Significance
Kinesins are major transporters of cargos toward the cell periphery. They are highly expressed in the CNS, and their dysfunction leads to a wide range of human pathologies, including neurodevelopmental and neurodegenerative diseases, ciliopathies, epilepsy, and birth defects. We have discovered that the widely used general anesthetic propofol shortens the distance that kinesins travel, but their velocity remains unchanged. These results suggest that propofol is not binding at the ATP site or allosteric sites that affect ATP turnover, leading to the conclusion that the allosteric sites form on microtubule association. We postulate that general anesthetics bind specifically to transport kinesins and/or the kinesin–β-tubulin interface, and diminish their ability to transport critical cargos, thereby contributing to the pleiotropic state of anesthesia.
Keywords: anesthesia, allosteric inhibitor, microtubule, etomidate, ketamine
Abstract
Propofol is the most widely used i.v. general anesthetic to induce and maintain anesthesia. It is now recognized that this small molecule influences ligand-gated channels, including the GABAA receptor and others. Specific propofol binding sites have been mapped using photoaffinity ligands and mutagenesis; however, their precise target interaction profiles fail to provide complete mechanistic underpinnings for the anesthetic state. These results suggest that propofol and other common anesthetics, such as etomidate and ketamine, may target additional protein networks of the CNS to contribute to the desired and undesired anesthesia end points. Some evidence for anesthetic interactions with the cytoskeleton exists, but the molecular motors have received no attention as anesthetic targets. We have recently discovered that propofol inhibits conventional kinesin-1 KIF5B and kinesin-2 KIF3AB and KIF3AC, causing a significant reduction in the distances that these processive kinesins can travel. These microtubule-based motors are highly expressed in the CNS and the major anterograde transporters of cargos, such as mitochondria, synaptic vesicle precursors, neurotransmitter receptors, cell signaling and adhesion molecules, and ciliary intraflagellar transport particles. The single-molecule results presented show that the kinesin processive stepping distance decreases 40–60% with EC50 values <100 nM propofol without an effect on velocity. The lack of a velocity effect suggests that propofol is not binding at the ATP site or allosteric sites that modulate microtubule-activated ATP turnover. Rather, we propose that a transient propofol allosteric site forms when the motor head binds to the microtubule during stepping.
Propofol (2,6-diisopropylphenol) is the most widely used i.v. general anesthetic to induce and maintain anesthesia (1, 2). The consensus to date has been that general anesthetics, like propofol, are small hydrophobic molecules that bind to ligand- or voltage-gated channels, and it is the change in their activities that elicits the end points of anesthesia, including behavioral immobility, amnesia, and loss of consciousness. Propofol influences ligand-gated channels, including the GABAA receptor as well as other ligand-gated channels and receptors (3–8). Moreover, specific propofol binding sites have been mapped using photoaffinity ligands and mutagenesis. However, their precise target interaction profiles have not yet satisfied the criteria of being both necessary and sufficient to produce the complete anesthetic state (4–9). This body of work suggests that propofol and other common anesthetics, such as etomidate and ketamine (10–14), may target additional protein networks of the CNS, including potential interactions with the cytoskeleton to contribute to the desired and undesired anesthesia end points. Some evidence for anesthetic interactions with the cytoskeleton exist (15–18). For example, different classes of general anesthetics bind specifically to tubulin and alter its stability (17, 18), and entire theories of anesthetic action have been constructed around microtubule (MT) properties (19). However, the molecular motors that travel along MTs have received no attention as anesthetic targets. We have recently discovered that propofol inhibits three processive kinesins (kinesin-1 KIF5B, kinesin-2 KIF3AB, and kinesin-2 KIF3AC) using single-molecule motility assays. These in vitro assays are powerful in that they can assess the ability of a single dimeric motor to step along a MT. Propofol caused a significant reduction in the potential distance that these kinesins can travel. These MT-based motors are highly expressed in the CNS and the major anterograde transporters of cargos, such as mitochondria, synaptic vesicle precursors, neurotransmitter receptors, cell signaling and cell adhesion molecules, mRNA particles, and ciliary intraflagellar transport particles (20–25). The results presented provide a compelling inhibition profile to hypothesize that kinesins may also be targets of general anesthetics, such as propofol, etomidate, and/or ketamine, and thereby, contribute to the state of general anesthesia.
Results
Propofol Decreases the Persistence of MT Gliding but Does Not Alter the Velocity of Movement.
To test the hypothesis that propofol can affect kinesin motility, a MT gliding assay was used in conjunction with total internal reflection fluorescence (TIRF) microscopy (26, 27). The advantage of this assay is that it evaluates the ability of the motor population to propel and sustain MT gliding across a lawn of kinesin motors. The first experiment tested the well-characterized homodimeric kinesin-1 K560 (27–29). K560 was bacterially expressed from human kinesin-1 KIF5B, encoding the first 560 amino acid residues. A 10-μL perfusion chamber was constructed, and antibodies to the C-terminal His tag of K560 were applied followed by perfusion of MT–K560 complexes. This approach ensures tight binding of the C terminus of K560 to the coverslip. Subsequently, K560 motility was initiated by 1 mM MgATP. The results in Fig. 1 show that, in the presence of 5 μM propofol, the MTs initially glided across the K560 coverslip as in the DMSO control, but the number of K560-associated MTs decreased dramatically as a function of time as MTs detached from the coverslip and were no longer illuminated in the TIRF field (Movies S1 and S2). Moreover, MT stalling on the coverslip was rarely observed in the presence of propofol. After 5 min, ∼80% of MTs glide persistently, but by 20 min, the population remaining on the kinesin-coated surface dropped to 59.3%. These results were in stark contrast to the DMSO control, where ∼95% of the MT population was observed to glide continuously across the K560 coverslip (Fig. 1G).
Fig. 1.
Propofol disrupts the persistence of MT gliding but does not significantly alter the velocity of MT gliding. (A–F) Histograms of velocities (A–C) in control conditions (3% DMSO) and (D–F) at 5 μM propofol for each population of motors. A Gaussian fit provides the average velocity ±SEM for each dataset. The average velocities were not statistically significant between DMSO controls and 5 μM propofol datasets (P > 0.5). All experiments were conducted in the presence of 1 mM MgATP. Representative Movies S1 and S2 show K560. (G–I) Persistence of MT gliding as a function of time in 3% DMSO (blue) or 5 μM propofol (red). For all panels, N represents the number of MTs analyzed for each condition. (A, D, and G) Kinesin-1 K560, (B, E, and H) kinesin-2 KIF3AC, and (C, F, and I) kinesin-2 KIF3AB. (J–M) Anesthetics used in this report: (J) propofol, 2,6-diisopropylphenol; (K) fropofol, 2-fluoro-1,3-diisopropylbenzene; (L) ketamine, 2-(2-chlorophenyl)-2-(methylamino)cyclohexanone; and (M) etomidate, 1-(α-methylbenzyl)imidazole-5-carboxylic acid ethyl ester.
