Abstract
Viridibacillus arenosi strain IHB B 7171 identified based on 16S rRNA gene sequence produced colony forming units (cfu/ml) ranging from 3.3 × 104 to 1.2 × 1010 under pH 5–11, 2.2 × 102 to 1.4 × 1010 for temperature 5–40 °C, 2.4 × 102 to 1.1 × 1010 for PEG 6000 10–30%, 2.2 × 102 to 1.4 × 1010 for 2.5–10% NaCl, 3.1 × 103 to 1.7 × 109 for 2.5–7.5 mM CaCl2, 2.2 × 102 to 1.4 × 107 for 2.5–7.5 mM AlCl3, and 3.2 × 102 to 1.2 × 107 for 2.5–7.5 mM FeCl3. The activities of plant growth-promoting attributes with the increasing acidity, desiccation and salinity ranged from 408 to 101, 20 to 8, 14 to 5 µg/ml P-liberated from tri-calcium phosphate, aluminium phosphate and iron phosphate, 20–9% siderophore units, 14–4 µg/ml IAA and 190–16 α-ketobutyrate h/mg protein ACC-deaminase activity. Plant height, leaf number, and leaf weight on treatment with bacterial inoculum showed an increment of 9.5, 17.6, 54.5 and 31.0% in tea seedlings, respectively. The bacterium also enhanced plant height and yield by 10 and 13% in pea and 2.8 and 13.9% in wheat. The results exhibited stress-tolerance and plant growth-promoting activities by the strain under stressed growth-conditions with potential as a broad-spectrum plant growth-promoting rhizobacterium.
Electronic supplementary material
The online version of this article (doi:10.1007/s12088-017-0642-8) contains supplementary material, which is available to authorized users.
Keywords: Viridibacillus arenosi, Abiotic stress-tolerance, PGPR activities, Plant growth promotion, Broad-spectrum PGPR
Introduction
Exploring plant growth-promoting rhizobacteria (PGPR) as a useful biological tool has gained an immense interest for improving growth and productivity of plants [1, 2]. Their metabolic versatility manipulates the rhizosphere for plant growth promotion mainly by liberation of bound P into utilizable forms by plants, secretion of auxins involved directly in plant growth and development, production of siderophores which facilitate iron-uptake by plants, and synthesis of ACC (1-aminocyclopropane-1-carboxylase) deaminase responsible for lowering the levels of plant stress-hormone ethylene [3, 4]. However, the outcome of PGPR application is impacted by environmental conditions and soil properties. The stress-tolerance against acidity/alkalinity, temperature, desiccation, salinity, and metal ions has been tested only for a few PGPR to ensure their field performance as the microbial inoculants [5, 6]. This paper reports the efficacy of Viridibacillus arenosi strain IHB B 7171 selected for abiotic stress-tolerace and multiple plant growth-promoting attributes as a broad-spectrum microbial inoculant.
Materials and Methods
Isolation and Characterization
The isolate IHB B 7171 purified on tryptone soya agar (TSA) from the several hundred bacterial colonies obtained by spread plating the serial soil dilutions of tea rhizosphere soils collected from a depth of 15–30 cm from Zen Tea Estate Gopalpur (latitude 32°11′30″N and longitude 76°20′54″E) in the Kangra valley located in the Western Himalayas. The isolate was identified by amplifying 16S rRNA gene using the standard methods.
Stress Tolerance and PGPR Activities
The effect of stress parameters on culture growth was studied in TSB: temperature—5, 10, 15, 30, 35 and 40 °C at pH 7; acidity/alkalinity—pH 4, 5, 6, 7, 8, 9, 10, 11 and 12 at 28 °C; desiccation—10, 20 and 30% PEG 6000 at pH 7 under 28 °C; salinity—2.5, 5.0, 7.5 and 10% NaCl at pH 7 under 28 °C; and salt tolerance—2.5, 5.0, 7.5, and 10.0 mM CaCl2, FeCl3 or AlCl3 at pH 7 under 28 °C. The growth was measured by determining cfu/ml by plating the serial dilutions after 24 h incubation under various conditions, excepting the cultures subjected to 5 °C were incubated up to 72 h. All cfu values represent 1 ml of the culture.
