Abstract
Background: Continuous positive airway pressure (CPAP) is considered the treatment of choice for obstructive sleep apnea (OSA), and studies have shown that there is a correlation between patient adherence and treatment outcomes. Newer CPAP machines can track adherence, hours of use, mask leak, and residual apnea–hypopnea index (AHI). Such data provide a strong platform to examine OSA outcomes in a chronic disease management model. However, there are no standards for capturing CPAP adherence data, scoring flow signals, or measuring mask leak, or for how clinicians should use these data.
Methods: American Thoracic Society (ATS) committee members were invited, based on their expertise in OSA and CPAP monitoring. Their conclusions were based on both empirical evidence identified by a comprehensive literature review and clinical experience.
Results: CPAP usage can be reliably determined from CPAP tracking systems, but the residual events (apnea/hypopnea) and leak data are not as easy to interpret as CPAP usage and the definitions of these parameters differ among CPAP manufacturers. Nonetheless, ends of the spectrum (very high or low values for residual events or mask leak) appear to be clinically meaningful.
Conclusions: Providers need to understand how to interpret CPAP adherence tracking data. CPAP tracking systems are able to reliably track CPAP adherence. Nomenclature on the CPAP adherence tracking reports needs to be standardized between manufacturers and AHIFlow should be used to describe residual events. Studies should be performed examining the usefulness of the CPAP tracking systems and how these systems affect OSA outcomes.
Keywords: CPAP adherence, sleep apnea, CPAP tracking systems
Contents
Executive Summary
Introduction
Methods
Committee Process
Literature Search
CPAP Adherence Tracking Systems
CPAP Adherence Tracking Transmission Systems
Barriers to Using CPAP Adherence Tracking Systems in Clinical Practice
Metrics to Determine CPAP Effectiveness
Future Directions: Research Strategies Needed to Address CPAP Tracking and Outcomes
Conclusions
Executive Summary
Obstructive sleep apnea (OSA) is an extremely common condition and is associated with significant morbidity and mortality (1–18). Optimal treatment of sleep apnea is critical because sleep-disordered breathing is associated with the increased risk of vehicular crashes and cardiovascular morbidity and mortality (6, 9–18). Continuous positive airway pressure (CPAP) is the treatment of choice for sleep apnea (19–22). Increased CPAP adherence has been shown to improve outcomes such as daytime sleepiness, quality of life, and mortality (23–25). OSA should be considered in a chronic disease management model in which CPAP adherence is tracked over time. To assess CPAP adherence and efficacy, tracking systems have been implemented. Such systems monitor CPAP efficacy (residual sleep-disordered breathing), hours of CPAP use, mask leak, and a number of different flow signals. However, there are no standards on how to use the data from these new CPAP tracking systems nor do we have evidence that these systems ultimately improve outcomes. See the online supplement for full discussion of the literature on the accuracy of the residual AHI and mask leak.
The purpose of this document is to (1) review the data (adherence, leak, efficacy, flow signals) obtained from CPAP adherence tracking systems and the reliability of these data; (2) examine the use of CPAP tracking systems in clinical practice; (3) discuss outcomes that satisfy payer reimbursement criteria for chronic CPAP use; and (4) propose research questions to address important issues in relation to CPAP tracking and OSA outcomes.
Major conclusions of this clinical statement:
-
•
CPAP adherence tracking systems intuitively seem useful; however, there are few studies that provide data that show that these systems improve CPAP usage or OSA outcomes. Notwithstanding, CPAP adherence tracking systems provide a strong platform to generate outcome data in a chronic disease management model, which is how the treatment of OSA should be considered.
-
•
CPAP usage can be reliably determined from CPAP tracking systems (although there can be technical failures with card transmission of data) and such data should be routinely examined in patients with OSA. CPAP adherence needs to be monitored sequentially over time.
-
•
To optimally interpret residual events on the CPAP adherence tracking reports, providers need to understand the different definitions for apneas and hypopneas from each manufacturer of CPAP tracking systems.
-
•
The residual apneas and hypopneas and mask leak data from CPAP tracking systems are not easy to interpret and the definitions of these parameters differ among each of the CPAP adherence tracking system manufacturers.
-
•
The value of CPAP adherence monitoring for clinical decision making based solely on intermediate values for the residual apnea–hypopnea index (AHI), residual apnea index, or mask leak is unclear and, therefore, additional research is indicated.
-
•
Very high or low values for residual apneas/hypopneas or mask leak appear to be clinically useful in chronically managing patients with sleep apnea.
-
•
Current clinical care systems are not optimally configured for examining data from CPAP adherence tracking systems.
-
•
Documentation of CPAP adherence should be accepted between 7 and 90 days, rather than the current 31- to 90-day requirement, and CPAP adherence should be monitored long term (for as long as the patient is using CPAP).
-
•
Nomenclature on the CPAP adherence tracking reports needs to be standardized between manufacturers and AHIFlow should be used to describe residual events.
-
•
Studies should be performed to examine the usefulness of the CPAP tracking systems and how these systems affect OSA outcomes.
Introduction
Obstructive sleep apnea (OSA) is an extremely common condition and is associated with considerable morbidity and mortality. The Wisconsin Sleep Cohort Study found that 4% of middle-aged males and 2% of middle-aged females had OSA (1). The prevalence of OSA is much higher (on the order of 80%) in certain populations (overweight type 2 diabetics, bariatric surgery patients) (2–5). Consequences of OSA can be broadly divided into those related to neurocognitive function (e.g., excessive sleepiness) and those related to the cardiovascular and metabolic systems. Excessive daytime sleepiness produces a number of different problems for patients with OSA, the most serious of which is motor vehicular accidents (MVAs). Studies in driving simulators indicate that OSA impairs driving ability (6), and crashes are increased among individuals who have OSA compared with matched individuals who do not have OSA (7). Patients with OSA can be as impaired in driving skills as those who are over the legal blood alcohol concentration limit (6). The Sleep Heart Health and other studies have demonstrated that patients with OSA are at increased risk for hypertension (9–11), myocardial infarction (MI) (12, 13), stroke (14), and death (15–18). Nocturnal cardiac arrhythmias including atrial fibrillation, sinus bradycardia/tachycardia, supra/ventricular tachycardias, heart block, and sinus pauses have all been reported during apneic episodes (26–29). Mild-to-moderate pulmonary hypertension can develop in patients with OSA (30–32) although overt right heart failure is uncommon in the absence of other contributing factors. Studies have shown that patients with moderate-to-severe OSA have increased mortality (10 to 20 yr after the diagnosis has been made) (16–18). On the basis of the public health impact of the increased risk of vehicular crashes and the cardiovascular morbidity and mortality, optimal treatment of OSA is clearly important.
Continuous positive airway pressure (CPAP) is the treatment of choice for OSA (19–22). CPAP has been shown to improve sleep architecture, decrease risk of MVAs (33–35), improve quality of life (23, 36), and decrease neurocognitive and cardiovascular consequences associated with OSA (12, 22, 37–47). Several consensus statements have examined the efficacy of CPAP in the treatment of OSA (22, 47, 48). Moreover, CPAP adherence (measured as hours of use per night) has been shown to modify outcomes (23–25). The importance of adherence to treatment has been shown by studies showing the return of sleepiness and impairments in simulated driving ability in as little as one night off CPAP (49, 50). Moreover, CPAP withdrawal resulted in a rapid recurrence of apneic events, daytime sleepiness, increased blood pressure, and increased heart rate (51).