The MT gliding assay was repeated for kinesin-2 KIF3AC (Fig. 1 B, E, and H) and KIF3AB (Fig. 1 C, F, and I). These kinesins are distinctive in that they exist physiologically as heterodimers formed from three different gene products: KIF3A, KIF3B, and KIF3C (21–24, 30). The KIF3AC and KIF3AB heterodimers, characterized previously, were engineered to include the N-terminal native motor domain sequence, neck linker, and native helix α7 followed by a dimerization motif to stabilize the native coiled coil (27, 31). The results in Fig. 1 also show that KIF3AC and KIF3AB were similarly affected by 5 μM propofol, such that the persistence of MT gliding decreased to 52.3% of the MT population for KIF3AC and 51.9% of the MT population for KIF3AB. Although propofol altered the persistence of MT gliding by K560, KIF3AC, and KIF3AB, it surprisingly did not alter MT gliding velocity (Fig. 1 A–F). These results indicate that propofol did not affect ATP turnover but rather, altered the processivity of individual kinesin motors. To test this hypothesis directly, single-molecule quantum dot (Qdot) motility assays were pursued with TIRF microscopy (27).
Propofol Shortens the Run Length Potential of Kinesin-1 K560 in a Concentration-Dependent Manner but Does Not Alter the Velocity of Movement.
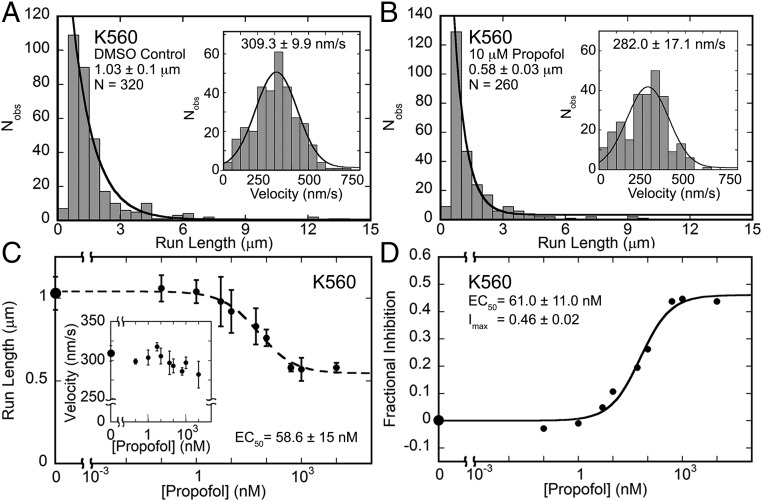
In the absence of propofol, single-molecule experiments show that K560 steps along the MT at a rate of 309.3 nm/s with a run length of 1.03 μm (Fig. 2). Note that, in the presence of 3–5% DMSO, the concentration used as the propofol vehicle, the motility properties of K560, KIF3AC, or KIF3AB were not noticeably altered (Table S1). In contrast, the run length decreased to 0.58 μm in 10 μM propofol, but the velocity was similar at 282 nm/s (Fig. 2 A and B and Movies S3 and S4). This run length change is significant, because for processive kinesins, each 8-nm step is coupled to one ATP turnover. Thereby, propofol decreased kinesin processivity from ∼129 to 72 steps per run. Subsequent experiments evaluated a propofol concentration dependence, with each data point in Fig. 2 C and D representing the average run length and velocity from the Gaussian fit to each histogram dataset as shown for 10 μM propofol in Fig. 1 A and B. Fig. 2C shows that the average run length decreased as a function of propofol concentration, with the decrease becoming statistically significant at 5 nM propofol (P < 0.002), but the velocity at each propofol concentration was unaffected. Furthermore, the Hill–Slope model fit to the data provided the EC50 at 58.6 nM.
Fig. 2.
Propofol shortens the mean run length of kinesin-1 K560 but does not alter velocity. (A and B) K560 run length and (Insets) velocity data in (A) 5% DMSO control and (B) 10 μM propofol. Statistical comparison of these data shows that the impact on run length is highly significant (P < 0.0001) but that the effect on velocity is not significant (P > 0.9). All experiments were conducted in the presence of 1 mM MgATP (Movies S3 and S4). (C) Mean run length and (Inset) velocity from K560 single-molecule motility assays plotted as a function of increasing propofol concentration over a range of 0 (5% DMSO control) to 106 nM propofol (log scale). The decrease in run length becomes statistically significant at 5 nM propofol (P < 0.002), whereas the variation in velocity is not statistically significant (P > 0.5). The EC50 was determined from fitting run length data to the Hill–Slope model. (D) Fractional inhibition of the run length data at each propofol concentration was plotted as a concentration dependence. The quadratic function provided the EC50 and the maximal fractional inhibition (Imax). All values are ±SEM.
Table S1.
Comparison of velocity and run length data in the presence and absence of 5% DMSO (related to Figs. 1–5)
| Kinesin | Velocity (nm/s) | Run length (μm) | ||
| —* | 5% DMSO | —* | 5% DMSO | |
| K560 | 305.1 ± 5.2 | 309.3 ± 9.9 | 1.26 ± 0.10 | 1.03 ± 0.10 |
| KIF3AC | 168.6 ± 5.6 | 182.1 ± 5.4 | 1.23 ± 0.09 | 1.16 ± 0.05 |
| KIF3AB | 246.2 ± 11.0 | 207.8 ± 2.3 | 1.62 ± 0.11 | 1.61 ± 0.33 |
| KIF3AA | 239.2 ± 2.2 | 286.1 ± 7.7 | 0.98 ± 0.05 | 1.05 ± 0.06 |
| KIF3BB | 327.6 ± 7.2 | 281.5 ± 11.0 | 1.51 ± 0.16 | 1.29 ± 0.17 |
| KIF3CC | 8.2 ± 0.3 | 6.9 ± 0.04 | 0.57 ± 0.03 | 0.79 ± 0.07 |
Data in the absence of DMSO were reported previously (27).
To determine the maximal fractional inhibition, the data were analyzed as follows:
where fractional inhibition, I, is defined as the difference between the run lengths (RL) of the DMSO control and each propofol concentration divided by the control run length (Fig. 2D). The following quadratic equation was fit to the data:
where Imax is the maximal fractional inhibition, and P is the propofol concentration. The EC50 from this fit at 61 nM is comparable with the Hill–Slope model estimation at 58.6 nM. The maximal fractional inhibition at 0.46 revealed a significant decrease in K560 run length potential.
Propofol Also Shortens the Run Length Potential of Kinesin-2 KIF3AC and KIF3AB.
