Abstract
Background:
It is well known that gastric mucosa dysplasia and intestinal metaplasia are gastric precancerous lesions (GPL). Moxibustion treatment of Liangmen (ST21) and Zusanli (ST36) alleviated the inflammatory response and dysplasia of gastric mucosa in our previous study. The purpose of this study was to further examine the underlying mechanism of moxibustion treatment of ST21 and ST36 on GPL.
Materials and Methods:
Sixty SD rats were divided into five groups and rats with GPL were treated with either moxibustion (ST), moxibustion (Sham), or vitacoenzyme. B-cell lymphoma 2 (bcl-2), tumor protein p53 (P53) and cellular Myc (C-MYC), which are related to cell apoptosis, proliferating cell nuclear antigen (PCNA), vascular endothelial growth factor (VEGF), argyrophilic nucleolar organizer region proteins (Ag-NORs), which are associated with cell proliferation, and cell signaling proteins, nuclear factor kappa B (NF-κB), epidermal growth factor receptor (EGFR) and phosphorylated extracellular signal regulated kinase (p-ERK), were measured after moxibustion treatment.
Results:
Compared with Control group, gastric mucosa in GPL group showed abnormal mucosal proliferation and pathological mitotic figure, the mRNA expression of bcl-2, P53 and C-MYC increased significantly (P < 0.01), the protein expression of PCNA, VEGF, Ag-NORs and the activity of NF-κβ as well as EGFR/ERK signaling proteins also increased significantly (P < 0.01). Moxibustion treatment decreased gastric mucosal proliferation and pathological mitotic figure, down-regulated the mRNA expression of bcl-2, P53, C-MYC (P < 0.01), decreased the protein expression of PCNA, VEGF, Ag-NORs and the activity of NF-κβ as well as EGFR/ERK signaling proteins significantly (P < 0.01). But moxibustion treatment of Sham didn’t show the same effect on GPL.
Conclusion:
Moxibustion treatment inhibited cell apoptosis and reduced gastric mucosa dysplasia by inhibiting the expression of bcl-2, P53, C-MYC and decreased the activity of NF-κβ as well as EGFR/ERK signaling proteins.
Keywords: Gastric precancerous lesions, Moxibustion treatment, Cell apoptosis, Cell proliferation, Signaling proteins
Introduction
The typical pathological features of gastric precancerous lesions (GPL) are gastric mucosa dysplasia and intestinal metaplasia, which is closely associated with gastric carcinogenesis (Marques-Silva et al., 2014; Yoon and Kim, 2015). Helicobacter pylori infection, dietary habits, immune factors and duodenal reflux were involved in the mechanism of GPL (Pan et al., 2014). Present medicine, including gastric mucosa protectant, proton pump inhibitors (PPIs) and antibiotic regimens for H. pylori eradication, are the most commonly used drugs for symptomatic treatment. However, the long-term use of PPIs is associated with side effects and may even increase the risk of carcinogenesis (Bahat, 2014; Florentin and Elisaf, 2012), and whether or not GPL are reversible by H. pylori eradication remains controversial (Park and Kim, 2015). Thus, an agent or therapy inhibiting GPL or preventing it worsening and accompanied by less side effects may represent an effective strategy to decrease morbidity from gastric cancer.
Nuclear factor kappa B (NF-κB) and epidermal growth factor receptor (EGFR)/extracellular signal regulated kinase (ERK) activation have been proved to be the important step in gastric carcinogenesis progress (Osaki et al., 2011; Wang et al., 2004). Overexpression of NF-κ B contributes to enhanced gastric mucosal intestinal metaplasia and promoting the expression of B-cell lymphoma 2 (bcl-2), tumor protein p53 (P53) and cellular Myc (C-MYC) (Xu et al., 2001; Yang et al., 2004). EGFR is involved in control of gastric cell proliferation through activation of mitogen-activated protein kinase (MAPK) /ERK signaling pathway (Osaki et al., 2011), EGFR/ERK signaling activation will up-regulate the expression of cell proliferation-related proteins in gastric mucosa, such as proliferating cell nuclear antigen (PCNA), vascular endothelial growth factor (VEGF) and argyrophilic nucleolar organizer region proteins (Ag-NORs) (Moraes et al., 2013). Traditional Chinese medical therapies (including herbs, acupuncture and moxibustion) have been used to treat gastric diseases and its classical symptoms for two thousand years (Deng et al., 2012; Gao et al., 2005). Our previous study found that moxibustion of Liangmen (ST21) and Zusanli (ST36) alleviated the inflammatory response, down-regulated the expression of cell proliferative factors and decreased the level of serum carcino-embryonic antigen (CEA) in rats with GPL, suggesting a potentially protective role (Chen et al., 2013; Yang et al., 2015). Electro-acupuncture of ST21 and ST36 could inhibit the expression of bcl-2 and P53 and decrease the apoptotic index, thereby play an important role in inhibiting cell apoptosis of gastric mucosa cells in rats with GPL (Yang et al., 2016). The aim of this study is to further investigate whether or not moxibustion of ST21 and ST36 protects gastric mucosa from carcinogenesis by targeting NF-κB and EGFR/ERK signaling pathway. Vitacoenzyme is used as a positive control, which is a compound preparation derived from soybean fermentation, the main ingredients of which include riboflavin and its derivatives. It has been used as standard care to treat GPL and has been shown to inhibit the expression of epidermal growth factor receptor (EGFR), tumor protein p53 (P53) and C-MYC in the gastric mucosa (Shen et al., 2008).
