Abstract
Background: The relation between α-linolenic acid (ALA), a plant-derived omega-3 (n–3) fatty acid, and age-related macular degeneration (AMD) is unclear. European researchers reported that ≤40% of ALA can be present as trans forms.
Objective: We aimed to evaluate the associations between intake of ALA and intermediate and advanced AMD.
Design: Seventy-five thousand eight hundred eighty-nine women from the Nurses’ Health Study and 38,961 men from Health Professionals Follow-Up Study were followed up from 1984 to 2012 and from 1986 to 2010, respectively. We assessed dietary intake by a validated food-frequency questionnaire at baseline and every 4 y thereafter. One thousand five hundred eighty-nine incident intermediate and 1356 advanced AMD cases (primarily neovascular AMD) were confirmed by medical record review.
Results: The multivariable-adjusted HR for intermediate AMD comparing ALA intake at the top quintile to the bottom quintile was 1.28 (95% CI: 1.05, 1.56; P-trend = 0.01) in the analyses combining 2 cohorts. The HR in each cohort was in the positive direction but reached statistical significance only in the women. However, the positive association was apparent only in the pre-2002 era in each cohort and not afterward (P-time interaction = 0.003). ALA intake was not associated with advanced AMD in either time period. Using gas-liquid chromatography, we identified both cis ALA (mean ± SD: 0.13% ± 0.04%) and trans ALA isomers (0.05% ± 0.01%) in 395 erythrocyte samples collected in 1989–1990. In stepwise regression models, mayonnaise was the leading predictor of erythrocyte concentrations of cis ALA and one isomer of trans ALA. We also found trans ALA in mayonnaise samples.
Conclusions: A high intake of ALA was associated with an increased risk of intermediate AMD before 2002 but not afterward. The period before 2002 coincides with the same time period when trans ALA was found in food and participants’ blood; this finding deserves further study.
Keywords: α-linolenic acid, omega-3 fatty acids, trans fat, age-related macular degeneration, prospective cohort study, food-frequency questionnaire
INTRODUCTION
Age-related macular degeneration (AMD)12 is a chronic, progressive disease that can cause irreversible blindness (1). According to the 2005–2008 US NHANES, the estimated prevalence in persons aged ≥40 y was 5.7% for early or intermediate AMD compared with 0.8% for advanced AMD (2). Because the number of patients with AMD is expected to grow exponentially in the next few decades in the United States and worldwide as populations age (3–5), the identification of potential strategies for primary prevention would have marked public health significance.
Marine long-chain omega-3 fatty acids, including EPA and DHA, have been associated with a lower risk of AMD in multiple observational studies (6–10). However, epidemiologic research on the shorter-chain ω-3 fatty acid, α-linolenic acid (ALA), has yielded inconsistent findings. Some studies reported a positive association between the risk of AMD and intakes of ALA (6, 11) and vegetable-fat foods (12–14) that contain ALA whereas others did not (7, 8, 15, 16). Additional studies of ALA’s effects are needed because this plant-derived fatty acid accounts for ≥85% of total ω-3 fatty acid intake in the United States (17) and presumably even more in parts of the world where access to fish is limited.
trans ALA is formed during partial hydrogenation, deep frying, and industrial deodorization (18, 19) and is hypothesized to interfere with the crucial biological functions of cis ALA. trans ALA has not received much attention in the United States. However, European researchers have reported the presence of trans isomers of ALA in food such as vegetable oils (18, 20), low-calorie spreads (21), and infant formulas (22, 23). Up to 40% of ALA can be present as trans isomers (19, 20). trans ALA also occurs in human serum (24) and maternal milk (25, 26).
We previously reported a positive association between the intake of ALA and AMD (6). With a 16-y-longer follow-up and 2500 more AMD cases than our previous study, our primary objective was to further evaluate this association. Our secondary aim was to examine trans ALA isomers in both foods and human bodies in the United States.
METHODS
Study population
The NHS (Nurses’ Health Study), initiated in 1976, is an ongoing prospective cohort that includes 121,700 US female registered nurses aged between 30 and 55 y at baseline. The HPFS (Health Professionals Follow-up Study), initiated in 1986, includes 51,529 US male health professionals aged between 40 and 75 y at baseline. Both cohorts are predominantly white. Participants are mailed biennial questionnaires about lifestyle factors and disease outcomes and food frequency questionnaires (FFQs) every 4 y to assess diet in the preceding years. The study protocol was approved by the Institutional Review Boards at the Brigham and Women’s Hospital and Harvard T.H. Chan School of Public Health.
We restricted the study population to those who were ≥50 y old at baseline and then added participants to the analysis once they reached age 50 y. Participants were censored at age 90 y to alleviate the concern of lower ascertainment of AMD cases (NHS, n = 15; HPFS, n = 528). At baseline, we excluded participants who did not return the initial FFQ, left >70 food items blank in the FFQ, reported implausible dietary intake (<600 or >3500 kcal/d for the NHS and <800 or >4200 kcal/d for the HPFS), or had prevalent AMD, cancer (except nonmelanoma skin cancer), diabetes, and cardiovascular disease. To minimize detection bias, we also excluded participants who never reported an eye exam over the entire follow-up period and skipped the person-time during any 2-y questionnaire interval in which they did not report an eye exam. After exclusions, a total of 75,889 women and 38,961 men contributed to the analysis. The flowchart of the study participants can be found at the Supplemental Figure 1.
AMD ascertainment
Our case validation and definition have been previously described (27). When a participant reported a diagnosis of AMD on a biennial questionnaire, we requested informed consent to review his or her medical record and then contacted the participant’s eye doctor to either complete a standardized questionnaire or send us copies of ocular records to confirm the diagnosis. The questionnaire requested information from the medical record on the date of initial diagnosis, treatment received for AMD, clinical signs of AMD, best corrected visual acuity, and whether in the opinion of the treating doctor the visual acuity loss was primarily due to AMD. Photos and/or optical coherence tomography were also reviewed when available to confirm clinical AMD lesions. Cases with only small hard drusen (drusen size <63-μm diameter circle) were excluded. We defined intermediate AMD as having ≥1 of the following signs in ≥1 eye: intermediate drusen (≥63 and <125 μm), pigment abnormalities, large drusen (≥125 μm), or any noncentral geographic atrophy (GA). We defined neovascular AMD as having any of the following signs in ≥1 eye: retinal pigment epithelium detachment, subretinal neovascular membrane, disciform scar, or history of treatment with laser, photodynamic, or anti–vascular endothelial growth factor therapy for AMD. Central GA was defined as having a central GA lesion involving the center of the macula in ≥1 eye. Advanced AMD included both neovascular AMD and central GA. Additionally, all case definitions, except those recent neovascular AMD cases that had anti–vascular endothelial growth factor therapy, included a visual acuity of 20/30 or worse primarily due to AMD. This magnitude of vision loss is not only of clinical significance but also is severe enough to warrant medical attention so as to minimize potential detection bias arising from differential health consciousness. The person was used as the unit of analysis, and the worst eye was used for classification.
Dietary assessment
We began follow-up in 1986 for the HPFS and 1984 for the NHS, when the first comprehensive FFQ with 131 items was administered, and assessed dietary intake approximately every 4 y thereafter. Intake of ALA is from many foods, but the major sources among FFQ items include 1) mayonnaise or other creamy salad dressing (1 tablespoon or 15 mL); 2) oil and vinegar dressing (e.g., Italian; 1 tablespoon or 15 mL); 3) beef, pork, or lamb as a main dish (4–6 ounces or 113–170 g); 4) margarine (1 pat or 5 g); and 5) other cheese (e.g., American, cheddar; 1 slice, 1 ounce or 28 g). These foods collectively accounted for 46% of ALA intake in the NHS and 38% in the HPFS at baseline. However, the total contribution from these foods decreased to 16% in 2010 in the NHS and 12% in 2006 in the HPFS. Walnut consumption (1 ounce or 28 g) was included in the 1998 FFQ and onward, which became the top contributor to ALA intake (18% in the NHS and 17% in the HPFS). Low-fat mayonnaise consumption (1 tablespoon or 15 mL) was included in the 1994 FFQ and onward. We started asking about the intake of flax seed oil and flax seed (yes or no) from the 2006 FFQ in the NHS and further inquired about the frequency of intake for flax seed (1 tablespoon or 14 g) in the 2010 FFQ in both the NHS and HPFS. Participants were asked to report how often, on average over the past year, they had consumed each food item (9 possible responses ranging from “≤1 time/mo” to “≥6 times/d”). The FFQs specifically inquired about the usual type of fat used for frying, sautéing, and baking. It also inquired about the usual brand and type of margarine by using an open-ended question. Such information was taken into account when calculating ALA intake. The daily nutrient intake was calculated by multiplying the consumption frequency of each food by its nutrient content and then summing across all foods. The fatty acid composition of food fried, baked, or sautéed at home was modified by the type of fat reported. The nutrient composition data were primarily based on the USDA Nutrient Database supplemented with information from manufacturers and published reports. We adjusted all the nutrient intakes for total energy using the residual method to reflect the composition of the diet (28).