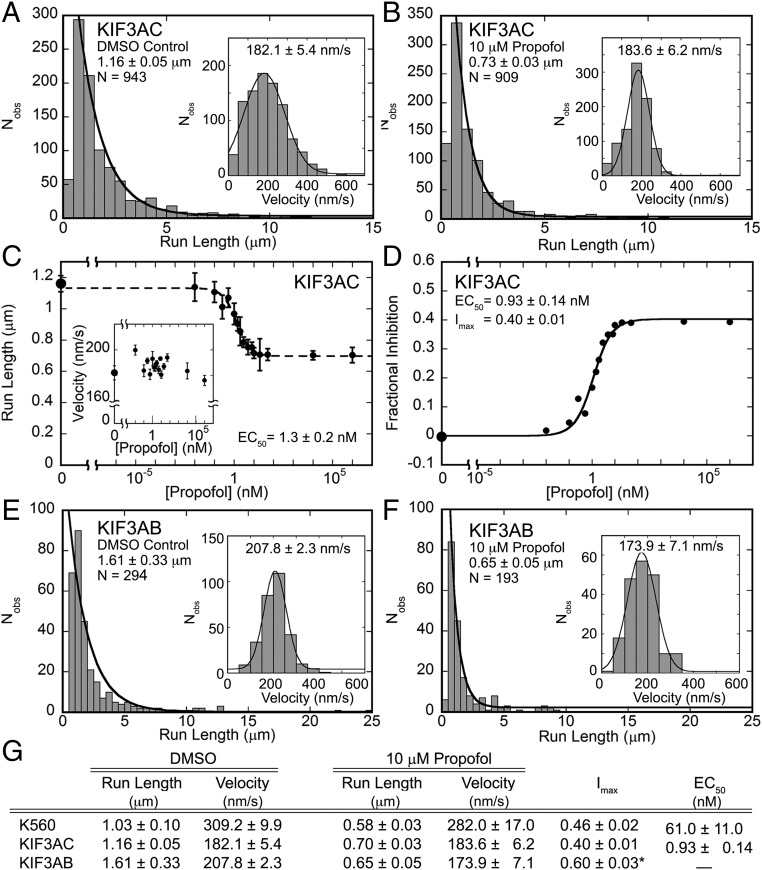
Fig. 3 A–D shows the results for the KIF3AC single-molecule studies (Movies S5 and S6). Note that, in the presence of 10 μM propofol, the run length decreased significantly from 1.16 to 0.7 μm (P < 0.0001), but the velocities were unaffected. Like K560, the KIF3AC single-molecule experiments were repeated as a function of propofol concentration (Fig. 3C). The difference in run length became statistically significant at 0.25 nM propofol (P < 0.0001), whereas the velocity remained unchanged. The Hill–Slope model fit to the run length data provided an EC50 at 1.3 nM. Fig. 3D shows the data presented as the fractional inhibition at each propofol concentration. The quadratic fit to these data provided an EC50 at 0.93 nM and the maximal fractional inhibition of 0.40, indicating that propofol shortened the run length potential significantly. Moreover, the EC50 value at ∼1 nM is very close to the concentration of the KIF3AC heterodimers in the perfusion chamber at 2 nM Qdot-bound KIF3AC, suggesting the possibility that KIF3A or KIF3C binds propofol more tightly than its partner motor head.
Fig. 3.
Propofol affects K560, KIF3AC, and KIF3AB motility similarly. (A and B) KIF3AC run length and (Insets) velocity data in (A) 5% DMSO control vs. (B) 10 μM propofol (Movies S5 and S6). (C) Average run length and (Inset) velocity data from KIF3AC single-molecule motility assays plotted as a function of increasing concentration from 0 (5% DMSO control) to 106 nM propofol (log scale). The difference in run length becomes statistically significant at 0.25 nM propofol (P < <0.0001), whereas there is no statistical significance between average velocities (P > 0.4). Run length data were fit to the Hill–Slope model, which provided the EC50. (D) Fractional inhibition of the KIF3AC run length data at each propofol concentration was plotted as a concentration dependence. The quadratic function fit to the data provided an EC50 and the maximal fractional inhibition Imax. (E and F) KIF3AB run length and (Insets) velocity data in (E) 5% DMSO control vs. (F) 10 μM propofol (Movies S7 and S8). The decrease in run length is highly statistically significant (P < <0.0001), whereas velocity does not exhibit a statistical difference in the presence of propofol (P > 0.2). (G) Compiled single-molecule motility data from Fig. 2 and this figure. All experiments were conducted in the presence of 1 mM MgATP, and all values are ±SEM. *Imax for KIF3AB is calculated based on the fractional inhibition at 10 μM propofol (E and F).
The impact of propofol on KIF3AB was also evaluated (Fig. 3 E and F and Movies S7 and S8). The results show that the 0.65-μm run length at 10 μM propofol was significantly less (P < 0.0001) than the run length in the absence of propofol at 1.61 μm. A propofol concentration dependence for KIF3AB was not pursued to determine the EC50, but the 10 μM results clearly show that, as with K560 and KIF3AC, propofol also decreases the run length of KIF3AB considerably (Imax = 0.60) without impacting velocity (P > 0.2).
These results illustrate the remarkable impact that propofol has on the performance of these processive kinesins with EC50 values in the nanomolar range (Fig. 3G). Furthermore, the 40–60% decrease in run length potential suggests a common mechanism, especially based on the overall sequence homology between the catalytic core of the processive kinesin motor domains. Equally intriguing is that each of these processive kinesins could sustain sequential stepping at very high concentrations of propofol and maintain their normal velocity (Fig. 3G).
Propofol Inhibition Is Dependent on the Propofol Hydroxyl.
Propofol is a fairly simple hydrophobic compound (Fig. 1J), but it contains the 1-hydroxyl that has been linked to molecular recognition within targets that contribute to anesthesia end points. Woll et al. (32) synthesized a compound named fropofol, in which the 1-hydroxyl was substituted with fluoride, dramatically reducing the ability to hydrogen bond (Fig. 1K). This analog maintains a similar molecular volume as propofol with a small increase in hydrophobicity, and fropofol also binds some of the molecular targets of propofol, such as apoferritin (32). With the 1-hydroxyl substitution, fropofol failed to induce loss of mobility end points in Xenopus laevis tadpoles and mice and does not enhance GABAA receptor activity. However, fropofol does retain the propofol-like ability to depress myocardial contractility (8, 32). Therefore, fropofol can be used to separate the desired from some undesired end points of anesthesia. To test the hypothesis that the 1-hydroxyl was necessary for the propofol effect on processive kinesins and thereby, potentially attribute this effect to desired end points of anesthesia, we pursued single-molecule experiments with KIF3AC at 10 and 100 μM fropofol. Fig. 4 shows that, even at 100 μM fropofol, neither the run length of KIF3AC (P > 0.6) nor the KIF3AC velocity (P > 0.3) were significantly diminished. These results clearly show that the propofol 1-hydroxyl is critical for molecular recognition and/or kinesin run length inhibition and that kinesins may contribute to propofol-induced unconsciousness as reflected by loss of mobility end points.
Fig. 4.
Fropofol does not inhibit KIF3AC run length or velocity. KIF3AC run length and (Insets) velocity data in the presence of (A) 5% DMSO control and (B) 100 μM fropofol. Neither the difference in run length nor the velocity were statistically significant (P > 0.3). All values are reported as the average from the fit of the histogram ±SEM.
Propofol Shortens the Run Length Potential of Homodimeric KIF3BB and KIF3CC, but No Effect Is Observed for KIF3AA.
We were intrigued by the EC50 at ∼1 nM propofol for KIF3AC, because the single-molecule experiments were performed at 2 nM KIF3AC dimer. We questioned whether this constant may reflect a difference in propofol binding affinity to KIF3A compared with KIF3C. To explore this hypothesis further, single-molecule experiments at 10 μM propofol were pursued with engineered homodimers of KIF3AA, KIF3BB, and KIF3CC (Fig. 5). The homodimer design was similar to that of the heterodimers, where each polypeptide included the native catalytic motor domain, the neck linker, helix α7 followed by a dimerization domain, the Tobacco Etch Virus site, and the His8 tag (27). The results were surprising. Although 10 μM propofol shortened the run length potentials of homodimeric KIF3BB (Imax = 0.56) and KIF3CC (Imax = 0.38), propofol seemed to have negligible effect on KIF3AA (Imax = 0.02).
Fig. 5.
Propofol does not affect homodimeric KIF3AA single-molecule motility. (A–F) Single-molecule run length and (Insets) velocity data for (A and B) KIF3AA, (C and D) KIF3BB, and (E and F) KIF3CC comparing control conditions at (A, C, and E) 5% DMSO vs. (B, D, and F) 10 μM propofol. All experiments were conducted at 1 mM MgATP. (G) Compiled single-molecule motility data. Imax is calculated based on the fractional inhibition at 10 μM propofol.
