Abstract
Background
Constipation is common during opioid therapy and can compromise analgesia.
Aim
The aim of this article is to determine the prevalence and clinical characteristics of opioid-induced constipation (OIC) in France.
Methods
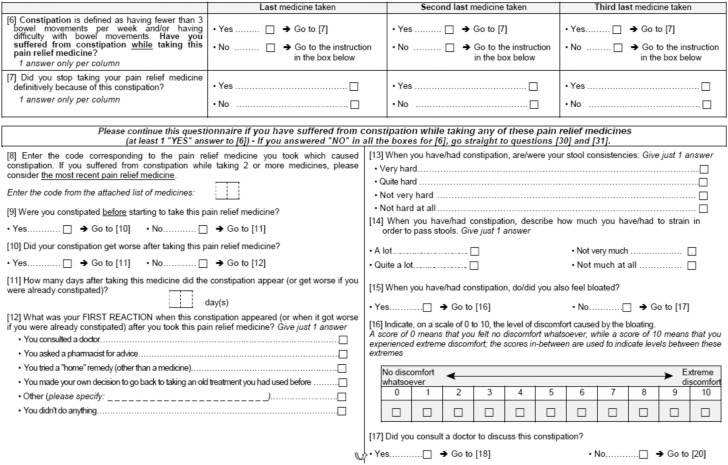
A questionnaire study was conducted in a representative sample of the French general population. Participants completed a 31-item questionnaire covering opioid use during the previous six months, and the occurrence of constipation (defined as <3 bowel movements per week, straining during defaecation, or both) during opioid treatment.
Results
Data were obtained from 15,213 participants, of whom 4753 (31.2%) reported opioid use. Most analgesics (96.5%) were classified as World Health Organization step II analgesics, and the remainder were step III. The most common indications for opioids were bone or joint pain, and soft tissue pain. Overall, 414/4753 (8.7%) opioid users reported OIC while the prevalence of OIC reached 21% in case of regular or prolonged (>1 month) opioid use. Other characteristics associated with OIC included female gender, age ≥50 years and use of step III opioids. Only 177/414 (42.8%) participants with OIC had used medications (most commonly osmotic laxatives) to treat constipation, and satisfaction with constipation medication was moderate (mean (SD) score 7.2 (1.3) on a scale of 0–10).
Conclusions
Approximately one-third of a representative French population had used opioids within the previous six months, and 9% of users had experienced OIC, which is more frequent in case of regular use. OIC appears to be under-treated, and participants’ satisfaction with their constipation medications was only moderate, suggesting that significant unmet need remains in OIC management.
Keywords: Constipation, opioids, side effects, pain, observational study
Introduction
Opioid analgesics have a central place in current guidelines for the management of chronic pain of both malignant1 and benign2 origin, and also form a mainstay of treatment in patients with moderate-to-severe acute pain.3 However, the use of opioids is often limited by the development of opioid-induced bowel dysfunction (OIBD) due to activation of peripheral µ-opioid receptors, which leads to disturbances in gut motility and secretion, and in sphincter function.3,4 OIBD is characterised by diverse gastrointestinal symptoms, affecting all regions of the gastrointestinal tract, and has been reported to occur in a majority of patients taking opioids for chronic pain; in contrast to other opioid-related adverse effects, such as sedation, the development of tolerance to OIBD is rare.5
The most common component of OIBD is opioid-induced constipation (OIC), which has been reported to occur in up to 87% of patients with chronic pain.3–5 OIC can have a significant impact on the patient, potentially compromising effective analgesia.6 In the Patient Reports of Opioid-Related Bothersome Effects (PROBE-1) study, OIC was the adverse effect most likely to be rated as severe, and was considered by patients to be the most bothersome.5 In the same study, 33% of patients had reduced or discontinued treatment because of adverse effects, with 92% of these patients reporting that they had experienced more severe pain as a result.5 Similarly, in a meta-analysis of 11 trials of an opioid therapy indicated for a chronic non-cancer pain, 24% of the patients receiving opioids discontinued treatment because of adverse effects, particularly constipation, compared with only 15% of placebo-treated patients.7 Furthermore, OIC is associated with significant financial costs to health care providers, principally due to staffing costs.8
Although OIC is recognised as a common complication of opioid therapy, the reported prevalence varies markedly depending on the definition used and the patient population studied.3 Variability in the definition of constipation may partly explain the findings that OIC is often under-diagnosed in clinical practice,9 and that health care professionals often under-estimate the severity of constipation as perceived by the patient.10 Similarly, estimates of the prevalence of chronic constipation in the general population have varied between 2% and 27%, depending on the definition used.4 In view of these substantial variations, the present study was undertaken to determine the prevalence of OIC defined by a low stool frequency and/or straining, in the general population in France, and the clinical characteristics of opioid users with OIC, in considering the type of opioid use, the duration of treatment and the existence or not of a pre-existing constipation. Secondary objectives were to determine the most common reasons for opioid use, and to identify unmet needs relating to the treatment of OIC.