The reproducibility and validity of FFQs in measuring fat intake has recently been assessed in the Women’s Lifestyle Validation Study (29), an extensive validation study that involved >700 women from the NHS and NHS II. After correcting for random within-person error in the diet records, the Spearman correlation between the intake of energy-adjusted ALA from the FFQ and from two 1-wk diet records was 0.57 (95% CI: 0.48, 0.65). Similarly, the de-attenuated correlation for ALA between the FFQ and four 24-h dietary recalls was 0.58.
Measurement of trans ALA and cis ALA
To confirm the presence of trans ALA in food supplies and in human bodies, we measured trans ALA among erythrocyte samples (n = 395) from the NHS participants by gas-liquid chromatography and reanalyzed some existing chromatography of mayonnaise samples collected before and after 2000. The methods were described elsewhere (30). Each trans ALA peak was identified by comparison with trans ALA standards (linolenic acid methyl ester isomer mix; Sigma-Aldrich), and the amount was expressed as the percentage of the total peak area. We identified 4 trans ALA isomers: trans ALA-A and -B have 2 trans double bonds whereas trans ALA-C and -D have 1 trans double bond. The concentration of erythrocyte trans ALA-A was extremely low and below the meaningful concentration (mean <0.01%) and was thus not included in the analysis. The overall CVs for trans ALA-B, -C, and -D were 23.6%, 33.4% and 40.8%, respectively. Higher CVs for trans ALA-C and -D were likely caused by the overlapping of those 2 peaks with 11t-Eicosenoic acid (20:1n-9t) and 8c-Eicosenoic acid (20:1n-12c), respectively. However, because the within-batch CV for each peak was <20% (an acceptable limit), we statistically accounted for the high overall CVs by recalibrating the concentration of each trans ALA isomer from all batches to its average batch according to a method outlined by Rosner et al. (31). Because trans ALA-C and -D peaks usually overlapped in chromatography, we combined those 2 in the analysis to reduce measurement error. We estimated the reproducibility of erythrocyte trans ALA by reanalyzing existing chromatography from an earlier within-person stability study in which 40 postmenopausal women gave 3 blood samples over 2–3 y (32). In that study, the intraclass correlation for cis ALA was 0.52. The intraclass correlation we calculated was 0.53 for trans ALA-B, 0.56 for trans ALA-C, and 0.43 for trans ALA-D, all indicating fair-to-good reproducibility.
To more accurately determine food sources of blood cis ALA, we also obtained previous measurements of erythrocyte and plasma cis ALA from participants of nested case-control studies of cardiovascular disease in the NHS and HPFS. The details on blood collection and measurements have been previously described (33, 34).
Statistical analysis
Participants contributed person-time to the analysis from the return of the baseline questionnaire if >50 y of age at baseline or from reaching 50 y old to the confirmed diagnosis of AMD, death, loss to follow-up, or the end of follow-up (31 May 2012 for the NHS and 31 January 2010 for the HPFS), whichever occurred first. To best represent long-term intake and minimize measurement error (35), we calculated the cumulative average intake of ALA by averaging all available FFQs up to the start of each 2-y risk interval. The cumulative average value was categorized into quintiles based on the distribution in each cohort. Likewise, we calculated the cumulative average intakes of foods and categorize them into prespecified groups.
We used time-varying Cox proportional hazards models to estimate the HRs and 95% CIs associated with intermediate and advanced AMD, respectively. To control as finely as possible for confounding by age and calendar time and any possible 2-way interactions between these 2 time scales, we stratified the model jointly by age in months at the start of follow-up and calendar year of the current questionnaire cycle. We controlled for established and probable risk factors including race, BMI (in kg/m2), pack-years of smoking, physical activity, aspirin use, history of hypertension, history of hypercholesterolemia, menopausal status and postmenopausal hormone use (in the NHS only), and dietary variables including intakes of total energy, linoleic acid (LA), and DHA.
Because of a change of major food sources to ALA intake around the early 2000s, which would potentially introduce a varying degree of confounding, we conducted a stratified analysis to evaluate the associations between intakes of ALA and AMD in the pre-2002 and post-2002 era. The early 2000s also marked a potential change in trans ALA content over time because there was an increasing public awareness of the adverse health effects by trans fatty acids and food industries started to reduce or eliminate them (36, 37). Several studies found a downward trend of total trans intake (38) and plasma concentrations (39) around that time. We calculated the cumulative average of ALA intake separately in each time period. We created product terms between the time-varying ALA variable and the binary indicator for time period and used a likelihood ratio test to test the significance of interaction by time comparing models with and without the product terms. A similar approach was used to test interactions between the intake of ALA and prespecified risk factors including age, smoking status, and menopausal hormone use.
We used stepwise linear regressions to identify foods that were significantly predictive of the blood measurements of cis and trans ALA (P < 0.05). This method has been described previously (27, 40). Briefly, we used the average of food intake from the 1986 and 1990 FFQs in the NHS and from the 1990 and 1994 FFQs in the HPFS to reduce the within-person variation of intake and to correspond with the time of blood draw. We developed the linear regression model by the biomarker type (erythrocyte and plasma) in each cohort.
We performed the statistical analyses separately in each cohort using SAS 9.3 (SAS Institute). To derive a pooled HR, we first combined the 2 cohorts and then applied a Cox proportional hazards model in the pooled data stratified by the cohort. Interpretation of the data was mainly based on pooled HRs unless otherwise specified. All hypothesis tests were 2-sided and used an α level of 0.05.
RESULTS
During 28 y of follow-up in the NHS and 24 y in the HPFS, we confirmed 2219 incident AMD cases (1209 intermediate and 1010 advanced cases) in women and 726 cases (380 intermediate and 346 advanced cases) in men. The advanced AMD cases were predominantly neovascular AMD (96%).
In 1998 (the middle of the follow-up period), participants’ demographic and lifestyle characteristics did not markedly vary across quintiles of ALA intake except that those in the higher-intake category were more likely to smoke cigarettes and have a higher BMI and were less likely to have hypercholesterolemia (Table 1). In terms of dietary intake, participants with higher intake of ALA were also more likely to have higher intakes of DHA, LA, trans fat, and saturated fat but overall a higher healthy-eating score.
TABLE 1.