To assess CPAP adherence and efficacy, tracking systems have been implemented by CPAP manufacturers. Such systems monitor CPAP efficacy (residual sleep-disordered breathing), hours of CPAP use, mask leak, and a number of different flow signals. However, there are no guidelines on how to use the data from these new CPAP tracking systems nor do we have evidence that these systems ultimately improve outcomes. Furthermore, methods of measuring and reporting the parameters from CPAP adherence downloads are inconsistent between manufacturers and not well validated. CPAP adherence tracking systems are available to most providers who care for patients with OSA. Yet there are few studies examining the usefulness of CPAP tracking systems even though they are commonly used in clinical practice to manage patients with OSA (52). Technological advances rather than evidence-based medicine are driving the increased use of CPAP tracking systems in this rapidly changing environment. CPAP adherence tracking systems have not been tested to show improved outcomes but their use is intuitively logical, such that CPAP adherence tracking is now a requirement for Medicare and other payers to continue reimbursement for CPAP beyond the first 3 months of treatment.
CPAP adherence tracking systems provide a strong platform to generate outcome data in a chronic disease management model, which is how the treatment of OSA should be considered. We have the ability to link patterns of use to salient disease outcomes and responses to treatment. It is much easier to determine CPAP adherence outcomes for OSA treatment than it is to determine treatment outcomes for most chronic disorders including asthma, hypertension, arthritis, or chronic obstructive pulmonary disease. As such, the OSA field has the opportunity to take a leading role in understanding interventions to improve adherence to therapy. Such strategies may be critical to improving clinical outcomes. We can track CPAP use in timeframes ranging from hours of use during one night, to use over months to even years. This ability permits the linking of patterns of use to cardiovascular outcomes and can help direct clinical decision making.
The goals of this statement are as follows:
-
•
To review CPAP adherence tracking systems, including the data that they track (adherence, leak, efficacy, flow signals) and the reliability of these data.
-
•
To examine the use of CPAP tracking systems in clinical practice.
-
•
To discuss clinical outcomes (CPAP usage and others) that could be measured to satisfy payer reimbursement criteria for chronic CPAP use.
-
•
To address important research questions in relation to CPAP tracking and OSA outcomes.
Methods
Committee Process
The Statement was conceived by the planning committee of the Sleep and Respiratory Neurobiology Assembly of the ATS, and was chair (Richard J. Schwab) initiated. Committee members were invited on the basis of their scientific/clinical expertise in investigation/management of OSA and CPAP monitoring. Most of the committee members were from academic institutions within the United States, because the focus of this document was directed toward providers within the United States. Meetings were held at the American Thoracic Society annual international conference and consensus on all conclusions was reached among members of the committee by discussion. All members reviewed and approved the entire final draft by e-mail. All committee members were required to disclose potential conflicts of interest, which were vetted according to the policies of the ATS. See Methods Table in the online supplement.
Literature Search
Committee members compiled reference material germane to the assessment of CPAP monitoring, adherence, and outcomes in OSA. In addition, a literature review was conducted, based primarily on PubMed (from 2000 to November 2012), medical library catalog searches, and manual reviews of the bibliographic and abstract sections for the annual meetings of the American Thoracic Society, the Associated Professional Sleep Societies, other relevant professional societies, and reference lists of selected papers and chapters. Key words for the literature search included the following: CPAP adherence tracking systems, CPAP adherence, CPAP compliance, CPAP use, and CPAP tracking systems. This clinical statement focused on peer-reviewed articles, reviews, and editorials, in which primary data, conclusions, and/or positions were available. However, because there were sparse primary data on CPAP adherence tracking systems, conclusions were primarily based on uncontrolled observations and the clinical experience of our expert committee.
CPAP Adherence Tracking Systems
Why do we care about CPAP adherence and hours of use? Although CPAP improves both the neurobehavioral (daytime sleepiness [23, 24, 41, 42], MVA [33–35, 53]) and cardiovascular consequences of OSA (hypertension [9–11, 39, 40, 43, 44, 46–48], MI [12, 13], stroke [14], atrial fibrillation [26–29]), the important question is whether or not increased CPAP hours of use further improve these outcomes. Weaver and colleagues and Antic and colleagues have independently shown that increasing the number of hours of CPAP use results in better outcomes (23, 24). Specifically in these studies, increased hours of CPAP use improved measures of daytime sleepiness (e.g., the Epworth Sleepiness Scale [ESS], the Multiple Sleep Latency Test [MSLT], and scores in the Functional Outcomes of Sleep Questionnaire [FOSQ]). Likewise, cardiovascular and mortality outcomes correlated with the amount of CPAP use (12, 25, 54–56). Thus, it is important to track CPAP use so that patients with OSA who use their device for only a short duration may be intervened on.
Objectively measured CPAP adherence is more reliable than self-reported adherence, because self-reported CPAP use has been shown to correlate poorly with actual hours of use (57). Refill rates of CPAP accessories have also be used as a surrogate measure for long-term CPAP adherence (58) but such data may be more difficult to obtain than CPAP adherence downloads. These tracking systems can be used in patients receiving conventional CPAP; auto-CPAP; or bilevel, auto-bilevel, or adaptive servo-ventilation. The specific pressure data reported in each of these systems differ, but regardless of the type of positive airway pressure unit the reports provide information on adherence, residual AHI, and mask leak. Table 1 reviews the adherence data that can be tracked from CPAP units, Table 2 (52, 59–62) examines the specific event detection methodology for each CPAP manufacturer, Table 3 (63) defines how leaks and large leaks are measured, and Figure 1 provides a clinical algorithm for the use of CPAP adherence data. The specific details regarding performance evaluation of CPAP device–derived data are described extensively in the online supplement.
TABLE 1.
ADHERENCE DATA DERIVED FROM CONTINUOUS POSITIVE AIRWAY PRESSURE TRACKING SYSTEMS
| Date ranges of device usage |
| Total number of nights the CPAP was used |
| Total number of nights the CPAP was not used |
| Percentage of nights with CPAP usage |
| Percentage of nights with CPAP usage ≥ 4 h/night |
| Percentage of nights with CPAP usage < 4 h/night |
| Average usage on nights when CPAP was used |
| Average usage on all nights |
Definition of abbreviation: CPAP = continuous positive airway pressure.
TABLE 2.
EVENT DETECTION ALGORITHMS
| Manufacturer | Apnea Event Detection | Hypopnea Event Detection |
|---|---|---|
| ResMed unit (S9 model) | Apnea is defined when the 2-s moving average root mean square ventilation (based on a pneumotachograph) falls below 25% of the long-term ventilation for 10 s | Hypopnea is defined when all of the following conditions are met: |
| 1. The 12-s moving average root mean square ventilation falls below 50% of the long-term ventilation | ||
| 2. The hypopnea is not immediately followed by an apnea | ||
| 3. The hypopnea contains one or more partially obstructed breaths | ||
| Phillips Respironics unit (System One model) | Apnea is detected after a moving window of 3-4 min is established and flow decreases by more than 80% for at least 10 s | Hypopnea is detected when moving window of 3-4 min is established and flow decreases by 40–80% for at least 10 s |
| DeVilbiss Healthcare IntelliPAP unit (SmartCode remote data retrieval system) | A reduction in a flow signal of >90% of the baseline flow for 10 s | A reduction in a flow signal of >50% of the baseline flow for 10 s |
| Fisher & Paykel InfoSmart software | >80% reduction in flow relative to a baseline determined from previous breaths | >40% reduction in flow relative to a baseline determined from previous breaths |
TABLE 3.
