Abstract
Background:
Morin is a flavanoid which exhibits potent antioxidant activity in various oxidative stress related diseases. The current study was attempted to scrutinize the preclinical bio-efficacy of morin on focal ischemia.
Methods:
The animal model of focal cerebral ischemic injury was done by midbrain carotid artery occlusion (MCAO) method, followed by Morin (30mg/kg) administration for seven days.
Results:
The outcome of the study showed that treatment with morin displayed positive effects in reducing the focal cerebral ischemia. This effect was evident with the improvements in neurological deficits, reduction in MDA content and elevation of antioxidant levels (SOD, GSH and Gpx). Furthermore, protein expression of Bax and caspase-3 were effectively down-regulated, whilst the expression of Bcl-2 was significantly elevated. On the other hand, the mRNA expression of proinflammatory cytokines was significantly reduced in focal cerebral ischemic rats upon morin intervention.
Conclusion:
Thus, the beneficial effects of morin on cerebral ischemia assault may result from the reduction of oxidative stress, inhibition of apoptosis and inflammation. The neuroprotective effects of morin supplement may serve as potent adjuvant in the amelioration of ischemic stroke.
Keywords: Focal cerebral ischemia, oxidative stress, apoptosis, inflammation, lipid peroxidation, morin
Introduction
Globally, stroke is the prominent element of fatality and cardinal factor in the adult disability. The swift obstruction of blood flow mediated by thrombus or embolism elicits an ischemic status in many of the stroke patients (Donnan et al., 2008; Lakhan et al., 2009). Albeit, tissue plasminogen activator is available for stroke treatment, it elicits minimal therapeutic window and efficacy (Hacke et al., 2008; Zhang et al., 2011). Thus, an effective clinical moiety for the amelioration of stroke is highly essential. Earlier, research studies underscore that, array of biological mechanism such as deprivation of ionic status, loss of ATP, rampant release of excitatory neurochemical transmitters, increased generation of reactive oxygen species (ROS) and apoptosis are the mainstay in the pathology of cerebral ischemic damage (Durukan and Tatlisumak, 2007; Lapchak and Araujo, 2007). Further, the above mentioned toxic events may aggravate to form irreversible brain damage.
In cerebral ischemia, oxidative stress is the predominant condition in the destruction of neuronal cell viability and tissue damage (Chan, 1996; Chen et al., 2011). During oxidative assault, vital bio molecules like DNA, lipids and protein will be affected by rampant release of free radicals and cause cell injury.
The prime noxious process during the tissue damage provoked by cerebral ischemia is mainly mediated by apoptosis induced DNA damage (Kawaguchi, et al., 2004; Liu et al., 2008; Wang et al., 2010; Lu et al., 2010). During cerebral ischemia, exorbitant oxidative stress status triggers the release of cytochrome C from mitochondria which overture to Caspase-3 activation (Broughton et al., 2009). Further, the released cytochrome c initiates the formation of apoptosome complex which encompasses apoptotic-protease activating factor-1, procaspase-9 and ATP (Wang, 2001). Then the apoptosome act as an activator of procaspase-9 initially and then procaspase-3 (Li et al., 1997). Thus, in the apoptotic cascade, the caspase-3 in its activated form regulates the process of DNA fragmentation leading to cell damage (Broughton et al., 2009).
The Bcl-2 family related genes are chiefly implicated in the regulation of apoptotic process by activation/inactivation of protein systems (Elmore, 2007). The Bcl-2 protein blocks the apoptosis process in various cell systems involved in the antithetic regulation proapoptotic factors overexpression (Gross et al., 1999; Shigeomi et al., 1999). The proapoptotic protein Bax integrates pivotal functions in inducing apoptosis (Yi-Te et al., 1997; Martinou et al., 2011).
Inflammation of cerebral cortex is the crucial scenario in the progression of ischemic stroke (Amantea et al., 2009; Kleinig and Vink, 2009). Mammoth reports, indicate that during cerebral ischemia there is an accelerated elevation in the levels of cytokines such as, TNF-α, IL-α and IL-6 (Wang et al., 2007; del Zoppo et al., 2000). In this context, agents which can limit ROS formation have been implicated to ameliorate brain damage following stroke-like events. Recently, plant derived flavanoid molecules gaining more attention for the mitigation of oxidative stress mediated pathologies. In this scenario, Morin a natural flavanoid ingredient found in wide array of Chinese medicinal plant displays potential anti-inflammatory and antioxidant activity (Wang et al., 2006). The neuroprotective effect of morin have been reported in Parkinsonism disease model (Zhang et al., 2010), however its beneficial effect on cerebral ischemia mediated neurotoxicity is not reported till date. Hence, we investigated neuroprotective efficacy of morin in alleviating cerebral ischemic insult in a murine model of middle cerebral artery occlusion (MCAO).
Materials and Methods
Drugs and Chemicals
Morin (> 98 %) was procured form Sigma Aldrich, USA. All other chemicals and reagents used in the study were analytical grade.