Materials and Methods
Animals
The animal experimental protocol was approved by the ethics committee for administration of experimental animals at the Medical College of Xiamen University. All animal procedures were performed in accordance with the regulations for the administration of affairs concerning experimental animals of the People’s Republic of China (November 14, 1988), which is consistent with the National Institute of Health “Guide for the Care and Use of Laboratory Animals”. Sixty healthy male specific pathogen free (SPF) grade Sprague-Dawley rats (100 ± 20 g, 4 weeks old) were provided by Xiamen University Laboratory Animal Center (certificate no. SCXK(Min)2008-0002) and housed in an air-conditioned atmosphere at 22°C and 50% relative humidity under a 12h dark-light cycle. Rats were allowed free access to food and water.
Main reagents and instruments
N-methyl-N’-nitro-N-nitrosoguanidine (MNNG, Cat.No. M0527) was bought from Tokyo Kasei Kogyo Co., Ltd. (Tokyo, Japan). Anti-rat ELISA kits, including PCNA (Cat.No. BM0104), VEGF (Cat.No. EK0540) and Ag-NORs (Cat.No. EK0583), were bought from Wuhan boster biotechnology co., Ltd. (Wuhan, China). Rabbit anti rat EGFR (Cat.No. #2232) and phosphorylated ERK (p-ERK, Cat.No. #4376) were purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA). Goat anti rabbit IgG/HRP antibody (Cat.No. SAB3700946) was acquired from Sigma-Aldrich Co. (St. Louis, MO, USA). Instruments used in this experiment were as follow: Microplate reader (type 352, LabSystems Multiskan MS, Thermo Fisher Scientific Inc., Waltham, MA, United States), Plate washer (type AC8, LabSystems Multiskan MS, Thermo Fisher Scientific Inc., Waltham, MA, United States), electrophoretic apparatus (Bio-Rad Laboratories, Inc., Hercules, California, USA), polymerase chain reaction system (Applied Biosystems co., Ltd., Waltham, MA, USA), electrophoresis groove (Beijing Donglin Changsheng biotechnology co., Ltd., Beijing, China).
Rat model of GPL
Model replication method was as follow: MNNG was mixed into the drinking water to arrive the final concentration of 150μg/ml. The bottle with MNNG solution was wrapped with aluminum foil in order to protect it from light. Rats drink the fresh prepared MNNG solution freely for each day. At the same time, rat’s tail was clipped everyday. Last, irregular diet was carried out that rats got 2 days full feeding and 1 day no feeding recurrently. One rat was sacrificed every 2 weeks. The stomach section was stained with Hematoxylin and Eosin (HE) for routine pathological examination by light microscopy.
Study design
Figure 1 shows the study design for moxibustion treatment of GPL rats. Sixty rats were randomly assigned to two groups: Control group (n = 10) and Model replication group (n = 50). One rat of model replication group was sacrificed every 2 weeks to confirm the establishment of GPL model until the end of 20th week, and 10 rats had used in total. The other 40 rats were further randomly divided into 4 subgroups: GPL group (n = 10), GPL+Moxa(ST) group (n = 10), GPL+Moxa(Sham) group (n = 10) and GPL+vitacoenzyme group (n = 10). Moxibustion or drug treatment began from the second day of model replication and lasted for a total of 20 weeks. After the entire treatment course, all rats were killed simultaneously. The assessments including B-cell lymphoma 2 (bcl-2), P53, C-MYC, PCNA, VEGF, Ag-NORs, nuclear factor kappa B (NF-κΕ), EGFR and p-ERK measurement were performed on the next day.
Figure 1.
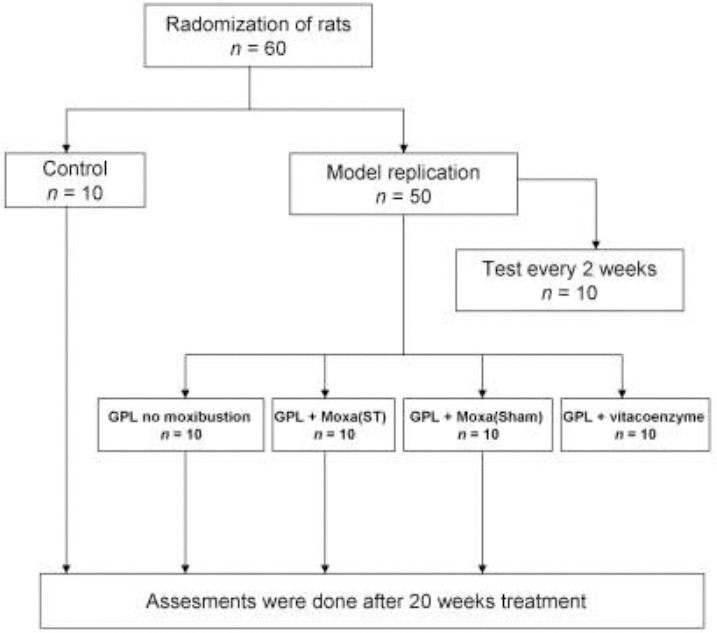
Study design of moxibustion treatment in gastric precancerous lesions rats
Control indicates the normal rats that their gastric mucosa doesn’t receive injury factors. Model replication is the rat model of gastric precancerous lesions (GPL). Some of them were used to test whether the model was established successfully every 2 weeks. The other rats were divided into four groups: GPL rats without moxibustion treatment, GPL rats with moxibustion treatment on ST21 and ST36, GPL rats with moxibustion treatment on Sham, and GPL rats with vitacoenzyme treatment. The whole treatment course lasted for 20 weeks and then evaluated their effect.