The plant growth-promoting activities were measured using Barton’s reagent for P-liberated in Pikovskaya’s broth (PVK) supplemented with 0.5% TCP, Al–P or Fe–P [7], CAS reagent for siderophore production in succinate broth [8], Salkowski reagent for IAA (indole-3-acetic acid)-like auxins in nutrient broth (NB) supplemented with 0.1% tryptophan [9], and 2,4-dinitrophenylhydrazine reagent for ACC-deaminase activity in NB supplemented with ACC [10]. Organic acids produced during phosphate solubilization were quantified using Lichrosphere RP-18 column (Merck, Germany) on Waters 996 High Performance Liquid Chromatography system (HPLC) equipped with PDA detector and Waters 717 Plus Autosampler. The mobile phase consisted of 0.1% ortho-phosphoric acid (Merck, Germany) with gradient flow of 0.4 ml min−1 for 0–8 min, 0.5 ml min−1 for 8–4 min and 1.2 ml min−1 for 14–25 min [7].
Plant Growth Promotion Under Controlled Environment and Field Conditions
The carrier-based inoculum on activated charcoal at approximately 108 cfu/g was applied to the surface-sterilized uniformly pre-germinated seeds of maize (Zea mays var. Girija) sown in the pots kept in a Randomized Block Design (RBD) under temperature 25 ± 2 °C, R.H. 65–70% and photoperiod 16 h in Controlled Environment Chamber. Data on growth parameters were recorded after 30 days of treatment. Likewise, the seeds collected from bushes of chinary tea (Camellia sinensis var. HPKV1) were germinated, selected for uniform radicle growth, and treated with the inoculum. The inoculum treated seeds along with the seeds treated with the carrier-base without inoculum to serve as the control were grown in polysleeves in RBD in the Tea Plantation Nursery, State Agriculture Department of Himachal Pradesh at Diffarpatt. Data on growth parameters were recorded after 1 year of inoculum application.
The field evaluations were done in the Experimental Farms of Krishi Vigyan Kendra, CSKHPKV at Bajaura in RBD in the plots measuring 4.08 m2 with 90 plants each for pea (Pisum sativum var. Punjab 89) and 9.9 m2 with 900 plants each for wheat (Triticum aestivum var. HPW-155). The inter-row and intra-plant distances were 45 and 10 cm for pea and 22 and 5 cm for wheat, respectively. Data were recorded for growth parameters for 10 plants randomly taken from each plot and for yield for all the plants plot-wise.
Statistical Analysis
The STATISTICA data analysis software system version 7 (StatSoft Inc., Tulsa, OK, USA 2004) was used for the analysis of variance (ANOVA).
Results and Discussion
Isolation and Characterization
The isolate was identified as V. arenosi strain IHB B 7171 based on 99.5% identity of 16S rRNA gene sequence with V. arenosi LMG 22166 reported from a soil sample from the Netherlands [11]. The phylogenetic analysis revealed close relatedness with V. arenosi LMG 22166 and V. arvi LLP-44 (ESM_1).
Stress Tolerance and PGPR Activities
Viridibacillus arenosi IHB B 7171 exhibited tolerance to various abiotic stresses as evidenced by the culture growth under stressful regimes of acidity/alkalinity, temperature, desiccation, salinity, and Ca, Al and Fe salts (Fig. 1). Growth over the wide pH range of 5–11 suggested its suitability for application under both acidic and alkaline soils [12–14]. High cfu values of 1.8 × 104 to 1.4 × 1010 of the cultures under 10–40 °C further indicated its tolerance to diurnal and seasonal temperature regimes through the different cropping seasons [15]. Endurance to desiccation revealed by the high cfu of 2.4 × 104 to 1.1 × 1010 under 10–30% PEG 6000 was comparable to the high desiccation tolerance reported for Bacillus pumilus, B. firmus and Pseudomonas poae strains [13, 16]. The culture growth producing cfu of 1.4 × 1010 under 2.5% NaCl and 2.3 × 105 under 5% NaCl suggested its tolerance to the saline growth conditions prevailing in the alkaline soils. Likewise, the culture growth of 3.1 × 103 to 1.7 × 109, 2.2 × 102 to 1.4 × 107 and 3.2 × 102 to 1.2 × 107 cfu/ml under the increasing concentrations of 2.5–7.5 mM CaCl2, AlCl3 and FeCl3, respectively, revealed tolerance of the strain to high concentrations reported for Ca in the alkaline soils and Al and Fe in the acidic soils [17, 18].
