Abstract
During their life cycle Plasmodium parasites rely upon an arsenal of proteins that establish key interactions with the host or vector, and between the parasite sexual stages, with the purpose of ensuring infection, reproduction and proliferation. Among these is a group of secreted or membrane-anchored proteins known as the six-cysteine (6-cys) family. This is a small but important family with only 14 members thus far identified, each stage-specifically expressed during the parasite life cycle. 6-cys proteins often localize at the parasite surface or interface with the host and are conserved in different Plasmodium species. The unifying feature of the family is the s48/45 domain, presumably involved in adhesion and structurally related to Ephrins, the ligands of Eph receptors. The most prominent s48/45 members are currently under functional investigation and are being pursued as vaccine candidates. In this review, we examine what is known about the 6-cys family, their structure and function, and discuss future research directions.
Keywords: Malaria, Plasmodium, s48/45, 6-cys proteins, Adhesion proteins, Host-pathogen interactions, Malaria vaccine
Graphical Abstract
1. Introduction
Malaria affects millions of people throughout the world and, according to the World Health Organization (WHO), nearly half a million lives were lost to malaria in 2015 (WHO, 2015). This life-threatening disease is caused mainly by Plasmodium falciparum and Plasmodium vivax parasites. The infection cycle of Plasmodium is complex, with multiple stages of development occurring in a vertebrate host and an anopheline mosquito vector (Fig. 1A). Plasmodium parasites possess multiple families of adhesion proteins, which have evolved either with the apicomplexan parasite lineages since their descent from free living ancestors or which were acquired by the parasite from an ancient host through horizontal gene transfer. Many of these proteins mediate key interactions by the parasites’ extracellular invasive stages and intracellular replicative stages either with host cells, for infection and proliferation or between sexual stages of the parasite for mating. These types of critical interactions seem to be among the functions of the members of the six-cysteine (6-cys) protein family, which in P. falciparum has 14 members expressed in different stages throughout the parasite cycle. These are: Pfs230, Pfs48/45, Pfs230p, Pfs47 and PfPSOP12 expressed in the sexual stages; Pf52, Pf36, PfLISP2 and PfB9 in the pre-erythrocytic stages; and Pf12, Pf12p, Pf41, Pf38, and Pf92 in the asexual erythrocytic stages (Fig. 1A, Table 1). 6-cys proteins are often expressed on the surface of the parasite and are conserved across Plasmodium spp. with most of the members having orthologues in human, non-human primate and rodent malaria parasites (PlamoDB (Aurrecoechea et al., 2009), attesting to the universal importance of the 6-cys family in survival and propagation of Plasmodium. The common feature of the family is the s48/45 domain, a structural element with six cysteines that form disulfide bonds, which can be found as multiple modules in most of the family members (Carter et al., 1995; Gerloff et al., 2005; Arredondo et al., 2012).
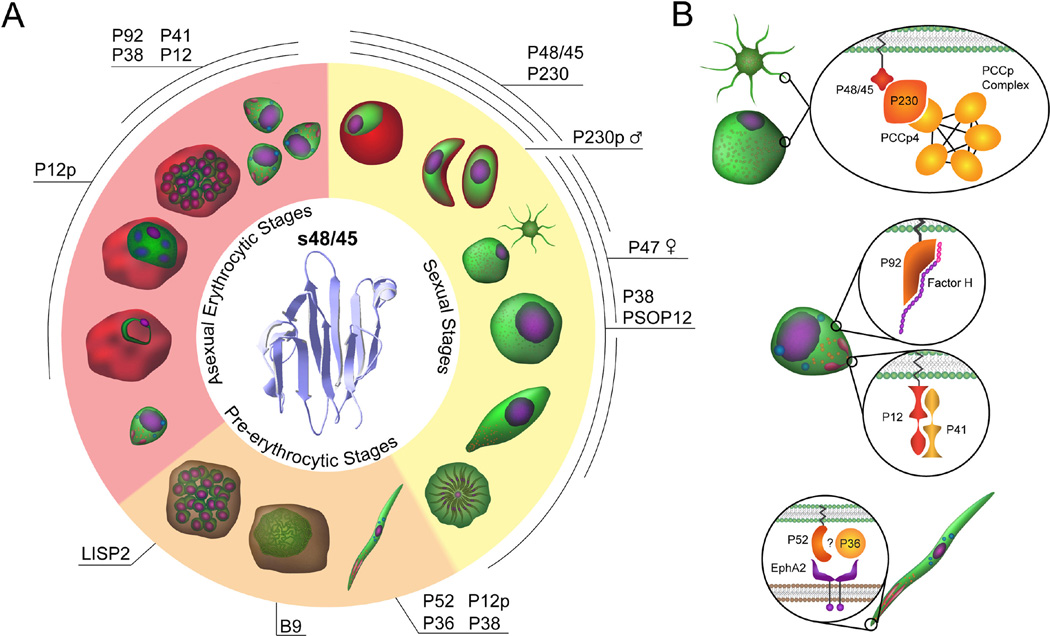
Fig. 1.
The s48/45 six-cysteine protein family in the Plasmodium falciparum life cycle. (A) Development of Plasmodium in the mosquito vector and vertebrate host through the three main stages of infection (clockwise: sexual stages shaded in yellow (gametocyte stage II, gametocytes stage V, gametes, zygote, ookinete, oocyst), pre-erythrocytic stages shaded in orange (sporozoite, mid-liver stage, late liver stage), and asexual erythrocytic stages shaded in pink (merozoite, ring, trophozoite, late schizont, merozoites). Proteins that contain at least one s48/45 domain (the molecular structure is shown in ribbon mode (Protein Data Bank (PDB) ID: 2LOE)) are expressed in the different stages of the parasite life cycle as labeled. PSOP12, putative secreted ookinete protein 12; LISP2, liver stage-specific protein 2. (B) Protein interactions of the s48/45 family. The interactions that have been confirmed or proposed based on the literature are depicted in the insets on the corresponding parasite stages. From top to bottom: the confirmed interaction of the PfCCp-Pfs230 (14 s48/45 domains) complex with Pfs48/45 (three domains) on the surface of gametes; the confirmed interactions of Pf92 (one domain) with Factor H, and of Pf12 with Pf41 (two domains each) on the merozoite; and the suspected interactions of P52 and P36 (two domains each) with EphA2 in the sporozoite. Figure reproduced with permission, ©Adriana Lippy.
Table 1.
Features of the known members of the Plasmodium six-cysteine (6-cys) s48/45 protein family. Data were extracted from PlasmoDB (Aurrecoechea et al., 2009), except for columns listing the number of s48/45 domains, Stage, Subcellular Localization and Function which were summarized from the reviewed literature.
| Name | PlasmoDB Accession No. |
Chromosome | No. of amino acidsa |
MW (kDa)a |
s48/45 Domains |
Orthologues | Stage | Subcellular Localization |
Function |
|---|---|---|---|---|---|---|---|---|---|
| Pfs230 | PF3D7_0209000 | 2 | 3135 | 363.2 | 14 | Pv, Pk, Pc, Pr, Pb, Py, Pch |
M and F gametocyte and gametes |
Plasma membrane of gametocytes and gametes |
Male fertility, exflagellation center formation, cross fertilization. |
| Pfs230p | PF3D7_0208900 | 2 | 2508 | 293.7 | 12 | Pv, Pk, Pc, Pr, Pb, Py, Pch |
M stage IV-V gametocytes |
Gametocyte cytoplasm |
ND |
| Pfs48/45 | PF3D7_1346700 | 13 | 448 | 51.5 | 3 | Pv, Pk, Pc, Pr, Pb, Py, Pch |
M and F gametocyte and gametes |
Plasma membrane of gametocytes and gametes |
Male fertility, cross fertilization. |
| Pfs47 | PF3D7_1346800 | 13 | 439 | 50.8 | 3 | Pv, Pk, Pc, Pr, Pb, Py, Pch |
F gametocytes and gametes; ookinete |
Surface of gametocytes, gametes and ookinetes |
Evasion of the mosquito immune system |
| PfPSOP12 | PF3D7_0513700 | 5 | 735 | 87.3 | 1 | Pv, Pk, Pc, Pr, Pb, Py, Pch |
M and F gametocytes, ookinetes, oocyts |
Surface gametocytes, gametes and ookinetes |
Possibly related to transmission |
| Pf36 | PF3D7_0404400 | 4 | 379 | 44.6 | 2 | Pv, Pk, Pc, Pr, Pb, Py, Pch |
Sporozoite | ND | Hepatocyte invasion |
| Pf52 | PF3D7_0404500 | 4 | 478 | 56.3 | 2 | Pv, Pk, Pc, Pr, Pb, Py, Pch |
Sporozoite | Micronemes | Hepatocyte invasion, PVM formation |
| PfLISP2 | PF3D7_0405300 | 4 | 1964 | 233.7 | 2 | Pv, Pk, Pc, Pr, Pb, Py, Pch |
Mid-late liver |
Vacuolar space/ exported to host cytoplasm |
Schizogony in the Liver |
| PfB9 | PF3D7_0317100 | 3 | 969 | 114.7 | 1 | Pv, Pk, Pc, Pr, Pb, Py, Pch |
Early liver | Plasma membrane |
Maintenance of PVM |
| Pf12 | PF3D7_0612700 | 6 | 347 | 39.4 | 2 | Pv, Pk, Pc, Pr, Pb, Py, Pch |
Late schizont/ merozoite |
Rhoptries, merozoite surface |
ND |
| Pf12p | PF3D7_0612800 | 6 | 371 | 43.5 | 2 | Pv, Pk, Pc, Pr, Pb, Py, Pch |
Blood stage and sporozoite |
ND | ND |
| Pf41 | PF3D7_0404900 | 4 | 378 | 43.1 | 2 | Pv, Pk, Pc, Pr, Pb, Py, Pch |
Late schizont/ merozoite |
Merozoite surface and apical organelles |
ND |
| Pf92 | PF3D7_1364100 | 13 | 796 | 92.7 | 1 | Pv, Pk, Pc, Pr |
Late schizont/ merozoite |
Merozoite surface |
Recruitment of Factor H for complement lysis protection |
| Pf38 | PF3D7_0508000 | 5 | 349 | 40.6 | 2 | Pv, Pk, Pc, Pr, Pb, Py, Pch |
Schizont, merozoites, sporozoites, gametocytes |
Merozoite surface and apical organelles. Sporozoite micronemes. Gametocyte surface. |
Suggested erythrocyte invasion. |
Includes signal peptide
Pf, Plasmodium falciparum; Pv, Plasmodium vivax; Pk, Plasmodium knowlesi; Pc, Plasmodium cynomolgi; Pr, Plasmodium reichenowi; Pb, Plasmodium berghei; Py, Plasmodium yoelii; Pch, Plasmodium chabaudi; M, male; F, female; ND, not determined; PVM, parasitophorous vacuole membrane.
2. The s48/45 domain
Plasmodium parasites have co-evolved with their vertebrate hosts for more than 150 million years (Carter and Mendis, 2002), during which time they acquired protein domains from their hosts by horizontal gene transfer (Aravind et al., 2003). In general, these domains seem to derive more often from proteins with extracellular interaction functions, implying that they were acquired by Plasmodium particularly due to their adhesion capabilities (Templeton et al., 2004). Some examples of adhesion domains that are known to have originated from animal hosts and transferred into apicomplexans are Sushi, Notch, Fibronectin-type II and Thrombospondin-1 (TSP-1) (Anantharaman et al., 2007); the latter are found in essential proteins such as Thrombospondin-related anonymous protein (TRAP), where the TSP-1 domain is critical for cell invasion, and circumsporozoite protein (CSP), where the domain also appears to have an important role in hepatocyte invasion (Menard, 2001; Tucker, 2004; Plassmeyer et al., 2009). More recently, the s48/45 domain has been proposed to derive from a vertebrate host protein domain involved in cell-cell interactions, an ephrin-like precursor that was probably acquired by a common ancestor of the coccidians and aconoidasidans and that eventually evolved into the SAG1-Related Sequence (SRS) and s48/45 structural domains in Toxoplasma and Plasmodium, respectively (Fig. 2A) (Arredondo et al., 2012). Initially, the s48/45 domain was believed to exist only in Plasmodium, however, although structural data is only currently available for Plasmodium proteins, the domain is reportedly present in all members of the Aconoidasida which includes haemosporidians and piroplasms (Fig. 2A) (Arredondo et al., 2012; Punta et al., 2012). In the Plasmodium genome the s48/45 domain expanded before speciation, resulting in the 6-cys family with distinct proteins that are conserved across species and have important roles in the different life cycle stages of the parasite, containing anywhere from one to 14 s48/45 domains (Fig. 2B). Gene duplication also played a part in the expansion of the s48/45 domain as some members of the family are found in tandem-arranged pairs (Templeton and Kaslow, 1999; Thompson et al., 2001). Nevertheless, only 17–36% overall amino acid sequence identity is observed among proteins.
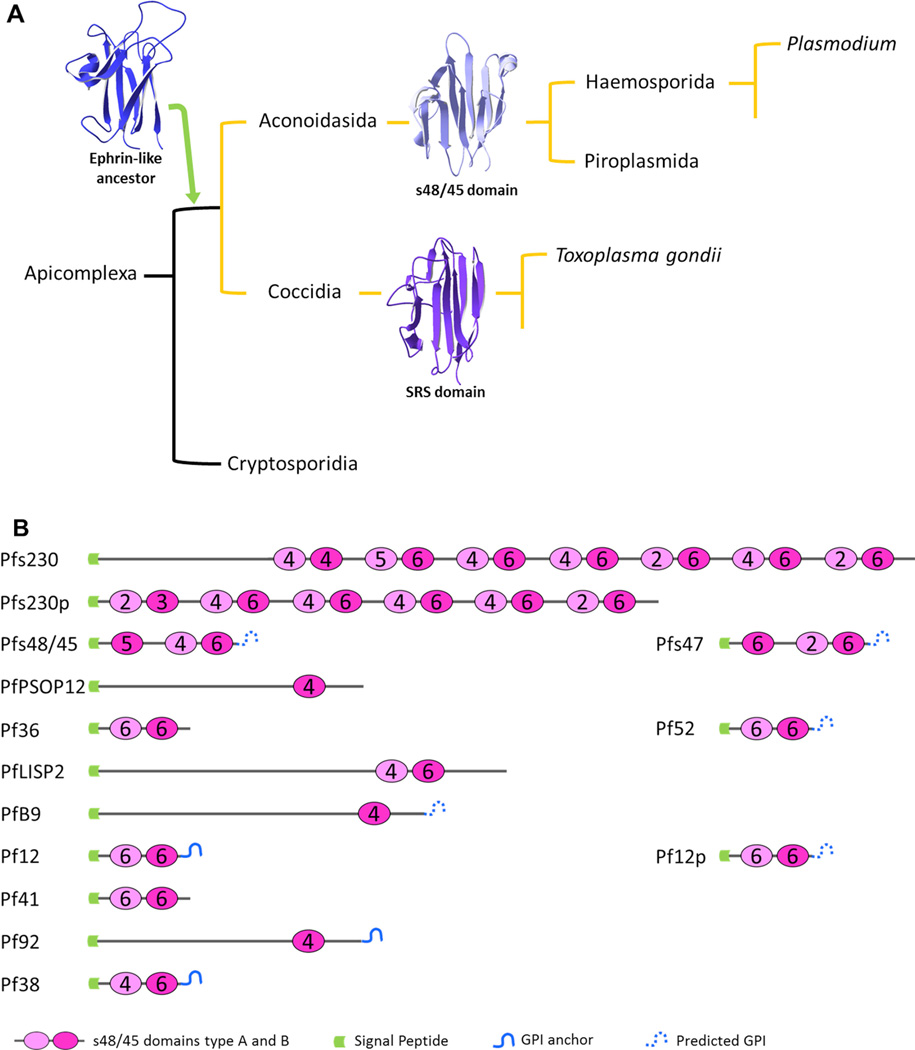
Fig. 2.
The evolution and distribution of the s48/45 six-cysteine protein domain. (A) The s48/45 domain is suggested to derive from an ancient Ephrin-like domain that was acquired by an ancestor of the aconoidasidans and coccidians that eventually evolved into the current s48/45 domain in Plasmodium and the SAG1-Related Sequence (SRS) domain in Toxoplasma (ribbon structures shown: Protein Data Bank (PDB) ID: 3WO3-b for Ephrin-like; 2LOE for s48/45; 1KZQ-a D1 for SRS), (figure adapted from Arredondo et al. (2012). (B) Illustration of the Plasmodium falciparum s48/45 family members showing the arrangement of the domains in each protein, the number of cysteines present in each domain and the predicted signal sequences and glycosylphosphatidylinositol (GPI) anchors.
