Abstract
Solid tumors, beyond mere accumulation of cancer cells, form a complex ecosystem consisting of normal epithelial cells, fibroblasts, blood and lymphatic vessels, structural components, and infiltrating hematopoietic cells including myeloid and lymphoid elements that impact tumor growth, tumor spreading, and clinical outcome. The composition of the immune microenvironment is diverse, including various populations of T cells, B cells, dendritic cells, natural killer cells, myeloid-derived suppressor cells, neutrophils, or macrophages. The immune contexture describes the density, location, and organization of these immune cells within solid tumors. In lung cancer, which is the deadliest type of cancer, and particularly in non–small cell lung cancer, its most prevalent form, reports have described some of the interactions between the tumor and the host. These data, in addition to articles on various types of tumors, provide a greater understanding of the tumor–host microenvironment interaction and stimulate the development of prognostic and predictive biomarkers, the identification of novel target antigens for therapeutic intervention, and the implementation of tools for long-term management of patients with cancer.
Keywords: non–small cell lung cancer, tumor immunology, immunotherapy, prognosis
Worldwide, lung cancer is the most common cause of cancer-related deaths (1). There are several histological subtypes of lung cancer, which differ in their relative frequency, location within the lung, and tendency to metastasize. Small cell lung cancer (SCLC), which often presents with widespread disease at the time of diagnosis, represents about 15% of all lung cancer cases (2). Non–small cell lung cancer (NSCLC) represents the remaining 85% of lung cancers, and the 5-year overall survival (OS) rate is about 15% (3). At present, NSCLC staging is based on the seventh tumor/lymph node/metastasis (TNM) classification (4, 5). This classification provides a standardized description of the disease and describes the severity of cancer based on the size and/or extent of the primary tumor, and whether or not it has spread to lymph nodes and/or distant organs. TNM stage grouping is currently considered the best determinant of the prognosis of patients with NSCLC (6, 7).
We have witnessed a change of view of cancer as an autonomous cellular disease comprising six biological capabilities (8) to that of a regulated disease involving the immune components of their microenvironment, called immune contexture. To their original hallmarks of cancer published in 2000 (8), Hanahan and Weinberg added two emerging hallmarks and two enabling characteristics, including “evading immune destruction” and “tumor-promoting inflammation” (9). This major evolution in the view of neoplastic disease reflects that cancer is now considered a complex ecosystem with many players that could impact tumor growth, spread, and clinical outcome (10, 11). During cancer development, tumor cells interact with their microenvironment and the immune contexture shapes their eventual destiny. Increasing evidence has established the potential key contribution of tumor-infiltrating immune cells in the development and progression of cancers and in the tumor response to therapy (12, 13). In colorectal cancer, a strong association between the quality, quantity, and coordination of intratumoral immune cell infiltration and patient survival has been elegantly reported (14, 15). Strikingly, the type and density of T cells infiltrating these tumors were found to be more powerful prognostic factors than standard pathological criteria, underscoring the need for clinicians to consider infiltrating immune cells when determining the prognosis of patients with cancer (16). Moreover, the organization of the immune cells and more particularly the presence of local lymph node–like structures seem to be of major importance in shaping protective antitumor immune responses (17–19).
There is now growing evidence that innate and adaptive immune cells interact in the lung tumor microenvironment and that the immune contexture of these events (i.e., the structural and functional links between local immunity and other variables in the tumor microenvironment) impacts cancer cells and clinical outcome. Here, we review the association between NSCLC and immune surveillance, immune escape, and immune subversion mechanisms as well as the prognostic value of the tumor immune microenvironment.
Immunosurveillance of Lung Cancer
General Concept
The immune-editing concept proposed by Dunn and colleagues (20), reflecting the evolution of the interactions between immune and tumor cells during cancer development, can be applied to NSCLC. Although it is difficult to provide proper proof of immunosurveillance in humans (21), a large body of evidence supports the idea that developing cancers are not ignored by the immune system. Evidence for immunosurveillance of lung cancer can be found in patients with HIV infection who have an increased frequency of virally induced malignancies (22, 23), and in immunosuppressed organ transplant recipients who have a higher risk of developing NSCLC (24). Further support of immunosurveillance in NSCLC can be found in studies that have demonstrated the ability of the immune system to spontaneously recognize tumor-associated antigens (TAAs) (25). In addition, immune-mediated paraneoplastic syndromes are often encountered in lung cancer (26). These syndromes occur in up 15% of patients with cancer, and lung cancer was found to be the malignancy most frequently associated with paraneoplastic syndrome (27). Paraneoplastic neurologic disorders develop in patients with cancer when an efficient antitumor immune reaction attacks the nervous system, because targeting antigens expressed by cancer cells share antigenic properties with healthy neurons (28). Autoimmune responses against Hu proteins have been described, and HuD, a Hu family member normally expressed in neurons, is frequently observed as aberrantly expressed in lung cancer cells. There is other evidence that patients with lung cancer may also produce antibodies against other antigens. One example, among others, is found in studies showing the presence of SOX antibodies in the sera of patients with lung cancer with and without paraneoplastic neurologic disorders (29). Together, these results show that lung cancers can be immunogenic tumors eliciting different serum antibody responses. Moreover, as reported for other solid tumors, the immune microenvironment of NSCLC has a strong prognostic value (12). Altogether, these data support the idea that the immune system is able to recognize and eliminate malignant cells.
Actors in Immunosurveillance
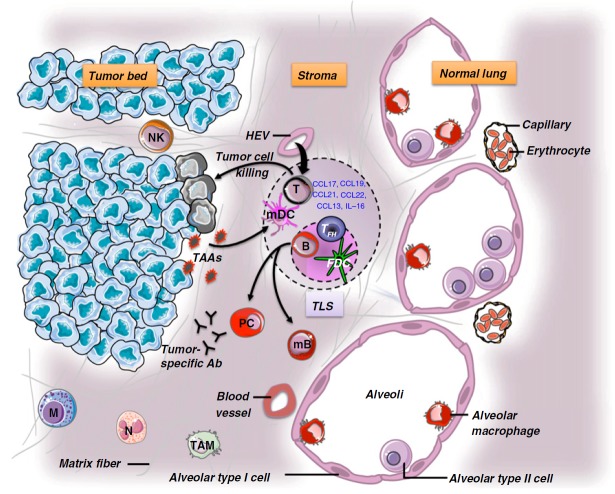
In lung tumors, the immune infiltrate comprises adaptive and innate immune cell populations (12, 30, 31). These immune cells are not randomly distributed within the tumor but rather are highly organized (Figure 1) (18). This high degree of organization is reflected by the presence of ectopic lymph node–like structures or tertiary lymphoid structures (TLSs). As reported for infectious diseases (32), autoimmune disorders (33), and allograft rejection (34), the intratumor ectopic lymphoid structures are composed of a B-cell follicle containing follicular helper T cells, tingible body macrophages and a network of follicular dendritic cells, and a T-cell area with mature dendritic cells (DCs), and are surrounded by high endothelial venules (Figure 1) (18, 35). Direct evidence of class switching has not been reported yet, but it has been observed that activation-induced cytidine deaminase, an indispensable enzyme in both class switch recombination and somatic hypermutation, is expressed by germinal center B cells of some ectopic lymphoid structures (36). Several integrins, adhesion molecules, and chemokines are likely to play a role in the recruitment of T cells from the blood to TLSs (35). As previously shown in secondary lymphoid organs (37), it has been hypothesized that this process implicates rolling and transmigration via high endothelial venules expressing peripheral node addressin (35). The role of TLSs has been well documented in respiratory immunity during viral infections. Notably, Moyron-Quiroz and colleagues have shown in two elegant studies that mice lacking spleen, lymph nodes, and Peyer’s patches were able to mount robust primary but also secondary B- and T-cell responses to inhaled influenza, due to compensatorily developed TLSs (38, 39). A body of evidence suggests that the presence of TLSs impacts on the local immune microenvironment in the lung. First, high densities of TLSs are associated with high numbers of activated and effector-memory CD8+ T cells inside the tumor (40). Another argument for a role of TLSs in the antitumor immune reaction is the presence of measurable IgG and/or IgA to tumor antigens from B cells purified from TLS-containing lung tumor tissues (Figure 1). Indeed, Germain and colleagues have reported that among 34 patients with NSCLC tested, more than 40% developed antibody reactivity against up to seven different TAAs (including LAGE-1, melanoma antigen [MAGE] family antigens, TP53, and NY-ESO-1) (36). In lung cancer, the positive correlation between high densities of TLSs and improved survival of patients supports the idea that these ectopic lymphoid structures are important in shaping protective immune responses (18, 36, 40). Interestingly, we found that patients with few TLSs had poor survival despite high numbers of infiltrating CD8+ T cells, suggesting that CD8+ T cells not educated in situ within TLSs are inefficient in controlling tumor progression in early- and late-stage NSCLC treated or not with neoadjuvant chemotherapy (40; R. Remark and D. Damotte, unpublished data). These data suggest that an immune reaction might occur within lung tumors independently of the secondary lymphoid organs. However, further analyses on the potential association between the presence of TLSs and the immunogenicity of tumors, as well as the specificity of the immune response, are needed to know the drivers leading to the formation of such structures and whether they are specific to tumor cells.
Figure 1.
The immune contexture of non–small cell lung cancer (NSCLC). The immune microenvironment of lung tumors is composed of T cells, B cells, natural killer (NK) cells, mature and immature dendritic cells (DCs), tumor-associated macrophages (TAMs), neutrophils, and mast cells. The great majority of immune cells are found at the interface between the tumor and the normal tissue, and some of them are organized in tertiary lymphoid structures (TLSs). The latter are considered a gateway for the entrance of immune cells from the blood to the tumor (via peripheral node addressin–expressing high endothelial venules [HEVs]). This process is highly regulated through chemokine/chemokine receptors, interleukins, integrins, and adhesion molecule expression or secretion. Ab = antibody; B = B cell; FDC = follicular dendritic cell; M = mast cell; mB = memory B cell; mDC = mature dendritic cell; N = neutrophil; PC = plasma cell; T = T cell; TAAs = tumor-associated antigens; TFH = follicular helper T cell.