Fig. 1.
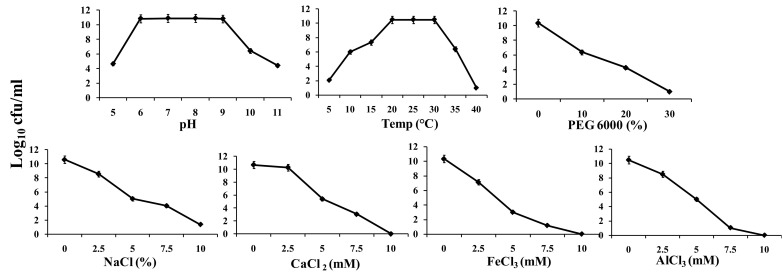
Growth of Viridibacillus arenosi strain IHB B 7171 cultures under different levels of pH, temperature, PEG 6000, NaCl (%), CaCl2, FeCl3 and AlCl3 (mM). Values are the mean of three replicates, error bars indicate standard deviation
Viridibacillus arenosi IHB B 7171 also exhibited the multiple plant growth-promoting functions of phosphate solubilization, siderophore production, production of IAA-like auxins, and ACC-deaminase activity similar to the efficient PGPR strains of Acinetobacter rhizosphaerae, Arthrobacter sp., Bacillus sp., Burkholderia phytofirmans, P. putida, Pseudomonas sp. and Serratia liquefaciens (Fig. 2) [19–21]. The solubilization of different inorganic phosphates indicated its suitability for application in the acidic soils rich in Al and Fe-bound P and the alkaline soils rich in Ca-bound P [20, 21]. In particular, the solubilization of Al–P and Fe–P shown has been reported among the rhizobacteria only for Arthrobacter sp., Bacillus sp., Microccocus sp. and P. fluorescens [22–24]. Under the culture growth at pH 5 which prevails in the acidic soils of tea plantations, the PGPR activities recorded P-liberation of 101.2, 21.2 and 14.2 µg/ml from TCP, Al–P and Fe–P solubilization, respectively, 9% siderophore units, 4.2 µg/ml IAA-like auxins and 16 nM α-ketobutyrate h/mg protein ACC-deaminase activity. Likewise, the culture growth at pH 8 which occurs widely in the cultivable alkaline soils showed PGPR activities of 105, 20 and 14 µg/ml P-liberation from TCP, Al–P and Fe–P solubilization, respectively, 16% siderophore units, 11 µg/ml IAA-like auxins and 595 nM α-ketobutyrate h/mg protein ACC-deaminase activity (14). The test strain also exhibited the PGPR activities to varying extent under the stressed growth conditions (Fig. 2), recording 109 µg/ml TCP solubilization, 20% siderophore units, 5 µg/ml IAA-like auxins and 190 nM α-ketobutyrate h/mg protein ACC-deaminase activity under the salinity of 2.5% NaCl, and 408 µg/ml P-liberation from TCP and 20% siderophore units under the desiccation regime of 10% PEG 6000. Only TCP solubilization has been reported under the stressed culture conditions of acidity and alkalinity for Arthrobacter sp. and Bacillus sp., acidity, alkalinity and temperature for Acenitobacter sp., and acidity and salinity for Pseudomonas spp. [21, 22].
Fig. 2.
