Abstract
Methylmercury (MeHg) is an environmental neurotoxicant of public health concern. It readily accumulates in exposed humans, primarily in neuronal tissue. Exposure to MeHg, either acutely or chronically, causes severe neuronal dysfunction in the central nervous system and spinal neurons; dysfunction of susceptible neuronal populations results in neurodegeneration, at least in part through Ca2+-mediated pathways. Biochemical and morphologic changes in peripheral neurons precede those in central brain regions, despite the fact that MeHg readily crosses the blood-brain barrier. Consequently, it is suggested that unique characteristics of spinal cord afferents and efferents could heighten their susceptibility to MeHg toxicity. Transient receptor potential (TRP) ion channels are a class of Ca2+-permeable cation channels that are highly expressed in spinal afferents, among other sensory and visceral organs. These channels can be activated in numerous ways, including directly via chemical irritants or indirectly via Ca2+ release from intracellular storage organelles. Early studies demonstrated that MeHg interacts with heterologous TRPs, though definitive mechanisms of MeHg toxicity on sensory neurons may involve more complex interaction with, and among, differentially-expressed TRP populations. In spinal efferents, glutamate receptors of the N-methyl-D-aspartate (NMDA), α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), and possibly kainic acid (KA) classes are thought to play a major role in MeHg-induced neurotoxicity. Specifically, the Ca2+-permeable AMPA receptors, which are abundant in motor neurons, have been identified as being involved in MeHg-induced neurotoxicity. In this review, we will describe the mechanisms that could contribute to MeHg-induced spinal cord afferent and efferent neuronal degeneration, including the possible mediators, such as uniquely expressed Ca2+-permeable ion channels.
1.Introduction
1.1 Methylmercury (MeHg) neurotoxicity
Mercury is an environmental toxicant derived from both natural and anthropogenic sources. In the environment, elemental mercury (Hg0) originates from the Earth’s crust and volcanic emissions. Anthropogenic sources include burning of coal, waste incineration and small-scale gold mining. Hg0 enters the atmosphere as a vapor, where it then becomes part of the water cycle. Once in the atmosphere, Hg0 can remain in its vaporous state and move throughout the hemisphere, or become oxidized to Hg2+ and recycled to the Earth in rain (Clarkson and Magos, 2006, Horowitz et al., 2014). Accumulated Hg2+ is a substrate for methylation by sulfate-reducing bacteria in bodies of water, generating MeHg. Given its relative lipophilic properties, MeHg then enters the aquatic food chain through the process of bioaccumulation. Humans are exposed to MeHg primarily through the consumption of contaminated seafood or marine mammals (Clarkson, 1995). A relatively recent contributor to MeHg exposure and subsequent toxicity, is the use of mercury amalgam for extracting gold in artisanal gold mining (Schmidt, 2012). This may represent the most frequent contemporary route of exposure to MeHg, especially in South America (Ashe, 2012; Fraser, 2016) and Africa (Odumo et al., 2014, Rajaee et al., 2015).
MeHg is the most prevalent form of organic Hg encountered, and it is of environmental and public health concern due to its prevalence and persistence in the environment. Two major MeHg poisoning episodes triggered the many studies and subsequent discoveries regarding MeHg toxicity: Minamata, Japan (1953–1956) and Iraq (1970s) (Bakir et al., 1973, Clarkson and Magos, 2006, Eto, 1997). Studies from these two poisoning events led to the following findings relevant to this review: 1) there is a latent period between the exposure to MeHg and the onset of symptoms; 2) severity of symptoms is MeHg dose-dependent; and 3) the first and most prevalent symptom reported is paresthesia, followed, often in succession, by ataxia, muscle weakness, tremor, dysarthria, and hearing and visual impairment (Bakir, et al., 1973, Eto, 1997). Patients presenting with these clinical signs and symptoms as a result of environmental exposure to MeHg were diagnosed with the neurologic syndrome referred to as Minamata Disease (MD) (McAlpine and Araki, 1958).
Developmental neurotoxicity to MeHg was especially apparent in these two major poisoning episodes (Bakir, et al., 1973, Eto, 1997). However, MeHg exposure occurs over the life span for anyone consuming certain species of fish or marine mammals, and the consequences of chronic, low-level adult onset exposures are an underexplored area. Ongoing studies of chronic dietary exposure to MeHg in the Seychelle (Davidson et al., 2000) and Faroe Islands (Grandjean et al., 1999), in which the primary food source is fish and/or marine mammals have been following the development of neurological sequellae.
Another more recently discovered region under critical examination is the Peruvian Amazon (Ashe, 2012, Fraser, 2016, Gardner, 2012), where Hg is used extensively for artisanal gold mining. MeHg toxicity under these conditions is somewhat unique, in that potentially multiple routes of exposure to Hg exist, and exposure to multiple forms of Hg can occur. Hg vapors are released to the environment during extraction of gold using Hg0-based amalgams and Hg0 waste is subsequently disposed into water sources, where it is converted into MeHg. Gold miners are not only exposed to Hg0 dermally and through vapors, but also to MeHg because of their common dietary practices of fish consumption (Ashe, 2012, Fraser, 2016, Gardner, 2012, Wade, 2013). South American exposure to mercurials through mining has made it the current largest known source of Hg pollution in the world (Wade, 2013), and one of the most extensive known adult Hg and MeHg exposures. As many as 48,000 people are estimated to have been affected so far; neurological effects levels of exposure have not yet been reported (Fraser, 2016). In 2012, it was reported that approximately 11% of the population at Madre de Dios mining zones had hair-Hg concentrations exceeding 16 mg Hg/g dry hair, a symptomatic concentration as described by the World Health Organization (Ashe, 2012). Recent reports indicating if and how these levels have changed are lacking. However, based on the reports we discuss in this review, if exposure to MeHg continues in Madre de Dios, Peru in a similar manner as has been reported since 2012, it is expected that this population will present neurological signs identical to those observed in MD.
Neurologic signs of MD correlate with degeneration of susceptible neuronal populations, including cerebellar granule cells and somatosensory neurons (Al-Saleem, 1976, Bakir, et al., 1973, Eto, 1997, Eto et al., 2002, Takeuchi et al., 1962). Although the precise sequence of cellular events leading to MeHg-induced neurodegeneration remains elusive, dysregulation of intracellular Ca2+ concentration ([Ca2+]i) appears to be a critical and early-onset component. MeHg exposure in vitro leads to a time- and concentration-dependent increase in [Ca2+]i in multiple types of primary, and native neurons as well as immortalized cells (Bradford et al., 2016, Edwards et al., 2005, Hare et al., 1993, Johnson et al., 2011, Marty and Atchison, 1997b, Ramanathan and Atchison, 2011, Yuan and Atchison, 2007).
MeHg-induced alterations in [Ca2+]i can be measured using single-cell Ca2+ microfluorimetry. Cells in culture are loaded with the fluorophore fura-2-acetoxymethyl ester which readily crosses the cell membrane, after which it is deesterified by endogenous esterases and capable of binding free Ca2+. Changes in [Ca2+]i are then measured at 505 nm by calculating the ratio of fluorescence obtained using alternating excitation at 340 nm and 380 nm, corresponding to fura-2 bound and unbound Ca2+i, respectively (Grynkiewicz et al., 1985, Limke and Atchison, 2005). MeHg increases [Ca2+]i in two kinetically-distinct phases: the first (referred to as Phase 1) results from release of Ca2+ from intracellular stores whereas the second (Phase 2) corresponds with the influx of extracellular Ca2+ in primary neurons in culture (Edwards et al., 2005, Limke et al., 2004a,b, Marty and Atchison, 1997, 1998; Ramanathan and Atchison, 2011), transformed cells (Hare et al, 1993, Hare and Atchison, 1995a,b), and using recombinant channels in heterologous expression systems (unpublished observation). For brain slices such as those of cerebellum, confocal microscopy is used in place of single cell fluorimetry (Bradford et al., 2016, Johnson et al., 2011, Yuan and Atchison, 2009), because confocal lasers cannot attain the spectral levels needed for excitation of fura-2. Using confocal microscopy, it is impossible to identify distinct phases of elevation of Ca2+i in these preparations. On the other hand, confocal microscopy allows examination of comparative spatial sensitivity of distinct neuronal populations to MeHg-induced [Ca2+]i dysregulation. For example, Yuan and Atchison (2009) showed a clear differential sensitivity to MeHg-induced changes in fluo-4 fluorescence between cerebellar granule and Purkinje cells in freshly prepared cerebellar slices. Moreover, in cerebellar organotypic slice culture, elevations of [Ca2+]i are also observed in response to low concentrations of MeHg (Bradford et al., 2016), in a developmental migratory stage-dependent manner in granule neurons. Thus MeHg-induced elevations of [Ca2+]i occur in both isolated cells, and intact circuits.
Consistent results also occur at the whole animal level, or following in vivo dosing. Chronic exposure to MeHg in drinking water, at concentrations which do not of themselves cause overt neurotoxic effects, causes elevations of [Ca2+]i in brainstem slices of mice harboring a mutation in superoxide dismutase-1 (SOD1-G93A) (Johnson et al., 2011). Moreover, in rats treated with MeHg (5 mg/kg/day, p.o., 12 days), and concomitantly with L-type (Cav1.3) Ca2+ channel blockers, there was a reduced incidence of gross signs of MeHg toxicity, compared with treats treated with MeHg alone (Sakamoto et al., 1996). Finally, in mice, dietary supplementation with the L-type Ca2+ channel antagonist nimodipine, delayed, or precluded MeHg-induced behavioral toxicity (Bailey et al., 2013). Taken together, results from isolated cells, brain slices, and in vivo exposures provide compelling evidence for the pivotal role of elevations in intracellular Ca2+ concentration. Additional effects, including generation of reactive oxygen species, and mitochondrial damage occur in neurons following MeHg exposure. However, two studies have shown that, at least for cerebellar granule cells in culture, or synaptosomes derived from striatum, elevation of [Ca2+]i is the sine qua non in MeHg-induced cytotoxicity (Dreiem and Seegal, 2007, Sarafian and Verity, 1991).
MeHg accumulates in different neuronal cell types; however, that does not necessarily contribute to their degeneration. In comparative studies, MeHg toxicity in cerebellar granule cells was not dependent on MeHg accumulation, as it accumulates in greater amounts (Leyshøn Sorland et al., 1994), yet is less cytotoxic in the cerebellar Purkinje cells, a larger cell type (Edwards et al., 2005). However, Purkinje cells express high levels of Ca2+ binding proteins such as calbindin that cerebellar granule cells do not. This apparently confers relative protection against the damage triggered by Ca2+ dysregulation. The absence of Ca2+ binding proteins and the presence of certain ligand- and voltage-gated ion channels appear to be important contributing factors to MeHg toxicity (Limke et al., 2004)
1.2 Organization of the spinal somatosensory and motor systems
Somatosensory information is carried from the periphery to the brain via sensory afferents, or dorsal root ganglia (DRG) neurons. Four somatosensory modalities (mechanoreception, proprioception, thermoreception, and nociception) are differentially transmitted by distinct DRG neuron types (Aα, Aβ, Aδ, C) (Boron and Boulpaep, 2009). These neurons can be generally characterized by axon fiber diameter (Lee et al., 1986, Scroggs and Fox, 1992), immunoreactivity (Dodd et al., 1983, Hjerling-Leffler et al., 2007, Hökfelt et al., 1976), action potential duration (Djouhri et al., 1998, Fang et al., 2005, Villière and McLachlan, 1996), and several other electrical properties (Harper and Lawson, 1985, Lawson, 2002, Murali et al., 2015, Scott and Edwards, 1980). It is these unique physical and electrical attributes of afferent neurons, in addition to diverse expression of specialized peripheral receptors, localization within the dorsal horn, ascending spinal pathways, and central targets, which contribute to modality segregation (Uddenberg, 1968, Wall and Dubner, 1972, Willis and Coggeshall, 2004). Consequently, somatosensory modalities can be loosely related to discrete DRG neuron types. Aδ and C fibers are the smaller of the DRG neurons and are canonically categorized as nociceptive (Boron and Boulpaep, 2009, Zigmond et al., 1999). While Aδ fibers transmit sharp, pricking pain sensations, C fibers mediate dull and burning pain; the distinct quality of pain carried by these neurons can be attributed to axon diameter and, thus, conduction velocity, in addition to receptor expression, and fiber myelination. Aα and Aβ are the larger of the DRG neurons that are extensively myelinated; they transmit mechanoreceptive and proprioceptive signals (Lawson, 2002).