Few studies have been performed on the immune contexture in metastases or lymph nodes. Unpublished data from our group revealed that an immune contexture is present and organized in primary lung tumors, liver metastases from lung cancer, and lung metastases of various origins (colorectal, renal cell, and breast cancers, as well as melanoma). We also reported that whereas the immune contexture differs between lung metastases from colorectal cancer and lung metastases from renal cell carcinomas, there was a significant correlation in the density of immune cell infiltrates between primary and metastatic sites in the same patient, conferring similar prognostic value (41). This comparative analysis of metastases from colorectal and renal cell cancers within the same organ, the lung, in relation to the primary site suggested that the malignant cell rather that the host tissue influences the establishment of a specific immune contexture driving clinical outcome.
Immunosuppressive Cells in the Immune Microenvironment
Myeloid-derived suppressor cells (MDSCs) are important components of the immune suppressive network and can inhibit host protective antitumor immunity (42). By producing vascular endothelial growth factor, basic fibroblast growth factor, hypoxia-induced factor 1, tumor growth factor (TGF)-β, matrix metalloproteinase 9, and generating reactive oxygen species, MDSCs create a favorable environment for tumor growth and metastasis, as well as neoangiogenesis (42–44). In the lung, these myeloid cells expressing l-arginase and inducible nitric oxide synthase are implicated in the suppression of CD8+ T-cell proliferation and decrease CD3ζ expression (45, 46). MDSC numbers are associated negatively with responsiveness to chemotherapy and positively with shorter survival (45, 46). In a murine model of lung cancer, the targeting of MDSCs enhanced effector and memory CD8+ T-cell responses, as well as natural killer (NK) cell and antigen-presenting cell activity (47).
Regulatory T cells (Tregs) are also found in NSCLC and play a role in the control of antitumor immune reactions by the following mechanisms: (1) inducing the expression of B7-H4 by antigen-presenting cells (APCs) (48), (2) promoting the direct killing of T cells and APCs (49, 50), (3) inducing the expression of indoleamine 2,3-dioxygenase by APCs (51), (4) disturbing cell metabolism via the production of adenosine, or (5) secreting IL-10, IL-35, and TGF-β (52). In lung tumors, these cells are likely to play a role in suppressing cytotoxic T-cell responses (30). As suggested in breast cancer, the location of Tregs in the tumor microenvironment seems of major importance in the inhibition of antitumor immune responses (53).
Tumor Intrinsic Factors with Immunosuppressive or Immunogenic Potential
As described in many neoplastic diseases, lung tumor cells develop escape mechanisms. It has been demonstrated that many effector immune cells are anergic and have reduced functions in the lung tumor microenvironment (23, 31). In addition, lung tumor cells may induce a loss or down-regulation of HLA class I molecules during tumor progression, modulating the susceptibility of tumor cells to lysis by cytotoxic CD8+ T lymphocytes and NK cells (54). It has also been reported that lung cancer cells are able to produce immunosuppressive factors in the tumor microenvironment. For example, they can secrete a soluble form of MHC class I chain–related molecule A, inducing down-regulation of NKG2D expression of CD8+ T cells and NK cells, thereby impairing lysis of cancer cells by these effector cells (55). Tumor cells are also known to secrete immunosuppressive cytokines such as IL-10 or TGF-β or the tryptophan catabolic enzyme indoleamine 2,3-dioxygenase, which are key players involved in the inhibition of immune responses and enhancing the proliferation of cancer cells (56, 57).
Lung cancer cells, among other components of the tumor microenvironment, express chemokine receptors and produce chemokines that regulate the trafficking of immune and cancer cells. CXCR4, which promotes tumor cell survival, proliferation, invasion, and metastasis, was reported as being overexpressed in tumor cells as compared with normal cells (58). The role of the CXCR4–CXCL12 axis has been highlighted in the metastatic process in NSCLC (59). It has also been suggested that the CCL20–CCR6 axis promotes NSCLC disease progression via their proinflammatory and proliferative effects (60). Finally, CXCR1 and CXCR2 as well as their ligand CXCL8 are expressed and secreted by NSCLC cells (61). CXCL8 could act as a growth factor for tumor cells in an autocrine and/or paracrine manner.
It is also important to note that some genetic alterations have been described in lung cancers (62). The most frequently encountered in lung tumors involve mutations in KRAS, TP53, FHIT, EGFR, CDKN2, LKB1, RB, and MYC genes. Epigenetic modifications leading to changes in the degree of methylation or acetylation of histone and inducing a modification in gene expression have also been reported, such as hypermethylation of TP16, CDH13, or adenomatous polyposis coli (63, 64). There is now increasing evidence that oncogenes impact the tumor microenvironment to promote immune escape. For example, Akbay and colleagues have demonstrated in a murine lung tumor model that signaling via mutant epidermal growth factor receptor in tumor cells directly up-regulates tumor programmed cell death ligand 1 (PD-L1) expression (65). It has also been shown that the loss of Lkb1 and Pten leads to lung squamous cell carcinoma with elevated PD-L1 expression (66). In a model of pancreatic cancer, it has been proposed that oncogenic Kras restrained the antitumor immune response through the production of granulocyte-macrophage colony-stimulating factor and the subsequent suppression of T-cell immunity (67). In the lung, several tumor antigens have been found: antigens encoded by cancer germline genes or mutated antigens. The presence of these cancer germline antigens was found to be associated with poor prognosis (68). Yet, antibodies and/or circulating effector T cells can spontaneously develop against alterations observed in NSCLC. For example, at the time of surgery, 7% of patients with NSCLC have circulating serum antibodies to NY-ESO-1 and another 7% have antibodies to TP53 (S. Gnjatic and N. Altorki, unpublished data). The presence of naturally occurring antibody responses to NY-ESO-1 usually correlates with the presence of circulating specific CD4+ and CD8+ T cells (69). TAAs such as MAGE-A3 have been used to vaccinate patients with NSCLC in the adjuvant setting (70), although unfortunately no clinical benefit was observed in a randomized phase 3 trial. Indeed, this antigen-specific cancer immunotherapeutic (MAGRIT) failed to hit first and second coprimary end points, as it did not significantly extend disease-free survival when compared with placebo.
Immune Checkpoint Expression and Immunotherapeutic Intervention in NSCLC
Several reports have shown that tumors are able to coopt certain immune checkpoint pathways to escape control by the immune system, particularly by T cells that are specific for tumor antigens (71). Indeed, effective antitumor immunity depends at least in part on cytotoxic T lymphocytes, whose fate and killing activity are the results of a balance between positive and negative signals conferred through interactions between various coregulatory receptors and ligands. They include inhibitory molecules such as programmed cell death protein-1 (PD-1), cytotoxic T-lymphocyte–associated protein-4 (CTLA-4), and T-cell immunoglobulin and mucin domain-3 (TIM-3) that can be expressed by T cells, and their ligands (PD-L1, PD-L2, B7-H2, etc.) that are expressed by APCs and cancer cells (71).
In NSCLC, it has been reported that 20–60% of tumors were positive for PD-L1 and/or PD-L2 at lower frequency (72). The expression of PD-L1 and PD-L2 on tumor cells was demonstrated on the cell membrane, in the cytoplasm, or both, in a focal or scattered pattern. The expression levels of both ligands were usually lower on tumor cells than on activated monocytes. High expression of PD-1 is frequent on activated and exhausted TILs (73). In the lung, high expression of PD-1 on tumor-infiltrating CD8+ T cells was associated with impaired T-cell function. Blocking the PD-1/PD-L1 pathway induced increased T-cell proliferation and cytokine production. High PD-L1 tumor expression has been correlated with poor prognosis in some studies (74, 75). However, some studies have reported a lack of association with clinical outcomes or even with improved influx into and survival of lymphocytes in the tumor microenvironment (72). Velcheti and colleagues have shown an association of PD-L1 protein expression with increased TILs and longer survival (76). One explanation among others is that the blockade of PD-1/PD-L1 interactions affects the development, maintenance, and function of PD-1–expressing Tregs (77). CTLA-4 was found on the cell surface and in the cytoplasm of tumor cells in about 50% of patients with NSCLC. The expression pattern was heterogeneous. CTLA-4 overexpression was found to be associated with good survival (78). This unexpected result was explained by the expression of CTLA-4 by tumor cells that can mediate negative signals, comparable with those observed in T cells, and possibly leading to a reduced inflammatory microenvironment.
Because most of the immune checkpoints are initiated by ligand–receptor interactions, they can be blocked or modulated by specific antibodies (79, 80). Ipilimumab, a monoclonal blocking antibody (IgG1) against CTLA-4, was the first of this class of cancer immunotherapeutics to be approved by the U.S. Food and Drug Administration (FDA) and more recently, the FDA also approved pembrolizumab, a monoclonal antibody (IgG4) against PD-1. Although NSCLC has not traditionally been considered an immunogenic disease, a better understanding of immunosurveillance and the identification of new targets of immunomodulation have led to the development of several clinical trials of checkpoint blockade therapies (81) to enhance natural antitumor immunity and induce strong and durable clinical responses in patients with NSCLC (Table 1). Interestingly, it has been suggested that individuals with preexisting antitumor immune responses may be those who are more likely to experience clinical benefit from checkpoint-blocking immunotherapies (82). In patients with melanoma treated with immune checkpoint blockade agents, it has been suggested that a preexisting T cell–rich tumor microenvironment could be a predictive marker of good response to therapy (83). Similar analyses are still missing in NSCLC. PD-L1 expression on tumor cells has been reported as a potential predictive biomarker for PD-1 blockade (84), but data are still limited. Although some other associations between tumor microenvironment biomarkers and clinical activity have been reported (85, 86), they still need to be more intensively investigated to validate them as predictive markers of response or for selection of patients most likely to respond to the treatment. These new immunotherapeutic interventions represent a major breakthrough in oncology, especially for the treatment of patients with advanced or metastatic lung cancer who fail to respond to chemotherapy or who relapse. Checkpoint inhibition stands in contrast to a variety of previous trials targeting the immune system in patients with NSCLC who have largely failed in the clinic (IFN, IL-2, vaccination, etc.) (22). One of the largest phase 3 studies in NSCLC with MAGE-A3 protein–based immunotherapy did not meet its primary end points, and a planned subanalysis using a predictive immune gene signature as a surrogate for immune contexture unfortunately could not be done. The limited success of these clinical trials could be explained by the immunosuppressive properties of the tumor microenvironment that counteract T-cell activity, but also by weak mobilization of immunity, immunoselection, or immune escape. Checkpoint blockade may, however, revive some of these failed strategies through future rational combinations to further improve clinical efficacy.