Methods
This study was conducted between 11 March and 16 April 2014 using a representative sample of the French non-institutionalised population (19,670 adults aged ≥ 18 years) from a mail panel (TNS-Sofres panel). This panel consists of 29,000 households representative of the French general population, based on distributions of the Institut national de la statistique et des études économiques (INSEE),11 in terms of age, gender, occupational class, region and size of city of residence. The panel is refreshed regularly (one-third per year), and is audited by independent statisticians every two to three years to ensure that it remains representative of the French general population. Study participants (one per household) were selected at random by means of a Kish selection table.12 The employment survey (‘enquête emploi’, which supplements the global population poll) is an ongoing survey among individuals who are at least 15 years of age and stands as the most accurate and most comprehensive survey used and referenced in France. These data serve as the basis for sample structure data used by all polling companies in France.
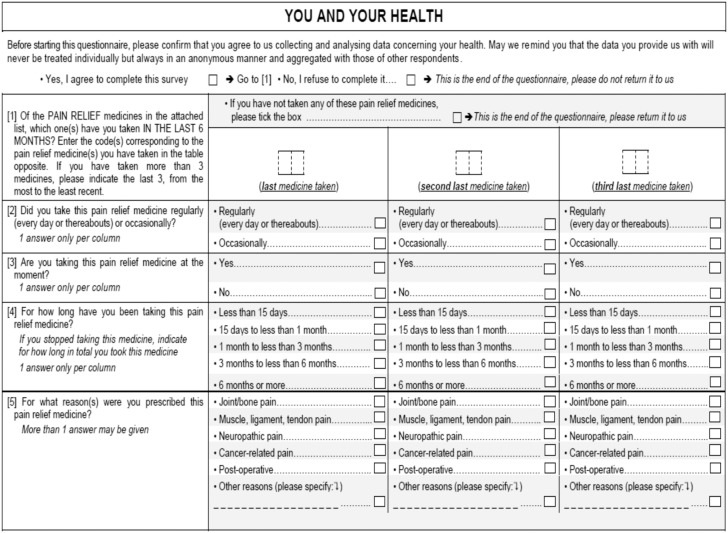
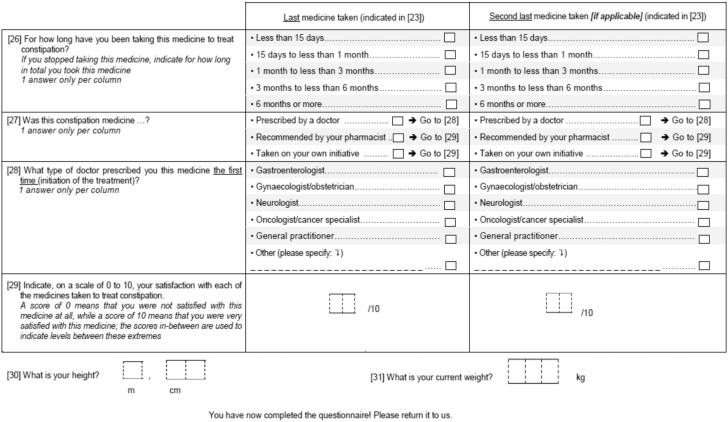
All participants were sent a self-administered postal questionnaire (see Appendix) consisting of 31 questions covering opioid use, whatever the duration of treatment, during the previous six months and opioid-related adverse effects, including previous experience of constipation; for participants who reported using opioids, details of the most recent use were recorded. In addition, participants reporting constipation (defined as fewer than three bowel movements per week, straining during bowel movements, or both) during opioid treatment answered questions on symptoms associated with constipation and medications used to treat constipation.
Statistical methods
The sample size was derived from a pilot feasibility study involving 2000 participants selected from the TNS-Sofres panel. The response rate in this study was 76.7%, resulting in a sample size of 1534. Of these, approximately 37% had used opioids in the previous six months, and 8.3% reported OIC. On the basis of this experience, it was assumed that a sample size of 20,000 would result in approximately 15,000 respondents, of whom 5000–6000 would be expected to be using opioids and 400–500 would have OIC.