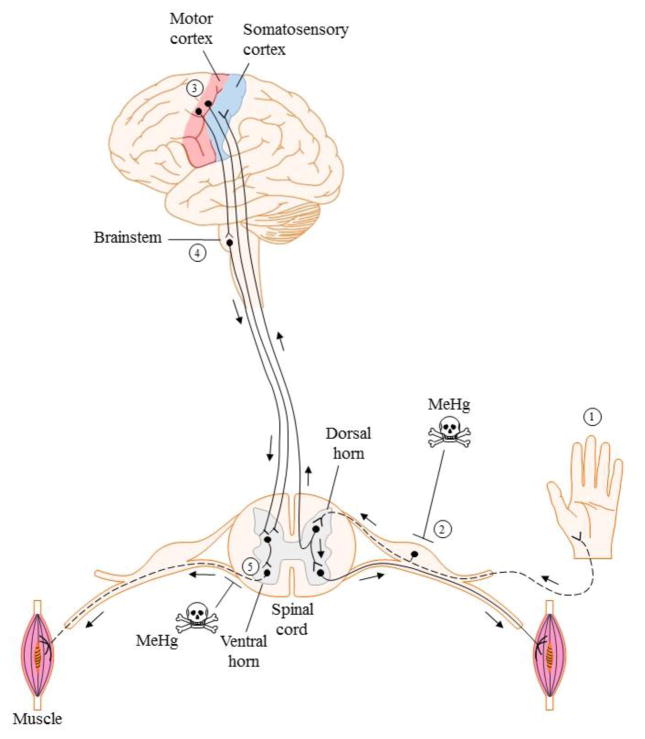
Motor information is transmitted from the primary motor cortex (via upper motor neurons (MNs)) to the muscles via lower MNs (Figure 1), permitting essential functions such as respiration, digestion, and voluntary movement to occur. Classification of MNs is based on the type and contractile properties of innervated muscle fibers. The three main MN classifications are: α, β and γ. α MNs are responsible for skeletal muscle contraction and innervate extrafusal skeletal muscle, β MNs are involved in muscle contraction as well, and innervate the intra- and extrafusal fibers. γ MNs are responsible for the muscle spindle and thus innervate intrafusal muscle fibers (Burke, 1980, Burke et al., 1973). β MNs are not as well characterized as α and γ MNs. Thus, a comparison of β MNs with the other two types is not yet possible. However, several studies have identified unique characteristics in α and γ MNs that can make them uniquely susceptible to neuronal degeneration as a result of exposure to environmental toxicants or neurodegenerative diseases (Kanning et al., 2010). α MNs are larger in size than γ MNs, resulting in a greater volume of axoplasm and membrane surface area to be maintained, and consequently, having a higher threshold for activation. The axon caliper of α MNs is also larger, and they have more dendrites and make deep synaptic folds in the neuromuscular junction, as opposed to the γ MNs, which have fewer synaptic inputs and shallow synaptic folds. This contributes to the difference in synaptic communication of these two cell types. Because α MNs are the most abundant MNs, they can be further subdivided as: α fast-twitch fatigable (FF), fast-twitch fatigue resistant (FR) and slow twitch fatigue resistant (S) (Burke, et al., 1973). These three classes of α MNs vary in size, arborization and the response of the muscle fiber they innervate (Kanning, et al., 2010).
Figure 1. Simplified schematic of organization and integration of somatosensory and motor systems.
Dashed lines indicate MeHg susceptible neuronal populations.
Sensory and MNs work cooperatively to enable environmental perception and movement (Fig. 1). Sensory neurons receive information through receptors from the skin and send the signal through the dorsal column to the thalamus, where it is then relayed to the primary somatosensory cortex. There the sensory signal is sent to three different association areas: 1) the unimodal and 2) multimodal sensory association area, and 3) the multimodal motor association area. Within the sensory association regions, specific sensory signals are sorted and integrated into a single sensory response, which is then interpreted and converted into a motor response by the multimodal motor association area. The motor response is then sent to the premotor and primary motor cortices. MNs that originate in the primary motor cortex descend though two major pathways, the lateral pathway, which controls voluntary movement, or the ventromedial pathway, which controls posture and locomotion. The ventromedial pathway is controlled by the brainstem. This pathway originates in the primary motor cortex, synapses with lower MNs in the brainstem superior colliculus or reticular nuclei and, in turn, their axons make a connection with the α MNs in the spinal cord (Bear et al., 2006). Subclasses of DRG neurons also form synapses with interneurons and the α MNs from the spinal cord to modulate reflexive motor movement without central integration (Boron and Boulpaep, 2009).
Interactions between sensory afferents and motor efferents are essential for their mutual function in the nervous system. Dysfunction or degeneration of one could lead to a similar defect in the other. Also, sensory and MN diversity in morphology, electrical properties and receptor expression could be an important factor in the susceptibility of these cell populations to MeHg toxicity. This is the focus of the present review.
2. Effects of MeHg on sensory afferents
Paresthesia is the preeminent clinical sign of toxicity in individuals poisoned by MeHg (Takaoka et al., 2008). Onset of sensory disturbances in poisoned adults can occur at hair-Hg concentrations of 100 ppm, whereas other clinical signs typically do not arise until hair-Hg concentrations approach 200 ppm (Bakir, et al., 1973, Clarkson and Magos, 2006). Clinical assessment and nerve biopsies of MeHg-poisoned adults suggest that MeHg-related paresthesia is associated with degeneration of DRG neurons (Eto, 1997, Eto, et al., 2002), though experimental studies, reviewed here, demonstrate that distinct changes in cell morphology and electrical properties precede cell death. Interestingly, severe perturbations in DRG function and neurodegeneration have been reported in only Aα and Aβ fibers, placing an onus on not only understanding the mechanisms preceding DRG neuronal degeneration, but also why large-fiber afferents are especially susceptible to MeHg neurotoxicity.
2.1 Morphologic changes and selective degeneration
To understand better the mechanisms of MeHg neurotoxicity in DRG neurons, rodent models are commonly used in conjunction with in vivo, subacute and subchronic exposure paradigms. Findings under these conditions align with spinal nerve biopsies and autopsies of patients diagnosed with MD. Adult rats administered MeHg (5 mg/kg/day ip, 10 days) present with severe changes in afferent morphology after as few as 8 days. The earliest signs of MeHg toxicity in the DRG of these animals include loss of Nissl staining, cytoplasmic vacuolization, swelling of cisternal organelles, and proliferation of satellite cells. These morphologic changes are predominantly present in large-diameter DRG neurons, corresponding to the Aα and Aβ fiber types, with only occasional degeneration of myelinated neurons (Cavanagh and Chen, 1971, Delio et al., 1992).
In a separate set of experiments, adult rats were administered MeHg (2 mg/kg/day, p.o.) for 19 days, followed by a 32-day recovery period. Despite the lower dose and opportunity for recovery and repair, a marked reduction occurred in the number and mean somal volume of large-fiber DRG neurons, whereas small-fiber DRG neurons were relatively spared from MeHg-related damage (Schiønning et al., 1998). Following MeHg exposure, Wallerian degeneration occurs at both the level of the dorsal roots and spinal cord fasciculus gracilis, and is thought to be related to MeHg-related microtubule disruption in these neurons (Abe et al., 1975, Miura et al., 2000). This widespread degeneration of DRG neurons precedes any detectable morphologic alterations in motor afferents or the cerebellum (Cao et al., 2013, Eto, 1997, Schiønning, et al., 1998). Autopsy and biopsy material from patients diagnosed with MD show consequences of MeHg exposure at protracted time points, up to 11 years following onset of sensory disturbances; samples from MD patients show endoneurial fibrosis, Büngner’s bands, and proliferation of fibroblasts and Schwann cells in the sural nerve tissue, suggesting an attempt to repair the damaged nerve tissue, albeit an abnormal and incomplete regeneration (Eto, 1997, Eto, et al., 2002). Thus, it has been suggested that early-stage ataxia, muscle weakness, and abnormal reflexes coinciding with MeHg-induced paresthesia are more related to degeneration of proprioceptive Aα and Aβ neurons and loss of sensory input than to central lesions (Yip and Riley, 1987).
Damage at the somatosensory cortex by MeHg is ill-defined. A proteomic study of the somatosensory cortices of juvenile rats revealed that MeHg (40 μg/kg/day, p.o. 12 weeks) induced a state of metabolic deficit through downregulation of essential metabolic proteins and components of neurotransmission (Freire et al., 2007, Kong et al., 2013). Though the impact of MeHg-induced damage to the somatosensory cortex may not be as immediately detectable as damage to peripheral afferents, it seems that alterations in this brain region may contribute to persistent paresthesia long after cessation of exposure to MeHg. When assessed 30 years following MeHg exposure in Minamata, patients with MD perceived sensations of touch, though the threshold for sensation is elevated at both distal and proximal sites. This indicates that long-lasting sensory dysfunction caused by MeHg may not be due to direct injury of peripheral nerves, but rather to acquired dysfunction of central mechanisms (Ninomiya et al., 2005).
2.2 Alteration of electrical and biochemical properties
In addition to morphologic abnormalities, MeHg-induced paresthesia may be explained by electrical changes to DRG neurons. Studies in isolated neonatal rat DRG neurons revealed that MeHg (0.25 – 50 μM) blocks current carried through voltage-activated ion channels to varying extents, as measured by IC50, or concentration of MeHg required to reduce the response by half; potency of MeHg at voltage-activated Ca2+ and K+ channels (IC50 = 2.6 and 2.2 μM, respectively) is much higher than at voltage-activated Na+ channels (IC50 = 12.3 μM) (Leonhardt et al., 1996a,b). However, in cerebellar granule neurons, the comparative sensitivity of voltage-activated Ca2+ and K+ channels is reversed, with Ca2+ channels being much more sensitive to block by MeHg (Yuan et al., 2005). Thus sensitivity of specific classes of voltage-gated ion channels to MeHg is cell-type dependent, and likely due to relative expression levels of specific subtypes of Ca2+ and K+ channels (Peng et al., 2002, Hajela et al., 2003, Sirois and Atchison, 2000). Despite the inhibitory action of MeHg on voltage-activated ion channels in vitro, DRG neurons from adult rats administered MeHg (5 mg/kg/day i.p. for 10 days) show no significant alteration in resting membrane potential, or action potential duration, amplitude, or kinetics (Delio et al., 1992). However, conduction velocity of Aβ and Aδ afferents is slowed by 150 – 200%, resulting in a significant delay in action potential onset following nerve stimulation. Aβ and Aδ afferents from MeHg-treated rats also display ectopic repetitive action potential discharge from single nerve stimulation in approximately 25% of cells sampled, whereas repetitive discharge in Aα and C fibers is a rare occurrence (Delio et al., 1992). Similar action potential trains are present in organotypic slices of the somatosensory cortex from rats exposed to MeHg in utero through weaning, when the dam had continuous access to 0.375 mg/kg/day MeHg ad libitum in drinking water beginning at the mating period; the increase in the number of action potential spikes evoked from single nerve stimulation may be related to the reduced spike threshold in MeHg-exposed weanlings (Világi et al., 2000).
In addition to MeHg, Hg2+ alters ion channel function and, in turn, electrical properties of sensory afferents. HgCl2 (1 μM), applied acutely to neonatal rat DRG neurons, blocks peak and sustained currents carried through voltage-gated Ca2+ channels to the same extent as 5 μM MeHg (Leonhardt et al., 1996b). A comparative study of the action of mercurial compounds on GABA-induced currents in neonatal rat DRG neurons found similar results in that HgCl2 had a higher potency than did MeHg. However, the discrete actions of HgCl2 and MeHg on GABA-induced currents are opposite; acute application of 10 μM HgCl2 greatly enhanced GABA currents to nearly 140% of control, whereas 100 μM MeHg reduced GABA currents to approximately 80% of control (Arakawa et al., 1991). The mechanism for HgCl2 potentiation of GABA-induced currents includes G protein and protein kinase A-coupled pathways, whereas the mechanism of MeHg reduction of these currents has not been elucidated (Huang and Narahashi, 1997a,b). When applied alone, both organic and inorganic mercury induce a slow, inward current in isolated DRG neurons that is not mediated by voltage-activated cation channels or GABA-receptor activated Cl− channels, suggesting the mercurials also act upon non-specific cation channels (Arakawa, et al., 1991, Huang and Narahashi, 1997a,b, Narahashi et al., 1994). A similar response has been observed in cerebellar slices following acute application of MeHg (Yuan and Atchison, 2005). Given the importance of ion gradients in maintaining membrane excitability and propagating action potentials, the distinct effects of both MeHg and HgCl2 on ion channels may contribute to abnormal electrical properties observed in afferents exposed to mercurials.
Ionic balance is not only important for maintaining electrical homeostasis in afferents, but also biochemical homeostasis. Although MeHg blocks current carried through voltage-gated Ca2+ channels (Hajela et al., 2003, Leonhardt, et al., 1996b, Peng et al., 2002, Shafer and Atchison, 1991, Sirois and Atchison, 2000, Tarabová et al., 2006), it also stimulates release of Ca2+ from intracellular stores to increase cytosolic Ca2+ levels (Castoldi et al., 1997, Hare and Atchison, 1995a, Harris and Baum, 1980, Limke and Atchison, 2002, Limke et al., 2003, Marty and Atchison, 1997, 1998, Sarafian, 1993). Regulation of intracellular Ca2+ is critical given its central role in basic neuronal functions, including protein synthesis, growth and differentiation, gene expression and synaptic transmission. Indeed, protein synthesis is reduced by 60% in the DRG of animals treated with MeHg (10 mg/kg/day s.c., 7 days). The reduction in protein synthesis persists beyond cessation of MeHg treatments and into the symptomatic period, or approximately day 15 of exposure (Omata et al., 1982). However, the reduction in protein synthesis is not uniform and depends upon the gene product; production of a majority of proteins is suppressed, though some are upregulated (Kasama et al., 1989). Alterations in protein synthesis within MeHg-exposed DRG neurons can lead to phenotypic changes of these neurons. Primary cultures of DRG obtained from adult mice and exposed to MeHg (0.1 – 1 μM, 24 hr) exhibit increased substance P and calcitonin gene-related peptide immunoreactivity (Baxter and Smith, 1998), both of which are immunologic markers for discrete subtypes of small-fiber nociceptive neuron (Jeftinija and Jeftinija, 1990, Otten et al., 1980). These alterations in DRG neuronal immunoreactivity can contribute to abnormal nociceptive responses in MeHg poisoning (Baxter and Smith, 1998). Additionally, neurite outgrowth is inhibited in both chick (Nakada et al., 1981) and embryonic rat DRG cultures (Wilke et al., 2003), with the median toxic concentrations (IC50) of 2 μM and 0.5 μM, respectively.