Animals
Male Adult Sprague-Dawley rats, aged 12-14 weeks and weighing 160-200 g were accessed from the Binzhou City Central Hospital, Binzhou, Shandong, China. The animals were housed in well maintained concrete structures with specified laboratory and humidity conditions under 12-h light/12-h dark cycles.
The rats were randomly divided into 3 groups and each group consists of 10 rats. The grouping of animals were as follows,
Group 1-Sham rats were given anaesthesia by 10% chloral hydrate (350mg/kg; b.wt; i.p) and carotid artery was exposed but did not undergo MCAO surgery.
Group 2-MCAO operated groups were treated with normal saline.
Group 3-MCAO groups were administered with Morin (30 mg/kg b.wt) through oral route. The Morin treatment was carried out for seven days daily after MCAO.
Animal model of MCAO
The MCAO mediated transient cerebral ischemia focal cerebral ischemic injury was induced by intraluminal filament surgical procedure (Longa et al., 1989). Anaesthesia of rats was done by intra peritoneal administration of 10% chloral hydrate (350mg/kg; b.wt). Briefly the right carotid artery was exposed, and a sterile nylon thread (15 mm length and 0.15 mm in diameter) was inserted into from the external to internal carotid artery and thus the origin of the left middle cerebral artery (MCA) was occluded. The procedure was terminated when mild resistance was felt.
Neurological test
The neurological deficts evaluation was done at 24 hours after MCAO surgery. The scoring was done by the method of Garcia et al (1995). The behavioural parameters like spontaneous action, equilibrium movement of fore limbs, stretching of fore paw, climbing, body voluntary movements, and response to vibrissae touch were measured. The scoring for the six tests was done from 0 to 3. The score ranges from a minimum score of 0 to a maximum of 18. The minimal score depicts serious neurological deficits.
Evaluation of oxidative stress
Briefly, the cortex affected with ischemia (n = 7) were homogenized in ice-chilled Tris buffer (pH 7.4). Then the homogenate was centrifuged at 12,000 X rpm at 4°C for 15 min. Then the resultant supernatant was separated to evaluate the malodialdehyde (MDA) level, superoxide dismutase (SOD) and glutathione peroxidase (GPx) content and activity of reduced glutathione (GSH) by spectrophotometer as per the instructions provided by the assay kit (Nanjing Jiancheng, China).
Measurement of apoptotic protein using Western blot analysis
Cortex affected with ischemia (n = 3, for each group) were isolated and homogenized in ice cold RIPA buffer (Applygen Technologies Inc., China). Then 20μg of protein samples were set on by 10% sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) for apoptotic proteins Caspase-3, Bax, and Bcl-2. The respective primary antibodies used in the analysis were, Caspase-3,1:1,000; Bax, 1:1,000; Bcl-2, 1:750 (Proteintech Group, USA). The blot was freed from unbounded primary antibodies and incubated with goat anti-mouse IgG conjugated to peroxidase (Santa Cruz, USA). β-actin (1:5,000, Santa Cruz, USA) used as an internal control. The binding of antibody was detected by ECL detection kit (Applygen Technologies Inc, China) and the protein bands were visualized using Gel Doc XR system (Bio-Rad, USA)
Analysis of pro-inflammatory cytokine gene expression by RT-PCR
From the frozen samples of rat ischemic cortex, the RNA was isolated and purified using the protocol provided in the kit (TRIzol, Gibco). The cDNAs was prepared from 1 μg of the purified RNA sample. The primers used for the RT-PCT analysis were shown in Table 1. The reaction was done for 30 cycles at using the following steps: 30s denaturing at 95 °C followed by a 30-sannealing step at 57 °C. Finally, the extension step was carried out for 1 min at 72 °C. The band intensity measurement was done by computerized image analysis procedure (Motic Images Advanced 3.2).
Table 1.
Primer used in the RT-PCR study
| S/No | Gene | Primer Sequence |
|---|---|---|
| 1 | TNF-α | Forward:5’-ATGAGCACGGAAAGCATGATCCGA-3’ |
| Reverse: 5’-CCAAAGTAGACCTGCCCGGACTC-3’ | ||
| 2 | IL-6 | Forward: 5’-CCAGTTGCCTTCTTGGGACTGATG-3’ |
| Reverse: 5’-ATTTTCTGACCACAGTGAGGAATG-3’ | ||
| 3 | β-actin | Forward: 5’ GCA CCA CAC CTT CTA CAA TG -3’ |
| Reverse: 5’-TGC TTG CTG ATC CAC ATC TG-3’ |
Statistical Analysis
The data were shown as mean ± S.E.M. The data analysis was done by using SPSS v 13.0. All data were subjected to one-way ANOVA and the comparison between the groups was dome by Tukey’s test. The value of P < 0.05 was considered as statistically significant.
Results
Morin improved the neuro behavorial score provoked by cerebral ischemia
The neurological score was diminished significantly (p< 0.05) in the MCAO animals as compared to the sham operated groups. After Morin treatment, neurological deficits were reduced compared to that of the MCAO (p< 0.05). The sham operated rats doesn’t elicited any evidence of neurological deficit (Fig 1).
Figure 1.