Separate analyses were performed in patients with and without pre-existing constipation. To reduce the risk of non-response bias, participants’ data were weighted (by age, gender, socioeconomic status, geographical region crossed with type and size of city/town) according to 2013 demographic data11 using the raking adjusted statistics method;13 the mean weighting efficiency was 86.6%, and the maximum and minimum weightings were 4.54 and 0.35, respectively. All analyses were performed using these weighted data. Data analysis was essentially descriptive, and was conducted using SAS (version 9.1) software (SAS Institute, Cary, NC, USA).
Results
Of the 19,670 individuals surveyed, 15,262 (77.6%) returned their questionnaires, and 15,213 (77.3%) were included in the analysis; 49 questionnaires were excluded from the analysis, mainly due to insufficient completion.
Opioid use in the general population
Among the 15,213 individuals included in the analysis, 4753 (31.2%) had used opioids in the previous six months. The mean (SD) number of treatments was 1.4 (0.7): Sixty-six per cent of participants had used one medication, 23% had used two, and 11% had used three. Forty-four per cent of participants had used opioids for fewer than 15 days, while the duration of treatment exceeded three months in 32% and six months in 25%. The demographic characteristics of opioid users and non-users are presented in Table 1. Compared with non-users, opioid users were more likely to be female (56% versus 50%, p < 0.05) and employed (60% versus 57%, p < 0.05), and were slightly younger (mean age 47.5 years versus 49.4 years, p < 0.05).
Table 1.
Demographic characteristics of opioid users and non-users
| Users | Non-users | |
|---|---|---|
| N (weighted) | 4753 | 10,460 |
| Gender | ||
| Male | 2068 (43.5%) | 5193 (49.6%) |
| Female | 2685 (56.5%) | 5267 (50.4%)a |
| Age | ||
| Mean (SD) age (years) | 47.5 (17.5) | 49.4 (18.2)a |
| <25 years | 478 (10.1%) | 1104 (10.6%) |
| 25–34 years | 867 (18.2%) | 1509 (14.4%)a |
| 35–49 years | 1255 (26.4%) | 2658 (25.4%) |
| 50–64 years | 1210 (25.5%) | 2595 (24.8%) |
| ≥65 years | 943 (19.8%) | 2594 (24.8%)a |
| Socioeconomic status | ||
| Employed | 2,866 (60.3%) | 5,909 (56.5%)a |
| Non-employed | 1,887 (39.7%) | 4,551 (43.5%) |
Data are presented as numbers and percentages of weighted totals, unless otherwise indicated.
p < 0.05.
Of the 7452 opioids taken by participants, 7190 (96.5%) were classified as step II analgesics on the World Health Organization (WHO) analgesic scale,14 and 262 (3.5%) were step III analgesics. The most common step II opioids were codeine-containing preparations, which accounted for 62% of preparations used, and tramadol-containing preparations, which accounted for 22%. For the step III analgesics, morphine or morphine sulphate both accounted for 1% of opioids, and other medications accounted for 2%.
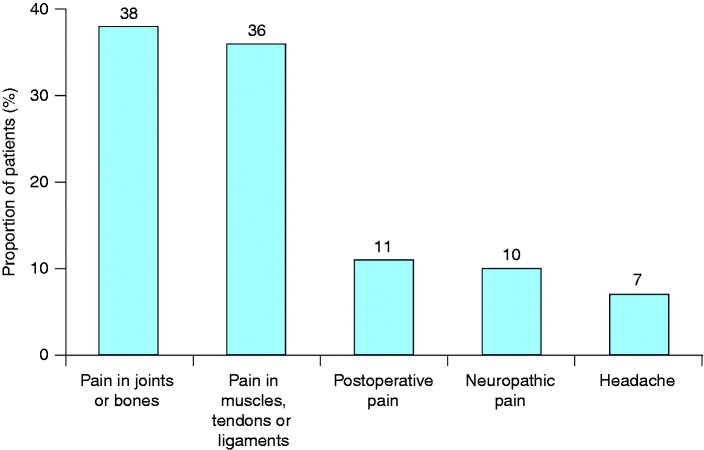
Overall, 76% of opioids were used on an occasional basis, while 23% were used regularly (every day or almost every day). The frequency of use was unknown in 1%. The most common reasons for using opioids were joint or bone pain, which were present in 38% of users, and pain in the muscles, tendons or ligaments (36%) (Figure 1): The median number of reasons for opioid use was 1.2. Compared with step II analgesics, step III analgesics were more likely to be used on a regular basis (22% versus 50%, p < 0.05) or to be in current use (25% versus 36%, p < 0.05). Similarly, compared with step II analgesics, step III medications were more likely to be taken for more than six months (25% versus 35%, p < 0.05), and were less likely to be taken for fewer than 15 days (45% versus 27%, p < 0.05).