Disruption of electrical and biochemical homeostasis in sensory afferents leads to apoptosis (Wilke, et al., 2003), and eventual neurodegeneration (Chang and Hartmann, 1972). Putative cellular targets through which MeHg exerts neurotoxicity include mitochondria (Hare, et al., 1993, Levesque et al., 1992, Limke and Atchison, 2002, Limke, et al., 2003b), inositol 1,4,5-trisphosphate-sensitive stores (Hare and Atchison, 1995a, Limke et al., 2003b), and both voltage-gated (Peng, et al., 2002, Hajela, et al., 2003, Hare and Atchison, 1995b, Ramanathan and Atchison, 2011, Shafer and Atchison, 1991, Shafer et al., 1990) and ligand-gated ion channels (Arakawa, et al., 1991, Herden et al., 2008, Ramanathan and Atchison, 2011, Yuan and Atchison, 2003, 2005). Mechanistic studies indicate a complexity in the actions of MeHg, and dysfunction at multiple cellular targets may collaborate to elicit toxicity. Yet, none of these identified molecular targets are exclusive to DRG neurons, let alone large-diameter DRG neurons. The fact that Aβ afferents are uniquely susceptible to MeHg suggests that a molecular feature of these neurons could also be involved in events leading to cell death and neurodegeneration. Transient receptor potential (TRP) channels, are a class of ion channels implicated with critical roles in sensory function (Hjerling-Leffler, 2007, Liedtke and Heller, 2007, Numazaki and Tominaga, 2004, Vennekens et al., 2002). As the understanding of the physiologic role of these sensory ion channels expands, questions arise as to how their dysfunction may contribute to pathological states.
2.3 Transient receptor potential (TRP) channels as mediators of MeHg-induced Ca2+ dysregulation
The TRP family of ion channels is organized into 6 distinct subgroups and consists of at least 30 known gene products. Many of these nonspecific cation channels have permeability to Ca2+, the extent of which varies across channel subtypes. Roles of TRP channels in cellular physiology differ as widely as the range of chemical, mechanical, and thermal stimuli that activate them. In some cases, TRP channel function or means of activation remain unknown. Because of their high level of expression in DRG and sensory organs, TRP channels have been implicated as critical components of sensory transmission. Within DRG neurons, TRP channel expression is localized to the peripheral and central terminals, as well as the soma (Islam, 2011, Liedtke and Heller, 2007). If MeHg targets TRP channels, their dysfunction could cause perturbations in receiving, integrating, and transmitting sensory information.
Perhaps the most well characterized TRP channels are those of the canonical subgroup, TRPC, which are Ca2+ store-operated (Funayama et al., 1996, Wes et al., 1995). TRPC channels are activated through at least 3 intracellular mechanisms: 1) generation of, and presumably direct interaction with, diacylglycerol; 2) conformational coupling with inositol 1,4,5-trisphosphate receptors; and 3) interaction with membrane-translocated stromal interaction molecule 1 (Islam, 2011, Liedtke and Heller, 2007). Given that MeHg stimulates release of Ca2+ from intracellular stores, it is not unreasonable to postulate that exposure to the toxicant may cause TRPC channel activation via the latter two mechanisms.
The only investigation into the action of mercurials on TRPs was performed using heterologously-expressed TRPC isoforms (Xu et al., 2012). TRPC channels were transfected into human embryonic kidney cells and acutely exposed to either MeHg or HgCl2 (0.1 – 10 μM). The study revealed that the influx of Ca2+ through TRPC4 and TRPC5 channels is increased by both MeHg (EC50 = 2.0 μM) and HgCl2 (EC50 = 3.1 μM), through interaction at two critical cysteine residues within the channel pore. The interaction between the channel and mercurials is believed to be specific, as other divalent heavy metals, including Cd2+ and Zn2+, have no effect on Ca2+ influx. Similarly, mercurials do not elicit a stimulatory effect on all TRPs; TRPC3, TRPC6, TRPV1, and TRPM2 channels are among the TRP isoforms for which exposure to either MeHg or HgCl2 does not enhance nor abolish Ca2+ entry through the channel. The prevalence of mercury-induced cell death of TRPC5-expressing cells is reduced following treatment with specific channel blockers or siRNA against TRPC5 (Xu et al., 2012). Though this provides reasonable evidence that this less extensively studied class of ion channels may be a target of MeHg, studies need to be performed in native tissues in which the full complement of receptors and ion channels are available to interact with and modulate one another. As shown in Figure 2, enhancement of Ca2+ entry through at least some TRP isoforms by mercurials could result in hyperexcitability of DRG neurons, despite block of some classes of voltage-gated ion channels (Hajela, et al., 2003, Leonhardt et al., 1996a,b, Peng, et al., 2002, Shafer and Atchison, 1991, Sirois and Atchison, 2000). MeHg-induced Ca2+ influx through discrete TRP isoforms in combination with the well-characterized release of Ca2+ from intracellular storage organelles (Hare and Atchison, 1995a, Hare, et al., 1993, Limke and Atchison, 2002, Limke et al., 2004a,b, Limke, et al., 2003b), could lead to perturbations in sensory function and Ca2+-mediated cell death.
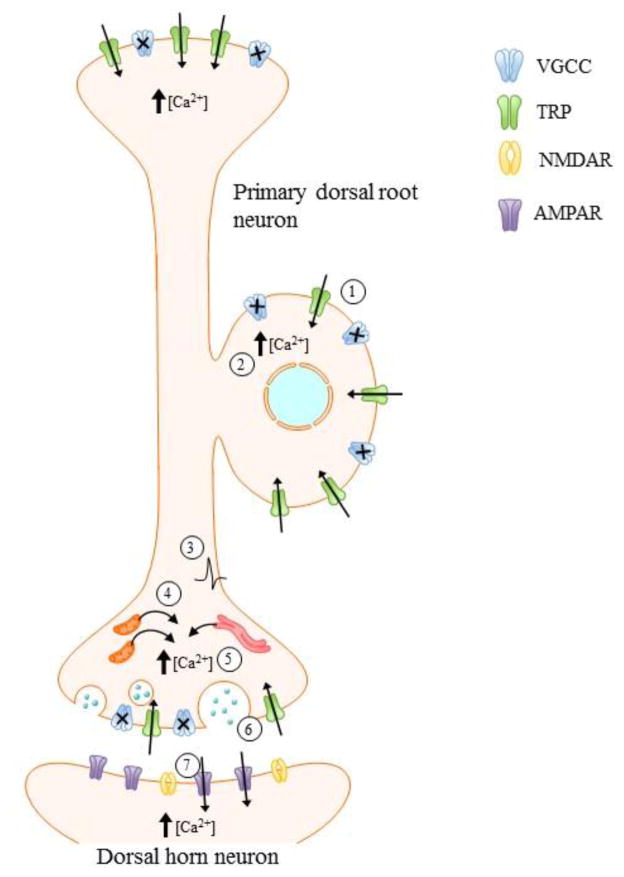
Figure 2. Consequences of stimulatory action of MeHg on TRPs in sensory afferents.
Although MeHg blocks current carried through VGCCs (1), its ability to stimulate current through specific TRPC isoforms suggests these channels contribute to MeHg-induced Ca2+i dysregulation (1). At the peripheral terminal and soma, elevations in [Ca2+]i (2) may result in a lowered threshold for channel activation and, thus, hyperexcitability of the DRG (3). At the central terminal, MeHg-induced Ca2+ release from internal stores (4) may further add to [Ca2+]i elevations mediated by TRPs (5). Such increases in [Ca2+]i at the presynaptic terminal results in greater release of neurotransmitter (6) and, in turn, activation of postsynaptic receptors (7). MeHg-induced [Ca2+]i dysregulation may ultimately lead to neuronal death. It is unclear whether dorsal horn neurons are affected secondarily through elevated [Ca2+]i or through loss of synaptic input from the degenerating primary afferent.
3. Effects of MeHg on motor efferents
Effects of MeHg on motor efferents have received little attention. However, the onset of motor dysfunction in adults can occur at hair Hg concentrations of 200 ppm (Bakir et al., 1973, Clarkson and Magos, 2006), and MN degeneration occurs after both acute and chronic exposure to MeHg in Minamata (Eto, 1997, Eto et al., 2002). In the Iraqi episode of poisoning, ~14% of the patients presented with a neuromuscular weakness resembling myasthenia gravis; its origin was not determined Bakir et al., 1980), nor was a definitive diagnosis of myasthenia gravis made. Motor dysfunction in MeHg-poisoned humans includes ataxia, muscle weakness, and inability to walk; these observations are consistent with clinical signs of neuromuscular-like disorders, suggesting that MeHg impacts MN function to some extent (Eto, 1997, Eto et al., 2002; Dietrich et al., 2005, Rustam et al., 1975). Additionally, MN-directed disease, including amyotrophic lateral sclerosis, has been reported after exposure to inorganic Hg (Adams et al., 1983; Praline et al., 2007; Rooney, 2011). In this section we review the studies that have focused on MeHg or HgCl2 toxicity as it relates to MNs.
3.1 Morphological changes and degeneration
In an effort to understand the mechanism of MeHg induced-toxicity and degeneration of MNs, early studies focused on examining the correlation between the pathways of MeHg exposure and its presence in the central nervous system. Exposure of adult Wistar rats to MeHg (100 or 200 μg, i.p., 2 – 50) revealed accumulation of MeHg in the cerebellum and upper cervical segments of the spinal cord after 10 days of exposure. The Hg level at these sites was ~ 2,000 mg. Interestingly, MeHg accumulated primarily in the anterior horn of the spinal cord, where effector MNs reside. Rats exposed to the highest MeHg concentration (200 mg/day) developed progressive motor coordination dysfunction such as hind leg weakness (Møller-Madsen, 1990). As previously observed in human exposures, there was a latent period between the onset of MeHg exposure and the onset of the motor dysfunction (Bakir et al., 1973, Eto, 1997, Møller-Madsen, 1990). Oral MeHg exposure ad libitum in water to 20 mg/L for 6 to 84 days produced similar results; MeHg deposits were visible in the spinal cord at day 16 for neurons and day 20 for glia (Møller-Madsen, 1991). Although there has been no correlation between MeHg accumulation and neuronal degeneration, when the spinal cord tissue was saturated at day 28, the majority of MeHg was deposited in the anterior horn, and ataxia was observed (Møller-Madsen, 1991). This could reflect degeneration of MNs present in the anterior horns. That was not the focus of the previous studies, however, and consequently was not assessed.
In order to identify if MeHg indeed caused the degeneration of MNs in the spinal cord, adult Wistar rats were exposed to MeHg (10 mg/kg/day p.o., 10 days) with equimolar amounts of L-cysteine. As demonstrated previously by Møller-Madsen, (1990, 1991) MeHg accumulated preferentially in the anterior horn at around 14 days (4 days after the final dose). Lumbar spinal cord sections as well as spinal anterior roots exhibited neurophagia, atrophy and degeneration of large MNs (20 – 50 mm diameter) and myelinated fibers at days 14 – 18 (Su et al., 1998). Small and medium size MNs did not exhibit damage or MeHg accumulation. Although these observations directly support previous findings of MeHg toxicity in α MNs, it is important to note that the concentrations used in this study are approaching the MeHg LD50, lethal dose to fifty percent of the population, and the co-administration of L-cysteine enhances MeHg uptake (Aschner et al., 1990).
MN degeneration after MeHg exposure also occurs in vitro in a MN-like cell line, NSC34. These cells are a hybrid of mouse embryonic spinal cord MNs and neuroblastoma cells (Cashman et al., 1992). They have been used extensively as a model for studies of MN diseases (Muyderman et al., 2009), although they lack certain important characteristics of native MNs. When exposed in vitro to MeHg (0.25 – 16 mM) for 48 hr, NSC34 cells undergo a concentration-dependent cell death (Chapman and Chan, 1999). Exposure to HgCl2 (15 – 200 mM) for 48 hr also resulted in a concentration-dependent incidence of cell death of NSC34 cells. However, the calculated LD50 of MeHg (1.7 μM) and HgCl2 (8.0 μM) suggests that MeHg is more toxic than HgCl2 in NSC34 cells (Chapman and Chan, 1999).