Age-standardized characteristics of participants in the NHS and HPFS according to cumulative average intake of ALA in 1998 (the middle of follow-up)1
| ALA intake |
|||||
| Q1 | Q2 | Q3 | Q4 | Q5 | |
| NHS | |||||
| ALA, mg/d | 738 ± 64 | 860 ± 25 | 943 ± 24 | 1036 ± 32 | 1242 ± 153 |
| Participants, n | 13,257 | 13,246 | 13,264 | 13,258 | 13,245 |
| Age, y | 64 ± 7 | 63 ± 7 | 63 ± 7 | 64 ± 7 | 64 ± 7 |
| BMI, kg/m2 | 26.1 ± 5.0 | 26.6 ± 5.1 | 26.8 ± 5.2 | 27.0 ± 5.4 | 27.1 ± 5.5 |
| Caucasian, % | 96 | 98 | 98 | 98 | 96 |
| Current smoker, % | 10 | 9 | 10 | 10 | 12 |
| Never smoker, % | 47 | 47 | 46 | 45 | 41 |
| Physical activity, MET-h/wk | 11 (4–24) | 11 (4–24) | 11 (4–23) | 10 (4–23) | 10 (3–22) |
| Hypertension, % | 44 | 44 | 44 | 44 | 44 |
| Hypercholesterolemia, % | 59 | 58 | 57 | 56 | 54 |
| Postmenopausal, % | 94 | 94 | 94 | 94 | 94 |
| Current menopausal hormone use,2 % | 44 | 45 | 46 | 45 | 45 |
| Current aspirin use, % | 47 | 49 | 49 | 49 | 47 |
| Dietary intake | |||||
| DHA, mg/d | 133 ± 83 | 137 ± 81 | 140 ± 82 | 139 ± 84 | 143 ± 91 |
| LA, g/d | 7.3 ± 1.5 | 8.1 ± 1.4 | 8.7 ± 1.3 | 9.3 ± 1.4 | 10.8 ± 1.9 |
| trans Fat, g/d | 2.5 ± 0.7 | 2.7 ± 0.7 | 2.9 ± 0.8 | 2.9 ± 0.8 | 3.0 ± 0.9 |
| Saturated fat, g/d | 17.7 ± 3.6 | 19.0 ± 3.5 | 19.7 ± 3.8 | 20.4 ± 4.0 | 21.3 ± 4.5 |
| Monounsaturated fat, g/d | 19.2 ± 3.6 | 20.8 ± 3.4 | 21.7 ± 3.6 | 22.5 ± 3.7 | 23.7 ± 4.0 |
| Alternative healthy-eating index | 50.6 ± 9.7 | 50.8 ± 9.3 | 51.0 ± 9.2 | 51.2 ± 9.3 | 52.1 ± 9.4 |
| Total energy intake, kcal/d | 1695 ± 434 | 1743 ± 436 | 1770 ± 433 | 1770 ± 433 | 1732 ± 441 |
| HPFS | |||||
| ALA, mg/d | 840 ± 79 | 987 ± 30 | 1085 ± 28 | 1196 ± 38 | 1445 ± 196 |
| Participants, n | 6148 | 6115 | 6161 | 6091 | 6180 |
| Age, y | 64 ± 9 | 64 ± 9 | 64 ± 9 | 64 ± 9 | 64 ± 9 |
| BMI, kg/m2 | 25.5 ± 3.2 | 26.0 ± 3.5 | 26.3 ± 3.6 | 26.4 ± 3.7 | 26.4 ± 3.7 |
| Caucasian, % | 94 | 95 | 96 | 96 | 95 |
| Current smoker, % | 5 | 5 | 4 | 5 | 6 |
| Never smoker, % | 52 | 51 | 51 | 49 | 48 |
| Physical activity, MET-h/wk | 24 (9–47) | 24 (10–47) | 24 (10–47) | 23 (10–47) | 23 (9–47) |
| Hypertension, % | 38 | 38 | 37 | 36 | 37 |
| Hypercholesterolemia, % | 51 | 50 | 48 | 47 | 45 |
| Current aspirin use, % | 61 | 63 | 63 | 62 | 58 |
| Dietary intake | |||||
| DHA, mg/d | 206 ± 142 | 206 ± 127 | 203 ± 130 | 206 ± 133 | 213 ± 158 |
| LA, g/d | 9.3 ± 2.1 | 10.2 ± 1.9 | 10.8 ± 1.8 | 11.6 ± 1.8 | 13.2 ± 2.4 |
| trans Fat, g/d | 2.8 ± 1.1 | 3.1 ± 1.0 | 3.3 ± 1.0 | 3.4 ± 1.1 | 3.4 ± 1.1 |
| Saturated fat, g/d | 19.7 ± 4.8 | 21.7 ± 4.5 | 22.9 ± 4.7 | 23.8 ± 4.9 | 24.9 ± 5.5 |
| Monounsaturated fat, g/d | 23.3 ± 5.1 | 25.6 ± 4.6 | 26.9 ± 4.5 | 28.1 ± 4.6 | 29.5 ± 5.1 |
| Alternative healthy-eating index | 54.0 ± 10.4 | 53.9 ± 9.8 | 53.6 ± 9.9 | 53.8 ± 9.9 | 55.3 ± 10.1 |
| Total energy intake, kcal/d | 1934 ± 520 | 1985 ± 520 | 1997 ± 527 | 2009 ± 528 | 1968 ± 543 |
Values are means ± SDs or medians (IQRs) unless otherwise indicated. ALA, α-linolenic acid; HPFS, Health Professionals Follow-up Study; LA, linoleic acid; MET-h, hours of metabolic equivalent tasks; NHS, Nurses’ Health Study; Q, quintile.
Current menopausal hormone use among postmenopausal women.
ALA intake was moderately correlated (average Pearson r = 0.62) with LA intake, the analogous ω-6 fatty acid that shares many food sources with ALA, but weakly correlated with intake of food-sourced EPA (r = 0.10) or DHA (r = 0.06). During the follow-up, the age-adjusted intake of ALA increased ∼50% in the NHS from 1984 (median intake, 1.03 g/d) to 2010 (1.58 g/d) and 29% in the HPFS from 1986 (1.12 g/d) to 2006 (1.44 g/d). In contrast, the intake of food-sourced EPA and DHA did not appreciably change during the follow-up (data not shown).
In a pooled analysis between the NHS and HPFS comparing extreme quintiles, intakes of ALA had a statistically significant positive association with intermediate AMD (comparing extreme quintiles, HR: 1.31; 95% CI: 1.21, 1.53; P-trend <0.001), and this association was essentially unaltered after further adjusting for LA or saturated, monounsaturated, and trans fatty acids (Table 2). The multivariable-adjusted HR for ALA was in the positive direction in each cohort, although it was statistically significant only in the NHS. For the baseline 1986 FFQ in the HPFS, we further separated the intake of ALA by animal and plant sources (such information was not available in the NHS). The median baseline intake was 350 mg/d for animal-sourced ALA and 700 mg/d for plant-sourced ALA, and they were weakly negatively correlated (Pearson r = −0.22). By comparing extreme quintiles, plant-sourced ALA (adjusted HR: 1.54; 95% CI 1.04, 2.28; P-trend = 0.03) was more strongly associated with intermediate AMD than the animal-sourced counterpart was (adjusted HR: 1.17; 95% CI: 0.85, 1.62; P-trend = 0.22). When they were mutually adjusted for each other, the HR did not materially change for plant-sourced ALA (adjusted HR: 1.60; 95% CI: 1.08, 2.38; P-trend = 0.02) or for animal-sourced ALA (adjusted HR: 1.23; 95% CI: 0.89, 1.70; P-trend = 0.13). By comparing extreme quintiles, the intake of LA also had a positive association with intermediate AMD (adjusted HR: 1.22; 95% CI: 1.05, 1.42; P-trend = 0.006); however, after further adjusting for ALA, the association was attenuated and no longer significant (adjusted HR: 1.06; 95% CI: 0.88, 1.27; P-trend = 0.50).
TABLE 2.