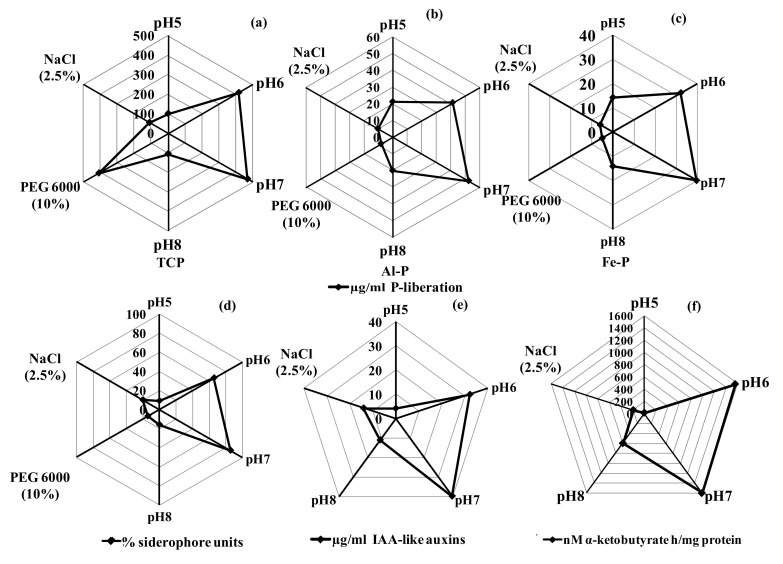
Radar pattern illustrating the PGPR activities of Viridibacillus arenosi strain IHB B 7171 under pH 5–8, PEG 6000 10% and 2.5% NaCl. TCP, Al–P and Fe–P solubilization (a–c), siderophore production (d), IAA-like auxins production (e), and ACC-deaminase activity (f). Values are the mean of three replicates. Scale-bars represent activity levels
Gluconic acid and oxalic acids commonly detected during the solubilization of these phosphate substrates by V. arenosi strain IHB B 7171 strain have been reported among the major organic acids produced during the microbial solubilization of phosphate substrates (Table 1). Detection of lactic and succinic acids only during TCP solubilization corroborated the influence of phosphate substrates on the nature of organic acids produced by microorganisms [25, 26]. However, only quantitative decrease was marked in the production of various organic acids by the test strain during solubilization of TCP, Al–P and Fe–P under the stressed growth conditions. The production of organic acids accompanied by the pH drop of cultures corroborated the involvement of organic acids in phosphate solubilization [7, 25, 26].
Table 1.
Production of organic acids by Viridibacillus arenosi strain IHB B 7171 during TCP, Al–P and Fe–P solubilization under different stress parameters
| Parameter | Organic acids production (µg/ml) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TCP | Al–P | Fe–P | ||||||||
| Final pH | GA | LA | OA | SA | GA | OA | GA | OA | ||
| pH | 5 | 3.9 | 217.8 | 629.3 | 117.8 | 434.3 | 16.2 | 125.3 | 24.8 | 14.8 |
| 6 | 4.1 | 1022.1 | 952.4 | 122.1 | 46.4 | 22.4 | 806.5 | 24.7 | 24.7 | |
| 7 | 3.9 | 1125.2 | 722.1 | 125.2 | 172.1 | 55.7 | 822.1 | 34.4 | 24.2 | |
| PEG 6000 (%) | 10 | 4.2 | 16.2 | 345.3 | 342.4 | ND | 16.8 | 111.3 | ND | ND |
| NaCl (%) | 2.5 | 4.1 | 211.9 | 132.3 | 56.6 | ND | ND | ND | ND | ND |
| CaCl2 (mM) | 2.5 | 4.0 | 121.9 | 154.3 | 111.3 | ND | ND | ND | ND | ND |
| AlCl3 (mM) | 2.5 | 3.8 | 168.8 | 111.7 | 14.9 | ND | ND | ND | ND | ND |
| FeCl3 (mM) | 2.5 | 3.9 | 145.9 | 15.4 | 29.4 | ND | ND | ND | ND | ND |
Values are the mean of three replicates and significantly different from the control at p < 0.05
ND not detected, TCP tricalcium phosphate, Al–P aluminium phosphate, Fe–P iron phosphate. OA oxalic acid, GA gluconic acid, SA succinic acid, LA lactic acid
Plant Growth Promotion Under Controlled Environment and Field Conditions
A significant improvement in plant growth with an increment of 9, 49.3, 30.7 and 93.3% of plant height, shoot dry weight, root length, and root dry weight in maize employed as the quick screen demonstrated plant growth-promotion potential of the strain (Table 2). Likewise, a significant improvement in various growth parameters in tea seedlings exhibited the usefulness of the strain for promoting growth in tea [27, 28]. This is the first stress-tolerant PGPR with multiple plant growth-promoting traits with the ability to solubilize Al and Fe bound P for utilization by tea plants for their growth limited by the binding of applied P with Al and Fe cations in the acidic soils required for tea cultivation [28]. A significant increment in plant height, fresh and dry weight and yield to the extent of 10, 16.9, 20.7 and 13% in pea and 2.8, 25, 27 and 13.9% in wheat demonstrated the broad-spectrum growth-promotion by the test strain considered important for undertaking bulk production at commercial-scale (Table 2).