Only one study has focused on identifying the effects of MeHg on primary MNs in vitro. Spinal cord cultures isolated from mice at postnatal day 4 – 6 were exposed to 0.1 – 1 μM MeHg in vitro. MeHg-induced alterations in [Ca2+]i were measured using Ca2+ microfluorimetry (Ramanathan and Atchison, 2011). At concentrations as low as 0.1 μM, MeHg-induced biphasic, temporally distinct increases in MN [Ca2+]i, in a manner similar to those occurring at identical concentrations in cerebellar granule cells (Limke et al., 2003a, Marty and Atchison, 1997). Thus MNs appear to be a highly sensitive target to MeHg. Disruption of [Ca2+]i in MNs was mediated in part by N-methyl-D-aspartate (NMDA), α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and kainate (KA), glutamate receptors. The onset of MeHg-induced disruption in [Ca2+]i was slowed by concomitant application of AMPA/KA antagonist CNQX and the NMDA receptor antagonist dizocilpine (MK-801) (Ramanathan and Atchison, 2011).
Alterations in synaptic transmission at neuromuscular junctions, the effector arm of motor output (Fig. 3), are some of the earliest identified characteristics of MeHg toxicity (Atchison, 2005, Juang, 1976, Juang and Yonemura, 1975). They could be one mechanisms by which MeHg causes MN damage. When applied acutely to amphibian (Juang, 1976; Juang and Yonemura, 1975) or mammalian neuromuscular junctions (Atchison and Narahashi, 1982), MeHg causes a consistent pattern of concentration-dependent effects. In phrenic-nerve-diaphragm preparations from adult rats, in vitro bath-applied MeHg (20 – 100 μM) irreversibly decreased the amplitude of evoked release of ACh (the end-plate potential, EPP) and first stimulated, then suppressed spontaneous release. The onset of these effects was MeHg concentration-dependent, with higher concentrations causing a more rapid onset of effect. Frequency of miniature end plate potentials (MEPPs), which reflect the spontaneous release of individual vesicles of ACh, increased after 15 – 40 min at 20 μM MeHg, before proceeding to complete block; at 100 μM, MeHg caused similar effects after 5 – 15 min (Atchison and Narahashi, 1982). These findings reflect, at the macroscopic functional level, block of action potential-evoked Ca2+ entry into the MN terminal, and increased release of [Ca2+]i, presumably due to release of intracellular Ca2+stores. This was corroborated by studies in which release of mitochondrial or smooth endoplasmic reticulum Ca2+ stores were blocked pharmacologically, and the ensuing response to MeHg was markedly obtunded (Levesque and Atchison, 1987, 1988). Evidence for block of Ca2+ entry was obtained through a series of studies in which the role of voltage-gated Ca2+ channels on evoked release of ACh was examined in the presence of MeHg (Atchison, 1986, 1987, Atchison et al., 1986, Traxinger and Atchison, 1987). This pattern of effects on synaptic transmission has been replicated at central synapses as well (Yuan and Atchison, 1999, 2003).
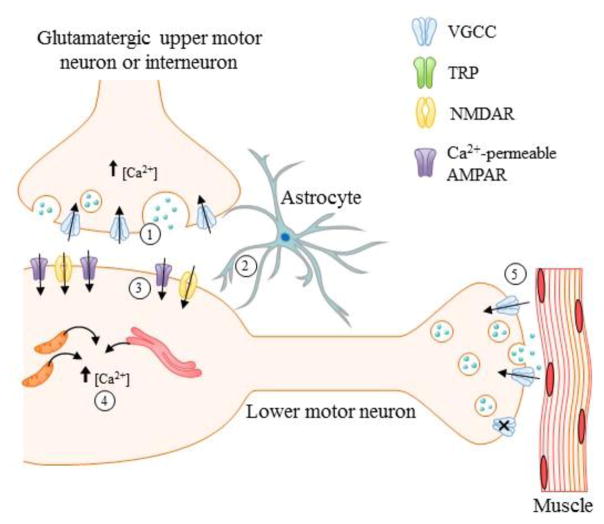
Figure 3. Potential mechanisms of MeHg-induced degeneration of motor efferents. MeHg leads to MN degeneration through Ca2+ mediated pathways.
Ionotrophic glutamate receptors NMDA and Ca2+ permeable AMPAR have been identified as important contributors to these alterations. However, studies in other cell types have shown that MeHg leads to increase glutamate release (1) and impaired function of excitatory amino acid transporter EAAT2 (impaired glutamate uptake) (2) which leads to increase glutamate in the synapse and could be contributing to the observed increased expression of NMDA receptors (3). These alterations could be contributing to the observed increase in [Ca2+]i in lower MNs (4) as well as the alterations in synaptic transmission observed at the level of the neuromuscular junction (5). However, most of these observations have not been studied in MNs and should be the focus of future studies.
In a study focused on understanding effects of MeHg on the innervation of motor and sensory neurons on muscle, adult rats were exposed to MeHg (2 mg/kg/day, p.o.) chronically for 5-weeks. Wallerian degeneration occurred in the extensor digitorum longus muscle (Yip and Riley, 1987). This observation is consistent with findings from patients from Minamata and Iraq, as well as the rodent studies previously discussed in this review. Degeneration of 13.7% of motor end plates occurred, however, no atrophy of muscle fibers occurred as a result of the motor end plate degeneration. Degeneration occurred at the level of the sensory fiber (Yip and Riley, 1987). Thus it is possible that sensory degeneration leads to altered MN communication and subsequently degeneration.
In addition to MeHg, inorganic Hg accumulates and leads to motor dysfunction in large MNs (Dietrich et al., 2005, Møller-Madsen, 1990, 1991, Pamphlett and Waley, 1996). Though Hg2+ is not as toxic as MeHg, it is important to identify its effects at the cellular level, because low concentrations of MeHg can be de-methylated in the brain of monkeys (Vahter et al., 1994) and rodents, resulting in small potential contributions of Hg2+ to the overall toxicity (Suda and Hirayama, 1992, Suda et al., 1992). HgCl2 can also move in a retrograde fashion from the motor nerve terminal into MN soma (Arvidson, 1992). In adult rats, HgCl2 (30 mg, i.m.) caused sensory and lower MN Hg accumulation. Although inorganic Hg accumulated in MN cell bodies, no cell damage was detected (Arvidson, 1992). At the neuromuscular junction, HgCl2 also inhibits synaptic transmission (Juang, 1976, Røed and Herlofson, 1994). Although HgCl2 cannot cross the blood brain barrier as readily as MeHg, once it does, it causes similar effects, most importantly MN degeneration. MN dysfunction has been observed in the form of amyotrophic lateral sclerosis (ALS)-like symptoms after HgCl2 poisoning (Praline et al., 2007). There has been no evidence of it being a causative agent of the disease, so it is suggested that gene X environment interactions could play a role in the development of such disease (Callaghan et al., 2011, Johnson and Atchison, 2009). The same has been the case for MeHg (Johnson, et al., 2011, Roos et al., 2006, Sutedja et al., 2009).
ALS patients present symptoms that are similar to those observed after mercurial poisoning. Most importantly, several of the mechanisms involved in ALS-induced MN degeneration are similar to those observed after MeHg poisoning. Other important factors that can contribute to MN susceptibility to degeneration after environmental exposures and in ALS are: 1) low abundance of Ca2+ binding proteins such as calbindin D-28K and parvalbumin (Ince et al., 1993, Palecek et al., 1999); and 2) increased expression/contribution of Ca2+-permeable AMPA receptors, which also occurs in normal human nervous system (Kawahara et al., 2003). Therefore, if MNs have a predisposition to cell death by Ca2+-mediated pathways, exposure to an environmental toxicant such as MeHg, which causes toxicity through Ca2+ dysregulation, would likely cause these cells to degenerate at a faster rate or exhibit toxicity earlier. This might have been the case in a study in which mice carrying the SOD1-G93A mutation, were exposed to chronic low dose (1 and 3 ppm) MeHg, ad libitum in water, and an earlier onset of ALS-like symptoms was observed (Johnson et al., 2011). The SOD-1 is a free radical scavenger protein that is present in mammals. In ALS, mutations in the SOD1 enzyme are present in both familial and sporadic forms of the disease. The SOD1-G93A mutation leads to an increase in expression of this enzyme. This overexpression causes impaired function of the protein and a cascade of downstream events that cause MN cell death (Gurney et al., 1994, Ripp et al., 1995, Wong et al., 1995). SOD1-G93A animals have been well characterized and are used extensively as a model for ALS-related studies. As the mechanism of pathogenesis in the sporadic and the familial forms of ALS is indistinguishable, the SOD1-G93A mouse represents a plausible model with which to study both sporadic and familial ALS.
In order to identify how MeHg was causing alterations in MNs in the SOD1-G93A mouse model, Ca2+ microfluorimetry experiments were performed in MNs from brainstem hypoglossal nucleus. MeHg-induced increases in [Ca2+]i were mediated in part by Ca2+-permeable AMPA and KA receptors, because they were delayed by the antagonist of Ca2+-permeable AMPA receptor, 1-naphthyl acetyl spermine trihydrochloride (NAS), as well as by the non-selective AMPA receptor antagonist, CNQX (Johnson et al., 2011).
3.2. Glutamate receptors as probable contributors of MeHg-induced Ca2+ dysregulation
Glutamate is the most abundant excitatory amino acid in the central nervous system. It acts on both ionotropic and metabotropic receptors. The two general classes of ionotropic receptors are the AMPA/kainate (AMPA/KA) and NMDA classes. The mGluR receptors comprise the metabotropic class. There are eight separate classes of mGluR, subdivided into classes I, II and III. They play important roles in many neurological functions including pain perception, synaptic plasticity, including both long term potentiation and long term depression, and modulation of the function of ionotropic glutamate receptors, principally NMDA receptors. Despite the obvious important roles that mGluRs play in neurological function, our focus in this review is on the ionotropic glutamate receptors, as they have been implicated in the degeneration of MNs in ALS (Kwak et al., 2010, Spalloni et al., 2012) and after MeHg exposure (Johnson, et al., 2011, Ramanathan and Atchison, 2011).
NMDA receptors are unique, as they have both ligand-gated and voltage-gated properties. They are not activated at strongly negative membrane potentials. This is due to the presence of Mg2+ in a specific binding site in the receptor pore (Mayer et al., 1984, Nowak et al., 1984). In order for NMDA receptors to activate, they need depolarization of the membrane, produced by activation of co-localized AMPA/KA receptors, leading to removal of Mg2+-induced block of the pore. Upon binding of glutamate or NMDA and a glycine molecule, the NMDA receptor-activated channel opens, allowing the flow of Na+, K+, and Ca2+, making them essential for excitatory neurotransmission. NMDA receptors also contain binding sites for an obligatory co-agonist, glycine, whose binding is essential for maximal receptor function (Bowery, 1987, Johnson and Ascher, 1987). NMDA receptors are heterotetramers comprised of two NR1 and two NR2 (NR2A-D) subunits. In certain neuronal populations the receptor composition can be two NR1 and two NR3 subunits (Paoletti et al., 2013).
NMDA receptors became the targets of study because they are the principal type of ion channel involved in synaptic plasticity which is essential for learning and memory, cognitive functions that are both affected in rodent models after MeHg exposure. Treatment of rats were treated with a single dose of MeHg (8 mg/kg, p.o.) caused alterations in cognitive functions. To determine if NMDA receptors were affected, mRNA expression of all the NMDA subunits was measured. The NR2B subunit was up-regulated in the hippocampus but not the frontal cortex, suggesting that alterations in cognitive functions could be associated with alterations in expression of specific NMDA receptor subunits in the hippocampus (Baraldi et al., 2002). In another study that focused on understanding MeHg effects on spatial learning and memory, exposure (5 mg/kg/day, p.o., 7 days) caused downregulation of the NR2A and NR2B subunits and increased expression of NR2C subunits in the hippocampus (Liu et al., 2009). Effects of MeHg on NMDA gene expression differ depending on the time of exposure, concentration and duration as demonstrated by Baraldi and Zanoli (2002) and Liu and Wang (2009). However, the seminal observation is that MeHg exposure causes altered expression of NMDA receptor subunits. An analogous effect could occur in other regions of the central nervous system, thereby contributing to MeHg-induced cytotoxicity.
NMDA receptors also mediate cell death after MeHg exposure in the occipital cortex, hippocampus, cerebellum and brainstem (Miyamoto et al., 2001). Postnatal days (PND) 2, 16 and 60 rats were treated with MeHg (10 mg/kg/day, p.o., 7 days), and concomitantly with the NMDA antagonist MK801 intraperitoneally. Mild levels of neuron degeneration occurred in the brainstem at PND2 and 16. However co-administration of MK801 decreased the extent of MeHg-induced effects (Miyamoto et al., 2001). This study demonstrated that in the brainstem, a region rich in MNs, MeHg causes cell death by mechanisms that involve NMDA receptors.
An in vitro study, in which human neuroblastoma SH-SY-5Y cells were exposed to 0.25 – 5 μM MeHg for 4 hr showed that MeHg causes necrotic cell death by Ca2+-mediated pathways regulated, in part, by NMDA receptors. Exposure to MK801 or the NMDA antagonist memantine (1-amino-3,5-dimethyl-adamantane) decreased the incidence of MeHg-induced cell death (Ndountse and Chan, 2008). Thus, the previous studies demonstrate that NMDA receptors can contribute to MeHg-induced cell death as a result of the alterations in the expression of specific receptor subunits and/or by contribution of NMDA-mediated Ca2+ influx.
