Abstract
Each presynaptic bouton is densely packed with many vesicles, only a small fraction of which are available for immediate release. These vesicles constitute the readily releasable pool (RRP). The RRP size, and the probability of release of each vesicle within the RRP, together determine synaptic strength. Here, we discuss complications and recent advances in determining the size of the physiologically relevant RRP. We consider molecular mechanisms to generate and regulate the RRP, and discuss the relationship between vesicle docking and the RRP. We conclude that many RRP vesicles are docked, that some docked vesicles may not be part of the RRP, and that undocked vesicles can contribute to the RRP by rapid recruitment to unoccupied, molecularly activated ready-torelease sites.
Definitions and measurements of RRP
The readily releasable pool (RRP) is functionally defined as a small subset of the many vesicles in a presynaptic bouton that is more readily released than other vesicles. An action potential evokes neurotransmitter release that depends upon the size of the RRP and on the initial probability of release of a vesicle (vesicular release probability p). During physiological patterns of presynaptic activity, many additional factors regulate synaptic responses. The RRP is depleted and replenished from a reserve pool of vesicles, and this replenishment is vital to sustaining responses. At many synapses, p is dynamically regulated by processes such as facilitation. In addition, synaptic transmission can be mediated by multiple pools of vesicles that differ in initial p, facilitation, and replenishment. Although such properties have been incorporated into complex models [1–5], it is difficult to experimentally determine the many parameters of such models. Consequently, the most widely used approaches to measure RRP size rely on a number of simplifying assumptions [6*, 7, 8*].
All methods of determining RRP require the quantification of vesicle fusion or neurotransmitter release, which is done in 3 general ways (Fig. 1). The most widely used approach is to quantify neurotransmitter release by measuring postsynaptic currents. This method is sensitive and readily applied to many types of synapses, but it can be complicated by nonlinearities arising from neurotransmitter spillover and pooling, receptor saturation and desensitization. Such difficulties can be overcome by using drugs that relieve receptor saturation and desensitization. Although such drugs are widely used to study AMPA receptors [9,10], and to a lesser extent for GABAA receptors [11*], it is difficult to prevent saturation and desensitization for synapses that use other neurotransmitters. Many published estimates of RRP size are inaccurate because they do not deal with receptor saturation and desensitization. A second approach, which has been applied to large synapses which are amenable to presynaptic patch recordings, is to measure the capacitance change associated with the addition of membrane during vesicle fusion. Finally, optical approaches can be used to measure exocytosis by detecting fluorescence associated with vesicle fusion [12,13]. This approach requires labeling vesicles with a fluorophore that changes fluorescence in response to membrane fusion. It does not require electrical recording from presynaptic terminals, and does not suffer from problems associated with postsynaptic receptor saturation and desensitization. These methods are suited for studying synapses between cultured cells, where background fluorescence is low and synapses are located within a single plane.
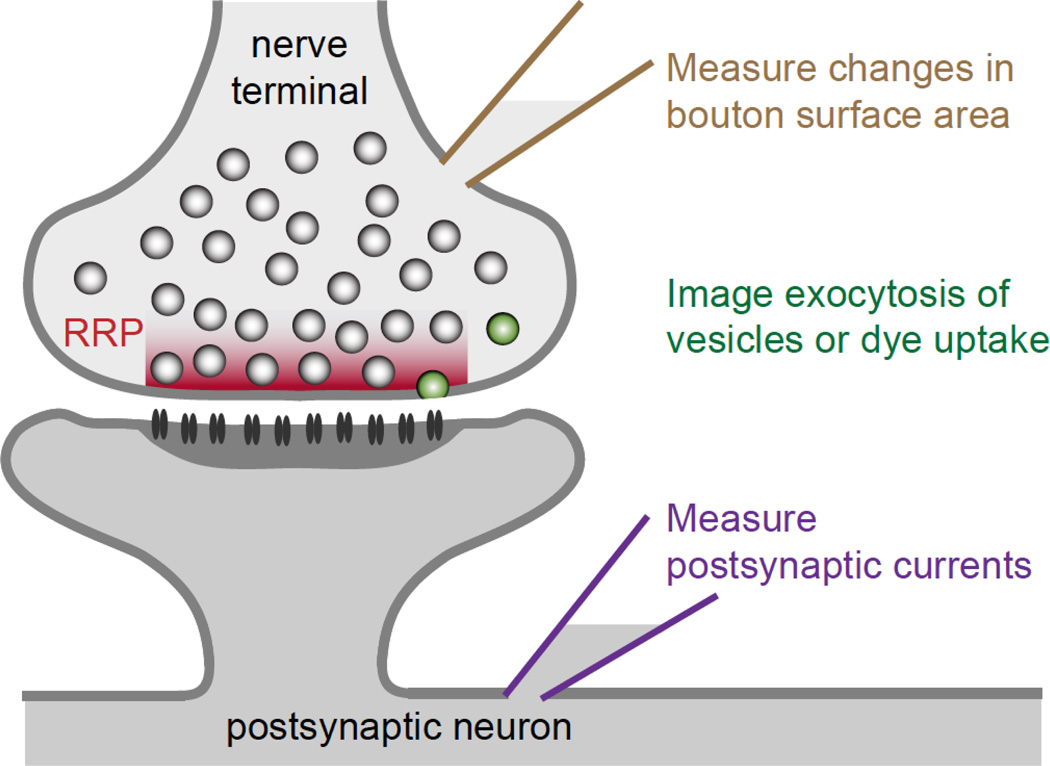
Figure 1. Measurements of RRP.
