Abstract
Background
Fibrotic change is one of the important reasons for the poor prognosis of patients with acute respiratory distress syndrome (ARDS). The present study investigated the effects of hydrogen-rich saline, a selective hydroxyl radical scavenger, on lipopolysaccharide (LPS)-induced pulmonary fibrosis.
Material/Methods
Male ICR mice were divided randomly into 5 groups: Control, LPS-treated plus vehicle treatment, and LPS-treated plus hydrogen-rich saline (2.5, 5, or 10 ml/kg) treatment. Twenty-eight days later, fibrosis was assessed by determination of collagen deposition, hydroxyproline, and type I collagen levels. Development of epithelial-to-mesenchymal transition (EMT) was identified by examining protein expressions of E-cadherin and α-smooth muscle actin (α-SMA). Transforming growth factor (TGF)-β1 content, total antioxidant capacity (T-AOC), malondialdehyde (MDA) content, catalase (CAT), and superoxide dismutase (SOD) activity were determined.
Results
Mice exhibited increases in collagen deposition, hydroxyproline, type I collagen contents, and TGF-β1 production in lung tissues after LPS treatment. LPS-induced lung fibrosis was associated with increased expression of α-SMA, as well as decreased expression of E-cadherin. In addition, LPS treatment increased MDA levels but decreased T-AOC, CAT, and SOD activities in lung tissues, indicating that LPS induced pulmonary oxidative stress. Hydrogen-rich saline treatment at doses of 2.5, 5, or 10 ml/kg significantly attenuated LPS-induced pulmonary fibrosis. LPS-induced loss of E-cadherin in lung tissues was largely reversed, whereas the acquisition of α-SMA was dramatically decreased by hydrogen-rich saline treatment. In addition, hydrogen-rich saline treatment significantly attenuated LPS-induced oxidative stress.
Conclusions
Hydrogen-rich saline may protect against LPS-induced EMT and pulmonary fibrosis through suppressing oxidative stress.
MeSH Keywords: Oxidative Stress, Pulmonary Fibrosis, Transforming Growth Factor beta1
Background
Acute respiratory distress syndrome (ARDS) is a life-threatening medical condition characterized by widespread inflammation in the lungs [1]. Typically, the appearance of this damage is divided into 3 successive phases: exudative, proliferative, and fibrotic [2]. The presence of fibrosis is significantly correlated with the duration of ARDS. Moreover, fibrotic change seems to an important reason for the poor prognosis of ARDS patients, because it leads to decreases in lung compliance and oxygenation [3,4].
Pulmonary fibrosis is characterized by activation of myofibroblasts, which can originate from epithelial cells via the formation of epithelial-to-mesenchymal transition (EMT). Although myofibroblasts are integral to normal repair mechanisms, the persistence of the myofibroblast beyond a period of normal repair has been associated with destruction of alveocapillary units and excessive extracellular matrix (ECM) deposition, finally resulting in structural remodeling and pulmonary fibrosis [5,6]. There are accumulating studies showing that lipopolysaccharide (LPS), a major pathogenic factor potentially provoking acute lung injury and ARDS, can promote the formation of EMT in various cell types, including melanoma cells [7], intrahepatic biliary epithelial cells [8], and hepatocytes [9]. Our recent study also showed that LPS induces EMT and pulmonary fibrosis in animal models [10].
It has been reported that oxidative stress is closely related with the EMT [11]. The epithelial surface of the lung is known to directly contact with the environment, thus lung tissues are highly susceptible to oxidative stress [12]. Exogenous pollutants, oxidants, and inflammatory stimuli can further enhance the formation of reactive oxygen species (ROS) [13]. EMT is known to be induced by fibrogenic signaling of transforming growth factor-β1 (TGF-β1) [14]. There are accumulating studies showing that ROS can activate TGF-β signaling pathway and is involved in the fibrogenic effects of TGF-β [15,16]. Thus, agents that have antioxidant properties may prevent the formation of EMT, and have protective effects against pulmonary fibrosis.
