Summary
A resident of interior Alaska, was diagnosed with an Orthopoxvirus infection. Phylogenetic analysis revealed it is a novel, previously undescribed Orthopoxvirus species. Phylogenetically, the virus is sister to recognized Old World orthopoxviruses, rather than North American Orthopoxvirus species.
Keywords: Alaska, lesion, North America, Orthopoxvirus, phylogenetics.
Abstract
Background.
Human infection by orthopoxviruses is being reported with increasing frequency, attributed in part to the cessation of smallpox vaccination and concomitant waning of population-level immunity. In July 2015, a female resident of interior Alaska presented to an urgent care clinic with a dermal lesion consistent with poxvirus infection. Laboratory testing of a virus isolated from the lesion confirmed infection by an Orthopoxvirus.
Methods.
The virus isolate was characterized by using electron microscopy and nucleic acid sequencing. An epidemiologic investigation that included patient interviews, contact tracing, and serum testing, as well as environmental and small-mammal sampling, was conducted to identify the infection source and possible additional cases.
Results.
Neither signs of active infection nor evidence of recent prior infection were observed in any of the 4 patient contacts identified. The patient’s infection source was not definitively identified. Potential routes of exposure included imported fomites from Azerbaijan via the patient’s cohabiting partner or wild small mammals in or around the patient’s residence. Phylogenetic analyses demonstrated that the virus represents a distinct and previously undescribed genetic lineage of Orthopoxvirus, which is most closely related to the Old World orthopoxviruses.
Conclusions.
Investigation findings point to infection of the patient after exposure in or near Fairbanks. This conclusion raises questions about the geographic origins (Old World vs North American) of the genus Orthopoxvirus. Clinicians should remain vigilant for signs of poxvirus infection and alert public health officials when cases are suspected.
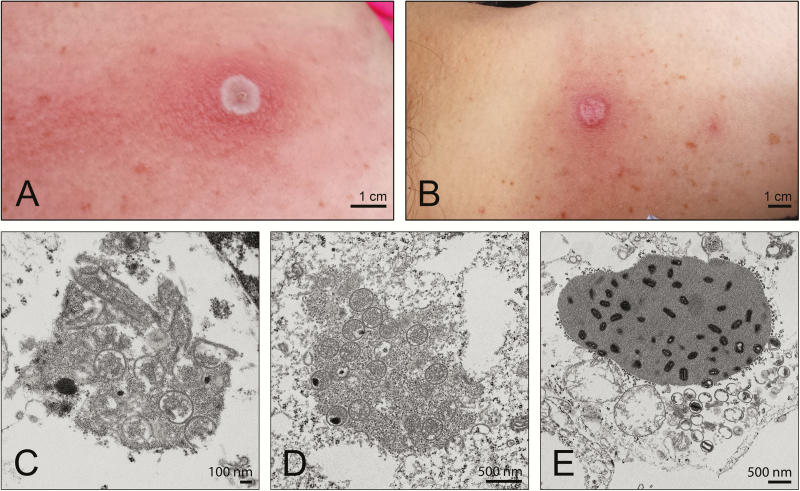
On 29 July 2015, a middle-aged woman (specific details about the index patient, her residence, and contacts identified during the investigation have been generalized or removed in the interest of maintaining privacy) presented to an urgent care clinic in Fairbanks, Alaska, with the chief complaint of a suspected spider bite on her right shoulder. She reported having experienced fever, fatigue, malaise, and tender lymph nodes during the 5 days before seeking care. Her medical history was significant only for hypothyroidism. She denied any recent contact with other sick persons, out-of-state travel, or recurrent skin infections. A physician examination confirmed a superficial ulceration, approximately 1 cm in diameter, and 2 smaller adjacent vesicles, approximately 2 mm in diameter, on posterior upper aspect of the patient’s right shoulder. The ulceration was associated with localized induration, warmth, and tenderness but had no fluctuance or discharge (Figure 1A). A single linear streak of erythema extended anteriorly over the patient’s right shoulder and back to the upper part of her chest on the right side, without crossing the midline. Her physician concluded that the distribution of erythema was consistent with a fifth cervical nerve root dermatome and raised concern for a viral infection. He deroofed and swabbed a vesicle and sent the swab sample (in universal transport media) to the Alaska State Public Health Virology Laboratory for culture and diagnostic testing.