AMPA receptor-type glutamate receptors have also been associated with MeHg-induced toxicity. AMPA receptors are heterotetramers comprised of combinations of GluA1, GluA2, GluA3, and GluA4 subunits. Each subunit is encoded by a distinct gene. AMPA receptors are composed of dimeric dimers. Each receptor subunit confers a distinct kinetic property to the AMPA receptor, as do posttranscriptional modifications, such as RNA editing and alternative splicing (Bettler and Mulle, 1995, Bleakman and Lodge, 1998). AMPA receptors are activated by glutamate, or the specific exogenous analog, AMPA. They are typically permeable to Na+ and K+, but not Ca2+; this property is regulated by post-transcriptional RNA editing of the GluA2 subunit. Another post-transcriptional process which AMPA receptors undergo is alternative splicing. This markedly affects their kinetic properties following receptor activation. Alternative splicing on AMPA receptors leads to two splice variants, “Flip” or “Flop”. The “Flip” form of the receptor desensitizes slowly while the “Flop” form desensitizes rapidly (Bettler and Mulle, 1995). Thus receptors containing the “Flip” splice will exhibit prolonged receptor activation, and subsequent membrane depolarization. Normally the GluA2 subunit is edited, at the so-called “Q/R editing site”, yielding AMPA receptors with low permeability to Ca2+. When the AMPA receptor lacks the GluA2 subunit, or contains the unedited form of the GluA2 (GluA2Q), they exhibit high Ca2+ permeability. This alteration results in pronounced Ca2+entry, making MNs susceptible to Ca2+-mediated excitotoxic degeneration (Vandenberghe et al., 2000). When coupled with the “Flip” alternative splice, AMPA receptors can maintain sustained Ca2+ entry, exacerbating the potential for excitotoxicity.
Alterations in glutamate reuptake and Ca2+ regulation in ALS and after MeHg exposures have been well documented (Aschner et al., 2007, Atchison, 2005, Brookes, 1992, Grosskreutz et al., 2010, Limke et al., 2004b, Mutkus et al., 2005), although effects on spinal efferents or afferents have received little attention. Most importantly, MeHg-induced alterations in [Ca2+]i in MNs lead to early onset ALS-like phenotype in the superoxide dismutase 1 (SOD1-G93A) mouse (Johnson, et al., 2011). Using Ca2+-sensitive fluorophores and confocal microscopy, it was determined that MeHg induced alterations in [Ca2+]i in brainstem MNs from the SOD1-G93A mice, which were moderated in part by Ca2+-permeable AMPA receptors. Although these findings implicated AMPA receptors as contributors to MeHg-induced toxicity on MNs, important gaps remain in determining how these alterations occur. Thus, further studies are needed in order to elucidate the role of AMPA receptors in MeHg-induced toxicity in MNs.
KA receptors are another group of ionotropic glutamate receptors that mediate fast excitatory neurotransmission in the central nervous system. They are composed of GriK1, GriK2, GriK3, GriK4 and GriK5 subunits arranged as a tetramer. Although KA receptors are less commonly distributed in the nervous system than are AMPA and NMDA receptors, they nonetheless play an important role in synaptic plasticity (Bettler and Mulle, 1995).
KA receptors had not been identified as potential contributors on MeHg-induced toxicity until recently. Ramanathan and Atchison (2011) and Johnson et al. (2011) demonstrated that exposure to MeHg causes increases in [Ca2+]i in MNs that are mediated in part by KA receptors. Inorganic Hg was previously identified as an antagonist for KA receptors; Xenopus oocytes expressing the cloned human KA receptors were superfused with 1 μM Hg2+ Block of KA current was observed (Umbach and Gundersen, 1989). Thus Hg2+ can disrupt the function of KA receptors. However, no further studies have examined the interaction of either Hg2+ or MeHg with KA receptors, or the role they may play in MeHg-induced toxicity in neuronal cells.
The previously discussed studies summarized the state of the literature with respect to MeHg-induced toxicity in sensory and MNs. As shown here, MNs undergo alterations in [Ca2+]i that are mediated by NMDA, AMPA and KA ionotropic glutamate receptors. How these alterations occur is not understood and should be the focus of future studies. It will be also important to identify if alterations in other ion channels found in MNs, such as the TRP channels, GABAA receptors (Herden et al, 2008, Tsai et al., 2016, Yuan and Atchison, 1997, 2003) muscarinic (Limke et al., 2003) and IP3 receptors (Hare and Atchison, 1995b, Limke et al., 2004) occur, as they have been consistently identified as MeHg targets in other cell types. These receptors could also play a role in the degeneration of spinal cord MNs (Fig. 3).
4. Summary
There is a paucity of studies on the effects of MeHg on sensory function. Sensory disturbances are typically the first clinical symptom of MeHg poisoning, whether it be developmental or post-developmental. Thus understanding the basis for the development of sensory impairment is an important goal towards understanding the mechanism underlying MeHg neurotoxicity, identifying factors that predispose cells to MeHg-induced neurotoxicity and deciphering the basis for development of the preeminent symptoms of MeHg poisoning.
To summarize the state of the literature with respect to MeHg toxicity in sensory and MNs, we demonstrated: 1) that sensory and MNs are a target of MeHg toxicity and 2) that ion channels other than the well studied GABAA receptor and voltage-gated Ca2+ channels contribute to MeHg-induced toxicity in neuronal cells, especially spinal sensory and MNs, and merit further characterization.
MeHg clearly targets sensory afferents and motor efferents. In both cell types MeHg causes degeneration, mainly through Ca2+-mediated pathways. MNs and DRG neurons are both quite sensitive to MeHg-induced disruption of [Ca2+]i and subsequent cytotoxicity- on par with the highly-sensitive cerebellar granule cells. Although these studies have illustrated potential mechanisms underlying the observed sensory and motor dysfunction which attend MeHg exposure, there are still many gaps that need to be filled. Some factors that should be considered in future studies include: 1) the relative contribution of the different types of sensory and MNs, 2) the role of MeHg-exposure time, and 3) the comparative contribution of the different types of ion channels present on these sensory and MNs to MeHg-induced cell death.
Just as in cerebellum where there are clear differences in sensitivity to MeHg, spinal cells likely exhibit a similar disparity in sensitivity. Types and levels of expression of ligand- and voltage-gated channels will likely play an essential role in sensitivity. MNs, though not typically a primary target of MeHg toxicity, nonetheless remain an essential prospective target for gene X environment interactions in MN disease. Additionally, the increased metabolic strain placed on α MNs by the extensive axoplasm and large membrane surface area make them especially susceptible to disruption of [Ca2+]i secondary to impedance of mitochondrial function. Also, known actions of MeHg to facilitate Ca2+ entry, whether by TRP channels, glutamate-activated channels, or voltage-gated Ca2+ channels will exacerbate this effect.
MeHg-induced neurotoxicity displays a characteristic delay, or “silent phase” (Rice, 1996) and current Hg0 and MeHg exposures are occurring in adult populations (Ashe, 2012, Fraser, 2016, Gardner, 2012, Wade, 2013). Therefore, it will be important to focus on understanding MeHg effects on sensory and MNs in adult and asymptomatic subjects. MN-directed diseases such as ALS have been reported in adults after Hg2+ exposure (Adams et al. , 1983, Praline, Guennoc, 2007, Rooney, 2011). For MeHg, gene X environment interactions have been proposed as contributing to the development of ALS. In adult SOD1-G93A mice, MeHg exposure led to an early-onset phenotype of the disease (Johnson et al., 2011). Thus, longitudinal studies should be conducted in populations which are exposed to Hg0 and MeHg to determine if this exposures to these toxicants hastens the onset of MN-directed disease. However, because of the relatively low prevalence of ALS, many epidemiological studies of this nature are likely to be underpowered, so this potential interaction may be difficult to identify conclusively
In conclusion, both spinal efferents and afferents are definitive targets of mercurial toxicity, and sensory dysfunction remains the most common complaint of mercury poisoning, especially for MeHg. Despite this, identifying the potential targets of MeHg on spinal neurons has lagged far behind studies of effects of MeHg on other neuronal and non-neuronal targets. By studying spinal neuron sensitivity to MeHg one may begin to unravel not only valuable information regarding disruption of sensory function, but also identify other heretofore unidentified, targets of MeHg in other regions of the brain.
Highlights for review.
The contribution of cellular targets including ion channels to methylmercury-induced disruption of sensory function is reviewed.
Acknowledgments
We would like to thank Dr. Ravindra K. Hajela for his valuable comments on this review. The assistance of Ms. Alana Chapman with word processing is greatly appreciated. Supported by NIH grants NIEHS R01ES3299, R01ES024064, and T32007255.
Keywords
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- DRG
dorsal root ganglia
- ([Ca2+]i)
intracellular Ca2+ concentration
- KA
kainic acid
- MeHg
methylmercury
- MN
motor neurons
- NMDA
N-methyl-D-aspartate
- TRPs
transient receptor potential channels
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abe T, Haga T, Kurokawa M. Blockage of axoplasmic transport and depolymerisation of reassembled microtubules by methyl mercury. Brain Res. 1975;86:504–8. doi: 10.1016/0006-8993(75)90904-x. [DOI] [PubMed] [Google Scholar]
- Adams CR, Ziegler DK, Lin J. Mercury intoxication simulating amyotrophic lateral sclerosis. JAMA. 1983;250:642–3. [PubMed] [Google Scholar]
- Al-saleem T. Levels of mercury and pathological changes in patients with organomercury poisoning. Bull World Health Organ. 1976;53(Suppl):99–104. [PMC free article] [PubMed] [Google Scholar]
- Arakawa O, Nakahiro M, Narahashi T. Mercury modulation of GABA-activated chloride channels and non-specific cation channels in rat dorsal root ganglion neurons. Brain Res. 1991;551:58–63. doi: 10.1016/0006-8993(91)90913-g. [DOI] [PubMed] [Google Scholar]
- Arvidson B. Inorganic mercury is transported from muscular nerve terminals to spinal and brainstem motoneurons. Muscle Nerve. 1992;15:1089–94. doi: 10.1002/mus.880151006. [DOI] [PubMed] [Google Scholar]
- Aschner M, Eberle NB, Goderie S, Kimelberg HK. Methylmercury uptake in rat primary astrocyte cultures: the role of the neutral amino acid transport system. Brain Res. 1990;521:221–8. doi: 10.1016/0006-8993(90)91546-s. [DOI] [PubMed] [Google Scholar]
- Aschner M, Syversen T, Souza DO, Rocha JB, Farina M. Involvement of glutamate and reactive oxygen species in methylmercury neurotoxicity. Braz J Med Biol Res. 2007;40:285–91. doi: 10.1590/s0100-879x2007000300001. [DOI] [PubMed] [Google Scholar]
- Ashe K. Elevated mercury concentrations in humans of Madre de Dios, Peru. PLoS One. 2012;7:1–6. doi: 10.1371/journal.pone.0033305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atchison WD. Effects of activation of sodium and calcium entry on spontaneous release of acetylcholine induced by methylmercury. J Pharmacol Exp Ther. 1987;241:131–9. [PubMed] [Google Scholar]
- Atchison WD. Extracellular calcium-dependent and -independent effects of methylmercury on spontaneous and potassium-evoked release of acetylcholine at the neuromuscular junction. J Pharmacol Exp Ther. 1986;237:672–80. [PubMed] [Google Scholar]