When the sequences of the motor domain were compared, KIF3B and KIF3C show 71% identity, but the identity between KIF3A and KIF3B or KIF3C is less at 69 and 57%, respectively. Kinesin-1 KIF5B was clearly affected by propofol, but its sequence identity compared with KIF3A, KIF3B, and KIF3C is less than 50%. However, structurally kinesins share a highly conserved Walker nucleotide binding fold that consists of a central twisted β-sheet and three nucleotide binding loops designated switch-1, switch-2, and the P loop (33–37). Kinesins also share a similar MT binding interface and a series of structural transitions in response to the nucleotide binding state that coordinates MT association and detachment (38–41). To sustain a processive run, the domains must be coordinated, so that one is always in a MT strongly bound state to prevent motor detachment from the MT (42–44). Therefore, these results suggest that, despite the relatively high homology between these motor domains, small sequence differences in the motor domain have resulted in KIF3A either not binding propofol and/or propofol not promoting premature motor detachment from the MT.
Etomidate and Ketamine Also Inhibit the Run Length Potential of KIF3AC.
General anesthetics have multiple functional targets and overlapping binding sites within their target proteins, in part because they are small hydrophobic molecules. To determine if processive kinesins are affected by other i.v. general anesthetics, we tested whether kinesin motility of KIF3AC was altered by ketamine or etomidate (Fig. 1 L and M). Etomidate, like propofol, enhances the GABAA-mediated inhibitory response, whereas ketamine acts primarily as an antagonist of NMDA receptors, although it too has multiple targets, including a subset of the G protein (heterotrimeric guanine nucleotide binding protein)-coupled receptors that are distributed throughout the CNS (10–14). The results in Fig. S1 show that, like propofol, 10 μM etomidate or ketamine shortened the run length potential of kinesin-2 KIF3AC: from 1.19 to 0.57 μm by etomidate and from 1.19 to 0.52 μm by ketamine (P < 0.0001). Also, like propofol, neither drug altered the velocity of movement significantly (P > 0.7). These results implicate processive kinesins as anesthetic targets that may contribute to modulation of the anesthetic state.
Fig. S1.
The general anesthetics ketamine and etomidate decrease the run length potential of KIF3AC (related to Fig. 3). (A–C) Run length and (Insets) velocity data collected for KIF3AC in the presence of (A) 5% DMSO control, (B)10 μM ketamine•HCl, S-(+)-2-(2-chlorophenyl)-2-(methylamino)cyclohexanone hydrochloride, and (C) 10 μM etomidate, (R)-1-(α-methylbenzyl)imidazole-5-carboxylic acid ethyl ester. The difference in run length is statistically significant (P < 0.0001) between the control and ketamine or etomidate, whereas the difference in velocity showed no statistical significance (P > 0.7).
Discussion
There Is a Common Mechanism to Shorten Run Length Potential.
Because propofol did not alter the velocity of movement for K560, KIF3AC, or KIF3AB in the MT gliding or single-molecule experiments, we propose that propofol is not binding at allosteric sites within the catalytic motor domain that would alter MT-activated ATP turnover. Moreover, because the concentration of MTs is at 0.1 μM in the single-molecule assays and the EC50 values are <100 nM propofol, it is unlikely that propofol is saturating binding sites along the MT where the kinesin head would step during a processive run. Moreover, the lack of a propofol effect on KIF3AA renders a common MT site unlikely. Rather, these results indicate that propofol and likely, etomidate and ketamine allosteric binding sites form transiently in kinesin when the motor domain binds to the MT lattice during stepping, reducing kinesin MT affinity. This outcome, in essence, implies a druggable allosteric site that, when occupied, promotes detachment of the kinesin motor from the MT. There may exist multiple transient propofol and therefore, etomidate and ketamine binding sites on the kinesin motor domain, requiring only anesthetic binding to kinesin to impact the motor’s interactions with the MT. Note too that the maximal fractional inhibition promoted by propofol, etomidate, and ketamine was 0.4–0.6, suggesting a common mechanism for shortening the run length of these kinesins. This inhibition profile is very different from the loop L5-targeting small-molecule drugs in either the monastrol family of inhibitors for human kinesin-5 KSP/Eg5 (45–48) or the kinesin-specific inhibitor GSK923295 for kinesin-7 CENP-E (49). Monastrol family inhibitors stabilize ADP at the active site and therefore, destabilize the MT–KSP interaction. GSK923295 inhibits the release of inorganic phosphate and stabilizes the interaction of CENP-E with the MT. However, both inhibitors alter MT-activated ATPase activity as well as kinesin-promoted motility, which is very different from the results presented here.
One can reason based on kinesin X-ray crystal structures and site-specific mutations that small, hydrophobic anesthetic molecules have the potential to bind at multiple allosteric sites on the kinesin motor domain or at residues of the MT•kinesin interface to alter kinesin binding affinity to the MT and promote motor detachment (38, 39, 50–54).
Are Processive Kinesins Targets of General Anesthetics, and Thereby, Do They Contribute to the Anesthetic State?
The binding of general anesthetics to ligand- or voltage-gated channels and receptors is known to elicit both desired and undesired end points of anesthesia. However, it is also recognized that none of these molecular targets have satisfied the criteria of being both necessary and sufficient to produce the complete anesthetic state. Other plausible targets have included mitochondria, tubulin, and the synaptic vesicle transport and release machinery (17, 55–58). For example, SNAP-25 and syntaxin specifically bind propofol (7). Therefore, it is reasonable that there are other targets that interact with anesthetics and contribute to the state of anesthesia. Our results expand the field by providing evidence for a largely overlooked but critically positioned set of targets, molecular motors, which could influence acute and/or chronic end points observed with some general anesthetics.
The EC50 values for kinesin “derailing” are at least 10-fold lower than those associated with propofol-induced immobility, suggesting that these effects may underlie other subclinical actions of propofol, such as amnesia and postural instability. Alternatively, it is possible that EC50 values will be very different in the crowded intracellular milieu and when the motors are loaded with cargo. Nevertheless, these results clearly indicate that kinesin motors will be influenced during propofol anesthesia in vivo, and it seems unlikely that such important intracellular movers will not contribute to components of anesthetic action.
The human kinesin superfamily includes 45 genes, 38 of which are expressed in brain, with three subfamilies of kinesins that are predominantly responsible for cargo transport to the cell periphery (i.e., the synapse in neurons) (21, 22, 24, 25, 30, 59, 60). Kinesins are the major anterograde transporters of cargos that have been established as anesthetic targets, including mitochondria, GABAA receptors, syntaxin, and SNAP-25 (7, 9, 57, 58, 61–64). The critical neurological role of kinesins is further indicated by the lethality of many mutations, an anesthetic-like immobility phenotype in others, and numerous kinesin dysfunctions linked to a wide range of human pathologies, including neurodevelopmental and neurodegenerative diseases, ciliopathies, epilepsy, and birth defects (21, 24, 64–67).
In summary, the flux of cellular substrates is a balance between the collective anterograde transport by kinesins and retrograde transport by dynein. Even a modest depletion of kinesin-1 or -2 processivity would create an opportunity for retrograde motors, like dynein, to drive cargo transport back to the cell body, leading to a critical imbalance of cargo distribution. Therefore, kinesins are critically positioned to underlie specific anesthesia end points, and this report has revealed a dramatic impact of three different anesthetics on processive kinesins at concentrations used in routine clinical care.