A schematic of a synapse is shown with a presynaptic nerve terminal containing many vesicles. Some of these vesicles are close to the active zone and make up the RRP. To quantify the RRP size it is necessary to quantify neurotransmitter release, which is done in several different ways. It is possible to record directly from some types of presynaptic boutons (top), and this allows control of the presynaptic potential for large voltage steps, allows control of the intracellular milieu, and makes it possible to measure the change in surface area in response to vesicle fusion. It is also possible to quantify fusion using optical methods (middle, illustrated by vesicles colored in green). The most common method to quantify RRP size is to record postsynaptic currents (bottom).
To accurately measure the RRP, the major challenge is to release the entire RRP while accounting for contributions from replenishment. The RRP size is underestimated if RRP depletion is incomplete, and overestimated if replenished vesicles contribute to the measure of RRP. One approach that has been used extensively in cultured cells is to apply high osmolarity solutions (usually 500 mM sucrose) to release the RRP [14–17]. It is thought that high osmolarity solutions result in fusion of the RRP. Although the release mechanism of this method is unclear, it has provided important insights because it has been used extensively to study roles for specific proteins in the control of the RRP using knockout mice. A second approach is to depolarize the presynaptic terminal with a prolonged voltage step. A third approach is to use caged calcium to increase presynaptic calcium levels. These methods have proven very useful in characterizing roles of proteins and chemical messengers that regulate the RRP. However, they often provide larger estimates of the RRP than estimates based on release evoked by action potential trains. It seems likely that these strong stimuli release some vesicles that cannot be released by high frequency stimulus trains [18*].
There are several ways of using action potential trains (Fig. 2a) to determine RRP size [6*, 8*]. These approaches all measure synchronous release that occurs in the milliseconds following each presynaptic action potential and they do not account for asynchronous release that does not contribute to peak EPSCs. The most common approach is to plot the cumulative EPSC amplitude as a function of stimulus number (Fig. 2b). In the absence of replenishment, the plateau of the response would correspond to the liberation of all of the vesicles in the RRP. But during prolonged stimulation, steadystate responses are a consequence of vesicle replenishment. A linear extrapolation is used to correct for replenishment and the intercept of the y-axis corresponds to the RRP size [19]. However, this method assumes constant replenishment throughout the train, and it has been shown that replenishment becomes faster during the train as more empty sites become available [7] (this is the case when replenishment is approximated by the same exponential recovery regardless of the extent of depression). Consequently, this method tends to underestimate the RRP. A second method, referred to as the EQ method after Elmqvist and Quastel [20], plots the EPSC amplitude as a function of the cumulative EPSC and linearly extrapolates to determine the RRP (Fig. 2c). However, responses early in the train that are most important for this method, and replenishment during the train will lead to overestimates of the RRP. Facilitation can also complicate the application of this method. As a practical matter, it is difficult to identify the best region for linear extrapolation. A third approach, though not widely used, can also provide insight into p and the size of the RRP. This method assumes that depression is a consequence of depletion such that RRP is reduced by a single stimulation to (1-p)RRP and then recovers exponentially [8*]. During a high frequency stimulation the amplitudes of synaptic responses vs. stimulus number dereases exponentially with a constant λ that is determined by the probability of release such that pdecay=1−exp(−1/λ) (Fig. 2d). This approach provides a useful test of whether the decrement in EPSC amplitude is consistent with release arising from depletion of a single pool of vesicles with the same p. Finally, it is possible to fit the data to a model. A simple depletion model works well for synapses with high p and sets replenishment proportional to the number of unoccupied release sites (which corresponds to an exponential recovery from depression [7,8*]).
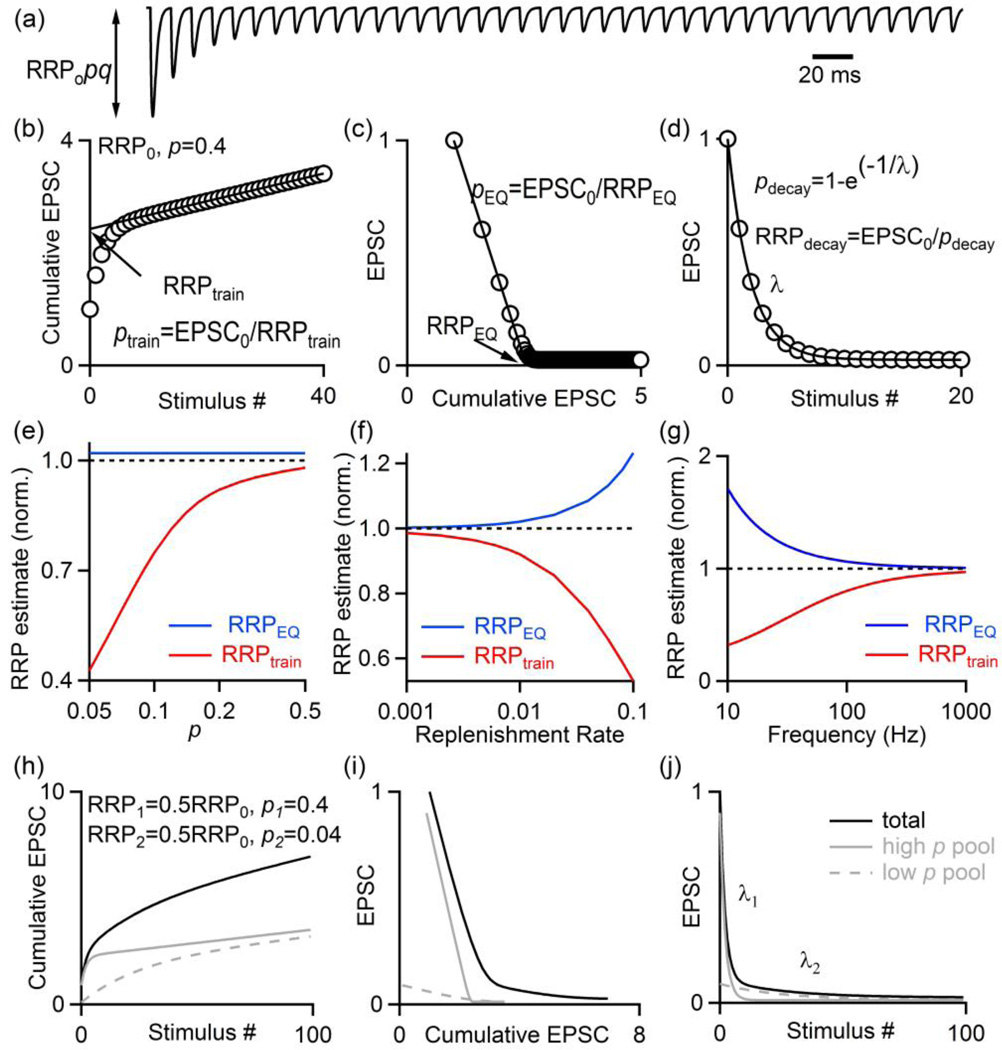
Figure 2. Using synaptic responses evoked by high-frequency stimulus trains to estimate synaptic parameters.