CONTINUOUS POSITIVE AIRWAY PRESSURE MASK LEAK MEASUREMENTS
| CPAP Manufacturer | How Leak Is Measured | Large Leak Threshold |
|---|---|---|
| Phillips Respironics | Intentional leak subtracted from total flow | Leak condition where the leak level exceeds a preset “flow vs. pressure” curve (the averaged leak through all mask exhalation ports at various pressure) |
| ResMed | Unintentional leak (device flow-intentional leak) + mouth leak | 95th percentile leak (<24 L/min with nasal interface and <36 L/min with full face interface) |
| Fisher & Paykel | Total leak, including mask and exhaust flow from mask | A leak value of >60 L/min |
| DeVilbiss Healthcare IntelliPAP | Records high leak flow time as a percentage of the time the leak was above 95 L/min | A leak value of >95 L/min |
Definition of abbreviation: CPAP = continuous positive airway pressure.
Figure 1.
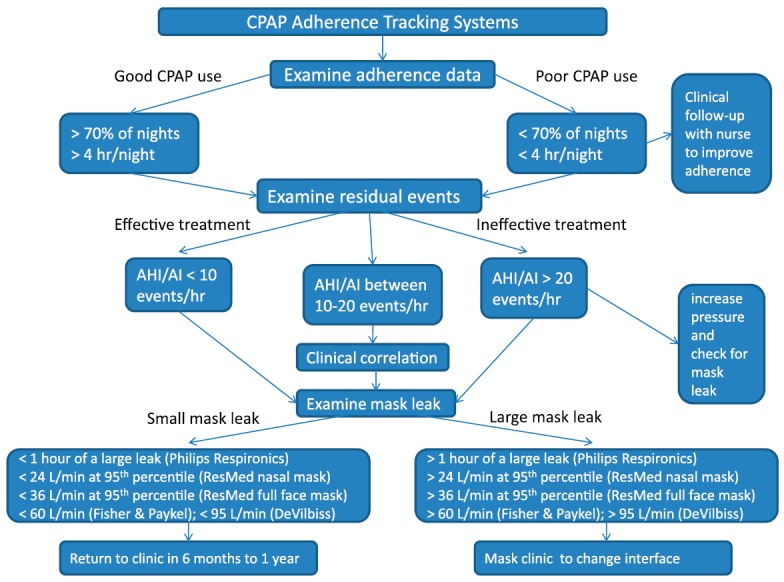
Clinical algorithm for using continuous positive airway pressure adherence tracking systems.
CPAP Adherence Tracking Transmission Systems
There are several different methods to transmit CPAP adherence tracking data. Most systems use cards (smart cards or SD cards), memory sticks, or wireless transmission. Several studies (64, 65) have demonstrated that these transmission systems may be better than routine practice (see the online supplement for details about these studies). Although wireless transmission of CPAP adherence data is the future, a major problem with wireless systems is having the resources to retrieve the data. It is not clear how, when, and who should monitor the wireless transmissions. Furthermore, there are known problems with loss of data and failure of recording systems (primarily smart cards) to further confound tracking systems (66). The electronic transfer of the CPAP adherence monitoring reports also raises privacy and safety issues. Health Insurance Portability and Accountability Act (HIPAA) violations could easily occur with these reports whether they are faxed or transmitted wirelessly. Moreover, a robust level of information security is required for transmission, storage, and access of the data and at the website where the data reside. Systems need to be developed to protect patient privacy when CPAP adherence data reports are reviewed and maintained on servers.
Barriers to Using CPAP Adherence Tracking Systems in Clinical Practice
There are other barriers to incorporating CPAP adherence tracking systems routinely in clinical practice. The lack of standardization precludes interoperability with existing electronic medical records. Data profiles are not standardized between the different proprietary tracking systems and the reports are not yet easily exportable to electronic medical records (presently the reports need to be scanned into electronic medical records, which takes time). Customized software should be written to allow more transparent input of CPAP adherence data to a given electronic medical record. Connectivity to server databases can be suboptimal (particularly when using multiple homecare providers and device companies). Current care delivery systems are not configured for this type of data management and examining the CPAP tracking reports can slow down patient flow in a busy sleep practice. Faxed reports are cumbersome and provide a black-and-white background, which is problematic because many of the reports need to be displayed in color.
There are also possible medical/legal ramifications of CPAP tracking systems. For instance, if a school bus driver is using CPAP and has a motor vehicle accident, CPAP tracking data could be examined during a legal proceeding. The bus driver’s CPAP use the night before an accident could be examined as well as CPAP use during the week before the motor vehicle accident. The physician taking care of this bus driver could also face medical/legal risk. If the bus driver’s CPAP use was not ideal, if there was a high residual AHI or a large CPAP mask leak, it could be argued that the physician should have acted on these data. However, in such a scenario it is not clear how much CPAP use is enough or what level of residual AHI matters.
Metrics to Determine CPAP Effectiveness
Although there are important concerns with CPAP tracking systems, third-party payers are mandating the use of CPAP adherence tracking systems to objectively document therapeutic use to reimburse CPAP. The Centers for Medicare and Medicaid Services (CMS) have developed specific guidelines on CPAP (or bilevel) reimbursement. Initial CPAP reimbursement is limited to 12 weeks (67). Continued coverage of a CPAP device beyond the first 3 months of therapy requires that, no sooner than Day 31 but no later than Day 91 after initiating therapy, the treating physician must conduct a clinical reevaluation and document that the patient is benefiting from CPAP therapy. Clinical benefit is demonstrated by a face-to-face clinical reevaluation by the treating provider with documentation that symptoms of OSA are improved with objective evidence of CPAP adherence. Adherence is defined as use of CPAP for at least 4 hours/night on 70% of nights during a consecutive 30-day period any time during the first 3 months of initial usage. However, there is insufficient evidence to support this definition of CPAP adherence as a threshold for improved neurocognitive and cardiovascular outcomes. As described previously, there is a dose–response relationship between amount of nightly CPAP use and clinical outcomes (23–25). These data indicate that even subjects who use CPAP for only 2 hours show improvement in measures of some outcomes (ESS, FOSQ, MSLT). Moreover, several studies (some randomized controlled trials) have shown improvements in daytime sleepiness, functional outcomes, cognitive function, and blood pressure in patients treated with CPAP for less than 4 hours/night, 70% of the nights (23, 44, 67–69). The CMS criteria assume that CPAP treatment has a threshold effect and therefore do not address whether outcomes may have a linear response with much lower levels of CPAP use (23). The CMS requirements also mandate an in-laboratory polysomnogram if CPAP was prescribed on the basis of portable monitoring and the patient subsequently fails the 90-day use criteria. The validity of this requirement is unclear, especially in the patient whose portable study demonstrates unequivocal severe OSA.
What specific clinical outcomes independent of the data from CPAP tracking systems should be measured to ascertain a salutary response to CPAP? Such outcomes could include (1) subjective daytime sleepiness (ESS [it should be noted, however, that the ESS is highly variable when administered sequentially to a clinical OSA population (70)], etc.); (2) objective daytime sleepiness (PVT [Psychomotor Vigilance Test], MSLT, MWT [Maintenance of Wakefulness Test]); (3) self-reported improvement in the presenting symptom (i.e., nocturia, headache, sleep fragmentation, insomnia); (4) blood pressure; (5) cardiovascular outcomes (MI, hypertension, cardiovascular accident, heart failure, arrhythmias, improved insulin resistance or diabetic control); (6) cognitive functioning (memory, neurocognitive testing); (7) quality of life (FOSQ, SF [Short Form Health Survey] 36, Calgary Sleep Apnea Specific Quality of Life Instrument [SAQLI] [71, 72], depression scales); (8) sexual function; (9) spousal outcomes; and (10) MVAs. It is likely that specific outcomes (e.g., cardiovascular vs. cognitive) in various populations (e.g., old vs. young, etc.) will be dependent on a range of CPAP durations.