Figure 1.
A, B, Patient’s lesion on 29 July 2015 (A) and 20 August 2015 (B). C–E, Electron microscopic images of the virus isolated from the patient showing crescents (C), spherical immature virus in virus factories (D), and A-type inclusion bodies occluded with mature virus in infected cells (E ).
The swab sample was placed into culture in MRC5, HEp-2, and RMK cell lines on 3 August, and by 10 August cytopathic effects were observed in all 3 tissue cultures. Results of both a direct fluorescent antibody test for herpes simplex virus and a polymerase chain reaction (PCR) test for varicella zoster virus (performed at the California Department of Public Health) were negative. On 17 August, an isolate from the MRC5 cell line was sent to the Alaska State Public Health Laboratory where results of non-Variola and Variola-specific Orthopoxvirus PCR tests were negative; however, a generic Orthopoxvirus PCR test had positive results. On 24 August, the original swab sample and 3 cell culture isolates (1 from each of the 3 cell lines) were sent to the Poxvirus Laboratory of the Centers for Disease Control and Prevention, where an Orthopoxvirus-generic PCR assay showed positive results on 27 August for all 4 submitted samples.
METHODS
An investigation was initiated to characterize the virus and identify the patient’s infection source. The phylogenetic position of the virus was determined by using DNA sequence analysis. Phylogenetic inference was based on 9 genes located within the central, conserved region of the genome (Supplementary Methods). Virus morphological characteristics were observed by using transmission electron microscopy (Supplementary Methods).
Methods used to identify the patient’s infection source included interviews with the patient, contact tracing and analysis of serum samples for the presence of anti-Orthopoxvirus immunoglobulin (Ig) G and IgM, and a visit to the patient’s residence to conduct environmental sampling and peridomestic small-mammal trapping. Given the uncertainty about an incubation period for this novel poxvirus, a conservative threshold of 4 weeks was assumed based on the incubation period of human monkeypox [1]. The patient was asked to identify persons with whom she had regular or close contact during the 4 weeks before and after symptom onset. The patient and each contact were interviewed to ascertain whether they had ever received a smallpox vaccination, had traveled recently, or had experienced any unusual health events during the presumptive incubation period. Serum samples were obtained from these persons and tested with enzyme-linked immunosorbent assays to determine the presence of anti-Orthopoxvirus IgM and IgG antibodies [2].
On 8 September, environmental samples were collected in and around the patient’s home during a site visit. Household surfaces that the patient indicated had been contacted by wild small mammals that periodically entered the home and possible fomites associated with international travel by the patient’s partner were swabbed by using HydraFlock Dacron swab samples (Puritan Medical). Feces of wild small mammals found around the home’s perimeter were also collected. Approximately 6 weeks later, small mammals were trapped around the perimeter of the patient’s home and at a site approximately 1 km away where she and her partner were building a new home (Supplementary Methods). Environmental and nonblood small-mammal samples (oral swab, liver tissue, and feces) were tested using real-time PCR assays that target specific orthopoxvirus generic sequences [3–5]; small-mammal blood samples were tested using enzyme-linked immunosorbent assays for anti-Orthopoxvirus IgG [6].
