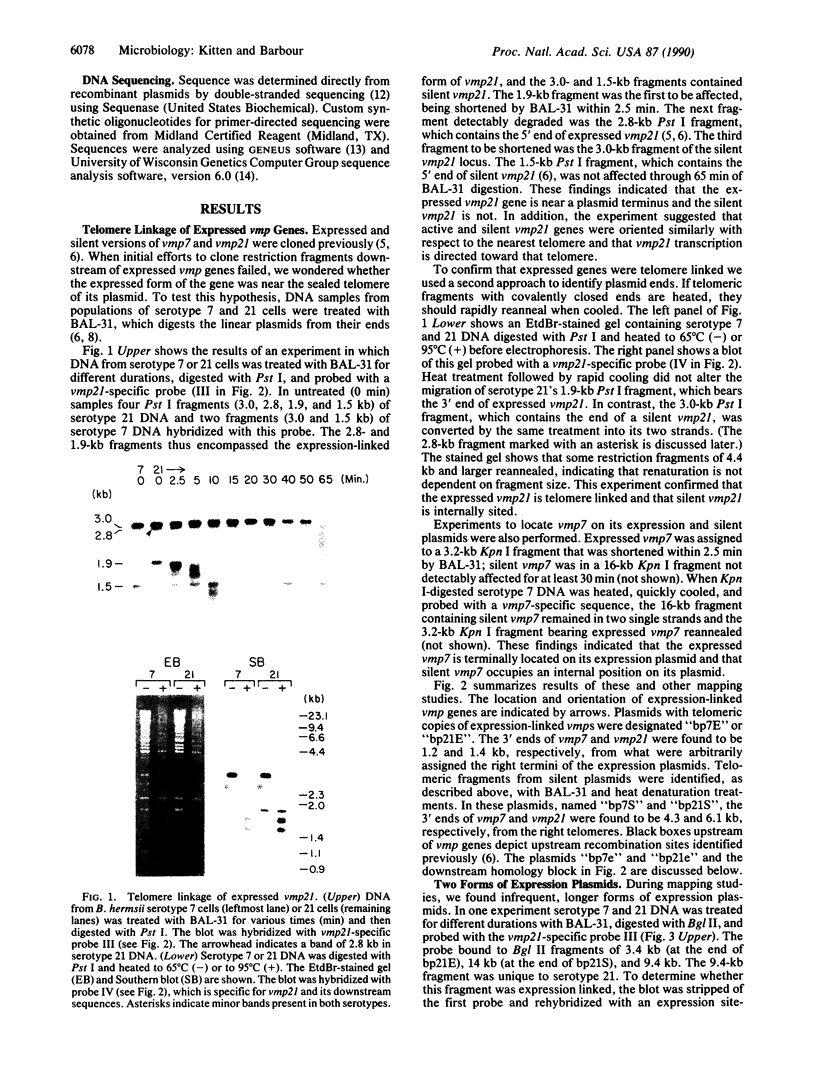
Abstract
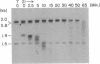
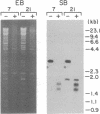
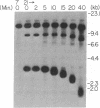
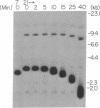
Borrelia hermsii, an agent of relapsing fever, survives in mammals through antigenic variation. Change in serotype-specific variable outer membrane proteins (Vmps) occurs when a Vmp gene at an expression site is replaced with a previously silent gene for another Vmp. Silent and active genes are on separate linear plasmids. The upstream site for a nonreciprocal recombination between two linear plasmids is near the 5' ends of the expressed and silent genes. In the present study we sought the downstream recombination sites in two serotypes, 7 and 21. Restriction fragments containing plasmid telomeres were identified by susceptibility to digestion with BAL-31 and rapid reannealment following denaturation. Whereas both silent genes and a minority population of both expression-linked genes were several kilobases from the telomeres, the predominant population of both expressed genes had 3' ends near plasmid telomeres. Sequence analysis of the predominant expression plasmids revealed that the telomeric sequences were the same in serotypes 7 and 21. Identical sequence was also downstream of silent Vmp genes. Switching of Vmp genes appears to occur by recombination that involves both upstream and downstream sites. The expression plasmid's telomere is preserved in the recombination event.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbour A. G., Garon C. F. Linear plasmids of the bacterium Borrelia burgdorferi have covalently closed ends. Science. 1987 Jul 24;237(4813):409–411. doi: 10.1126/science.3603026. [DOI] [PubMed] [Google Scholar]
- Barbour A. G., Garon C. F. The genes encoding major surface proteins of Borrelia burgdorferi are located on a plasmid. Ann N Y Acad Sci. 1988;539:144–153. doi: 10.1111/j.1749-6632.1988.tb31847.x. [DOI] [PubMed] [Google Scholar]
- Barbour A. G., Hayes S. F. Biology of Borrelia species. Microbiol Rev. 1986 Dec;50(4):381–400. doi: 10.1128/mr.50.4.381-400.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour A. G. Isolation and cultivation of Lyme disease spirochetes. Yale J Biol Med. 1984 Jul-Aug;57(4):521–525. [PMC free article] [PubMed] [Google Scholar]