Table 1.
Summary of Clinical Trials Evaluating Various Immune Checkpoint Mediators for Patients with Non–Small Cell Lung Cancer
| Target | State of Clinical Development | Trial Design | Conclusions |
|---|---|---|---|
| CTLA-4 | Phase 1 trial (NCT02040064) | Open-label, safety, tolerability, and efficacy study of tremelimumab plus gefitinib in EGFR-mutated patients with advanced or metastatic NSCLC. The expected enrollment is 24 patients | NA |
| Phase 2 trial (NCT00312975) | Randomized trial comparing tremelimumab with best supportive care after first-line platinum-based therapy in patients with locally advanced or metastatic NSCLC. The 87 patients enrolled received tremelimumab (n = 44) or best supportive care (n = 43) | Tremelimumab was tolerable, with safety consistent with prior studies. Among patients receiving tremelimumab, there were 4.8% partial responses and 16.6% stable diseases, compared with 0% and 14.3% patients receiving best supportive care, respectively. However, progression-free survival analysis did not demonstrate superiority of tremelimumab over best supportive care | |
| Phase 2 study (NCT01998126) | Trial assessing the toxicity of ipilimumab and erlotinib in EGFR-mutated patients and toxicity of ipilimumab and crizotinib in ALK-mutated patients with stage IV NSCLC. Estimated enrollment: 46 subjects | NA | |
| Phase 2 study (NCT00527735) | Multicenter trial including 204 chemotherapy-naive patients with stage IIIB–IV NSCLC. Subjects were assigned to a control arm (up to six doses of placebo plus chemotherapy—paclitaxel and carboplatin), concurrent anti–CTLA-4 antibody (ipilimumab) regimen (four doses of anti–CTLA-4 antibody plus the same combination of chemotherapeutic drugs followed by two doses of placebo plus chemotherapy), or phased ipilimumab regimen (two doses of placebo plus chemotherapy followed by four doses of ipilimumab plus chemotherapy) | The authors have reported that phased ipilimumab plus chemotherapy improved progression-free survival. However, the clinical benefit was only slight | |
| Phase 2 study (2006-000568-95) | Randomized, noncomparative study using anti–CTLA-4 (tremelimumab) or best supportive care in patients with NSCLC (stage IIIB or IV disease) that has responded or remained stable after platinum-based therapy | NA | |
| Phase 3 trial (NCT01285609) | Randomized, multicenter, double-blind trial in patients with stage IV/recurrent squamous NSCLC comparing the effect of ipilimumab plus paclitaxel and carboplatin vs. placebo plus paclitaxel and carboplatin is currently recruiting patients. Estimated enrollment: 920 subjects | NA | |
| PD-1 | Phase 1 trial (NCT01295827) | Exploration of the clinical activity and efficacy of anti–PD-1 antibody (MK-3475, lambrolizumab) in subjects with locally advanced or metastatic NSCLC. The efficacy of low, medium, and high doses of the drug in combination with standard chemotherapy in participants with locally advanced or metastatic NSCLC and the effects of low and high doses of blocking antibody in treatment-naive and previously treated participants with NSCLC with PD-L1 gene expression were also assessed | Some adverse events have been reported, but most of them were low grade. In this trial, the potential clinical benefits of the anti–PD-1 antibody are still being explored |
| Phase 1 trial (NCT01840579) | Study using pembrolizumab (MK-3475) alone or in combination with cisplatin/pemetrexed or carboplatin/paclitaxel in patients with advanced NSCLC. Estimated enrollment: 30 subjects | NA | |
| Phase 1 study (NCT02007070) | Open-label, nonrandomized, multicenter study of MK-3475 in subjects with PD-L1+ advanced NSCLC. Estimated enrollment: 24 subjects | NA | |
| Phase 1 trial (NCT01454102) | Multiarm safety study of nivolumab in combination with platinum doublet, bevacizumab maintenance, erlotinib, ipilimumab, or nivolumab alone in subjects with stage IIIB/IV NSCLC. Estimated enrollment: 412 patients | NA | |
| Phase 1/2 trial (NCT02039674) | Trial assessing the safety, tolerability, and efficacy of pembrolizumab (MK-3475) in combination with chemotherapy or immunotherapy in participants with locally advanced or metastatic NSCLC. Estimated enrollment: 320 subjects | NA | |
| Phase 2 trial (NCT01721759) | Assessment of the objective response rate in patients with advanced or metastatic squamous NSCLC treated with nivolumab (BMS-936558) after failure of two prior systemic regimens. Study ongoing (estimated enrollment: 100 patients) | NA | |
| Phase 2 trial (NCT01928576) | Nivolumab monotherapy after azacitidine + entinostat or after oral azacitidine (epigenetic priming study). Estimated enrollment: 120 subjects | NA | |
| Phase 2/3 trial (NCT01905657) | Study of low/high doses of MK-3475 (pembrolizumab) vs. docetaxel in patients with NSCLC who have experienced disease progression after platinum-based chemotherapy. The estimated enrollment is 920 subjects with PD-L1–positive tumor | NA | |
| Phase 3 trial (NCT00527735) | Randomized analysis of the clinical value of BMS-936558 (nivolumab) in 122 patients with NSCLC after failure of prior therapy | Objective and durable responses have been reported in patients with squamous (response rate, 33%) as well as in nonsquamous histological type (response rate, 12%) | |
| Phase 3 trial (NCT01642004) | Study of nivolumab (BMS-936558) compared with docetaxel in subjects with advanced or metastatic squamous NSCLC after failure of prior platinum-based chemotherapy. Estimated enrollment: 264 subjects | NA | |
| Phase 3 trial (NCT01673867) | Nivolumab (BMS-936558) monotherapy vs. docetaxel in metastatic nonsquamous cell NSCLC after failure of prior platinum-based chemotherapy. Estimated enrollment: 582 subjects | NA | |
| PD-L1 | Phase 1 study (NCT01846416) | Multicenter, single-arm study evaluating the efficacy and safety of MPDL3280A in advanced or metastatic PD-L1–positive NSCLC. This study is ongoing, but not recruiting participants (n = 128) | NA |
| Phase 1 trial (NCT02013219) | Open-label, multicenter study assessing the safety, tolerability, and pharmacokinetics of MPDL3280A and erlotinib administered in combination to patients with NSCLC. Thirty-two patients are expected to be enrolled in this trial | NA | |
| Phase 1 trial (NCT00729664) | Safety, clinical activity, and tolerability assessment of anti–PD-L1 antibody (BMS-936559) in 75 patients with advanced NSCLC. Other patients with cancer were also included in this trial (55 with melanoma, 18 with colorectal cancer, 17 with renal cell cancer, 17 with ovarian cancer, 14 with pancreatic cancer, 7 with gastric cancer, and 4 with breast cancer) | Among 75 patients, 49 were included in the efficacy analysis. Five objective responses were observed at doses of 3 and 10 mg/kg, with response rates of 8 and 16%, respectively | |
| Phase 1 study (NCT01375842) | Assessment of MPDL3280A, in 85 pretreated patients with advanced or metastatic NSCLC | The treatment was well tolerated and often yielded rapid, durable responses (overall response rate, 23%). Objective response was particularly pronounced in the population with the highest level of PD-L1 expression | |
| Phase 1 study (NCT02000947) | Open-label study evaluating the tolerability and safety of MEDI4736 in combination with tremelimumab in subjects with advanced NSCLC. Estimated enrollment: 208 patients | NA | |
| Phase 2 trial (NCT02031458) | Multicenter, single-arm study evaluating the efficacy and safety of MPD L3280A in patients with PD-L1+ locally advanced or metastatic NSCLC. Estimated enrollment: 635 subjects | NA | |
| Phase 2 trial (NCT01903993) | Multicenter, open-label, randomized trial evaluating the efficacy and safety of MPDL3280A compared with docetaxel in patients with advanced or metastatic NSCLC that failed to respond to platinum-based treatment. Enrollment: 287 subjects | NA | |
| Phase 3 study (NCT02008227) | Randomized trial evaluating the efficacy and safety of MPDL3280A compared with docetaxel in patients with locally advanced or metastatic NSCLC after failure with platinum-based chemotherapy treatment. Estimated enrollment: 850 patients | NA |
Definition of abbreviations: ALK = anaplastic lymphoma kinase; CTLA-4 = cytotoxic T-lymphocyte–associated protein-4; EGFR = epidermal growth factor receptor; NA = not available; NSCLC = non–small cell lung cancer; PD-1 = programmed cell death protein-1; PD-L1 = programmed cell death ligand-1.
Prognostic Immune Markers in NSCLC
In a large array of various primary and metastatic tumors, it has been reported that the immune microenvironment is often associated with the clinical outcome of patients (87, 88). A worldwide task force is currently working to establish the immunoscore as an independent prognostic marker for colorectal cancer (89).