Moxibustion treatment
In the GPL+Moxa(ST) group, moxibustion was applied at ST21 and ST36, located according to the textbook “Experimental Acupuncture & Moxibustion” (Li, 2003). ST21 was located at the intersection of midclavicular line and horizontal line of 3/4 distance from the upper edge of the sternum to the external genital organs. ST36 was located ~5mm below the capitulum fibulae. In the GPL+Moxa(Sham) group, moxibustion was applied at 1cm lateral to ST21 and 1cm medial to ST36 (Figure S1). Rats in the GPL+Moxa(ST) and GPL+Moxa(Sham) groups were treated unilaterally with lit moxa respectively, from the second day of model replication. Prior to treatment, rats were fixed on boards and hair was clipped around the site of stimulations. Cottons with isoflurane were put into 50ml tubes, and rats inhaled isoflurane during moxibustion treatment. Lit moxa cones (width 1.75cm) were fixed using self-made moxibustion equipment to ensure the lit ends were 0.5cm away from the surface of the skin. Moxibustion was applied for 15 minutes every other day for 20 weeks in total and laterality was alternated with each session.
Drug treatment
Rats in the GPL+vitacoenzyme group received 216mg/kg vitacoenzyme (Cat.No. 010401; Hebei Huanhai Medicine Industry Co., Ltd, Cangzhou, China), which is equivalent to a clinical dose of 2.4g per day (by conversion according to body surface area). Vitacoenzyme tablets were dissolved in distilled water and administered intragastrically every day for 20 weeks.
Specimen collection
Keeping from food for 24h after 20weeks treatment, all rats were anesthetized intraperitoneally with 10% chloral hydrate, then were sacrificed and their stomachs were dissected. Each stomach was opened along the greater curvature and washed with 1%DEPC ice-cold saline. Stomach specimens of curvatura gastrica minor were used for histopathological examination. Stomach specimens of sinus ventriculi with the size of 0.5cm*0.5cm was cut out and homogenized in saline, then the homogenate was put in refrigerator with the temperature of -20°C. Cell membrane was damaged by repeated freezing and thawing twice and the homogenate was centrifuged for 5 minutes (5000rpm/min, 2~8°C), then the supernatant fluid was used for protein test.
Reverse transcription polymerase chain reaction (RT-PCR)
Total RNA was extracted from gastric mucosal cells using TRIzol reagent and reverse-transcribed to cDNA using TOYOBO reverse transcription kits with cDNA Eraser, according to the manufacturer’s instructions. Then cDNA was synthesized using polymerase chain reaction system (Applied Biosystems co., Ltd., Waltham, MA, USA) at 30°C for 10min, 42°C for 60min, 99°C for 5min, 4°C for 5min. The full-length mRNA sequences of bcl-2, P53, C-MYC and NF-κB, were obtained from GenBank (http://www.ncbi.nlm.nih.gov/nucleotide), the sequence of primers were designed by using Primer 5.0 software (Applied Biosystems co., Ltd., Waltham, MA, USA) and synthesized by Beijing Dingguo Changsheng biotechnology co., Ltd. (Beijing, China) (see Table 1). Primers were used for a total of 35 cycles of PCR under the following parameters: pre-denaturation at 94°C for 2 min, denaturation at 94°C for 30 sec, annealing at 55°C for 30 sec, extension at 72°C for 30 sec, extension at 72°C for 10 min. PCR product quality was monitored by post-PCR melt curve analysis. Each PCR product was subjected to electrophoresis on 2% agarose gel for 30 min, then ethidium bromide-stained bands were scanned and analysed.
Table 1.
The primer sequence of GAPDH, bcl-2, P53, C-MYC and NF-κB
| Name | Transcription | Primer sequence |
|---|---|---|
| GAPDH | Forward | 5’ -AGAAGGCTGGGGCTCATTTG-3’ |
| Reverse | 5’ -AGGGGCCATCCACAGTCTTC-3’ | |
| bcl-2 | Forward | 5’ -GGCATCTTCTCCTTCCA-3’ |
| Reverse | 5’ -AGTTCCTCCACCACCGT-3’ | |
| P53 | Forward | 5’ -GCTTTGAGGTTCGTGTT-3’ |
| Reverse | 5’ -CCTTCTTGGTCTTCGGG-3’ | |
| C-MYC | Forward | 5’ -TCCAGCGAGAGACAGAG-3’ |
| Reverse | 5’ -GCAGAGGCAGAGAACAC-3’ | |
| NF-κB | Forward | 5’ -AATTTGGCTTCCTTTCTTGGCT-3’ |
| Reverse | 5’ -CTGCGATACCTTAATGACAGCG-3’ |
Enzyme-linked immunosorbent assay (ELISA)
Levels of the following proteins involved in cellular proliferation were measured in gastric tissue: PCNA, VEGF and Ag-NORs using rat-specific ELISA kits according to the manufacturer’s instructions (Wuhan Boster Biotechnology Co., Ltd., Wuhan, China). Briefly, samples were incubated for 30 min at 37°C, then were exposed to biotin-conjugated detection antibody and streptavidin-HRP respectively for 60 min at 37°C. Stabilised chromogen and stop solution were added to terminate the reaction, then plates were read at 450 nm (OD values) within 2 hours using a spectrophotometer.
Western blotting
Protein extracts were isolated from gastric mucosa in radio-immunoprecipitation assay buffer and protein concentrations were measured using the bicinchoninic acid (BCA) method. Protein supernatants (80μl) were separated on 10% SDS-polyacrylamide gel (SDS-PAGE) and transferred to nitrocellulose membranes. Rabbit anti-rat EGFR and p-ERK antibodies (1:1000; Cell Signaling Technology, Inc., Danvers, MA, USA) and goat anti-rabbit-IgG (H+L) antibody conjugated with horseradish peroxidase (1:5000; Sigma-Aldrich Co., St. Louis, MO, USA) were used as primary and secondary antibodies, respectively. The enhanced chemiluminescence (ECL) imaging method was used to facilitate the detection of protein bands.