Methods
Standard MT gliding and single-molecule kinesin Qdot assays and TIRF imaging techniques were used throughout. Detailed descriptions of kinesin motor construct design, expression, and purification and microscopy methods are provided in SI Methods. MT concentrations are reported as paclitaxel-stabilized α,β-tubulin concentration.
SI Methods
Kinesin-1 K560 Homodimers.
Human kinesin-1 K560 was expressed from Addgene plasmid K560HTR 24444, which encodes the first 560 amino acid residues of KIF5B followed by a C-terminal His6 tag (28). The plasmid was transformed into Escherichia coli BL21-CodonPlus (DE3)-RIL cells (Stratagene Corp.) with selection on LB plates containing 100 μg/mL ampicillin and 10 μg/mL chloramphenicol. Positive clones were selected and grown in LB medium with antibiotics at 37 °C until the cultures reached an A600 of ∼0.35. The temperature was shifted to 16 °C on ice, and the cultures were subsequently induced at 0.1 mM isopropyl-1-thio-β-d-galactopyranoside followed by incubation with shaking for 16–18 h at 16 °C. Cell pellets were collected by centrifugation and resuspended at 5 mL lysis buffer per 1 g cells at 4 °C. Lysis buffer contained 20 mM sodium phosphate buffer, pH 6.9, 100 mM NaCl, 2 mM MgCl2, 0.1 mM EGTA, 0.05 mM ATP, 1 mM DTT, and 5 mM PMSF. One SIGMAFAST Protease Inhibitor Mixture Tablet (Sigma-Aldrich) was added to the resuspended cells followed by adjusting to 1 mg/mL lysozyme (Sigma-Aldrich). The cells were stirred in an ice bath at 4 °C for 60 min and then lysed by three cycles of freeze (liquid N2) and thaw (37 °C water bath with tube inversion). Cell lysates were clarified by ultracentrifugation, and the supernatant was allowed to equilibrate to room temperature.
The supernatant enriched in K560 was adjusted to 0.1 mM GTP, 40 μM paclitaxel, 5 μM MT, and 0.1 mM Adenosine 5′-(β,γ-imido)triphosphate (AMPPNP) followed by incubation at room temperature for 20 min to promote MT•K560 complex formation. After centrifugation (38,000 × g for 35 min at 25 °C), the supernatant was discarded. The MT–K560 pellet was resuspended in 5 mL of lysis buffer without PMSF; adjusted to 1 mM MgATP, 150 mM KCl, and 40 μM paclitaxel; and incubated for 20 min to release active kinesin to the supernatant. After centrifugation, the K560-enriched supernatant was transferred to a fresh tube and adjusted to 300 mM NaCl plus 20 mM imidazole, pH 6.9.
The K560 supernatant was loaded onto a HisTrap FF Ni2+-NTA column (GE Healthcare), which was equilibrated in Ni2+-NTA Binding Buffer at 4 °C (20 mM sodium phosphate buffer, pH 6.9, 300 mM NaCl, 2 mM MgCl2, 0.1 mM EGTA, 1 mM DTT, 0.02 mM ATP, 20 mM imidazole). The column was washed until the absorbance returned to baseline. K560 was eluted using a linear gradient of 20–400 mM imidazole, pH 6.9, in Ni2+-NTA Binding Buffer. K560-enriched fractions were identified by SDS/PAGE, pooled, and dialyzed overnight against dialysis buffer (20 mM Hepes, pH 7.2, 250 mM NaCl, 0.1 mM EGTA, 0.1 mM EDTA, 5 mM magnesium acetate, 50 mM potassium acetate, 1 mM DTT). The dialysis buffer was changed to 20 mM Hepes, pH 7.2, 250 mM NaCl, 0.1 mM EDTA, 0.1 mM EGTA, 5 mM magnesium acetate, 50 mM potassium acetate, and 1 mM DTT plus 5% sucrose, and the K560 was dialyzed for an additional 2 h followed by ultracentrifugation to remove protein aggregates. The supernatant was concentrated, aliquoted, and flash frozen in liquid nitrogen before storage at −80 °C. Based on SDS/PAGE analysis, this purification scheme resulted in high-purity protein without fragmentation. The protein yield was ∼3 mg per 10 g cells, with the predicted molecular weight per polypeptide chain at 64,557 and a functional dimer molecular weight at 129,114.
Kinesin-2 KIF3 Heterodimeric and Homodimeric Motors.
The Mus musculus KIF3A, KIF3B, and KIF3C plasmids for expression of the KIF3AB (31) and KIF3AC (27) heterodimers as well as homodimers of KIF3AA, KIF3BB, and KIF3CC were described previously in detail along with their expression and purification (27). The construct for KIF3A when expressed as a heterodimer with KIF3C includes the native sequence of the motor domain, the neck linker, and helix α7 (M1-L374) followed by the dimerization motif of EB1 (bold), the C-terminal Tobacco Etch Virus (TEV) protease-cleavable site (italics), linker (plain font) and StrepII tag (underlined): KIF3A(M1-L374)-DFYFGKLRNIELICQENEGENDPVLQRIVDILYATDETTSENLYFQGASNWSHPQFEK. The KIF3C construct for KIF3AC includes the native sequence of the KIF3C motor domain, the neck linker, and helix α7 (M1-L374) followed by the dimerization motif of EB1 (bold), the TEV protease-cleavable site (italics), and His8 tag (underlined) with the linker residues in plain font: KIF3C(M1-L374)-DFYFGKLRNIELICQENEGENDPVLQRIVDILYATDETTSENLYFQGASHHHHHHHH.
To generate a stable heterodimer of KIF3AB, a synthetic heterodimerization domain motif containing either an acidic heterodimerization domain (AHD) or a basic fusion helix heterodimerization domain (BHD) was used (31, 68, 69). The KIF3A-AHD polypeptide consisted of the KIF3A motor domain, the neck linker, and three heptads of native helix sequence followed by the AHD helix (bold), a TEV protease site (italics), linker residues (plain font), and StrepII tag (underlined): KIF3A(M1-E376)-LEKEIAALEKEIAALEKTTSENLYFQGASNWSHPQFEK. The KIF3B-BHD polypeptide contained the KIF3B motor domain, the neck linker, and three heptads of native helix sequence followed by the BHD helix (bold) and a TEV protease site (italics) with linker residues (plain font) and a His8 tag (underlined): KIF3B(M1-K371)-LKEKIAALKEKIAALKETTSENLYFQGASHHHHHHHH.
For the KIF3AA, KIF3BB, and KIF3CC homodimers, each native N-terminal motor domain sequence, neck linker, and α7 helix was C-terminally fused to an in-register segment of the dimerization motif from EB1 (bold) followed by the TEV protease site (italics) with linker residues (plain font) and a His8 tag (underlined): KIF3A: KIF3A(Met1-Leu374)-DFYFGKLRNIELICQENEGENDPVLQRIVDILYATDETTSENLYFQGASHHHHHHHH; KIF3B: KIF3B(Met1-Leu369)-DFYFGKLRNIELICQENEGENDPVLQRIVDILYATDETTSENLYFQGASHHHHHHHH; KIF3C: KIF3C(M1-L374)-DFYFGKLRNIELICQENEGENDPVLQRIVDILYATDETTSENLYFQGASHHHHHHHH.
Note that the EB1 motif is a dimerization domain only and does not interact with MTs as shown previously (27, 70, 71). For all of the experiments reported here, the TEV-cleavable purification tags were left intact.
KIF3 Protein Expression and Purification.