In NSCLC, several studies have investigated the association between the presence and density of adaptive or innate immune cell populations and patient survival (Table 2). In the majority of studies, high CD3+, CD8+, or CD4+ T-cell infiltrations were associated with a favorable prognosis. Tregs were associated with poor survival in NSCLC. The ratio of stromal FoxP3 to CD3-positive cells was also significantly associated with a higher risk of relapse (Table 2). It is important to highlight the absence of specific markers of regulatory T cells in immunohistochemistry, and most of the investigators used FoxP3 or CD25 (also expressed at low levels by activated T cells) as surrogate markers of Tregs. As previously stated, the location of Tregs and their presence within TLSs could be of major importance in their impact on the survival of patients with NSCLC (90). One report demonstrated an inverse clinical correlation between the density of IL-17 (a cytokine produced mainly by Th17 cells)–positive cells and patient survival (Table 2). This finding is in accordance with the proinflammatory properties of Th17 cells. In contrast to T cells, the clinical impact of B cells is poorly understood. Every stage of B-cell differentiation has been observed in NSCLC. In general, high densities of B cells were correlated with increased survival (Table 2). We can hypothesize that B cells play a role in antitumor immunity, perhaps by capturing and presenting tumor antigens to T cells directly or by generating tumor antigen–specific antibodies that target tumor antigens in the form of immune complexes to professional APCs. As previously stated, NK cells are also present in the microenvironment of NSCLC. As nonspecific markers have been used in the majority of studies, the clinical impact of NK cells is poorly understood. However, using the NKp46 marker, it has been suggested that the density of NK cells does not correlate with clinical outcome in early-stage NSCLC (Table 2). The altered phenotype of NK cells in the tumor microenvironment could explain this absence of clinical impact. As reported for other solid cancers (11), tumor-associated macrophages (TAMs) represent another major component of the immune microenvironment of NSCLC. In an overly simplistic manner, TAMs have been classified as “proinflammatory” M1 macrophages with antitumor activity, and “proangiogenic and immunosuppressive” M2 macrophages with protumor activity. In NSCLC, TAMs with a M1 phenotype were associated with good survival and high M2 macrophage number correlated with a poor prognosis (Table 2). However, a more comprehensive analysis of the clinical impact of TAMs is still needed. Neutrophils represent a significant portion of infiltrating inflammatory cells, but little is known about their role in NSCLC. It has been suggested that high neutrophil counts are associated with a higher risk of relapse but not with survival (Table 2). Their potentially different, specialized functional phenotype has never been taken into account in these analyses. DCs are major regulators of immune response and can elicit T-cell responses unlike any other APCs. In NSCLC, mature DCs are located exclusively within TLSs and are associated with a good prognosis (Table 2). Because the density of mature DCs and the number of TLSs correlate with each other, it has been proposed that the presence of TLSs is associated with a good prognosis in patients with NSCLC (18).
Table 2.
Summary of Prognostic Immune Markers in Non–Small Cell Lung Cancer
| Marker | Author and Year | Patients (n) | Stage(s) | Conclusions |
|---|---|---|---|---|
| T cells | Johnson et al. (2000) (91) | 95 | I (57%) | High CD3+ in tumor correlated with longer OS |
| II (18%) | ||||
| III (21%) | ||||
| Hiraoka et al. (2006) (92) | 109 | I (61%) | Concurrent high CD4+ and CD8+ in stroma correlated with longer survival | |
| II–III (39%) | ||||
| Kikuchi et al. (2007) (93) | 161 | I (59%) | HLA class I expression correlates with longer OS in stage I | |
| II–IV (41%) | HLA class I expression correlated with CD8+ cells | |||
| Ruffini et al. (2009) (94) | 1,290 | I (55%) | TILs (mostly CD8+ cells) in tumor correlated with better OS | |
| II (21%) | ||||
| IIIA (17%) | ||||
| Wakabayashi et al. (2003) (95) | 178 | I (60%) | High CD4+ in stroma correlated with longer OS | |
| II (13%) | High CD8+ in tumor correlated with shorter OS | |||
| IIIA (27%) | ||||
| Al-Shibli et al. (2008) (96) | 335 | I (63%) | High CD4+ in stroma correlated with longer DSS | |
| II (27%) | High CD8+ in stroma correlated with longer DSS | |||
| IIIA (10%) | ||||
| Kawai et al. (2008) (97) | 199 | IV (100%) | Predominant distribution of CD8+ T cells in cancer nests as opposed to cancer stroma correlated with longer OS | |
| Suzuki et al. (2013) (98) | 956 | I (100%) | Densities of CD3+, CD4+, CD8+, CD45RO+ are not associated with RFP | |
| Relative proportion of stromal FoxP3+ to CD3+ correlated with RFP | ||||
| Goc et al. (2014) (40) | 376 | I (44%) | High CD8+ T cells correlated with longer survival | |
| II (27%) | ||||
| III (28%) | ||||
| IV (0.5%) | ||||
| ND (0.5%) | ||||
| Remark and Damotte, unpublished data | 161 | III (100%) | High CD8+ T cells correlated with longer survival | |
| B cells | Germain et al. (2014) (36) | 196 | I (32%) | High density of follicular B cells correlated with longer survival |
| II (6%) | ||||
| III (62%) | ||||
| Pelletier et al. (2001) (99) | 113 | I (58%) | Peritumoral CD20+ correlated with longer survival | |
| II (18%) | ||||
| III (24%) | ||||
| Al-Shibli et al. (2008) (96) | 335 | I (63%) | High CD20+ in stroma correlated with longer DSS | |
| II (27%) | ||||
| IIIA (10%) | ||||
| Suzuki et al. (2013) (98) | 956 | I (100%) | Densities of CD20+ are not associated with RFP | |
| Th17 cells | Chen et al. (2010) (100) | 52 | I–II (63%) | High IL-17+ cell densities correlated with poor survival |
| III (37%) | ||||
| Tregs | Suzuki et al. (2013) (98) | 956 | I (100%) | Densities of FoxP3+ cells are not associated with RFP |
| Relative proportion of stromal FoxP3+ to CD3+ correlated with RFP | ||||
| Tao et al. (2012) (101) | 87 | ND | High Treg densities correlated with poor OS and DFS | |
| Shimizu et al. (2010) (102) | 100 | I (68%) | High FoxP3+ correlated with shorter time to recurrence | |
| II (14%) | COX-2 expression correlated with shorter time to recurrence | |||
| III (18%) | COX-2 expression correlated with FoxP3+ infiltration | |||
| Petersen et al. (2006) (103) | 64 | I (100%) | High proportion of FoxP3+ among TILs in tumor correlated with shorter DFS | |
| NK cells | Platonova et al. (2011) (104) | 86 | I (83%) | Presence of NKp46+ NK cells did not impact the clinical outcome |
| II (10%) | ||||
| IV (7%) | ||||
| Johnson et al. (2000) (91) | 95 | I (57%) | High CD57+ NK cell density tended to correlate with longer OS (P = 0.07) | |
| II (18%) | ||||
| III (21%) | ||||
| Takanami et al. (2001) (105) | 150 | I (55%) | High CD57+ NK cells correlated with longer OS in patients with adenocarcinoma | |
| II (14%) | ||||
| III (31%) | ||||
| Al-Shibli et al. (2009) (106) | 335 | I (63%) | High stromal CD56+ cells correlated with improved DSS | |
| II (27%) | ||||
| III (10%) | ||||
| Villegas et al. (2002) (107) | 50 | I (82%) | CD57+ cells correlated with longer survival in patients with squamous cell carcinoma | |
| II (12%) | ||||
| III (6%) | ||||
| DCs | Dieu-Nosjean et al. (2008) (18) | 74 | I (84%) | High DC-LAMP+ mature DCs correlated with longer survival |
| II (16%) | ||||
| Inoshima et al. (2002) (108) | 132 | I (51%) | High S-100+ DCs correlated with longer survival | |
| II (17%) | ||||
| III (25%) | ||||
| IV (7%) | ||||
| Goc et al. (2014) (40) | 376 | I (44%) | High DC-LAMP+ mature DCs correlated with prolonged survival | |
| II (27%) | ||||
| III (28%) | ||||
| IV (0.5%) | ||||
| ND (0.5%) | ||||
| Zeid et al. (1993) (109) | 130 | ND | High S-100+ Langerhans cells (LCs) in tumor were associated with enhanced survival | |
| Germain et al. (2014) (36) | 196 | I (32%) | DC-LAMP+ mature DCs correlated with longer survival | |
| II (6%) | ||||
| III (62%) | ||||
| Johnson et al. (2000) (91) | 95 | I (57%) | High S-100+ LCs in tumor correlated with longer OS | |
| II (18%) | ||||
| III (21%) | ||||
| Sautès-Fridman et al. (2011) (110) | 74 | I (84%) | High CD1a+ LCs and CD14+ CD68low interstitial DCs were associated with longer DSS | |
| II (16%) | ||||
| Al-Shibli et al. (2009) (106) | 335 | I (63%) | High stromal CD1a+ DCs correlated with improved DSS | |
| II (27%) | ||||
| III (10%) | ||||
| Remark and Damotte, unpublished data | 161 | III (100%) | High DC-LAMP+ mature DCs correlated with longer survival | |
| TAMs | Chen et al. (2003) (111) | 35 | I (40%) | TAMs in stroma correlated with shorter OS |
| II (11%) | ||||
| III (49%) | ||||
| Kawai et al. (2008) (97) | 199 | IV (100%) | Predominant infiltration of TAMs in cancer nests was a significant predictor of poor survival | |
| Zeni et al. (2007) (112) | 47 | I (51%) | IL-10-high TAMs associated with shorter OS | |
| II–IV (49%) | ||||
| Ohri et al. (2009) (113) | 40 | I (65%) | High number of M1 macrophages correlated with longer survival | |
| II (20%) | ||||
| III (15%) | ||||
| Ho et al. (2008) (114) | 68 | I (35%) | TREM-1 expression in macrophages correlated with shorter DFS and OS | |
| II (22%) | ||||
| III (43%) | ||||
| Takanami et al. (1999) (115) | I (50%) | High number of CD68+ TAMs correlated with longer OS | ||
| II (4%) | ||||
| III (36%) | ||||
| IV (10%) | ||||
| Al-Shibli et al. (2009) (106) | 335 | I (63%) | High CD68+ were not associated with DSS | |
| II (27%) | ||||
| III (10%) | ||||
| Welsh et al. (2005) (116) | 162 | I (49%) | High stromal and tumor islet CD68+ TAMs correlated with OS | |
| II (27%) | ||||
| IIIa (22%) | ||||
| IIIb/IV (2%) | ||||
| Kim et al. (2008) (117) | 144 | I (55%) | TAMs in tumor correlated with prolonged OS | |
| II (17%) | ||||
| III (26%) | ||||
| IV (2%) | ||||
| Ohtaki et al. (2010) (118) | 170 | IA (56%) | Significant association between high numbers of CD204+ macrophages and poor outcome | |
| IB–IIIA (44%) | ||||
| Ma et al. (2010) (119) | 100 | I (35%) | High CD68+ HLA-DR+ M1 macrophages were associated with good outcome. CD68+ CD163+ M2 macrophages had no prognostic value | |
| II (20%) | ||||
| III (35%) | ||||
| IV (10%) | ||||
| Carus et al. (2013) (120) | 335 | I (65%) | CD163+ macrophage density was not correlated with survival | |
| II (20%) | ||||
| III (15%) | ||||
| Neutrophils | Ilie et al. (2012) (121) | 632 | I (44%) | High CD66+ neutrophil densities were associated with higher risk of relapse |
| II (29%) | ||||
| III (27%) | ||||
| Carus et al. (2013) (120) | 335 | I (65%) | CD66b+ neutrophil count was not correlated with survival | |
| II (20%) | ||||
| III (15%) |
Definition of abbreviations: COX-2 = cyclooxygenase-2; DC-LAMP = dendritic cell lysosome-associated membrane protein; DFS = disease-free survival; DSS = disease-specific survival; ND = not determined; NK = natural killer; OS = overall survival; RFP = relapse-free period; TAMs = tumor-associated macrophages; TILs = tumor-infiltrating lymphocytes; Treg = regulatory T cells.