Synaptic responses are described by N0 (the size of the readily releasable pool, RRP), p (the vesicular release probability), R (the rate of replenishment of the RRP from a reserve pool) and q (the size of a quantal response).
(a). Simulated EPSCs in response to a 100 Hz stimulus train.
(b, c). Two extrapolation methods commonly used to estimate synaptic parameters are illustrated: one referred to as the train method (b) and the other as the Elmqvist and Quastel (EQ) method.
(d). If depression of synaptic responses is due to RRP depletion, the dependence of the EPSC amplitude on number of stimuli can be used to estimate p and determine the RRP (from [8*]).
(e–f) Simulations based on a depletion model were used to determine EPSC amplitudes during a train and the cumulative train method and EQ methods were used to estimate the RRP from these simulated responses (from [8*]). The dashed line corresponds to the RRP size used in the simulations.
(h–i) Simulations with a depletion model were made for a synapse with 50% of release having p=0.4 and 50% having p=0.04. Plots were made as in B–D that highlight complications associated with having nonuniform p.
A comparison of several methods at the calyx of Held indicates that they agree with each other very well when p is high and when the rate of replenishment is low compared to the stimulus frequency [7, 8*]. However, they deviate from each other in predictable ways when this is not the case. This is illustrated by determining RRPtrain and RRPEQ for simulations based on a depletion model (Figs. 2e–f). When p is low, RRPEQ slightly overestimates RRP and RRPtrain greatly underestimates RRP (Fig. 2e). Rapid replenishment of the RRP from the reserve pool also compromises both methods (Fig. 2f). It is also well established that high frequency stimulation provides better RRP estimates (Fig. 2g), but there are practical limitations on how rapidly presynaptic axons can be stimulated.
One of the major assumptions of these methods is that all release occurs with the same p, but this is not the case at all synapses [6*,11*,18*,21*]. Consider the case where 50% of the vesicles have p=0.4 and 50% have p=0.04. The EPSC amplitude as a function of stimulus frequency is no longer approximated by a single exponential decay, there are 2 components from 2 different pools of vesicles (Fig. 2h). In this case it is not possible to determine RRPtrain, because there is no obvious region that is appropriate for linear extrapolation. This sort of behavior is seen at many synapses. It is also difficult to apply the EQ method (Fig. 2i). These simulations illustrate how multiple heterogeneous pools of vesicles can complicate the determination of the RRP. Many other factors can make it difficult to reliably estimate RRP, including use-dependent changes in replenishment [22], decreased replenishment arising from depletion of the reserve pool, and use-dependent synaptic plasticity such as facilitation [6*,8*,11*].
Several alternative approaches take advantage of the stochastic nature of synaptic responses to estimate synaptic parameters. Methods such as variance-mean analysis allow determination of RRP size without reliance on spike trains, but they require stable measurements of synaptic properties in at least three different experimental conditions [23]. Recently a new approach that relies on the statistics of responses evoked by stimulus trains has been developed to quantify and characterize the RRP [24]. Another approach is to use irregular spike trains to evoke synaptic responses that are used to determine synaptic parameters associated with a model. This approach can be used at synapses with prominent facilitation and a low probability of release. It is possible to either use averaged responses and traditional fitting methods, or to use the statistics of synaptic transmission [25–27]. It will be important to determine how well such methods estimate RRP size, and to determine if this approach can be adapted to synapses where release is mediated by multiple pools of vesicles with different properties.
In summary, a number of strategies are used to quantify the RRP. Even though hypertonic sucrose and prolonged presynaptic voltage steps have limitations in providing insight into release under physiological conditions, such strong stimuli will continue to provide an important means of quantifying RRP size and will allow the comparison of effects arising from different molecular manipulations. It is also likely that optical methods, which avoid many complications associated with other methods, will become more widely used if their application can be extended to more intact preparations. Finally, action potential trains will continue to provide invaluable insight into vesicle pools under physiological conditions at synapses that fulfill the strict requirements for the validity of these measurements [6*, 8*]. In some cases, such as when the properties of release are heterogeneous, it will be necessary to pair detailed and extensive electrophysiological characterization with models to properly describe synaptic transmission.
Molecular mechanisms to generate RRP vesicles
The prevalent model is that synaptic vesicles interact with presynaptic proteins to become part of an RRP of vesicles. This molecular process is called vesicle priming, and studies using knockout animals and in vitro fusion assays revealed that Munc13 takes a central role in priming [28–33]. We will discuss how Munc13 participates in multiple steps during synaptic vesicle exocytosis (Fig. 3) and then analyze specific roles for Munc13 in generating the RRP.