RESULTS
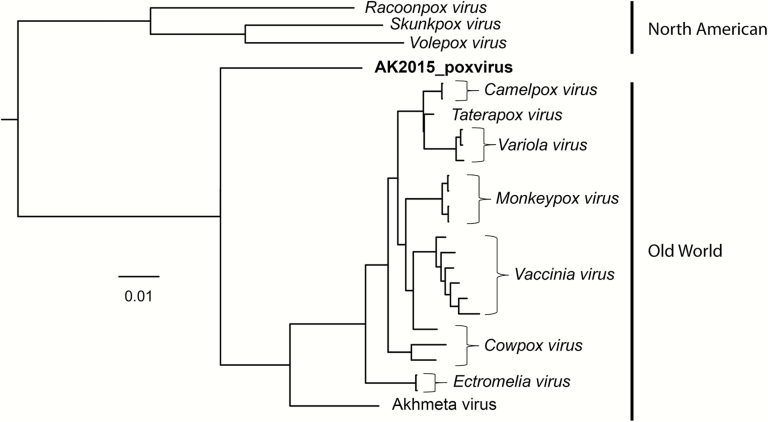
Phylogenetic analyses of the concatenated sequence alignment (28037 base pairs in length) indicate that the virus isolate represents a distinct genetic lineage of orthopoxvirus that is highly divergent from congeners included in our analysis. We will hereafter refer to the virus as AK2015_poxvirus. A well-supported topology was recovered in which the grouping of Taterapox virus and Variola virus as sister taxa (Bayesian posterior probability, 0.5983) was the only node with a Bayesian posterior probability <0.999 (Figure 2, Supplementary Figure 1, and Supplementary_table_1). AK2015_poxvirus was grouped within the genus Orthopoxvirus and recovered as sister to a monophyletic clade containing all Old World orthopoxviruses with high support; it was estimated to be 6.1%–7.3% divergent from different species of Old World orthopoxviruses, and 12.3%–12.6% divergent from isolates within the North American clade. The genetic distances estimated between examined isolates of recognized Old World orthopoxvirus species varied from 0.6% (Taterapox virus to Camelpox virus) to 3.2% (Ectromelia virus to Variola virus).
Figure 2.
Results of Bayesian phylogenetic inference, indicating the position of AK2015_poxvirus within the genus Orthopoxvirus. Analysis was based on 9 genes located within the central, conserved region of the genome (Vaccinia virus Copenhagen strain homologues A7L, A10L, A24R, D1R, D5R, E6R, E9L, H4L, and J6R).
Electron microscopic observation of cells infected with AK2015_poxvirus demonstrated the presence of different morphological forms, including crescents (Figure 1C) and immature virus particles (Figure 1D). In addition, infected cells had inclusion bodies formed by accumulation of A-type inclusion proteins. The inclusion bodies were embedded with mature virus particles and surrounded by ribosomes (Figure 1E).
During interviews, the patient reiterated a lack of sick contacts during the 4 weeks before symptom onset. During the 4 weeks after symptom onset, she reported contact with 4 persons. Of 3 household contacts (adult male partner and 2 teenaged children), all had regular, direct physical contact with her and with common household items, but none reported any unusual health events. One social contact (adult female friend) reported a rash on her chest within 1 week of being in physical contact with the patient in the days after the patient’s symptom onset. A history of smallpox vaccination was reported by both adult contacts (vaccination before 2003) but neither adolescent contact. The patient did not recall being vaccinated and did not have a vaccination scar. Serological tests performed on specimens collected from these persons did not identify evidence of recent exposure among the contacts; anti-Orthopoxvirus IgM was only detected in serum from the patient (Table 1). Consistent with their self-reported smallpox vaccination histories, anti-Orthopoxvirus IgG was detected in the serum from both adult contacts but neither adolescent contact. The serum sample from the patient also tested positive for anti-Orthopoxvirus IgG.
Table 1.