The conserved cysteine motifs of the s48/45 domain that gave its name to the 6-cys family of proteins in Plasmodium was first identified in 1993 by Williamson et al. (1993) while studying Pfs230. The authors also noticed that the pattern was present in another protein, Pf12. As more P. falciparum genes were sequenced, other proteins were found to contain similar cysteine motifs and the family was established (Carter et al., 1995; Templeton and Kaslow, 1999; Thompson et al., 2001). A more detailed analysis of the arrangement of the characteristic cysteines gave rise to a prediction of disulfide bond connectivity that also proposed folding into structurally similar discrete domains (Carter et al., 1995), and eventually led to the accurate computational structural modeling of double 6-cys domains (types A and B) defined as PGSH (Plasmodium gamete surface homology) fragments (Gerloff et al., 2005; Gerloff, D.L., Draizen, E., 2016. 6-Cys Domain Model Database. Foundation for Applied Molecular Evolution (FfAME), UC Santa Cruz Biomolecular Engineering, USA and University of Edinburgh, Scotland. http://pgsh.soe.ucsc.edu/).
The importance of the formation of disulfide bonds for the proper folding and conformation of the 6-cys proteins was evident early on, as most of the monoclonal antibodies (mAbs) raised against native Pfs230 and Pfs48/45 only recognized the non-reduced proteins (Quakyi et al., 1987; Carter et al., 1990, 1995; Read et al., 1994). However, due to the many challenges presented by the expression of natively folded 6-cys proteins in heterologous expression systems throughout the years, the experimentally determined molecular conformation of the 6-cys domain was not obtained until recently with the solution nuclear magnetic resonance (NMR) structure of the C-terminal domain of Pf12, shown in Fig. 2A (Arredondo et al., 2012). The molecular structure of the first s48/45 domain, as termed by the Pfam Protein Families Database (Punta et al., 2012), confirmed the original predictions in terms of disulfide connectivity, and the similarity of its structure to the SRS domain from Toxoplasma (Fig. 2A) (He et al., 2002; Gerloff et al., 2005). The SRS superfamily comprises a group of more than 180 genes found in different stages of the life cycle of Toxoplasma that also tend to arrange in tandem and share the structural SRS domain (Jung et al., 2004; Wasmuth et al., 2012). The SRS proteins that have been characterized to date show that these are glycosylphosphatidylinositol (GPI)-anchored surface proteins with important functions in cell adhesion, immune evasion or virulence attenuation (Dzierszinski et al., 2000; Jacquet et al., 2001; Lekutis et al., 2001; Jung et al., 2004; Radke et al., 2004; Crawford et al., 2010; Wasmuth et al., 2012).
The s48/45 domain is characterized by a β-sandwich formed by a mix of parallel and antiparallel β-sheets (Fig. 2A). The domain is held together by two disulfide bonds resulting from the pairing of Cys1-Cys2 and Cys3-Cys6, and there is a third disulfide bond (Cys4-Cys5) outside the core of the domain as originally predicted (Carter et al., 1995; Gerloff et al., 2005). The Cys3-Cys6 disulfide bond is very well conserved compared with the structures of the SRS domains and it is suggested to be essential for the structural stability and proper conformation of the domain (Arredondo et al., 2012). In the early studies of the 6-cys family, some of the proteins were described to have “degenerate” domains containing only two or four cysteines in addition to the canonical 6-cys domains (Templeton and Kaslow, 1999). More recently, new members of the family were identified using a consensus 4-cys minimal motif (Ø×Ø×C×nC[F,S,T] ×n[F,I,L]×C×C; Ø = hydrophobic residue) which was assembled based on the MEME analysis of previously established 6-cys domains (Annoura et al., 2014). This is not unexpected since some of the SRS proteins also have only four cysteines in their domains and it is possible that, in some cases, only one disulfide bond might be enough to hold the β-sandwich in the necessary conformation to maintain function. In addition to the presence of at least one consensus motif containing the positionally conserved cysteines, the prediction to fold as an s48/45 domain and the prediction of a signal peptide were also constraints for inclusion of these proteins in the family.
To date, only the molecular structures for Pf12 and Pf41 have been solved and, as anticipated, each domain of these proteins superimposes well with the original s48/45 structure (Arredondo et al., 2012; Tonkin et al., 2013; Parker et al., 2015). However, outside the main core of the domains, interesting flexible features are observed as seen by NMR in the C-terminus of Pf12, which might also be the case for an insertion domain in Pf41 that is necessary for binding to Pf12 (Arredondo et al., 2012; Parker et al., 2015). Unfortunately, no function has been assigned to Pf12 and Pf41. Therefore, structure-function associations relevant to parasite biology cannot be derived yet. In the meantime, molecular models based on the solved structures, are available online for a subset of the other s48/45 proteins (http://pgsh.soe.ucsc.edu/).
3. 6-cys proteins in the sexual stages
The Plasmodium life cycle has an obligate sexual reproduction stage that begins with the commitment to produce merozoites which, after invasion and following five stages of development (I-V), become mature male or female gametocytes (reviewed in Josling and Llinas (2015)). When stage V gametocytes are ingested by a mosquito taking a blood meal, they activate in the midgut and emerge from the erythrocyte; males exflagellate forming microgametes and fertilize female gametes to produce zygotes which then transform into motile ookinetes (Fig. 1A) (reviewed in Kuehn and Pradel (2010);Bennink et al. (2016). During traversal through the midgut epithelial cells, ookinetes activate the mosquito immune response through the JNK pathway and some are “tagged” for complement lysis and perish as they come into contact with the hemolymph (Han and Barillas-Mury, 2002; Garver et al., 2013). Surviving ookinetes, however, transform into oocysts which eventually produce thousands of sporozoites. In this section, we will discuss the 6-cys proteins found in the sexual stages of the parasite.
3.1. P230
Pfs230 is the founding member of the 6-Cys family (Williamson et al., 1993). It was originally identified by immunoprecipitation of radiolabeled gametes using transmission-blocking mouse mAbs obtained from immunizations with P. falciparum-purified gametes. It was named according to its molecular weight in SDS-PAGE under non-reducing conditions (Rener et al., 1983; Vermeulen et al., 1985a). Pfs230 has been extensively studied for more than 30 years due to the early recognition of its potential as a transmission-blocking vaccine antigen (Quakyi et al., 1987). For a more extensive review on earlier research on this protein alone see Williamson (2003).
There are 14 cysteine motif domains in Pfs230, some of which have four or five cysteines per domain instead of six, but they are all predicted and modeled to fold as s48/45 domains (Fig. 2B). The s48/45 domains are preceded by a glutamate-rich region and a predicted signal sequence with no GPI anchor predicted on the C-terminus (Carter et al., 1995; Gerloff et al., 2005; http://pgsh.soe.ucsc.edu/). Polymorphisms have been reported for the Pfs230 gene and it is suggested to be under positive selection (Williamson and Kaslow, 1993; Niederwieser et al., 2001; Tonkin et al., 2013). The original immunoprecipitation studies in combination with many other transcriptional, proteomic and immunofluorescence studies, agree that P230 is expressed only in male and female sexual stages starting at stage II gametocytes and continuing until fertilization is complete (Rener et al., 1983; Vermeulen et al., 1985a; Williamson et al., 1993, 1995; Florens et al., 2002; Le Roch et al., 2003; Khan et al., 2005; Eksi et al., 2006; van Schaijk et al., 2006; Pradel, 2007; Silvestrini et al., 2010; van Dijk et al., 2010). In addition, Pfs230 was confirmed to be on the plasma membrane of gametocytes and gametes by immune electron microscopy (Williamson et al., 1996). Pfs230 was also found localized on the recently described membranous protrusions on activated gametocytes that are hypothesized to mediate their interactions (Rupp et al., 2011). As mentioned above, P230 does not have a GPI-anchoring signal, however, early studies determined that P230 forms a complex with P48/45 which is predicted to be GPI-anchored (Kumar, 1987; Kumar and Wizel, 1992); and deletion experiments showed that, in ΔPfs48/45 gametes, Pfs230 is expressed but it is not associated with the gamete surface after emergence from the red blood cell (Eksi et al., 2006) implying that P230 localizes on the membrane through its interaction with P48/45 (Fig. 1B). During gamete emergence, P230 undergoes two independent proteolytic cleavage events at the N-terminus, at sites N-terminal to the first s48/45 domain, resulting in two processed versions of the protein (300 and 307 kDa) that remain associated with the gamete (Williamson et al., 1996; Brooks and Williamson, 2000).
Disruption of P230 shed light on the crucial role of this protein in fertilization. ΔP230 parasites have a defect in male fertility that leads to significantly reduced mosquito infection and, consequently, reduced oocyst production (Eksi et al., 2006; van Dijk et al., 2010). Furthermore, in P. falciparum, exflagellating males are unable to bind to erythrocytes to establish exflagellation centers, which are important for fertilization (Eksi et al., 2006) and, in Plasmodium berghei, ΔP230 male gametes do not recognize female gametes, whereas ΔP230 female gametes have no apparent phenotype (van Dijk et al., 2010). Interestingly, the deletion of either P230 or P48/45 in P. berghei female gametes increased their hybridization with Plasmodium yoelii male gametes (Ramiro et al., 2015).
Recently, Pfs230 together with Pfs48/45 have been shown to be part of a multimeric protein complex that includes the PfCCp family members and Pfs25 (Fig. 1B), all with roles in parasite sexual reproduction and development in the mosquito (Tomas et al., 2001; Pradel et al., 2004; Scholz et al., 2008; Simon et al., 2009, 2016). Thus far, this is the only report of s48/45 proteins as part of a larger complex. A series of co-immunoprecipitation experiments showed that, in nonactivated gametocytes, Pfs230 already interacts directly with Pfs48/45 and PfCCp4 (which is in complex with other PfCCp proteins). Following gametocyte activation, Pfs230 also establishes an interaction with the other PfCCp proteins stabilizing the complex on the membrane. Absence of Pfs230 or inhibition of its proteolytic cleavage results in degradation of PfCCp proteins (Simon et al., 2016). A different study also reports the interaction of Pfs230 with PfWL1, a newly identified WD40-repeat protein that might also be part of the PfCCp complex (von Bohl et al., 2015).
It is well established that both Pfs230 and Pfs48/45 are recognized by antibodies from naturally infected individuals from malaria-endemic regions (Vermeulen et al., 1985a; Graves et al., 1988; Carter et al., 1990; Targett, 1990; Roeffen et al., 1996; Healer et al., 1999; Drakeley et al., 2004; Bousema et al., 2010; Ouedraogo et al., 2011; Stone et al., 2016;), and the response to these antigens seems to be boosted after subsequent natural infections (Skinner et al., 2015). In addition, high antibody titers are correlated with transmission reduction (Stone et al., 2016). From the beginning, Pfs230 was defined as a transmission-blocking vaccine candidate since antibodies against this protein act in a complement-dependent manner to lyse the parasite and prevent further development in the mosquito midgut (Quakyi et al., 1987; Read et al., 1994; Roeffen et al., 1995; Healer et al., 1997; Bustamante et al., 2000). The minimal effective antigenic region of Pfs230 was originally mapped to amino acids 443–1132 referred to as region C, which contains 13 cysteines (Bustamante, 2000). These cysteines need to be properly paired for the epitopes to attain native conformation and elicit full transmission-blocking immunity, making P230 a challenging protein to produce in heterologous expression systems (Riley et al., 1995; Williamson et al., 1995; Vincent et al., 1999; Bustamante et al., 2000; Williamson, 2003; Farrance et al., 2011; Tachibana et al., 2011; Kapulu et al., 2015). However, more recently, the prodomain N-terminal to the first s48/45 domain alone was reported to be sufficient to induce transmission-blocking antibodies (Tachibana et al., 2011). As of 2015, Pfs230 has entered clinical trials in the US as an EPA (Pseudomonas aeruginosa exoprotein A) fusion in combination of Pfs25-EPA (Draper et al., 2015).
The P. vivax orthologue Pvs230 has also been evaluated as a transmission-blocking candidate. It was reported to have a similar expression and localization pattern on gametes as Pfs230, and antibody raised against a region comparable with Pfs230 region C significantly reduced oocyst numbers in the mosquito midgut (Tachibana et al., 2011). The analysis of 113 Pvs230 sequences from around the world showed that it also is under purifying selection with very low nucleotide diversity (Doi et al., 2011; Tachibana et al., 2011).
3.2. P48/45
Pfs48/45 was identified in a search for gametocyte antigens that are recognized by antibodies capable of blocking parasites within the mosquito midgut, and it was named Pfs48/45 because it migrated as two bands of 48 and 45 kDa in SDS-PAGE under non-reducing conditions (Rener et al., 1983; Vermeulen et al., 1985a). Pfs230 and Pfs48/45 were initially co-eluted with these transmission-blocking mAbs, and thus the two proteins were characterized in parallel in the early experiments and were soon found to interact with each other (Kumar, 1987; Kumar and Wizel, 1992). P48/45 joined the 6-cys family as the first prediction of disulfide connectivity for the s48/45 domain was made; it has three s48/45 domains, with four, five and six cysteines, respectively (Carter et al., 1995; Gerloff et al., 2005), and it is predicted to have a signal peptide and a GPI anchor (Fig. 2B) (Kocken et al., 1993). P48/45 RNA and protein expression profiles are similar to P230 with expression in gametocytes and gametes (Rener et al., 1983; Vermeulen et al., 1985a; Kocken et al., 1993; van Dijk et al., 2001, 2010; Florens et al., 2002; Le Roch et al., 2003; Khan et al., 2005; van Schaijk et al., 2006; Pradel, 2007; Silvestrini et al., 2010) and its subcellular localization mirrors Pfs230 (van Dijk et al., 2001; Eksi et al., 2006; Rupp et al., 2011). Gene deletion in P. falciparum and P. berghei and cross-fertilization experiments in rodent species showed an essential role for P48/45 in male, but not female, gametes during fertilization, with production of ookinetes drastically reduced. Motile PbΔP48/45 male gametes were able to adhere to erythrocytes to form exflagellation centers but were unable to attach to females and fertilize, as reported for PbΔ230 (van Dijk et al., 2001, 2010; Ramiro et al., 2015). It is not clear whether the observed phenotype in ΔP48/45 mutants results from the loss of a direct role of P48/45 itself or from the lack of P230 on the plasma membrane (Eksi et al., 2006). Besides P48/45 being part of the PfCCp protein complex mentioned in Section 3.1, due to its interaction with P230 (Simon et al., 2016), no other direct protein interaction has been reported for this protein to date. There is evidence that Pfs48/45 is under positive selection, and extreme fixation of variation in this protein has been reported (Escalante et al., 1998; Conway et al., 2001; Escalante et al., 2002; Anthony et al., 2007; Tonkin et al., 2013).
Similar to Pfs230, Pfs48/45 is a transmission-blocking vaccine candidate and correct epitope conformation is also very important for eliciting effective blocking antibodies, however, protection is not complement-dependent (Vermeulen et al., 1985b; Carter et al., 1990; Targett, 1990; Roeffen et al., 1996; Healer et al., 1997; Milek et al., 1998b; Merino et al., 2016). The best epitope for blocking transmission was originally mapped to the third s48/45 domain at the C-terminus, however more recent constructs include different regions or even the full-length protein (Carter et al., 1990; Outchkourov et al., 2007, 2008; Chowdhury et al., 2009; Kapulu et al., 2015; Singh et al., 2015). Despite multiple challenges related to protein expression through the years (Milek et al., 1998a, 2000; Outchkourov et al., 2008; Chowdhury et al., 2009; Mamedov et al., 2012; Jones et al., 2013), a fusion of the C-terminus of Pfs48/45 with the N-terminus of P. falciparum glutamate-rich protein (PfGLURP) is currently a vaccine candidate under clinical development (Theisen et al., 2014; Draper et al., 2015; Singh et al., 2015).
Pvs48/45 is also under investigation as a transmission-blocking vaccine candidate. As for Pfs48/45, the P. vivax orthologue was found on the surface of gametes (Tachibana et al., 2015) and two independent groups reported significant transmission-blocking activity for antibodies raised against Pvs48/45 recombinant protein (Arevalo-Herrera et al., 2015; Tachibana et al., 2015). Population analyses report very little sequence diversity for Pvs48/45 in several parasite populations around the world (Woo et al., 2013; Feng et al., 2015; Tachibana et al., 2015; Vallejo et al., 2016).