The HRs (95% CIs) of AMD according to quintiles of the cumulative average intake of α-linolenic acid1
| Q1 | Q2 | Q3 | Q4 | Q5 | P-trend | |
| Intermediate AMD | ||||||
| NHS | ||||||
| Median, mg/d | 760 | 875 | 963 | 1065 | 1252 | |
| Cases/person-years | 211/300,584 | 237/301,701 | 203/302,772 | 273/301,809 | 285/302,175 | |
| Age-adjusted model | 1 (Ref) | 1.19 (0.99, 1.43) | 1.02 (0.84, 1.24) | 1.36 (1.14, 1.63) | 1.38 (1.15, 1.64) | <0.001 |
| Multivariate model 1 | 1 (Ref) | 1.17 (0.97, 1.41) | 1.01 (0.83, 1.22) | 1.33 (1.11, 1.60) | 1.34 (1.12, 1.61) | <0.001 |
| Multivariate model 2 | 1 (Ref) | 1.21 (1.00, 1.47) | 1.05 (0.85, 1.29) | 1.38 (1.12, 1.69) | 1.31 (1.05, 1.64) | 0.02 |
| HPFS | ||||||
| Median, mg/d | 850 | 990 | 1093 | 1213 | 1425 | |
| Cases/person-years | 74/122,869 | 76/123,263 | 67/122,309 | 75/123,512 | 88/122,996 | |
| Age-adjusted model | 1 (Ref) | 1.06 (0.77, 1.47) | 0.95 (0.68, 1.32) | 1.06 (0.77, 1.47) | 1.22 (0.90, 1.67) | 0.20 |
| Multivariate model 1 | 1 (Ref) | 1.04 (0.76, 1.44) | 0.93 (0.67, 1.30) | 1.03 (0.75, 1.43) | 1.20 (0.88, 1.64) | 0.24 |
| Multivariate model 2 | 1 (Ref) | 1.03 (0.75, 1.43) | 0.92 (0.65, 1.30) | 1.01 (0.72, 1.43) | 1.18 (0.82, 1.69) | 0.37 |
| Pooled (NHS + HPFS) | ||||||
| Multivariate model 1 | 1 (Ref) | 1.15 (0.97, 1.34) | 0.99 (0.84, 1.17) | 1.26 (1.07, 1.47) | 1.31 (1.12, 1.53) | <0.001 |
| Multivariate model 2 | 1 (Ref) | 1.16 (0.99, 1.37) | 1.01 (0.85, 1.21) | 1.28 (1.07, 1.52) | 1.27 (1.05, 1.54) | 0.01 |
| Multivariate model 3 | 1 (Ref) | 1.17 (0.99, 1.38) | 1.02 (0.85, 1.22) | 1.28 (1.07, 1.54) | 1.28 (1.05, 1.56) | 0.01 |
| Advanced AMD | ||||||
| NHS | ||||||
| Median, mg/d | 760 | 875 | 963 | 1065 | 1252 | |
| Cases/person-years | 189/300,571 | 196/301,736 | 212/302,750 | 195/301,877 | 218/302,246 | |
| Age-adjusted model | 1 (Ref) | 1.10 (0.90, 1.35) | 1.20 (0.99, 1.46) | 1.09 (0.89, 1.33) | 1.19 (0.98, 1.44) | 0.14 |
| Multivariate model 1 | 1 (Ref) | 1.07 (0.88, 1.31) | 1.15 (0.94, 1.40) | 1.04 (0.85, 1.27) | 1.12 (0.92, 1.36) | 0.40 |
| Multivariate model 2 | 1 (Ref) | 1.06 (0.86, 1.30) | 1.13 (0.91, 1.40) | 1.01 (0.81, 1.27) | 1.07 (0.84, 1.36) | 0.77 |
| HPFS | ||||||
| Median, mg/d | 850 | 990 | 1093 | 1213 | 1425 | |
| Cases/person-years | 66/122,877 | 73/123,272 | 67/122,316 | 66/123,518 | 74/123,014 | |
| Age-adjusted model | 1 (Ref) | 1.15 (0.82, 1.60) | 1.05 (0.75, 1.48) | 1.02 (0.73, 1.44) | 1.14 (0.81, 1.58) | 0.66 |
| Multivariate model 1 | 1 (Ref) | 1.11 (0.80, 1.55) | 1.02 (0.73, 1.44) | 0.97 (0.69, 1.36) | 1.08 (0.77, 1.51) | 0.88 |
| Multivariate model 2 | 1 (Ref) | 1.09 (0.78, 1.53) | 0.98 (0.69, 1.40) | 0.90 (0.63, 1.31) | 1.02 (0.69, 1.49) | 0.81 |
| Pooled (NHS + HPFS) | ||||||
| Multivariate model 1 | 1 (Ref) | 1.09 (0.92, 1.29) | 1.12 (0.94, 1.32) | 1.02 (0.86, 1.22) | 1.11 (0.94, 1.32) | 0.40 |
| Multivariate model 2 | 1 (Ref) | 1.07 (0.90, 1.28) | 1.09 (0.91, 1.31) | 0.98 (0.81, 1.19) | 1.06 (0.86, 1.30) | 0.88 |
| Multivariate model 3 | 1 (Ref) | 1.06 (0.89, 1.27) | 1.08 (0.89, 1.30) | 0.97 (0.80, 1.18) | 1.05 (0.85, 1.30) | 0.92 |
Multivariate Cox regression model 1 included age (continuous), race (Caucasian or not), BMI (in kg/m2: <18.5, 18.5–23, 23–25, 25–30, 30–35, or >35), pack-years of smoking (never, 1–9, 10–24, 25–44, 45–64, or ≥65 y), physical activity (<3, 3–8.9, 9–17.9, 18–26.9, or ≥27 h of metabolic equivalent tasks/wk), current aspirin use (≥1 tablet/wk or none), history of hypertension and hypercholesterolemia, DHA (quintiles), and total energy intake (quintiles). In the NHS, models were additionally adjusted for postmenopausal status and menopausal hormone use (never, current, and past). Multivariate Cox regression model 2 = multivariate model 1 + linoleic acid (quintiles). Multivariate Cox regression model 3 = multivariate model 1 + linoleic acid + monounsaturated fat + saturated fat + trans fat (all in quintiles). P-trend was calculated by modeling the median value of each category as a continuous variable. AMD, age-related macular degeneration; HPFS, Health Professionals Follow-up Study; NHS, Nurses’ Health Study; Ref, reference.
In contrast, we did not find any significant association between advanced AMD and the intake of ALA after multivariable adjustment (Table 2) or for LA (pooled HR for comparing extreme quintiles: 1.08; 95% CI: 0.91, 1.28; P-trend = 0.25).
To understand whether the association with intermediate AMD was specific to ALA, we examined the intakes of several other correlated fatty acids including cis 18:1, trans 18:1, cis 18:2, trans 18:2, saturated fat, and total trans fatty acids. By comparing extreme quintiles, intakes of cis 18:1, cis 18:2, trans 18:2, and saturated fat each had a significant positive association with intermediate AMD (Supplemental Figure 2). However, all the associations were attenuated and no longer statistically significant after adjusting for ALA, whereas in all scenarios ALA persisted with a significant positive association (Supplemental Figure 2). On the other hand, intakes of trans 18:1 (P-trend = 0.05), trans 18:2 (P-trend = 0.04), and total trans fatty acids (P-trend = 0.03) had a suggestively significant association with advanced AMD after adjusting for ALA (Supplemental Figure 3). The association between the intake of ALA and intermediate AMD was apparent only among never smokers (pooled HR comparing extreme quintiles: 1.66; 95% CI: 1.23, 2.22; P-trend = 0.001) compared with ever smokers (pooled HR comparing extreme quintiles: 1.07; 95% CI: 0.83, 1.37; P-trend = 0.51) (P-interaction = 0.10) (Supplemental Table 1). In postmenopausal women, the HR for the association between intake of ALA and intermediate AMD among current users of exogenous hormones was 1.84 (95% CI: 1.16, 2.92; P-trend = 0.04; n = 315 cases), whereas the HR among non–current users was 1.17 (95% CI: 0.89, 1.54; P-trend = 0.17; n = 789 cases) and P-interaction = 0.01. When we further restricted this analysis to the pre-2002 era, the HR among current users of exogenous hormones was 2.20 (95% CI: 1.27, 3.82; P-trend = 0.03; n = 235 cases) and non–current users was 1.12 (95% CI: 0.76, 1.63; P-trend = 0.35; n = 380 cases) and P-interaction = 0.005. There was no significant interaction by age (Supplemental Table 2).
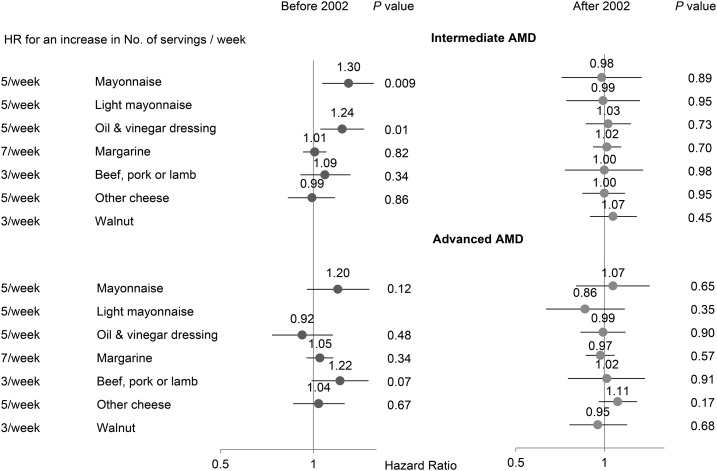
In the stratified analysis by time, by comparing extreme quintiles the intake of ALA was positively associated with intermediate AMD before 2002 but not after, and the interaction by time period was significant (P = 0.003) (Table 3). There was no significant association between the intake of ALA and advanced AMD in either time period. Mayonnaise and oil-and-vinegar salad dressing were both positively associated with intermediate AMD before 2002, but neither was after 2002, nor did any other major ALA-bearing foods (Figure 1). In a sensitivity analysis, we used 1998 instead of 2002 as the cutoff for the 2 time periods. The association between the intake of ALA and intermediate AMD in each time period did not materially change; the HR comparing extreme quintiles was 1.36 (95% CI: 1.00, 1.83; P-trend = 0.02) before 1998 and 1.00 (95% CI: 0.78, 1.26; P-trend = 0.91) after 1998, but the P-interaction was slightly attenuated (P = 0.08).