Statistical analysis
All data were normally distributed and were expressed as mean ± standard deviation. Groups were compared by one-way analysis of variance (ANOVA) using the Statistical Package for the Social Sciences (SPSS) version 19.0 (SPSS Inc., Chicago, IL, USA). Fisher’s test of least significant difference was used for post-hoc individual comparisons. P < 0.05 or 0.01 (two-sided) was considered to be statistically significant.
Results
Effect of moxibustion treatment on gastric mucosal pathology
In order to investigate whether or not moxibustion treatment protected gastric mucosa in GPL rats effectively, we compared the routine tissue slice of gastric mucosal tissue stained by HE from five groups and observed by light microscopy. At 12 weeks, the stomach of model replication group represents less gastric mucosal folds than that of control group, HE staining of the stomach showed gastric mucosal glands atrophy and decrease (Figure S2). At 20 weeks, the structure of the gastric mucosa in Control rats was intact and visible lesions were not evident microscopically. However, in untreated GPL rats, the structure of gastric mucosa was incomplete, irregular glands and divided cellular nuclei were evident under high magnification. Both moxibustion at ST21 and ST36 and vitacoenzyme treatment appeared to attenuate the severity of gastric mucosal lesions, because the arrangement of the gastric gland was more regular and glandular atrophy was not observed. By contrast, moxibustion at Sham points did not appear to protect the gastric mucosa from pathological lesions (Figure 2).
Figure 2.
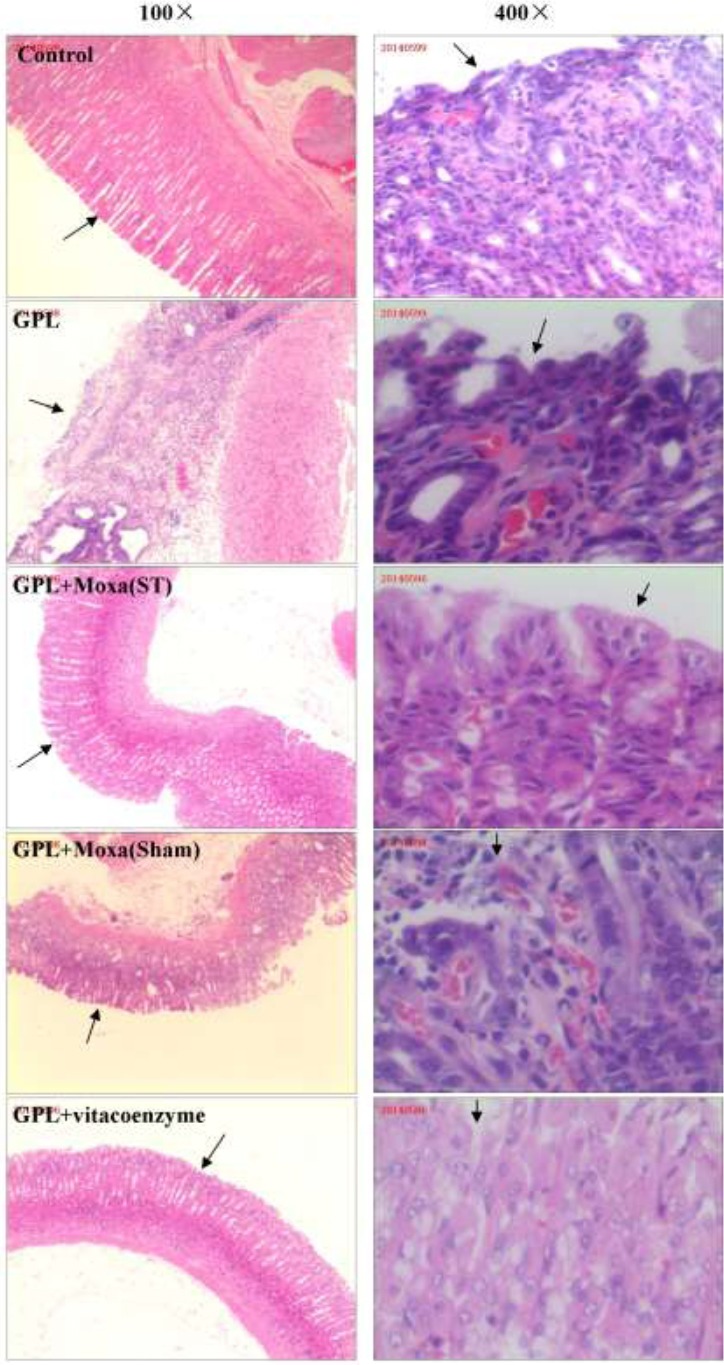
Histological evaluation of stomach sections of rats from different groups
Figures in the left column show the low amplification (100×) of HE staining of representative gastric mucosa section from five groups: Control, gastric precancerous lesions (GPL), GPL+Moxa(ST), GPL+Moxa(Sham) and GPL+vitacoenzyme. Figures in the right column show the high amplification (400×) of five groups respectively. Black arrow point to representive features of gastric mucosa of each group.