Some studies have highlighted that the positive impact of T cells, DCs, or B cells might be limited to more advanced stages of disease (stages II–IV). This interesting observation could be linked to the good efficacy of curative surgery at early stages and the potential beneficial effect of the immune response in inducing systemic protection when hematogenous dissemination occurs at later stages.
In conclusion, the immune infiltrates are of major importance in the clinical outcome of patients with NSCLC (Table 2), and the immune contexture should be taken into account in the clinical management of patients with cancer.
Conclusions
During lung cancer progression, tumor cells interact with their microenvironment, where local cancer antigen–specific immune responses shape their eventual destiny. There is now increasing evidence that the NSCLC immune contexture, defined as the nature, location, density, organization, and functional orientation of a natural in situ immune reaction, represents a major player in the development and progression of malignant disease. Some elements of this immune ecosystem and particularly the presence of lymphoid-like structures correlate with survival. Tumors in compensation develop escape mechanisms to avoid destruction. This offers potential targets for therapies. Blocking antibodies targeting the checkpoint inhibitors have shown promising results in several solid tumors. Their efficacy is being tested actively in several clinical trials in NSCLC. We are also actively pursuing strategies to alter the microenvironment of NSCLC by injection of Toll-like receptor agonists at the tumor site, followed by evaluation of immune contexture from surgical specimens. We believe that evaluating the immune contexture represents an important tool to identify subsets of patients with high risk of relapse, and to predict outcome in the context of treatment. Standardized immunopathological assessment in the form of a prognostic and/or predictive score should now be validated in large consortium-driven studies and implemented to help clinicians to decide which therapeutic strategy to choose.
Footnotes
Supported by the Cancer Vaccine Collaborative funded jointly by the Cancer Research Institute and the Ludwig Institute for Cancer Research.
Author Contributions: R.R., C.B., M.M., and S.G. conceived, designed, and drafted the initial manuscript; R.R., C.B., J.E.G., D.D., M.-C.D.-N., C.S.-F., W.-H.F., C.A.P., N.K.A., M.M., and S.G. edited the manuscript; and all authors agreed on the final submitted version.
Originally Published in Press as DOI: 10.1164/rccm.201409-1671PP on November 4, 2014
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.van Meerbeeck JP, Fennell DA, De Ruysscher DK. Small-cell lung cancer. Lancet. 2011;378:1741–1755. doi: 10.1016/S0140-6736(11)60165-7. [DOI] [PubMed] [Google Scholar]
- 3.Goldstraw P, Ball D, Jett JR, Le Chevalier T, Lim E, Nicholson AG, Shepherd FA. Non-small-cell lung cancer. Lancet. 2011;378:1727–1740. doi: 10.1016/S0140-6736(10)62101-0. [DOI] [PubMed] [Google Scholar]
- 4.Goldstraw P, Crowley J, Chansky K, Giroux DJ, Groome PA, Rami-Porta R, Postmus PE, Rusch V, Sobin L International Association for the Study of Lung Cancer International Staging Committee; Participating Institutions. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol. 2007;2:706–714. doi: 10.1097/JTO.0b013e31812f3c1a. [DOI] [PubMed] [Google Scholar]
- 5.Lababede O, Meziane M, Rice T. Seventh edition of the cancer staging manual and stage grouping of lung cancer: quick reference chart and diagrams. Chest. 2011;139:183–189. doi: 10.1378/chest.10-1099. [DOI] [PubMed] [Google Scholar]
- 6.Shepherd FA, Crowley J, Van Houtte P, Postmus PE, Carney D, Chansky K, Shaikh Z, Goldstraw P International Association for the Study of Lung Cancer International Staging Committee and Participating Institutions. The International Association for the Study of Lung Cancer lung cancer staging project: proposals regarding the clinical staging of small cell lung cancer in the forthcoming (seventh) edition of the tumor, node, metastasis classification for lung cancer. J Thorac Oncol. 2007;2:1067–1077. doi: 10.1097/JTO.0b013e31815bdc0d. [DOI] [PubMed] [Google Scholar]
- 7.Detterbeck FC, Boffa DJ, Tanoue LT. The new lung cancer staging system. Chest. 2009;136:260–271. doi: 10.1378/chest.08-0978. [DOI] [PubMed] [Google Scholar]
- 8.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 9.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 10.Swartz MA, Iida N, Roberts EW, Sangaletti S, Wong MH, Yull FE, Coussens LM, DeClerck YA. Tumor microenvironment complexity: emerging roles in cancer therapy. Cancer Res. 2012;72:2473–2480. doi: 10.1158/0008-5472.CAN-12-0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21:309–322. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 12.Fridman WH, Pagès F, Sautès-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12:298–306. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- 13.Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol. 2004;22:329–360. doi: 10.1146/annurev.immunol.22.012703.104803. [DOI] [PubMed] [Google Scholar]
- 14.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, Tosolini M, Camus M, Berger A, Wind P, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 15.Bindea G, Mlecnik B, Tosolini M, Kirilovsky A, Waldner M, Obenauf AC, Angell H, Fredriksen T, Lafontaine L, Berger A, et al. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity. 2013;39:782–795. doi: 10.1016/j.immuni.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 16.Broussard EK, Disis ML. TNM staging in colorectal cancer: T is for T cell and M is for memory. J Clin Oncol. 2011;29:601–603. doi: 10.1200/JCO.2010.32.9078. [DOI] [PubMed] [Google Scholar]
- 17.Di Caro G, Bergomas F, Grizzi F, Doni A, Bianchi P, Malesci A, Laghi L, Allavena P, Mantovani A, Marchesi F. Occurrence of tertiary lymphoid tissue is associated with T-cell infiltration and predicts better prognosis in early-stage colorectal cancers. Clin Cancer Res. 2014;20:2147–2158. doi: 10.1158/1078-0432.CCR-13-2590. [DOI] [PubMed] [Google Scholar]
- 18.Dieu-Nosjean MC, Antoine M, Danel C, Heudes D, Wislez M, Poulot V, Rabbe N, Laurans L, Tartour E, de Chaisemartin L, et al. Long-term survival for patients with non-small-cell lung cancer with intratumoral lymphoid structures. J Clin Oncol. 2008;26:4410–4417. doi: 10.1200/JCO.2007.15.0284. [DOI] [PubMed] [Google Scholar]
- 19.Cipponi A, Mercier M, Seremet T, Baurain JF, Théate I, van den Oord J, Stas M, Boon T, Coulie PG, van Baren N. Neogenesis of lymphoid structures and antibody responses occur in human melanoma metastases. Cancer Res. 2012;72:3997–4007. doi: 10.1158/0008-5472.CAN-12-1377. [DOI] [PubMed] [Google Scholar]
- 20.Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 21.Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ. Natural innate and adaptive immunity to cancer. Annu Rev Immunol. 2011;29:235–271. doi: 10.1146/annurev-immunol-031210-101324. [DOI] [PubMed] [Google Scholar]
- 22.Tartour E, Zitvogel L. Lung cancer: potential targets for immunotherapy. Lancet Respir Med. 2013;1:551–563. doi: 10.1016/S2213-2600(13)70159-0. [DOI] [PubMed] [Google Scholar]
- 23.Prado-Garcia H, Romero-Garcia S, Aguilar-Cazares D, Meneses-Flores M, Lopez-Gonzalez JS. Tumor-induced CD8+ T-cell dysfunction in lung cancer patients. Clin Dev Immunol. 2012;2012:741741. doi: 10.1155/2012/741741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Engels EA, Pfeiffer RM, Fraumeni JF, Jr, Kasiske BL, Israni AK, Snyder JJ, Wolfe RA, Goodrich NP, Bayakly AR, Clarke CA, et al. Spectrum of cancer risk among US solid organ transplant recipients. JAMA. 2011;306:1891–1901. doi: 10.1001/jama.2011.1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ichiki Y, Takenoyama M, Mizukami M, So T, Sugaya M, Yasuda M, So T, Hanagiri T, Sugio K, Yasumoto K. Simultaneous cellular and humoral immune response against mutated p53 in a patient with lung cancer. J Immunol. 2004;172:4844–4850. doi: 10.4049/jimmunol.172.8.4844. [DOI] [PubMed] [Google Scholar]
- 26.Darnell R. Tumor immunity in small-cell lung cancer. J Clin Oncol. 2004;22:762–764. doi: 10.1200/JCO.2004.12.936. [DOI] [PubMed] [Google Scholar]
- 27.Richardson GE, Johnson BE. Paraneoplastic syndromes in lung cancer. Curr Opin Oncol. 1992;4:323–333. doi: 10.1097/00001622-199204000-00014. [DOI] [PubMed] [Google Scholar]