Figure 1.
Principal indications for opioid treatment.
Prevalence of OIC
Among the 4753 opioid users, 414 (8.7%) reported constipation following their most recent use; this figure corresponds to 2.7% of the general study population. However, in regular users, the prevalence of constipation reached 21%. Among the 414 participants reporting constipation, 221 (53.4%) had pre-existing constipation prior to their most recent analgesic use. Constipation worsened following analgesic use in 147 of these 221 (67%) patients. The mean (SD) delay for constipation worsening was 7.2 (8.3) days. The mean (SD) number of analgesics taken was 1.7 (0.8) in participants with pre-existing constipation and 2.0 (0.8) in participants without pre-existing constipation. Compared with participants who had not suffered from constipation before using analgesics, those constipated prior to their last analgesic treatment were more likely to have taken one pain relief medication (48% versus 37%) and to have taken two medications for constipation (23% versus 14%), and were less likely to report being highly satisfied with their constipation treatment (45% versus 50%). The median level of laxative treatment satisfaction (rated from 0 to 10, with higher values indicating greater satisfaction) in participants with pre-existing constipation was 7.0, compared with 8.0 in those without pre-existing constipation.
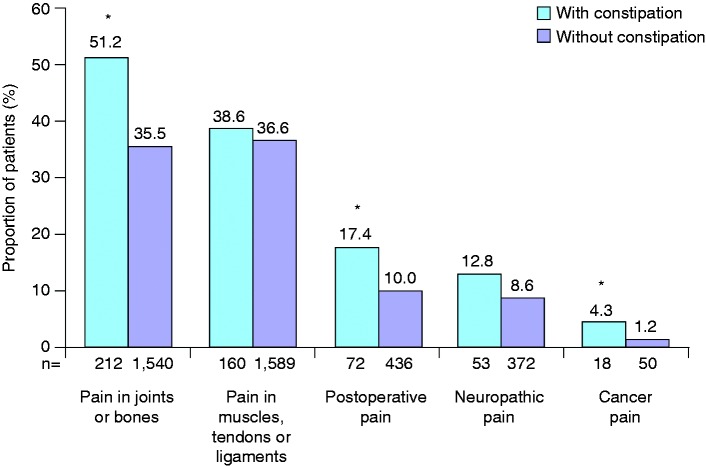
Compared with those without constipation, opioid users with constipation were more likely to be female, older, and non-employed (Table 2). In addition, participants with OIC were more likely than non-constipated opioid users to be taking step III medications, and were more likely to have been taking opioids for more than six months (Table 2). The indications for opioid use among participants with and without constipation are shown in Figure 2. The most common reasons for taking opioids among participants with OIC were joint or bone pain (51.2%), and pain in the muscles, tendons or ligaments (38.6%). Compared with corresponding opioid-using participants without constipation, the proportion of participants with OIC was increased (p < 0.05) in those with joint or bone pain, postoperative pain, or cancer pain (Figure 2).
Table 2.
Demographic and clinical characteristics of opioid users with and without constipation
| With constipation | Without constipation | |
|---|---|---|
| N (weighted) | 414 | 4339 |
| Gender | ||
| Male | 119 (28.7%) | 1949 (44.9%) |
| Female | 294 (71.0%) | 2390 (55.1%)a |
| Age | ||
| Mean (SD) age (years) | 54.0 (16.7) | 46.9 (17.5)a |
| <25 years | 15 (3.6%) | 463 (10.7%)a |
| 25–34 years | 46 (11.1%) | 821 (18.9%)a |
| 35–49 years | 96 (23.2%) | 1159 (26.7%) |
| 50–64 years | 145 (35.0%) | 1065 (24.5%)a |
| ≥65 years | 112 (27.1%) | 831 (19.2%)a |
| Socioeconomic status | ||
| Employed | 218 (52.7%) | 2647 (61.0%) |
| Non-employed | 195 (47.1%) | 1692 (39.0%)a |
| Mean (SD) body mass index (kg/m2) | 26.7 (6.0) | 26.0 (5.5) |
| Analgesic grade | ||
| Step II | 371 (89.6%) | 4266 (98.3%) |
| Step III | 43 (10.4%) | 73 (1.7%)a |
| Frequency of opioid use | ||
| Regular | 236 (57.0%) | 873 (20.1%)a |
| Occasional | 174 (42.0%) | 3440 (79.3%) |
| Current use | 232 (56.0%) | 1156 (26.6%)a |
| Duration of opioid treatment | ||
| <15 days | 90 (21.7%) | 2152 (49.6%)a |
| 15 days−<1 month | 56 (13.5%) | 424 (9.8%) |
| 1–<3 months | 53 (12.8%) | 327 (7.5%)a |
| 3–<6 months | 31 (7.5%) | 286 (6.6%) |
| ≥6 months | 177 (42.8%) | 1044 (24.1%)a |
Data are presented as numbers and percentages of weighted totals, unless otherwise indicated.
p < 0.05.