Anti-Orthopoxvirus IgG and IgM Results for Serum Samples from the Patient and 4 Contactsa
| Person | Smallpox vaccination | IgM Valueb | IgM Interpretation | IgG Valueb | IgG interpretation |
|---|---|---|---|---|---|
| Patient | Unlikelyc | 0.091 | Positive | 0.183 | Positive |
| Patient’s partner (household contact) | Yes | −0.024 | Negative | 0.426 | Positive |
| Patient’s older child (household contact) | No | 0.032 | Equivocald | −0.201 | Negative |
| Patient’s younger child (household contact) | No | −0.020 | Negative | −0.194 | Negative |
| Patient’s friend (social contact) | Yes | −0.034 | Negative | 0.483 | Positive |
Abbreviation: Ig, immunoglobulin.
aSerology was performed using enzyme-linked immunosorbent assays.
bSerum optical density (OD) cutoff values (OD value − 3 standard deviations of negative control) at 1:50 and 1:100 dilutions were considered positive for IgM and IgG, respectively.
cThe patient did not recall being vaccinated and did not have a vaccination scar.
dThe sample from the patient’s older child was positive for anti-Orthopoxvirus IgM, but because this value was low and the corresponding anti-Orthopoxvirus IgG result was negative, the result was interpreted as equivocal.
The patient reiterated that no out-of-state travel occurred during the 4 weeks before symptom onset. She reported working intermittently in the petroleum industry in the North Slope region of Alaska, during February–April 2015. Her cohabitating partner was also employed in the petroleum industry and had worked intermittently (5 weeks on and 5 weeks off) on oil-drilling platforms in Azerbaijan, from October 2013 to March 2015. He had no other out-of-state travel during this period and while overseas he remained almost exclusively on oil platforms with limited in-country travel. He returned to Alaska from his last trip to Azerbaijan in March 2015 (approximately 4 months before the patient’s symptom onset) and did not report any unusual health events during or within 4 weeks after the trip. When he returned from a trip to Azerbaijan in October 2014 (approximately 9 months before the patient’s symptom onset), he brought back souvenirs for the patient (a wooden jewelry box containing a cloth jewelry pouch) and a collared work jacket that he wore regularly during the trip and that the patient wore periodically after his return to Alaska. Swab samples of the surfaces of these items were tested to determine whether any might have served as fomites. No evidence of orthopoxvirus DNA was detected.
The home that the patient shared with her partner and 2 children was located in a forested, low-density area, within 50 miles of Fairbanks. She reported that wild small mammals (eg, shrews, voles, squirrels) were abundant in this boreal forest environment, were regularly observed around the home’s perimeter, and entered the home on occasion. She also reported that her children periodically handled the carcasses of squirrels that they shot near the home using a pellet rifle. When asked about construction of her new home, the patient reported that she and her partner had used scrap wood from an abandoned shed located near her home that had been occupied by wild small mammals. All 23 environmental samples collected during the site visit, including swab samples of scrap wood from the abandoned shed at the new home construction site, tested negative for orthopoxviruses by PCR (Supplementary_table_2). At the time of the site visit to the patient’s residence (approximately 45 days after symptom onset), her symptoms included an active lesion that had decreased in size since initial presentation but remained raised, tender, and warm (approximated in Figure 1B). Thirty-one small-mammal samples, collected from 12 individual animals belonging to 2 species (Sorex cinereus, n = 3; Myodes rutilus, n = 9), tested negative for orthopoxviruses by PCR (Supplementary_table_3).
DISCUSSION
An Alaska resident was infected by a previously undescribed genetic lineage of Orthopoxvirus. The patient reported that the lesion took approximately 6 months to fully resolve. No evidence of transmission from the index patient, or fatalities associated with infection, was reported.