Hydrogen (H2), the most abundant element in the universe, has been shown to be a potent antioxidant and can effectively protect against tissue damage such as liver injury, renal injury, and lung injury [17–19]. Xu et al. confirm that hydrogen-rich saline treatment reduces kidney fibrosis and infiltration of macrophages induced by unilateral ureteric obstruction (UUO) in rats [18]. However, whether hydrogen-rich saline can affect the pathogenesis of pulmonary fibrosis remains largely unknown. Thus, the present study investigated the effects of hydrogen-rich saline on EMT and pulmonary fibrosis induced by LPS in mice.
Material and Methods
Hydrogen-rich saline
To reach the supersaturated level, hydrogen was dissolved in saline under high pressure (0.4 MPa) for 2 h, using equipment provided by the Department of Diving Medicine, the Second Military Medical University (Shanghai, China) [20]. The hydrogen-rich saline was freshly prepared every week, sterilized by γ-radiation, and was stored under atmospheric pressure at 4°C in an aluminum bag without dead volume. Thus, the hydrogen concentration was maintained at a level above 0.8 mmol/L.
Animals
Male adult ICR mice were obtained from Shanghai SLAC Laboratory Animal Co. (Shanghai, China). Mice were housed in an antigen- and virus-free room with water and a pelleted stock diet ad libitum. All experiments were performed in accordance with the animal experimental guidelines issued by the Animal Care and Use Committee at Shanghai University of Sports.
LPS-induced lung fibrosis and hydrogen-rich saline treatment
Mice were anesthetized with ketamine (70 mg/kg) and xylazine (10 mg/kg). A total of 105 mice were used. These mice were first randomly distributed into control (n=21) and LPS (n=84) groups. Then, a single dose of purified LPS (Escherichia coli 0111: B4, Sigma-Aldrich, St. Louis, MO) at 5 mg/kg in 50 μl sterile saline was intratracheally administered as previously described [21]. Control mice were administered with 50 μl saline. Mice treated with LPS were then randomly distributed into 4 groups (n=21 for each group) that were treated with vehicle or hydrogen-rich saline (2.5, 5, or 10 ml/kg, i.p.) once daily. Lung tissues were harvested 28 days after hydrogen-rich saline treatment. Among these samples, 35 lung tissue samples (n=7 for each group) were used for histopathologic and immunohistochemical examination; 35 samples (n=7 for each group) were used for Western blot analysis; 35 samples (n=7 for each group) were used for determination of pulmonary levels of malondialdehyde, hydroxyproline, total antioxidant capacity, catalase and superoxide dismutase activities, TGF-β1, and type I collagen.
Lung histopathologic and immunohistochemical examination
Lung tissues was fixed with 10% formalin and embedded in paraffin, and 4-μm sections were stained with Masson’s trichrome staining (Sigma-Aldrich) according to the manufacturer’s instructions. For immunohistochemical analysis, lung sections were incubated with primary antibodies against E-cadherin (1: 200, Santa Cruz Biotechnology, Santa Cruz, CA) or α-SMA (1: 200, Abcam, Cambridge, UK). Binding was eventually detected with a biotin-streptavidin-peroxidase system (Santa Cruz) using 3,5-diaminobenzidine as chromogen. For negative controls, primary antibodies were substituted with the same concentration of normal IgG.
Western blot analysis
Protein samples of lung tissues or MLVECs were collected and processed according to the standard protocols. Briefly, 30 μg of protein was separated by 10% SDS-PAGE and subsequently transferred to nitrocellulose membranes (Millipore Corp, Bedford, MA). After blocking, immunoblots were incubated with primary antibody against collagen I (Abcam), E-cadherin (Santa Cruz), α-SMA (Abcam), TGF-β (Cell Signaling, Beverly, MA) or β-actin (Sigma-Aldrich) at 4°C overnight, followed by incubation with a secondary HRP-conjugated IgG (Santa Cruz) for 1 h at room temperature. Immunoreactive proteins were visualized using the enhanced chemiluminescence Western blotting detection system (Santa Cruz).
Determination of malondialdehyde levels
Malondialdehyde (MDA) levels were determined as previously described [22]. Briefly, lung tissues were homogenized in 10 vol of 1.15% KCl solution containing 0.85% NaCl. After centrifuging at 1500 g for 15 min, the homogenates were added to a reaction mixture consisting of 0.8% thiobarbituric acid, 8.1% sodium dodecyl sulfate, and 20% acetic acid (adjusted to pH 3.5 with NaOH). The mixture was then heated at 95°C for 40 min, and was cleared by centrifugation at 10 000g for 10 min. The absorbance was examined at 532 nm.