Effect of moxibustion treatment on cell apoptosis-related proteins
To explore the mechanism of moxibustion treatment on gastric precancerous lesions in vivo, we further study the cell apoptosis-related proteins by using RT-PCR. As shown in Figure 3, the mRNA expression of bcl-2, P53 and C-MYC in gastric mucosa of GPL group was significantly up-regulated when compared with the healthy Control group (2.95 ± 0.14 vs 2.18 ± 0.13, 0.79 ± 0.05 vs 0.35 ± 0.03, 0.72 ± 0.10 vs 0.18 ± 0.01, respectively, P < 0.01). Moxibustion treatment at ST21 and ST36 significantly down-regulated the expression of bcl-2, p53 and C-MYC in GPL rats relative to no treatment (1.18 ± 0.07 vs 2.95 ± 0.14, 0.40 ± 0.05 vs 0.79 ± 0.05, 0.39 ± 0.03 vs 0.72 ± 0.10, respectively, P < 0.01), which was lower than that of moxibustion treatment at Sham points (1.18 ± 0.07 vs 2.73 ± 0.15, 0.40 ± 0.05 vs 0.79 ± 0.07, respectively, P < 0.01, and 0.40 ± 0.03 vs 0.52 ± 0.08, P < 0.05). And drug treatment also showed the similar down-regulating effect on the mRNA expression of these cell apoptosis proteins.
Figure 3.
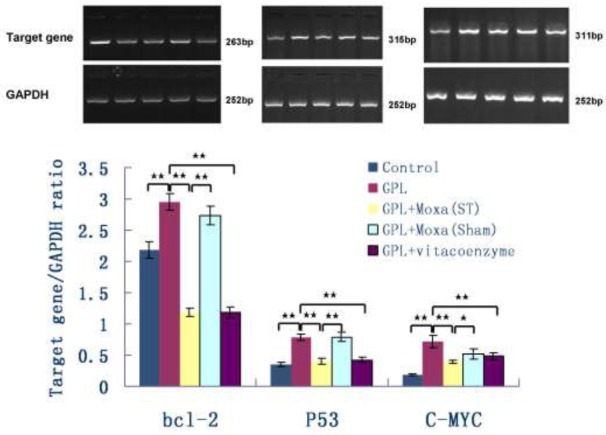
Effect of moxibustion treatment on the mRNA expression of apoptosis-related proteins in gastric precancerous lesions After 20 weeks of treatment, the mRNA expression of B-cell lymphoma 2 (bcl-2), tumor protein p53 (P53) and cellular Myc (C-MYC) in gastric mucosa of five groups: Control, GPL, GPL+Moxa(ST), GPL+Moxa(Sham) and GPL+vitacoenzyme (n=10), were detected by RT-PCR, the ratio of target gene and GAPDH was calculated. Results were compared by one-way analysis of variance and following with Fisher’s Least Significant Difference test for individual comparisons. *p < 0.05, **p < 0.01.
Effect of moxibustion treatment on oncogenes relating to cell proliferation
To explore the cell proliferation in gastric mucosa, we detected oncogenes relating to cell proliferation by using ELISA, RT-PCR and western blotting. Figure 4 showed the expression of various cell proliferation-related proteins in gastric mucosa measured by ELISA. Compared with Control group, the expression of PCNA, VEGF and Ag-NORs in GPL group increased about 1.5-fold (2.92 ± 0.06 vs 1.76 ± 0.04 ng/ml, 235.29 ± 4.71 vs 150.28 ± 3.13 ng/ml, and 116.57 ± 2.72 vs 76.20 ± 2.65 ng/ml, respectively, P < 0.01). Moxibustion treatment at ST21 and ST36 for 20 weeks significantly reduced gastric mucosal expression of them (2.06 ± 0.09 vs 2.92 ± 0.06 ng/ml, 170.10 ± 4.31 vs 235.29 ± 4.71 ng/ml, 87.23 ± 2.91 vs 116.57 ± 2.72 ng/ml, respectively, P < 0.01). Furthermore, their levels of GPL+Moxa (ST) group were significantly lower than that of GPL+Moxa (Sham) group (2.06 ± 0.09 vs 2.30 ± 0.09 ng/ml, 170.10 ± 4.31 vs 189.45 ± 5.04 ng/ml, 87.23 ± 2.91 vs 93.76 ± 3.75 ng/ml, respectively, P < 0.01).
Figure 4.
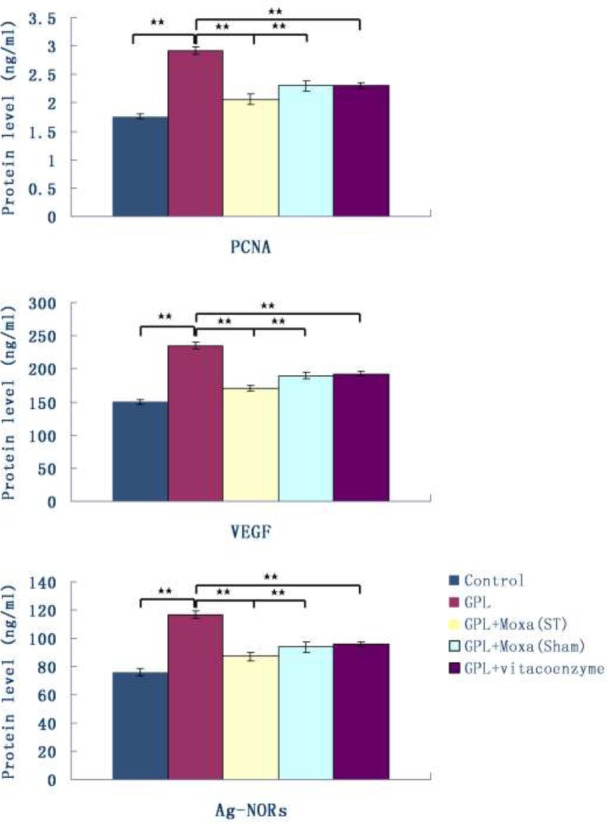
Effect of moxibustion treatment on cell proliferation-related proteins in gastric precancerous lesions After 20 weeks of treatment, the expression of proliferating cell nuclear antigen (PCNA), vascular endothelial growth factor (VEGF) and argyrophilic nucleolar organizer region proteins (Ag-NORs) in gastric mucosa of five groups: Control, GPL, GPL+Moxa(ST), GPL+Moxa(Sham) and GPL+vitacoenzyme (n=10), were measured by ELISA. Results were compared by one-way analysis of variance and following with Fisher’s Least Significant Difference test for individual comparisons. **p<0.01.