Epidemiologic information gathered during the investigation provides inconclusive evidence for 2 general hypotheses concerning the patient’s route of exposure. The first involves importation of an Old World orthopoxvirus into Alaska, either as an active infection in a person with whom the patient came into contact or by way of one or more fomites. The limited number of contacts identified by the patient, and the apparent absence of additional cases, indicates that the probability of unidentified secondary spread is remote. Work-related travel to Azerbaijan by the patient’s partner represents a possible fomite-associated importation scenario, although sampling and testing of travel-related fomites returned negative results. Although the recent discovery of a novel, zoonotic orthopoxvirus in the nearby Republic of Georgia [7] indicates that unidentified orthopoxviruses might be circulating in this region, the delay between the arrival of these fomites in Alaska and the timing of onset of the patient’s symptoms indicates that infection by this route is unlikely. The duration of viability of AK2015_poxvirus on fomites is uncertain; however, a laboratory study of Vaccinia virus demonstrated retention of viability on environmental surfaces for up to 56 days [8]. The presence of A-type inclusion bodies occluded with mature virus in the AK2015_poxvirus isolate observed by electron microscopy might be indicative of enhanced environmental resilience and prolonged viability [9].
Alternatively, AK2015_poxvirus might be endemic to Alaska, perhaps circulating within one or more wildlife reservoir population, and infection might have occurred through an animal exposure. Different species of wild small mammals occur in the boreal forest environment surrounding the patient’s residence, and infection by indirect animal contact (eg, contact with household surfaces, squirrels shot by the patient’s children, or handling of potentially contaminated wood from the shed occupied by wild small mammals during construction of the new home) might be implicated. Rodents and other small mammals are known or suspected reservoirs for multiple orthopoxviruses [10], and evidence of infection has been produced by serosurveys in Eurasia [11–14], Africa [5, 15], South America [16, 17], and the continental United States [18]. Among the limited number of wildlife serosurveys of terrestrial mammals conducted in Alaska, the majority have focused on large mammals [19–21], and those that sampled small mammals did not test for evidence of a poxvirus infection or exposure [22–24]. Small-mammal serosurveys in regions of northern Europe that are ecologically similar to Alaska have identified seropositive animals [25–27]. Although results of our small-mammal trapping and testing were negative, the sample was limited and taxonomically restricted.
Phylogenetic analyses indicate that AK2015_poxvirus is more closely related to the Old World orthopoxviruses than to North American congeners. However, that the virus is known only from North America creates a discordance that precludes its assignment to one or the other of these geographically defined groups with confidence. The global distribution of other genera and unassigned isolates within Chordopoxvirinae, and incomplete sampling of potential reservoir taxa as part of our investigation, create additional uncertainty regarding this assignment. Nevertheless, given the inconsistent timelines of contact travel and patient symptom onset, negative results of travel-associated fomite testing, and the potential for regular and close contact with wild small mammals in and around the patient’s home, the most parsimonious explanation of infection is exposure to AK2015_poxvirus near Fairbanks. Evidence of virus circulation in Alaska or elsewhere in North America (ie, infections in persons or reservoir species) would indicate either a New World origin of orthopoxviruses or an Old World origin with multiple introductions to the New World. Both scenarios run counter to the present characterization of North American and Old World orthopoxviruses as representing reciprocally monophyletic lineages, and challenge the currently accepted hypothesis of an Old World origin of the genus Orthopoxvirus with a solitary introduction of the New World orthopoxviruses to North America [28].