Determination of hydroxyproline, total antioxidant capacity, and catalase and superoxide dismutase activities
The hydroxyproline content, total antioxidant capacity (T-AOC), catalase (CAT), and superoxide dismutase (SOD) activities in lung tissue homogenates were measured according to the manufacturer’s instructions (Winching, Nanjing, China) [23].
Determination of TGF-β1 and type I collagen
The levels of TGF-β1 and type I collagen in lung tissues homogenates were examined by ELISA kits purchased from R&D (Minneapolis, MN) and Southern Biotech (Birmingham, AL) according to the manufacturer’s instructions, respectively.
Statistical analysis
Values are shown as mean ±SEM. Normal distribution of the data was assessed with a Kolmogorov-Smirnov test. Comparisons involving all experimental groups were performed with one-way analysis of variance (ANOVA), followed by the Student-Newman-Keuls post hoc tests. A P value <0.05 was considered to be statistically significant.
Results
Hydrogen-rich saline alleviates LPS-induced lung fibrosis
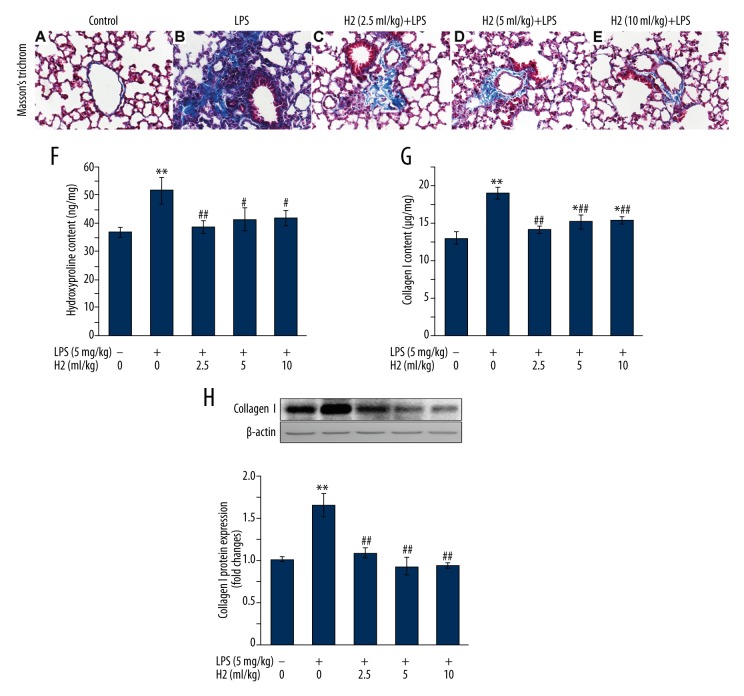
We have previously investigated the time-course of LPS-induced lung fibrosis [10], finding that lung fibrosis is the most serious on day 28 after intratracheal administration of LPS [10]. Based on these findings, the present study examined whether LPS-induced lung fibrosis was improved by hydrogen-rich saline treatment for 4 weeks. By using Masson’s trichrome staining, the collagen deposition appeared diffusively in the interstitium, and was accompanied with the destruction of normal pulmonary architecture 28 days after LPS treatment (Figure 1A, 1B). As shown in Figure 1C–1E, the extensive fibrillar collagen deposition observed in LPS-treated mice was significantly alleviated by hydrogen-rich saline (at 2.5, 5, or 10 ml/kg) treatment. To quantify the amount of collagen deposited in the lung, the effects of hydrogen-rich saline on pulmonary contents of collagen I and hydroxyproline were further determined. We found that hydrogen-rich saline treatment profoundly reduced the LPS-induced increases in pulmonary hydroxyproline (Figure 1F) and collagen I contents (Figure 1G, 1H).
Figure 1.