3.3. P230p
Pfs230p, also known as PfMR5 (male-related, stage 5 antigen), was first identified as a paralog of Pfs230 when the P. falciparum chromosome 2 sequence became available (Gardner et al., 1998) and was predicted to have a signal peptide and five 6-cys domains in addition to some intervening “degenerate” domains with two or four cysteines (Templeton and Kaslow, 1999). Structural models indicate that P230p has 12 s48/45 domains, five of which have six cysteines with all others having two, three or four cysteines (Fig. 2B) (Gerloff et al., 2005; http://pgsh.soe.ucsc.edu/). A GPI anchor was not predicted for P230p. Transcripts were reported in P. berghei and P. falciparum in gametocytes only (Thompson et al., 2001; Le Roch et al., 2003; Hall et al., 2005; van Dijk et al., 2010; Schneider et al., 2015), particularly in stages II-V, and were absent in gametes (Eksi and Williamson, 2002). Proteomic studies, western blot analysis and a GFP reporter study indicated that P230p expresses only in male gametocytes stages IV-V (Eksi and Williamson, 2002; Khan et al., 2005; Eksi et al., 2008; Silvestrini et al., 2010). Immunofluorescence data showed Pfs230p with a cytoplasmic distribution within the gametocyte (Eksi and Williamson, 2002).
The function of P230p has not yet been described, however a role in rapid DNA replication or cytoskeletal reorganization necessary for exflagellation has been hypothesized (Williamson, 2003). Deletion mutants in P. berghei and P. yoelii have shown no effect on the parasite life cycle and in fact the P230p locus has even been used as a reporter insertion site (Janse et al., 2006; van Dijk et al., 2010; Lin et al., 2011; Hart et al., 2014). Functional redundancy with Pfs230 has been ruled out since the expression pattern of these proteins is different and no compensating upregulation was observed for Pfs230p in a ΔPfs230 mutant (Eksi and Williamson, 2002; Eksi et al., 2006). Interestingly, P230p is the only member of the family, among those with known subcellular localization, which is not found on the surface or in an invasion-related organelle.
3.4. P47
P47, the paralog of P48/45, was originally identified in a BLAST search based on 6-cys domains from previously known members of the family and it was determined to have two domains with six cysteines separated by a linker region, as well as a predicted signal sequence and GPI anchor (Templeton and Kaslow, 1999). The linker region is actually a “degenerate” s48/45 domain with only two cysteines (Fig. 2B) (Gerloff et al., 2005; http://pgsh.soe.ucsc.edu/). Multiple transcriptional and proteomic experiments showed P47 to be expressed only in female gametocytes, starting at stage II, and on female gametes following emergence from red blood cells, both in P. falciparum and P. berghei (van Dijk et al., 2001, 2010; Florens et al., 2002; Lasonder et al., 2002; Le Roch et al., 2003; Hall et al., 2005; Khan et al., 2005; van Schaijk et al., 2006; Silvestrini et al., 2010). In addition, Pfs47 was localized to the surface of female gametocytes and gametes by immunofluorescence assays and immunoprecipitation of labeled gametes (Eksi et al., 2006; van Schaijk et al., 2006). Earlier experiments did not find a critical function for P47 in P. falciparum gamete fertilization and transmission-blocking activity was not observed for α-P47 antibodies (van Schaijk et al., 2006). Since Pfs47 was deemed to be dispensable, several reporter parasites were created by inserting transgenes in the P47 locus (Talman et al., 2010; Vaughan et al., 2012; Lu et al., 2016). In P. berghei, however, P47 was reported to have an important role in female fertility (van Dijk et al., 2010).
More recently, Pfs47 transcripts were found in ookinetes and the protein was localized to the surface by immunofluorescence. Furthermore, in this study, through a combination of genetic mapping, linkage and functional genomics applied to progeny from a P. falciparum strains 7G8 × GB4 genetic cross, the authors determined an essential role for Pfs47 in the evasion of the Anopheles gambiae innate immune response to infection (Molina-Cruz et al., 2013). Pfs47 on the surface of the ookinete mediated inactivation of JNK signaling in mosquito midgut cells that eventually resulted in inhibition of binding of the complement-like system protein TEP1 to the ookinete which in turn prevents lysis of the parasite (Ramphul et al., 2015). The identity of the mosquito receptor interacting with Pfs47 remains to be determined. Interestingly, the immune evasion function of Pfs47 is allele-dependent, and different alleles allow survival in mosquitoes from different geographical locations (Molina-Cruz and Barillas-Mury, 2014; Molina-Cruz et al., 2015), however it has been suggested that Pfs47 is not uniquely responsible for this phenomenon and that there might be other factors involved, particularly in parasite field isolates (Eldering et al., 2016). Pfs47 is very polymorphic and it is suggested to be under positive selection based on the analysis of laboratory and field isolates showing a marked geographic genetic structure (Anthony et al., 2007; Manske et al., 2012; Molina-Cruz et al., 2013; Molina-Cruz and Barillas-Mury, 2014). Most of the polymorphisms in Pfs47 and Pbs47 are found in the second s48/45 domain (van Dijk et al., 2010; Molina-Cruz et al., 2015), suggesting a role for this domain in the parasite-vector interactions required for immune evasion.
In P. vivax, P47 is also expressed in gametocytes but reportedly localized to the cytoplasm. Nevertheless, antibodies against Pvs47 significantly reduced oocyst numbers in mosquito feeding assays with P. vivax-infected blood. Population analysis of Pvs47 isolates form Thailand, Vanuatu and Colombia reported very few polymorphisms (Tachibana et al., 2015) but multiple polymorphisms were reported for Pvs47 in Korean isolates (Woo et al., 2013). In rodent malaria species, P47 is one of the fastest evolving proteins and it is under strong positive selection in P. berghei and P. yoelii (van Dijk et al., 2010; Ramiro et al., 2015).
3.5. PSOP12
Putative secreted ookinete protein 12 (PSOP12) was identified in a reverse genetics screen of P. berghei proteins expressed by the ookinete (Ecker et al., 2008). This protein has one C-terminal 4-cys s48/45 domain and a predicted signal sequence but no GPI anchor (Fig. 2B) (Ecker et al., 2008; Annoura et al., 2014). Mass spectrometry data indicates it is expressed in gametocytes, ookinetes and oocyts in P. berghei and in P. falciparum (Florens et al., 2002; Hall et al., 2005; Ecker et al., 2008; Silvestrini et al., 2010). Live and immunofluorescence microscopy of PSOP12-GFPtagged parasites detected the protein on the surface of male and female gametocytes, gametes and ookinetes (Sala et al., 2015). Plasmodium berghei ΔPSOP12 parasites showed only a mild, non-significant reduction in oocyst production, resulting in normal numbers of sporozoites and normal transmission to mice (Ecker et al., 2008). Nevertheless, a recent vaccine study using baculovirus-expressed PbPSOP12 reported a small but significant transmission-blocking activity for antibodies to this protein both in vitro and in mice (Sala et al., 2015). Further characterization of SOP12 in P. falciparum and P. yoelii is required to determine whether robust blocking transmission activity can be achieved by targeting this protein.
4. 6-cys proteins in the pre-erythrocytic stages
After traversal of the mosquito midgut epithelium, the ookinete attaches to the basal lamina of the midgut and transforms into an oocyst which grows, undergoes sporogony and produces sporozoites. Escape from the oocyst allows the sporozoites to disperse in the hemolymph and eventually reach and recognize specific receptors on the salivary gland epithelium, prompting them to actively invade and take up residence within the gland ducts. The next time the infected mosquito takes a blood meal, sporozoites are deposited in the host skin where, by means of gliding motility, they will make their way to a blood vessel and enter it. They are then transported to the liver, where they cross the liver sinusoidal endothelium to reach the parenchyma. Following the traversal of multiple hepatocytes, and the successful identification of key receptors on a suitable host hepatocyte, the sporozoite invades by establishing a parasitophorous vacuole. Within this protective compartment, the sporozoite transforms into a trophozoite, enters schizogony and eventually differentiates into thousands of exo-erythrocytic merozoites ready to egress and start the asexual cycle of red blood cell infection (reviewed in Vaughan et al., 2008; Aly et al., 2009). It is evident that during the pre-erythrocytic phase of infection there are multiple points at which parasite-host cell interactions are necessary for effective life cycle progression. In this section we discuss the members of the 6-cys family which fulfill important roles in these interactions.
4.1. P36
P36 was identified in the above-mentioned BLAST search (Section 3.4) as having two 6-cys domains and it is predicted to have a secretory signal peptide but not a GPI anchor sequence (Fig. 2B) (Templeton and Kaslow, 1999). This protein was reported to be expressed in P. berghei gametocytes by northern blot analyses (van Dijk et al., 2010; Thompson et al., 2001) and by proteomic studies (Hall et al., 2005; Khan et al., 2005); however, deletion of P36 in P. berghei did not have detrimental effects on fertilization (van Dijk et al., 2010). In addition, P36 expression in P. falciparum and P. yoelii sporozoites was demonstrated by proteomic experiments (Lasonder et al., 2008; Lindner et al., 2013) and reverse transcription (RT) -PCR (Labaied et al., 2007; VanBuskirk et al., 2009). Western blot analysis suggested that P36 is expressed in salivary gland sporozoites and not in oocyst sporozoites (Ishino et al., 2005). The actual subcellular localization of this protein has not been reported to date. Pf36 does not seem to be very polymorphic, as population studies of 11 P. falciparum isolates showed non-significant results for polymorphisms in this protein (Anthony et al., 2007).
Deletion of P36 in P. falciparum or rodent malaria parasites did not show any apparent defect in blood stage replication, development in the mosquito or sporozoite infection of the salivary glands (Ishino et al., 2005; Labaied et al., 2007; VanBuskirk et al., 2009). PbΔP36 sporozoites demonstrated an important role for this protein in hepatocyte invasion as these mutants were not able to productively invade hepatocytes and could only infrequently establish a productive infection in mice (Ishino et al., 2005). Similar results were reported for PfΔP36 parasites in hepatocyte invasion assays in vitro, where parasite developmental arrest occurred early inside the host cell (VanBuskirk et al., 2009). Moreover, rapid clearance of PyΔP36 parasites from mouse livers has been recently described (Kaushansky et al., 2015). P36 (together with P52, Section 4.2) is critical for the establishment and/or maintenance of the parasitophorous vacuole during invasion as PyΔP36 parasites can enter hepatocytes but then are observed free in the cytoplasm of the host cell (Labaied et al., 2007; Ploemen et al., 2012). Although there was abundant evidence that P36 is important for successful invasion of hepatocytes by sporozoites, the exact role of the protein had not been determined. However, a recent study points towards the interaction of P36 with host EphA2, a hepatocyte-expressed receptor tyrosine kinase that naturally binds GPI-anchored EphrinA1 to mediate cell-cell interactions (Kaushansky et al., 2015). This is an intriguing finding in light of the structural similarity of s48/45 domains with the Ephrin fold. Although binding of P36 to EphA2 was not directly demonstrated, the work showed that recombinant P36 interfered with EphrinA1 binding to EphA2, indicating an interaction (Kaushansky et al., 2015). The importance of EphA2 in pre17 erythrocytic infection was corroborated by the observation that EphA2 knockout mice were dramatically less susceptible to sporozoite infection compared with wildtype mice and that sporozoites require hepatocytes with high expression of EphA2 to support invasion with parasitophorous vacuole formation (Kaushansky et al., 2015). It is tempting to hypothesize that the structural similarities between the host Ephrin fold and parasite s48/45 domains have been exploited by the parasite to allow interaction with Eph receptors for host cell engagement.
4.2. P52
P52 (named according to molecular mass) was identified in the first comprehensive sporozoite transcriptome study (Kappe et al., 2001) and in a comparative genomics study as a paralog of P36 and therefore named P36p (Thompson et al., 2001). It has two 6-cys domains and it is predicted to have a signal sequence and a GPI anchor (Fig. 2B) (Kappe et al., 2001; Thompson et al., 2001). Sequence analysis of 13 P. falciparum isolates suggested that Pf52 is under positive selection (Tonkin et al., 2013). Transcriptional and proteomic analyses in P. berghei, P. yoelii and P. falciparum agree and show that P52 is only expressed in sporozoites (Kappe et al., 2001; Le Roch et al., 2003; Ishino et al., 2005; van Dijk et al., 2005, 2010; Labaied et al., 2007; Lasonder et al., 2008; VanBuskirk et al., 2009; Lindner et al., 2013). While P52 transcripts were reported in both oocyst sporozoites and salivary gland sporozoites (Kappe et al., 2001), western blot analysis showed expression only in salivary gland sporozoites of P. berghei (Ishino et al., 2005). Immunofluorescence and immune electron microscopy (EM) tentatively localized P52 to the sporozoite micronemes (Ishino et al., 2005; VanBuskirk et al., 2009). In addition, the protein was reported to be translocated to the surface of the sporozoite during gliding motility (Ishino et al., 2005).
Deletion of P52 resulted in a similar phenotype as observed for ΔP36 mutants in vitro and in vivo, where sporozoites arrest soon after entering hepatocytes and are not able to achieve productive invasion (Ishino et al., 2005; van Dijk et al., 2005; van Schaijk et al., 2008; VanBuskirk et al., 2009). Simultaneous deletion of P36 and P52 had a more profound infection phenotype than the single deletion mutants (Labaied et al., 2007; VanBuskirk et al., 2009). The strong phenotype is caused by the mutant’s inability to form and/or maintain a parasitophorous vacuole very early in/after invasion (Labaied et al., 2007; Ploemen et al., 2012). Because ΔP36ΔP52 parasites are severely attenuated in vitro and in vivo, they can be used for immunization and this conferred sterile immunity against wildtype sporozoite challenge in mice (Labaied et al., 2007). PfΔP36ΔP52 parasites were also shown to be severely attenuated in a controlled human malaria infection study (Spring et al., 2013) and thus deletions of Pf36 and Pf52 alongside a third gene deletion are now part of a Genetically Attenuated Parasite (GAP) vaccine candidate that is undergoing clinical testing (Mikolajczak et al., 2014).
Considering the very similar phenotypes observed in deletion mutants of P36 and P52, and the precedence set by the P230-P48/45 complex in gametocytes, it is possible that P36 and P52 are forming a complex where the GPI-anchored P52 would attach to the parasite plasma membrane and bind secreted P36. The P52/P36 complex could establish contact with the EphA2 receptor on the hepatocyte surface and this interaction would initiate the formation of the parasitophorous vacuole and productive invasion (Fig. 1B). However, a direct interaction between P36 and P52 has not yet been reported. It is also possible that the two proteins function in separate, but closely related, pathways or are part of a larger complex that fully disassembles only when both proteins are absent, resulting in the stronger phenotype of the double gene knockout.
4.3. LISP2 (Sequestrin)
Plasmodium falciparum Liver stage-Specific Protein 2 (LISP2) was first identified by immunoprecipitation from parasite-infected erythrocytes and was originally named Sequestrin (Ockenhouse et al., 1991); however, it was only recently recognized as a 6-cys protein (Arredondo et al., 2012; Annoura et al., 2014). LISP2 is predicted to have a signal sequence but not a GPI anchor. Two independent groups reported two s48/45 domains for this protein, one domain with four cysteines and the other with six (Fig. 2B) (Arredondo et al., 2012; http://pgsh.soe.ucsc.edu/), and two other groups report that LISP2 only has the one 6-cys domain (Orito et al., 2013; Annoura et al., 2014). Across Plasmodium spp., the most conserved region contains the 6-cys domain (Orito et al., 2013), implying the essentiality of this domain for the function of the protein.
The original study reported LISP2 on the surface of cytoadherent P. falciparum-infected erythrocytes as observed by immunofluorescence assay. In addition, it was reported to bind to CD36 (Ockenhouse et al., 1991), however no other studies characterizing this interaction have been reported to date and its role in blood stages remains unknown. On the other hand, transcripts of LISP2, as well as the protein, were identified in abundance in a proteomics study of late P. yoelii liver stages (Tarun et al., 2008) and more recently in a expressed sequence tag analysis of P. berghei liver stages (Orito et al., 2013). These studies also determined that LISP2, named after its expression pattern comparable with LISP1 (Tarun et al., 2008), is exclusively expressed in the mid-to-late liver stages stages. Another group has independently confirmed these results by characterizing stage-specific expression using the promoter region of P. berghei LISP2, in conjunction with a dual luminescence system (Helm et al., 2010; De Niz et al., 2015). Immunofluorescence assays of liver stages showed that LISP2 is transported to the vacuolar space and then, after processing, exported into the hepatocyte cytoplasm (Orito et al., 2013; Itani et al., 2014).
Targeted disruption of LISP2 in P. berghei demonstrated an important role for this protein at the time of liver stage schizogony since the majority of the ΔLISP2 parasites formed aberrant exo-erythrocytic merozoites with a defect in their release, delaying onset of blood stage patency (Orito et al., 2013). A second ΔLISP2 parasite, created by a different group, was characterized and shown to have lower levels of parasite liver stage burden in mice at late developmental time points. Further analysis in vitro showed drastically reduced merozoite surface protein 1 (MSP-1) expression, a merozoite surface marker that is normally expressed late in liver stage schizogony (Annoura et al., 2014), confirming an important role for LISP2 in late liver stages, particularly in the formation of merozoites. Furthermore, a very recent study reported another P. berghei ΔLISP2 parasite to be more severely attenuated in mouse infections than previously reported, attributing the difference to the full deletion of the gene including the N-terminus (Kumar et al., 2016). It is possible that this protein has a dual role; first in exo-erythrocytic schizogony and then, after processing and translocation to the hepatocyte cytoplasm, a second function perhaps related to parasite egress or immune evasion.