TABLE 3.
The HRs (95% CIs) of AMD according to quintiles of cumulative average intake of ALA before and after 20021
| Q1 | Q2 | Q3 | Q4 | Q5 | P-trend | P-interaction | |
| Intermediate AMD | |||||||
| Beginning to 2002 | |||||||
| NHS (n = 683 cases) | |||||||
| Median, mg/d | 748 | 865 | 957 | 1060 | 1242 | ||
| Multivariate model 2 | 1 (Ref) | 1.18 (0.91, 1.53) | 1.00 (0.75, 1.33) | 1.36 (1.03, 1.80) | 1.33 (0.98, 1.80) | 0.05 | |
| HPFS (n = 249 cases) | |||||||
| Median, mg/d | 830 | 970 | 1075 | 1197 | 1410 | ||
| Multivariate model 2 | 1 (Ref) | 1.05 (0.69, 1.59) | 0.90 (0.57, 1.40) | 1.16 (0.75, 1.80) | 1.50 (0.95, 2.38) | 0.06 | |
| 2002 to end | |||||||
| NHS (n = 499 cases) | |||||||
| Median, mg/d | 710 | 875 | 1010 | 1190 | 1620 | ||
| Multivariate model 2 | 1 (Ref) | 0.94 (0.71, 1.24) | 1.10 (0.83, 1.45) | 0.89 (0.66, 1.20) | 0.85 (0.62, 1.17) | 0.25 | |
| HPFS (n = 124 cases) | |||||||
| Median, mg/d | 870 | 1050 | 1200 | 1380 | 1790 | ||
| Multivariate model 2 | 1 (Ref) | 1.05 (0.60, 1.83) | 1.14 (0.66, 1.99) | 1.23 (0.70, 2.17) | 0.81 (0.43, 1.54) | 0.57 | |
| Pooled (NHS + HPFS) | |||||||
| Beginning to 2002 | 1 (Ref) | 1.13 (0.91, 1.41) | 0.96 (0.76, 1.22) | 1.30 (1.03, 1.64) | 1.36 (1.06, 1.75) | 0.008 | 0.003 |
| 2002 to end | 1 (Ref) | 0.96 (0.75, 1.23) | 1.12 (0.87, 1.43) | 0.95 (0.73, 1.24) | 0.85 (0.64, 1.13) | 0.21 | |
| Advanced AMD | |||||||
| Beginning to 2002 | |||||||
| NHS (n = 435 cases) | |||||||
| Median, mg/d | 748 | 865 | 957 | 1060 | 1242 | ||
| Multivariate model 2 | 1 (Ref) | 1.17 (0.84, 1.64) | 1.44 (1.03, 2.01) | 1.10 (0.77, 1.59) | 1.12 (0.76, 1.64) | 0.91 | |
| HPFS (n = 189 cases) | |||||||
| Median, mg/d | 830 | 970 | 1075 | 1197 | 1410 | ||
| Multivariate model 2 | 1 (Ref) | 1.01 (0.64, 1.60) | 1.01 (0.62, 1.63) | 0.97 (0.58, 1.59) | 1.03 (0.60, 1.79) | 0.96 | |
| 2002 to end | |||||||
| NHS (n = 546 cases) | |||||||
| Median, mg/d | 710 | 875 | 1010 | 1190 | 1620 | ||
| Multivariate model 2 | 1 (Ref) | 0.78 (0.60, 1.02) | 0.93 (0.71, 1.22) | 1.07 (0.82, 1.40) | 0.82 (0.60, 1.11) | 0.54 | |
| HPFS (n = 147 cases) | |||||||
| Median, mg/d | 870 | 1050 | 1200 | 1380 | 1790 | ||
| Multivariate model 2 | 1 (Ref) | 0.69 (0.39, 1.21) | 1.21 (0.73, 1.99) | 0.83 (0.48, 1.44) | 0.95 (0.55, 1.65) | 0.93 | |
| Pooled (NHS + HPFS) | |||||||
| Beginning to 2002 | 1 (Ref) | 1.11 (0.85, 1.46) | 1.28 (0.97, 1.68) | 1.05 (0.78, 1.41) | 1.09 (0.79, 1.49) | 0.96 | 0.14 |
| 2002 to end | 1 (Ref) | 0.76 (0.59, 0.96) | 0.98 (0.78, 1.24) | 1.01 (0.79, 1.29) | 0.84 (0.65, 1.10) | 0.59 |
Multivariate Cox regression model 2 was the same as in Table 2. The number of cases does not add up to the number in Table 2 because participants missing ALA intake in 2002 were excluded from the analysis of the post-2002 era. P-trend was calculated by modeling the median value of each category as a continuous variable. P-interaction was calculated by creating product terms between the time-varying ALA variable and the binary indicator for time period and testing the significance by a likelihood ratio test. ALA, α-linolenic acid; AMD, age-related macular degeneration; HPFS, Health Professionals Follow-up Study; NHS, Nurses’ Health Study; Q, quintile; Ref, reference.
FIGURE 1.
The associations of primary α-linolenic acid–containing foods with intermediate and advanced AMD. Each food was modeled as a continuous variable in a multivariate Cox regression model that included age (continuous), race (Caucasian or not), BMI (in kg/m2: <18.5, 18.5–23, 23–25, 25–30, 30–35, or >35), pack-years of smoking (never, 1–9, 10–24, 25–44, 45–64, or ≥65 y), physical activity (<3, 3–8.9, 9–17.9, 18–26.9, or ≥27 h metabolic equivalent tasks/wk), current aspirin use (≥1 tablet/wk or none), history of hypertension, history of hypercholesterolemia, and total calories (quintiles). In the Nurses’ Health Study, models were additionally adjusted for postmenopausal status and menopausal hormone use (never, current, and past). AMD, age-related macular degeneration.
We further assessed the interplay between DHA and ALA by examining their joint effects. Within each tertile of DHA, the HR increased as the intake of ALA increased from the bottom to the top tertile (Supplemental Figure 4). Compared with the intake amount at the top tertile of DHA but the bottom tertile of ALA, the intake at the bottom tertile of DHA and the top tertile of ALA had a 46% (HR: 1.46; 95% CI: 1.17, 1.83) increased risk of intermediate AMD, but the interaction between ALA and DHA was not statistically significant (P = 0.16). There was no apparent interaction between ALA and LA (P-interaction = 0.69).