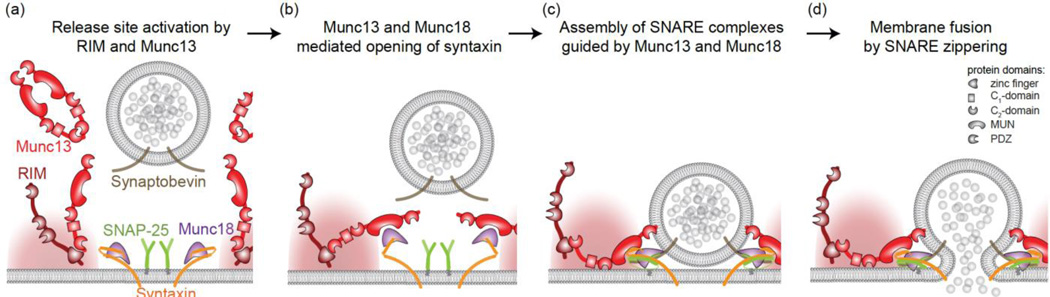
Figure 3. Simplified, Munc13-centered model of exocytosis.
Munc13 participates in multiple steps of exocytosis, which raises the question at which step a vesicle becomes part of the RRP.
(a) RIM recruits and monomerizes Munc13 to activate a release site.
(b) Munc13, together with Munc18, opens syntaxin-1 to allow for the assembly of the SNARE complex.
(c) SNARE complexes may partially assemble under the molecular control of Munc13 and Munc18, and this assembly may be regulated by complexin, synaptotagmin, or other SNARE-binding proteins.
(d) Fusion proceeds when SNARE proteins fully assemble into a four-alpha-helical bundle that forces the vesicular and target membranes to fuse.
Munc13 is a modular protein that is inactive as a dimer. It is monomerized and anchored at the active zone through interactions between the RIM zinc finger and Munc13 C2A domains [34–36] (Fig. 3a). Downstream of this activation, the MUN domain, a sequence element within Munc13 that is common to other tethering factors, is required for synaptic vesicle exocytosis [37,38]. Recent studies using in vitro fusion assays led to a model in which the MUN domain of Munc13 plays a central role in assembling the SNARE complex that mediates fusion [39,40*,41*]. The t-SNARE protein syntaxin-1 begins in a closed, inactive confirmation bound to Munc18, a protein that is structurally unrelated to Munc13. The Munc13 MUN domain, together with Munc18, activates syntaxin-1 by opening it to expose the syntaxin-1 SNARE motif (Fig. 3b), while the Munc13 C2B and C2C domains are thought to bind to synaptic vesicles and target membranes. This membrane bridging activity could occur before, during or after opening of syntaxin. SNARE complex assembly is initiated under the control of Munc18 and the Munc13 MUN domain (Fig. 3c), bringing the t-SNAREs syntaxin-1 and SNAP-25 close to the v-SNARE synaptobrevin-2/VAMP-2. Fusion is executed by complete zippering of SNAREs into a four alphahelical bundle to fuse the vesicular and target membranes (Fig. 3d). Additional proteins, for example complexin and synaptotagmin [30,42–44], bind to SNARE complexes to pause or activate SNARE complex assembly and fusion through several distinct mechanisms.
Of particular interest is whether there is a defined "priming" step in this molecular chain reaction that adds a vesicle to the RRP. From a functional viewpoint, one could ask what the rate-limiting step during replenishment is, considering an RRP vesicle as one that has passed this rate-limiting step for replenishment. The state at which the fusion process is paused before calcium triggering could be at any point after this rate-limiting step.
Insight into this question came from gene knockout studies. Disruption of the initial steps in this model, by deleting RIM proteins, significantly impairs vesicle priming [36,45,46] by preventing anchoring and activation of Munc13 [34,36]. These data indicate that processes upstream of syntaxin-1 opening and SNARE complex assembly are required for the RRP. All genetic manipulations that impair fusion downstream of RIM-mediated activation and recruitment of Munc13 (Figs. 3b–d) affect the measurement of RRP size. This could either reflect a role of the manipulated gene in generating the RRP, or a role for fusion of vesicles after they have been added to the RRP. Thus, these genetic experiments suggest that generating RRP vesicles requires processes upstream of SNARE complex assembly that cannot be compensated for by replenishment, and we propose that it entails activation of a release site by mechanisms that include Munc13 and RIM (Fig. 3a).
It is important to point out that the protein machinery mediating RRP and fusion is more complex than outlined in our simplified, Munc13-centered model. It is likely that additional proteins including CAPS [47], ELKS [48], complexin [49], and synaptotagmin [50*] also contribute to generation of the RRP.
Morphological correlates of RRP vesicles
Studies of synaptic ultrastructure provided additional insights. These studies addressed whether RRP vesicles could be identified based on their morphology. Because there was a good correlation between the number of docked vesicles and the vesicles released by a 20-Hz 40-action potential stimulus train, it was proposed that docked vesicles are the RRP [51]. This hypothesis is supported by studies of RIM mutants, in which reductions in vesicle docking using glutaraldehyde-fixed tissue are paralleled by reductions in the RRP at hippocampal synapses and in the calyx of Held [45,46]. However, the same experiments did not reveal a docking phenotype in Munc13 deficient hippocampal neurons [28,29] or in neurons that lack SNARE proteins. Technical improvements have addressed some of these discrepancies by employing rapid freezing under high pressure and electron tomography, which enhanced resolution of the docking process. A recent study performed a precise morphological analysis of synapses in organotypic slice cultures of various knockout mice [52**]. Interestingly, mutant mice for Munc13, syntaxin-1, SNAP-25 or synaptobrevin-2 had strong reductions in docked vesicles within 2 nm of the target membrane. At the same time, vesicle numbers at 5–20 nm away from the presynaptic plasma membrane increased. These data, together with a previous study [53], indicate that Munc13 and SNARE proteins, which are essential for fusion, mediate the tight membrane attachment of synaptic vesicles. Another study determined the number of docked vesicles by combining optogenetics and rapid freezing before and tens of milliseconds after presynaptic stimulation [54]. Brief optogenetic activation decreased the number of docked vesicles by ~30% in cultured hippocampal neurons, suggesting that docked vesicles are released upon stimulation.