Table 2.
Plant growth promotion by Viridibacillus arenosi strain IHB B 7171 in maize and tea under controlled conditions and pea and wheat in field conditions
| Growth parameters | Control | Inoculated | Increase over control (%) |
|---|---|---|---|
| Maize seedlings (controlled environment)a | |||
| Plant height (cm) | 55 | 60 | 9.0 |
| Shoot dry weight (g) | 2.59 | 3.86 | 49.3 |
| Root length (cm) | 13 | 17 | 30.7 |
| Root dry weight (g) | 0.15 | 0.29 | 93.3 |
| Tea (nursery conditions)b | |||
| Plant height (cm) | 21 | 23 | 9.5 |
| Leaves (No.) | 17 | 20 | 17.6 |
| leaf fresh weight (g) | 11 | 17 | 54.5 |
| leaf dry weight (g) | 0.29 | 0.38 | 31.0 |
| Pea (field conditions)c | |||
| Plant height (cm) | 40 | 44.3 | 10.7 |
| Yield (q/ha) | 46 | 52 | 13.0 |
| Fresh weight (q/ha) | 12.4 | 14.5 | 16.9 |
| Dry weight (q/ha) | 8.2 | 9.9 | 20.7 |
| Wheat (field conditions)d | |||
| Plant height (cm) | 91 | 93.6 | 2.8 |
| Yield (q/ha) | 25.8 | 29.4 | 13.9 |
| Fresh weight (q/ha) | 56.18 | 70.01 | 25 |
| Dry weight (q/ha) | 11.0 | 14.07 | 27 |
Values are the mean of three replicates and significantly different from the control at p < 0.05
aTemperature 25 ± 2 °C, R.H. 65–70% and photoperiod 16 h
bAverage minimum and maximum temperatures 15–34 °C and R.H. 45–75%, and overall rainfall 290–1250 mm
cAverage minimum and maximum temperatures 1–27 °C and R.H. 35–94%, and overall rainfall 9.5–153.8 mm
dAverage minimum and maximum temperatures 1–32 °C and R.H. 35–95%, and overall rainfall 95–290 mm
The stress-tolerant V. arenosi strain IHB B 7171 with multiple plant growth-promoting activities and broad-spectrum plant growth promotion is a prospective microbial inoculant for improving productivity in tea plantations and agricultural crops under stressed farming systems.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Authors acknowledge the Director of the CSIR-IHBT for the laboratory facilities and the Vice-Chancellor of CSKHPKV for his permission for conducting the field trials at the Krishi Vigyan Kendra, Bajaura, Himachal Pradesh. The Technical Officer Tea is also acknowledged for the support for conducting the experiments at the Tea Plantation Nursery at Diffarpatt, State Agriculture Department, Himachal Pradesh. This work was supported by the funding from the CSIR Network Project “Plant–Microbe-Soil Interactions” (BSC0117).
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s12088-017-0642-8) contains supplementary material, which is available to authorized users.