Figure 2.
Indications for opioid treatment in opioid users with (n = 414) and without (n = 4339) constipation. ap < 0.05 versus opioid users without constipation.
Of the 4637 participants treated with step II analgesics, 371 (8%) suffered from constipation, whereas there were 43 (37%) sufferers of constipation among the 116 users of step III analgesics.
OIC symptoms
Symptoms reported by the 414 participants with OIC are summarised in Table 3. Approximately 83% of participants experienced hard or very hard stool consistency, and a similar proportion reported ‘severe’ or ‘very severe’ straining during defaecation. In addition, approximately two-thirds reported intestinal bloating, of whom 42% reported a high level of discomfort as a result. The mean (SD) level of discomfort, measured on a scale of 0–10, was 6.9 (1.8) in participants using step II analgesics and 7.1 (1.8) among those using step III analgesics.
Table 3.
Symptoms associated with opioid-induced constipation according to the type of opioids
| Step II opioids | Step III opioids | |
|---|---|---|
| N (weighted) | 354 | 60 |
| Stool consistency | ||
| Very hard | 150 (42.4%) | 35 (58.3%)a |
| Quite hard | 143 (40.4%) | 13 (21.7%)a |
| Not very hard | 17 (4.8%) | 3 (5.0%) |
| Not hard at all | 6 (1.7%) | 1 (1.7%) |
| Unknown | 38 (10.7%) | 8 (13.3%) |
| Straining during defaecation | ||
| A lot | 144 (40.7%) | 36 (60.0%)a |
| Quite a lot | 142 (40.1%) | 14 (23.3%)a |
| Not very much | 28 (7.9%) | 5 (8.3%) |
| Not much at all | 3 (0.8%) | 1 (1.7%) |
| Unknown | 37 (10.5%) | 4 (6.7%) |
| Intestinal bloating and discomfort | 235 (66.4%) | 43 (71.7%) |
| Mean (SD) level of discomfortb | 6.9 (1.8) | 7.1 (1.8) |
| High level of discomfortc (8–10) | 102 (43.4%) | 16 (37.2%) |
| Mild level of discomfortc (4–7) | 120 (51.1%) | 27 (62.8%) |
| Low level of discomfortc (0–3) | 10 (4.3%) | 0 |
Data are presented as numbers and percentages of weighted totals, unless otherwise indicated.
p < 0.05. bReported on a scale of 0–10. cProportion of participants reporting bloating.
OIC symptoms had an impact on compliance with pain treatment, leading nearly one-third of participants to reduce the number of pills taken for treating pain.
Treatment of OIC
Overall, 168 of 354 (47.5%) participants taking step II opioids and reporting OIC, and 39 of 60 (65.0%) taking step III opioids and reporting OIC (p < 0.05), consulted a physician (usually a primary care physician) because of constipation following their most recent use of opioids. The mean (SD) time from onset of symptoms to consultation was shorter (p < 0.05) in participants taking step III analgesics than in those taking step II medications: 8.2 (7.0) days versus 17.0 (18.5) days, respectively.