These studies establish that docked vesicles contribute to the RRP. However, it remains uncertain whether all docked vesicles are part of the RRP, and whether all RRP vesicles are docked (Fig. 4). Recent data support alternative models. For example, pHluorin imaging experiments suggest that the average RRP in cultured hippocampal neurons contains ~4 vesicles per synapse [12,13], whereas precise morphological measurements revealed ~15 docked vesicles if the active zone is considered a circular structure with a diameter of 350 nm [52**]. Thus, at least some measurements suggest that the RRP is smaller than the number of docked vesicles (Fig. 4b). It has also been found that undocked vesicles can be released by RRP-depleting stimuli. This was first observed in experiments that measured vesicle release after labeling the RRP during one round of recycling [55]. It may also be the case in CAPS mutants, which have a dramatic reduction in vesicles within 5 nm of the plasma membrane [52**], but release quite efficiently in high extracellular calcium or after short stimulus trains [47]. The simultaneous knockout of RIM and ELKS abolished vesicle docking and also strongly reduced vesicles within 100 nm of the presynaptic membrane [56*], but only reduced neurotransmitter release by approximately a factor of two in response to hypertonic sucrose, short stimulus trains or in elevated extracellular calcium. This suggested that vesicles located at some distance from the release site contribute to the RRP.
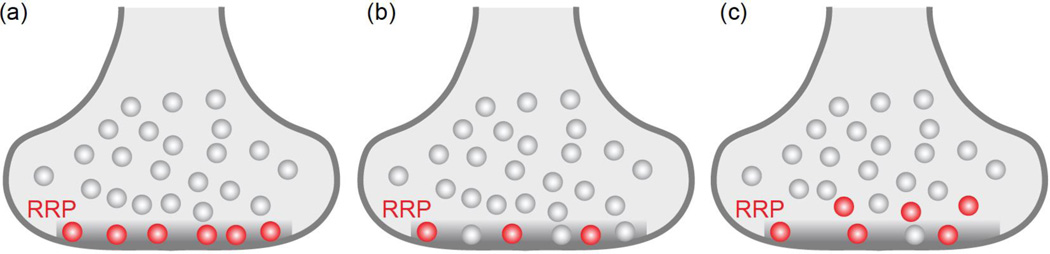
Figure 4. Morphological correlates of RRP.
The RRP consits of docked vesicles. The questions that arise are: Are all RRP vesicles docked? Are all docked vesicles in the RRP?
(a) One model posits that all docked vesicles are part of the RRP and all RRP vesicles are docked.
(b) Another possiblity is that only a subset of docked vesicles is the RRP.
(c) A third model is that many RRP vesicles are docked, but additional vesicles may contribute to RRP through rapid recruitment to empty, activated release sites.
In (a) – (c), RRP vesicles are illustrated in red and the active zone is the grey shaded area.
In aggregate, all data are consistent with a model in which some or many docked vesicles are part of the RRP, and some undocked vesicles can be rapidly released as RRP vesicles (Fig. 4c). We propose that release site activation is rate limiting for generating RRP vesicles (Fig. 3a), docked vesicles associated with activated sites can be released immediately, and vesicles can be rapidly recruited to unoccupied activated sites to contribute to the RRP. An interesting possibility that arises from this model is that it accounts for previous reports that the RRP and vesicle docking are not static [57]. Activation and inactivation of release sites and recruitment of vesicles to these sites allows for dynamic changes in RRP. Future studies should continue to dissect molecular mechanisms for RRP generation in reduced systems and rigorous measurements of RRP at specific synapses in intact preparations should complement these approaches to test and further develop these models.
Highlights.
Methods to measure RRP are reviewed and their assumptions and limitations discussed
Action potential trains can be used to measure the physiologically relevant RRP
Activation of a release site by Munc13 and RIM is necessary for RRP generation
RRP is comprised of docked vesicles and rapidly recruited undocked vesicles
Linking ultrastructure, molecular mechanisms and RRP measurements remains a challenge
Acknowledgments
This work was supported by NIH/NINDS R01NS083898 to PSK, Harvard Brain Initiative Bipolar Disorder Seed Grant to PSK, NIH/NINDS R01NS032405 to WGR and NIH/NINDS R35NS097284 to WGR. We thank J. Rizo, M. Thanawala, R. Held and S. Wang for comments on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Guo J, Ge J-L, Hao M, Sun Z-C, Wu X-S, Zhu J-B, Wang W, Yao P-T, Lin W, Xue L. A three-pool model dissecting readily releasable pool replenishment at the calyx of held. [Internet] Sci. Rep. 2015;5:9517. doi: 10.1038/srep09517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mahfooz K, Singh M, Renden R, Wesseling JF. A Well-Defined Readily Releasable Pool with Fixed Capacity for Storing Vesicles at Calyx of Held. PLoS Comput. Biol. 2016;12:1–38. doi: 10.1371/journal.pcbi.1004855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qiu X, Zhu Q, Sun J. Quantitative analysis of vesicle recycling at the calyx of Held synapse. [Internet] Proc. Natl. Acad. Sci. U. S. A. 2015;112:4779–4784. doi: 10.1073/pnas.1424597112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pan B, Zucker RS. A general model of synaptic transmission and short-term plasticity [Internet] Neuron. 2009;62:539–554. doi: 10.1016/j.neuron.2009.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dittman JS, Kreitzer AC, Regehr WG. Interplay between facilitation, depression, and residual calcium at three presynaptic terminals. J. Neurosci. 2000;20:1374–1385. doi: 10.1523/JNEUROSCI.20-04-01374.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Neher E. Merits and Limitations of Vesicle Pool Models in View of Heterogeneous Populations of Synaptic Vesicles [Internet] Neuron. 2015;87:1131–1142. doi: 10.1016/j.neuron.2015.08.038. This reference provides a comprehensive review of the literature regarding RRP measurements that we are unable to provide here because of space constraints. It provides an overview of the different methods used to estimate RRP and considers their different assumptions and their limitations. There is a particular focus on the heterogeneity of release properties and the implications for measuring RRP.