This discovery of a novel orthopoxvirus is the latest in a growing number of reports of human poxvirus infection published in recent years. These include the emergence of novel poxviruses [7, 29–31] and the increased incidence of human monkeypox, an orthopoxvirus illness historically associated with relatively low incidence [32]. Because smallpox vaccination has been demonstrated to provide cross-protection against other orthopoxviruses [33, 34], these observations have been attributed to the cessation of routine smallpox vaccination after eradication of Variola virus in 1980 and the subsequent waning of population-level vaccine-derived immunity [7, 32, 35]. Continued emergence and reemergence of orthopoxviruses is expected. To effectively treat persons infected by orthopoxviruses, clinicians should remain vigilant for signs of poxvirus infections and immediately alert public health officials when infection is suspected so that prompt diagnostic testing and appropriate control measures can be implemented. Within Alaska, populations that might represent foci for surveillance include persons, such as the patient’s partner, who travel to geographic regions associated with the emergence or reemergence of orthopoxviruses, and persons with regular direct or indirect contact with wildlife (eg, residents of rural settings, scientists, environmental consultants, hunters, and adventure guides). The latter population in Alaska might be relatively large, given the high proportion of state residents and visitors who live, work, or recreate in wilderness areas.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Acknowledgments. We thank the patient and contacts for their cooperation during the investigation. Contributions from J. Butler, T. Gardner, A. M. Gunderson, T. Hennessy, and staff of the Alaska State Public Health Virology and Public Health laboratories are gratefully acknowledged.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Financial support. This work was supported by funds from the state of Alaska and the US federal government.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. McCollum AM, Damon IK. Human monkeypox. Clin Infect Dis 2014; 58:260–7. [DOI] [PubMed] [Google Scholar]
- 2. Karem KL, Reynolds M, Braden Z, et al. Characterization of acute-phase humoral immunity to monkeypox: use of immunoglobulin M enzyme-linked immunosorbent assay for detection of monkeypox infection during the 2003 North American outbreak. Clin Diagn Lab Immunol 2005; 12:867–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kondas AV, Olson VA, Li Y, et al. Variola virus-specific diagnostic assays: characterization, sensitivity, and specificity. J Clin Microbiol 2015; 53:1406–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Li Y, Olson VA, Laue T, Laker MT, Damon IK. Detection of monkeypox virus with real-time PCR assays. J Clin Virol 2006; 36:194–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Reynolds MG, Carroll DS, Olson VA, et al. A silent enzootic of an orthopoxvirus in Ghana, West Africa: evidence for multi-species involvement in the absence of widespread human disease. Am J Trop Med Hyg 2010; 82:746–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lederman ER, Reynolds MG, Karem K, et al. Prevalence of antibodies against orthopoxviruses among residents of Likouala region, Republic of Congo: evidence for monkeypox virus exposure. Am J Trop Med Hyg 2007; 77:1150–6. [PubMed] [Google Scholar]
- 7. Vora NM, Li Y, Geleishvili M, et al. Human infection with a zoonotic orthopoxvirus in the country of Georgia. N Engl J Med 2015; 372:1223–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wood J, Choi Y, Wendling M, Rogers J, Chappie D. Environmental persistence of vaccinia virus on materials. Lett Appl Microbiol 2013; 57:399–404. [DOI] [PubMed] [Google Scholar]
- 9. Kastenmayer RJ, Maruri-Avidal L, Americo JL, Earl PL, Weisberg AS, Moss B. Elimination of A-type inclusion formation enhances cowpox virus replication in mice: implications for orthopoxvirus evolution. Virology 2014; 452:59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shchelkunov SN. An increasing danger of zoonotic orthopoxvirus infections. PLoS Pathog 2013; 9:e100376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Begon M, Hazel SM, Baxby D, et al. Transmission dynamics of a zoonotic pathogen within and between wildlife host species. Proc Biol Sci 1999; 266:1939–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kinnunen PM, Henttonen H, Hoffmann B, et al. Orthopox virus infections in Eurasian wild rodents. Vector Borne Zoonotic Dis 2011; 11:1133–40. [DOI] [PubMed] [Google Scholar]