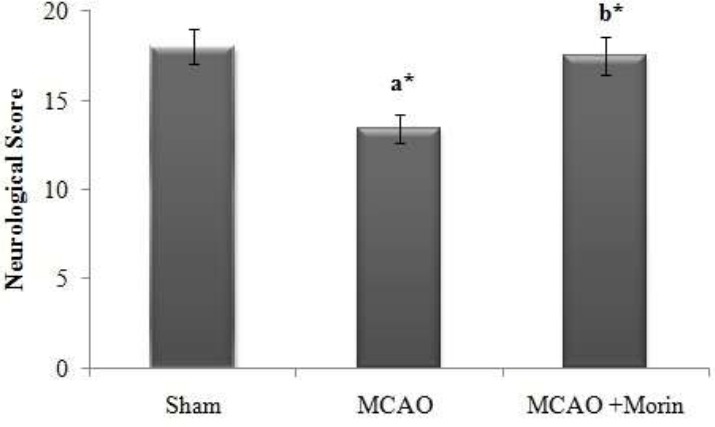
Morin improved the neuro behavorial score provoked by cerebral ischemia. The neurological assessment was done by 18 point Garcia scale system. The results were shown as mean ±SEM for 10 rats in each group. Comparisons are made between: (a) Sham and MCAO; (b) MCAO + Morin. *Statistically significant (p<0.05).
Morin significantly attenuated the lipid peroxidation in MCAO rats
Lipid peroxidation in the ischemic hemisphere was analysed by estimating the level of MDA (Fig. 2). In MCAO rats, the MDA value was significantly (p < 0.05) elevated as that of the sham rats. Thus, the elevated MDA level reflects the state of oxidative stress in MCAO rats, which was effectively reduced by Morin intervention (p <0.05).
Figure 2.
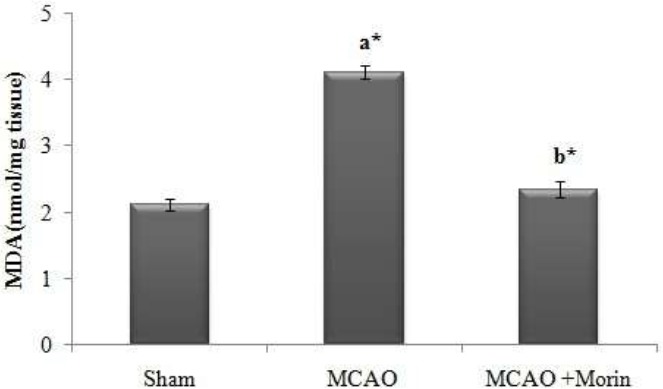
Morin significantly attenuated the lipid peroxidation in MCAO rats. MDA serves as a prominent marker of lipid peroxidation and it is expressed in terms of nmole/mg tissue. The results were shown as mean ±SEM for 7 rats in each group. Comparisons are made between: (a) Sham and MCAO; (b) MCAO and MCAO +Morin *Statistically significant (p<0.05).
Morin boosted the antioxidant level in cerebral ischemia rats
In the present study, post MCAO there was a significant (p < 0.05) decline in the level of antioxidants (GSH, SOD and Gpx) when compared to the sham rats. Morin intervention significantly (p < 0.05) elevated the level of antioxidant in brain through its anti lipid peroxidative effect (Fig 3).
Figure 3.
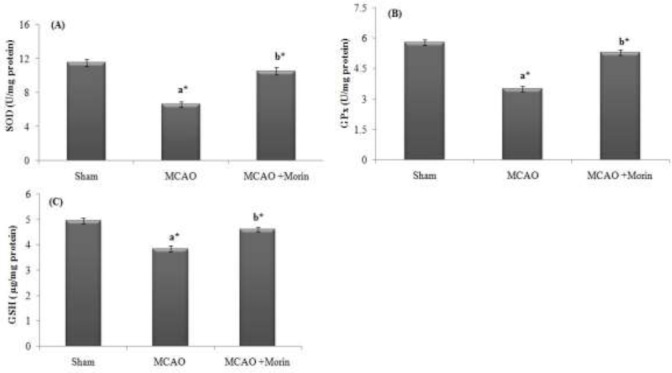
Morin boosted the antioxidant level in cerebral ischemia rats. (A): SOD (U/mg protein); (B) Gpx (U/mg protein); (C) GSH (μg/mg protein). The results were shown as mean ±SEM for 7 animals in each group. Comparisons are made between: (a) Sham and MCAO; (b) MCAO and MCAO +Morin. *Statistically significant (p<0.05).
Influence of Morin and MCAO on apoptotic protein markers assessed by Western Blot
The protein expression of apoptotic markers Caspase-3, Bax and Bcl-2 in cortex tissues affected with ischemia was done by Western blot. There was a significant upregulation of protein expression of Caspase-3 and Bax and downregulation of Bcl-2 protein expression in ischemic group. However, Morin treated rats displayed significant downregulation of Caspase-3 and Bax and upregulation of Bcl-2 protein expression (Fig 4A). Further, the relative protein expression of apoptotic markers (Bcl-2, Bax and Caspase-3) were significantly (p<0.05) altered in MCAO group as that of the control and Morin administered rats elicited effective restoration in the expression level apoptotic proteins (Fig 4B, 4C & 4D).
