiety disorders and posttraumatic stress disorder. In: Kryger MH, Roth T, Dement WC, eds. Principles and Practice of Sleep Medicine. 6th ed Philadelphia, PA: Elsevier; 2016: 1341–1351. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.