We believe optimal clinical practices (largely based on clinical experience) for chronically managing CPAP in patients with OSA should include the following:
-
•
We encourage patients to use CPAP whenever they are asleep (during the day or night).
-
•
We consider patients adherent if they regularly use CPAP for more than 4 h/night or if they use CPAP for more than 2 h/night and are making progress toward improved daytime sleepiness as measured by the ESS, subjective improvement in quality of life, or improvement of other OSA-associated health impairments (e.g., diabetes, hypertension). This reflects our belief that partial use is better than no use, although our goal is always to achieve full-time CPAP use during sleep.
-
•
We assess these outcomes soon after the initiation of CPAP therapy, because data demonstrate that CPAP adherence is typically established early in the course of treatment, perhaps as early as the first 3–7 days (73–80). We measure the outcomes after 1 week, 4–6 weeks, 12 weeks, 6 months, 1 year after the initiation of CPAP, and then monitor them yearly thereafter.
Regardless of the specific time frame these outcomes need to be measured longitudinally because OSA should be viewed and treated as a chronic disease. Addressing CPAP intolerance early may improve CPAP adherence, whereas waiting for the requisite minimum 30 days may allow entrenched problems to result in abandonment of, or suboptimal adherence to, CPAP. The current 31- to 90-day requirement for documentation of CPAP adherence is arbitrary and not supported by evidence. The committee members prefer to document CPAP adherence earlier (i.e., 7–90 d) because there is evidence that addressing CPAP intolerance early may improve long-term adherence (81–83). Moreover, CPAP adherence needs to be monitored long term (for as long as the patient is using CPAP).
Finally, there are potentially ethical issues associated with the use of CPAP adherence monitoring systems as a requirement for Medicare payment. CPAP adherence has been shown to be related to socioeconomic class, marital status, race, and psychiatric disease (84–86). These patients may have a difficult time achieving the Medicare adherence patterns and thus certain segments of the population are potentially targets of government-mandated reimbursement discrimination (87, 88).
Future Directions: Research Strategies Needed to Address CPAP Tracking and Outcomes
There are many unanswered research questions involving CPAP tracking systems. First, does tracking CPAP adherence improve clinical outcomes associated with OSA, and if so, which specific outcomes? It is important to perform studies examining the validity, reliability, and usefulness of CPAP tracking event detection (flow signals) and mask leak data. It would also be important to study which CPAP adherence system is most accurate. Studies need to determine the clinically significant leak threshold and whether mask leak decreases CPAP adherence. Finally, it is important to determine the usefulness of measuring additional signals, such as vibratory snoring, periodic breathing (Cheyne-Stokes pattern), RERA (respiratory effort–related arousal), flow limitation, and clear airway apnea (central sleep apnea). The validity of these signals has yet to be determined. Studies need to be performed that evaluate whether the use of these data can improve CPAP adherence. Other potentially helpful signals would include heart rate variability (ECG), oximetry (which would allow the event detection algorithms to more closely reflect the standard scoring criteria on polysomnograms for hypopneas), body and neck position, actigraphy, quantification of snoring, blood pressure, quantification of periodic limb movements, and a measure of sleep architecture.
The second critical issue is to develop standard definitions of events across different systems (i.e., apnea, hypopnea, flow limitation, AHI, and leak parameters). For example, CPAP downloads typically report residual “AHI,” which is confusing both to providers and to patients. Because hypopnea scoring, when adhering to a widely accepted standard, requires either oxygen desaturation or arousal to be scored (89), these reports may be misleading because this “AHI” is only based on a reduction in airflow. We believe that the terminology for residual AHI assessment should be standardized, and suggest that it be reported as residual AHIFlow. Moreover, studies need to determine a minimum threshold for clinically meaningful residual AHIFlow based on outcome data. Similarly, mask leak may impair CPAP effectiveness, but its measurement and reporting terminology are inconsistent. For example, typical CPAP downloads may report “average leak,” “large leak,” and other information, but do not typically include information about total leak minus expected leak or artifact-free leak. It would be helpful for leak to be reported as total leak minus expected leak in a standardized manner across all manufacturers. Mouth leak should be quantified in patients with a nasal mask as compared with leaking around the nasal mask.
Not only should the terminology for CPAP adherence tracking measurements be standardized but there also needs to be the development and acceptance of common report formats among industry partners. Data safety considerations need to be incorporated into these systems. Finally, the medical/legal ramifications of CPAP adherence tracking systems need to be determined.
Conclusions
OSA is a chronic disease and needs to be managed accordingly (i.e., CPAP adherence should be monitored consistently over time). Increased CPAP usage has been shown to improve OSA outcomes. Third-party payers are requiring documented use of therapy with CPAP tracking systems. CPAP adherence can be reliably determined from CPAP tracking systems. However, the residual events (apnea/hypopnea) and leak data from CPAP tracking systems are not as easy to interpret and the definitions of these parameters differ among the manufacturers of CPAP adherence tracking systems. Nonetheless, ends of the spectrum (very high or low values for residual events or mask leak) appear to be clinically meaningful. The health risks of patients with residual OSA on the event detection algorithms are essentially unknown and need to be studied. CPAP tracking systems intuitively seem useful, although there are few studies showing that CPAP adherence tracking systems improve CPAP adherence or OSA outcomes. Current clinical care systems are not optimally configured for this technology, yet most providers are using CPAP tracking systems to monitor their patients with OSA. Use of CPAP adherence monitoring in real time for clinical decision making based solely on intermediate values for residual AHI or mask leak is unclear, and therefore additional studies are indicated. At present, the data are limited but the technology is young and evolving quickly. Because usage patterns for CPAP are often determined in the first week (74, 90) it seems reasonable that documentation of CPAP adherence should be accepted between 7 and 90 days, rather than the current 31- to 90-day requirement, and CPAP adherence needs to be monitored long term (for as long as the patient is using CPAP). Nomenclature on the CPAP tracking reports needs to be standardized and AHIFlow should be used to describe residual events. It is clear that studies should be performed to examine the usefulness of the CPAP tracking systems and how these systems affect OSA outcomes.
Acknowledgments
This official statement was prepared by an ad hoc subcommittee of the Sleep and Respiratory Neurobiology Assembly.
Members of the committee:
Richard J. Schwab, M.D. (Chair)
Safwan M. Badr, M.D.
Lawrence J. Epstein, M.D.
Peter C. Gay, M.D.
David Gozal, M.D.
Malcolm Kohler, M.D.
Patrick Lévy, M.D.
Atul Malhotra, M.D.
Barbara A. Phillips, M.D., M.P.H.
Ilene M. Rosen, M.D., M.S.C.E.
Kingman P. Strohl, M.D.
Patrick J. Strollo, M.D.
Edward M. Weaver, M.D., M.P.H.
Terri E. Weaver, Ph.D., R.N.