Hydrogen-rich saline treatment attenuates LPS-induced pulmonary fibrosis. Mice were instilled intratracheally with LPS (5 mg/kg). Hydrogen-rich saline was administered at a dose of 2.5, 5. or 10 ml/kg/d i.p. Lungs were harvested at the end of 28-day hydrogen-rich saline treatment therapy. (A–E) Collagen deposition was assessed with Masson’s trichrome staining on paraffin-fixed lung sections. Original magnification, ×400. Scale bars correspond to 20 μm. Fibrosis was also quantified by determination of hydroxyproline (F) and type I collagen (G, H) levels in lung tissues as described in “Material and Methods”. Data are expressed as means ±SEM (n=7 per each group). * p<0.05, ** p<0.01 vs. control group. # p<0.05, ## p<0.01 vs. LPS group. H2 represents hydrogen-rich saline.
Hydrogen-rich saline ameliorates LPS-induced epithelial-to-mesenchymal transition
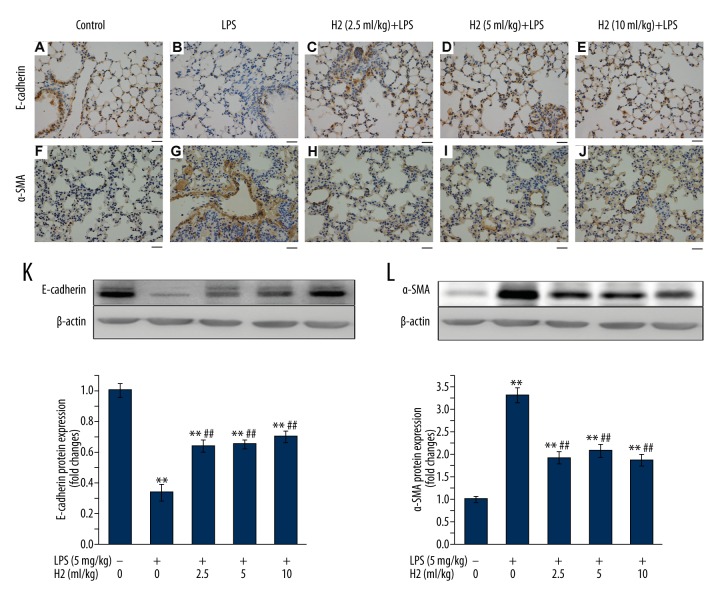
To investigate the role of hydrogen-rich saline in regulating the formation of EMT, both epithelial marker E-cadherin and myofibroblast marker α-SMA were examined by using immunohistochemistry staining in the lung sections. As shown in Figure 2, LPS treatment resulted in decreases of E-cadherin immunostaining (Figure 2B) and increases of α-SMA immunostaining (Figure 2G). Hydrogen-rich saline therapy (2.5, 5, or 10 ml/kg) for 4 weeks significantly increased the number of epithelial cells expressing E-cadherin (Figure 2C–2E), but decreased the number of myofibroblasts expressing α-SMA in pulmonary interstitium (Figure 2H–2J) of LPS-treated mice. We next evaluated the protein expressions of E-cadherin and α-SMA. As shown in Figure 2K, 2L, Western blot analysis showed the loss of epithelial marker E-cadherin, accompanied by the acquisition of mesenchymal cell marker α-SMA after LPS treatment, which were reversed by hydrogen-rich saline therapy (2.5, 5, or 10 ml/kg). These findings demonstrated the protective effect of hydrogen-rich saline against EMT and lung fibrosis induced by LPS treatment.
Figure 2.
Hydrogen-rich saline treatment attenuates LPS-induced EMT. Mice were instilled intratracheally with LPS (5 mg/kg). Hydrogen-rich saline was administered at a dose of 2.5, 5, or 10 ml/kg/d i.p. Lungs were harvested at the end of 28-day hydrogen-rich saline treatment therapy. (A–J) Lung sections were subjected to immunohistochemical analysis using antibodies against E-cadherin (A–E) or α-SMA (F–J). Original magnification, ×400. Scale bars correspond to 20 μm. (K, L) Protein expression of E-cadherin (K) or α-SMA (L) in lung homogenates was determined by Western blot analysis. Data are expressed as means ±SEM (n=7 per each group). ** p<0.01 vs. control group. ## p<0.01 vs. LPS group. H2 represents hydrogen-rich saline.