Figure 4.
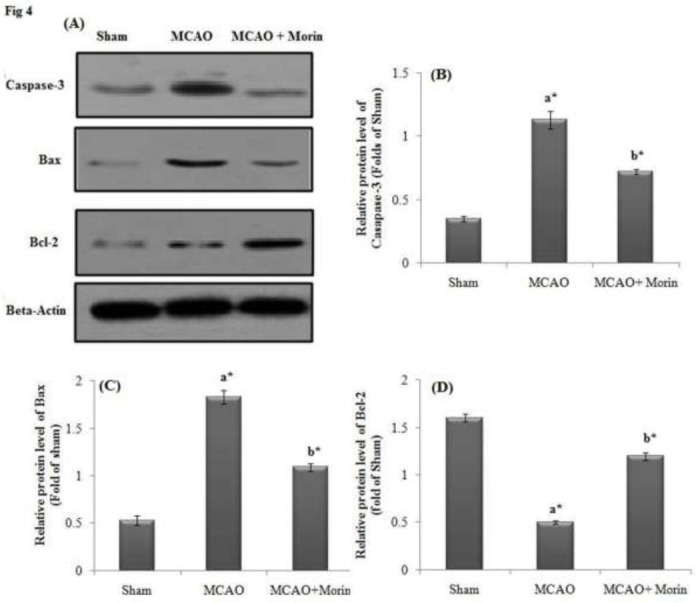
Influence of Morin on Apoptosis-Related Protein after Cerebral Ischemic injury assessed by Western Blot. Β-actin served as a standard. (A) MCAO injured rats displayed upregulated expression of Bax and caspase-3, whilst the expression of Bcl-2 was decreased. Morin significantly attenuated the apoptosis and restored the markers level to normalcy. The Relative protein expression levels were shown as (B) Caspase-3; (C) Bax and (D) Bcl-2. The results were shown as mean ±SEM for 3 rats in each group. Comparisons are made between: (a) Sham and MCAO; (b) MCAO and MCAO +Morin *Statistically significant (p<0.05).
Morin inhibited the cytokine mRNA expression profile in MCAO rats
In the present study, MCAO rats displayed significant upregulated mRNA gene expression of proinflammatory cytokines (TNF-α and IL-6) and Morin treated rats significantly attenuated the inflammation by downregulating the mRNA expression of TNF-α and IL-6 (Fig 5A). Further, the relative expression of TNF-α and IL-6 were significantly (p<0.05) higher in the MCAO group as that of the control and Morin intervention elicited significant (p<0.05) reduction in the expression level of proinflammatory cytokines (Fig 5B).
Figure 5.
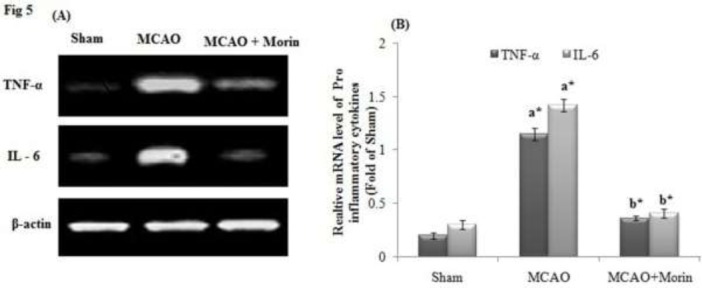
Morin inhibited the cytokine mRNA expression profile in MCAO rats. β-actin served as a standard. (A) After MCAO the upregulated mRNA expression of TNF-α and IL-6 was observed and Morin treatment effectively reduced the expression. (B) Relative mRNA level of TNF-α and IL-6. Values are mean ±SD for 3 rats in each group. Comparisons are made between: (a) Sham and MCAO; (b) MCAO and MCAO +Morin. *Statistically significant (p<0.05).
The preclinical MCAO model performed insertion of thread is the most widely used stroke model, since the pathological features closely resembles the conditions in stroke patients affected from focal cerebral arterial thrombosis (Longa et al., 1989). The restriction of blood flow to brain during ischemic conditions may exaggerate the necrosis of neuronal and brain cells (Traystman, 2003; Park et al., 2009). Array of bio mechanisms like elevated level of ROS, reduced state of detoxification cascade and blockade of antioxidant reserve enzymes have been involved in the pathology of cerebral ischemia (Kutsuna et al., 2010; Pratap et al., 2011). The present study is focused to delineate the neuroprotective role of Morin on focal cerebral ischemic injury.
In our study, MCAO rats displayed significant reduction in the voluntary motor activity and the flexion tests and affects the behavioural activity. Thus, the neurological deficts in ischemia conditions is mainly due to excessive free radical generation in hippocampus and cortical areas responsible for motor activities (Nakamura et al., 2004). However, Morin administration significantly attenuated the neurological deficts, restored the brain motor activities which is in corroboration with the previous report (Zhang et al., 2010).