All KIF3 motors were expressed in the E. coli BL21-CodonPlus (DE3)-RIL cell line (Stratagene) with heterodimers resulting from cotransformation of two plasmids and selection on LB plates containing 100 μg/mL ampicillin, 50 μg/mL kanamycin, and 10 μg/mL chloramphenicol (27, 31). To achieve the purification of KIF3AC and KIF3AB, sequential affinity columns were used. The HisTrap FF Ni+2-NTA column (GE Healthcare) first selected for the C-terminally His8-tagged KIF3B or KIF3C followed by the StrepTactin column (GE Healthcare), which selected for the Strep-tagged KIF3A. The purified KIF3AC and KIF3AB motors were evaluated by analytical gel filtration chromatography (Superose 10/300; GE Healthcare) and SDS/PAGE to confirm purification of stable heterodimers with a 1:1 stoichiometry of KIF3A to either KIF3C or KIF3B. The predicted molecular weight based on amino acid sequence of KIF3AC is 98,317, and the predicted molecular weight based on amino acid sequence of KIF3AB is 92,131.
The KIF3 proteins were dialyzed against ATPase buffer containing 20 mM Hepes, pH 7.2, with KOH, 5 mM magnesium acetate, 0.1 mM EDTA, 0.1 mM EGTA, 50 mM potassium acetate, and 1 mM DTT plus 5% sucrose followed by ultracentrifugation to remove aggregates and then frozen as aliquots in liquid N2 for storage at −80 °C.
To purify homodimeric KIF3AA, KIF3BB, or KIF3CC, the supernatant of the cell lysate was loaded onto the HisTrap FF Ni+2-NTA column (GE Healthcare) to select for the His8 tag followed by additional purification using gel filtration on an HPLC Superose 10/300 gel filtration column (GE Healthcare) with elution into the 20 mM Hepes dialysis buffer described above plus 200 mM NaCl. The purity of the KIF3AA, KIF3BB, or KIF3CC homodimers was subsequently analyzed by analytical gel filtration and SDS/PAGE. The predicted molecular weight based on amino acid sequence of KIF3AA is 97,000, the predicted molecular weight based on amino acid sequence of KIF3BB is 96,022, and the predicted molecular weight based on amino acid sequence of KIF3CC is 99,518.
Before each experiment, KIF3 protein aliquots were thawed and clarified for 10 min at 4 °C (313,000 × g; TLA-100 rotor; Beckman Coulter TLX Optima Ultracentrifuge), and the protein concentration was determined using the Bio-Rad protein assay with IgG as a protein standard.
In Vitro MT Gliding Assays.
Polarity marked X-rhodamine–labeled MTs were polymerized as described (26, 27, 69) and stabilized with 20 μM paclitaxel. MT•motor complexes were performed with a final concentration of 0.5 μM tubulin (paclitaxel-stabilized α,β-tubulin) and 2.5 μM kinesin dimer. Perfusion chambers were constructed using acid-washed coverslips and incubated first with 40 μg/mL Penta-His antibodies (Qiagen) for 10 min followed by a 5-min incubation of blocking buffer (PME80; 1.5 mM magnesium acetate, 500 μg/mL casein, 25 mM glucose, 0.2 mg/mL glucose oxidase, 175 μg/mL catalase, 0.3 mg/mL creatine phosphokinase, 2 mM phosphocreatine, 0.5% β-mercaptoethanol, 15 μM paclitaxel). MT•motor complexes were then introduced into the chamber and allowed to bind for 5 min. Unbound complexes were removed with excess blocking buffer. Gliding via the surface-bound motors was activated by introducing Activity Buffer (blocking buffer supplemented with 1 mM MgATP and either 3% DMSO or 5 μM propofol at 3% DMSO). MT gliding was imaged by TIRF microscopy at 564 nm using a 180-ms exposure time and a 10- (K560) or 20-s (KIF3) interval for 20 min at 25 °C. The velocity of gliding MTs (Fig. 1) was determined by tracking the leading edge of the moving polarity-marked MTs using the tracking algorithm on AxioVision 4.8.2 software (Carl Zeiss Microscopy, Inc.). MTs were scored that moved at least eight frames (≥80 s for K560 and ≥160 s for KIF3AC and KIFAB) and met the following criteria: MTs were entirely in the field of view, were ≥3 but ≤8 μm in length, and traveled in a clearly defined path. MT gliding velocity data were then compiled, and a Gaussian fit of the histogram provided the mean velocity ±SEM. Persistence of MT gliding was defined as the percentage of scorable MTs that remained attached to the surface-bound kinesins and continued to glide. The data reported in Fig. 1 G–I represent a minimum of 3 different experimental days, with six to eight movies collected per day. Within each field of observation, ∼10–60 MTs were scored for persistence of gliding.
Qdot Motor Attachment and Single-Molecule Processivity Assays.
Streptavidin-coated Qdots (Qdot 525-Streptavidin conjugate; Life Technologies) at 200 nM were preincubated in a 1:1 ratio with biotinylated-Penta-His antibody (Qiagen) for 60 min at room temperature in PME80 buffer (80 mM Pipes, pH 6.9, with KOH, 5 mM MgCl2, 1 mM EGTA). His-tagged motors were then added to the Qdot–antibody complex to a final concentration of 20 nM dimer and incubated for 60 min at 4 °C to give a 1:10 ratio of dimer:Qdots (20 nM dimer, 200 nM Qdot–antibody complex). This procedure results in a working stock, in which according to a Poisson distribution, 9% of the Qdots are estimated to have one motor bound and 0.5% of the Qdots are estimated to have two or more motors bound. Previous KIF3 experiments confirmed that a 1:10 ratio of kinesin motors to Qdots was sufficient for single-molecule conditions (27).
Perfusion chambers were formed from a silanized coverslip mounted on a glass slide separated by strips of double-sided tape to generate a 10-μL flow cell. The chamber was first incubated with 0.4% rat anti–α-tubulin antibody (ABD Serotec) for 5 min followed by surface blocking with 5% Pluronic F-127 (Sigma-Aldrich) for 5 min. X-rhodamine MTs (30 μM stock diluted 1:300 in PME80 supplemented with paclitaxel to 22 μM; final MT concentration at 0.1 μM) were then introduced into the chamber and incubated for 10 min. Unbound MTs were removed by flowing into the chamber PME80 supplemented with 10 mM DTT and 20 μM paclitaxel. The previously described working stock of Qdot–motor complexes (20 nM dimer, 200 nM Qdot–antibody complex) was further diluted 10× in Activity Buffer (PME80; 0.5% Pluronic F-127, 30 μM paclitaxel, 125 μg/mL BSA, 50 μM DTT, 25 mM glucose, 0.2 mg/mL glucose oxidase, 175 μg/mL catalase, 0.3 mg/mL creatine phosphokinase, 2 mM phosphocreatine, 1 mM MgATP, 5% DMSO with or without propofol) to give a final concentration of 2 nM Qdot–motor complex in the chamber. Flowing in the Activity Buffer activated motor activity, and chambers were imaged immediately.
TIRF Microscopy and Image Acquisition.
Chambers were imaged by TIRF microscopy at 25 °C using a Zeiss Inverted Axio Observer Z1 MOT fluorescence microscope with the 100× oil 1.46 N.A. Plan-Apochromat objective (Carl Zeiss Microscopy, Inc.) and an incubation hood as described (27). Digital images were collected through a Hamamatsu electron multiplier EM-CCD digital camera using the AxioVision 4.8.2 software package. This imaging method yielded 512 × 512-pixel images with 0.16 μm per pixel in both x and y planes. The Qdot complexes were tracked by imaging at 488 nm (5% laser power) every ∼0.6 s for 5 min using 100-ms exposure. Reference images of the X-rhodamine MT tracks were taken at 564 nm (2% laser power) with 300-ms exposure both before and after acquisition of the Qdot channel. Qdot videos were then overlaid with the MT image using NIH ImageJ software.