Hydrogen-rich saline suppresses pulmonary TGF-β production after LPS treatment
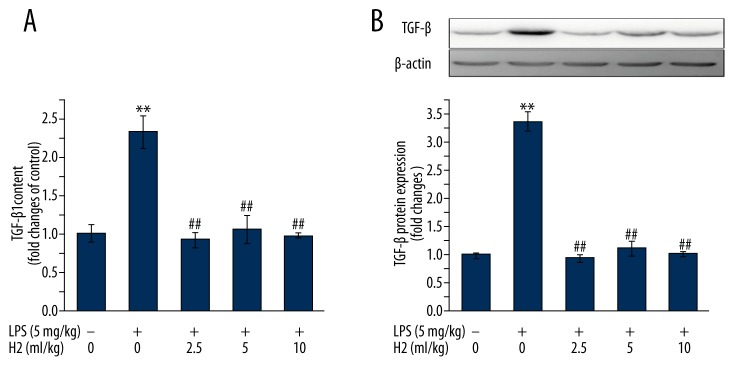
TGF-β family signaling plays a predominant role in the reprogramming of gene expression during EMT [24]. As shown in Figure 3, hydrogen-rich saline (2.5, 5, or 10 ml/kg) treatment significantly inhibited pulmonary TGF-β1 production (Figure 3A) and TGF-β protein expression (Figure 3B) induced by LPS treatment.
Figure 3.
(A, B) Hydrogen-rich saline treatment suppresses LPS-induced TGFβ production. Mice were instilled intratracheally with LPS (5 mg/kg). Hydrogen-rich saline was administered at a dose of 2.5, 5, or 10 ml/kg/d i.p. Lungs were harvested at the end of 28-day hydrogen-rich saline treatment therapy. TGFβ-1 content and TGFβ protein expression in lung tissues were analyzed by ELISA and Western blot analysis, respectively. Data are expressed as means ±SEM (n=7 per each group). ** p<0.01 vs. control group. ## p<0.01 vs. LPS group. H2 represents hydrogen-rich saline.
Hydrogen-rich saline decreases LPS-induced oxidative stress
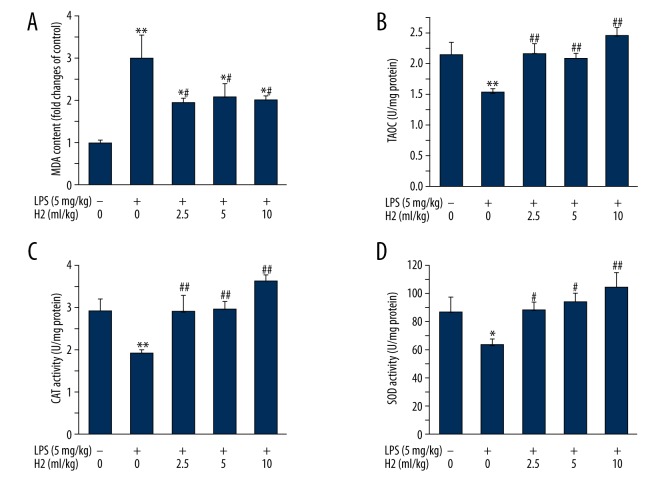
As shown in Figure 4, hydrogen-rich saline therapy (2.5, 5, or 10 ml/kg) resulted in a significant decrease in the MDA level (Figure 4A). On the other hand, hydrogen-rich saline therapy led to increases in the levels of T-AOC, SOD, and CAT activities in lung tissues of LPS-treated mice (Figure 4B–4D).
Figure 4.
Hydrogen-rich saline treatment attenuates LPS-induced lung oxidative stress. Mice were instilled intratracheally with LPS (5 mg/kg). Hydrogen-rich saline was administered at a dose of 2.5, 5, or 10 ml/kg/d i.p. Lungs were harvested at the end of 28-day hydrogen-rich saline therapy. MDA level (A), T-AOC level (B), CAT activity (C), and SOD activity (D) were determined as described in “Material and Methods”. Data are expressed as means ±SEM (n=7 per each group). * p<0.05, ** p<0.01 vs. control group. # p<0.05, ## p<0.01 vs. LPS group. H2 represents hydrogen-rich saline.