4.4. B9
B9 was recently identified as a 6-cys family member through a genome search for proteins containing a minimal 4-cysteine motif derived from the already known s48/45 domains (Annoura et al., 2014). B9 has a single 4-cys s48/45 domain at its C-terminus, and it is predicted to have a signal sequence and a GPI anchor (Fig. 2B). Immunofluorescence assays suggested that B9 localizes to the plasma membrane of liver stages. In sporozoites, however, the protein seems to be transcriptionally repressed as RT-PCR data shows the presence of transcripts but protein expression was not observed until after hepatocyte infection, peaking at 5 h p.i. in P. berghei and starting at 24 h p.i. in in vitro P. falciparum liver stages (Annoura et al., 2014). In contrast, peptides corresponding to B9 were identified in a proteomic study of P. falciparum sporozoites (Lindner et al., 2013). The analysis of P. berghei, P. yoelii and P. falciparum ΔB9 parasites showed developmental arrest early after invasion of hepatocytes with a very small number of parasites able to continue development. Neither the arrested nor the developing ΔB9 parasites were positive for parasitophorous vacuole membrane markers, indicating a critical role for B9 in its integrity (Annoura et al., 2014). Deletion of B9 is part of a second GAP vaccine candidate that was shown to induce sterile protective immunity in the P. berghei model (van Schaijk et al., 2014) and the P. falciparum version will likely undergo clinical testing in the near future.
5. 6-cys proteins in asexual erythrocytic stages
The clinically symptomatic asexual cycle of replication of Plasmodium in red blood cells begins with a loose association of the merozoite with the erythrocyte surface, followed by reorientation to establish apical contact with the cell, followed by irreversible attachment of the merozoite and creation of a tight junction that allows the progressive entrance into the cell, and ultimately the establishment of the parasitophorous vacuole; each one of these steps requiring multiple protein-protein interactions of the parasite with the erythrocyte (reviewed in Cowman et al., 2012; Koch and Baum, 2016). Once the merozoite has sealed itself inside the erythrocyte, protein export and remodeling of the cell begins. The parasite progresses through the ring and trophozoite stages, finally differentiating into schizonts that produce a new generation of merozoites, ready to invade erythrocytes and start another asexual replication cycle. In the trophozoite stages of P. falciparum, knob-like structures containing parasite cytoadhesion molecules allow the parasites to bind to the vascular endothelium and sequester, causing severe malaria (reviewed in Maier et al., 2009). In this section we will review the 6-cys proteins expressed in the asexual erythrocytic stages.
5.1. P12
P12 is the smallest member of the 6-cys family and was first identified in a screen of a P. falciparum genomic library expressed on the surface of mammalian cells and tested for reactivity against human immune sera from P. falciparum-exposed African adults (Elliott et al., 1990). P12 has two 6-cysteine motifs similar to those detected in Pfs230 and it was deemed to be the structural archetype of the family (Williamson et al., 1993; Carter et al., 1995; Gerloff et al., 2005); its NMR analysis provided the first experimentally determined molecular structure for the 6-cys family (Fig. 2B) (Arredondo et al., 2012; Tonkin et al., 2013). Pf12 was shown to be a GPI-anchored protein by the proteomic analysis of radioactive glucosamine-labeled late schizont membranes (Gilson et al., 2006). Although transcripts have been detected in late schizonts, sporozoites and gametocytes in P. falciparum and P. berghei (Bozdech et al., 2003; Le Roch et al., 2003; Hall et al., 2005; Gilson et al., 2006; van Dijk et al., 2010), protein expression has only been confirmed for schizonts/merozoites by western blot (Taechalertpaisarn et al., 2012; Tonkin et al., 2013) and membrane proteome analyses (Sanders et al., 2005; Gilson et al., 2006). Immunofluorescence microscopy indicated that Pf12 is localized on the surface of the merozoite with a more prominent signal on the apical end that colocalizes significantly with the rhoptry neck protein RON4 (Taechalertpaisarn et al., 2012; Tonkin et al., 2013) and electron microscopy studies localized Pf12 to the rhoptry organelles of the merozoite (Silvia Arredondo, unpublished data). However, it has been suggested that P12 is shed from the merozoite following proteolytic cleavage (Taechalertpaisarn et al., 2012).
Based on its expression profile and localization, Pf12 was anticipated to be involved in erythrocyte invasion, however ΔP12 P. falciparum parasites did not show any deficiency in invasion or other apparent phenotypes during the asexual replication cycle (Taechalertpaisarn et al., 2012). In addition, neither native Pf12 from culture supernatants nor recombinant protein showed direct binding to red blood cells (Taechalertpaisarn et al., 2012), in contrast to earlier reports of Pf12 synthetic peptides binding and displaying modest invasion inhibition activity (Garcia et al., 2009). Antibodies raised against recombinant Pf12 did not show any invasion inhibition activity either (Taechalertpaisarn et al., 2012). Co-immunoprecipitation experiments established an interaction of Pf12 with Pf41 and this has been confirmed by biochemical and structural experiments using recombinant protein (Fig. 1B) (Taechalertpaisarn et al., 2012; Crosnier et al., 2013; Tonkin et al., 2013; Parker et al., 2015). Although the molecular structures for Pf12 and Pf41 have been solved (discussed in Sections 2 and 5.3), it has not been possible to derive functional insights from these structures.
Pf12 appears to be a strong natural immunogen; in addition to its initial identification by immune sera from African populations (Elliott et al., 1990) and immunoprecipitation by immune sera from Papua New Guinea (Sanders et al., 2005), studies have reported high seroprevalence against Pf12 in Kenyan and Papua New Guinean children with a weak association with protection from symptomatic malaria in the latter (Richards et al., 2013; Osier et al., 2014). However, P12 is remarkably conserved and believed to be under purifying selection (Tetteh et al., 2009; Tonkin et al., 2013), an indication of a crucial function for this protein that has yet to be determined.
Pv12, the Pf12 orthologue in P. vivax, was shown to be transcribed in schizonts and localized to the surface and rhoptries of merozoites by immunofluorescence (Li et al., 2012; Cheng et al., 2013; Moreno-Perez et al., 2013). The interaction of Pv12 and Pv41 has recently been reported in addition to a novel interaction with PVX_110945 (Hostetler et al., 2015). Low genetic diversity was observed for Pv12 in a Colombian parasite population (Forero-Rodriguez et al., 2014) but a higher number of polymorphisms was found in parasite populations from the China-Myanmar border (Wang et al., 2014). Pv12 is recognized by sera of naturally infected patients (Chen et al., 2010; Moreno-Perez et al., 2013; Franca et al., 2016) and a strong association in reduced risk of clinical disease and antibody levels was recently reported (Franca et al., 2016).
5.2. P12p
P12p is a paralog of P12 found in tandem with p12 on chromosome 6, and it was first reported to be a member of the family by Gerloff and co-workers (Gerloff et al., 2005). Similar to P12, there are two 6-cys domains in P12p, and a signal sequence and GPI anchor are predicted (Fig. 2B) (Gilson et al., 2006). P12p was reported to be transcribed in the blood stages in P. falciparum by microarray and in P. berghei by northern blot analysis (Gilson et al., 2006; van Dijk et al., 2010). However, mass spectrometry data indicates P12p is also present in P. falciparum and P. yoelii sporozoites (Lasonder et al., 2008; Lindner et al., 2013). Deletion of P12p in P. yoelii did not show any detectable phenotype at any stage of the infection cycle (our unpublished results). Thus, the function of P12p remains unknown and requires further investigation.
5.3. P41
P41 was identified in the same BLAST search as Pf36 and Pfs47 and it was described to have a secretory signal peptide, two 6-cysteine domains and an “intervening region” that was recently shown to be an important element within the first domain (Fig. 2B) (Templeton and Kaslow, 1999; Parker et al., 2015). P41 lacks a GPI anchor but, as mentioned in Section 5.1, it is part of the second confirmed heterodimer within the family, interacting with P12 (Fig. 1B) (Taechalertpaisarn et al., 2012; Crosnier et al., 2013; Tonkin et al., 2013; Hostetler et al., 2015; Parker et al., 2015). Correspondingly, the expression and localization profiles for Pf41 are similar to Pf12 (Hall et al., 2005; Sanders et al., 2005; van Dijk et al., 2010). Pf41 was found in the proteome of merozoite surface membranes and antibody against the inter-domain region localized it to the surface of merozoites within mature schizonts and in the apical organelles in free merozoites by immunofluorescence microscopy (Sanders et al., 2005). Biochemical studies and targeted deletion for Pf41, carried out in parallel with Pf12, showed no critical function for this protein in erythrocyte binding and invasion (Taechalertpaisarn et al., 2012), contrary to the reported inhibitory activity of P41 peptides (Garcia et al., 2009). Pf41 is the second 6-cys protein for which the molecular structure has been solved (Parker et al., 2015). Initial biochemical studies on the Pf12/Pf41 complex suggested the proteins to have an anti-parallel arrangement (Tonkin et al., 2013). The actual structure of Pf41 revealed a more interesting feature: what was originally described as an interdomain region is actually an intra-domain insertion that is essential for binding to Pf12 and is protected from proteolytic cleavage upon interaction of the two proteins (Parker et al., 2015).
Pf41, seemingly under positive selection (Tonkin et al., 2013), is recognized by sera from naturally infected individuals with reported seroprevalences of 32–88% (Sanders et al., 2005; Richards et al., 2013; Osier et al., 2014). In addition, high correlation with malaria disease protection was reported in a study of a cohort of Kenyan children (Osier et al., 2014), while only weak association with protection from symptomatic malaria was observed in a population of Papua New Guinean children (Richards et al., 2013).
The expression and subcellular localization profiles for Pv41 are similar to the P. falciparum orthologue, however the apical localization in the merozoite was not reported (Angel et al., 2008; Chen et al., 2010; Cheng et al., 2013). Positive balancing selection was suggested for Pv41, particularly in the C-terminus of parasites from the China-Myanmar border (Wang et al., 2014). Alternatively, low genetic diversity was found for Pv41 in parasites from Colombia with a few substitutions in the C-terminus under positive selection (Forero-Rodriguez et al., 2014). Pv41 is also recognized by sera from naturally infected individuals and a strong association for protection is reported for Pv41 antibodies in a Papua New Guinean cohort (Chen et al., 2010; Cheng et al., 2013; Franca et al., 2016).
5.4. P92
Pf92 was initially identified in a proteomic study of detergent-resistant membranes of mature asexual blood stages (Sanders et al., 2005). Interestingly, P92 is the only protein in the family that does not have an orthologue in rodent Plasmodium spp. The 6-cys model database indicates it has a double s48/45 domain, however it has also been reported to have a single C-terminal s48/45 domain with only four cysteines (Arredondo et al., 2012; Annoura et al., 2014; http://pgsh.soe.ucsc.edu/). It is predicted to have a signal sequence and it has been validated as a GPI-anchored protein in the same proteomics study as Pf12 (Fig. 2B) (Gilson et al., 2006). Transcriptional analysis indicated that Pf92 is expressed in blood stage schizonts (Bozdech et al., 2003; Le Roch et al., 2003; Sanders et al., 2005; Gilson et al., 2006). Expression of Pf92 in late schizonts and merozoites has also been confirmed by proteomic experiments (Sanders et al., 2005, 2007; Gilson et al., 2006), western blot analysis (Obando-Martinez et al., 2010; Kennedy et al., 2016) and immunofluorescence microscopy, which showed localization on the surface of the merozoites using a GFP-tagged version of the protein (Sanders et al., 2005). In addition, Pf92 peptides were reported in oocyst-derived sporozoites (Lasonder et al., 2008).
Among the most abundant proteins found in schizont-derived membranes (Sanders et al., 2007), Pf92 was recently described to have an important role in preventing complement-dependent lysis of merozoites. Kennedy et al. (2016) showed that Factor H, a complement regulator that normally binds to cell receptors to prevent “self” destruction by the complement cascade, is recruited by merozoites, representing yet another immune evasion strategy of the Plasmodium parasite. Immunoprecipitation experiments identified Pf92 as the main protein recruiting Factor H to the surface of merozoites (Fig. 1B). Deletion mutants of Pf92 were not able to bind to Factor H and showed significant growth reduction after four in vitro replication cycles. Although the binding domains in Factor H were successfully mapped, the corresponding interacting regions in Pf92 remain to be determined (Kennedy et al., 2016). As mentioned above, Pf92 is not found in rodent malaria species, however Factor H is part of the mouse complement system and human Factor H is capable of regulating mouse complement (Pouw et al., 2015), raising questions regarding the identity of the rodent Plasmodium protein or proteins that may be functionally substituting for Pf92.
Pf92 has been reported to be under positive selection in the analysis of 13 isolates from PlasmoDB sequences (Tonkin et al., 2013) and under weak negative selection in the P. falciparum Gambian parasite population (Tetteh et al., 2009). While Pf92 elicits an immune response in individuals from endemic regions, with ~80% seropositivity observed in a cohort of Kenyan children, there was no significant correlation with protection from clinical disease episodes (Osier et al., 2014).
5.5. P38
Pf38 was identified as a member of the 6-cys family in a comparative genomics study (Thompson et al., 2001). This protein has two s48/45 domains with four and six cysteines, respectively, with a predicted signal peptide, and it has been determined to have a GPI anchor (Fig. 2B) (Thompson et al., 2001; Gilson et al., 2006; http://pgsh.soe.ucsc.edu/). P38 is reported to be expressed in schizonts, sexual stages and sporozoites by several transcriptomic and proteomic studies (Bozdech et al., 2003; Le Roch et al., 2003; Gilson et al., 2006; Silvestrini et al., 2010; Lindner et al., 2013). In addition, northern blot analysis showed transcripts for P38 in gametocytes and blood stages of P. berghei (Thompson et al., 2001; van Dijk et al., 2010). Pf38 was found in detergent-resistant membranes from late schizont/merozoites (Sanders et al., 2005, 2007; Gilson et al., 2006) and confirmed to localize on the surface of merozoites by immunofluorescence microscopy in both P. yoelii and P. falciparum blood stages (Feller et al., 2013; Harupa, A., 2015. Identification and functional analysis of novel sporozoite surface proteins in the rodent malaria parasite Plasmodium yoelii, Doctoral thesis, Freie Univeritat Berlin, Germany). However, live fluorescence microscopy using a Pf38-GFP fusion showed this protein mainly in the apical end of merozoites (Sanders et al., 2005). P38 has also been reported to localize to sporozoite micronemes, using an hemagglutinin- (HA) tagged P38 in P. yoelii (Harupa, A., 2015. Doctoral thesis, cited earlier), and on gametocytes, macrogametes and zygotes (Feller, 2013).
Deletion mutants of P38 have shown no obvious phenotypic differences compared with wildtypes both in P. berghei and P. yoelii (van Dijk et al., 2010; Harupa, A., 2015. Doctoral thesis, cited earlier). However, Pf38-derived peptides were reported to show a 20–33% inhibition of erythrocyte invasion in vitro (Garcia et al., 2009); and anti-Pf38 antibody significantly inhibited the growth of P. falciparum blood stages, in addition to inhibiting zygote development (Feller et al., 2013). P38 is the only member of the family that has been shown to have a phosphorylated serine residue in proteomic studies (Aurrecoechea et al., 2009; Treeck et al., 2011; Pease et al., 2013; Lasonder et al., 2015) but whether this residue modification is important for function remains to be determined.
Population analyses showed that, in the Gambian and Papua New Guinean parasite populations, Pf38 is a polymorphic gene and it seems to be under modest balancing selection with a stronger signature of balancing selection for Domain I of the protein in New Guinea (Tetteh et al., 2009; Reeder et al., 2011). Analysis of laboratory isolates, however, did not yield significant results regarding polymorphisms for this gene (Anthony et al., 2007). Pf38 is recognized by antibodies of naturally infected individuals from different populations with high seroprevalence (Sanders et al., 2005; Richards et al., 2013; Osier et al., 2014). The study of a cohort of Papua New Guinean children showed that antibodies against Pf38 were correlated with intermediate protection from symptomatic malaria (Richards et al., 2013), and in a different cohort of infected Kenyan children, Pf38 also ranked highly in the association of protection from clinical episodes (Osier et al., 2014).