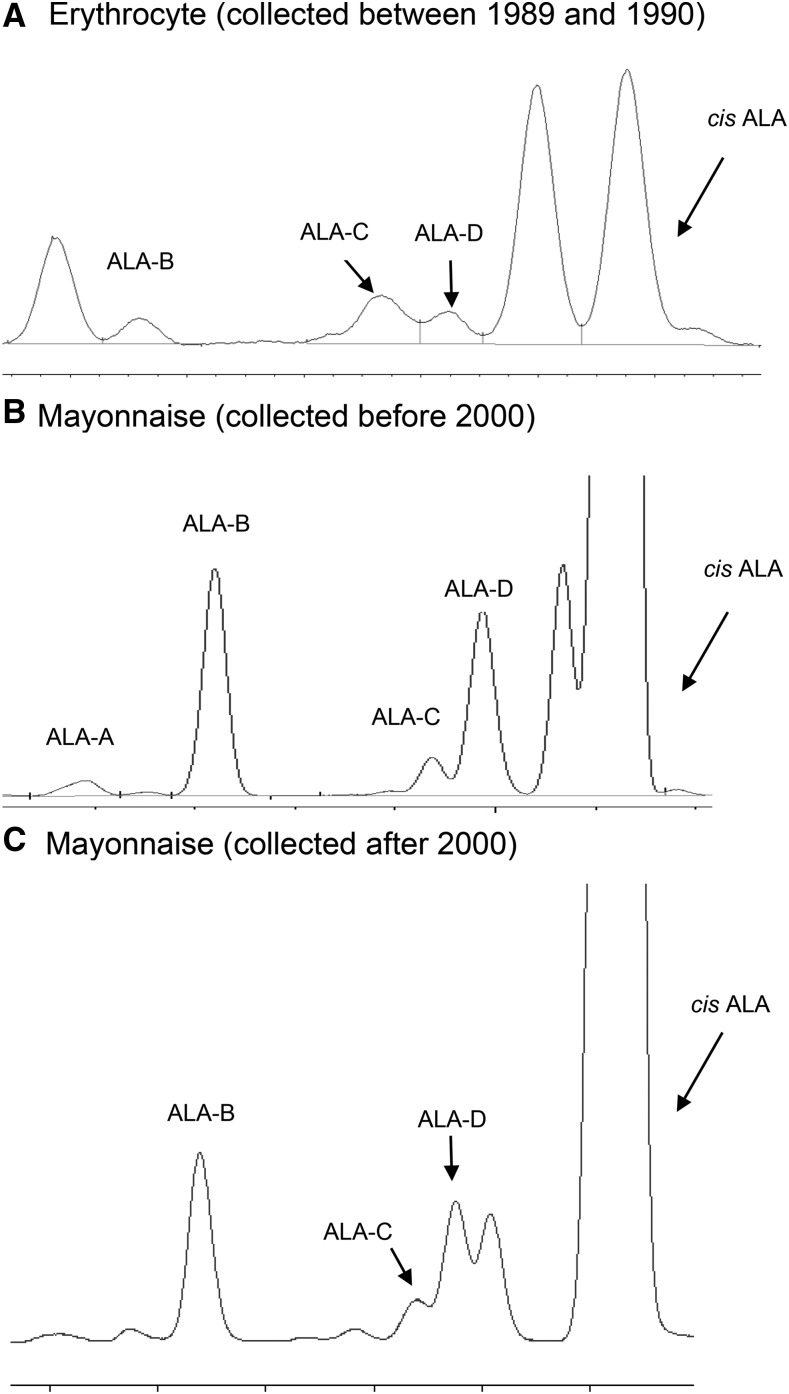
We identified 4 trans ALA isomers in erythrocytes and mayonnaise: trans ALA-A, -B, -C, and -D (Figure 2). The amount of total trans ALA (mean ± SD: 0.05% ± 0.01%) was lower compared with cis ALA (0.13% ± 0.04%) in erythrocytes (Supplemental Table 3). Among individual isomers of trans ALA, the correlation with cis ALA was much stronger for trans ALA-B (Pearson r = 0.65) than for trans ALA-C/-D (r = 0.17) (Supplemental Table 4). Mayonnaise was the leading significant predictor for both cis ALA and trans ALA-B (Supplemental Table 3). Mayonnaise was also positively related to the plasma and erythrocyte concentrations of cis ALA among previously measured samples from our cohorts (Supplemental Table 5). When the 2 representative mayonnaise samples collected before and after 2000 were further compared, the percentage of trans ALA among total ALA (cis and trans) decreased from 11.8% to 5.0%. Comparatively, the percentage of trans 18:2 among total 18:2 (cis and trans) decreased from 0.88% to 0.69%.
FIGURE 2.
Partial gas-liquid chromatography of trans and cis ALA isomers. ALA-A and ALA-B are trans ALAs with 2 trans bonds; ALA-C and ALA-D are trans ALAs with 1 trans bond. In all erythrocyte (A) and mayonnaise (B and C) samples, the amount of trans ALA-A was extremely low and could not always be identified. ALA, α-linolenic acid.
DISCUSSION
In this large, prospective study with 24–28 y of follow-up, a higher intake of ALA was associated with a modest, increased risk of visually significant intermediate AMD but not with advanced AMD. This association was independent of intakes of other correlated fatty acids and could not be attributed to obvious sources of confounding. However, when further stratified by the time period, the ALA intake only in the pre-2002 era seemed harmful. No increase in risk of AMD was seen after that time. In accordance with European studies, we confirmed the presence of a small amount of trans ALA isomers in both food and human blood samples in the United States that were collected in the pre-2002 era when partially hydrogenated oils were more prevalent.
The intake of ALA was positively associated only with the risk of intermediate AMD but not with advanced AMD. Correspondingly, intakes of EPA and DHA were also associated only with intermediate AMD but in the opposite direction (41). The reasons for the differential associations have been described (41). Briefly, we speculate that advanced AMD and a subset of intermediate AMD are 2 different disease processes, not just a continuum of progression, given the diverse clinical manifestations and underlying genetic profiles of AMD. Our AMD ascertainment scheme may have accrued mostly those intermediate AMD cases that were slow progressing or not destined to progress further.
Among the previous 6 epidemiologic studies (6–8, 11, 14, 16) that had specific intake information for ALA, 2 studies with dietary data collected before 2002 (6, 11) showed a significant positive association between the intake of ALA and intermediate AMD. Measurement error arising from a single assessment of diet and/or a small number of cases may have obscured a modest association for ALA in the other studies. No prior studies (7, 14, 16) have showed any significant association with advanced AMD, and the numbers of cases were very small (<80 cases). In 2 studies (12, 13), a higher intake of vegetable fat was related to an elevated risk of advanced AMD, but information on ALA was not available.
The lack of association between ALA intake and AMD after ∼2002 strongly suggests that ALA is not the primary causal factor. We considered the reduction in trans ALA as a possible explanation. In accordance with European literature, we were able to confirm trans ALA in foods and blood samples of US participants. Furthermore, one isomer of trans ALA shared the same food, mayonnaise, with the cis ALA. Information on the trans ALA intake in the United States is virtually nonexistent likely because the intake of its precursor cis ALA is already much lower compared with oleic acid and LA, the precursors to major trans fatty acids in the US diet. For example, according to NHANES 2009–2010 data, the mean intakes are 1.50 g ALA/d, 26 g oleic acid/d, and 15 g LA/d (42). However, studies suggested that ALA is 12–15 times more easily isomerized into trans forms than LA is because of the more unsaturated nature of ALA and ≤40% of ALA can be present as trans isomers in edible oils (19, 20). Based on a few US food samples (e.g., mayonnaise, white bread, muffins), we crudely estimated the ratio of trans ALA to cis ALA was 1:7.5. By assuming that this ratio is generalizable to other trans ALA-containing foods, this translates into an intake of 0.2 g trans ALA/d on the basis of a mean intake of 1.5 g ALA/d in the US population (17), which is slightly lower but in the same magnitude as the estimated intake among Dutch and Scottish (0.5–0.7 g/d) and French participants (0.2–0.4 g/d) (24). Our study and the European study (24) both suggested a low concentration of erythrocyte trans ALA (∼0.05% of total fatty acids) under a habitual diet. However, a study among 50 Indian participants with a high use (86%) of ALA-rich cooking oils, canola and mustard, and a high consumption of fried snacks and sweets (>3 d/wk) showed a markedly high concentration of trans ALA in serum, adipose, and cheek epithelium tissues (content ranging from 1.2% to 1.8%) (43).
trans ALA seems to be a plausible explanation for our findings. Long-term feeding rats of a diet high in trans ALA severely disturbed visual function and resulted in a significant linear increase in trans DHA and a decrease in cis DHA (44, 45). In humans, trans ALA is absorbed and incorporated into tissue lipids (24). Small amounts of trans EPA and DHA are present in human platelets (46). We speculate that the intake of ALA may mostly reflect the intake of trans ALA, particularly in the pre-2002 era when partially hydrogenated oils were more prevalent. The positive association between the intake of ALA and intermediate AMD in the pre-2002 era may be mediated by trans ALA that was converted to trans DHA in vivo, which interfered with the function of natural cis DHA in the retina. This hypothesized mechanism is consistent with our finding that a higher intake of ALA was related to a much greater risk of intermediate AMD among postmenopausal women taking exogenous hormones. Exogenous hormones are known to enhance Δ6 desaturase activities and were found to increase the conversion from ALA to DHA by 62% in women (47, 48). One recent study also showed an increased erythrocyte concentration of DHA in women using hormone replacement therapy compared with women who were not (49). On the other hand, trans ALA may affect the risk of AMD by its own metabolic effects, rather than through conversion to trans DHA. In a European study where 88 healthy men were randomly assigned to receive either a diet high (1.41 g/d) or low (0.06 g/d) in trans ALA for 6 wk, the concentration of trans ALA in plasma lipids was significantly increased compared with that of the control group (24). This study further showed a statistically significant increase in ratio of HDL to LDL in the high–trans ALA group (50).