- Atchison WD. Is chemical neurotransmission altered specifically during methylmercury-induced cerebellar dysfunction? Trends Pharmacol Sci. 2005;26:549–57. doi: 10.1016/j.tips.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Atchison WD, Joshi U, Thornburg JE. Irreversible suppression of calcium entry into nerve terminals by methylmercury. J Pharmacol Exp Ther. 1986;238:618–24. [PubMed] [Google Scholar]
- Atchison WD, Narahashi T. Methylmercury-induced depression of neuromuscular transmission in the rat. Neurotoxicology. 1982;3:37–50. [PubMed] [Google Scholar]
- Bailey JM, Hutsell BA, Newland MC. Dietary nimodipine delays the onset of methylmercury neurotoxicity in mice. Neurotoxicology. 2013;37:108–17. doi: 10.1016/j.neuro.2013.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakir F, Damluji SF, Amin-Zaki L, Murtadha M, Khalidi A, al-Rawi NY, Tikriti S, Dahahir HI, Clarkson TW, Smith JC, Doherty RA. Methylmercury poisoning in Iraq. Science. 1973;181:230–41. doi: 10.1126/science.181.4096.230. [DOI] [PubMed] [Google Scholar]
- Baraldi M, Zanoli P, Tascedda F, Blom JM, Brunello N. Cognitive deficits and changes in gene expression of NMDA receptors after prenatal methylmercury exposure. Environ Health Perspect. 2002;110:855–58. doi: 10.1289/ehp.02110s5855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter GJ, Smith RA. Changes in neuropeptide immunoreactivity in cultured adult mouse sensory neurons following methylmercury chloride treatments. Neurosci Lett. 1998;246:13–6. doi: 10.1016/s0304-3940(98)00210-9. [DOI] [PubMed] [Google Scholar]
- Bear MF, Connors BW, Paradiso MA. Neuroscience: Exploring the Brain. 3. Philadelphia: Lipppincott Williams and Wilkins; 2006. [Google Scholar]
- Bettler B, Mulle C. Review: neurotransmitter receptors. II. AMPA and kainate receptors. Neuropharmacology. 1995;34:123–39. doi: 10.1016/0028-3908(94)00141-e. [DOI] [PubMed] [Google Scholar]
- Bleakman D, Lodge D. Neuropharmacology of AMPA and kainate receptors. Neuropharmacology. 1998;37:1187–204. doi: 10.1016/s0028-3908(98)00139-7. [DOI] [PubMed] [Google Scholar]
- Boron WF, Boulpaep EL. Medical Physiology. 2. Philadelphia, PA: Saunders; 2009. [Google Scholar]
- Bowery NG. Glycine-binding sites and NMDA receptors in brain. Nature. 1987;326:338. doi: 10.1038/326338a0. [DOI] [PubMed] [Google Scholar]
- Bradford AB, Mancini JD, Atchison WD. Methylmercury-dependent increases in fluo4 fluorescence in neonatal rat cerebellar slices depend on granule cell migrational stage and GABAA receptor modulation. J Pharmacol Exp Ther. 2016;356:2–12. doi: 10.1124/jpet.115.226761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookes N. In vitro evidence for the role of glutamate in the CNS toxicity of mercury. Toxicology. 1992;76:245–56. doi: 10.1016/0300-483x(92)90193-i. [DOI] [PubMed] [Google Scholar]
- Burke RE. Motor unit types: functional specializations in motor control. Trends Neurosci. 1980;3:255–8. [Google Scholar]
- Burke RE, Levine DN, Tsairis P, Zajac FE., III Physiological types and histochemical profiles in motor units of the cat gastrocnemius. J Physiol (Lond) 1973;234:723–48. doi: 10.1113/jphysiol.1973.sp010369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan B, Feldman D, Gruis K, Feldman E. The association of exposure to lead, mercury, and selenium and the development of amyotrophic lateral sclerosis and the epigenetic implications. Neurodegener Dis. 2011;8:1–8. doi: 10.1159/000315405. [DOI] [PubMed] [Google Scholar]
- Cao B, Lv W, Jin S, Tang J, Wang S, Zhao H, et al. Degeneration of peripheral nervous system in rats experimentally induced by methylmercury intoxication. Neurol Sci. 2013;34:663–9. doi: 10.1007/s10072-012-1100-3. [DOI] [PubMed] [Google Scholar]
- Cashman NR, Durham HD, Blusztajn JK, Oda K, Tabira T, Shaw IT, et al. Neuroblastoma x spinal cord (NSC) hybrid cell lines resemble developing motor neurons. Dev Dyn. 1992;194:209–21. doi: 10.1002/aja.1001940306. [DOI] [PubMed] [Google Scholar]
- Candura SM, D'Agostino G, Castoldi AF, Messori E, Liuzzi M, Manzo L, Tonini M. Effects of mercuric chloride and methyl mercury on cholinergic neuromuscular transmission in the guinea-pig ileum. Pharmacol Toxicol. 1997;80:218–24. doi: 10.1111/j.1600-0773.1997.tb01963.x. [DOI] [PubMed] [Google Scholar]
- Cavanagh JB, Chen FC. The effects of methyl-mercury-dicyandiamide on the peripheral nerves and spinal cord of rats. Acta Neuropathol. 1971;19:208–15. doi: 10.1007/BF00684597. [DOI] [PubMed] [Google Scholar]
- Chang LW, Hartmann HA. Ultrastructural studies of the nervous system after mercury intoxication. II. Pathological changes in the nerve fibers. Acta Neuropathol. 1972;20:316–34. doi: 10.1007/BF00691749. [DOI] [PubMed] [Google Scholar]
- Chapman LA, Chan HM. Inorganic mercury pre-exposures protects against methyl mercury toxicity in NSC-34 (neuron x spinal cord hybrid) cells. Toxicology. 1999;132:167–78. doi: 10.1016/s0300-483x(98)00151-6. [DOI] [PubMed] [Google Scholar]
- Clarkson TW. Environmental contaminants in the food chain. Am J Clin Nutr. 1995;61:682S–6S. doi: 10.1093/ajcn/61.3.682S. [DOI] [PubMed] [Google Scholar]
- Clarkson TW, Magos L. The toxicology of mercury and its chemical compounds. Crit Rev Toxicol. 2006;36:609–62. doi: 10.1080/10408440600845619. [DOI] [PubMed] [Google Scholar]
- Davidson PW, Palumbo D, Myers GJ, Cox C, Shamlaye CF, Sloane-Reeves J, Cernichiari E, Wilding GE, Clarkson TW. Neurodevelopmental outcomes of Seychellois children from the pilot cohort at 108 months following prenatal exposure to methylmercury from a maternal fish diet. Environ Res. 2000;84:1–11. doi: 10.1006/enrs.2000.4084. [DOI] [PubMed] [Google Scholar]
- Delio DA, Reuhl KR, Lowndes HE. Ectopic impulse generation in dorsal root ganglion neurons during methylmercury intoxication: an electrophysiological and morphological study. Neurotoxicology. 1992;13:527–39. [PubMed] [Google Scholar]
- Dietrich MO, Mantese CE, Anjos GD, Souza DO, Farina M. Motor impairment induced by oral exposure to methylmercury in adult mice. Environ Toxicol Pharmacol. 2005;19:169–75. doi: 10.1016/j.etap.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Djouhri L, Bleazard L, Lawson SN. Association of somatic action potential shape with sensory receptive properties in guinea-pig dorsal root ganglion neurones. J Physiol (Lond) 1998;513:857–72. doi: 10.1111/j.1469-7793.1998.857ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd J, Jahr CE, Hamilton PN, Heath MJ, Matthew WD, Jessell TM. Cytochemical and physiological properties of sensory and dorsal horn neurons that transmit cutaneous sensation. Cold Spring Harb Symp Quant Biol. 1983;48:685–95. doi: 10.1101/sqb.1983.048.01.072. [DOI] [PubMed] [Google Scholar]
- Dreiem A, Seegal RF. Methylmercury-induced changes in mitochondrial function in striatal synaptosomes are calcium-dependent and ROS-independent. Neurotoxicology. 2007;28:720–6. doi: 10.1016/j.neuro.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards JR, Marty MS, Atchison WD. Comparative sensitivity of rat cerebellar neurons to dysregulation of divalent cation homeostasis and cytotoxicity caused by methylmercury. Toxicol Appl Pharmacol. 2005;208:222–32. doi: 10.1016/j.taap.2005.02.015. [DOI] [PubMed] [Google Scholar]
- Eto K. Pathology of Minamata disease. Toxicol Pathol. 1997;25:614–23. doi: 10.1177/019262339702500612. [DOI] [PubMed] [Google Scholar]
- Eto K, Tokunaga H, Nagashima K, Takeuchi T. An autopsy case of Minamata disease (methylmercury poisoning)- pathological viewpoints of peripheral nerves. Toxicol Pathol. 2002;30:714–22. doi: 10.1080/01926230290166805. [DOI] [PubMed] [Google Scholar]
- Fang X, McMullan S, Lawson SN, Djouhri L. Electrophysiological differences between nociceptive and non-nociceptive dorsal root ganglion neurones in the rat in vivo. J Physiol (Lond) 2005;565:927–43. doi: 10.1113/jphysiol.2005.086199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser B. Peru's gold rush raises health fears. Nature. 2016;534:162. doi: 10.1038/nature.2016.19999. [DOI] [PubMed] [Google Scholar]
- Freire MA, Oliveira RB, Picanço-Diniz CW, Pereira A., Jr Differential effects of methylmercury intoxication in the rat's barrel field as evidenced by NADPH diaphorase histochemistry. Neurotoxicology. 2007;28:175–81. doi: 10.1016/j.neuro.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Funayama M, Goto K, Kondo H. Cloning and expression localization of cDNA for rat homolog of TRP protein, a possible store-operated calcium (Ca2+) channel. Brain Res Mol Brain Res. 1996;43:259–66. doi: 10.1016/s0169-328x(96)00208-2. [DOI] [PubMed] [Google Scholar]
- Gardner E. Peru battles the golden curse of Madre de Dios. Nature. 2012;486:306–7. doi: 10.1038/486306a. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Budtz-Jørgensen E, White RF, Jørgensen PJ, Weihe P, Debes F, Keiding N. Methylmercury exposure biomarkers as indicators of neurotoxicity in children aged 7 years. Am J Epidemiol. 1999;150:301–5. doi: 10.1093/oxfordjournals.aje.a010002. [DOI] [PubMed] [Google Scholar]
- Grosskreutz J, Van Den Bosch L, Keller BU. Calcium dysregulation in amyotrophic lateral sclerosis. Cell Calcium. 2010;47:165–74. doi: 10.1016/j.ceca.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–50. [PubMed] [Google Scholar]
- Gurney ME, Pu H, Chiu AY, Dal Canto MC, Polchow CY, Alexander DD, Caliendo J, Hentati A, Kwon YW, Deng HX, Chen W, Zhai P, Sufit RL, Siddique T. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science. 1994;174:1772–5. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- Hajela RK, Peng SQ, Atchison WD. Comparative effects of methylmercury and Hg2+ on human neuronal N- and R-type high-voltage activated calcium channels transiently expressed in human embryonic kidney 293 cells. J Pharmacol Exp Ther. 2003;306:1129–36. doi: 10.1124/jpet.103.049429. [DOI] [PubMed] [Google Scholar]
- Hare MF, Atchison WD. Methylmercury mobilizes Ca++ from intracellular stores sensitive to inositol 1,4,5-trisphosphate in NG108–15 cells. J Pharmacol Exp Ther. 1995a;272:1016–23. [PubMed] [Google Scholar]
- Hare MF, Atchison WD. Nifedipine and tetrodotoxin delay the onset of methylmercury-induced increase in [Ca2+]i in NG108-15 cells. Toxicol Appl Pharmacol. 1995b;135:299–307. doi: 10.1006/taap.1995.1236. [DOI] [PubMed] [Google Scholar]
- Hare MF, McGinnis KM, Atchison WD. Methylmercury increases intracellular concentrations of Ca++ and heavy metals in NG108-15 cells. J Pharmacol Exp Ther. 1993;266:1626–35. [PubMed] [Google Scholar]
- Harper AA, Lawson SN. Electrical properties of rat dorsal root ganglion neurones with different peripheral nerve conduction velocities. J Physiol (Lond) 1985;359:47–63. doi: 10.1113/jphysiol.1985.sp015574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris EJ, Baum H. Mitochondrial Ca2+ efflux: stimulation by mercurial reversed by ubiquinone. Biochem Soc Trans. 1980;8:336–7. doi: 10.1042/bst0080336. [DOI] [PubMed] [Google Scholar]
- Hjerling-Leffler J, AlQatari M, Ernfors P, Koltzenburg M. Emergence of functional sensory subtypes as defined by transient receptor potential channel expression. J Neurosci. 2007;27:2435–43. doi: 10.1523/JNEUROSCI.5614-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hökfelt T, Elde R, Johansson O, Luft R, Nilsson G, Arimura A. Immunohistochemical evidence for separate populations of somatostatin-containing and substance P-containing primary afferent neurons in the rat. Neuroscience. 1976;1:131–6. doi: 10.1016/0306-4522(76)90008-7. [DOI] [PubMed] [Google Scholar]
- Horowitz HM, Jacob DJ, Amos HM, Streets DG, Sunderland EM. Historical mercury releases from commercial products: global environmental implications. Environ Sci Tech. 2014;48:10242–50. doi: 10.1021/es501337j. [DOI] [PubMed] [Google Scholar]