Data Analysis.
Single-molecule Qdot motility was analyzed using the MultipleKymograph plugin for ImageJ (J. Rietdorf and A. Seitz, European Molecular Biology Laboratory, Heidelberg, Germany). Velocity histograms were plotted, and a Gaussian function was applied to determine the mean velocity ±SEM. Run lengths were plotted as histograms using a single-exponential decay fit to the data to determine mean run length:
where A is the maximum amplitude, and l is the mean run length reported ±SEM. The first bin of run-length histograms was masked from the fit because of the resolution limit of the TIRF microscope (<0.25 μm). Statistical analyses for run lengths and velocities were performed using the StatPlus plugin for Microsoft Excel (AnalystSoft Inc.). The Comparing Means (t test assuming different variances) algorithm with an α-reliability level of 5% was used for both parameters, with velocities compared with a two-tailed t test, whereas run lengths were compared with a one-tailed t test.
To ensure that the maximum run length potential of the Qdot–motor complexes was visualized, Qdots were excluded from the analysis if they fell off at the end of the MT, paused at the MT end, or began or ended a run outside the timescale of the experiment. Only long MT tracks were examined to allow for the collection of both long and short runs from the same MT tracks, thereby avoiding data bias. Pausing and stalling occurred for all motors tested in both the absence and presence of propofol but represented only a minor fraction (2–6%) of each dataset and showed no apparent correlation between pause frequency or duration and propofol concentration. The data that included pausing events were eliminated from additional analysis.
Supplementary Material
Acknowledgments
We thank Pei Tang (Department of Anesthesiology, University of Pittsburgh) for her initial suggestion that propofol might affect kinesin motility and Nicole Stoddard, who initiated this project as her Rensselaer Senior Research Thesis. This work was supported, in part, by an award from the Rensselaer Office of Research, National Science Foundation Graduate Research Fellowship Program Grant DGE-1321851 (to K.A.W.), and NIH Grants P01-GM55876 (to R.G.E.) and R37-GM054141 (to S.P.G.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1701482114/-/DCSupplemental.
References
- 1.Kotani Y, Shimazawa M, Yoshimura S, Iwama T, Hara H. The experimental and clinical pharmacology of propofol, an anesthetic agent with neuroprotective properties. CNS Neurosci Ther. 2008;14:95–106. doi: 10.1111/j.1527-3458.2008.00043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bateman BT, Kesselheim AS. Propofol as a transformative drug in anesthesia: Insights from key early investigators. Drug Discov Today. 2015;20:1012–1017. doi: 10.1016/j.drudis.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 3.Krasowski MD, et al. General anesthetic potencies of a series of propofol analogs correlate with potency for potentiation of gamma-aminobutyric acid (GABA) current at the GABA(A) receptor but not with lipid solubility. J Pharmacol Exp Ther. 2001;297:338–351. [PubMed] [Google Scholar]
- 4.Stewart DS, et al. p-(4-Azipentyl)propofol: A potent photoreactive general anesthetic derivative of propofol. J Med Chem. 2011;54:8124–8135. doi: 10.1021/jm200943f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiara DC, et al. Photoaffinity labeling the propofol binding site in GLIC. Biochemistry. 2014;53:135–142. doi: 10.1021/bi401492k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jayakar SS, et al. Multiple propofol-binding sites in a γ-aminobutyric acid type A receptor (GABAAR) identified using a photoreactive propofol analog. J Biol Chem. 2014;289:27456–27468. doi: 10.1074/jbc.M114.581728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Woll KA, et al. A novel bifunctional alkylphenol anesthetic allows characterization of γ-aminobutyric acid, type A (GABAA), receptor subunit binding selectivity in synaptosomes. J Biol Chem. 2016;291:20473–20486. doi: 10.1074/jbc.M116.736975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meng T, et al. Molecular mechanism of anesthetic-induced depression of myocardial contraction. FASEB J. 2016;30:2915–2925. doi: 10.1096/fj.201600290RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Woll KA, Dailey WP, Brannigan G, Eckenhoff RG. Shedding light on anesthetic mechanisms: Application of photoaffinity ligands. Anesth Analg. 2016;123:1253–1262. doi: 10.1213/ANE.0000000000001365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rudolph U, Antkowiak B. Molecular and neuronal substrates for general anaesthetics. Nat Rev Neurosci. 2004;5:709–720. doi: 10.1038/nrn1496. [DOI] [PubMed] [Google Scholar]
- 11.Brown EN, Purdon PL, Van Dort CJ. General anesthesia and altered states of arousal: A systems neuroscience analysis. Annu Rev Neurosci. 2011;34:601–628. doi: 10.1146/annurev-neuro-060909-153200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erdoes G, Basciani RM, Eberle B. Etomidate–a review of robust evidence for its use in various clinical scenarios. Acta Anaesthesiol Scand. 2014;58:380–389. doi: 10.1111/aas.12289. [DOI] [PubMed] [Google Scholar]
- 13.Ho J, et al. Molecular recognition of ketamine by a subset of olfactory G protein-coupled receptors. Sci Signal. 2015;8:ra33. doi: 10.1126/scisignal.2005912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li L, Vlisides PE. Ketamine: 50 Years of modulating the mind. Front Hum Neurosci. 2016;10:612. doi: 10.3389/fnhum.2016.00612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaech S, Brinkhaus H, Matus A. Volatile anesthetics block actin-based motility in dendritic spines. Proc Natl Acad Sci USA. 1999;96:10433–10437. doi: 10.1073/pnas.96.18.10433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turina D, Bjornstrom K, Sundqvist T, Eintrei C. Propofol alters vesicular transport in rat cortical neuronal cultures. J Physiol Pharmacol. 2011;62:119–124. [PubMed] [Google Scholar]
- 17.Emerson DJ, et al. Direct modulation of microtubule stability contributes to anthracene general anesthesia. J Am Chem Soc. 2013;135:5389–5398. doi: 10.1021/ja311171u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Craddock TJ, et al. Computational predictions of volatile anesthetic interactions with the microtubule cytoskeleton: Implications for side effects of general anesthesia. PLoS One. 2012;7:e37251. doi: 10.1371/journal.pone.0037251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Craddock TJ, Priel A, Tuszynski JA. Keeping time: Could quantum beating in microtubules be the basis for the neural synchrony related to consciousness? J Integr Neurosci. 2014;13:293–311. doi: 10.1142/S0219635214400019. [DOI] [PubMed] [Google Scholar]
- 20.Miki H, Setou M, Kaneshiro K, Hirokawa N. All kinesin superfamily protein, KIF, genes in mouse and human. Proc Natl Acad Sci USA. 2001;98:7004–7011. doi: 10.1073/pnas.111145398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hirokawa N, Niwa S, Tanaka Y. Molecular motors in neurons: Transport mechanisms and roles in brain function, development, and disease. Neuron. 2010;68:610–638. doi: 10.1016/j.neuron.2010.09.039. [DOI] [PubMed] [Google Scholar]