In P. vivax, Pv38 has two s48/45 domains, however the N-terminal domain has five cysteines rather than four (Wang et al., 2014; http://pgsh.soe.ucsc.edu/). Pv38 was reported to be present in membranes from asexual blood stages by western blot analysis and localized on schizonts with a speckled pattern (Mongui et al., 2008). Low genetic diversity has been reported for Pv38 in parasites from Colombia and, similarly to the P. falciparum orthologue, balancing selection was suggested for the N-terminal domain of the protein (Forero-Rodriguez et al., 2014). Parasites from the China-Myanmar border were reported to have significant genetic differentiation in comparison to the Colombian population, and in this case positive balancing selection was suggested for Domain II of Pv38 (Wang et al., 2014).
6. Concluding remarks
It appears as if the suggested acquisition of the s48/45 domain by the malaria parasite from its host, its expansion in the 6-cys family and its preservation through millions of years of evolution, have conferred multiple and significant advantages to Plasmodium parasites. To date, there are three main categories in which the adhesive functions of the s48/45 proteins cluster to ensure parasite survival and life cycle progression: fertilization in the sexual stages (P230, P48/45 and PSOP12), establishment or maintenance of the parasitophorous vacuole membrane to sustain infection of hepatocytes (P36, P52, B9) and immune evasion (P47, P92 and perhaps LISP2, P12, P41, P38, P12p). Another common theme is becoming apparent: a GPI-anchored family member attaches to the parasite plasma membrane and binds a secreted member by forming a heterodimer, raising the question as to whether some members serve only as scaffolds while others perform effector/binding functions. The non-conserved regions outside the main s48/45 domains of the proteins deserve closer analysis as well. Could the flexible loops and extensions away from the main domain core hold the key to the adhesive properties of these proteins, as may be the case of the intra-domain region in Pf41 or the N-terminus to the first s48/45 domain in Pfs230Δ Is the intrinsic value of the s48/45 domain in its versatility in allowing extensive peripheral flexibility while maintaining robust stability within the core? The solution of the molecular structures of the 6-cys family members for which functions and host partners are known, for example the co-crystal of Pf92 and Factor H, or P36 and EphA2, will be very useful in answering these questions. It is reasonable to ask whether P38 is a scaffold throughout different parasite stages, stabilizing unrelated or other s48/45 proteins. Is there redundancy among “scaffold” members? Proteolytic cleavage to create functional protein might also be a more general feature of 6-cys proteins, as proteolysis has been reported for P230, LISP2, P12 and perhaps P41 which upon cleavage loses the ability to bind to P12. Is the host-interaction functionality only activated after proteolysis? To better understand the interactions of these proteins we must also look into loss/gain of function for the same protein across species, for example for P47 and P230 in P. berghei and P. falciparum. It would be very informative to define the specific protein regions involved in female fertilization or male exflagellation, respectively. P230 remains a very interesting protein; with its 14 domains it is tempting to imagine the opportunities for multiple functions and to date it appears to have at least three separate functions: fertilization and exflagellation in males and mating barriers in females. Is this multiplicity of function what makes P230 a strong transmission-blocking vaccine candidate? If so, we may have to look at the s48/45 family from a more global perspective and perhaps through a combination of in silico and in vitro approaches define minimal conserved conformational epitopes that may elicit strong cross-reactive antibody responses that can interfere with the function of multiple s48/45 proteins at once, attacking parasite progression at multiple points in the life cycle. Finally, the advent of new and more robust parasite transgenesis techniques such as the CRISPR/Cas9 system will allow for precise genetic manipulations to introduce mutations at specific residues, delete full domains or even swap domains between members of the family or across species. This will accelerate the functional characterization of the s48/45 proteins and the identification of essential epitopes for more effective vaccines, addressing the many questions we still have about this intriguing family in the near future.
Highlights.
The Plasmodium 6-cys protein family has 14 members that share the s48/45 domain.
s48/45 proteins are found in all parasite stages and are conserved across species.
s48/45 proteins have functions in fertilization, parasitophorous vacuole membrane fitness and immune evasion.
Some of the s48/45 proteins are under investigation as vaccine candidates.
Acknowledgments
Work by the authors is funded by the US National Institute of Allergy and Infectious Diseases (NIAID), USA, the Malaria Vaccine Initiative (MVI), USA and the Bill & Melinda Gates Foundation (BMGF), USA. We apologize to all colleagues whose work could not be cited due to space constraints.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aly AS, Vaughan AM, Kappe SH. Malaria parasite development in the mosquito and infection of the mammalian host. Annu Rev Microbiol. 2009;63:195–221. doi: 10.1146/annurev.micro.091208.073403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anantharaman V, Iyer LM, Balaji S, Aravind L. Adhesion molecules and other secreted host-interaction determinants in Apicomplexa: insights from comparative genomics. International review of cytology. 2007;262:1–74. doi: 10.1016/S0074-7696(07)62001-4. [DOI] [PubMed] [Google Scholar]
- Angel DI, Mongui A, Ardila J, Vanegas M, Patarroyo MA. The Plasmodium vivax Pv41 surface protein: identification and characterization. Biochem Biophys Res Commun. 2008;377:1113–1117. doi: 10.1016/j.bbrc.2008.10.129. [DOI] [PubMed] [Google Scholar]
- Annoura T, van Schaijk BC, Ploemen IH, Sajid M, Lin JW, Vos MW, Dinmohamed AG, Inaoka DK, Rijpma SR, van Gemert GJ, Chevalley-Maurel S, Kielbasa SM, Scheltinga F, Franke-Fayard B, Klop O, Hermsen CC, Kita K, Gego A, Franetich JF, Mazier D, Hoffman SL, Janse CJ, Sauerwein RW, Khan SM. Two Plasmodium 6-Cys family-related proteins have distinct and critical roles in liver-stage development. FASEB J. 2014;28:2158–2170. doi: 10.1096/fj.13-241570. [DOI] [PubMed] [Google Scholar]
- Anthony TG, Polley SD, Vogler AP, Conway DJ. Evidence of non-neutral polymorphism in Plasmodium falciparum gamete surface protein genes Pfs47 and Pfs48/45. Molecular and biochemical parasitology. 2007;156:117–123. doi: 10.1016/j.molbiopara.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Aravind L, Iyer LM, Wellems TE, Miller LH. Plasmodium biology: genomic gleanings. Cell. 2003;115:771–785. doi: 10.1016/s0092-8674(03)01023-7. [DOI] [PubMed] [Google Scholar]
- Arevalo-Herrera M, Vallejo AF, Rubiano K, Solarte Y, Marin C, Castellanos A, Cespedes N, Herrera S. Recombinant Pvs48/45 antigen expressed in E. coli generates antibodies that block malaria transmission in Anopheles albimanus mosquitoes. PLoS One. 2015;10:e0119335. doi: 10.1371/journal.pone.0119335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arredondo SA, Cai M, Takayama Y, MacDonald NJ, Anderson DE, Aravind L, Clore GM, Miller LH. Structure of the Plasmodium 6-cysteine s48/45 domain. Proc Natl Acad Sci U S A. 2012;109:6692–6697. doi: 10.1073/pnas.1204363109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aurrecoechea C, Brestelli J, Brunk BP, Dommer J, Fischer S, Gajria B, Gao X, Gingle A, Grant G, Harb OS, Heiges M, Innamorato F, Iodice J, Kissinger JC, Kraemer E, Li W, Miller JA, Nayak V, Pennington C, Pinney DF, Roos DS, Ross C, Stoeckert CJ, Jr, Treatman C, Wang H. PlasmoDB: a functional genomic database for malaria parasites. Nucl Acids Res. 2009;37:D539–D543. doi: 10.1093/nar/gkn814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennink S, Kiesow MJ, Pradel G. The development of malaria parasites in the mosquito midgut. Cell Microbiol. 2016;18:905–918. doi: 10.1111/cmi.12604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousema T, Roeffen W, Meijerink H, Mwerinde H, Mwakalinga S, van Gemert GJ, van de Vegte-Bolmer M, Mosha F, Targett G, Riley EM, Sauerwein R, Drakeley C. The dynamics of naturally acquired immune responses to Plasmodium falciparum sexual stage antigens Pfs230 & Pfs48/45 in a low endemic area in Tanzania. PLoS One. 2010;5:e14114. doi: 10.1371/journal.pone.0014114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozdech Z, Llinas M, Pulliam BL, Wong ED, Zhu J, DeRisi JL. The transcriptome of the intraerythrocytic developmental cycle of Plasmodium falciparum. PLoS Biol. 2003;1:E5. doi: 10.1371/journal.pbio.0000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks SR, Williamson KC. Proteolysis of Plasmodium falciparum surface antigen, Pfs230, during gametogenesis. Mol Biochem Parasitol. 2000;106:77–82. doi: 10.1016/s0166-6851(99)00201-7. [DOI] [PubMed] [Google Scholar]
- Bustamante PJ, Woodruff DC, Oh J, Keister DB, Muratova O, Williamson KC. Differential ability of specific regions of Plasmodium falciparum sexual-stage antigen, Pfs230, to induce malaria transmission-blocking immunity. Parasite Immunol. 2000;22:373–380. doi: 10.1046/j.1365-3024.2000.00315.x. [DOI] [PubMed] [Google Scholar]
- Carter R, Coulson A, Bhatti S, Taylor BJ, Elliott JF. Predicted disulfide-bonded structures for three uniquely related proteins of Plasmodium falciparum, Pfs230, Pfs48/45 and Pf12. Mol Biochem Parasitol. 1995;71:203–210. doi: 10.1016/0166-6851(94)00054-q. [DOI] [PubMed] [Google Scholar]
- Carter R, Graves PM, Keister DB, Quakyi IA. Properties of epitopes of Pfs 48/45, a target of transmission blocking monoclonal antibodies, on gametes of different isolates of Plasmodium falciparum. Parasite Immunol. 1990;12:587–603. doi: 10.1111/j.1365-3024.1990.tb00990.x. [DOI] [PubMed] [Google Scholar]
- Carter R, Mendis KN. Evolutionary and historical aspects of the burden of malaria. Clin Microbiol Rev. 2002;15:564–594. doi: 10.1128/CMR.15.4.564-594.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JH, Jung JW, Wang Y, Ha KS, Lu F, Lim CS, Takeo S, Tsuboi T, Han ET. Immunoproteomics profiling of blood stage Plasmodium vivax infection by high-throughput screening assays. J Proteome Res. 2010;9:6479–6489. doi: 10.1021/pr100705g. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Lu F, Tsuboi T, Han ET. Characterization of a novel merozoite surface protein of Plasmodium vivax Pv41. Acta Trop. 2013;126:222–228. doi: 10.1016/j.actatropica.2013.03.002. [DOI] [PubMed] [Google Scholar]
- Chowdhury DR, Angov E, Kariuki T, Kumar N. A potent malaria transmission blocking vaccine based on codon harmonized full length Pfs48/45 expressed in Escherichia coli. PLoS One. 2009;4:e6352. doi: 10.1371/journal.pone.0006352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway DJ, Machado RL, Singh B, Dessert P, Mikes ZS, Povoa MM, Oduola AM, Roper C. Extreme geographical fixation of variation in the Plasmodium falciparum gamete surface protein gene Pfs48/45 compared with microsatellite loci. Mol Biochem Parasitol. 2001;115:145–156. doi: 10.1016/s0166-6851(01)00278-x. [DOI] [PubMed] [Google Scholar]
- Cowman AF, Berry D, Baum J. The cellular and molecular basis for malaria parasite invasion of the human red blood cell. J Cell Biol. 2012;198:961–971. doi: 10.1083/jcb.201206112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford J, Lamb E, Wasmuth J, Grujic O, Grigg ME, Boulanger MJ. Structural and functional characterization of SporoSAG: a SAG2-related surface antigen from Toxoplasma gondii. J Biol Chem. 2010;285:12063–12070. doi: 10.1074/jbc.M109.054866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosnier C, Wanaguru M, McDade B, Osier FH, Marsh K, Rayner JC, Wright GJ. A library of functional recombinant cell-surface and secreted P. falciparum merozoite proteins. Mol Cell Proteo. 2013;12:3976–3986. doi: 10.1074/mcp.O113.028357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Niz M, Helm S, Horstmann S, Annoura T, Del Portillo HA, Khan SM, Heussler VT. In vivo and in vitro characterization of a Plasmodium liver stage-specific promoter. PLoS One. 2015;10:e0123473. doi: 10.1371/journal.pone.0123473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi M, Tanabe K, Tachibana S, Hamai M, Tachibana M, Mita T, Yagi M, Zeyrek FY, Ferreira MU, Ohmae H, Kaneko A, Randrianarivelojosia M, Sattabongkot J, Cao YM, Horii T, Torii M, Tsuboi T. Worldwide sequence conservation of transmission-blocking vaccine candidate Pvs230 in Plasmodium vivax. Vaccine. 2011;29:4308–4315. doi: 10.1016/j.vaccine.2011.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drakeley CJ, Eling W, Teelen K, Bousema JT, Sauerwein R, Greenwood BM, Targett GA. Parasite infectivity and immunity to Plasmodium falciparum gametocytes in Gambian children. Parasite Immunol. 2004;26:159–165. doi: 10.1111/j.0141-9838.2004.00696.x. [DOI] [PubMed] [Google Scholar]
- Draper SJ, Angov E, Horii T, Miller LH, Srinivasan P, Theisen M, Biswas S. Recent advances in recombinant protein-based malaria vaccines. Vaccine. 2015;33:7433–7443. doi: 10.1016/j.vaccine.2015.09.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzierszinski F, Mortuaire M, Cesbron-Delauw MF, Tomavo S. Targeted disruption of the glycosylphosphatidylinositol-anchored surface antigen SAG3 gene in Toxoplasma gondii decreases host cell adhesion and drastically reduces virulence in mice. Mol Microbiol. 2000;37:574–582. doi: 10.1046/j.1365-2958.2000.02014.x. [DOI] [PubMed] [Google Scholar]
- Ecker A, Bushell ES, Tewari R, Sinden RE. Reverse genetics screen identifies six proteins important for malaria development in the mosquito. Mol Microbiol. 2008;70:209–220. doi: 10.1111/j.1365-2958.2008.06407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eksi S, Czesny B, van Gemert GJ, Sauerwein RW, Eling W, Williamson KC. Malaria transmission-blocking antigen, Pfs230, mediates human red blood cell binding to exflagellating male parasites and oocyst production. Mol Microbiol. 2006;61:991–998. doi: 10.1111/j.1365-2958.2006.05284.x. [DOI] [PubMed] [Google Scholar]
- Eksi S, Suri A, Williamson KC. Sex- and stage-specific reporter gene expression in Plasmodium falciparum. Mol Biochem Parasitol. 2008;160:148–151. doi: 10.1016/j.molbiopara.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eksi S, Williamson KC. Male-specific expression of the paralog of malaria transmission-blocking target antigen Pfs230, PfB0400w. Mol Biochem Parasitol. 2002;122:127–130. doi: 10.1016/s0166-6851(02)00091-9. [DOI] [PubMed] [Google Scholar]