The free radicals released during cerebral ischemia vulnerably oxidize the biomolecules like lipids which further exasperate cell toxicity and brain dysfunction (Akinmoladun et al., 2015). The noxious effects of free radical on lipids prelude to the formation of lipid peroxidation end product, MDA, a toxic product which educe neuronal cell death and loss of brain function (Serteser et al., 2002). In our study, Morin treatment reduced the MDA level in MCAO rats, which is highly attributed to inhibitory potential of Morin on the oxidative insult (Subash and Subramanian, 2009). GSH, an effective non-protein thiol, act as potent quencher of ROS. Previous research studies indicate that cerebral ischemia induced lipid peroxidation is concurrent with GSH depletion in brain tissue (Chan, 2001; Al-Omar et al., 2006; Saleem et al., 2006). Further, studies reveal that antioxidant enzymes like SOD and GPx mediate pivotal function a prime role in the regulation of redox homeostasis in tissues (Gilgun-Sherki et al., 2002; Lo et al., 2003). In the present study, MCAO groups displayed reduced level of GSH, SOD and GPx in the brain ischemic tissue. Treatment with Morin restored the depleted antioxidants level due to its potent antioxidant action and anti lipid peroxidative effect. The occurrence of a phenolic group with an aromatic base conjugation in the structure of morin contributes to the reduction of ROS (Wang et al., 2006; Hou et al., 2003).
The TNF-α and IL-1β levels, as a result of inflammation were considerably elevated during ischemic stroke and also augment the process of brain damage in ischemic conditions (Hallenbeck,2002; Rothwell et al., 1997). Further, the elevated level of proinflammatory cytokines increases the protein level of ICAM-1 and E-selectin in leukocytes, and enhances the adhesion and transendothelial migration of leukocytes. Finally, these mechanisms, activates the inflammatory cascade damage of BBB, edema in brain and neuronal cell death (Huang et al., 2006). In our study MCAO rats displayed upregulation of TNF-α and IL-1β mRNA expression in ischemic brain tissue. The reports highlighted in the present study are in corroboration with the previous reports that aggravated TNF-α and IL-1β levels are one of the cardinal factors in brain damage after transient brain ischemia (Tang et al., 2010). However, Morin intervention effectively downregulated the expression of proinflammatory cytokines mediated through its anti-inflammatory activity (Fang et al., 2003; Galvez et al., 2001; Lee et al., 2008). Apoptosis mediate a central role in the progression of cerebral ischemia injury through activation apoptotic related protein cascade located within the apoptotic cells (Galluzzi et al., 2009; Nakka et al., 2008). The Bcl-2 family related proteins are the mainstay in the regulation of major apoptotic signal transduction pathways and elicit irreversible cell damage (Weyhenmeyer et al., 2012; Yang et al., 2009). The Bcl-2/Bax ratio is the hallmark in the process of apoptotic cell death (Bar-Am et al., 2004). Furthermore, Caspase-3, is the key protein involved in the apoptosis, which is highly vital for DNA damage and the noxious cell morphology alteration in relation to apoptosis (Cohen, 1997; Janicke et al., 1998). In our study, MCAO rats displayed upregulated protein expression of Caspase 3, Bax and downregulated protein level of Bcl-2 in ischemic tissue of brain. Whilst, the morin intervention restored the expression of apoptotic markers to normal and thus prevented the apoptosis. The anti-apoptotic activity of Morin is documented in the previous research reports (Poonam and Radhika, 2012). Further in our study, no negative effect of morin has been documented and receptor based studies and molecular level studies are cardinal to study the negative effect of morin.
Conclusion
The outcome of the current study shows that the neuro-protection rendered by Morin on cerebral ischemic damage provoked by MCAO rats mainly due to inhibition of neurological deficits and lipid peroxidation, restoration of antioxidants followed by the mitigation of inflammation and apoptosis responses. Thus, based on the preclinical results Morin may be a suitable clinical molecule for the amelioration of array of brain ischemic conditions as a result of oxidative damage. Furthermore, molecular mechanism research studies are highly warranted to explore the anti-stroke efficacy of Morin.