- 28.Pignolet BS, Gebauer CM, Liblau RS. Immunopathogenesis of paraneoplastic neurological syndromes associated with anti-Hu antibodies: A beneficial antitumor immune response going awry. OncoImmunology. 2013;2:e27384. doi: 10.4161/onci.27384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maddison P, Thorpe A, Silcocks P, Robertson JF, Chapman CJ. Autoimmunity to SOX2, clinical phenotype and survival in patients with small-cell lung cancer. Lung Cancer. 2010;70:335–339. doi: 10.1016/j.lungcan.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 30.Ganesan AP, Johansson M, Ruffell B, Yagui-Beltrán A, Lau J, Jablons DM, Coussens LM. Tumor-infiltrating regulatory T cells inhibit endogenous cytotoxic T cell responses to lung adenocarcinoma. J Immunol. 2013;191:2009–2017. doi: 10.4049/jimmunol.1301317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Platonova S, Cherfils-Vicini J, Damotte D, Crozet L, Vieillard V, Validire P, André P, Dieu-Nosjean MC, Alifano M, Régnard JF, et al. Profound coordinated alterations of intratumoral NK cell phenotype and function in lung carcinoma. Cancer Res. 2011;71:5412–5422. doi: 10.1158/0008-5472.CAN-10-4179. [DOI] [PubMed] [Google Scholar]
- 32.Shomer NH, Fox JG, Juedes AE, Ruddle NH. Helicobacter-induced chronic active lymphoid aggregates have characteristics of tertiary lymphoid tissue. Infect Immun. 2003;71:3572–3577. doi: 10.1128/IAI.71.6.3572-3577.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barone F, Bombardieri M, Manzo A, Blades MC, Morgan PR, Challacombe SJ, Valesini G, Pitzalis C. Association of CXCL13 and CCL21 expression with the progressive organization of lymphoid-like structures in Sjögren’s syndrome. Arthritis Rheum. 2005;52:1773–1784. doi: 10.1002/art.21062. [DOI] [PubMed] [Google Scholar]
- 34.Thaunat O, Field AC, Dai J, Louedec L, Patey N, Bloch MF, Mandet C, Belair MF, Bruneval P, Meilhac O, et al. Lymphoid neogenesis in chronic rejection: evidence for a local humoral alloimmune response. Proc Natl Acad Sci USA. 2005;102:14723–14728. doi: 10.1073/pnas.0507223102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Chaisemartin L, Goc J, Damotte D, Validire P, Magdeleinat P, Alifano M, Cremer I, Fridman WH, Sautès-Fridman C, Dieu-Nosjean MC. Characterization of chemokines and adhesion molecules associated with T cell presence in tertiary lymphoid structures in human lung cancer. Cancer Res. 2011;71:6391–6399. doi: 10.1158/0008-5472.CAN-11-0952. [DOI] [PubMed] [Google Scholar]
- 36.Germain C, Gnjatic S, Tamzalit F, Knockaert S, Remark R, Goc J, Lepelley A, Becht E, Katsahian S, Bizouard G, et al. Presence of B cells in tertiary lymphoid structures is associated with a protective immunity in patients with lung cancer. Am J Respir Crit Care Med. 2014;189:832–844. doi: 10.1164/rccm.201309-1611OC. [DOI] [PubMed] [Google Scholar]
- 37.Girard JP, Moussion C, Förster R. HEVs, lymphatics and homeostatic immune cell trafficking in lymph nodes. Nat Rev Immunol. 2012;12:762–773. doi: 10.1038/nri3298. [DOI] [PubMed] [Google Scholar]
- 38.Moyron-Quiroz JE, Rangel-Moreno J, Kusser K, Hartson L, Sprague F, Goodrich S, Woodland DL, Lund FE, Randall TD. Role of inducible bronchus associated lymphoid tissue (iBALT) in respiratory immunity. Nat Med. 2004;10:927–934. doi: 10.1038/nm1091. [DOI] [PubMed] [Google Scholar]
- 39.Moyron-Quiroz JE, Rangel-Moreno J, Hartson L, Kusser K, Tighe MP, Klonowski KD, Lefrançois L, Cauley LS, Harmsen AG, Lund FE, et al. Persistence and responsiveness of immunologic memory in the absence of secondary lymphoid organs. Immunity. 2006;25:643–654. doi: 10.1016/j.immuni.2006.08.022. [DOI] [PubMed] [Google Scholar]
- 40.Goc J, Germain C, Vo-Bourgais TK, Lupo A, Klein C, Knockaert S, de Chaisemartin L, Ouakrim H, Becht E, Alifano M, et al. Dendritic cells in tumor-associated tertiary lymphoid structures signal a Th1 cytotoxic immune contexture and license the positive prognostic value of infiltrating CD8+ T cells. Cancer Res. 2014;74:705–715. doi: 10.1158/0008-5472.CAN-13-1342. [DOI] [PubMed] [Google Scholar]
- 41.Remark R, Alifano M, Cremer I, Lupo A, Dieu-Nosjean MC, Riquet M, Crozet L, Ouakrim H, Goc J, Cazes A, et al. Characteristics and clinical impacts of the immune environments in colorectal and renal cell carcinoma lung metastases: influence of tumor origin. Clin Cancer Res. 2013;19:4079–4091. doi: 10.1158/1078-0432.CCR-12-3847. [DOI] [PubMed] [Google Scholar]
- 42.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murdoch C, Muthana M, Coffelt SB, Lewis CE. The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer. 2008;8:618–631. doi: 10.1038/nrc2444. [DOI] [PubMed] [Google Scholar]
- 44.Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol. 2009;182:4499–4506. doi: 10.4049/jimmunol.0802740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu CY, Wang YM, Wang CL, Feng PH, Ko HW, Liu YH, Wu YC, Chu Y, Chung FT, Kuo CH, et al. Population alterations of L-arginase- and inducible nitric oxide synthase-expressed CD11b+/CD14−/CD15+/CD33+ myeloid-derived suppressor cells and CD8+ T lymphocytes in patients with advanced-stage non-small cell lung cancer. J Cancer Res Clin Oncol. 2010;136:35–45. doi: 10.1007/s00432-009-0634-0. [DOI] [PubMed] [Google Scholar]
- 46.Feng PH, Lee KY, Chang YL, Chan YF, Kuo LW, Lin TY, Chung FT, Kuo CS, Yu CT, Lin SM, et al. CD14(+)S100A9(+) monocytic myeloid-derived suppressor cells and their clinical relevance in non-small cell lung cancer. Am J Respir Crit Care Med. 2012;186:1025–1036. doi: 10.1164/rccm.201204-0636OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sawant A, Schafer CC, Jin TH, Zmijewski J, Tse HM, Roth J, Sun Z, Siegal GP, Thannickal VJ, Grant SC, et al. Enhancement of antitumor immunity in lung cancer by targeting myeloid-derived suppressor cell pathways. Cancer Res. 2013;73:6609–6620. doi: 10.1158/0008-5472.CAN-13-0987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kryczek I, Wei S, Zhu G, Myers L, Mottram P, Cheng P, Chen L, Coukos G, Zou W. Relationship between B7-H4, regulatory T cells, and patient outcome in human ovarian carcinoma. Cancer Res. 2007;67:8900–8905. doi: 10.1158/0008-5472.CAN-07-1866. [DOI] [PubMed] [Google Scholar]
- 49.Cao X, Cai SF, Fehniger TA, Song J, Collins LI, Piwnica-Worms DR, Ley TJ. Granzyme B and perforin are important for regulatory T cell-mediated suppression of tumor clearance. Immunity. 2007;27:635–646. doi: 10.1016/j.immuni.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 50.Grossman WJ, Verbsky JW, Barchet W, Colonna M, Atkinson JP, Ley TJ. Human T regulatory cells can use the perforin pathway to cause autologous target cell death. Immunity. 2004;21:589–601. doi: 10.1016/j.immuni.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 51.Fallarino F, Grohmann U, Hwang KW, Orabona C, Vacca C, Bianchi R, Belladonna ML, Fioretti MC, Alegre ML, Puccetti P. Modulation of tryptophan catabolism by regulatory T cells. Nat Immunol. 2003;4:1206–1212. doi: 10.1038/ni1003. [DOI] [PubMed] [Google Scholar]
- 52.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6:295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- 53.Gobert M, Treilleux I, Bendriss-Vermare N, Bachelot T, Goddard-Leon S, Arfi V, Biota C, Doffin AC, Durand I, Olive D, et al. Regulatory T cells recruited through CCL22/CCR4 are selectively activated in lymphoid infiltrates surrounding primary breast tumors and lead to an adverse clinical outcome. Cancer Res. 2009;69:2000–2009. doi: 10.1158/0008-5472.CAN-08-2360. [DOI] [PubMed] [Google Scholar]
- 54.So T, Takenoyama M, Mizukami M, Ichiki Y, Sugaya M, Hanagiri T, Sugio K, Yasumoto K. Haplotype loss of HLA class I antigen as an escape mechanism from immune attack in lung cancer. Cancer Res. 2005;65:5945–5952. doi: 10.1158/0008-5472.CAN-04-3787. [DOI] [PubMed] [Google Scholar]
- 55.Salih HR, Rammensee HG, Steinle A. Cutting edge: down-regulation of MICA on human tumors by proteolytic shedding. J Immunol. 2002;169:4098–4102. doi: 10.4049/jimmunol.169.8.4098. [DOI] [PubMed] [Google Scholar]
- 56.Massagué J. TGFbeta in Cancer. Cell. 2008;134:215–230. doi: 10.1016/j.cell.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Itakura E, Huang RR, Wen DR, Paul E, Wünsch PH, Cochran AJ. IL-10 expression by primary tumor cells correlates with melanoma progression from radial to vertical growth phase and development of metastatic competence. Mod Pathol. 2011;24:801–809. doi: 10.1038/modpathol.2011.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wald O, Shapira OM, Izhar U. CXCR4/CXCL12 axis in non small cell lung cancer (NSCLC) pathologic roles and therapeutic potential. Theranostics. 2013;3:26–33. doi: 10.7150/thno.4922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Su L, Zhang J, Xu H, Wang Y, Chu Y, Liu R, Xiong S. Differential expression of CXCR4 is associated with the metastatic potential of human non-small cell lung cancer cells. Clin Cancer Res. 2005;11:8273–8280. doi: 10.1158/1078-0432.CCR-05-0537. [DOI] [PubMed] [Google Scholar]