- Eldering M, Morlais I, van Gemert GJ, van de Vegte-Bolmer M, Graumans W, Siebelink-Stoter R, Vos M, Abate L, Roeffen W, Bousema T, Levashina EA, Sauerwein RW. Variation in susceptibility of African Plasmodium falciparum malaria parasites to TEP1 mediated killing in Anopheles gambiae mosquitoes. Sci Rep. 2016;6:20440. doi: 10.1038/srep20440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott JF, Albrecht GR, Gilladoga A, Handunnetti SM, Neequaye J, Lallinger G, Minjas JN, Howard RJ. Genes for Plasmodium falciparum surface antigens cloned by expression in COS cells. Proc Natl Acad Sci U S A. 1990;87:6363–6367. doi: 10.1073/pnas.87.16.6363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escalante AA, Grebert HM, Chaiyaroj SC, Riggione F, Biswas S, Nahlen BL, Lal AA. Polymorphism in the gene encoding the Pfs48/45 antigen of Plasmodium falciparum XI. Asembo Bay Cohort Project. Mol Biochem Parasitol. 2002;119:17–22. doi: 10.1016/s0166-6851(01)00386-3. [DOI] [PubMed] [Google Scholar]
- Escalante AA, Lal AA, Ayala FJ. Genetic polymorphism and natural selection in the malaria parasite Plasmodium falciparum. Genetics. 1998;149:189–202. doi: 10.1093/genetics/149.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrance CE, Rhee A, Jones RM, Musiychuk K, Shamloul M, Sharma S, Mett V, Chichester JA, Streatfield SJ, Roeffen W, van de Vegte-Bolmer M, Sauerwein RW, Tsuboi T, Muratova OV, Wu Y, Yusibov V. A plant-produced Pfs230 vaccine candidate blocks transmission of Plasmodium falciparum. Clin Vaccine Immunol. 2011;18:1351, 1357. doi: 10.1128/CVI.05105-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feller T, Thom P, Koch N, Spiegel H, Addai-Mensah O, Fischer R, Reimann A, Pradel G, Fendel R, Schillberg S, Scheuermayer M, Schinkel H. Plant-based production of recombinant Plasmodium surface protein pf38 and evaluation of its potential as a vaccine candidate. PLoS One. 2013;8:e79920. doi: 10.1371/journal.pone.0079920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng H, Gupta B, Wang M, Zheng W, Zheng L, Zhu X, Yang Y, Fang Q, Luo E, Fan Q, Tsuboi T, Cao Y, Cui L. Genetic diversity of transmission-blocking vaccine candidate Pvs48/45 in Plasmodium vivax populations in China. Parasit Vectors. 2015;8:615. doi: 10.1186/s13071-015-1232-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florens L, Washburn MP, Raine JD, Anthony RM, Grainger M, Haynes JD, Moch JK, Muster N, Sacci JB, Tabb DL, Witney AA, Wolters D, Wu Y, Gardner MJ, Holder AA, Sinden RE, Yates JR, Carucci DJ. A proteomic view of the Plasmodium falciparum life cycle. Nature. 2002;419:520–526. doi: 10.1038/nature01107. [DOI] [PubMed] [Google Scholar]
- Forero-Rodriguez J, Garzon-Ospina D, Patarroyo MA. Low genetic diversity and functional constraint in loci encoding Plasmodium vivax P12 and P38 proteins in the Colombian population. Malaria J. 2014;13:58. doi: 10.1186/1475-2875-13-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franca CT, Hostetler JB, Sharma S, White MT, Lin E, Kiniboro B, Waltmann A, Darcy AW, Li Wai Suen CS, Siba P, King CL, Rayner JC, Fairhurst RM, Mueller I. An Antibody Screen of a Plasmodium vivax Antigen Library Identifies Novel Merozoite Proteins Associated with Clinical Protection. PLoS Negl Trop Dis. 2016;10:e0004639. doi: 10.1371/journal.pntd.0004639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia J, Curtidor H, Pinzon CG, Vanegas M, Moreno A, Patarroyo ME. Identification of conserved erythrocyte binding regions in members of the Plasmodium falciparum Cys6 lipid raft-associated protein family. Vaccine. 2009;27:3953–3962. doi: 10.1016/j.vaccine.2009.04.039. [DOI] [PubMed] [Google Scholar]
- Gardner MJ, Tettelin H, Carucci DJ, Cummings LM, Aravind L, Koonin EV, Shallom S, Mason T, Yu K, Fujii C, Pederson J, Shen K, Jing J, Aston C, Lai Z, Schwartz DC, Pertea M, Salzberg S, Zhou L, Sutton GG, Clayton R, White O, Smith HO, Fraser CM, Adams MD, Venter JC, Hoffman SL. Chromosome 2 sequence of the human malaria parasite Plasmodium falciparum. Science. 1998;282:1126–1132. doi: 10.1126/science.282.5391.1126. [DOI] [PubMed] [Google Scholar]
- Garver LS, de Almeida Oliveira G, Barillas-Mury C. The JNK pathway is a key mediator of Anopheles gambiae antiplasmodial immunity. PLoS pathogens. 2013;29:e1003622. doi: 10.1371/journal.ppat.1003622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerloff DL, Creasey A, Maslau S, Carter R. Structural models for the protein family characterized by gamete surface protein Pfs230 of Plasmodium falciparum. Proc Natl Acad Sci U S A. 2005;102:13598–13603. doi: 10.1073/pnas.0502378102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilson PR, Nebl T, Vukcevic D, Moritz RL, Sargeant T, Speed TP, Schofield L, Crabb BS. Identification and stoichiometry of glycosylphosphatidylinositol-anchored membrane proteins of the human malaria parasite Plasmodium falciparum. Mol Cell Proteo. 2006;5:1286–1299. doi: 10.1074/mcp.M600035-MCP200. [DOI] [PubMed] [Google Scholar]
- Graves PM, Wirtz RA, Carter R, Burkot TR, Looker M, Targett GA. Naturally occurring antibodies to an epitope on Plasmodium falciparum gametes detected by monoclonal antibody-based competitive enzyme-linked immunosorbent assay. Infect Immun. 1988;56:2818–2821. doi: 10.1128/iai.56.11.2818-2821.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall N, Karras M, Raine JD, Carlton JM, Kooij TW, Berriman M, Florens L, Janssen CS, Pain A, Christophides GK, James K, Rutherford K, Harris B, Harris D, Churcher C, Quail MA, Ormond D, Doggett J, Trueman HE, Mendoza J, Bidwell SL, Rajandream MA, Carucci DJ, Yates JR, 3rd, Kafatos FC, Janse CJ, Barrell B, Turner CM, Waters AP, Sinden RE. A comprehensive survey of the Plasmodium life cycle by genomic, transcriptomic, and proteomic analyses. Science. 2005;307:82–86. doi: 10.1126/science.1103717. [DOI] [PubMed] [Google Scholar]
- Han YS, Barillas-Mury C. Implications of Time Bomb model of ookinete invasion of midgut cells. Insect Biochem Mol Biol. 2002;32:1311–1316. doi: 10.1016/s0965-1748(02)00093-0. [DOI] [PubMed] [Google Scholar]
- Hart RJ, Lawres L, Fritzen E, Ben Mamoun C, Aly AS. Plasmodium yoelii vitamin B5 pantothenate transporter candidate is essential for parasite transmission to the mosquito. Sci Rep. 2014;4:5665. doi: 10.1038/srep05665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He XL, Grigg ME, Boothroyd JC, Garcia KC. Structure of the immunodominant surface antigen from the Toxoplasma gondii SRS superfamily. Nat Struct Biol. 2002;9:606–611. doi: 10.1038/nsb819. [DOI] [PubMed] [Google Scholar]
- Healer J, McGuinness D, Carter R, Riley E. Transmission-blocking immunity to Plasmodium falciparum in malaria-immune individuals is associated with antibodies to the gamete surface protein Pfs230. Parasitology. 1999;119(Pt 5):425–433. doi: 10.1017/s0031182099005041. [DOI] [PubMed] [Google Scholar]
- Healer J, McGuinness D, Hopcroft P, Haley S, Carter R, Riley E. Complement-mediated lysis of Plasmodium falciparum gametes by malaria-immune human sera is associated with antibodies to the gamete surface antigen Pfs230. Infect Immun. 1997;65:3017–3023. doi: 10.1128/iai.65.8.3017-3023.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helm S, Lehmann C, Nagel A, Stanway RR, Horstmann S, Llinas M, Heussler VT. Identification and characterization of a liver stage-specific promoter region of the malaria parasite Plasmodium. PLoS One. 2010;5:e13653. doi: 10.1371/journal.pone.0013653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostetler JB, Sharma S, Bartholdson SJ, Wright GJ, Fairhurst RM, Rayner JC. A Library of Plasmodium vivax Recombinant Merozoite Proteins Reveals New Vaccine Candidates and Protein-Protein Interactions. PLoS Negl Trop Dis. 2015;9:e0004264. doi: 10.1371/journal.pntd.0004264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishino T, Chinzei Y, Yuda M. Two proteins with 6-cys motifs are required for malarial parasites to commit to infection of the hepatocyte. Mol Microbiol. 2005;58:1264–1275. doi: 10.1111/j.1365-2958.2005.04801.x. [DOI] [PubMed] [Google Scholar]
- Itani S, Torii M, Ishino T. D-Glucose concentration is the key factor facilitating liver stage maturation of Plasmodium. Parasitol Int. 2014;63:584–590. doi: 10.1016/j.parint.2014.03.004. [DOI] [PubMed] [Google Scholar]
- Jacquet A, Coulon L, De Neve J, Daminet V, Haumont M, Garcia L, Bollen A, Jurado M, Biemans R. The surface antigen SAG3 mediates the attachment of Toxoplasma gondii to cell-surface proteoglycans. Mol Biochem Parasitol. 2001;116:35–44. doi: 10.1016/s0166-6851(01)00297-3. [DOI] [PubMed] [Google Scholar]
- Janse CJ, Franke-Fayard B, Mair GR, Ramesar J, Thiel C, Engelmann S, Matuschewski K, van Gemert GJ, Sauerwein RW, Waters AP. High efficiency transfection of Plasmodium berghei facilitates novel selection procedures. Mol Biochem Parasitol. 2006;145:60–70. doi: 10.1016/j.molbiopara.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Jones CS, Luong T, Hannon M, Tran M, Gregory JA, Shen Z, Briggs SP, Mayfield SP. Heterologous expression of the C-terminal antigenic domain of the malaria vaccine candidate Pfs48/45 in the green algae Chlamydomonas reinhardtii. Appl Microbiol Biotechnol. 2013;97:1987–1995. doi: 10.1007/s00253-012-4071-7. [DOI] [PubMed] [Google Scholar]
- Josling GA, Llinas M. Sexual development in Plasmodium parasites: knowing when it's time to commit. Nat Rev Microbiol. 2015;13:573–587. doi: 10.1038/nrmicro3519. [DOI] [PubMed] [Google Scholar]
- Jung C, Lee CY, Grigg ME. The SRS superfamily of Toxoplasma surface proteins. Int J Parasitol. 2004;34:285–296. doi: 10.1016/j.ijpara.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Kappe SH, Gardner MJ, Brown SM, Ross J, Matuschewski K, Ribeiro JM, Adams JH, Quackenbush J, Cho J, Carucci DJ, Hoffman SL, Nussenzweig V. Exploring the transcriptome of the malaria sporozoite stage. Proc Natl Acad Sci U S A. 2001;98:9895–9900. doi: 10.1073/pnas.171185198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapulu MC, Da DF, Miura K, Li Y, Blagborough AM, Churcher TS, Nikolaeva D, Williams AR, Goodman AL, Sangare I, Turner AV, Cottingham MG, Nicosia A, Straschil U, Tsuboi T, Gilbert SC, Long CA, Sinden RE, Draper SJ, Hill AV, Cohuet A, Biswas S. Comparative assessment of transmission-blocking vaccine candidates against Plasmodium falciparum. Sci Rep. 2015;5:11193. doi: 10.1038/srep11193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushansky A, Douglass AN, Arang N, Vigdorovich V, Dambrauskas N, Kain HS, Austin LS, Sather DN, Kappe SH. Malaria parasites target the hepatocyte receptor EphA2 for successful host infection. Science. 2015;350:1089–1092. doi: 10.1126/science.aad3318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy AT, Schmidt CQ, Thompson JK, Weiss GE, Taechalertpaisarn T, Gilson PR, Barlow PN, Crabb BS, Cowman AF, Tham WH. Recruitment of Factor H as a Novel Complement Evasion Strategy for Blood-Stage Plasmodium falciparum Infection. J Immunol. 2016;196:1239–1248. doi: 10.4049/jimmunol.1501581. [DOI] [PubMed] [Google Scholar]
- Khan SM, Franke-Fayard B, Mair GR, Lasonder E, Janse CJ, Mann M, Waters AP. Proteome analysis of separated male and female gametocytes reveals novel sex-specific Plasmodium biology. Cell. 2005;121:675–687. doi: 10.1016/j.cell.2005.03.027. [DOI] [PubMed] [Google Scholar]
- Koch M, Baum J. The mechanics of malaria parasite invasion of the human erythrocyte - towards a reassessment of the host cell contribution. Cell Microbiol. 2016;18:319–329. doi: 10.1111/cmi.12557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocken CH, Jansen J, Kaan AM, Beckers PJ, Ponnudurai T, Kaslow DC, Konings RN, Schoenmakers JG. Cloning and expression of the gene coding for the transmission blocking target antigen Pfs48/45 of Plasmodium falciparum. Mol Biochem Parasitol. 1993;61:59–68. doi: 10.1016/0166-6851(93)90158-t. [DOI] [PubMed] [Google Scholar]
- Kuehn A, Pradel G. The coming-out of malaria gametocytes. J Biomed Biotechnol. 2010;2010:976827. doi: 10.1155/2010/976827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar H, Sattler JM, Singer M, Heiss K, Reinig M, Hammerschmidt-Kamper C, Heussler V, Mueller AK, Frischknecht F. Protective efficacy and safety of liver stage attenuated malaria parasites. Sci Rep. 2016;6:26824. doi: 10.1038/srep26824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar N. Target antigens of malaria transmission blocking immunity exist as a stable membrane bound complex. Parasite Immunol. 1987;9:321–335. doi: 10.1111/j.1365-3024.1987.tb00511.x. [DOI] [PubMed] [Google Scholar]
- Kumar N, Wizel B. Further characterization of interactions between gamete surface antigens of Plasmodium falciparum. Mol Biochem Parasitol. 1992;53:113–120. doi: 10.1016/0166-6851(92)90013-a. [DOI] [PubMed] [Google Scholar]
- Labaied M, Harupa A, Dumpit RF, Coppens I, Mikolajczak SA, Kappe SH. Plasmodium yoelii sporozoites with simultaneous deletion of P52 and P36 are completely attenuated and confer sterile immunity against infection. Infect Immun. 2007;75:3758–3768. doi: 10.1128/IAI.00225-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasonder E, Green JL, Grainger M, Langsley G, Holder AA. Extensive differential protein phosphorylation as intraerythrocytic Plasmodium falciparum schizonts develop into extracellular invasive merozoites. Proteomics. 2015;15:2716–2729. doi: 10.1002/pmic.201400508. [DOI] [PubMed] [Google Scholar]
- Lasonder E, Ishihama Y, Andersen JS, Vermunt AM, Pain A, Sauerwein RW, Eling WM, Hall N, Waters AP, Stunnenberg HG, Mann M. Analysis of the Plasmodium falciparum proteome by high-accuracy mass spectrometry. Nature. 2002;419:537–542. doi: 10.1038/nature01111. [DOI] [PubMed] [Google Scholar]
- Lasonder E, Janse CJ, van Gemert GJ, Mair GR, Vermunt AM, Douradinha BG, van Noort V, Huynen MA, Luty AJ, Kroeze H, Khan SM, Sauerwein RW, Waters AP, Mann M, Stunnenberg HG. Proteomic profiling of Plasmodium sporozoite maturation identifies new proteins essential for parasite development and infectivity. PLoS Pathog. 2008;4:e1000195. doi: 10.1371/journal.ppat.1000195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Roch KG, Zhou Y, Blair PL, Grainger M, Moch JK, Haynes JD, De La Vega P, Holder AA, Batalov S, Carucci DJ, Winzeler EA. Discovery of gene function by expression profiling of the malaria parasite life cycle. Science. 2003;301:1503–1508. doi: 10.1126/science.1087025. [DOI] [PubMed] [Google Scholar]
- Lekutis C, Ferguson DJ, Grigg ME, Camps M, Boothroyd JC. Surface antigens of Toxoplasma gondii : variations on a theme. Int J Parasitol. 2001;31:1285–1292. doi: 10.1016/s0020-7519(01)00261-2. [DOI] [PubMed] [Google Scholar]
- Li J, Ito D, Chen JH, Lu F, Cheng Y, Wang B, Ha KS, Cao J, Torii M, Sattabongkot J, Tsuboi T, Han ET. Pv12, a 6-Cys antigen of Plasmodium vivax is localized to the merozoite rhoptry. Parasitol Int. 2012;61:443–449. doi: 10.1016/j.parint.2012.02.008. [DOI] [PubMed] [Google Scholar]