References
- 1.Akinmoladun AC, Akinrinola BL, Olaleye MT, Farombi EO. Kolaviron, a Garcinia kola bioflavonoid complex, protects against ischemia/reperfusion injury: pertinent mechanistic insights from biochemical and physical evaluations in rat brain. Neurochem. Res. 2015;40:777–787. doi: 10.1007/s11064-015-1527-z. [DOI] [PubMed] [Google Scholar]
- 2.Al-Omar FA, Nagi MN, Abdul gadir MM, Al Joni KS, Al-Majed AA. Immediate and delayed treatments with curcumin prevent forebrain ischemia-induced neuronal damage and oxidative insult in the rat hippocampus. Neurochem. Res. 2006;31:611–618. doi: 10.1007/s11064-006-9059-1. [DOI] [PubMed] [Google Scholar]
- 3.Amantea D, Nappi G, Bernardi G, Bagetta G, Corasaniti MT. Post-ischemic brain damage: pathophysiology and role of inflammatory mediators. FEBS. J. 2009;276:13–26. doi: 10.1111/j.1742-4658.2008.06766.x. [DOI] [PubMed] [Google Scholar]
- 4.Bar-Am O, Weinreb O, Amit T, Youdim MB. Regulation of Bcl-2 family proteins, neurotrophic factors and APP processing in the neuro rescue activity of propargylamine. FASEB. J. 2005;19:1899–1901. doi: 10.1096/fj.05-3794fje. [DOI] [PubMed] [Google Scholar]
- 5.Broughton BR, Reutens D.C, Sobey CG. Apoptotic mechanisms after cerebral ischemia. Stroke. 2009;40:331–339. doi: 10.1161/STROKEAHA.108.531632. [DOI] [PubMed] [Google Scholar]
- 6.Chan PH. Role of oxidants in ischemic brain damage. Stroke. 1996;27:1124–1129. doi: 10.1161/01.str.27.6.1124. [DOI] [PubMed] [Google Scholar]
- 7.Chan PH. Reactive oxygen radicals in signaling and damage in the ischemic brain. J. Cereb. Blood. Metab. 2001;21:2–14. doi: 10.1097/00004647-200101000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Chen H, Yoshioka H, Kim GS, Jung JE, Okami N, Sakata H, Maier CM, Narasimhan P, Goeders CE, Chan PH. Antioxid. Redox Sig. 2011;14:1505–1517. doi: 10.1089/ars.2010.3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen GM. Caspases: the executioners of apoptosis. Biochem. J. 1997;326:1–16. doi: 10.1042/bj3260001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.del Zoppo G, Ginis I, Hallenbeck JM, Iadecola C, Wang X, Feuerstein GZ. Inflammation and stroke: putative role for cytokines, adhesion molecules and iNOS in brain response to ischemia Brain. Pathol. 2000;10:95–112. doi: 10.1111/j.1750-3639.2000.tb00247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donnan GA, Fisher M, Macleod M, Davis SM. Stroke. Lancet. 2008;371:1612–1623. doi: 10.1016/S0140-6736(08)60694-7. [DOI] [PubMed] [Google Scholar]
- 12.Durukan A, Tatlisumak T. Acute ischemic stroke: overview of major experimental rodent models, pathophysiology, and therapy of focal cerebral ischemia. Pharmacol. Biochem. Behav. 2007;87:179–197. doi: 10.1016/j.pbb.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 13.Elmore S. Apoptosis: a review of programmed cell death. Toxicol. Pathol. 2007;35:495–16. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fang SH, Hou YC, Chang WC, Hsiu SL, Chao PD, Chiang BL. Morin sulfates/glucuronides exert anti-inflammatory activity on activated macrophages and decreased the incidence of septic shock. Life Sci. 2003;74:743–756. doi: 10.1016/j.lfs.2003.07.017. [DOI] [PubMed] [Google Scholar]
- 15.Galluzzi L, Morselli E, Kepp O, Kroemer G. Targeting post-mitochondrial effectors of apoptosis for neuroprotection. Biochem. Biophys. Acta. 2009;1787:402–413. doi: 10.1016/j.bbabio.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 16.Galvez J, Coelho G, Crespo ME, Cruz T, Rodriguez Cabezas ME, Concha A, Gonzalez M, Zarzuelo A. Intestinal anti-inflammatory activity of morin on chronic experimental colitis in the rat. Aliment. Pharmacol. Ther. 2001;15:2027–2039. doi: 10.1046/j.1365-2036.2001.01133.x. [DOI] [PubMed] [Google Scholar]
- 17.Garcia J, Wagner S, Liu KF, Hu XJ. Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats: statistical validation. Stroke. 1995;26:627–635. doi: 10.1161/01.str.26.4.627. [DOI] [PubMed] [Google Scholar]
- 18.Gilgun-Sherki Y, Rosenbaum Z, Melamed E, Offen D. Antioxidant therapy in acute central nervous system injury: current state. Pharmacol.Rev. 2002;54:271–284. doi: 10.1124/pr.54.2.271. [DOI] [PubMed] [Google Scholar]
- 19.Gross A, McDonnell JM, Korsmeyer SJ. BCL-2 family members and the mitochondria in apoptosis. Genes. Dev. 1999;13:1899–1911. doi: 10.1101/gad.13.15.1899. [DOI] [PubMed] [Google Scholar]