- 7.Thanawala MS, Regehr WG. Presynaptic calcium influx controls neurotransmitter release in part by regulating the effective size of the readily releasable pool [Internet] J. Neurosci. 2013;33:4625–4633. doi: 10.1523/JNEUROSCI.4031-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Thanawala MS, Regehr WG. Determining Synaptic Parameters Using High-Frequency Activation [Internet] J. Neurosci. Methods. 2016 doi: 10.1016/j.jneumeth.2016.02.021. This study examines the use of synaptic currents evoked by stimulus trains to determine the size of the RRP at the calyx of Held. It provides a detailed comparison of the different approaches to measure RRP. The EQ, cumulative train and decay tau methods all work well when p is high and replenishment is slow relative to the stimulus frequency. Simulations based on a depletion model fit the data well and could be used to estimate p, RRP and the rate of replenishment.
- 9.Wadiche JI, Jahr CE. Multivesicular release at climbing fiber-Purkinje cell synapses [Internet] Neuron. 2001;32:301–313. doi: 10.1016/s0896-6273(01)00488-3. [DOI] [PubMed] [Google Scholar]
- 10.Raman IM, Trussell LO. The kinetics of the response to glutamate and kainate in neurons of the avian cochlear nucleus. Neuron. 1992;9:173–186. doi: 10.1016/0896-6273(92)90232-3. [DOI] [PubMed] [Google Scholar]
- 11. Turecek J, Jackman SL, Regehr WG. Synaptic specializations support frequency-independent Purkinje cell output from the cerebellar cortex. Cell Rep. doi: 10.1016/j.celrep.2016.11.081. in press. This study uses a low affinity antagonist to demonstrate a role for GABAA receptor saturation at the synapse between Purkinje cells and targets in deep nuclei. It also shows that transmission at this synapse is mediated by two pools of vesicles with different properties: one of these pools specialized to maintained transmission during high frequency stimulation in a manner that leads to frequency independent charge transfer.
- 12.Ariel P, Ryan TA. Optical mapping of release properties in synapses. [Internet] Front. Neural Circuits. 2010;4:1–10. doi: 10.3389/fncir.2010.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ariel P, Hoppa MB, Ryan Ta. Intrinsic variability in Pv, RRP size, Ca2+ channel repertoire, and presynaptic potentiation in individual synaptic boutons [Internet] Front. Synaptic Neurosci. 2013;5:1–18. doi: 10.3389/fnsyn.2012.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fatt P, Katz B. Spontaneous Subthreshold Activity at Motor Nerve Endings. J. Physiol. 1952;117:109–128. [PMC free article] [PubMed] [Google Scholar]
- 15.Rosenmund C, Stevens CF. Definition of the readily releasable pool of vesicles at hippocampal synapses [Internet] Neuron. 1996;16:1197–1207. doi: 10.1016/s0896-6273(00)80146-4. [DOI] [PubMed] [Google Scholar]
- 16.Moulder KL, Mennerick S. Reluctant vesicles contribute to the total readily releasable pool in glutamatergic hippocampal neurons [Internet] J. Neurosci. 2005;25:3842–3850. doi: 10.1523/JNEUROSCI.5231-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schotten S, Meijer M, Walter AM, Huson V, Mamer L, Kalogreades L, Ter Veer M, Ruiter M, Brose N, Rosenmund C, et al. Additive effects on the energy barrier for synaptic vesicle fusion cause supralinear effects on the vesicle fusion rate. Elife. 2015;2015:1–25. doi: 10.7554/eLife.05531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ritzau-Jost A, Delvendahl I, Rings A, Byczkowicz N, Harada H, Shigemoto R, Hirrlinger J, Eilers J, Hallermann S. Ultrafast action potentials mediate kilohertz signaling at a central synapse [Internet] Neuron. 2014;84:152–163. doi: 10.1016/j.neuron.2014.08.036. This paper provides a valuable comparison of different methods to quantify the RRP. They simultaneously record from a cerebellar mossy fiber bouton and a granule cell and measured the RRP for the whole bouton using capacitance (each mossy fiber synapses onto many granule cells), and the RRP for the contact onto a single granule cell using both the response to a voltage step and the with action potentials and the cumulative EPSC method. The voltage step evoked 2 components of release and the fastest component (time constant of 0.43 ms) corresponded to the RRP determined using the cumulative EPSC method.
- 19.Schneggenburger R, Meyer AC, Neher E. Released fraction and total size of a pool of immediately available transmitter quanta at a calyx synapse [Internet] Neuron. 1999;23:399–409. doi: 10.1016/s0896-6273(00)80789-8. [DOI] [PubMed] [Google Scholar]
- 20.Elmqvist BYD, Quastel DMJ. A quantitative study of end-plate potentials in isolated human muscle. J. Physiol. 1965;178:505–529. doi: 10.1113/jphysiol.1965.sp007639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lu H-W, Trussell LO. Spontaneous Activity Defines Effective Convergence Ratios in an Inhibitory Circuit [Internet] J. Neurosci. 2016;36:3268–3280. doi: 10.1523/JNEUROSCI.3499-15.2016. This study provides a comprehensive characterization of a synapse that has significant contributions from 2 pools of vesicles, one with low p and the other with a high p. The low p pool was particularly important for sustaining release during prolonged stimulation.