Effect of moxibustion treatment on NF-κB and EGFR/ERK signaling proteins
To explore the intracellular signal pathway of moxibustion treatment on GPL, we further focused on the mRNA expression of NF-κB after 20 weeks treatment. The result demonstrated that the mRNA expression of NF-κB was significantly up-regulated in GPL group compared to Control group (0.84±0.06 vs 0.26±0.02, P < 0.01). Moxibustion treatment of ST21 and ST36 down-regulated its expression in gastric mucosa significantly (0.43 ± 0.08 vs 0.84 ± 0.06, P < 0.01), and the effect was better than that of Sham points (0.43 ± 0.08 vs 0.76 ± 0.04, P < 0.01). Drug treatment didn’t show the effect of down-regulation of NF-κB in GPL (0.80 ± 0.08 vs 0.84 ± 0.06, P > 0.05) (Figure 5A).
Figure 5.
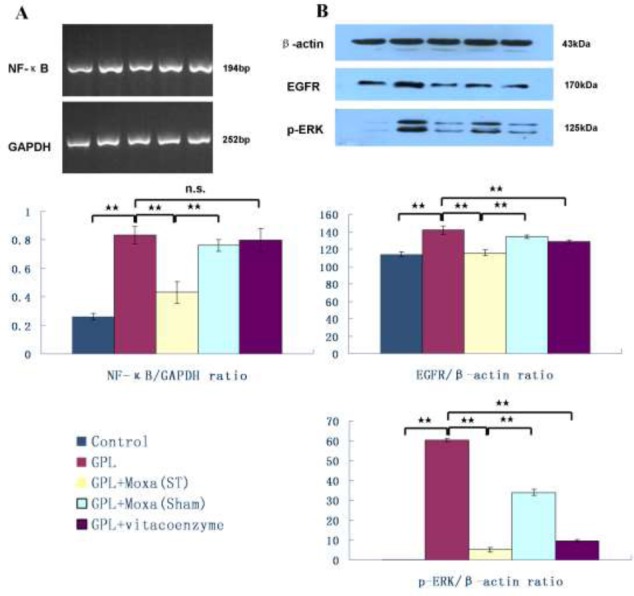
Effect of moxibustion treatment on intracellular signaling proteins in gastric precancerous lesions
After 20 weeks of treatment, the expression of nuclear factor kappa B (NF-κB), epidermal growth factor receptor (EGFR) and phosphorylated extracellular signal regulated kinase (p-ERK) in gastric mucosa of five groups: Control, GPL, GPL+Moxa(ST), GPL+Moxa(Sham) and GPL+vitacoenzyme (n=10), were detected by RT-PCR or Western blotting. Results were compared by one-way analysis of variance and following with Fisher’s Least Significant Difference test for individual comparisons. **p<0.01. n.s. means no significant difference. n.s. means no significant difference.
EGFR/ERK signaling pathway is involved in control of gastric cell proliferation (Osaki et al., 2011). Here, we found that the expression of EGFR and p-ERK in GPL was significant higher than that in normal gastric mucosa (141.54 ± 4.93 vs 113.94 ± 3.26, 60.46 ± 0.87 vs 0.17 ± 0.08, respectively, P < 0.01). Moxibustion treatment of ST21 and ST36 reduced their expression (115.83 ± 3.11 vs 141.54 ± 4.93, 5.28 ± 1.16 vs 60.46 ± 0.87, respectively, P < 0.01), this effect was significant better than that of Sham points (115.83 ± 3.11 vs 134.01 ± 1.84, 5.28 ± 1.16 vs 34.06 ± 1.47, respectively, P < 0.01) (Figure 5B).
Discussion
Gastric precancerous lesions include dysplasia and intestinal metaplasia of gastric mucosa, which is a high risk for gastric carcinogenesis in patients. The unique characteristics of gastric cancer are the intestinal and gastric types of cells in tumor. Gastric mucosal epithelial hyperplasia is considered as the earliest histological change in gastric carcinogenesis (Ming, 1998). Modern medical therapies, including H. pylori eradication, gastric acid inhibition and gastric mucosa protectant, are hard to cure this disease, and accompany with many side effects (Park and Kim, 2015). Moxibustion treatment is an important part of traditional Chinese therapies. Clinical studies have shown that moxibustion treatment have definite therapeutic effects on chronic atrophic gastritis, especially in improving the symptoms, and so can provide possibilities in reversing the pathologic changes of glandular atrophy and intestinal metaplasia (Gao et al., 2005). This study further provided evidence to prove that moxibustion treatment of ST21 and ST36 significant reduced gastric mucosa proliferation, pathological mitotic figure and glandular atrophy, the underlying mechanism was involved in promoting cell apoptosis and inhibiting oncogenes relating to cell proliferation by inhibiting the expression of bcl-2, P53, C-MYC and oncogenes relating to cell proliferation, the intracellular signaling pathway included NF-κB and EGFR/ERK signaling proteins.