Footnotes
This statement has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Author Disclosures: R.J.S. reported consulting for Apnex ($5,000–24,999) and ApniCure ($5,000–24,999). D.G. reported consulting for Galleon ($5,000–24,999) and research support from ResMed. A.M. reported consulting for Apnex ($10,000–49,999), ApniCure ($1,000–9,999), Galleon ($1,000–9,999), Philips Respironics ($10,001–49,999), Pfizer ($1,000–9,999), and SGS ($10,000–49,999). K.P.S. reported serving as president of iONSLEEP sleep medicine consultants and consulting for Inspire Medical Systems ($10,000–49,999); he served on advisory committees for SleepMed ($5,001–10,000) and Sleep Solutions, Inc. ($5,001–10,000), and received research support from Inspire Medical Systems ($10,000–49,999). P.J.S. reported research support from ResMed ($10,000–49,999). T.E.W. reported licensing agreements for FOSQ with Apnex, Cephalon, GlaxoSmithKline, Nova Nordisk, Nova Som, and Philips Respironics; she received royalties or license fees from Apnex ($5,000–24,999), Cephalon ($5,000–24,999), GlaxoSmithKline ($5,000–24,999), Nova Nordisk ($5000–24,999), Nova Som ($5,000–24,999), and Philips Respironics ($5,000–24,999); she received research support from Cephalon ($5,000–24,999), Nova Som ($5,000–24,999), and Philips Respironics ($5,000–24,999). S.M.B., L.J.E., P.C.G., M.K., P.L., B.A.P., I.M.R., and E.M.W. reported they had no relevant commercial interests.
References
- 1.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 2.Foster GD, Sanders MH, Millman R, Zammit G, Borradaile KE, Newman AB, Wadden TA, Kelley D, Wing RR, Sunyer FX, et al. Sleep AHEAD Research Group. Obstructive sleep apnea among obese patients with type 2 diabetes. Diabetes Care. 2009;32:1017–1019. doi: 10.2337/dc08-1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adams TD, Avelar E, Cloward T, Crosby RD, Farney RJ, Gress R, Halverson RC, Hopkins PN, Kolotkin RL, Lamonte MJ, et al. Design and rationale of the Utah Obesity Study: a study to assess morbidity following gastric bypass surgery. Contemp Clin Trials. 2005;26:534–551. doi: 10.1016/j.cct.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 4.O’Keeffe T, Patterson EJ. Evidence supporting routine polysomnography before bariatric surgery. Obes Surg. 2004;14:23–26. doi: 10.1381/096089204772787248. [DOI] [PubMed] [Google Scholar]
- 5.Sareli AE, Cantor CR, Williams NN, Korus G, Raper SE, Pien G, Hurley S, Maislin G, Schwab RJ. Obstructive sleep apnea in patients undergoing bariatric surgery—a tertiary center experience. Obes Surg. 2011;21:316–327. doi: 10.1007/s11695-009-9928-1. [DOI] [PubMed] [Google Scholar]
- 6.George CF, Boudreau AC, Smiley A. Simulated driving performance in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 1996;154:175–181. doi: 10.1164/ajrccm.154.1.8680676. [DOI] [PubMed] [Google Scholar]
- 7.Tregear S, Reston J, Schoelles K, Phillips B. Obstructive sleep apnea and risk of motor vehicle crash: systematic review and meta-analysis. J Clin Sleep Med. 2009;5:573–581. [PMC free article] [PubMed] [Google Scholar]
- 8.Vakulin A, Baulk SD, Catcheside PG, Antic NA, van den Heuvel CJ, Dorrian J, McEvoy RD. Effects of alcohol and sleep restriction on simulated driving performance in untreated patients with obstructive sleep apnea. Ann Intern Med. 2009;151:447–455. doi: 10.7326/0003-4819-151-7-200910060-00005. [DOI] [PubMed] [Google Scholar]
- 9.Shahar E, Whitney CW, Redline S, Lee ET, Newman AB, Nieto FJ, O’Connor GT, Boland LL, Schwartz JE, Samet JM. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163:19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 10.Nieto FJ, Young TB, Lind BK, Shahar E, Samet JM, Redline S, D’Agostino RB, Newman AB, Lebowitz MD, Pickering TG. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study: Sleep Heart Health Study. JAMA. 2000;283:1829–1836. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 11.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–1384. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 12.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea–hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–1053. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 13.Gottlieb DJ, Yenokyan G, Newman AB, O’Connor GT, Punjabi NM, Quan SF, Redline S, Resnick HE, Tong EK, Diener-West M, et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the Sleep Heart Health Study. Circulation. 2010;122:352–360. doi: 10.1161/CIRCULATIONAHA.109.901801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Redline S, Yenokyan G, Gottlieb DJ, Shahar E, O’Connor GT, Resnick HE, Diener-West M, Sanders MH, Wolf PA, Geraghty EM, et al. Obstructive sleep apnea–hypopnea and incident stroke: the Sleep Heart Health Study. Am J Respir Crit Care Med. 2010;182:269–277. doi: 10.1164/rccm.200911-1746OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Punjabi NM, Caffo BS, Goodwin JL, Gottlieb DJ, Newman AB, O’Connor GT, Rapoport DM, Redline S, Resnick HE, Robbins JA, et al. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med. 2009;6:e1000132. doi: 10.1371/journal.pmed.1000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gami AS, Howard DE, Olson EJ, Somers VK. Day–night pattern of sudden death in obstructive sleep apnea. N Engl J Med. 2005;352:1206–1214. doi: 10.1056/NEJMoa041832. [DOI] [PubMed] [Google Scholar]
- 17.Marshall NS, Wong KK, Liu PY, Cullen SR, Knuiman MW, Grunstein RR. Sleep apnea as an independent risk factor for all-cause mortality: the Busselton Health Study. Sleep. 2008;31:1079–1085. [PMC free article] [PubMed] [Google Scholar]
- 18.Young T, Finn L, Peppard PE, Szklo-Coxe M, Austin D, Nieto FJ, Stubbs R, Hla KM. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep. 2008;31:1071–1078. [PMC free article] [PubMed] [Google Scholar]
- 19.Ballester E, Badia JR, Hernández L, Carrasco E, de Pablo J, Fornas C, Rodriguez-Roisin R, Montserrat JM. Evidence of the effectiveness of continuous positive airway pressure in the treatment of sleep apnea/hypopnea syndrome. Am J Respir Crit Care Med. 1999;159:495–501. doi: 10.1164/ajrccm.159.2.9804061. [DOI] [PubMed] [Google Scholar]
- 20.Marshall NS, Barnes M, Travier N, Campbell AJ, Pierce RJ, McEvoy RD, Neill AM, Gander PH. Continuous positive airway pressure reduces daytime sleepiness in mild to moderate obstructive sleep apnoea: a meta-analysis. Thorax. 2006;61:430–434. doi: 10.1136/thx.2005.050583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gay P, Weaver T, Loube D, Iber C Positive Airway Pressure Task Force; Standards of Practice Committee; American Academy of Sleep Medicine. Evaluation of positive airway pressure treatment for sleep related breathing disorders in adults. Sleep. 2006;29:381–401. doi: 10.1093/sleep/29.3.381. [DOI] [PubMed] [Google Scholar]