- 22.Zucker RS, Regehr WG. Short-term synaptic plasticity [Internet] Annu. Rev. Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]
- 23.Silver RA. Estimation of nonuniform quantal parameters with multiple-probability fluctuation analysis: Theory, application and limitations. J. Neurosci. Methods. 2003;130:127–141. doi: 10.1016/j.jneumeth.2003.09.030. [DOI] [PubMed] [Google Scholar]
- 24.Miki T, Malagon G, Pulido C, Llano I, Neher E, Marty A. Actin- and Myosin-Dependent Vesicle Loading of Presynaptic Docking Sites Prior to Exocytosis [Internet] Neuron. 2016;91:808–823. doi: 10.1016/j.neuron.2016.07.033. [DOI] [PubMed] [Google Scholar]
- 25.Bhumbra GS, Beato M. Reliable evaluation of the quantal determinants of synaptic efficacy using Bayesian analysis. [Internet] J. Neurophysiol. 2013;109:603–620. doi: 10.1152/jn.00528.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Costa RP, Sjöström PJ, van Rossum MCW. Probabilistic inference of short-term synaptic plasticity in neocortical microcircuits. [Internet] Front. Comput. Neurosci. 2013;7:75. doi: 10.3389/fncom.2013.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barri A, Wang Y, Hansel D, Mongillo G. Quantifying Repetitive Transmission at Chemical Synapses: A Generative-Model Approach. [Internet] eNeuro. 1967;3(Suppl):1–40. doi: 10.1523/ENEURO.0113-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Augustin I, Rosenmund C, Sudhof TC, Brose N. Munc13-1 is essential for fusion competence of glutamatergic synaptic vesicles [Internet] Nature. 1999;400:457–461. doi: 10.1038/22768. [DOI] [PubMed] [Google Scholar]
- 29.Varoqueaux F, Sigler A, Rhee JS, Brose N, Enk C, Reim K, Rosenmund C. Total arrest of spontaneous and evoked synaptic transmission but normal synaptogenesis in the absence of Munc13-mediated vesicle priming [Internet] Proc. Natl. Acad. Sci. U. S. A. 2002;99:9037–9042. doi: 10.1073/pnas.122623799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jahn R, Fasshauer D. Molecular machines governing exocytosis of synaptic vesicles [Internet] Nature. 2012;490:201–207. doi: 10.1038/nature11320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Richmond JE, Weimer RM, Jorgensen EM. An open form of syntaxin bypasses the requirement for UNC-13 in vesicle priming [Internet] Nature. 2001;412:338–341. doi: 10.1038/35085583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Richmond JE, Davis WS, Jorgensen EM. UNC-13 is required for synaptic vesicle fusion in C. elegans [Internet] Nat. Neurosci. 1999;2:959–964. doi: 10.1038/14755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aravamudan B, Fergestad T, Davis WS, Rodesch CK, Broadie K. Drosophila UNC-13 is essential for synaptic transmission [Internet] Nat. Neurosci. 1999;2:965–971. doi: 10.1038/14764. [DOI] [PubMed] [Google Scholar]
- 34.Andrews-Zwilling YS, Kawabe H, Reim K, Varoqueaux F, Brose N. Binding to Rab3A-interacting molecule RIM regulates the presynaptic recruitment of Munc13-1 and ubMunc13-2 [Internet] J. Biol. Chem. 2006;281:19720–19731. doi: 10.1074/jbc.M601421200. [DOI] [PubMed] [Google Scholar]
- 35.Lu J, Machius M, Dulubova I, Dai H, Sudhof TC, Tomchick DR, Rizo J. Structural basis for a Munc13-1 homodimer to Munc13-1/RIM heterodimer switch [Internet] PLoS Biol. 2006;4:e192. doi: 10.1371/journal.pbio.0040192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deng L, Kaeser PS, Xu W, Sudhof TC. RIM Proteins Activate Vesicle Priming by Reversing Autoinhibitory Homodimerization of Munc13 [Internet] Neuron. 2011;69:317–331. doi: 10.1016/j.neuron.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Basu J, Shen N, Dulubova I, Lu J, Guan R, Guryev O, Grishin NV, Rosenmund C, Rizo J. A minimal domain responsible for Munc13 activity [Internet] Nat Struct Mol Biol. 2005;12:1017–1018. doi: 10.1038/nsmb1001. [DOI] [PubMed] [Google Scholar]
- 38.Li W, Ma C, Guan R, Xu Y, Tomchick DR, Rizo J. The crystal structure of a Munc13 C-terminal module exhibits a remarkable similarity to vesicle tethering factors [Internet] Structure. 2011;19:1443–1455. doi: 10.1016/j.str.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ma C, Su L, Seven AB, Xu Y, Rizo J. Reconstitution of the Vital Functions of Munc18 and Munc13 in Neurotransmitter Release [Internet] Science. 2013;339:421–425. doi: 10.1126/science.1230473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang X, Wang S, Sheng Y, Zhang M, Zou W, Wu L, Kang L, Rizo J, Zhang R, Xu T, et al. Syntaxin opening by the MUN domain underlies the function of Munc13 in synaptic-vesicle priming [Internet] Nat. Struct. Mol. Biol. 2015 doi: 10.1038/nsmb.3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Liu X, Seven AB, Camacho M, Esser V, Xu J, Trimbuch T, Quade B, Su L, Ma C, Rosenmund C, et al. Functional Synergy between the Munc13 C-terminal C1 and C2 domains [Internet] Elife. 2016;5:1–27. doi: 10.7554/eLife.13696. These studies (references 40 and 41) performed in vitro fusion assays and biophysical experiments to dissect to molecular mechanisms of Munc13 in fusion. Together with an earlier important study (ref. 39) they revealed that the MUN domain is an essential tethering domain that aides Munc18 in SNARE complex assembly, and they support that the C2 domains that flank the MUN domain support SNARE assembly by tethering the vesicular and the target membranes close to one another.