Cell apoptosis and the controlled genes have a profound effect on the malignant phenotype (Lowe and Lin, 2000). It is reported that over-expression of Bcl-2 protein is an early event in gastric tumorigenesis, before gastric dysplastic changes occur (Anagnostopoulos et al., 2005). P53 gene mutations were found in 35% of the gastritis samples and 45% of the intestinal metaplasia samples, suggesting a prominent role in gastic carcinogenesis (Morgan et al., 2003). And methylated pattern of C-MYC was abnormal in human gastric carcinogenesis (Fang et al., 1996). What’s more, the expression of bcl-2, P53 and C-MYC were negatively correlated with apoptotic index (Wu et al., 2012). As shown in our results, the mRNA expression of bcl-2, P53 and C-MYC in GPL group was up-regulated significantly. Moxibustion and drug treatment for 20 weeks significant down-regulated the expression of bcl-2, P53 and C-MYC in gastric mucosa, which would decrease the inhibition effect of bcl-2, P53 and C-MYC, and helped to promote cell apoptosis as well as prevented the development of gastric tumorigenesis.
The remarkable characteristics of cancer cells are malignant proliferation and invasive ability, PCNA, Ag-NORs and VEGF are usually recognized as useful markers for proliferation and invasion of cancer cells (Yu et al., 1992). PCNA and Ag-NORs are the intro-nuclear protein that are closely linked to the cell cycle, while VEGF contribute to form new vessels to nourish tumor or metastasize cancer cells (Moraes et al., 2013; Wang, 2014). Our results showed that the protein level of PCNA, Ag-NORs and VEGF increased significantly in gastric mucosa of rats fed with MNNG, moxibustion and drug treatment significant decreased their expression, suggesting that moxibustion treatment had the potential role of inhibiting malignant proliferation in GPL
We further investigated the intra-cellular signaling pathway correlated with cell apoptosis and cell proliferation in GPL development. The expression of NF-kappaB/p65 were up-regulated at the early stage of gastric carcinogenesis, suggesting NF-κB activation was an important step in carcinogenesis progress (Wang et al., 2004). In our study, we found that the mRNA expression of NF-κB was up-regulated in gastric mucosa of GPL, moxibustion treatment play a significant role in inhibiting NF-κB expression. Transforming growth factor alpha (TGF-a)and other ligands bind to EGFR and trigger signaling cascades, such as MAPK. ERK1/2 is one part of MAPK, ERK phosphorylation stimulates transcription factors and following cell proliferation, so EGFR/ERK signaling pathway play an important role in gastric cancer development (Lurje and Lenz, 2009; Osaki et al., 2011). The protein expression of EGFR and p-ERK increased in gastric precancerous lesions, moxibustion treatment of ST21 and ST36 significant inhibited their expression.
Our study had several limitations. First, although moxibustion of Sham point had down-regulated the level of C-MYC, PCNA, VEGF, Ag-NORs and p-ERK/β-actin ratio, the protective effect showed significant decrease than that of ST point. The reason for the phenomenon may come from close moxibustion distance, some of the heat in Sham point spread to ST point. Second, we didn’t detect cell apoptosis in gastric mucosa directly by TUNEL staining. Third, intracellular signaling pathway blockers or gene knockout technique should be used to prove molecular mechanism in the future.
In conclusion, this study provided evidence for that moxibustion treatment of ST21 and ST36 had a potent gastro-protective effect against gastric precancerous lesions, the underlying mechanism was involved in inhibiting the expression of bcl-2, P53, C-MYC and decreasing the activity of NF-κB as well as EGFR/ERK signaling proteins, indicated that moxibustion treatment might be beneficial in preventing against gastric carcinoma.
Acknowledgements
This work was supported by National Natural Science Foundation of China (No. 81260556 and No. 81473751), National Key Basic Research Development Plan of China (No. 2015CB554502), Natural Science Foundation of Guangdong Province of China (No. 2014A030313005 and No. 2016A030313860), and Natural Science Foundation of Fujian Province of China (No. 2016J01413).
References
- 1.Anagnostopoulos GK, Stefanou D, Arkoumani E, Sakorafas G, Pavlakis G, Arvanitidis D, Tsianos E, Agnantis NJ. Bax and Bcl-2 protein expression in gastric precancerous lesions: immunohistochemical study. J Gastroenterol Hepatol. 2005;20:1674–1678. doi: 10.1111/j.1440-1746.2005.04057.x. [DOI] [PubMed] [Google Scholar]
- 2.Bahat G. Risk of proton pump inhibitor-induced mild hyponatremia in older adults. J Am Geriatr Soc. 2014;62:1206–1207. doi: 10.1111/jgs.12861. [DOI] [PubMed] [Google Scholar]
- 3.Chen Y, Le YM, Yang ZB, Ye HP, Wang XM, Yi XQ, Zuo JS. Acupuncture and moxibustion on pathologic and serum CEA changes in rats with precancerous lesion induced by MNNG. Chinese Journal of Cancer Prevention and Treatment. 2013;20:1649–1652. [Google Scholar]
- 4.Deng X, Liu ZW, Wu FS, Li LH, Liang J. A clinical study of weining granules in the treatment of gastric precancerous lesions. J Tradit Chin Med. 2012;32:164–172. doi: 10.1016/s0254-6272(13)60006-0. [DOI] [PubMed] [Google Scholar]