- 22.Smith I, Lasserson TJ. Pressure modification for improving usage of continuous positive airway pressure machines in adults with obstructive sleep apnoea. Cochrane Database Syst Rev. 2009;(4):CD003531. doi: 10.1002/14651858.CD003531.pub3. [DOI] [PubMed] [Google Scholar]
- 23.Weaver TE, Maislin G, Dinges DF, Bloxham T, George CF, Greenberg H, Kader G, Mahowald M, Younger J, Pack AI. Relationship between hours of CPAP use and achieving normal levels of sleepiness and daily functioning. Sleep. 2007;30:711–719. doi: 10.1093/sleep/30.6.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Antic NA, Catcheside P, Buchan C, Hensley M, Naughton MT, Rowland S, Williamson B, Windler S, McEvoy RD. The effect of CPAP in normalizing daytime sleepiness, quality of life, and neurocognitive function in patients with moderate to severe OSA. Sleep. 2011;34:111–119. doi: 10.1093/sleep/34.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Campos-Rodriguez F, Peña-Griñan N, Reyes-Nuñez N, De la Cruz-Moron I, Perez-Ronchel J, De la Vega-Gallardo F, Fernandez-Palacin A. Mortality in obstructive sleep apnea–hypopnea patients treated with positive airway pressure. Chest. 2005;128:624–633. doi: 10.1378/chest.128.2.624. [DOI] [PubMed] [Google Scholar]
- 26.Mehra R, Benjamin EJ, Shahar E, Gottlieb DJ, Nawabit R, Kirchner HL, Sahadevan J, Redline S Sleep Heart Health Study. Association of nocturnal arrhythmias with sleep-disordered breathing: the Sleep Heart Health Study. Am J Respir Crit Care Med. 2006;173:910–916. doi: 10.1164/rccm.200509-1442OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kanagala R, Murali NS, Friedman PA, Ammash NM, Gersh BJ, Ballman KV, Shamsuzzaman AS, Somers VK. Obstructive sleep apnea and the recurrence of atrial fibrillation. Circulation. 2003;107:2589–2594. doi: 10.1161/01.CIR.0000068337.25994.21. [DOI] [PubMed] [Google Scholar]
- 28.Konecny T, Brady PA, Orban M, Lin G, Pressman GS, Lehar F, Tomas K, Gersh BJ, Tajik AJ, Ommen SR, et al. Interactions between sleep disordered breathing and atrial fibrillation in patients with hypertrophic cardiomyopathy. Am J Cardiol. 2010;105:1597–1602. doi: 10.1016/j.amjcard.2010.01.023. [DOI] [PubMed] [Google Scholar]
- 29.Hoyer FF, Lickfett LM, Mittmann-Braun E, Ruland C, Kreuz J, Pabst S, Schrickel J, Juergens U, Tasci S, Nickenig G, et al. High prevalence of obstructive sleep apnea in patients with resistant paroxysmal atrial fibrillation after pulmonary vein isolation. J Interv Card Electrophysiol. 2010;29:37–41. doi: 10.1007/s10840-010-9502-8. [DOI] [PubMed] [Google Scholar]
- 30.Sajkov D, Cowie RJ, Thornton AT, Espinoza HA, McEvoy RD. Pulmonary hypertension and hypoxemia in obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 1994;149:416–422. doi: 10.1164/ajrccm.149.2.8306039. [DOI] [PubMed] [Google Scholar]
- 31.Marrone O, Bonsignore MR. Pulmonary haemodynamics in obstructive sleep apnoea. Sleep Med Rev. 2002;6:175–193. doi: 10.1053/smrv.2001.0185. [DOI] [PubMed] [Google Scholar]
- 32.Arias MA, García-Río F, Alonso-Fernández A, Martínez I, Villamor J. Pulmonary hypertension in obstructive sleep apnoea: effects of continuous positive airway pressure: a randomized, controlled cross-over study. Eur Heart J. 2006;27:1106–1113. doi: 10.1093/eurheartj/ehi807. [DOI] [PubMed] [Google Scholar]
- 33.Tregear S, Reston J, Schoelles K, Phillips B. Continuous positive airway pressure reduces risk of motor vehicle crash among drivers with obstructive sleep apnea: systematic review and meta-analysis. Sleep. 2010;33:1373–1380. doi: 10.1093/sleep/33.10.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hack M, Davies RJ, Mullins R, Choi SJ, Ramdassingh-Dow S, Jenkinson C, Stradling JR. Randomised prospective parallel trial of therapeutic versus subtherapeutic nasal continuous positive airway pressure on simulated steering performance in patients with obstructive sleep apnoea. Thorax. 2000;55:224–231. doi: 10.1136/thorax.55.3.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Findley L, Smith C, Hooper J, Dineen M, Suratt PM. Treatment with nasal CPAP decreases automobile accidents in patients with sleep apnea. Am J Respir Crit Care Med. 2000;161:857–859. doi: 10.1164/ajrccm.161.3.9812154. [DOI] [PubMed] [Google Scholar]
- 36.Woodson BT, Steward DL, Weaver EM, Javaheri S. A randomized trial of temperature-controlled radiofrequency, continuous positive airway pressure, and placebo for obstructive sleep apnea syndrome. Otolaryngol Head Neck Surg. 2003;128:848–861. doi: 10.1016/S0194-59980300461-3. [DOI] [PubMed] [Google Scholar]
- 37.Buchner NJ, Sanner BM, Borgel J, Rump LC. Continuous positive airway pressure treatment of mild to moderate obstructive sleep apnea reduces cardiovascular risk. Am J Respir Crit Care Med. 2007;176:1274–1280. doi: 10.1164/rccm.200611-1588OC. [DOI] [PubMed] [Google Scholar]
- 38.Harsch IA, Schahin SP, Radespiel-Tröger M, Weintz O, Jahreiss H, Fuchs FS, Wiest GH, Hahn EG, Lohmann T, Konturek PC, et al. Continuous positive airway pressure treatment rapidly improves insulin sensitivity in patients with obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2004;169:156–162. doi: 10.1164/rccm.200302-206OC. [DOI] [PubMed] [Google Scholar]
- 39.Mansfield DR, Gollogly NC, Kaye DM, Richardson M, Bergin P, Naughton MT. Controlled trial of continuous positive airway pressure in obstructive sleep apnea and heart failure. Am J Respir Crit Care Med. 2004;169:361–366. doi: 10.1164/rccm.200306-752OC. [DOI] [PubMed] [Google Scholar]
- 40.Becker HF, Jerrentrup A, Ploch T, Grote L, Penzel T, Sullivan CE, Peter JH. Effect of nasal continuous positive airway pressure treatment on blood pressure in patients with obstructive sleep apnea. Circulation. 2003;107:68–73. doi: 10.1161/01.cir.0000042706.47107.7a. [DOI] [PubMed] [Google Scholar]
- 41.Henke KG, Grady JJ, Kuna ST. Effect of nasal continuous positive airway pressure on neuropsychological function in sleep apnea–hypopnea syndrome: a randomized, placebo-controlled trial. Am J Respir Crit Care Med. 2001;163:911–917. doi: 10.1164/ajrccm.163.4.9910025. [DOI] [PubMed] [Google Scholar]