- 42.Choi UB, Zhao M, Zhang Y, Lai Y, Brunger AT. Complexin induces a conformational change at the membrane-proximal C-terminal end of the SNARE complex [Internet] Elife. 2016;5:1689–1699. doi: 10.7554/eLife.16886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen X, Tomchick DR, Kovrigin E, Arac D, Machius M, Sudhof TC, Rizo J. Three-dimensional structure of the complexin/SNARE complex [Internet] Neuron. 2002;33:397–409. doi: 10.1016/s0896-6273(02)00583-4. [DOI] [PubMed] [Google Scholar]
- 44.Zhou Q, Lai Y, Bacaj T, Zhao M, Lyubimov AY, Uervirojnangkoorn M, Zeldin OB, Brewster AS, Sauter NK, Cohen AE, et al. Architecture of the synaptotagmin–SNARE machinery for neuronal exocytosis [Internet] Nature. 2015 doi: 10.1038/nature14975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kaeser PS, Deng L, Wang Y, Dulubova I, Liu X, Rizo J, Sudhof TC. RIM proteins tether Ca2+ channels to presynaptic active zones via a direct PDZ-domain interaction [Internet] Cell. 2011;144:282–295. doi: 10.1016/j.cell.2010.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Han Y, Kaeser PS, Sudhof TC, Schneggenburger R. RIM Determines Ca(2+) Channel Density and Vesicle Docking at the Presynaptic Active Zone [Internet] Neuron. 2011;69:304–316. doi: 10.1016/j.neuron.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jockusch WJ, Speidel D, Sigler A, Sørensen JB, Varoqueaux F, Rhee JS, Brose N. CAPS-1 and CAPS-2 are essential synaptic vesicle priming proteins [Internet] Cell. 2007;131:796–808. doi: 10.1016/j.cell.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 48.Held RG, Liu C, Kaeser PS. ELKS controls the pool of readily releasable vesicles at excitatory synapses through its N-terminal coiled-coil domains. Elife. 2016;1:1–20. doi: 10.7554/eLife.14862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kaeser-Woo YJ, Yang X, Sudhof TC. C-terminal complexin sequence is selectively required for clamping and priming but not for Ca2+ triggering of synaptic exocytosis [Internet] J. Neurosci. 2012;32:2877–2885. doi: 10.1523/JNEUROSCI.3360-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bacaj T, Wu D, Burré J, Malenka RC, Liu X, Südhof TC. Synaptotagmin-1 and-7 Are Redundantly Essential for Maintaining the Capacity of the Readily-Releasable Pool of Synaptic Vesicles [Internet] PLOS Biol. 2015;13:e1002267. doi: 10.1371/journal.pbio.1002267. This study found that simultaneous removal of the calcium sensors synaptotagmin 1 and synaptotagmin 7 decreased the size of the RRP but not vesicle docking in cultured hippocampal neurons. In contrast, removal of each protein alone did not impair the RRP. This study reveals redundancy between synaptotagmin 1 and synaptotagmin 7 in controlling the size of the RRP that is not present for the calcium sensing functions of these proteins.
- 51.Schikorski T, Stevens CF. Morphological correlates of functionally defined synaptic vesicle populations. Nat. Neurosci. 2001;4:391–395. doi: 10.1038/86042. [DOI] [PubMed] [Google Scholar]
- 52. Imig C, Min SW, Krinner S, Arancillo M, Rosenmund C, Südhof TC, Rhee JS, Brose N, Cooper BH. The Morphological and Molecular Nature of Synaptic Vesicle Priming at Presynaptic Active Zones. Neuron. 2014;84:416–431. doi: 10.1016/j.neuron.2014.10.009. This hallmark study characterized roles for presynaptic proteins in vesicle docking using organotypic slice cultures, high pressure freezing and electron tomography. These methods improved resolution of the docking process and they revealed that mutant mice for SNAREs, Munc13 and CAPS have a dramatic reduction in tightly docked vesicles, whereas synaptotagmin and complexin mutants had milder or no defects, respectively. Interestingly, the mutants with fewer docked vesicles had more vesicles in the bins 5–20 nm away from the presynaptic membrane.
- 53.Hammarlund M, Palfreyman MT, Watanabe S, Olsen S, Jorgensen EM. Open syntaxin docks synaptic vesicles [Internet] PLoS Biol. 2007;5:e198. doi: 10.1371/journal.pbio.0050198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Watanabe S, Rost BR, Camacho-Pérez M, Davis MW, Söhl-Kielczynski B, Rosenmund C, Jorgensen EM. Ultrafast endocytosis at mouse hippocampal synapses. [Internet] Nature. 2013;504:242–247. doi: 10.1038/nature12809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rizzoli SO, Betz WJ. The structural organization of the readily releasable pool of synaptic vesicles. [Internet] Science. 2004;303:2037–2039. doi: 10.1126/science.1094682. [DOI] [PubMed] [Google Scholar]
- 56. Wang SSH, Held RG, Wong MY, Liu C, Karakhanyan A, Kaeser PS. Fusion Competent Synaptic Vesicles Persist upon Active Zone Disruption and Loss of Vesicle Docking [Internet] Neuron. 2016;91:777–791. doi: 10.1016/j.neuron.2016.07.005. This study found that genetic deletion of ELKS and RIM leads to a strong structural disruption of the active zone, the protein network that forms release sites at the presynaptic membrane, including loss of Munc13. Active zone disruption led to a near complete loss of vesicle docking, a strong reduction in vesicles within 100 nm of the presynaptic membrane, and a strong decrease in vesicular release probability. Surprisingly, some fusion competent vesicles persisted despite loss of vesicle docking and tethering.
- 57.Zenisek D, Steyer Ja, Almers W. Transport, capture and exocytosis of single synaptic vesicles at active zones. Nature. 2000;406:849–854. doi: 10.1038/35022500. [DOI] [PubMed] [Google Scholar]