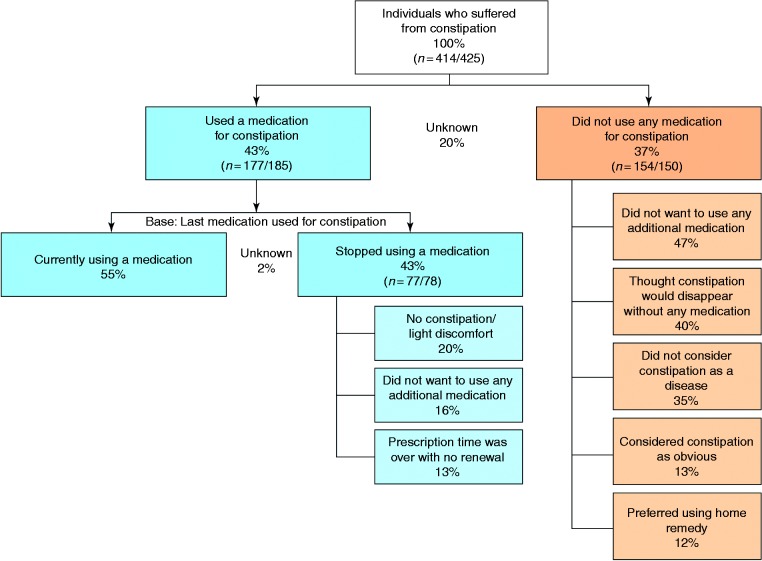
Of the 414 participants reporting OIC, 177 (42.8%) had used medications to treat constipation. The mean (SD) number of medications used was 1.6 (1.3): Sixty-one per cent of participants had used a single medication, and 32% had used two or more (no information was available for the remaining 7%). Older or non-employed participants were more likely to use medication to treat constipation, as were regular or current users of analgesics and participants using analgesics for more than six months (Table 4). Similarly, participants using medication to treat constipation were significantly more likely than non-users to have experienced constipation prior to opioid treatment, and to have had worsening of constipation after taking opioids (Table 4). Reasons for non-use of medication for constipation are shown in Figure 3. Among participants who did not use any medication for constipation, the most common reasons were a desire not to take further medication (47%), and beliefs that constipation would resolve spontaneously (40%) or that constipation is not a disease (35%). In participants who discontinued constipation medication (n = 77), the principal reasons for discontinuation were mildness of constipation or discomfort (20%), a desire not to take additional medication (16%), and non-renewal of the prescription (13%).
Table 4.
Demographic and clinical characteristics of participants with opioid-induced constipation according to use of medication to treat constipation
| Using medication for constipation | Not using medication for constipation | |
|---|---|---|
| N (weighted) | 177 | 154 |
| Gender | ||
| Male | 44 (24.9%) | 46 (29.9%) |
| Female | 133 (75.1%) | 108 (70.1%) |
| Age | ||
| Mean (SD) age (years) | 55.3 (16.7) | 49.5 (15.6)a |
| <25 years | 8 (4.5%) | 5 (3.2%) |
| 25–34 years | 14 (7.9%) | 27 (17.5%)a |
| 35–49 years | 37 (20.9%) | 42 (27.3%) |
| 50–64 years | 68 (38.4%) | 55 (35.7%) |
| ≥65 years | 50 (28.2%) | 25 (16.2%)a |
| Socioeconomic status | ||
| Employed | 84 (47.5%) | 100 (64.9%) |
| Non-employed | 93 (52.5%) | 54 (35.1%)a |
| Mean (SD) body mass index (kg/m2) | 26.7 (5.7) | 27.1 (6.2) |
| Opioid use | ||
| Regular use | 130 (73.4%) | 74 (48.1%)a |
| Current use | 107 (60.5%) | 74 (48.1%)a |
| Treatment >6 months | 89 (50.3%) | 50 (32.5%)a |
| Constipation history | ||
| Constipation leading to cessation of analgesia | 18 (10.2%) | 12 (7.8%) |
| Constipation before use of analgesia | 108 (61.0%) | 69 (44.8%)a |
| Prior constipation worsening after use of analgesia (% of above) | 81 (75.0%) | 36 (52.2%)a |
Data are presented as numbers and percentages of weighted totals, unless otherwise indicated.
p < 0.05.
Figure 3.
Reasons for non-use or discontinuation of medication for constipation among opioid users. n = weighted/unweighted values.
For patients who had stopped medication for constipation, we have given only the three main reasons of discontinuation; therefore, the overall percentage is not 100%. For the remaining patients, reasons of discontinuation were miscellaneous.
Overall, 80% of the individuals treated for constipation took at least one laxative. The most commonly used preparations were osmotic laxatives, which were used by 57% of participants using constipation treatments, followed by stimulant laxatives (9%), and lubricant laxatives (4%). Bulking agents were used by 7% of participants, while enemas and herbal preparations were used by 3% each. In 77% of the cases, the medication was prescribed by a doctor, whereas over-the-counter products were used by only 23% of participants. Constipation medication was most commonly prescribed by primary care physicians (69%) and gastroenterologists (15%); in the remaining cases, the medication was prescribed by other specialists, such as gynaecologists, neurologists, oncologists or rheumatologists.
Of the 177 participants with OIC who used medication for constipation, 108 (61%) had pre-existing constipation prior to the use of analgesics (Table 4). Of the latter, 81 (75%) experienced worsening constipation after using analgesics. By contrast, of 154 participants who did not take medication for constipation, only 69 (45%, p < 0.05 versus participants receiving constipation medication) had pre-existing constipation, and only 36 (52%, p < 0.05) of these experienced worsening constipation following analgesic use (Table 4).