References
- 1.Vessey JK. Plant growth promoting rhizobacteria as biofertilizers. Plant Soil. 2003;255:571–586. doi: 10.1023/A:1026037216893. [DOI] [Google Scholar]
- 2.Glick BR, Chen Z, Czarny J, Duan J. Promotion of plant growth by ACC deaminase-producing soil bacteria. Eur J Plant Pathol. 2007;119:329–339. doi: 10.1007/s10658-007-9162-4. [DOI] [Google Scholar]
- 3.Gulati A, Rahi P, Vyas P. Characterization of phosphate-solubilizing fluorescent pseudomonads from the rhizosphere of seabuckthorn growing in the cold deserts of Himalayas. Curr Microbiol. 2008;56:73–79. doi: 10.1007/s00284-007-9042-3. [DOI] [PubMed] [Google Scholar]
- 4.Gulati A, Vyas P, Rahi P, Kasana RC. Plant growth-promoting and rhizosphere-competent Acinetobacter rhizosphaerae strain BIHB 723 from the cold deserts of the Himalayas. Curr Microbiol. 2008;58:371–377. doi: 10.1007/s00284-008-9339-x. [DOI] [PubMed] [Google Scholar]
- 5.Dimpka C, Weinand T, Asch F. Plant-rhizobacteria interactions alleviate abiotic stress conditions. Plant Cell Environ. 2009;32:1682–1694. doi: 10.1111/j.1365-3040.2009.02028.x. [DOI] [PubMed] [Google Scholar]
- 6.Grover M, Ali SK, Sandhya V, Rasul A, Venkateswarlu B. Role of microorganisms in adaptation of agriculture crops to abiotic stresses. World J Microbiol Biotechnol. 2015;27:1231–1240. doi: 10.1007/s11274-010-0572-7. [DOI] [Google Scholar]
- 7.Vyas P, Gulati A. Organic acid production in vitro and plant growth promotion in maize under controlled environment by phosphate-solubilizing fluorescent Pseudomonas. BMC Microbiol. 2009;9:174. doi: 10.1186/1471-2180-9-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alexander DB, Zuberer DA. Use of chrome azurol S reagents to evaluate siderophore production by rhizosphere bacteria. Biol Fertil Soils. 1991;12:39–45. doi: 10.1007/BF00369386. [DOI] [Google Scholar]
- 9.Glickmann E, Dessaux Y. A critical examination of the specificity of the salkowski reagent for indolic compounds produced by phytopathogenic bacteria. Appl Environ Microbiol. 1995;61:793–796. doi: 10.1128/aem.61.2.793-796.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacobson BC, Pasternak JJ, Glick BR. Partial purification and characterization of 1-aminocyclopropane-1-carboxylate deaminase from the plant growth-promoting rhizobacterium Pseudomonas putida GR 12-2. Can J Microbiol. 1994;40:1019–1025. doi: 10.1139/m94-162. [DOI] [Google Scholar]
- 11.Albert RA, Archambault J, Lempa M, Hurst B, Richardson C, Gruenloh S, Duran M, Worliczek HL, Huber BE, Rossello-Mora R, Schumann P, Busse HJ. Proposal of Viridibacillus gen. nov. and reclassification of Bacillus arvi, Bacillus arenosi and Bacillus neidei as Viridibacillus arvi gen. nov., comb. nov., Viridibacillus arenosi comb. nov. and Viridibacillus neidei comb. nov. Int J Syst Evol Microbiol. 2007;57:2729–2737. doi: 10.1099/ijs.0.65256-0. [DOI] [PubMed] [Google Scholar]
- 12.Chaiharn M, Lumyong S. Phosphate solubilization potential and stress tolerance of rhizobacteria from rice soil in Northern Thailand. World J Microbiol Biotechnol. 2009;25:305–314. doi: 10.1007/s11274-008-9892-2. [DOI] [Google Scholar]
- 13.Vyas P, Rahi P, Gulati A. Stress tolerance and genetic variability of phosphate-solubilizing fluorescent Pseudomonas from the cold deserts of the trans-Himalayas. Microb Ecol. 2009;58:425–443. doi: 10.1007/s00248-009-9511-2. [DOI] [PubMed] [Google Scholar]
- 14.Karagöz K, Ates F, Karagöz H, Kotan R, Çakmakç R. Characterization of plant growth-promoting traits of bacteria isolated from the rhizosphere of grapevine grown in alkaline and acidic soils. Eur J Soil Biol. 2012;50:144–150. doi: 10.1016/j.ejsobi.2012.01.007. [DOI] [Google Scholar]