- 20.Hacke W, Kaste M, Bluhmki E, Brozman M, Davalos A, Guidetti D, Larrue V, Lees KR, Medeghri Z, Machnig T, Schneider D, von Kummer R, Wahlgren N, Toni DN. Thrombolysis with alteplase 4.5-6 hours after acute ischemic stroke. Engl. J. Med. 2008;359:1317–29. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 21.Hallenbeck JM. The many faces of tumor necrosis factor in stroke. Nat. Med. 2002;8:1363–1368. doi: 10.1038/nm1202-1363. [DOI] [PubMed] [Google Scholar]
- 22.Hou YC, Chao PD, Ho HJ, Wen CC, Hsiu SL. Profound difference in pharmacokinetics between morin and its isomer quercetin in rats. J Pharm Pharmacol. 2003;55(2):199–203. doi: 10.1211/002235702487. [DOI] [PubMed] [Google Scholar]
- 23.Huang J, Upadhyay UM, Tamargo RJ. Inflammation in stroke and focal cerebral ischemia. Surg. Neurol. 2006;66:232–45. doi: 10.1016/j.surneu.2005.12.028. [DOI] [PubMed] [Google Scholar]
- 24.Janicke RU, Sprengart ML, Wati MR, Porter AG. Caspase-3 is required for DNA fragmentation and morphological changes associated with apoptosis. J. Biol. Chem. 1998;273:9357–9360. doi: 10.1074/jbc.273.16.9357. [DOI] [PubMed] [Google Scholar]
- 25.Kawaguchi M, Drummond JC, Cole DJ, Kelly PJ, Spurlock MP, Patel PM. Effect of isoflurane on neuronal apoptosis in rats subjected to focal cerebral ischemia. Anesth. Analg. 2004;98:798–805. doi: 10.1213/01.ane.0000105872.76747.f6. [DOI] [PubMed] [Google Scholar]
- 26.Kleinig TJ, Vink R. Suppression of inflammation in ischemic and hemorrhagic stroke: therapeutic options. Curr.Opin. Neurol. 2009;22:294–301. doi: 10.1097/wco.0b013e32832b4db3. [DOI] [PubMed] [Google Scholar]
- 27.Kutsuna S, Tsuruta R, Fujita M, Todani M, Yagi T, Ogino Y, Igarashi M, Takahashi K, Izumi T, Kasaoka S, Yuasa M, Maekawa T. Brain Res. 2010;1313:242–249. doi: 10.1016/j.brainres.2009.11.077. [DOI] [PubMed] [Google Scholar]
- 28.Lakhan SE, Kirchgessner A, Hofer M. Inflammatory mechanisms in ischemic stroke: therapeutic approaches. J. Transl. Med. 2009;7:97. doi: 10.1186/1479-5876-7-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lapchak PA, Araujo DM. Advances in ischemic stroke treatment: neuroprotective and combination therapies. Exp.Opin.Emer. Drugs. 2007;12:97–112. doi: 10.1517/14728214.12.1.97. [DOI] [PubMed] [Google Scholar]
- 30.Lee HS, Jung KH, Hong SW, Park IS, Lee C, Han HK, Lee DH, Hong SS. Morin protects acute liver damage by carbon tetrachloride CCl4 in rat. Arch. Pharm. Res. 2008;31:1160–1165. doi: 10.1007/s12272-001-1283-5. [DOI] [PubMed] [Google Scholar]
- 31.Li P, Nijhawan D, Budihardio I. Cytochrome c and dATP dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 32.Liu R, Gao M, Yang ZH, Du GH. Pinocembrin protects rat brain against oxidation and apoptosis induced by ischemia-reperfusion both in vivo and in vitro. Brain. Res. 2008;1216:104–15. doi: 10.1016/j.brainres.2008.03.049. [DOI] [PubMed] [Google Scholar]
- 33.Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nat. Rev. Neurosci. 2003;4:399–415. doi: 10.1038/nrn1106. [DOI] [PubMed] [Google Scholar]
- 34.Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 35.Lu Y, Zhang J, Ma B, Li K, Li X, Bai H, Yang Q, Zhu X, Ben J, Chen Q. Glycine attenuates cerebral ischemia/reperfusion injury by inhibiting neuronal apoptosis in mice. Neurochem. Int. 2012;61:649–658. doi: 10.1016/j.neuint.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 36.Martinou JC, Youle RJ. Mitochondria in apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev. Cell. 2011;21:92–101. doi: 10.1016/j.devcel.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakamura T, Keep RF, Hua Y, Schallert T, Hoff JT, Xi G. Deferoxamine induced attenuation of brain edema and neurological deficits in a rat model of intracerebral hemorrhage. J. Neurosurg. 2004;100:672–678. doi: 10.3171/jns.2004.100.4.0672. [DOI] [PubMed] [Google Scholar]
- 38.Nakka VP, Gusain A, Mehta SL, Raghubir R. Molecular mechanisms of apoptosis in cerebral ischemia: multiple neuroprotective opportunities. Mol. Neurobiol. 2008;37:7–38. doi: 10.1007/s12035-007-8013-9. [DOI] [PubMed] [Google Scholar]
- 39.Park SJ, Nam KW, Lee HJ, Cho EY, Koo U, Mar W. Neuroprotective effects of an alkaloid-free ethyl acetate extract from the root of Sophora flavescens Ait. against focal cerebral ischemia in rats. Phytomed. 2009;16:1042–1051. doi: 10.1016/j.phymed.2009.03.017. [DOI] [PubMed] [Google Scholar]