- Huang CS, Narahashi T. The role of G proteins in the activity and mercury modulation of GABA-induced currents in rat neurons. Neuropharmacology. 1997a;36:1623–30. doi: 10.1016/s0028-3908(97)00173-1. [DOI] [PubMed] [Google Scholar]
- Huang CS, Narahashi T. The role of phosphorylation in the activity and mercury modulation of GABA-induced currents in rat neurons. Neuropharmacology. 1997b;36:1631–40. doi: 10.1016/s0028-3908(97)00172-x. [DOI] [PubMed] [Google Scholar]
- Ince P, Stout N, Shaw P, Slade J, Hunziker W, Heizmann CW, Baimbridge KG. Parvalbumin and calbindin D-28k in the human motor system and in motor neuron disease. Neuropathol Appl Neurobiol. 1993;19:291–9. doi: 10.1111/j.1365-2990.1993.tb00443.x. [DOI] [PubMed] [Google Scholar]
- Islam MS. Transient Receptor Potential Channels. New York: Springer; 2011. [Google Scholar]
- Jeftinija S, Jeftinija K. Calcitonin gene-related peptide immunoreactivity in neuronal perikarya in dorsal root. Brain Res. 1990;519:324–8. doi: 10.1016/0006-8993(90)90095-s. [DOI] [PubMed] [Google Scholar]
- Johnson FO, Atchison WD. The role of environmental mercury, lead and pesticide exposure in development of amyotrophic lateral sclerosis. Neurotoxicology. 2009;30:761–5. doi: 10.1016/j.neuro.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson FO, Yuan Y, Hajela RK, Chitrakar A, Parsell DM, Atchison WD. Exposure to an environmental neurotoxicant hastens the onset of amyotrophic lateral sclerosis-like phenotype in human Cu2+/Zn2+ superoxide dismutase 1 G93A mice: glutamate-mediated excitotoxicity. J Pharmacol Exp Ther. 2011;338:518–27. doi: 10.1124/jpet.110.174466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JW, Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature. 1987;325:529–31. doi: 10.1038/325529a0. [DOI] [PubMed] [Google Scholar]
- Juang M. Depression of frog muscle contraction by methylmercuric chloride and mercuric chloride. Toxicol Appl Pharmacol. 1976;35:183–5. doi: 10.1016/0041-008x(76)90124-1. [DOI] [PubMed] [Google Scholar]
- Juang M, Yonemura K. Increase spontaneous transmitter release from presynaptic nerve terminal by methylmercuric chloride. Nature. 1975;256:211–3. doi: 10.1038/256211a0. [DOI] [PubMed] [Google Scholar]
- Kanning KC, Kaplan A, Henderson CE. Motor neuron diversity in development and disease. Ann Rev Neurosci. 2010;33:409–40. doi: 10.1146/annurev.neuro.051508.135722. [DOI] [PubMed] [Google Scholar]
- Kasama H, Itoh K, Omata S, Sugano H. Differential effects of methylmercury on the synthesis of protein species in dorsal root ganglia of the rat. Arch Toxicol. 1989;63:226–30. doi: 10.1007/BF00316373. [DOI] [PubMed] [Google Scholar]
- Kawahara Y, Ito K, Sun H, Kanazawa I, Kwak S. Low editing efficiency of GluR2 mRNA is associated with a low relative abundance of ADAR2 mRNA in white matter of normal human brain. Eur J Neurosci. 2003;18:23–33. doi: 10.1046/j.1460-9568.2003.02718.x. [DOI] [PubMed] [Google Scholar]
- Kong HK, Wong MH, Chan HM, Lo SC. Chronic exposure of adult rats to low doses of methylmercury induced a state of metabolic deficit in the somatosensory cortex. J Proteome Res. 2013;12:5233–45. doi: 10.1021/pr400356v. [DOI] [PubMed] [Google Scholar]
- Kwak S, Hideyama T, Yamashita T, Aizawa H. AMPA receptor-mediated neuronal death in sporadic ALS. Neuropathology. 2010;30:182–8. doi: 10.1111/j.1440-1789.2009.01090.x. [DOI] [PubMed] [Google Scholar]
- Lawson SN. Phenotype and function of somatic primary afferent nociceptive neurones with C-, Aδ- or Aα/β-fibres. Exp Physiol. 2002;87:239–44. doi: 10.1113/eph8702350. [DOI] [PubMed] [Google Scholar]
- Lee KH, Chung K, Chung JM, Coggeshall RE. Correlation of cell body size, axon size, and signal conduction velocity for individually labelled dorsal root ganglion cells in the cat. J Comp Neurol. 1986;243:335–46. doi: 10.1002/cne.902430305. [DOI] [PubMed] [Google Scholar]
- Leonhardt R, Haas H, Büsselberg D. Methyl mercury reduces voltage-activated currents of rat dorsal root ganglion neurons. Na_nyn Schmied Arch Pharmacol. 1996a;354:532–8. doi: 10.1007/BF00168447. [DOI] [PubMed] [Google Scholar]
- Leonhardt R, Pekel M, Platt B, Haas HL, Büsselberg D. Voltage-activated calcium channel currents of rat DRG neurons are reduced by mercuric chloride (HgCl2) and methylmercury (CH3HgCl) Neurotoxicology. 1996b;17:85–92. [PubMed] [Google Scholar]
- Levesque PC, Atchison WD. Interactions of mitochondrial inhibitors with methylmercury on spontaneous quantal release of acetylcholine. Toxicol Appl Pharmacol. 1987;87:315–24. doi: 10.1016/0041-008x(87)90293-6. [DOI] [PubMed] [Google Scholar]
- Levesque PC, Atchison WD. Effect of alteration of nerve terminal Ca2+ regulation on increased spontaneous quantal release of acetylcholine by methyl mercury. Toxicol Appl Pharmacol. 1988;15:55–65. doi: 10.1016/0041-008x(88)90336-5. [DOI] [PubMed] [Google Scholar]
- Levesque PC, Hare MF, Atchison WD. Inhibition of mitochondrial Ca2+ release diminishes the effectiveness of methyl mercury to release acetylcholine from synaptosomes. Toxicol Appl Pharmacol. 1992;115:11–20. doi: 10.1016/0041-008x(92)90362-v. [DOI] [PubMed] [Google Scholar]
- Liedtke WB, Heller S. TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades. Boca Raton: CRC Press; 2007. [PubMed] [Google Scholar]
- Limke T, Otero-Montañez J, Atchison W. Evidence for interactions between intracellular calcium stores during methylmercury-induced intracellular calcium dysregulation in rat cerebellar granule neurons. J Pharmacol Exp Ther. 2003;304:949–58. doi: 10.1124/jpet.102.042457. [DOI] [PubMed] [Google Scholar]
- Limke TL, Atchison WD. Acute exposure to methylmercury opens the mitochondrial permeability transition pore in rat cerebellar granule cells. Toxicol Appl Pharmacol. 2002;178:52–61. doi: 10.1006/taap.2001.9327. [DOI] [PubMed] [Google Scholar]
- Limke TL, Atchison WD. Application of single-cell microfluorimetry to neurotoxicology assays. Curr Protoc Toxicol. 2009;42:12.15:12.5.1–.5.3. doi: 10.1002/0471140856.tx1215s42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limke TL, Bearss JJ, Atchison WD. Acute exposure to methylmercury causes Ca2+ dysregulation and neuronal death in rat cerebellar granule cells through an M3 muscarinic receptor-linked pathway. Toxicol Sci. 2004a;80:60–8. doi: 10.1093/toxsci/kfh131. [DOI] [PubMed] [Google Scholar]
- Limke TL, Heidemann SR, Atchison WD. Disruption of intraneuronal divalent cation regulation by methylmercury: are specific targets involved in altered neuronal development and cytotoxicity in methylmercury poisoning? Neurotoxicology. 2004b;25:741–60. doi: 10.1016/j.neuro.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Liu W, Wang X, Zhang R, Zhou Y. Effects of postnatal exposure to methylmercury on spatial learning and memory and brain NMDA receptor mRNA expression in rats. Toxicol Lett. 2009;188:230–5. doi: 10.1016/j.toxlet.2009.04.021. [DOI] [PubMed] [Google Scholar]
- Marty MS, Atchison WD. Pathways mediating Ca2+ entry in rat cerebellar granule cells following in vitro exposure to methyl mercury. Toxicol Appl Pharmacol. 1997;147:319–30. doi: 10.1006/taap.1997.8262. [DOI] [PubMed] [Google Scholar]
- Marty MS, Atchison WD. Elevations of intracellular Ca2+ as a probable contributor to decreased viability in cerebellar granule cells following acute exposure to methylmercury. Toxicol Appl Pharmacol. 1998;150:98–105. doi: 10.1006/taap.1998.8383. [DOI] [PubMed] [Google Scholar]
- Mayer ML, Westbrook GL, Guthrie PB. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature. 1984;309:261–3. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- McAlpine D, Araki S. Minamata disease: an unusual neurological disorder caused by contaminated fish. Lancet. 1958;2:629–31. doi: 10.1016/s0140-6736(58)90348-9. [DOI] [PubMed] [Google Scholar]
- Miura K, Himeno S, Koide N, Imura N. Effects of methylmercury and inorganic mercury on the growth of nerve fibers in cultured chick dorsal root ganglia. Tohoku J Exp Med. 2000;192:195–210. doi: 10.1620/tjem.192.195. [DOI] [PubMed] [Google Scholar]
- Miyamoto K, Nakanishi H, Moriguchi S, Fukuyama N, Eto K, Wakamiya K, et al. Involvement of enhanced sensitivity of N-methyl-D-aspartate receptors in vulnerability of developing cortical neurons to methylmercury neurotoxicity. Brain Res. 2001;901:252–8. doi: 10.1016/s0006-8993(01)02281-8. [DOI] [PubMed] [Google Scholar]
- Møller-Madsen B. Localization of mercury in CNS of the rat. II. Intraperitoneal injection of methylmercuric chloride (CH3HgCl) and mercuric chloride (HgCl2) Toxicol Appl Pharmacol. 1990;103:303–23. doi: 10.1016/0041-008x(90)90232-j. [DOI] [PubMed] [Google Scholar]
- Møller-Madsen B. Localization of mercury in CNS of the rat. III. Oral administration of methylmercuric chloride (CH3HgCl) Fundam Appl Toxicol. 1991;16:172–87. [PubMed] [Google Scholar]
- Murali SS, Napier IA, Mohammadi SA, Alewood PF, Lewis RJ, Christie MJ. High-voltage-activated calcium current subtypes in mouse DRG neurons adapt in a subpopulation-specific manner after nerve injury. J Neurophysiol. 2015;113:1511–9. doi: 10.1152/jn.00608.2014. [DOI] [PubMed] [Google Scholar]
- Mutkus L, Aschner JL, Syversen T, Aschner M. Methylmercury alters the in vitro uptake of glutamate in GLAST- and GLT-1-transfected mutant CHO-K1 cells. Biol Trace Elem Res. 2005;107:231–45. doi: 10.1385/BTER:107:3:231. [DOI] [PubMed] [Google Scholar]
- Muyderman H, Hutson PG, Matusica D, Rogers ML, Rush RA. The human G93A-superoxide dismutase-1 mutation, mitochondrial glutathione and apoptotic cell death. Neurochem Res. 2009;34:1847–56. doi: 10.1007/s11064-009-9974-z. [DOI] [PubMed] [Google Scholar]
- Nakada S, Saito H, Imura N. Effect of methylmercry and inorganic mercury on the nerve growth factor-induced neurite outgrowth in chick embryonic sensory ganglia. Toxicol Lett. 1981;8:23–8. doi: 10.1016/0378-4274(81)90132-6. [DOI] [PubMed] [Google Scholar]
- Narahashi T, Ma JY, Arakawa O, Reuveny E, Nakahiro M. GABA receptor-channel complex as a target site of mercury, copper, zinc, and lanthanides. Cell Mol Neurobiol. 1994;14:599–621. doi: 10.1007/BF02088671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndountse LT, Chan HM. Methylmercury increases N-methyl-D-aspartate receptors on human SH-SY 5Y neuroblastoma cells leading to neurotoxicity. Toxicology. 2008;249:251–5. doi: 10.1016/j.tox.2008.05.011. [DOI] [PubMed] [Google Scholar]
- Ninomiya T, Imamura K, Kuwahata M, Kindaichi M, Susa M, Ekino S. Reappraisal of somatosensory disorders in methylmercury poisoning. Neurotoxicol Teratol. 2005;27:643–53. doi: 10.1016/j.ntt.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Nowak L, Bregestovski P, Ascher P, Herbet A, Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984;307:462–5. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Numazaki M, Tominaga M. Nociception and TRP channels. Curr Drug Targets CNS Neurol Disord. 2004;3:479–85. doi: 10.2174/1568007043336789. [DOI] [PubMed] [Google Scholar]
- Odumo BO, Carbonell G, Angeyo HK, Patel JP, Torrijos M, Rodríguez Martín JA. Impact of gold mining associated with mercury contamination in soil, biota sediments and tailings in Kenya. Environ Sci Pollut Res Int. 2014;21:12426–35. doi: 10.1007/s11356-014-3190-3. [DOI] [PubMed] [Google Scholar]
- Omata S, Momose Y, Ueki H, Sugano H. In vivo effect of methylmercury on protein synthesis in peripheral nervous tissues of the rat. Arch Toxicol. 1982;49:203–14. doi: 10.1007/BF00347868. [DOI] [PubMed] [Google Scholar]
- Otten U, Goedert M, Mayer N, Lembeck F. Requirement of nerve growth factor for development of substance P-containing sensory neurones. Nature. 1980;287:158–9. doi: 10.1038/287158a0. [DOI] [PubMed] [Google Scholar]
- Palecek J, Lips MB, Keller BU. Calcium dynamics and buffering in motoneurones of the mouse spinal cord. J Physiol (Lond) 1999;520:485–502. doi: 10.1111/j.1469-7793.1999.00485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pamphlett R, Waley P. Motor neuron uptake of low dose inorganic mercury. J Neurol Sci. 1996;135:63–7. doi: 10.1016/0022-510x(95)00258-4. [DOI] [PubMed] [Google Scholar]