- 42.Montserrat JM, Ferrer M, Hernandez L, Farré R, Vilagut G, Navajas D, Badia JR, Carrasco E, De Pablo J, Ballester E. Effectiveness of CPAP treatment in daytime function in sleep apnea syndrome: a randomized controlled study with an optimized placebo. Am J Respir Crit Care Med. 2001;164:608–613. doi: 10.1164/ajrccm.164.4.2006034. [DOI] [PubMed] [Google Scholar]
- 43.Pepperell JC, Ramdassingh-Dow S, Crosthwaite N, Mullins R, Jenkinson C, Stradling JR, Davies RJ. Ambulatory blood pressure after therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised parallel trial. Lancet. 2002;359:204–210. doi: 10.1016/S0140-6736(02)07445-7. [DOI] [PubMed] [Google Scholar]
- 44.Faccenda JF, Mackay TW, Boon NA, Douglas NJ. Randomized placebo-controlled trial of continuous positive airway pressure on blood pressure in the sleep apnea–hypopnea syndrome. Am J Respir Crit Care Med. 2001;163:344–348. doi: 10.1164/ajrccm.163.2.2005037. [DOI] [PubMed] [Google Scholar]
- 45.Babu AR, Herdegen J, Fogelfeld L, Shott S, Mazzone T. Type 2 diabetes, glycemic control, and continuous positive airway pressure in obstructive sleep apnea. Arch Intern Med. 2005;165:447–452. doi: 10.1001/archinte.165.4.447. [DOI] [PubMed] [Google Scholar]
- 46.Haentjens P, Van Meerhaeghe A, Moscariello A, De Weerdt S, Poppe K, Dupont A, Velkeniers B. The impact of continuous positive airway pressure on blood pressure in patients with obstructive sleep apnea syndrome: evidence from a meta-analysis of placebo-controlled randomized trials. Arch Intern Med. 2007;167:757–764. doi: 10.1001/archinte.167.8.757. [DOI] [PubMed] [Google Scholar]
- 47.Durán-Cantolla J, Aizpuru F, Martínez-Null C, Barbé-Illa F. Obstructive sleep apnea/hypopnea and systemic hypertension. Sleep Med Rev. 2009;13:323–331. doi: 10.1016/j.smrv.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 48.Durán-Cantolla J, Aizpuru F, Montserrat JM, Ballester E, Terán-Santos J, Aguirregomoscorta JI, Gonzalez M, Lloberes P, Masa JF, De La Peña M, et al. Spanish Sleep and Breathing Group. Continuous positive airway pressure as treatment for systemic hypertension in people with obstructive sleep apnoea: randomised controlled trial. BMJ. 2010;341:c5991. doi: 10.1136/bmj.c5991. [DOI] [PubMed] [Google Scholar]
- 49.Kribbs NB, Pack AI, Kline LR, Getsy JE, Schuett JS, Henry JN, Maislin G, Dinges DF. Effects of one night without nasal CPAP treatment on sleep and sleepiness in patients with obstructive sleep apnea. Am Rev Respir Dis. 1993;147:1162–1168. doi: 10.1164/ajrccm/147.5.1162. [DOI] [PubMed] [Google Scholar]
- 50.Tregear S, Tiller M, Fontanarrosa J, Price N, Akafomo C. Washington, DC: Federal Motor Carrier Safety Administration; 2007. Executive summary: obstructive sleep apnea and commercial motor vehicle driver safety. [Google Scholar]
- 51.Kohler M, Stoewhas AC, Ayers L, Senn O, Bloch KE, Russi EW, Stradling JR. Effects of continuous positive airway pressure therapy withdrawal in patients with obstructive sleep apnea: a randomized controlled trial. Am J Respir Crit Care Med. 2011;184:1192–1199. doi: 10.1164/rccm.201106-0964OC. [DOI] [PubMed] [Google Scholar]
- 52.Berry RB, Kushida CA, Kryger MH, Soto-Calderon H, Staley B, Kuna ST. Respiratory event detection by a positive airway pressure device. Sleep. 2012;35:361–367. doi: 10.5665/sleep.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Douglas NJ, George CF. Treating sleep apnoea is cost effective. Thorax. 2002;57:93. doi: 10.1136/thorax.57.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Peker Y, Hedner J, Norum J, Kraiczi H, Carlson J. Increased incidence of cardiovascular disease in middle-aged men with obstructive sleep apnea: a 7-year follow-up. Am J Respir Crit Care Med. 2002;166:159–165. doi: 10.1164/rccm.2105124. [DOI] [PubMed] [Google Scholar]
- 55.Peker Y, Carlson J, Hedner J. Increased incidence of coronary artery disease in sleep apnoea: a long-term follow-up. Eur Respir J. 2006;28:596–602. doi: 10.1183/09031936.06.00107805. [DOI] [PubMed] [Google Scholar]
- 56.Barbé F, Durán-Cantolla J, Capote F, de la Peña M, Chiner E, Masa JF, Gonzalez M, Marín JM, Garcia-Rio F, de Atauri JD, et al. Spanish Sleep and Breathing Group. Long-term effect of continuous positive airway pressure in hypertensive patients with sleep apnea. Am J Respir Crit Care Med. 2010;181:718–726. doi: 10.1164/rccm.200901-0050OC. [DOI] [PubMed] [Google Scholar]
- 57.Kribbs NB, Pack AI, Kline LR, Smith PL, Schwartz AR, Schubert NM, Redline S, Henry JN, Getsy JE, Dinges DF. Objective measurement of patterns of nasal CPAP use by patients with obstructive sleep apnea. Am Rev Respir Dis. 1993;147:887–895. doi: 10.1164/ajrccm/147.4.887. [DOI] [PubMed] [Google Scholar]
- 58.Patel N, Sam A, Valentin A, Quan SF, Parthasarathy S. Refill rates of accessories for positive airway pressure therapy as a surrogate measure of long-term adherence. J Clin Sleep Med. 2012;8:169–175. doi: 10.5664/jcsm.1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bakker J, Campbell A, Neill A. Randomised controlled trial of auto-adjusting positive airway pressure in morbidly obese patients requiring high therapeutic pressure delivery. J Sleep Res. 2011;20:233–240. doi: 10.1111/j.1365-2869.2010.00846.x. [DOI] [PubMed] [Google Scholar]
- 60.Prasad B, Carley DW, Herdegen JJ. Continuous positive airway pressure device–based automated detection of obstructive sleep apnea compared to standard laboratory polysomnography. Sleep Breath. 2010;14:101–107. doi: 10.1007/s11325-009-0285-z. [DOI] [PubMed] [Google Scholar]
- 61.Desai H, Patel A, Patel P, Grant BJ, Mador MJ. Accuracy of autotitrating CPAP to estimate the residual apnea–hypopnea index in patients with obstructive sleep apnea on treatment with autotitrating CPAP. Sleep Breath. 2009;13:383–390. doi: 10.1007/s11325-009-0258-2. [DOI] [PubMed] [Google Scholar]
- 62.Ueno K, Kasai T, Brewer G, Takaya H, Maeno K, Kasagi S, Kawana F, Ishiwata S, Narui K. Evaluation of the apnea–hypopnea index determined by the S8 auto-CPAP, a continuous positive airway pressure device, in patients with obstructive sleep apnea–hypopnea syndrome. J Clin Sleep Med. 2010;6:146–151. [PMC free article] [PubMed] [Google Scholar]
- 63.Baltzan MA, Dabrusin R, Garcia-Asensi A, Sully JL, Parenteau M, Tansimat G, Kassissia I, Wolkove N. Leak profile inspection during nasal continuous positive airway pressure. Respir Care. 2011;56:591–595. doi: 10.4187/respcare.00977. [DOI] [PubMed] [Google Scholar]