- 60.Kirshberg S, Izhar U, Amir G, Demma J, Vernea F, Beider K, Shlomai Z, Wald H, Zamir G, Shapira OM, et al. Involvement of CCR6/CCL20/IL-17 axis in NSCLC disease progression. PLoS ONE. 2011;6:e24856. doi: 10.1371/journal.pone.0024856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhu YM, Webster SJ, Flower D, Woll PJ. Interleukin-8/CXCL8 is a growth factor for human lung cancer cells. Br J Cancer. 2004;91:1970–1976. doi: 10.1038/sj.bjc.6602227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ramalingam SS, Owonikoko TK, Khuri FR. Lung cancer: New biological insights and recent therapeutic advances. CA Cancer J Clin. 2011;61:91–112. doi: 10.3322/caac.20102. [DOI] [PubMed] [Google Scholar]
- 63.Belinsky SA. Gene-promoter hypermethylation as a biomarker in lung cancer. Nat Rev Cancer. 2004;4:707–717. doi: 10.1038/nrc1432. [DOI] [PubMed] [Google Scholar]
- 64.Tsou JA, Hagen JA, Carpenter CL, Laird-Offringa IA. DNA methylation analysis: a powerful new tool for lung cancer diagnosis. Oncogene. 2002;21:5450–5461. doi: 10.1038/sj.onc.1205605. [DOI] [PubMed] [Google Scholar]
- 65.Akbay EA, Koyama S, Carretero J, Altabef A, Tchaicha JH, Christensen CL, Mikse OR, Cherniack AD, Beauchamp EM, Pugh TJ, et al. Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discov. 2013;3:1355–1363. doi: 10.1158/2159-8290.CD-13-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xu C, Fillmore CM, Koyama S, Wu H, Zhao Y, Chen Z, Herter-Sprie GS, Akbay EA, Tchaicha JH, Altabef A, et al. Loss of Lkb1 and Pten leads to lung squamous cell carcinoma with elevated PD-L1 expression. Cancer Cell. 2014;25:590–604. doi: 10.1016/j.ccr.2014.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bayne LJ, Beatty GL, Jhala N, Clark CE, Rhim AD, Stanger BZ, Vonderheide RH. Tumor-derived granulocyte-macrophage colony-stimulating factor regulates myeloid inflammation and T cell immunity in pancreatic cancer. Cancer Cell. 2012;21:822–835. doi: 10.1016/j.ccr.2012.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gure AO, Chua R, Williamson B, Gonen M, Ferrera CA, Gnjatic S, Ritter G, Simpson AJ, Chen YT, Old LJ, et al. Cancer-testis genes are coordinately expressed and are markers of poor outcome in non-small cell lung cancer. Clin Cancer Res. 2005;11:8055–8062. doi: 10.1158/1078-0432.CCR-05-1203. [DOI] [PubMed] [Google Scholar]
- 69.Gnjatic S, Atanackovic D, Jäger E, Matsuo M, Selvakumar A, Altorki NK, Maki RG, Dupont B, Ritter G, Chen YT, et al. Survey of naturally occurring CD4+ T cell responses against NY-ESO-1 in cancer patients: correlation with antibody responses. Proc Natl Acad Sci USA. 2003;100:8862–8867. doi: 10.1073/pnas.1133324100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Atanackovic D, Altorki NK, Cao Y, Ritter E, Ferrara CA, Ritter G, Hoffman EW, Bokemeyer C, Old LJ, Gnjatic S. Booster vaccination of cancer patients with MAGE-A3 protein reveals long-term immunological memory or tolerance depending on priming. Proc Natl Acad Sci USA. 2008;105:1650–1655. doi: 10.1073/pnas.0707140104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Konishi J, Yamazaki K, Azuma M, Kinoshita I, Dosaka-Akita H, Nishimura M. B7-H1 expression on non-small cell lung cancer cells and its relationship with tumor-infiltrating lymphocytes and their PD-1 expression. Clin Cancer Res. 2004;10:5094–5100. doi: 10.1158/1078-0432.CCR-04-0428. [DOI] [PubMed] [Google Scholar]
- 73.Wang SF, Fouquet S, Chapon M, Salmon H, Regnier F, Labroquère K, Badoual C, Damotte D, Validire P, Maubec E, et al. Early T cell signalling is reversibly altered in PD-1+ T lymphocytes infiltrating human tumors. PLoS ONE. 2011;6:e17621. doi: 10.1371/journal.pone.0017621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mu CY, Huang JA, Chen Y, Chen C, Zhang XG. High expression of PD-L1 in lung cancer may contribute to poor prognosis and tumor cells immune escape through suppressing tumor infiltrating dendritic cells maturation. Med Oncol. 2011;28:682–688. doi: 10.1007/s12032-010-9515-2. [DOI] [PubMed] [Google Scholar]
- 75.Chen YB, Mu CY, Huang JA. Clinical significance of programmed death-1 ligand-1 expression in patients with non-small cell lung cancer: a 5-year-follow-up study. Tumori. 2012;98:751–755. doi: 10.1177/030089161209800612. [DOI] [PubMed] [Google Scholar]
- 76.Velcheti V, Schalper KA, Carvajal DE, Anagnostou VK, Syrigos KN, Sznol M, Herbst RS, Gettinger SN, Chen L, Rimm DL. Programmed death ligand-1 expression in non-small cell lung cancer. Lab Invest. 2014;94:107–116. doi: 10.1038/labinvest.2013.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol. 2012;24:207–212. doi: 10.1016/j.coi.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Salvi S, Fontana V, Boccardo S, Merlo DF, Margallo E, Laurent S, Morabito A, Rijavec E, Dal Bello MG, Mora M, et al. Evaluation of CTLA-4 expression and relevance as a novel prognostic factor in patients with non-small cell lung cancer. Cancer Immunol Immunother. 2012;61:1463–1472. doi: 10.1007/s00262-012-1211-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sharma P, Wagner K, Wolchok JD, Allison JP. Novel cancer immunotherapy agents with survival benefit: recent successes and next steps. Nat Rev Cancer. 2011;11:805–812. doi: 10.1038/nrc3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dougan M, Dranoff G. Immune therapy for cancer. Annu Rev Immunol. 2009;27:83–117. doi: 10.1146/annurev.immunol.021908.132544. [DOI] [PubMed] [Google Scholar]
- 81.Sundar R, Soong R, Cho BC, Brahmer JR, Soo RA. Immunotherapy in the treatment of non-small cell lung cancer. Lung Cancer. 2014;85:101–109. doi: 10.1016/j.lungcan.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dhodapkar KM, Gettinger SN, Das R, Zebroski H, Dhodapkar MV. SOX2-specific adaptive immunity and response to immunotherapy in non-small cell lung cancer. OncoImmunology. 2013;2:e25205. doi: 10.4161/onci.25205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ji RR, Chasalow SD, Wang L, Hamid O, Schmidt H, Cogswell J, Alaparthy S, Berman D, Jure-Kunkel M, Siemers NO, et al. An immune-active tumor microenvironment favors clinical response to ipilimumab. Cancer Immunol Immunother. 2012;61:1019–1031. doi: 10.1007/s00262-011-1172-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hamid O, Schmidt H, Nissan A, Ridolfi L, Aamdal S, Hansson J, Guida M, Hyams DM, Gómez H, Bastholt L, et al. A prospective phase II trial exploring the association between tumor microenvironment biomarkers and clinical activity of ipilimumab in advanced melanoma. J Transl Med. 2011;9:204. doi: 10.1186/1479-5876-9-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Berman DM, Wolchok J, Weber J, Hamid O, O’Day S, Chasalow SD. Association of peripheral blood absolute lymphocyte count (ALC) and clinical activity in patients (pts) with advanced melanoma treated with ipilimumab. J Clin Oncol. 2009;27:15s. [Google Scholar]
- 87.Giraldo NA, Becht E, Remark R, Damotte D, Sautès-Fridman C, Fridman WH. The immune contexture of primary and metastatic human tumours. Curr Opin Immunol. 2014;27:8–15. doi: 10.1016/j.coi.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 88.Fridman WH, Remark R, Goc J, Giraldo NA, Becht E, Hammond SA, Damotte D, Dieu-Nosjean MC, Sautès-Fridman C. The immune microenvironment: a major player in human cancers. Int Arch Allergy Immunol. 2014;164:13–26. doi: 10.1159/000362332. [DOI] [PubMed] [Google Scholar]
- 89.Galon J, Pagès F, Marincola FM, Angell HK, Thurin M, Lugli A, Zlobec I, Berger A, Bifulco C, Botti G, et al. Cancer classification using the Immunoscore: a worldwide task force. J Transl Med. 2012;10:205. doi: 10.1186/1479-5876-10-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ménétrier-Caux C, Gobert M, Caux C. Differences in tumor regulatory T-cell localization and activation status impact patient outcome. Cancer Res. 2009;69:7895–7898. doi: 10.1158/0008-5472.CAN-09-1642. [DOI] [PubMed] [Google Scholar]
- 91.Johnson SK, Kerr KM, Chapman AD, Kennedy MM, King G, Cockburn JS, Jeffrey RR. Immune cell infiltrates and prognosis in primary carcinoma of the lung. Lung Cancer. 2000;27:27–35. doi: 10.1016/s0169-5002(99)00095-1. [DOI] [PubMed] [Google Scholar]