- 5.Fang J, Zhu S, Xiao S, Shi Y, Jiang S, Zhou X, Qian L. Alterations of level of total genomic DNA methylation and pattern of c-myc, c-Ha-ras oncogene methylation in human gastric carcinogenesis. Chin Med J (Engl) 1996;109:787–791. [PubMed] [Google Scholar]
- 6.Florentin M, Elisaf MS. Proton pump inhibitor-induced hypomagnesemia: A new challenge. World J Nephrol. 2012;1:151–154. doi: 10.5527/wjn.v1.i6.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gao X, Rao H, Wang Y, Meng D, Wei Y. Protective action of acupuncture and moxibustion on gastric mucosa in model rats with chronic atrophic gastritis. J Tradit Chin Med. 2005;25:66–69. [PubMed] [Google Scholar]
- 8.Li ZR. Experimental Acupuncture & Moxibustion. 1 edn. Beijing: China Press of Traditional Chinese Medicine; 2003. [Google Scholar]
- 9.Lowe SW, Lin AW. Apoptosis in cancer. Carcinogenesis. 2000;21:485–495. doi: 10.1093/carcin/21.3.485. [DOI] [PubMed] [Google Scholar]
- 10.Lurje G, Lenz HJ. EGFR signaling and drug discovery. Oncology. 2009;77:400–410. doi: 10.1159/000279388. [DOI] [PubMed] [Google Scholar]
- 11.Marques-Silva L, Areia M, Elvas L, Dinis-Ribeiro M. Prevalence of gastric precancerous conditions: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2014;26:378–387. doi: 10.1097/MEG.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 12.Ming SC. Cellular and molecular pathology of gastric carcinoma and precursor lesions: A critical review. Gastric Cancer. 1998;1:31–50. doi: 10.1007/s101200050053. [DOI] [PubMed] [Google Scholar]
- 13.Moraes TM, Rozza AL, Kushima H, Pellizzon CH, Rocha LR, Hiruma-Lima CA. Healing actions of essential oils from Citrus aurantium and d-limonene in the gastric mucosa: the roles of VEGF PCNA, COX-2 in cell proliferation. J Med Food. 2013;16:1162–1167. doi: 10.1089/jmf.2012.0259. [DOI] [PubMed] [Google Scholar]
- 14.Morgan C, Jenkins GJ, Ashton T, Griffiths AP, Baxter JN, Parry EM, Parry JM. Detection of p53 mutations in precancerous gastric tissue. Br J Cancer. 2003;89:1314–1319. doi: 10.1038/sj.bjc.6601302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Osaki LH, Figueiredo PM, Alvares EP, Gama P. EGFR is involved in control of gastric cell proliferation through activation of MAPK and Src signalling pathways in early-weaned rats. Cell Prolif. 2011;44:174–182. doi: 10.1111/j.1365-2184.2011.00733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pan KF, Formichella L, Zhang L, Zhang Y, Ma JL, Li ZX, Liu C, Wang YM, Goettner G, Ulm K, Classen M, You WC, Gerhard M. Helicobacter pylori antibody responses and evolution of precancerous gastric lesions in a Chinese population. Int J Cancer. 2014;134:2118–2125. doi: 10.1002/ijc.28560. [DOI] [PubMed] [Google Scholar]
- 17.Park YH, Kim N. Review of atrophic gastritis and intestinal metaplasia as a premalignant lesion of gastric cancer. J Cancer Prev. 2015;20:25–40. doi: 10.15430/JCP.2015.20.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shen SW, Yuwen Y, Zhang ZL, Dong S, Liu JT, Wang XM. Effect of Jinguo Weikang Capsule on proto-oncogene expression of gastric mucosa in rats with gastric precancerous lesions. Chin J Integr Med. 2008;14:212–216. doi: 10.1007/s11655-008-0212-3. [DOI] [PubMed] [Google Scholar]
- 19.Wang SC. PCNA: a silent housekeeper or a potential therapeutic target? Trends Pharmacol Sci. 2014;35:178–186. doi: 10.1016/j.tips.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 20.Wang W, Luo HS, Yu BP. Expression of NF-kappaB and human telomerase reverse transcriptase in gastric cancer and precancerous lesions. World J Gastroenterol. 2004;10:177–181. doi: 10.3748/wjg.v10.i2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu X, Cai ZD, Lou LM, Zhu YB. Expressions of p53, c-MYC, BCL-2 and apoptotic index in human osteosarcoma and their correlations with prognosis of patients. Cancer Epidemiol. 2012;36:212–216. doi: 10.1016/j.canep.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 22.Xu AG, Li SG, Liu JH, Gan AH. Function of apoptosis and expression of the proteins Bcl-2, p53 and C-myc in the development of gastric cancer. World J Gastroenterol. 2001;7:403–406. doi: 10.3748/wjg.v7.i3.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang LQ, Fang DC, Wang RQ, Yang SM. Effect of NF-kappaB, survivin, Bcl-2 and Caspase3 on apoptosis of gastric cancer cells induced by tumor necrosis factor related apoptosis inducing ligand. World J Gastroenterol. 2004;10:22–25. doi: 10.3748/wjg.v10.i1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang Z, Wang C, Chen J, Wang H, Wang Y, Ma F. Effects of moxibustion on cell proliferative factors in gastric mucosa in rats with precancerous lesions of chronic atrophic gastritis. Zhongguo Zhen Jiu. 2015;35:1269–1273. [PubMed] [Google Scholar]
- 25.Yang ZB, Wang YD, Chang XR, Wang CG, Liu M. Study on mechanism of gastric mucosal cell apoptosis in rats with chronic atrophic gastritis inhibited by electro-acupuncture. Chin J Tradit Chin Med Pharm. 2016;31:3005–3008. [Google Scholar]
- 26.Yoon H, Kim N. Diagnosis and management of high risk group for gastric cancer. Gut Liver. 2015;9:5–17. doi: 10.5009/gnl14118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu CC, Fletcher CD, Newman PL, Goodlad JR, Burton JC, Levison DA. A comparison of proliferating cell nuclear antigen (PCNA) immunostaining, nucleolar organizer region (AgNOR) staining, and histological grading in gastrointestinal stromal tumours. J Pathol. 1992;166:147–152. doi: 10.1002/path.1711660210. [DOI] [PubMed] [Google Scholar]