- 40.Pratap R, Pillai KK, Khanam R, Islam F, Ahmad SJ, Akhtar M. Protective effect of irbesartan, an angiotensin II receptor antagonist, alone and in combination with aspirin on middle cerebral artery occlusion model of focal cerebral ischemia in rats. Hum. Exp. Toxicol. 2011;30:354–362. doi: 10.1177/0960327110371257. [DOI] [PubMed] [Google Scholar]
- 41.Radhika K, Poonam K. Protective Role of Morin, a Flavonoid, against high glucose induced oxidative stress mediated apoptosis in primary rat hepatocytes. PLoS One. 2012;7:e41663. doi: 10.1371/journal.pone.0041663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rothwell N, Allan S, Toulmond S. The role of interleukin-1 in acute neuro degeneration and stroke: Pathophysiological and therapeutic implications. J. Clin. Invest. 1997;100:2648–2652. doi: 10.1172/JCI119808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saleem S, Ahmad M, Ahmad AS, Yousuf S, Ansari MA, Khan MB, Ishrat T, Islam F. Behavioral and histologic neuroprotection of aqueous garlic extract after reversible focal cerebral ischemia. J. Med. Food. 2006;9:537–44. doi: 10.1089/jmf.2006.9.537. [DOI] [PubMed] [Google Scholar]
- 44.Serteser M, Ozben T, Gumuslu S, Balkan S, Balkan E. Lipid peroxidation in rat brain during focal cerebral ischemia: prevention of malondialdehyde and lipid conjugated diene production by a novel antiepileptic, lamotrigine. Neurotoxicol. 2002;23:111–119. doi: 10.1016/s0161-813x(02)00018-9. [DOI] [PubMed] [Google Scholar]
- 45.Shigeomi S, Yoshihide T. Bcl-2 family proteins regulate the release of apoptogenic cytochrome c by the mitochondrial channel VDAC. Nature. 1999;399:483–487. doi: 10.1038/20959. [DOI] [PubMed] [Google Scholar]
- 46.Subash S, Subramanian P. Morin a flavonoid exerts antioxidant potential in chronic hyperammonemic rats: a biochemical and histopathological study. Mol Cell Biochem. 2009;327:153–161. doi: 10.1007/s11010-009-0053-1. [DOI] [PubMed] [Google Scholar]
- 47.Tang NY, Liu CH, Hsieh C.T, Hsieh CL. The anti-inflammatory effect of paeoniflorin on cerebral infarction induced by ischemia-reperfusion injury in Sprague-Dawley rats. Am. J. Chin. Med. 2010;38:51–64. doi: 10.1142/S0192415X10007786. [DOI] [PubMed] [Google Scholar]
- 48.Traystman RJ. Animal models of focal and global cerebral ischemia. ILAR. J. 2003;44:85–95. doi: 10.1093/ilar.44.2.85. [DOI] [PubMed] [Google Scholar]
- 49.Wang L, Tu YC, Lian TW, Hung JT, Yen JH, Wu MJ. Distinctive antioxidant and antiinflammatory effects of flavonols. J. Agri. Food. Chem. 2006;54:9798–9804. doi: 10.1021/jf0620719. [DOI] [PubMed] [Google Scholar]
- 50.Wang L, Tu YC, Lian TW, Hung JT, Yen JH, Wu MJ. Distinctive antioxidant and anti-inflammatory effects of flavonols. J. Agric. Food. Chem. 2006;54:9798–9804. doi: 10.1021/jf0620719. [DOI] [PubMed] [Google Scholar]
- 51.Wang Q, Tang XN, Yenari MA. The inflammatory response in stroke. J. Neuro.Immunol. 2007;184:53–68. doi: 10.1016/j.jneuroim.2006.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang W, Xu J, Li L, Wang P, Ji X, Ai H, Zhang L, Li L. Neuroprotective effect of morroniside on focal cerebral ischemia in rats. Brain. Res. Bull. 2010;83:196–201. doi: 10.1016/j.brainresbull.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 53.Wang X. The expanding role of mitochondria in apoptosis. Genes. Dev. 2001;15:2922–2933. [PubMed] [Google Scholar]
- 54.Weyhenmeyer B, Murphy AC, Prehn JH, Murphyn BM. Targeting the anti-apoptotic Bcl-2 family members for the treatment of cancer. Exp. Oncol. 2012;34:192–199. [PubMed] [Google Scholar]
- 55.Yang T.M, Barbone D, Fennell DA, Broaddus VC. Bcl-2 family proteins contribute to apoptotic resistance in lung cancer multicellular spheroids. Am. J. Respir.Cell. Mol. Biol. 2009;41:14–23. doi: 10.1165/rcmb.2008-0320OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yi-Te H, Keith GW, Richard JY. Cytosol-to-membrane redistribution of Bax and Bcl-X (L) during apoptosis. Proc. Nat. Acad. Sci. USA. 1997;94:3668–3672. doi: 10.1073/pnas.94.8.3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang B, Sun XJ, Ju CH. Thrombolysis with alteplase 4.5-6 hours after acute ischemic stroke. Eur. Neurol. 2011;65:170–174. doi: 10.1159/000324291. [DOI] [PubMed] [Google Scholar]
- 58.Zhang ZT, Cao XB, Xiong N, Wang HC, Huang JS, Sun SG, Wang T. Morin exerts neuroprotective actions in Parkinson disease models in vitro and in vivo Acta. Pharmacol. Sin. 2010;8:900–906. doi: 10.1038/aps.2010.77. [DOI] [PMC free article] [PubMed] [Google Scholar]