- 13. Oldal M, Sironen T, Henttonen H, et al. Serologic survey of orthopoxvirus infection among rodents in Hungary. Vector Borne Zoonotic Dis 2015; 15:317–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tsanava SA, Sakvarelidze LA, Shelukhina EM. Serologic survey of wild rodents in Georgia for antibodies to orthopoxviruses. Acta Virol 1989; 33:91. [PubMed] [Google Scholar]
- 15. Orba Y, Sasaki M, Yamaguchi H, et al. Orthopoxvirus infection among wildlife in Zambia. J Gen Virol 2015; 96:390–4. [DOI] [PubMed] [Google Scholar]
- 16. Abrahão JS, Guedes MI, Trindade GS, et al. One more piece in the VACV ecological puzzle: could peridomestic rodents be the link between wildlife and bovine vaccinia outbreaks in Brazil? PLoS One 2009; 4:e7428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schatzmayr HG, Simonetti BR, Abreu DC, et al. Animal infections by vaccinia-like viruses in the state of Rio de Janeiro: an expanding disease. Pesquisa Veterinária Brasileira 2009; 29:509–14. [Google Scholar]
- 18. Emerson GL, Li Y, Frace MA, et al. The phylogenetics and ecology of the orthopoxviruses endemic to North America. PLoS One 2009; 4:e7666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rah H, Chomel BB, Follmann EH, et al. Serosurvey of selected zoonotic agents in polar bears (Ursus maritimus). Vet Rec 2005; 156:7–13. [DOI] [PubMed] [Google Scholar]
- 20. Zarnke RL. Serologic survey for selected microbial pathogens in Alaskan wildlife. J Wildl Dis 1983; 19:324–9. [DOI] [PubMed] [Google Scholar]
- 21. Zarnke RL, Ballard WB. Serologic survey for selected microbial pathogens of wolves in Alaska, 1975–1982. J Wild Dis 1987; 23:77–85. [DOI] [PubMed] [Google Scholar]
- 22. Rausch R. Studies on the helminth fauna of Alaska. XI. Helminth parasites of microtine rodents; taxonomic considerations. J Parasitol 1952; 38:415–44. [PubMed] [Google Scholar]
- 23. Schiller EL. Ecology and health of Rattus at Nome, Alaska. J Mammal 1956; 37:181–8. [Google Scholar]
- 24. Testut P, Renard CA, Terradillos O, et al. A new hepadnavirus endemic in arctic ground squirrels in Alaska. J Virol 1996; 70:4210–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Forbes KM, Voutilainen L, Jääskeläinen A, et al. Serological survey of rodent-borne viruses in Finnish field voles. Vector Borne Zoonotic Dis 2014; 14:278–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pelkonen PM, Tarvainen K, Hynninen A, et al. Cowpox with severe generalized eruption, Finland. Emerg Infect Dis 2003; 9:1458–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tryland M, Sandvik T, Mehl R, Bennett M, Traavik T, Olsvik O. Serosurvey for orthopoxviruses in rodents and shrews from Norway. J Wildl Dis 1998; 34:240–50. [DOI] [PubMed] [Google Scholar]
- 28. Babkin IV, Babkina IN. A retrospective study of the orthopoxvirus molecular evolution. Infect Genet Evol 2012; 12:1597–604. [DOI] [PubMed] [Google Scholar]
- 29. Hoffmann D, Franke A, Jenckel M, et al. Out of the reservoir: phenotypic and genotypic characterization of a novel cowpox virus isolated from a common vole. J Virol 2015; 89:10959–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lakis NS, Li Y, Abraham JL, et al. Novel poxvirus infection in an immune suppressed patient. Clin Infect Dis 2015; 61:1543–8. [DOI] [PubMed] [Google Scholar]
- 31. Osadebe LU, Manthiram K, McCollum AM, et al. Novel poxvirus infection in 2 patients from the United States. Clin Infect Dis 2015; 60:195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rimoin AW, Mulembakani PM, Johnston SC, et al. Major increase in human monkeypox incidence 30 years after smallpox vaccination campaigns cease in the Democratic Republic of Congo. Proc Natl Acad Sci U S A 2010; 107:16262–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Di Giulio DB, Eckburg PB. Human monkeypox: an emerging zoonosis. Lancet Infect Dis 2004; 4:15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mombouli JV, Ostroff SM. The remaining smallpox stocks: the healthiest outcome. Lancet 2012; 379:10–2. [DOI] [PubMed] [Google Scholar]
- 35. Nolen LD, Osadebe L, Katomba J, et al. Introduction of monkeypox into a community and household: risk factors and zoonotic reservoirs in the Democratic Republic of the Congo. Am J Trop Med Hyg 2015; 93:410–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.