- Paoletti P, Bellone C, Zhou Q. NMDA receptor subunit diversity: impact on receptor properties, synaptic plasticity and disease. Nat Rev Neurosci. 2013;14:383–400. doi: 10.1038/nrn3504. [DOI] [PubMed] [Google Scholar]
- Peng SQ, Hajela RK, Atchison WD. Effects of methylmercury on human neuronal L-type calcium channels transiently expressed in human embryonic kidney cells (HEK-293) J Pharmacol Exp Ther. 2002;302:424–32. doi: 10.1124/jpet.102.032748. [DOI] [PubMed] [Google Scholar]
- Praline J, Guennoc AM, Limousin N, Hallak H, de Toffol B, Corcia P. ALS and mercury intoxication: a relationship? Clin Neurol Neurosurg. 2007;109:880–3. doi: 10.1016/j.clineuro.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Rajaee M, Long RN, Renne EP, Basu N. Mercury exposure assessment and spatial distribution in a Ghanaian small-scale gold mining community. Int J Environ Res Public Health. 2015;12:10755–82. doi: 10.3390/ijerph120910755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanathan G, Atchison WD. Ca2+ entry pathways in mouse spinal motor neurons in culture following in vitro exposure to methylmercury. Neurotoxicology. 2011;32:742–50. doi: 10.1016/j.neuro.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice D. Evidence for delayed neurotoxicity produced by methylmercury. Neurotoxicology. 1996;17:583–96. [PubMed] [Google Scholar]
- Ripps ME, Huntley GW, Hof PR, Morrison JH, Gordon JW. Transgenic mice expressing an altered murine superoxide dismutase gene provide an animal model of amyotrophic lateral sclerosis. Proc Natl Acad Sci USA. 1995;92:689–93. doi: 10.1073/pnas.92.3.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Røed A, Herlofson BB. Inhibitory effects of HgCl2 on excitation-secretion coupling at the motor nerve terminal and excitation-contraction coupling in the muscle cell. Cell Mol Neurobiol. 1994;14:623–36. doi: 10.1007/BF02088672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney J. Further thoughts on mercury, epigenetics, genetics and amyotrophic lateral sclerosis. Neurodegener Dis. 2011;8:523–4. doi: 10.1159/000324518. [DOI] [PubMed] [Google Scholar]
- Roos PM, Vesterberg O, Nordberg M. Metals in motor neuron diseases. Exp Biol Med (Maywood) 2006;231:1481–7. doi: 10.1177/153537020623100906. [DOI] [PubMed] [Google Scholar]
- Rustam H, Von Burg R, Amin-Zaki L, El Hassani S. Evidence for a neuromuscular disorder in methylmercury poisoning. Arch Environ Health. 1975;30:190–5. doi: 10.1080/00039896.1975.10666674. [DOI] [PubMed] [Google Scholar]
- Sakamoto M1, Ikegami N, Nakano A. Protective effects of Ca2+ channel blockers against methyl mercury toxicity. Pharmacol Toxicol. 1996;78:193–9. doi: 10.1111/j.1600-0773.1996.tb00203.x. [DOI] [PubMed] [Google Scholar]
- Sarafian TA. Methyl mercury increases intracellular Ca2+ and inositol phosphate levels in cultured cerebellar granule neurons. J Neurochem. 1993;61:648–57. doi: 10.1111/j.1471-4159.1993.tb02169.x. [DOI] [PubMed] [Google Scholar]
- Sarafian T, Verity MA. Oxidative mechanisms underlying methyl mercury neurotoxicity. Int J Dev Neurosci. 1991;9:147–53. doi: 10.1016/0736-5748(91)90005-7. [DOI] [PubMed] [Google Scholar]
- Schiønning JD, Larsen JO, Tandrup T, Brændgaard H. Selective degeneration of dorsal root ganglia and dorsal nerve roots in methyl mercury-intoxicated rats: a stereological study. Acta Neuropathol. 1998;96:191–201. doi: 10.1007/s004010050881. [DOI] [PubMed] [Google Scholar]
- Schmidt CW. Quicksilver and gold: mercury pollution from rtisenal and small scale gold mining. Environ Hlth Perspect. 2012;120:A425–9. doi: 10.1289/ehp.120-a424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott BS, Edwards BA. Electric membrane properties of adult mouse DRG neurons and the effect of culture duration. J Neurobiol. 1980;11:291–301. doi: 10.1002/neu.480110307. [DOI] [PubMed] [Google Scholar]
- Scroggs RS, Fox AP. Calcium current variation between acutely isolated adult rat dorsal root ganglion neurons of different sizes. J Physiol (Lond) 1992;445:639–58. doi: 10.1113/jphysiol.1992.sp018944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer TJ, Atchison WD. Methylmercury blocks N- and L-type Ca++ channels in nerve growth factor-differentiated pheochromocytoma (PC12) cells. J Pharmacol Exp Ther. 1991;258:149–57. [PubMed] [Google Scholar]
- Shafer TJ, Contreras ML, Atchison WD. Characterization of interactions of methylmercury with Ca2+ channels in synaptosomes and pheochromocytoma cells: radiotracer flux and binding studies. Mol Pharmacol. 1990;38:102–13. [PubMed] [Google Scholar]
- Sirois JE, Atchison WD. Methylmercury affects multiple subtypes of calcium channels in rat cerebellar granule cells. Toxicol Appl Pharmacol. 2000;167:1–11. doi: 10.1006/taap.2000.8967. [DOI] [PubMed] [Google Scholar]
- Spalloni A, Nutini M, Longone P. Role of N-methyl-D-aspartate receptors complex in amyotrophic lateral sclerosis. Biochim Biophys Acta. 2012;1832:312–22. doi: 10.1016/j.bbadis.2012.11.013. [DOI] [PubMed] [Google Scholar]
- Su M, Wakabayashi K, Kakita A, Ikuta F, Takahashi H. Selective involvement of large motor neurons in the spinal cord of rats treated with methylmercury. J Neurol Sci. 1998;156:12–7. doi: 10.1016/s0022-510x(98)00030-6. [DOI] [PubMed] [Google Scholar]
- Suda I, Hirayama K. Degradation of methyl and ethyl mercury into inorganic mercury by hydroxyl radical produced from rat liver microsomes. Arch Toxicol. 1992;66:398–402. doi: 10.1007/BF02035129. [DOI] [PubMed] [Google Scholar]
- Suda I, Totoki S, Uchida T, Takahashi H. Degradation of methyl and ethyl mercury into inorganic mercury by various phagocytic cells. Arch Toxicol. 1992;66:40–4. doi: 10.1007/BF02307268. [DOI] [PubMed] [Google Scholar]
- Sutedja NA, Veldink JH, Fischer K, Kromhout H, Heederik D, Huisman MHB, et al. Exposure to chemicals and metals and risk of amyotrophic lateral sclerosis: A systematic review. Amyotroph Lateral Scler. 2009;10:302–9. doi: 10.3109/17482960802455416. [DOI] [PubMed] [Google Scholar]
- Takaoka S, Kawakami Y, Fujino T, Oh-ishi F, Motokura F, Kumagai Y, et al. Somatosensory disturbance by methylmercury exposure. Environ Res. 2008;107:6–19. doi: 10.1016/j.envres.2007.05.012. [DOI] [PubMed] [Google Scholar]
- Takeuchi T, Morikawa N, Matsumoto H, Shiraishi Y. A pathological study of Minamata disease in Japan. Acta Neuropathol. 1962;2:40–57. [Google Scholar]
- Tarabová B, Kurejová M, Sulová Z, Drabová M, Lacinová L. Inorganic mercury and methylmercury inhibit the Cav3.1 channel expressed in human embryonic kidney 293 cells by different mechanisms. J Pharmacol Exp Ther. 2006;317:418–27. doi: 10.1124/jpet.105.095463. [DOI] [PubMed] [Google Scholar]
- Traxinger DL, Atchison WD. Reversal of methylmercury-induced block of nerve-evoked release of acetylcholine at the neuromuscular junction. Toxicol Appl Pharmacol. 1987;90:23–33. doi: 10.1016/0041-008x(87)90302-4. [DOI] [PubMed] [Google Scholar]
- Tsai T, Yuan Y, Hajela RK, Philips SW, Atchison WD. Methylmercury induces an initial increase in GABA-evoked currents in Xenopus oocytes expressing α1 and α6 subunit-containing GABAA receptors. Neurotoxicology. 2016 Oct 6; doi: 10.1016/j.neuro.2016.10.003. pii: S0161-813X(16)30207-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddenberg N. Differential localization in dorsal funiculus of fibres originating from different receptors. Exp Brain Res. 1968;4:367–76. doi: 10.1007/BF00235701. [DOI] [PubMed] [Google Scholar]
- Umbach J, Gundersen C. Mercuric ions are potent non competitive antagonists of human brain kainate receptors expressed in xenopus oocytes. Mol Pharmacol. 1989;36:582–8. [PubMed] [Google Scholar]
- Vahter M, Mottet NK, Friberg L, Lind B, Shen DD, Burbacher T. Speciation of mercury in the primate blood and brain following long-term exposure to methyl mercury. Toxicol Appl Pharmacol. 1994;124:221–9. doi: 10.1006/taap.1994.1026. [DOI] [PubMed] [Google Scholar]
- Vandenberghe W, Robberecht W, Brorson JR. AMPA receptor calcium permeability, GluR2 expression, and selective motoneuron vulnerability. J Neurosci. 2000;20:123–32. doi: 10.1523/JNEUROSCI.20-01-00123.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vennekens R, Voets T, Bindels RJ, Droogmans G, Nilius B. Current understanding of mammalian TRP homologues. Cell Calcium. 2002;31:253–64. doi: 10.1016/s0143-4160(02)00055-6. [DOI] [PubMed] [Google Scholar]
- Világi I, Dóczi J, Banczerowski-Pelyhe I. Altered electrophysiological characteristics of developing rat cortical neurones after chronic methylmercury chloride treatment. Int J Dev Neurosci. 2000;18:493–9. doi: 10.1016/s0736-5748(00)00027-7. [DOI] [PubMed] [Google Scholar]
- Villière V, McLachlan EM. Electrophysiological properties of neurons in intact rat dorsal root ganglia classified by conduction velocity and action potential duration. J Neurophysiol. 1996;76:1924–41. doi: 10.1152/jn.1996.76.3.1924. [DOI] [PubMed] [Google Scholar]
- Wade L. Gold's dark side. Science. 2013;341:1448–9. doi: 10.1126/science.341.6153.1448. [DOI] [PubMed] [Google Scholar]
- Wall PD, Dubner R. Somatosensory pathways. Annu Rev Physiol. 1972;34:315–36. doi: 10.1146/annurev.ph.34.030172.001531. [DOI] [PubMed] [Google Scholar]
- Wes PD, Chevesich J, Jeromin A, Rosenberg C, Stetten G, Montell C. TRPC1, a human homolog of a Drosophila store-operated channel. Proc Natl Acad Sci USA. 1995;92:9652–6. doi: 10.1073/pnas.92.21.9652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilke RA, Kolbert CP, Rahimi RA, Windebank AJ. Methylmercury induces apoptosis in cultured rat dorsal root ganglion neurons. Neurotoxicology. 2003;24:369–78. doi: 10.1016/S0161-813X(03)00032-9. [DOI] [PubMed] [Google Scholar]
- Willis WD, Jr, Coggeshall RE. Sensory Mechanisms of the Spinal Cord. 3. New York: Springer; 2004. [Google Scholar]
- Wong PC, Pardo CA, Borchelt DR, Lee MK, Copeland NG, Jenkins NA, Sisodia SS, Cleveland DW, Price DL. An adverse property of a familial ALS-linked SOD1 mutation causes motor neuron disease characterized by vacuolar degeneration of mitochondria. Neuron. 1995;14:1105–16. doi: 10.1016/0896-6273(95)90259-7. [DOI] [PubMed] [Google Scholar]
- Xu SZ, Zeng B, Daskoulidou N, Chen GL, Atkin SL, Lukhele B. Activation of TRPC cationic channels by mercurial compounds confers the cytotoxicity of mercury exposure. Toxicol Sci. 2012;125:56–68. doi: 10.1093/toxsci/kfr268. [DOI] [PubMed] [Google Scholar]
- Yip RK, Riley DA. Effects of methylmercury on the motor and sensory innervation of the rat extensor digitorum longus muscle. Environ Res. 1987;43:85–96. doi: 10.1016/s0013-9351(87)80060-9. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Atchison WD. Comparative effects of methylmercury on parallel-fiber and climbing-fiber responses of rat cerebellar slices. J Pharmacol Exp Ther. 1999;288:1015–25. [PubMed] [Google Scholar]
- Yuan Y, Atchison WD. Methylmercury differentially affects GABAA receptor-mediated spontaneous IPSCs in Purkinje and granule cells of rat cerebellar slices. J Physiol (Lond) 2003;550:191–204. doi: 10.1113/jphysiol.2003.040543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y, Atchison WD. Methylmercury induces a spontaneous, transient slow inward chloride current in Purkinje cells of rat cerebellar slices. J Pharmacol Exp Ther. 2005;313:751–64. doi: 10.1124/jpet.104.080721. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Otero-Montañez JK, Yao A, Herden CJ, Sirois JE, Atchison WD. Inwardly rectifying and voltage-gated outward potassium channels exhibit low sensitivity to methylmercury. Neurotoxicology. 2005;26:439–54. doi: 10.1016/j.neuro.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Zigmond MJ, Bloom FE, Landis SC, Roberts JL, Squire LR. Fundamental Neuroscience. 1. Cambridge, MA: Academic Press; 1999. [Google Scholar]