Discussion
At the post-acute phase of ARDS patients, fibrotic changes are associated with poor prognosis [3,4]. This study demonstrates that administration of hydrogen-rich saline significantly attenuates the oxidative stress and TGF-β1 production induced by LPS treatment, thus reversing the formation of EMT and protecting against LPS-induced lung fibrosis.
Acute lung injury (ALI), or ARDS, is a severe and life-threatening medical condition. There are many factors that can cause acute lung injury. Previous studies have demonstrated that hydrogen-rich saline can protect against lung injuries caused by intestinal ischemia/reperfusion [19], hyperoxia [25], or extensive burn models [26] in rats. In the present study, our findings provide evidence showing that hydrogen-rich saline exhibits anti-fibrosis effects in an LPS-induced murine model of lung fibrosis.
Accumulating evidence has shown that ROS can enhance TGF-β1 production and collagen release from fibroblasts in the lung [27]. Furthermore, as lung epithelial surface is sensitive to oxidative stress, disorder is more often to occur due to the imbalance between pro-oxidants and antioxidant system [12]. Depending on these pathophysiologic mechanisms, antioxidant agents may have protective effects against lung fibrosis. Recent evidence has shown that molecular hydrogen is an effective antioxidant and may have potential medical applications [28]. Sun et al. [29] demonstrated that hydrogen-rich saline prevents ROS accumulation in various types of liver injury and liver fibrosis. Kawai et al. [17] reported that consumption of hydrogen-rich water can decrease the oxidative stress biomarker 8-hydroxydeoxyguanosine (8-OHdG) and thus may be an effective treatment for nonalcoholic steatohepatitis by reducing hepatic oxidative stress. Consistent with these findings, the present study provides evidence that hydrogen-rich saline significantly attenuates LPS-induced oxidative stress in lung tissues. Hydrogen-rich saline resulted in a decrease in pulmonary MDA level, whereas it significantly reversed the LPS-induced decreases in pulmonary anti-oxidative biomarkers, including T-AOC, CAT, and SOD activities. These findings indicate that the beneficial effects of hydrogen-rich saline in the treatment of LPS-induced lung fibrosis might be, at least partly, associated with its potent antioxidant property.
Activated myofibroblasts play a central role during the pathogenesis of pulmonary fibrosis by synthesizing and depositing ECM proteins [5,6]. Myofibroblasts are derived from various cells: (1) resident stromal fibroblasts, (2) bone-marrow-derived ‘fibrocytes’ and (3) trans-differentiation of alveolar type II epithelial cells into myofibroblasts via EMT [6,24]. Accumulating studies have demonstrated the critical role of EMT in pulmonary fibrosis. During EMT, epithelial cells lose apical-basal polarity, basement membrane attachment, and cell-cell contact. Epithelial cells gain mesenchymal characteristics associated with increased migratory behavior, cytoskeletal rearrangements, and their migration into the lung interstitium where they produce excess ECM [30]. It is well-recognized that the TGF-β family has a predominant role in the regulation of EMT [6,14]. Hydrogen-rich saline has been found to decrease the expression of TGF-β1 mRNA and suppress liver fibrogenesis in a thioacetamide-induced liver fibrosis model [31]. The present study shows that hydrogen-rich saline significantly inhibited pulmonary TGF-β1 production in LPS-treated mice. Moreover, hydrogen-rich saline significantly increased the epithelial marker E-cadherin, but decreased the myofibroblast marker α-SMA level, indicating that hydrogen-rich saline can reverse the formation of pulmonary EMT process in LPS-treated mice. Taken together, these findings demonstrate that the protective effect of hydrogen-rich saline against LPS-induced lung fibrosis may exerted through suppression of LPS-induced TGF-β1 production and reversal of EMT.
Conclusions
In summary, our data demonstrate that antioxidant hydrogen-rich saline significantly reverses the formation of the pulmonary EMT process and improves the pulmonary fibrotic changes in LPS-treated mice. The anti-fibrotic effects of hydrogen-rich saline are associated with modulation of the oxidative status and TGF-β1 production in the lung. Thus, hydrogen-rich saline shows promise as a useful adjuvant treatment for patients with ARDS-associated pulmonary fibrosis.
Footnotes
Statement
The authors have not disclosed any potential conflicts of interest.