- Barbour A. G., Tessier S. L., Stoenner H. G. Variable major proteins of Borrellia hermsii. J Exp Med. 1982 Nov 1;156(5):1312–1324. doi: 10.1084/jem.156.5.1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barstad P. A., Coligan J. E., Raum M. G., Barbour A. G. Variable major proteins of Borrelia hermsii. Epitope mapping and partial sequence analysis of CNBr peptides. J Exp Med. 1985 Jun 1;161(6):1302–1314. doi: 10.1084/jem.161.6.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- De Lange T., Borst P. Genomic environment of the expression-linked extra copies of genes for surface antigens of Trypanosoma brucei resembles the end of a chromosome. Nature. 1982 Sep 30;299(5882):451–453. doi: 10.1038/299451a0. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harr R., Fällman P., Häggström M., Wahlström L., Gustafsson P. GENEUS, a computer system for DNA and protein sequence analysis containing an information retrieval system for the EMBL data library. Nucleic Acids Res. 1986 Jan 10;14(1):273–284. doi: 10.1093/nar/14.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinnebusch J., Bergström S., Barbour A. G. Cloning and sequence analysis of linear plasmid telomeres of the bacterium Borrelia burgdorferi. Mol Microbiol. 1990 May;4(5):811–820. doi: 10.1111/j.1365-2958.1990.tb00651.x. [DOI] [PubMed] [Google Scholar]
- Liu A. Y., Van der Ploeg L. H., Rijsewijk F. A., Borst P. The transposition unit of variant surface glycoprotein gene 118 of Trypanosoma brucei. Presence of repeated elements at its border and absence of promoter-associated sequences. J Mol Biol. 1983 Jun 15;167(1):57–75. doi: 10.1016/s0022-2836(83)80034-5. [DOI] [PubMed] [Google Scholar]
- Meier J. T., Simon M. I., Barbour A. G. Antigenic variation is associated with DNA rearrangements in a relapsing fever Borrelia. Cell. 1985 Jun;41(2):403–409. doi: 10.1016/s0092-8674(85)80013-1. [DOI] [PubMed] [Google Scholar]
- Pays E., Steinert M. Control of antigen gene expression in African trypanosomes. Annu Rev Genet. 1988;22:107–126. doi: 10.1146/annurev.ge.22.120188.000543. [DOI] [PubMed] [Google Scholar]
- Pays E., Van Assel S., Laurent M., Darville M., Vervoort T., Van Meirvenne N., Steinert M. Gene conversion as a mechanism for antigenic variation in trypanosomes. Cell. 1983 Sep;34(2):371–381. doi: 10.1016/0092-8674(83)90371-9. [DOI] [PubMed] [Google Scholar]
- Plasterk R. H., Simon M. I., Barbour A. G. Transposition of structural genes to an expression sequence on a linear plasmid causes antigenic variation in the bacterium Borrelia hermsii. Nature. 1985 Nov 21;318(6043):257–263. doi: 10.1038/318257a0. [DOI] [PubMed] [Google Scholar]
- Stoenner H. G., Dodd T., Larsen C. Antigenic variation of Borrelia hermsii. J Exp Med. 1982 Nov 1;156(5):1297–1311. doi: 10.1084/jem.156.5.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]