Participants’ satisfaction with their constipation treatment was rated from 0 to 10. The mean (SD) level of satisfaction was 7.2 (1.3). Patients with pre-existing constipation prior to their most recent analgesic use were more likely than those without pre-existing constipation to report mild or high levels of satisfaction (95% versus 90%), and less likely to report low levels of satisfaction (2% versus 5%).
Discussion
This study was designed to assess the prevalence of OIC in a large representative group of the French general population, and to describe the clinical characteristics of constipation according to the type of opioid users and the indications for opioid treatment. The results showed that approximately one-third of participants of all ages had used some form of analgesia, principally step II analgesics, in the previous six months, and 37% of those using step III analgesics had experienced OIC. This is a consequence of the medical practice and prescriptions as French patients are not allowed to buy over the counter even grade II opioids. The overall prevalence of constipation among opioid users in this study (8.7%) was markedly lower than in previous studies, which have reported prevalence of up to 87% in patients with chronic pain.3–5 This lower figure presumably reflects the unselected nature of the study population, two-thirds of whom were not using any form of analgesia, and the fact that 44% of participants had used opioids for fewer than 15 days, while the duration of treatment exceeded six months in only 25%. As in previous studies,15,16 the prevalence of OIC tended to increase with age: Sixty-two per cent of participants who reported constipation following their last use of analgesic were aged 50 years or older. As might be anticipated, the incidence of OIC tended to increase with both analgesia step and the frequency and duration of opioid use.5,17 We confirmed this point with the demonstration of prevalence of OIC reaching 21% in regular users. Importantly, only 46% of analgesic medications associated with constipation were in current use when the survey was conducted. This is consistent with previous studies, which have shown that OIC is often sufficiently troublesome to cause patients to reduce analgesic doses or discontinue treatment completely.5–7
In this study, we planned to analyse epidemiological data on OIC by taking into account possible pre-existing constipation. More than 50% of the participants who reported constipation following opioid use had pre-existing constipation prior to their most recent analgesic usage, and two-thirds of these experienced worsening of constipation following opioid treatment. These patients tended to take more anti-constipation medications than those without pre-existing constipation (mean (SD) 1.8 (1.5) versus 1.3 (0.6)), but their median level of satisfaction with these medications was lower (7.0 versus 8.0).
Constipation was defined in this study as fewer than three bowel movements per week, straining during defaecation, or both. The use of two criteria to define constipation was intended to reduce the risk of bias resulting from the fact that no validated constipation scoring systems, such as the Bristol Stool Form Scale18 or the Rome III criteria for functional constipation,19 were used in this study. A more comprehensive definition has recently been proposed by a multidisciplinary working group, which defines OIC as ‘a change from baseline bowel habits when initiating opioid therapy that is characterised by any of the following: reduced bowel movement frequency, development or worsening of straining to pass bowel movements, a sense of incomplete rectal evacuation or harder stool frequency’.6
The mean participants’ satisfaction with anti-constipation medication of 7.2 (rated from 0 to 10) reflects the problematic nature of OIC treatment. First-line laxative treatment is often unsuccessful due to the non-specific mechanism of action of these agents, which do not affect peripheral µ-opioid receptors.3,20 Peripherally acting µ-opioid receptor antagonists such as naloxegol or methylnaltrexone offer a potential approach to the management of OIC that does not compromise central analgesic effects.4
Strengths of the present study include the large and representative population studied, and the high response rate (78%). In addition, the design of the questionnaire allowed the occurrence and symptoms of OIC to be related to the type of opioid used and to the presence or absence of pre-existing constipation. Limitations include the retrospective questionnaire design, which presents a risk of responder bias (although this has been compensated for to some extent by weighting the data according to current French population demographics), the lack of a validated questionnaire for OIC assessment and the fact that opioid users were taking opioids predominantly for non-cancer indications and in many cases for a short duration. Related to this latter point is the finding that 44% of users were taking opioids for fewer than 15 days; as the frequency of OIC is known to correlate with the duration of treatment, this might suggest that the incidence of OIC has been under-estimated.
In conclusion, this study has shown that approximately one-third of a representative French population had used opioid analgesics within the six months prior to surveying, that 9% of opioid users (3% of the general population) had experienced OIC, while OIC is more frequent in regular users who are the population of interest for the development of new treatments. OIC appears to be under-treated, as only 43% had used any medication for constipation. Furthermore, participants’ satisfaction with their constipation medications was only moderate. These findings would suggest that a significant unmet need remains in the management of OIC.
Supplementary Material
Acknowledgements
Conception and study design: PD, CS, CF and JM; study conduct: CF and JM; results analysis: PD, CS, CF and JM; revision of the manuscript: PD, CS and CF. All authors approved the final version of the manuscript, including the authorship list.
Writing support was provided by Anagram and funded by AstraZeneca.