Source of support: This work was supported by the National Natural Science Foundation of China (No. 81272144, No. 31371164, No. 31671213, and No. 81672266) and the Shanghai Sailing Program (No.16YF1407300)
References
- 1.Rubenfeld GD, Caldwell E, Peabody E, et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353:1685–93. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 2.Tomashefski JF., Jr Pulmonary pathology of acute respiratory distress syndrome. Clin Chest Med. 2000;21:435–66. doi: 10.1016/s0272-5231(05)70158-1. [DOI] [PubMed] [Google Scholar]
- 3.Burnham EL, Janssen WJ, Riches DW, et al. The fibroproliferative response in ARDS: Mechanisms and clinical significance. Eur Respir J. 2014;43:276–85. doi: 10.1183/09031936.00196412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rocco PR, Dos Santos C, Pelosi P. Lung parenchyma remodeling in acute respiratory distress syndrome. Minerva Anestesiol. 2009;75:730–40. [PubMed] [Google Scholar]
- 5.Cottin V. Interstitial lung disease. Eur Respir Rev. 2013;22:26–32. doi: 10.1183/09059180.00006812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chapman HA. Epithelial-mesenchymal interactions in pulmonary fibrosis. Annu Rev Physiol. 2011;73:413–35. doi: 10.1146/annurev-physiol-012110-142225. [DOI] [PubMed] [Google Scholar]
- 7.Chen MC, Chang WW, Kuan YD, et al. Resveratrol inhibits LPS-induced epithelial-mesenchymal transition in mouse melanoma model. Innate Immun. 2012;18:685–93. doi: 10.1177/1753425912436589. [DOI] [PubMed] [Google Scholar]
- 8.Zhao L, Yang R, Cheng L, et al. LPS-induced epithelial mesenchymal transition of intrahepatic biliary epithelial cells. J Surg Res. 2011;171:819–25. doi: 10.1016/j.jss.2010.04.059. [DOI] [PubMed] [Google Scholar]
- 9.Jing YY, Han ZP, Sun K, et al. Toll-like receptor 4 signaling promotes epithelial-mesenchymal transition in human hepatocellular carcinoma induced by lipopolysaccharide. BMC Med. 2012;10:98. doi: 10.1186/1741-7015-10-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang YQ, Liu YJ, Mao YF, et al. Resveratrol ameliorates lipopolysaccharide-induced epithelial mesenchymal transition and pulmonary fibrosis through suppression of oxidative stress and transforming growth factor-β1 signaling. Clin Nutr. 2015;34:752–60. doi: 10.1016/j.clnu.2014.08.014. [DOI] [PubMed] [Google Scholar]
- 11.Cannito S, Novo E, di Bonzo LV, et al. Epithelial mesenchymal transition: From molecular mechanisms, redox regulation to implications in human health and disease. Antioxid Redox Signal. 2010;12:1383–430. doi: 10.1089/ars.2009.2737. [DOI] [PubMed] [Google Scholar]
- 12.Langen RC, Korn SH, Wouters EF. ROS in the local and systemic pathogenesis of COPD. Free Radic Biol Med. 2003;35:226–35. doi: 10.1016/s0891-5849(03)00316-2. [DOI] [PubMed] [Google Scholar]
- 13.Khadaroo RG, Kapus A, Powers KA, et al. Oxidative stress reprograms lipopolysaccharide signaling via Src kinasedependent pathway in RAW 264.7 macrophage cell line. J Biol Chem. 2003;278:47834–41. doi: 10.1074/jbc.M302660200. [DOI] [PubMed] [Google Scholar]
- 14.Willis BC, Borok Z. TGF-beta-induced EMT: Mechanisms and implications for fibrotic lung disease. Am J Physiol Lung Cell Mol Physiol. 2007;293:L525–34. doi: 10.1152/ajplung.00163.2007. [DOI] [PubMed] [Google Scholar]
- 15.Hecker L, Vittal R, Jones T, et al. NADPH oxidase-4 mediates myofibroblast activation and fibrogenic responses to lung injury. Nat Med. 2009;15:1077–81. doi: 10.1038/nm.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ishikawa F, Kaneko E, Sugimoto T, et al. A mitochondrial thioredoxin-sensitive mechanism regulates TGF-beta-mediated gene expression associated with epithelial-mesenchymal transition. Biochem Biophys Res Commun. 2014;443:821–27. doi: 10.1016/j.bbrc.2013.12.050. [DOI] [PubMed] [Google Scholar]