- 15.Nautiyal CS, Bhadauria S, Kumar P, Lal H, Mondal R, Verma D. Stress induced phosphate solubilization in bacteria isolated from alkaline soils. FEMS Microbiol Lett. 2000;182:291–296. doi: 10.1111/j.1574-6968.2000.tb08910.x. [DOI] [PubMed] [Google Scholar]
- 16.Ngumbi E, Kloepper J. Bacterial-mediated drought tolerance: current and future prospects. Appl Soil Ecol. 2016;105:109–125. doi: 10.1016/j.apsoil.2016.04.009. [DOI] [Google Scholar]
- 17.Fung KF, Carr HP, Zhang J, Wong MH. Growth and nutrient uptake of tea under different aluminium concentrations. J Sci Food Agric. 2008;88:1582–1591. doi: 10.1002/jsfa.3254. [DOI] [Google Scholar]
- 18.Dutta J, Handique JP, Thakur D. Assessment of culturable tea rhizobacteria isolated from tea estates of Assam, India for growth promotion in commercial tea cultivars. Front Microbiol. 2015;6:1252. doi: 10.3389/fmicb.2015.01252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ashraf M, Berge SH, Mahmood OT. Inoculating wheat seedling with exopolysaccharide-producing bacteria restricts sodium uptake and stimulates plant growth under salt stress. Biol Fertil Soils. 2004;40:157–162. [Google Scholar]
- 20.Barka AE, Nowak J, Clement C. Enhancement of chilling resistance of inoculated grapevine plantlets with a plant growth promoting rhizobacterium, Burkholderia phytofirmans strain PsJN. Appl Environ Microbiol. 2006;72:7246–7252. doi: 10.1128/AEM.01047-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang WS, van de Mortel M, Nielsen L, de Guzman GN, Li X, Halverson LJ. Alginate production by Pseudomonas putida creates a hydrated microenvironment and contributes to biofilm architecture and stress tolerance under water-limiting conditions. J Bacteriol. 2007;189:8290–8299. doi: 10.1128/JB.00727-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Banerjee S, Palit R, Sengupta C, Standing D. Stress induced phosphate solubilization by Arthrobacter sp. and Bacillus sp. isolated from tomato rhizosphere. Aust J Crop Sci. 2010;4:378–383. [Google Scholar]
- 23.Panhwar QA, Radziah O, Sariah M, Ismail MR. Solubilization of different phosphate forms by phosphate solubilizing bacteria isolated from aerobic rice. Int J Agric Biol. 2009;11:667–673. [Google Scholar]
- 24.Panda B, Rahman H, Chatlod LR, Panda J. Isolation and characterization of phosphate solubilizing bacteria from the soils of Sikkim, India. Int J Stress Manag. 2013;4:255–262. [Google Scholar]
- 25.Henri F, Laurette N, Annette D, John Q, Wolfgang M, Francois-Xavier E, Dieudonné N. Solubilization of inorganic phosphates and plant growth promotion by strains of Pseudomonas fluorescens isolated from acidic soils of Cameroon. Afr J Microbiol Res. 2008;2:171–178. [Google Scholar]
- 26.Chen YP, Rekha PD, Arun AB, Shen FT, Lai WA, Young CC. Phosphate solubilizing bacteria from subtropical soil and their tricalcium phosphate solubilizing abilities. Appl Soil Ecol. 2006;34:33–41. doi: 10.1016/j.apsoil.2005.12.002. [DOI] [Google Scholar]
- 27.Bashan Y, Kamnev AA, de-Bashan LE. Tricalcium phosphate is inappropriate as a universal selection factor for isolating and testing phosphate-solubilizing bacteria that enhance plant growth: a proposal for an alternative procedure. Biol Fertil Soils. 2013;49:465–479. doi: 10.1007/s00374-012-0737-7. [DOI] [Google Scholar]
- 28.Chakraborty U, Chakraborty BN, Chakraborty AP, Sunar K, Dey PL. Plant growth promoting rhizobacteria mediated improvement of health status of tea plants. Indian J Biotechnol. 2013;12:20–31. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