- 64.Stepnowsky CJ, Palau JJ, Marler MR, Gifford AL. Pilot randomized trial of the effect of wireless telemonitoring on compliance and treatment efficacy in obstructive sleep apnea. J Med Internet Res. 2007;9:e14. doi: 10.2196/jmir.9.2.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Smith CE, Dauz ER, Clements F, Puno FN, Cook D, Doolittle G, Leeds W. Telehealth services to improve nonadherence: a placebo-controlled study. Telemed J E Health. 2006;12:289–296. doi: 10.1089/tmj.2006.12.289. [DOI] [PubMed] [Google Scholar]
- 66.Barnes M, Houston D, Worsnop CJ, Neill AM, Mykytyn IJ, Kay A, Trinder J, Saunders NA, Douglas McEvoy R, Pierce RJ. A randomized controlled trial of continuous positive airway pressure in mild obstructive sleep apnea. Am J Respir Crit Care Med. 2002;165:773–780. doi: 10.1164/ajrccm.165.6.2003166. [DOI] [PubMed] [Google Scholar]
- 67.Centers for Medicare & Medicaid Services. National coverage determination (NCD) for continuous positive airway pressure (CPAP) therapy for obstructive sleep apnea (OSA) (240.4). 2008 [accessed 2013 Aug 26]. Available from: http://www.cms.gov/medicare-coverage-database/details/ncd-details.aspx?NCDId=226&bc=AgAAQAAAAAAA&ncdver=3
- 68.Engleman HM, Martin SE, Deary IJ, Douglas NJ. Effect of CPAP therapy on daytime function in patients with mild sleep apnoea/hypopnoea syndrome. Thorax. 1997;52:114–119. doi: 10.1136/thx.52.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Aloia MS, Knoepke CE, Lee-Chiong T. The new local coverage determination criteria for adherence to positive airway pressure treatment: testing the limits? Chest. 2010;138:875–879. doi: 10.1378/chest.09-2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nguyen AT, Baltzan MA, Small D, Wolkove N, Guillon S, Palayew M. Clinical reproducibility of the Epworth Sleepiness Scale. J Clin Sleep Med. 2006;2:170–174. [PubMed] [Google Scholar]
- 71.Flemons WW, Reimer MA. Development of a disease-specific health-related quality of life questionnaire for sleep apnea. Am J Respir Crit Care Med. 1998;158:494–503. doi: 10.1164/ajrccm.158.2.9712036. [DOI] [PubMed] [Google Scholar]
- 72.Flemons WW, Reimer MA. Measurement properties of the Calgary sleep apnea quality of life index. Am J Respir Crit Care Med. 2002;165:159–164. doi: 10.1164/ajrccm.165.2.2010008. [DOI] [PubMed] [Google Scholar]
- 73.Weaver TE, Grunstein RR. Adherence to continuous positive airway pressure therapy: the challenge to effective treatment. Proc Am Thorac Soc. 2008;5:173–178. doi: 10.1513/pats.200708-119MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Weaver TE, Kribbs NB, Pack AI, Kline LR, Chugh DK, Maislin G, Smith PL, Schwartz AR, Schubert NM, Gillen KA, et al. Night-to-night variability in CPAP use over the first three months of treatment. Sleep. 1997;20:278–283. doi: 10.1093/sleep/20.4.278. [DOI] [PubMed] [Google Scholar]
- 75.McArdle N, Devereux G, Heidarnejad H, Engleman HM, Mackay TW, Douglas NJ. Long-term use of CPAP therapy for sleep apnea/hypopnea syndrome. Am J Respir Crit Care Med. 1999;159:1108–1114. doi: 10.1164/ajrccm.159.4.9807111. [DOI] [PubMed] [Google Scholar]
- 76.Krieger J. Long-term compliance with nasal continuous positive airway pressure (CPAP) in obstructive sleep apnea patients and nonapneic snorers. Sleep. 1992;15(6, Suppl):S42–S46. doi: 10.1093/sleep/15.suppl_6.s42. [DOI] [PubMed] [Google Scholar]
- 77.Rosenthal L, Gerhardstein R, Lumley A, Guido P, Day R, Syron ML, Roth T. CPAP therapy in patients with mild OSA: implementation and treatment outcome. Sleep Med. 2000;1:215–220. doi: 10.1016/s1389-9457(00)00012-5. [DOI] [PubMed] [Google Scholar]
- 78.Reeves-Hoche MK, Meck R, Zwillich CW. Nasal CPAP: an objective evaluation of patient compliance. Am J Respir Crit Care Med. 1994;149:149–154. doi: 10.1164/ajrccm.149.1.8111574. [DOI] [PubMed] [Google Scholar]
- 79.Sanders MH, Gruendl CA, Rogers RM. Patient compliance with nasal CPAP therapy for sleep apnea. Chest. 1986;90:330–333. doi: 10.1378/chest.90.3.330. [DOI] [PubMed] [Google Scholar]
- 80.Budhiraja R, Parthasarathy S, Drake CL, Roth T, Sharief I, Budhiraja P, Saunders V, Hudgel DW. Early CPAP use identifies subsequent adherence to CPAP therapy. Sleep. 2007;30:320–324. [PubMed] [Google Scholar]
- 81.Pamidi S, Knutson KL, Ghods F, Mokhlesi B. The impact of sleep consultation prior to a diagnostic polysomnogram on continuous positive airway pressure adherence. Chest. 2012;141:51–57. doi: 10.1378/chest.11-0709. [DOI] [PubMed] [Google Scholar]
- 82.Conwell W, Patel B, Doeing D, Pamidi S, Knutson KL, Ghods F, Mokhlesi B. Prevalence, clinical features, and CPAP adherence in REM-related sleep-disordered breathing: a cross-sectional analysis of a large clinical population. Sleep Breath. 2012;16:519–526. doi: 10.1007/s11325-011-0537-6. [DOI] [PubMed] [Google Scholar]
- 83.Kohler M, Smith D, Tippett V, Stradling JR. Predictors of long-term compliance with continuous positive airway pressure. Thorax. 2010;65:829–832. doi: 10.1136/thx.2010.135848. [DOI] [PubMed] [Google Scholar]
- 84.Joo MJ, Herdegen JJ. Sleep apnea in an urban public hospital: assessment of severity and treatment adherence. J Clin Sleep Med. 2007;3:285–288. [PMC free article] [PubMed] [Google Scholar]
- 85.Platt AB, Field SH, Asch DA, Chen Z, Patel NP, Gupta R, Roche DF, Gurubhagavatula I, Christie JD, Kuna ST. Neighborhood of residence is associated with daily adherence to CPAP therapy. Sleep. 2009;32:799–806. doi: 10.1093/sleep/32.6.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Simon-Tuval T, Reuveni H, Greenberg-Dotan S, Oksenberg A, Tal A, Tarasiuk A. Low socioeconomic status is a risk factor for CPAP acceptance among adult OSAS patients requiring treatment. Sleep. 2009;32:545–552. doi: 10.1093/sleep/32.4.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Brown LK. Adherence-based coverage of positive airway pressure treatment for sleep apnea: the “brave new world” of cost-saving strategies. Curr Opin Pulm Med. 2011;17:403–405. doi: 10.1097/MCP.0b013e32834bdf6e. [DOI] [PubMed] [Google Scholar]
- 88.Brown LK. Use it or lose it: Medicare’s new paradigm for durable medical equipment coverage? Chest. 2010;138:785–789. doi: 10.1378/chest.10-1168. [DOI] [PubMed] [Google Scholar]
- 89.Iber C, Ancoli-Israel S, Chesson A, Quan SF. Westchester, IL: American Academy of Sleep Medicine; 2007. The AASM manual for the scoring of sleep and associated events: rules, terminology, and technical specifications. [Google Scholar]
- 90.Ye L, Pack AI, Maislin G, Dinges D, Hurley S, McCloskey S, Weaver TE. Predictors of continuous positive airway pressure use during the first week of treatment. J Sleep Res. 2012;21:419–426. doi: 10.1111/j.1365-2869.2011.00969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