- Lin JW, Annoura T, Sajid M, Chevalley-Maurel S, Ramesar J, Klop O, Franke-Fayard BM, Janse CJ, Khan SM. A novel 'gene insertion/marker out' (GIMO) method for transgene expression and gene complementation in rodent malaria parasites. PLoS One. 2011;6:e29289. doi: 10.1371/journal.pone.0029289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindner SE, Swearingen KE, Harupa A, Vaughan AM, Sinnis P, Moritz RL, Kappe SH. Total and putative surface proteomics of malaria parasite salivary gland sporozoites. Mol Cell Proteo. 2013;12:1127–1143. doi: 10.1074/mcp.M112.024505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Tong Y, Pan J, Yang Y, Liu Q, Tan X, Zhao S, Qin L, Chen X. A redesigned CRISPR/Cas9 system for marker-free genome editing in Plasmodium falciparum. Parasit Vectors. 2016;9:198. doi: 10.1186/s13071-016-1487-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier AG, Cooke BM, Cowman AF, Tilley L. Malaria parasite proteins that remodel the host erythrocyte. Nat Rev Microbiol. 2009;7:341–354. doi: 10.1038/nrmicro2110. [DOI] [PubMed] [Google Scholar]
- Mamedov T, Ghosh A, Jones RM, Mett V, Farrance CE, Musiychuk K, Horsey A, Yusibov V. Production of non-glycosylated recombinant proteins in Nicotiana benthamiana plants by co-expressing bacterial PNGase. F. Plant Biotechnol J. 2012;10:773–782. doi: 10.1111/j.1467-7652.2012.00694.x. [DOI] [PubMed] [Google Scholar]
- Manske M, Miotto O, Campino S, Auburn S, Almagro-Garcia J, Maslen G, O'Brien J, Djimde A, Doumbo O, Zongo I, Ouedraogo JB, Michon P, Mueller I, Siba P, Nzila A, Borrmann S, Kiara SM, Marsh K, Jiang H, Su XZ, Amaratunga C, Fairhurst R, Socheat D, Nosten F, Imwong M, White NJ, Sanders M, Anastasi E, Alcock D, Drury E, Oyola S, Quail MA, Turner DJ, Ruano-Rubio V, Jyothi D, Amenga-Etego L, Hubbart C, Jeffreys A, Rowlands K, Sutherland C, Roper C, Mangano V, Modiano D, Tan JC, Ferdig MT, Amambua-Ngwa A, Conway DJ, Takala-Harrison S, Plowe CV, Rayner JC, Rockett KA, Clark TG, Newbold CI, Berriman M, MacInnis B, Kwiatkowski DP. Analysis of Plasmodium falciparum diversity in natural infections by deep sequencing. Nature. 2012;487:375–379. doi: 10.1038/nature11174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menard R. Gliding motility and cell invasion by Apicomplexa: insights from the Plasmodium sporozoite. Cell Microbiol. 2001;3:63–73. doi: 10.1046/j.1462-5822.2001.00097.x. [DOI] [PubMed] [Google Scholar]
- Merino KM, Bansal GP, Kumar N. Reduced immunogenicity of Plasmodium falciparum gamete surface antigen (Pfs48/45) in mice after disruption of disulphide bonds - evaluating effect of interferon-gamma-inducible lysosomal thiol reductase. Immunology. 2016;148:433–447. doi: 10.1111/imm.12621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikolajczak SA, Lakshmanan V, Fishbaugher M, Camargo N, Harupa A, Kaushansky A, Douglass AN, Baldwin M, Healer J, O'Neill M, Phuong T, Cowman A, Kappe SH. A next-generation genetically attenuated Plasmodium falciparum parasite created by triple gene deletion. Mol Ther. 2014;22:1707–1715. doi: 10.1038/mt.2014.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milek RL, DeVries AA, Roeffen WF, Stunnenberg H, Rottier PJ, Konings RN. Plasmodium falciparum: heterologous synthesis of the transmission-blocking vaccine candidate Pfs48/45 in recombinant vaccinia virus-infected cells. Exp Parasitol. 1998a;90:165–174. doi: 10.1006/expr.1998.4315. [DOI] [PubMed] [Google Scholar]
- Milek RL, Roeffen WF, Kocken CH, Jansen J, Kaan AM, Eling WM, Sauerwein RW, Konings RN. Immunological properties of recombinant proteins of the transmission blocking vaccine candidate, Pfs48/45, of the human malaria parasite Plasmodium falciparum produced in Escherichia coli. Parasite Immunol. 1998b;20:377–385. doi: 10.1046/j.1365-3024.1998.00171.x. [DOI] [PubMed] [Google Scholar]
- Milek RL, Stunnenberg HG, Konings RN. Assembly and expression of a synthetic gene encoding the antigen Pfs48/45 of the human malaria parasite Plasmodium falciparum in yeast. Vaccine. 2000;18:1402–1411. doi: 10.1016/s0264-410x(99)00392-8. [DOI] [PubMed] [Google Scholar]
- Molina-Cruz A, Barillas-Mury C. The remarkable journey of adaptation of the Plasmodium falciparum malaria parasite to New World anopheline mosquitoes. Mem Inst Oswaldo Cruz. 2014;109:662–667. doi: 10.1590/0074-0276130553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina-Cruz A, Canepa GE, Kamath N, Pavlovic NV, Mu J, Ramphul UN, Ramirez JL, Barillas-Mury C. Plasmodium evasion of mosquito immunity and global malaria transmission: The lock-and-key theory. Proc Natl Acad Sci U S A. 2015;112:15178–15183. doi: 10.1073/pnas.1520426112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina-Cruz A, Garver LS, Alabaster A, Bangiolo L, Haile A, Winikor J, Ortega C, van Schaijk BC, Sauerwein RW, Taylor-Salmon E, Barillas-Mury C. The human malaria parasite Pfs47 gene mediates evasion of the mosquito immune system. Science. 2013;340:984–987. doi: 10.1126/science.1235264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongui A, Angel DI, Guzman C, Vanegas M, Patarroyo MA. Characterisation of the Plasmodium vivax Pv38 antigen. Biochem Biophys Res Commun. 2008;376:326–330. doi: 10.1016/j.bbrc.2008.08.163. [DOI] [PubMed] [Google Scholar]
- Moreno-Perez DA, Areiza-Rojas R, Florez-Buitrago X, Silva Y, Patarroyo ME, Patarroyo MA. The GPI-anchored 6-Cys protein Pv12 is present in detergent-resistant microdomains of Plasmodium vivax blood stage schizonts. Protist. 2013;164:37–48. doi: 10.1016/j.protis.2012.03.001. [DOI] [PubMed] [Google Scholar]
- Niederwieser I, Felger I, Beck HP. Limited polymorphism in Plasmodium falciparum sexual-stage antigens. Am J Trop Med Hyg. 2001;64:9–11. doi: 10.4269/ajtmh.2001.64.9. [DOI] [PubMed] [Google Scholar]
- Obando-Martinez AZ, Curtidor H, Arevalo-Pinzon G, Vanegas M, Vizcaino C, Patarroyo MA, Patarroyo ME. Conserved high activity binding peptides are involved in adhesion of two detergent-resistant membrane-associated merozoite proteins to red blood cells during invasion. J Med Chem. 2010;53:3907–3918. doi: 10.1021/jm901474p. [DOI] [PubMed] [Google Scholar]
- Ockenhouse CF, Klotz FW, Tandon NN, Jamieson GA. Sequestrin, a CD36 recognition protein on Plasmodium falciparum malaria-infected erythrocytes identified by anti-idiotype antibodies. Proc Natl Acad Sci U S A. 1991;88:3175–3179. doi: 10.1073/pnas.88.8.3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orito Y, Ishino T, Iwanaga S, Kaneko I, Kato T, Menard R, Chinzei Y, Yuda M. Liver-specific protein 2: a Plasmodium protein exported to the hepatocyte cytoplasm and required for merozoite formation. Mol Microbiol. 2013;87:66–79. doi: 10.1111/mmi.12083. [DOI] [PubMed] [Google Scholar]
- Osier FH, Mackinnon MJ, Crosnier C, Fegan G, Kamuyu G, Wanaguru M, Ogada E, McDade B, Rayner JC, Wright GJ, Marsh K. New antigens for a multicomponent blood-stage malaria vaccine. Sci Transl Med. 2014;6:247ra102. doi: 10.1126/scitranslmed.3008705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouedraogo AL, Roeffen W, Luty AJ, de Vlas SJ, Nebie I, Ilboudo-Sanogo E, Cuzin-Ouattara N, Teleen K, Tiono AB, Sirima SB, Verhave JP, Bousema T, Sauerwein R. Naturally acquired immune responses to Plasmodium falciparum sexual stage antigens Pfs48/45 and Pfs230 in an area of seasonal transmission. Infect Immun. 2011;79:4957–4964. doi: 10.1128/IAI.05288-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Outchkourov N, Vermunt A, Jansen J, Kaan A, Roeffen W, Teelen K, Lasonder E, Braks A, van de Vegte-Bolmer M, Qiu LY, Sauerwein R, Stunnenberg HG. Epitope analysis of the malaria surface antigen pfs48/45 identifies a subdomain that elicits transmission blocking antibodies. J Biol Chem. 2007;282:17148–17156. doi: 10.1074/jbc.M700948200. [DOI] [PubMed] [Google Scholar]
- Outchkourov NS, Roeffen W, Kaan A, Jansen J, Luty A, Schuiffel D, van Gemert GJ, van de Vegte-Bolmer M, Sauerwein RW, Stunnenberg HG. Correctly folded Pfs48/45 protein of Plasmodium falciparum elicits malaria transmission-blocking immunity in mice. Proc Natl Acad Sci U S A. 2008;105:4301–4305. doi: 10.1073/pnas.0800459105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker ML, Peng F, Boulanger MJ. The Structure of Plasmodium falciparum Blood-Stage 6-Cys Protein Pf41 Reveals an Unexpected Intra-Domain Insertion Required for Pf12 Coordination. PLoS One. 2015;10:e0139407. doi: 10.1371/journal.pone.0139407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pease BN, Huttlin EL, Jedrychowski MP, Talevich E, Harmon J, Dillman T, Kannan N, Doerig C, Chakrabarti R, Gygi SP, Chakrabarti D. Global analysis of protein expression and phosphorylation of three stages of Plasmodium falciparum intraerythrocytic development. J Proteome Res. 2013;12:4028–4045. doi: 10.1021/pr400394g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plassmeyer ML, Reiter K, Shimp RL, Jr, Kotova S, Smith PD, Hurt DE, House B, Zou X, Zhang Y, Hickman M, Uchime O, Herrera R, Nguyen V, Glen J, Lebowitz J, Jin AJ, Miller LH, MacDonald NJ, Wu Y, Narum DL. Structure of the Plasmodium falciparum circumsporozoite protein, a leading malaria vaccine candidate. J Biol Chem. 2009;284:26951–26963. doi: 10.1074/jbc.M109.013706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploemen IH, Croes HJ, van Gemert GJ, Wijers-Rouw M, Hermsen CC, Sauerwein RW. Plasmodium berghei Deltap52&p36 parasites develop independent of a parasitophorous vacuole membrane in Huh-7 liver cells. PLoS One. 2012;7:e50772. doi: 10.1371/journal.pone.0050772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouw RB, Vredevoogd DW, Kuijpers TW, Wouters D. Of mice and men: The factor H protein family and complement regulation. Mol Immunol. 2015;67:12–20. doi: 10.1016/j.molimm.2015.03.011. [DOI] [PubMed] [Google Scholar]
- Pradel G. Proteins of the malaria parasite sexual stages: expression, function and potential for transmission blocking strategies. Parasitology. 2007;134:1911–1929. doi: 10.1017/S0031182007003381. [DOI] [PubMed] [Google Scholar]
- Pradel G, Hayton K, Aravind L, Iyer LM, Abrahamsen MS, Bonawitz A, Mejia C, Templeton TJ. A multidomain adhesion protein family expressed in Plasmodium falciparum is essential for transmission to the mosquito. J Exp Med. 2004;199:1533–1544. doi: 10.1084/jem.20031274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punta M, Coggill PC, Eberhardt RY, Mistry J, Tate J, Boursnell C, Pang N, Forslund K, Ceric G, Clements J, Heger A, Holm L, Sonnhammer EL, Eddy SR, Bateman A, Finn RD. The Pfam protein families database. Nucl Acids Res. 2012;40:D290–D301. doi: 10.1093/nar/gkr1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quakyi IA, Carter R, Rener J, Kumar N, Good MF, Miller LH. The 230-kDa gamete surface protein of Plasmodium falciparum is also a target for transmission-blocking antibodies. J Immunol. 1987;139:4213–4217. [PubMed] [Google Scholar]
- Radke JR, Gubbels MJ, Jerome ME, Radke JB, Striepen B, White MW. Identification of a sporozoite-specific member of the Toxoplasma SAG superfamily via genetic complementation. Mol Microbiol. 2004;52:93–105. doi: 10.1111/j.1365-2958.2003.03967.x. [DOI] [PubMed] [Google Scholar]
- Ramiro RS, Khan SM, Franke-Fayard B, Janse CJ, Obbard DJ, Reece SE. Hybridization and pre-zygotic reproductive barriers in Plasmodium. Proc Biol Sci. 2015;282:20143027. doi: 10.1098/rspb.2014.3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramphul UN, Garver LS, Molina-Cruz A, Canepa GE, Barillas-Mury C. Plasmodium falciparum evades mosquito immunity by disrupting JNK-mediated apoptosis of invaded midgut cells. Proc Natl Acad Sci U S A. 2015;112:1273–1280. doi: 10.1073/pnas.1423586112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read D, Lensen AH, Begarnie S, Haley S, Raza A, Carter R. Transmission-blocking antibodies against multiple, non-variant target epitopes of the Plasmodium falciparum gamete surface antigen Pfs230 are all complement-fixing. Parasite Immunol. 1994;16:511–519. doi: 10.1111/j.1365-3024.1994.tb00305.x. [DOI] [PubMed] [Google Scholar]
- Reeder JC, Wapling J, Mueller I, Siba PM, Barry AE. Population genetic analysis of the Plasmodium falciparum 6-cys protein Pf38 in Papua New Guinea reveals domain-specific balancing selection. Malaria J. 2011;10:126. doi: 10.1186/1475-2875-10-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rener J, Graves PM, Carter R, Williams JL, Burkot TR. Target antigens of transmission-blocking immunity on gametes of Plasmodium falciparum. J Exp Med. 1983;158:976–981. doi: 10.1084/jem.158.3.976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JS, Arumugam TU, Reiling L, Healer J, Hodder AN, Fowkes FJ, Cross N, Langer C, Takeo S, Uboldi AD, Thompson JK, Gilson PR, Coppel RL, Siba PM, King CL, Torii M, Chitnis CE, Narum DL, Mueller I, Crabb BS, Cowman AF, Tsuboi T, Beeson JG. Identification and prioritization of merozoite antigens as targets of protective human immunity to Plasmodium falciparum malaria for vaccine and biomarker development. J Immunol. 2013;191:795–809. doi: 10.4049/jimmunol.1300778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley EM, Williamson KC, Greenwood BM, Kaslow DC. Human immune recognition of recombinant proteins representing discrete domains of the Plasmodium falciparum gamete surface protein, Pfs230. Parasite Immunol. 1995;17:11–19. doi: 10.1111/j.1365-3024.1995.tb00961.x. [DOI] [PubMed] [Google Scholar]
- Roeffen W, Beckers PJ, Teelen K, Lensen T, Sauerwein RW, Meuwissen JH, Eling W. Plasmodium falciparum: a comparison of the activity of Pfs230-specific antibodies in an assay of transmission-blocking immunity and specific competition ELISAs. Exp Parasitol. 1995;80:15–26. doi: 10.1006/expr.1995.1003. [DOI] [PubMed] [Google Scholar]
- Roeffen W, Mulder B, Teelen K, Bolmer M, Eling W, Targett GA, Beckers PJ, Sauerwein R. Association between anti-Pfs48/45 reactivity and P. falciparum transmission-blocking activity in sera from Cameroon. Parasite Immunol. 1996;18:103–109. doi: 10.1046/j.1365-3024.1996.d01-54.x. [DOI] [PubMed] [Google Scholar]
- Rupp I, Sologub L, Williamson KC, Scheuermayer M, Reininger L, Doerig C, Eksi S, Kombila DU, Frank M, Pradel G. Malaria parasites form filamentous cell-to-cell connections during reproduction in the mosquito midgut. Cell Res. 2011;21:683–696. doi: 10.1038/cr.2010.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala KA, Nishiura H, Upton LM, Zakutansky SE, Delves MJ, Iyori M, Mizutani M, Sinden RE, Yoshida S, Blagborough AM. The Plasmodium berghei sexual stage antigen PSOP12 induces anti-malarial transmission blocking immunity both in vivo and in vitro. Vaccine. 2015;33:437–445. doi: 10.1016/j.vaccine.2014.11.038. [DOI] [PubMed] [Google Scholar]
- Sanders PR, Cantin GT, Greenbaum DC, Gilson PR, Nebl T, Moritz RL, Yates JR, 3rd, Hodder AN, Crabb BS. Identification of protein complexes in detergent-resistant membranes of Plasmodium falciparum schizonts. Mol Biochem Parasitol. 2007;154:148–157. doi: 10.1016/j.molbiopara.2007.04.013. [DOI] [PubMed] [Google Scholar]