- 92.Hiraoka K, Miyamoto M, Cho Y, Suzuoki M, Oshikiri T, Nakakubo Y, Itoh T, Ohbuchi T, Kondo S, Katoh H. Concurrent infiltration by CD8+ T cells and CD4+ T cells is a favourable prognostic factor in non-small-cell lung carcinoma. Br J Cancer. 2006;94:275–280. doi: 10.1038/sj.bjc.6602934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kikuchi E, Yamazaki K, Torigoe T, Cho Y, Miyamoto M, Oizumi S, Hommura F, Dosaka-Akita H, Nishimura M. HLA class I antigen expression is associated with a favorable prognosis in early stage non-small cell lung cancer. Cancer Sci. 2007;98:1424–1430. doi: 10.1111/j.1349-7006.2007.00558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ruffini E, Asioli S, Filosso PL, Lyberis P, Bruna MC, Macrì L, Daniele L, Oliaro A. Clinical significance of tumor-infiltrating lymphocytes in lung neoplasms. Ann Thorac Surg. 2009;87:365–371, discussion 371–372. doi: 10.1016/j.athoracsur.2008.10.067. [DOI] [PubMed] [Google Scholar]
- 95.Wakabayashi O, Yamazaki K, Oizumi S, Hommura F, Kinoshita I, Ogura S, Dosaka-Akita H, Nishimura M. CD4+ T cells in cancer stroma, not CD8+ T cells in cancer cell nests, are associated with favorable prognosis in human non-small cell lung cancers. Cancer Sci. 2003;94:1003–1009. doi: 10.1111/j.1349-7006.2003.tb01392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Al-Shibli KI, Donnem T, Al-Saad S, Persson M, Bremnes RM, Busund LT. Prognostic effect of epithelial and stromal lymphocyte infiltration in non-small cell lung cancer. Clin Cancer Res. 2008;14:5220–5227. doi: 10.1158/1078-0432.CCR-08-0133. [DOI] [PubMed] [Google Scholar]
- 97.Kawai O, Ishii G, Kubota K, Murata Y, Naito Y, Mizuno T, Aokage K, Saijo N, Nishiwaki Y, Gemma A, et al. Predominant infiltration of macrophages and CD8(+) T Cells in cancer nests is a significant predictor of survival in stage IV nonsmall cell lung cancer. Cancer. 2008;113:1387–1395. doi: 10.1002/cncr.23712. [DOI] [PubMed] [Google Scholar]
- 98.Suzuki K, Kadota K, Sima CS, Nitadori J, Rusch VW, Travis WD, Sadelain M, Adusumilli PS. Clinical impact of immune microenvironment in stage I lung adenocarcinoma: tumor interleukin-12 receptor β2 (IL-12Rβ2), IL-7R, and stromal FoxP3/CD3 ratio are independent predictors of recurrence. J Clin Oncol. 2013;31:490–498. doi: 10.1200/JCO.2012.45.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pelletier MP, Edwardes MD, Michel RP, Halwani F, Morin JE. Prognostic markers in resectable non-small cell lung cancer: a multivariate analysis. Can J Surg. 2001;44:180–188. [PMC free article] [PubMed] [Google Scholar]
- 100.Chen X, Wan J, Liu J, Xie W, Diao X, Xu J, Zhu B, Chen Z. Increased IL-17-producing cells correlate with poor survival and lymphangiogenesis in NSCLC patients. Lung Cancer. 2010;69:348–354. doi: 10.1016/j.lungcan.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 101.Tao H, Mimura Y, Aoe K, Kobayashi S, Yamamoto H, Matsuda E, Okabe K, Matsumoto T, Sugi K, Ueoka H. Prognostic potential of FOXP3 expression in non-small cell lung cancer cells combined with tumor-infiltrating regulatory T cells. Lung Cancer. 2012;75:95–101. doi: 10.1016/j.lungcan.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 102.Shimizu K, Nakata M, Hirami Y, Yukawa T, Maeda A, Tanemoto K. Tumor-infiltrating Foxp3+ regulatory T cells are correlated with cyclooxygenase-2 expression and are associated with recurrence in resected non-small cell lung cancer. J Thorac Oncol. 2010;5:585–590. doi: 10.1097/JTO.0b013e3181d60fd7. [DOI] [PubMed] [Google Scholar]
- 103.Petersen RP, Campa MJ, Sperlazza J, Conlon D, Joshi MB, Harpole DH, Jr, Patz EF., Jr Tumor infiltrating Foxp3+ regulatory T-cells are associated with recurrence in pathologic stage I NSCLC patients. Cancer. 2006;107:2866–2872. doi: 10.1002/cncr.22282. [DOI] [PubMed] [Google Scholar]
- 104.Platonova S, Cherfils-Vicini J, Damotte D, Crozet L, Vieillard V, Validire P, André P, Dieu-Nosjean MC, Alifano M, Régnard JF, et al. Profound coordinated alterations of intratumoral NK cell phenotype and function in lung carcinoma. Cancer Res. 2011;71:5412–5422. doi: 10.1158/0008-5472.CAN-10-4179. [DOI] [PubMed] [Google Scholar]
- 105.Takanami I, Takeuchi K, Giga M. The prognostic value of natural killer cell infiltration in resected pulmonary adenocarcinoma. J Thorac Cardiovasc Surg. 2001;121:1058–1063. doi: 10.1067/mtc.2001.113026. [DOI] [PubMed] [Google Scholar]
- 106.Al-Shibli K, Al-Saad S, Donnem T, Persson M, Bremnes RM, Busund LT. The prognostic value of intraepithelial and stromal innate immune system cells in non-small cell lung carcinoma. Histopathology. 2009;55:301–312. doi: 10.1111/j.1365-2559.2009.03379.x. [DOI] [PubMed] [Google Scholar]
- 107.Villegas FR, Coca S, Villarrubia VG, Jiménez R, Chillón MJ, Jareño J, Zuil M, Callol L. Prognostic significance of tumor infiltrating natural killer cells subset CD57 in patients with squamous cell lung cancer. Lung Cancer. 2002;35:23–28. doi: 10.1016/s0169-5002(01)00292-6. [DOI] [PubMed] [Google Scholar]
- 108.Inoshima N, Nakanishi Y, Minami T, Izumi M, Takayama K, Yoshino I, Hara N. The influence of dendritic cell infiltration and vascular endothelial growth factor expression on the prognosis of non-small cell lung cancer. Clin Cancer Res. 2002;8:3480–3486. [PubMed] [Google Scholar]
- 109.Zeid NA, Muller HK. S100 positive dendritic cells in human lung tumors associated with cell differentiation and enhanced survival. Pathology. 1993;25:338–343. doi: 10.3109/00313029309090853. [DOI] [PubMed] [Google Scholar]
- 110.Sautès-Fridman C, Cherfils-Vicini J, Damotte D, Fisson S, Fridman WH, Cremer I, Dieu-Nosjean MC. Tumor microenvironment is multifaceted. Cancer Metastasis Rev. 2011;30:13–25. doi: 10.1007/s10555-011-9279-y. [DOI] [PubMed] [Google Scholar]
- 111.Chen JJ, Yao PL, Yuan A, Hong TM, Shun CT, Kuo ML, Lee YC, Yang PC. Up-regulation of tumor interleukin-8 expression by infiltrating macrophages: its correlation with tumor angiogenesis and patient survival in non-small cell lung cancer. Clin Cancer Res. 2003;9:729–737. [PubMed] [Google Scholar]
- 112.Zeni E, Mazzetti L, Miotto D, Lo Cascio N, Maestrelli P, Querzoli P, Pedriali M, De Rosa E, Fabbri LM, Mapp CE, et al. Macrophage expression of interleukin-10 is a prognostic factor in nonsmall cell lung cancer. Eur Respir J. 2007;30:627–632. doi: 10.1183/09031936.00129306. [DOI] [PubMed] [Google Scholar]
- 113.Ohri CM, Shikotra A, Green RH, Waller DA, Bradding P. Macrophages within NSCLC tumour islets are predominantly of a cytotoxic M1 phenotype associated with extended survival. Eur Respir J. 2009;33:118–126. doi: 10.1183/09031936.00065708. [DOI] [PubMed] [Google Scholar]
- 114.Ho CC, Liao WY, Wang CY, Lu YH, Huang HY, Chen HY, Chan WK, Chen HW, Yang PC. TREM-1 expression in tumor-associated macrophages and clinical outcome in lung cancer. Am J Respir Crit Care Med. 2008;177:763–770. doi: 10.1164/rccm.200704-641OC. [DOI] [PubMed] [Google Scholar]
- 115.Takanami I, Takeuchi K, Kodaira S. Tumor-associated macrophage infiltration in pulmonary adenocarcinoma: association with angiogenesis and poor prognosis. Oncology. 1999;57:138–142. doi: 10.1159/000012021. [DOI] [PubMed] [Google Scholar]
- 116.Welsh TJ, Green RH, Richardson D, Waller DA, O’Byrne KJ, Bradding P. Macrophage and mast-cell invasion of tumor cell islets confers a marked survival advantage in non-small-cell lung cancer. J Clin Oncol. 2005;23:8959–8967. doi: 10.1200/JCO.2005.01.4910. [DOI] [PubMed] [Google Scholar]
- 117.Kim DW, Min HS, Lee KH, Kim YJ, Oh DY, Jeon YK, Lee SH, Im SA, Chung DH, Kim YT, et al. High tumour islet macrophage infiltration correlates with improved patient survival but not with EGFR mutations, gene copy number or protein expression in resected non-small cell lung cancer. Br J Cancer. 2008;98:1118–1124. doi: 10.1038/sj.bjc.6604256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ohtaki Y, Ishii G, Nagai K, Ashimine S, Kuwata T, Hishida T, Nishimura M, Yoshida J, Takeyoshi I, Ochiai A. Stromal macrophage expressing CD204 is associated with tumor aggressiveness in lung adenocarcinoma. J Thorac Oncol. 2010;5:1507–1515. doi: 10.1097/JTO.0b013e3181eba692. [DOI] [PubMed] [Google Scholar]
- 119.Ma J, Liu L, Che G, Yu N, Dai F, You Z. The M1 form of tumor-associated macrophages in non-small cell lung cancer is positively associated with survival time. BMC Cancer. 2010;10:112. doi: 10.1186/1471-2407-10-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Carus A, Ladekarl M, Hager H, Pilegaard H, Nielsen PS, Donskov F. Tumor-associated neutrophils and macrophages in non-small cell lung cancer: no immediate impact on patient outcome. Lung Cancer. 2013;81:130–137. doi: 10.1016/j.lungcan.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 121.Ilie M, Hofman V, Ortholan C, Bonnetaud C, Coëlle C, Mouroux J, Hofman P. Predictive clinical outcome of the intratumoral CD66b-positive neutrophil-to-CD8-positive T-cell ratio in patients with resectable nonsmall cell lung cancer. Cancer. 2012;118:1726–1737. doi: 10.1002/cncr.26456. [DOI] [PubMed] [Google Scholar]