- 17.Kawai D, Takaki A, Nakatsuka A, et al. Hydrogen-rich water prevents progression of nonalcoholic steatohepatitis and accompanying hepatocarcinogenesis in mice. Hepatology. 2012;56:912–21. doi: 10.1002/hep.25782. [DOI] [PubMed] [Google Scholar]
- 18.Xu B, Zhang Y, Li Z, et al. Hydrogen-rich saline ameliorates renal injury induced by unilateral ureteral obstruction in rats. Int Immunopharmacol. 2013;17:447–52. doi: 10.1016/j.intimp.2013.06.033. [DOI] [PubMed] [Google Scholar]
- 19.Mao YF, Zheng XF, Cai JM, et al. Hydrogen-rich saline reduces lung injury induced by intestinal ischemia/reperfusion in rats. Biochem Biophys Res Commun. 2009;381:602–5. doi: 10.1016/j.bbrc.2009.02.105. [DOI] [PubMed] [Google Scholar]
- 20.Cai J, Kang Z, Liu K, et al. Neuroprotective effects of hydrogen saline in neonatal hypoxia – ischemia rat model. Brain Res. 2009;1256:129–37. doi: 10.1016/j.brainres.2008.11.048. [DOI] [PubMed] [Google Scholar]
- 21.Wei W, Ma B, Li HY, et al. Biphasic effects of selective inhibition of transforming growth factor beta1 activin receptor-like kinase on LPS-induced lung injury. Shock. 2010;33:218–24. doi: 10.1097/shk.0b013e3181aef736. [DOI] [PubMed] [Google Scholar]
- 22.Yang T, Mao YF, Liu SQ, et al. Protective effects of the free radical scavenger edaravone on acute pancreatitis-associated lung injury. Eur J Pharmacol. 2010;630:152–57. doi: 10.1016/j.ejphar.2009.12.025. [DOI] [PubMed] [Google Scholar]
- 23.Li H, Du S, Yang L, et al. Rapid pulmonary fibrosis induced by acute lung injury via a lipopolysaccharide three-hit regimen. Innate Immun. 2009;15:143–54. doi: 10.1177/1753425908101509. [DOI] [PubMed] [Google Scholar]
- 24.Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial – mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15:178–96. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun Q, Cai J, Liu S, et al. Hydrogen-rich saline provides protection against hyperoxic lung injury. J Surg Res. 2011;165:e43–49. doi: 10.1016/j.jss.2010.09.024. [DOI] [PubMed] [Google Scholar]
- 26.Fang Y, Fu XJ, Gu C, et al. Hydrogen-rich saline protects against acute lung injury induced by extensive burn in rat model. J Burn Care Res. 2011;32:e82–91. doi: 10.1097/BCR.0b013e318217f84f. [DOI] [PubMed] [Google Scholar]
- 27.Qi S, den Hartog GJ, Bast A. Superoxide radicals increase transforming growth factor-beta1 and collagen release from human lung fibroblasts via cellular influx through chloride channels. Toxicol Appl Pharmacol. 2009;237:111–18. doi: 10.1016/j.taap.2009.02.019. [DOI] [PubMed] [Google Scholar]
- 28.Ohta S. Recent progress toward hydrogen medicine: Potential of molecular hydrogen for preventive and therapeutic applications. Curr Pharm Des. 2011;17:2241–52. doi: 10.2174/138161211797052664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun HY, Chen L, Zhou W, et al. The protective role of hydrogen-rich saline in experimental liver injury in mice. J Hepatol. 2011;54:471–80. doi: 10.1016/j.jhep.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 30.Balli D, Ustiyan V, Zhang Y, et al. Foxm1 transcription factor is required for lung fibrosis and epithelial-to-mesenchymal transition. EMBO J. 2013;32:231–44. doi: 10.1038/emboj.2012.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koyama Y, Taura K, Hatano E, et al. Effects of oral intake of hydrogen water on liver fibrogenesis in mice. Hepatol Res. 2014;44:663–77. doi: 10.1111/hepr.12165. [DOI] [PubMed] [Google Scholar]