Our study has several limitations. As with other observational studies, we cannot exclude confounding or chance in our findings. Measurements of some trans ALA isomers in the food and blood may lack precision given that they co-eluted with other fatty acids. The diagnosis of AMD, especially intermediate AMD, may be susceptible to misclassification because the initial detection was done by the participant’s eye physician in a clinical examination. We tried to reduce misclassification by requesting and reviewing photos and/or optical coherence tomography when available to confirm clinical AMD lesions. However, we expect the misclassification to be nondifferential with respect to the intake of ALA, which would lead to an underestimation of the true association.
In summary, the higher intake of the shorter-chain ω-3 fatty acid, ALA, was associated with an increased risk of intermediate AMD before 2002; the intake of ALA after 2002 does not appear to increase the risk of AMD. This finding is important because of other health benefits of this essential fatty acid. We found a small amount of trans ALA in US food and participant’s blood, particularly before 2002 when partially hydrogenated oils were prevalent, and we hypothesize that this may account for the different relations between the intake of ALA and the risk of AMD over time. This possible mechanism deserves further investigation because in many countries partially hydrogenated oils are widely used or the quality of industrial deodorization of vegetable oils is suboptimal.
Acknowledgments
We thank Stephanie Chiuve for kindly granting us the access to the blood samples for trans ALA analysis. We also thank Hannia Campos, Jeremy Furtado, and Ashley Drake at the Nutritional Biomarker Laboratory at Harvard T.H. Chan School of Public Health for analyzing the blood sample data.
The authors’ responsibilities were as follows—JW: analyzed the data; JW, DAS, and WCW: wrote the manuscript; JW: had primary responsibility for the final content; and all authors: designed the study, and read and approved the final manuscript. None of the authors reported a conflict of interest related to the study.
Footnotes
Abbreviations used: ALA, α-linolenic acid; AMD, age-related macular degeneration; FFQ, food-frequency questionnaire; GA, geographic atrophy; HPFS, Health Professionals Follow-up Study; LA, linoleic acid; NHS, Nurses’ Health Study.
REFERENCES
- 1.Coleman HR, Chan CC, Ferris FL III, Chew EY. Age-related macular degeneration. Lancet 2008;372:1835–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klein R, Chou CF, Klein BE, Zhang X, Meuer SM, Saaddine JB. Prevalence of age-related macular degeneration in the US population. Arch Ophthalmol 2011;129:75–80. [DOI] [PubMed] [Google Scholar]
- 3.Friedman DS, O’Colmain BJ, Munoz B, Tomany SC, McCarty C, de Jong PT, Nemesure B, Mitchell P, Kempen J. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol 2004;122:564–72. [DOI] [PubMed] [Google Scholar]
- 4.Wong WL, Su X, Li X, Cheung CM, Klein R, Cheng CY, Wong TY. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health 2014;2:e106–16. [DOI] [PubMed] [Google Scholar]
- 5.Rein DB, Wittenborn JS, Zhang X, Honeycutt AA, Lesesne SB, Saaddine J. Forecasting age-related macular degeneration through the year 2050: the potential impact of new treatments. Arch Ophthalmol 2009;127:533–40. [DOI] [PubMed] [Google Scholar]
- 6.Cho E, Hung S, Willett WC, Spiegelman D, Rimm EB, Seddon JM, Colditz GA, Hankinson SE. Prospective study of dietary fat and the risk of age-related macular degeneration. Am J Clin Nutr 2001;73:209–18. [DOI] [PubMed] [Google Scholar]
- 7.Tan JS, Wang JJ, Flood V, Mitchell P. Dietary fatty acids and the 10-year incidence of age-related macular degeneration: the blue mountains eye study. Arch Ophthalmol 2009;127:656–65. [DOI] [PubMed] [Google Scholar]
- 8.Christen WG, Schaumberg DA, Glynn RJ, Buring JE. Dietary omega-3 fatty acid and fish intake and incident age-related macular degeneration in women. Arch Ophthalmol 2011;129:921–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sangiovanni JP, Agron E, Meleth AD, Reed GF, Sperduto RD, Clemons TE, Chew EY. ω-3 Long-chain polyunsaturated fatty acid intake and 12-y incidence of neovascular age-related macular degeneration and central geographic atrophy: AREDS report 30, a prospective cohort study from the Age-Related Eye Disease Study. Am J Clin Nutr 2009;90:1601–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.SanGiovanni JP, Chew EY, Agron E, Clemons TE, Ferris FL III, Gensler G, Lindblad AS, Milton RC, Seddon JM, Klein R, et al. The relationship of dietary omega-3 long-chain polyunsaturated fatty acid intake with incident age-related macular degeneration: AREDS report no. 23. Arch Ophthalmol 2008;126:1274–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parekh N, Voland RP, Moeller SM, Blodi BA, Ritenbaugh C, Chappell RJ, Wallace RB, Mares JA. Association between dietary fat intake and age-related macular degeneration in the Carotenoids in Age-Related Eye Disease Study (CAREDS): an ancillary study of the Women’s Health Initiative. Arch Ophthalmol 2009;127:1483–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seddon JM, Cote J, Rosner B. Progression of age-related macular degeneration: association with dietary fat, transunsaturated fat, nuts, and fish intake. Arch Ophthalmol 2003;121:1728–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seddon JM, Rosner B, Sperduto RD, Yannuzzi L, Haller JA, Blair NP, Willett W. Dietary fat and risk for advanced age-related macular degeneration. Arch Ophthalmol 2001;119:1191–9. [DOI] [PubMed] [Google Scholar]
- 14.Chong EW, Robman LD, Simpson JA, Hodge AM, Aung KZ, Dolphin TK, English DR, Giles GG, Guymer RH. Fat consumption and its association with age-related macular degeneration. Arch Ophthalmol 2009;127:674–80. [DOI] [PubMed] [Google Scholar]
- 15.Seddon JM, George S, Rosner B. Cigarette smoking, fish consumption, omega-3 fatty acid intake, and associations with age-related macular degeneration: the US twin Study of age-related macular degeneration. Arch Ophthalmol 2006;124:995–1001. [DOI] [PubMed] [Google Scholar]
- 16.Merle B, Delyfer MN, Korobelnik JF, Rougier MB, Colin J, Malet F, Feart C, Le Goff M, Dartigues JF, Barberger-Gateau P, et al. Dietary omega-3 fatty acids and the risk for age-related maculopathy: the Alienor Study. Invest Ophthalmol Vis Sci 2011;52:6004–11. [DOI] [PubMed] [Google Scholar]
- 17.Kris-Etherton PM, Taylor DS, Yu-Poth S, Huth P, Moriarty K, Fishell V, Hargrove RL, Zhao G, Etherton TD. Polyunsaturated fatty acids in the food chain in the United States. Am J Clin Nutr 2000;71:179S–88S. [DOI] [PubMed] [Google Scholar]
- 18.Wolff RL. Heat-induced geometrical isomerization of alpha-linolenic acid: effect of temperature and heating time on the appearance of individual isomers. J Am Oil Chem Soc 1993;70:425–30. [Google Scholar]
- 19.Wolff RL. Further studies on artificial geometrical isomers of alpha-linolenic acid in edible linolenic acid-containing oils. J Am Oil Chem Soc 1993;70:219–24. [Google Scholar]
- 20.Wolff RL. trans-Polyunsaturated fatty acids in French edible rapeseed and soybean oils. J Am Oil Chem Soc 1992;69:106–10. [Google Scholar]