Special thanks go to Milka Maravic, an employee of AstraZeneca, for her critical review of the manuscript.
APPENDIX
Study questionnaire
Declaration of conflicting interests
PD has served as study coordinator and has received funding from AstraZeneca. CS and CF are employees of AstraZeneca. JM is an employee of Kantar Health.
Funding
This work was supported by AstraZeneca.
References
- 1.Caraceni A, Hanks G, Kaasa S, et al. Use of opioid analgesics in the treatment of cancer pain: Evidence-based recommendations from the EAPC. Lancet Oncol 2012; 13: e58–e68. [DOI] [PubMed] [Google Scholar]
- 2.Cheung CW, Qiu Q, Choi SW, et al. Chronic opioid therapy for chronic non-cancer pain: A review and comparison of treatment guidelines. Pain Physician 2014; 17: 401–414. [PubMed] [Google Scholar]
- 3.McCarberg BH. Overview and treatment of opioid-induced constipation. Postgrad Med 2013; 125: 7−17–7−17. [DOI] [PubMed] [Google Scholar]
- 4.Poulsen JL, Brock C, Olesen AE, et al. Clinical potential of naloxegol in the management of opioid-induced bowel dysfunction. Clin Exp Gastroenterol 2014; 7: 345–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bell TJ, Panchal SJ, Miaskowski C, et al. The prevalence, severity, and impact of opioid-induced bowel dysfunction: Results of a US and European Patient Survey (PROBE 1). Pain Med 2009; 10: 35–42. [DOI] [PubMed] [Google Scholar]
- 6.Camilleri M, Drossman DA, Becker G, et al. Emerging treatments in neurogastroenterology: A multidisciplinary working group consensus statement on opioid-induced constipation. Neurogastroenterol Motil 2014; 26: 1386–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalso E, Edwards JE, Moore RA, et al. Opioids in chronic non-cancer pain: Systematic review of efficacy and safety. Pain 2004; 112: 372–380. [DOI] [PubMed] [Google Scholar]
- 8.Wee B, Adams A, Thompson K, et al. How much does it cost a specialist palliative care unit to manage constipation in patients receiving opioid therapy? J Pain Symptom Manage 2010; 39: 644–654. [DOI] [PubMed] [Google Scholar]
- 9.McMillan SC, Tittle M, Hagan S, et al. Management of pain and pain-related symptoms in hospitalized veterans with cancer. Cancer Nurs 2000; 23: 327–336. [DOI] [PubMed] [Google Scholar]
- 10.Laugsand EA, Sprangers MA, Bjordal K, et al. Health care providers underestimate symptom intensities of cancer patients: A multicenter European study. Health Qual Life Outcomes 2010; 8: 104–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Institut national de la statistique et des études économiques (INSEE). Tableaux de l’économie Française, 2013, http://www.insee.fr/fr/ffc/tef/tef2013/tef2013.pdf (accessed 12 May 2015).
- 12.Kish L. A procedure for objective respondent selection within the household. J Am Stat Assoc 1949; 44: 380–387. [Google Scholar]
- 13.Deville JC, Särndal CE, Sautory O. Generalized raking procedures in survey sampling. J Am Stat Assoc 1993; 88: 1013–1020. [Google Scholar]
- 14.World Health Organization. Cancer pain relief: With a guide to opioid availability, 2nd ed Geneva: World Health Organization, 1996. [Google Scholar]
- 15.Williams RE, Bosnic N, Sweeney CT, et al. Prevalence of opioid dispensings and concurrent gastrointestinal medications in Quebec. Pain Res Manag 2008; 13: 395–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosti G, Gatti A, Costantini A, et al. Opioid-related bowel dysfunction: Prevalence and identification of predictive factors in a large sample of Italian patients on chronic treatment. Eur Rev Med Pharmacol Sci 2010; 14: 1045–1050. [PubMed] [Google Scholar]
- 17.Cook SF, Lanza L, Zhou X, et al. Gastrointestinal side effects in chronic opioid users: Results from a population-based survey. Aliment Pharmacol Ther 2008; 27: 1224–1232. [DOI] [PubMed] [Google Scholar]
- 18.Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol 1997; 32: 920–924. [DOI] [PubMed] [Google Scholar]
- 19.Drossman DA, Dumitrascu DL. Rome III: New standard for functional gastrointestinal disorders. J Gastrointestin Liver Dis 2006; 15: 237–241. [PubMed] [Google Scholar]
- 20.Pappagallo M. Incidence, prevalence, and management of opioid bowel dysfunction. Am J Surg 2001; 182(5A Suppl): 11S–18S. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.