- Sanders PR, Gilson PR, Cantin GT, Greenbaum DC, Nebl T, Carucci DJ, McConville MJ, Schofield L, Hodder AN, Yates JR, 3rd, Crabb BS. Distinct protein classes including novel merozoite surface antigens in Raft-like membranes of Plasmodium falciparum. J Biol Chem. 2005;280:40169–40176. doi: 10.1074/jbc.M509631200. [DOI] [PubMed] [Google Scholar]
- Schneider P, Reece SE, van Schaijk BC, Bousema T, Lanke KH, Meaden CS, Gadalla A, Ranford-Cartwright LC, Babiker HA. Quantification of female and male Plasmodium falciparum gametocytes by reverse transcriptase quantitative PCR. Mol Biochem Parasitol. 2015;199:29–33. doi: 10.1016/j.molbiopara.2015.03.006. [DOI] [PubMed] [Google Scholar]
- Scholz SM, Simon N, Lavazec C, Dude MA, Templeton TJ, Pradel G. PfCCp proteins of Plasmodium falciparum : gametocyte-specific expression and role in complement-mediated inhibition of exflagellation. Int J Parasitol. 2008;38:327–340. doi: 10.1016/j.ijpara.2007.08.009. [DOI] [PubMed] [Google Scholar]
- Silvestrini F, Lasonder E, Olivieri A, Camarda G, van Schaijk B, Sanchez M, Younis Younis S, Sauerwein R, Alano P. Protein export marks the early phase of gametocytogenesis of the human malaria parasite Plasmodium falciparum. Mol Cell Proteom. 2010;9:1437–1448. doi: 10.1074/mcp.M900479-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon N, Kuehn A, Williamson KC, Pradel G. Adhesion protein complexes of malaria gametocytes assemble following parasite transmission to the mosquito. Parasitol Int. 2016;65:27–30. doi: 10.1016/j.parint.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon N, Scholz SM, Moreira CK, Templeton TJ, Kuehn A, Dude MA, Pradel G. Sexual stage adhesion proteins form multi-protein complexes in the malaria parasite Plasmodium falciparum. J Biol Chem. 2009;284:14537–14546. doi: 10.1074/jbc.M808472200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SK, Roeffen W, Andersen G, Bousema T, Christiansen M, Sauerwein R, Theisen M. A Plasmodium falciparum 48/45 single epitope R0.6C subunit protein elicits high levels of transmission blocking antibodies. Vaccine. 2015;33:1981–1986. doi: 10.1016/j.vaccine.2015.02.040. [DOI] [PubMed] [Google Scholar]
- Skinner J, Huang CY, Waisberg M, Felgner PL, Doumbo OK, Ongoiba A, Kayentao K, Traore B, Crompton PD, Williamson KC. Plasmodium falciparum Gametocyte-Specific Antibody Profiling Reveals Boosting through Natural Infection and Identifies Potential Markers of Gametocyte Exposure. Infect Immun. 2015;83:4229–4236. doi: 10.1128/IAI.00644-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spring M, Murphy J, Nielsen R, Dowler M, Bennett JW, Zarling S, Williams J, de la Vega P, Ware L, Komisar J, Polhemus M, Richie TL, Epstein J, Tamminga C, Chuang I, Richie N, O'Neil M, Heppner DG, Healer J, O'Neill M, Smithers H, Finney OC, Mikolajczak SA, Wang R, Cowman A, Ockenhouse C, Krzych U, Kappe SH. First-in-human evaluation of genetically attenuated Plasmodium falciparum sporozoites administered by bite of Anopheles mosquitoes to adult volunteers. Vaccine. 2013;31:4975–4983. doi: 10.1016/j.vaccine.2013.08.007. [DOI] [PubMed] [Google Scholar]
- Stone WJ, Dantzler KW, Nilsson SK, Drakeley CJ, Marti M, Bousema T, Rijpma SR. Naturally acquired immunity to sexual stage P. falciparum parasites. Parasitology. 2016;143:187–198. doi: 10.1017/S0031182015001341. [DOI] [PubMed] [Google Scholar]
- Tachibana M, Suwanabun N, Kaneko O, Iriko H, Otsuki H, Sattabongkot J, Kaneko A, Herrera S, Torii M, Tsuboi T. Plasmodium vivax gametocyte proteins, Pvs48/45 and Pvs47, induce transmission-reducing antibodies by DNA immunization. Vaccine. 2015;33:1901–1908. doi: 10.1016/j.vaccine.2015.03.008. [DOI] [PubMed] [Google Scholar]
- Tachibana M, Wu Y, Iriko H, Muratova O, MacDonald NJ, Sattabongkot J, Takeo S, Otsuki H, Torii M, Tsuboi T. N-terminal prodomain of Pfs230 synthesized using a cell-free system is sufficient to induce complement-dependent malaria transmission-blocking activity. Clin Vacc Immunol. 2011;18:1343–1350. doi: 10.1128/CVI.05104-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taechalertpaisarn T, Crosnier C, Bartholdson SJ, Hodder AN, Thompson J, Bustamante LY, Wilson DW, Sanders PR, Wright GJ, Rayner JC, Cowman AF, Gilson PR, Crabb BS. Biochemical and functional analysis of two Plasmodium falciparum blood-stage 6-cys proteins: P12 and P41. PLoS One. 2012;7:e41937. doi: 10.1371/journal.pone.0041937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talman AM, Blagborough AM, Sinden RE. A Plasmodium falciparum strain expressing GFP throughout the parasite's life-cycle. PLoS One. 2010;5:e9156. doi: 10.1371/journal.pone.0009156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Targett G. Immunity to sexual stages of human malaria parasites: immune modulation during natural infections, antigenic determinants, and the induction of transmission-blocking immunity. Scand J Infect Dis Suppl. 1990;76:79–88. [PubMed] [Google Scholar]
- Tarun AS, Peng X, Dumpit RF, Ogata Y, Silva-Rivera H, Camargo N, Daly TM, Bergman LW, Kappe SH. A combined transcriptome and proteome survey of malaria parasite liver stages. Proc Natl Acad Sci U S A. 2008;105:305–310. doi: 10.1073/pnas.0710780104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Templeton TJ, Iyer LM, Anantharaman V, Enomoto S, Abrahante JE, Subramanian GM, Hoffman SL, Abrahamsen MS, Aravind L. Comparative analysis of apicomplexa and genomic diversity in eukaryotes. Genome Res. 2004;14:1686–1695. doi: 10.1101/gr.2615304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Templeton TJ, Kaslow DC. Identification of additional members define a Plasmodium falciparum gene superfamily which includes Pfs48/45 and Pfs230. Mol Biochem Parasitol. 1999;101:223–227. doi: 10.1016/s0166-6851(99)00066-3. [DOI] [PubMed] [Google Scholar]
- Tetteh KK, Stewart LB, Ochola LI, Amambua-Ngwa A, Thomas AW, Marsh K, Weedall GD, Conway DJ. Prospective identification of malaria parasite genes under balancing selection. PLoS One. 2009;4:e5568. doi: 10.1371/journal.pone.0005568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theisen M, Roeffen W, Singh SK, Andersen G, Amoah L, van de Vegte-Bolmer M, Arens T, Tiendrebeogo RW, Jones S, Bousema T, Adu B, Dziegiel MH, Christiansen M, Sauerwein R. A multi-stage malaria vaccine candidate targeting both transmission and asexual parasite life-cycle stages. Vaccine. 2014;32:2623–2630. doi: 10.1016/j.vaccine.2014.03.020. [DOI] [PubMed] [Google Scholar]
- Thompson J, Janse CJ, Waters AP. Comparative genomics in Plasmodium : a tool for the identification of genes and functional analysis. Mol Biochem Parasitol. 2001;118:147–154. doi: 10.1016/s0166-6851(01)00377-2. [DOI] [PubMed] [Google Scholar]
- Tomas AM, Margos G, Dimopoulos G, van Lin LH, de Koning-Ward TF, Sinha R, Lupetti P, Beetsma AL, Rodriguez MC, Karras M, Hager A, Mendoza J, Butcher GA, Kafatos F, Janse CJ, Waters AP, Sinden RE. P25 and P28 proteins of the malaria ookinete surface have multiple and partially redundant functions. EMBO J. 2001;20:3975–3983. doi: 10.1093/emboj/20.15.3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonkin ML, Arredondo SA, Loveless BC, Serpa JJ, Makepeace KA, Sundar N, Petrotchenko EV, Miller LH, Grigg ME, Boulanger MJ. Structural and biochemical characterization of Plasmodium falciparum 12 (Pf12) reveals a unique interdomain organization and the potential for an antiparallel arrangement with Pf41. J Biol Chem. 2013;288:12805–12817. doi: 10.1074/jbc.M113.455667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treeck M, Sanders JL, Elias JE, Boothroyd JC. The phosphoproteomes of Plasmodium falciparum and Toxoplasma gondii reveal unusual adaptations within and beyond the parasites' boundaries. Cell Host Microbe. 2011;10:410–419. doi: 10.1016/j.chom.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker RP. The thrombospondin type 1 repeat superfamily. Int J Biochem Cell Biol. 2004;36:969–974. doi: 10.1016/j.biocel.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Vallejo AF, Martinez NL, Tobon A, Alger J, Lacerda MV, Kajava AV, Arevalo-Herrera M, Herrera S. Global genetic diversity of the Plasmodium vivax transmission-blocking vaccine candidate Pvs48/45. Malaria J. 2016;15:202. doi: 10.1186/s12936-016-1263-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk MR, Douradinha B, Franke-Fayard B, Heussler V, van Dooren MW, van Schaijk B, van Gemert GJ, Sauerwein RW, Mota MM, Waters AP, Janse CJ. Genetically attenuated, P36p-deficient malarial sporozoites induce protective immunity and apoptosis of infected liver cells. Proc Natl Acad Sci U S A. 2005;102:12194–12199. doi: 10.1073/pnas.0500925102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk MR, Janse CJ, Thompson J, Waters AP, Braks JA, Dodemont HJ, Stunnenberg HG, van Gemert GJ, Sauerwein RW, Eling W. A central role for P48/45 in malaria parasite male gamete fertility. Cell. 2001;104:153–164. doi: 10.1016/s0092-8674(01)00199-4. [DOI] [PubMed] [Google Scholar]
- van Dijk MR, van Schaijk BC, Khan SM, van Dooren MW, Ramesar J, Kaczanowski S, van Gemert GJ, Kroeze H, Stunnenberg HG, Eling WM, Sauerwein RW, Waters AP, Janse CJ. Three members of the 6-cys protein family of Plasmodium play a role in gamete fertility. PLoS pPathog. 2010;6:e1000853. doi: 10.1371/journal.ppat.1000853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Schaijk BC, Janse CJ, van Gemert GJ, van Dijk MR, Gego A, Franetich JF, van de Vegte-Bolmer M, Yalaoui S, Silvie O, Hoffman SL, Waters AP, Mazier D, Sauerwein RW, Khan SM. Gene disruption of Plasmodium falciparum p52 results in attenuation of malaria liver stage development in cultured primary human hepatocytes. PLoS One. 2008;3:e3549. doi: 10.1371/journal.pone.0003549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Schaijk BC, Ploemen IH, Annoura T, Vos MW, Foquet L, van Gemert GJ, Chevalley-Maurel S, van de Vegte-Bolmer M, Sajid M, Franetich JF, Lorthiois A, Leroux-Roels G, Meuleman P, Hermsen CC, Mazier D, Hoffman SL, Janse CJ, Khan SM, Sauerwein RW. A genetically attenuated malaria vaccine candidate based on P. falciparum b9/slarp gene-deficient sporozoites. Elife. 2014;3 doi: 10.7554/eLife.03582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Schaijk BC, van Dijk MR, van de Vegte-Bolmer M, van Gemert GJ, van Dooren MW, Eksi S, Roeffen WF, Janse CJ, Waters AP, Sauerwein RW. Pfs47, paralog of the male fertility factor Pfs48/45, is a female specific surface protein in Plasmodium falciparum. Mol Biochem Parasitol. 2006;149:216–222. doi: 10.1016/j.molbiopara.2006.05.015. [DOI] [PubMed] [Google Scholar]
- VanBuskirk KM, O'Neill MT, De La Vega P, Maier AG, Krzych U, Williams J, Dowler MG, Sacci JB, Jr, Kangwanrangsan N, Tsuboi T, Kneteman NM, Heppner DG, Jr, Murdock BA, Mikolajczak SA, Aly AS, Cowman AF, Kappe SH. Preerythrocytic, live-attenuated Plasmodium falciparum vaccine candidates by design. Proc Natl Acad Sci U S A. 2009;106:13004–13009. doi: 10.1073/pnas.0906387106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan AM, Aly AS, Kappe SH. Malaria parasite pre-erythrocytic stage infection: gliding and hiding. Cell Host Microbe. 2008;4:209–218. doi: 10.1016/j.chom.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan AM, Mikolajczak SA, Camargo N, Lakshmanan V, Kennedy M, Lindner SE, Miller JL, Hume JC, Kappe SH. A transgenic Plasmodium falciparum NF54 strain that expresses GFP-luciferase throughout the parasite life cycle. Mol Biochem Parasitol. 2012;186:143–147. doi: 10.1016/j.molbiopara.2012.10.004. [DOI] [PubMed] [Google Scholar]
- Vermeulen AN, Ponnudurai T, Beckers PJ, Verhave JP, Smits MA, Meuwissen JH. Sequential expression of antigens on sexual stages of Plasmodium falciparum accessible to transmission-blocking antibodies in the mosquito. J Exp Med. 1985a;162:1460–1476. doi: 10.1084/jem.162.5.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeulen AN, Roeffen WF, Henderik JB, Ponnudurai T, Beckers PJ, Meuwissen JH. Plasmodium falciparum transmission blocking monoclonal antibodies recognize monovalently expressed epitopes. Dev Biol Standard. 1985b;62:91–97. [PubMed] [Google Scholar]
- Vincent AA, Fanning S, Caira FC, Williamson KC. Immunogenicity of malaria transmission-blocking vaccine candidate, y230.CA14 following crosslinking in the presence of tetanus toxoid. Parasite Immunol. 1999;21:573–581. doi: 10.1046/j.1365-3024.1999.00255.x. [DOI] [PubMed] [Google Scholar]
- von Bohl A, Kuehn A, Simon N, Ngongang VN, Spehr M, Baumeister S, Przyborski JM, Fischer R, Pradel G. A WD40-repeat protein unique to malaria parasites associates with adhesion protein complexes and is crucial for blood stage progeny. Malaria J. 2015;14:435. doi: 10.1186/s12936-015-0967-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Ma A, Chen SB, Yang YC, Chen JH, Yin MB. Genetic diversity and natural selection of three blood-stage 6-Cys proteins in Plasmodium vivax populations from the China-Myanmar endemic border. Infect Genet Evol. 2014;28:167–174. doi: 10.1016/j.meegid.2014.09.026. [DOI] [PubMed] [Google Scholar]
- Wasmuth JD, Pszenny V, Haile S, Jansen EM, Gast AT, Sher A, Boyle JP, Boulanger MJ, Parkinson J, Grigg ME. Integrated bioinformatic and targeted deletion analyses of the SRS gene superfamily identify SRS29C as a negative regulator of Toxoplasma virulence. MBio. 2012;3 doi: 10.1128/mBio.00321-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson KC. Pfs230: from malaria transmission-blocking vaccine candidate toward function. Parasite Immunol. 2003;25:351–359. doi: 10.1046/j.1365-3024.2003.00643.x. [DOI] [PubMed] [Google Scholar]
- Williamson KC, Criscio MD, Kaslow DC. Cloning and expression of the gene for Plasmodium falciparum transmission-blocking target antigen, Pfs230. Mol Biochem Parasitol. 1993;58:355–358. doi: 10.1016/0166-6851(93)90058-6. [DOI] [PubMed] [Google Scholar]
- Williamson KC, Fujioka H, Aikawa M, Kaslow DC. Stage-specific processing of Pfs230, a Plasmodium falciparum transmission-blocking vaccine candidate. Mol Biochem Parasitol. 1996;78:161–169. doi: 10.1016/s0166-6851(96)02621-7. [DOI] [PubMed] [Google Scholar]
- Williamson KC, Kaslow DC. Strain polymorphism of Plasmodium falciparum transmissionblocking target antigen Pfs230. Mol Biochem Parasitol. 1993;62:125–127. doi: 10.1016/0166-6851(93)90186-2. [DOI] [PubMed] [Google Scholar]
- Williamson KC, Keister DB, Muratova O, Kaslow DC. Recombinant Pfs230, a Plasmodium falciparum gametocyte protein, induces antisera that reduce the infectivity of Plasmodium falciparum to mosquitoes. Mol Biochem Parasitol. 1995;75:33–42. doi: 10.1016/0166-6851(95)02507-3. [DOI] [PubMed] [Google Scholar]
- Woo MK, Kim KA, Kim J, Oh JS, Han ET, An SS, Lim CS. Sequence polymorphisms in Pvs48/45 and Pvs47 gametocyte and gamete surface proteins in Plasmodium vivax isolated in Korea. Mem Inst Oswaldo Cruz. 2013;108 doi: 10.1590/S0074-02762013000300015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. World malaria report 2015. Geneva, Switzerland: World Health Organization; 2015. [Google Scholar]