- 21.Wolff RL, Sebedio JL. Geometrical isomers of linolenic acid in low-calorie spreads marketed in France. J Am Oil Chem Soc 1991;68:719–25. [Google Scholar]
- 22.Chardigny JM, Wolff RL, Mager E, Bayard CC, Sebedio JL, Martine L, Ratnayake WMN. Fatty acid composition of French infant formulas with emphasis on the content and detailed profile of trans fatty acids. J Am Oil Chem Soc 1996;73:1595–601. [Google Scholar]
- 23.Ratnayake WMN, Chardigny JM, Wolff RL, Bayard CC, Sebedio JL, Martine L. Essential fatty acids and their trans geometrical isomers in powdered and liquid infant formulas sold in Canada. J Pediatr Gastroenterol Nutr 1997;25:400–7. [DOI] [PubMed] [Google Scholar]
- 24.Sébédio JL, Vermunt SH, Chardigny JM, Beaufrère B, Mensink RP, Armstrong RA, Christie WW, Niemelä J, Hénon G, Riemersma RA. The effect of dietary trans alpha-linolenic acid on plasma lipids and platelet fatty acid composition: the TransLinE study. Eur J Clin Nutr 2000;54:104–13. [DOI] [PubMed] [Google Scholar]
- 25.Chardigny JM, Wolff RL, Mager E, Sebedio JL, Martine L, Juaneda P. Trans mono- and polyunsaturated fatty acids in human milk. Eur J Clin Nutr 1995;49:523–31. [PubMed] [Google Scholar]
- 26.Chen ZY, Pelletier G, Hollywood R, Ratnayake WMN. Trans fatty acid isomers in Canadian human milk. Lipids 1995;30:15–21. [DOI] [PubMed] [Google Scholar]
- 27.Wu J, Cho E, Willett WC, Sastry SM, Schaumberg DA. Intakes of lutein, zeaxanthin, and other carotenoids and age-related macular degeneration during 2 decades of prospective follow-up. JAMA Ophthalmol 2015;133:1415–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr 1997;65:1220S–8S; discussion 9S–31S. [DOI] [PubMed] [Google Scholar]
- 29.Yuan C, Spiegelman D, Rimm EB, Rosner BA, Stampfer MJ, Barnett JB, Chavarro JE, Subar AF, Sampson LK, Willett WC. Validity of a dietary questionnaire assessed by comparison with multiple weighed dietary records or 24-hour recalls. Am J Epidemiol 2017. Feb 25 (Epub ahead of print; DOI:10.1093/aje/kww104). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baylin A, Kim MK, Donovan-Palmer A, Siles X, Dougherty L, Tocco P, Campos H. Fasting whole blood as a biomarker of essential fatty acid intake in epidemiologic studies: comparison with adipose tissue and plasma. Am J Epidemiol 2005;162:373–81. [DOI] [PubMed] [Google Scholar]
- 31.Rosner B, Cook N, Portman R, Daniels S, Falkner B. Determination of blood pressure percentiles in normal-weight children: some methodological issues. Am J Epidemiol 2008;167:653–66. [DOI] [PubMed] [Google Scholar]
- 32.Kotsopoulos J, Tworoger SS, Campos H, Chung FL, Clevenger CV, Franke AA, Mantzoros CS, Ricchiuti V, Willett WC, Hankinson SE, et al. Reproducibility of plasma and urine biomarkers among premenopausal and postmenopausal women from the Nurses’ Health Studies. Cancer Epidemiol Biomarkers Prev 2010;19:938–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun Q, Ma J, Campos H, Hankinson SE, Hu FB. Comparison between plasma and erythrocyte fatty acid content as biomarkers of fatty acid intake in US women. Am J Clin Nutr 2007;86:74–81. [DOI] [PubMed] [Google Scholar]
- 34.Sun Q, Ma J, Campos H, Rexrode KM, Albert CM, Mozaffarian D, Hu FB. Blood concentrations of individual long-chain n-3 fatty acids and risk of nonfatal myocardial infarction. Am J Clin Nutr 2008;88:216–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu FB, Stampfer MJ, Rimm E, Ascherio A, Rosner BA, Spiegelman D, Willett WC. Dietary fat and coronary heart disease: a comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. Am J Epidemiol 1999;149:531–40. [DOI] [PubMed] [Google Scholar]
- 36.Food and Drug Administration; HHS. Food labeling: trans fatty acids in nutrition labeling, nutrient content claims, and health claims. Final rule. Fed Regist 2003;68:41433–506. [PubMed] [Google Scholar]
- 37.Eckel RH, Borra S, Lichtenstein AH, Yin-Piazza SY. Understanding the complexity of trans fatty acid reduction in the American diet: American Heart Association Trans Fat Conference 2006: report of the Trans Fat Conference Planning Group. Circulation 2007;115:2231–46. [DOI] [PubMed] [Google Scholar]
- 38.Honors MA, Harnack LJ, Zhou X, Steffen LM. Trends in fatty acid intake of adults in the Minneapolis-St Paul, MN Metropolitan area, 1980-1982 through 2007-2009. J Am Heart Assoc 2014;3:e001023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schwenke DC, Foreyt JP, Miller ER III, Reeves RS, Vitolins MZ. Plasma concentrations of trans fatty acids in persons with type 2 diabetes between September 2002 and April 2004. Am J Clin Nutr 2013;97:862–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hendrickson SJ, Willett WC, Rosner BA, Eliassen AH. Food predictors of plasma carotenoids. Nutrients 2013;5:4051–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu J, Cho E, Giovannucci EL, Rosner BA, Sastry SM, Willett WC, Schaumberg DA. Dietary intakes of eicosapentaenoic acid and docosahexaenoic acid and risk of age-related macular degeneration. Ophthalmology 2017. Jan 30 (Epub ahead of print; DOI: 10.1016/j.ophtha.2016.12.033). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marshall J, Carleton KL, Cronin T. Colour vision in marine organisms. Curr Opin Neurobiol 2015;34:86–94. [DOI] [PubMed] [Google Scholar]
- 43.Abraham RA, Bahl VK, Parshad R, Seenu V, Roy A, Golandaz S, Dorairaj P, Ramakrishnan L. Content of trans fatty acids in human cheek epithelium: comparison with serum and adipose tissue. Biomed Res Int 2013;2013:276174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Acar N, Chardigny JM, Bonhomme B, Almanza S, Doly M, Sebedio JL. Long-term intake of trans (n-3) polyunsaturated fatty acids reduces the b-wave amplitude of electroretinograms in rats. J Nutr 2002;132:3151–4. [DOI] [PubMed] [Google Scholar]
- 45.Acar N, Bonhomme B, Joffre C, Bron AM, Creuzot-Garcher C, Bretillon L, Doly M, Chardigny JM. The retina is more susceptible than the brain and the liver to the incorporation of trans isomers of DHA in rats consuming trans isomers of alpha-linolenic acid. Reprod Nutr Dev 2006;46:515–25. [DOI] [PubMed] [Google Scholar]
- 46.Chardigny JM, Sébédio JL, Juanéda P, Vatèle JM, Grandgirard A. Occurrence of N-3 trans polyunsaturated fatty acids in human platelets. Nutr Res 1993;13:1105–11. [Google Scholar]
- 47.Burdge GC, Wootton SA. Conversion of alpha-linolenic acid to eicosapentaenoic, docosapentaenoic and docosahexaenoic acids in young women. Br J Nutr 2002;88:411–20. [DOI] [PubMed] [Google Scholar]
- 48.Ottosson UB, Lagrelius A, Rosing U, von Schoultz B. Relative fatty acid composition of lecithin during postmenopausal replacement therapy–a comparison between ethinyl estradiol and estradiol valerate. Gynecol Obstet Invest 1984;18:296–302. [DOI] [PubMed] [Google Scholar]
- 49.Jin Y, Kim TH, Park Y. Association between erythrocyte levels of n-3 polyunsaturated fatty acids and depression in postmenopausal women using or not using hormone therapy. Menopause 2016;23:1012–8. [DOI] [PubMed] [Google Scholar]
- 50.Vermunt SH, Beaufrere B, Riemersma RA, Sebedio JL, Chardigny JM, Mensink RP. Dietary trans alpha-linolenic acid from deodorised rapeseed oil and plasma lipids and lipoproteins in healthy men: the TransLinE Study. Br J Nutr 2001;85:387–92. [DOI] [PubMed] [Google Scholar]