- 22.Verhey KJ, Kaul N, Soppina V. Kinesin assembly and movement in cells. Annu Rev Biophys. 2011;40:267–288. doi: 10.1146/annurev-biophys-042910-155310. [DOI] [PubMed] [Google Scholar]
- 23.Scholey JM. Kinesin-2: A family of heterotrimeric and homodimeric motors with diverse intracellular transport functions. Annu Rev Cell Dev Biol. 2013;29:443–469. doi: 10.1146/annurev-cellbio-101512-122335. [DOI] [PubMed] [Google Scholar]
- 24.Maday S, Twelvetrees AE, Moughamian AJ, Holzbaur EL. Axonal transport: Cargo-specific mechanisms of motility and regulation. Neuron. 2014;84:292–309. doi: 10.1016/j.neuron.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bentley M, Banker G. The cellular mechanisms that maintain neuronal polarity. Nat Rev Neurosci. 2016;17:611–622. doi: 10.1038/nrn.2016.100. [DOI] [PubMed] [Google Scholar]
- 26.Sardar HS, Luczak VG, Lopez MM, Lister BC, Gilbert SP. Mitotic kinesin CENP-E promotes microtubule plus-end elongation. Curr Biol. 2010;20:1648–1653. doi: 10.1016/j.cub.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guzik-Lendrum S, et al. Kinesin-2 KIF3AC and KIF3AB can drive long-range transport along microtubules. Biophys J. 2015;109:1472–1482. doi: 10.1016/j.bpj.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tomishige M, Vale RD. Controlling kinesin by reversible disulfide cross-linking. Identifying the motility-producing conformational change. J Cell Biol. 2000;151:1081–1092. doi: 10.1083/jcb.151.5.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yildiz A, Tomishige M, Gennerich A, Vale RD. Intramolecular strain coordinates kinesin stepping behavior along microtubules. Cell. 2008;134:1030–1041. doi: 10.1016/j.cell.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hirokawa N, Noda Y, Tanaka Y, Niwa S. Kinesin superfamily motor proteins and intracellular transport. Nat Rev Mol Cell Biol. 2009;10:682–696. doi: 10.1038/nrm2774. [DOI] [PubMed] [Google Scholar]
- 31.Albracht CD, Rank KC, Obrzut S, Rayment I, Gilbert SP. Kinesin-2 KIF3AB exhibits novel ATPase characteristics. J Biol Chem. 2014;289:27836–27848. doi: 10.1074/jbc.M114.583914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Woll KA, et al. Role for the propofol hydroxyl in anesthetic protein target molecular recognition. ACS Chem Neurosci. 2015;6:927–935. doi: 10.1021/acschemneuro.5b00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kull FJ, Sablin EP, Lau R, Fletterick RJ, Vale RD. Crystal structure of the kinesin motor domain reveals a structural similarity to myosin. Nature. 1996;380:550–555. doi: 10.1038/380550a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gigant B, et al. Structure of a kinesin-tubulin complex and implications for kinesin motility. Nat Struct Mol Biol. 2013;20:1001–1007. doi: 10.1038/nsmb.2624. [DOI] [PubMed] [Google Scholar]
- 35.Cao L, et al. The structure of apo-kinesin bound to tubulin links the nucleotide cycle to movement. Nat Commun. 2014;5:5364. doi: 10.1038/ncomms6364. [DOI] [PubMed] [Google Scholar]
- 36.Shang Z, et al. High-resolution structures of kinesin on microtubules provide a basis for nucleotide-gated force-generation. eLife. 2014;3:e04686. doi: 10.7554/eLife.04686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morikawa M, et al. X-ray and Cryo-EM structures reveal mutual conformational changes of Kinesin and GTP-state microtubules upon binding. EMBO J. 2015;34:1270–1286. doi: 10.15252/embj.201490588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Woehlke G, et al. Microtubule interaction site of the kinesin motor. Cell. 1997;90:207–216. doi: 10.1016/s0092-8674(00)80329-3. [DOI] [PubMed] [Google Scholar]
- 39.Klumpp LM, et al. Microtubule-kinesin interface mutants reveal a site critical for communication. Biochemistry. 2004;43:2792–2803. doi: 10.1021/bi035830e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Klumpp LM, Hoenger A, Gilbert SP. Kinesin’s second step. Proc Natl Acad Sci USA. 2004;101:3444–3449. doi: 10.1073/pnas.0307691101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rice S, et al. A structural change in the kinesin motor protein that drives motility. Nature. 1999;402:778–784. doi: 10.1038/45483. [DOI] [PubMed] [Google Scholar]
- 42.Asbury CL, Fehr AN, Block SM. Kinesin moves by an asymmetric hand-over-hand mechanism. Science. 2003;302:2130–2134. doi: 10.1126/science.1092985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaseda K, Higuchi H, Hirose K. Alternate fast and slow stepping of a heterodimeric kinesin molecule. Nat Cell Biol. 2003;5:1079–1082. doi: 10.1038/ncb1067. [DOI] [PubMed] [Google Scholar]
- 44.Yildiz A, Tomishige M, Vale RD, Selvin PR. Kinesin walks hand-over-hand. Science. 2004;303:676–678. doi: 10.1126/science.1093753. [DOI] [PubMed] [Google Scholar]
- 45.Mayer TU, et al. Small molecule inhibitor of mitotic spindle bipolarity identified in a phenotype-based screen. Science. 1999;286:971–974. doi: 10.1126/science.286.5441.971. [DOI] [PubMed] [Google Scholar]
- 46.Kapoor TM, Mayer TU, Coughlin ML, Mitchison TJ. Probing spindle assembly mechanisms with monastrol, a small molecule inhibitor of the mitotic kinesin, Eg5. J Cell Biol. 2000;150:975–988. doi: 10.1083/jcb.150.5.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yan Y, et al. Inhibition of a mitotic motor protein: Where, how, and conformational consequences. J Mol Biol. 2004;335:547–554. doi: 10.1016/j.jmb.2003.10.074. [DOI] [PubMed] [Google Scholar]
- 48.Ulaganathan V, et al. Structural insights into a unique inhibitor binding pocket in kinesin spindle protein. J Am Chem Soc. 2013;135:2263–2272. doi: 10.1021/ja310377d. [DOI] [PubMed] [Google Scholar]
- 49.Wood KW, et al. Antitumor activity of an allosteric inhibitor of centromere-associated protein-E. Proc Natl Acad Sci USA. 2010;107:5839–5844. doi: 10.1073/pnas.0915068107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Klumpp LM, Brendza KM, Rosenberg JM, Hoenger A, Gilbert SP. Motor domain mutation traps kinesin as a microtubule rigor complex. Biochemistry. 2003;42:2595–2606. doi: 10.1021/bi026715r. [DOI] [PubMed] [Google Scholar]
- 51.Tischfield MA, et al. Human TUBB3 mutations perturb microtubule dynamics, kinesin interactions, and axon guidance. Cell. 2010;140:74–87. doi: 10.1016/j.cell.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Djagaeva I, et al. Three routes to suppression of the neurodegenerative phenotypes caused by kinesin heavy chain mutations. Genetics. 2012;192:173–183. doi: 10.1534/genetics.112.140798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Niwa S, Takahashi H, Hirokawa N. β-Tubulin mutations that cause severe neuropathies disrupt axonal transport. EMBO J. 2013;32:1352–1364. doi: 10.1038/emboj.2013.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Minoura I, et al. Reversal of axonal growth defects in an extraocular fibrosis model by engineering the kinesin-microtubule interface. Nat Commun. 2016;7:10058. doi: 10.1038/ncomms10058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Falk MJ, Kayser EB, Morgan PG, Sedensky MM. Mitochondrial complex I function modulates volatile anesthetic sensitivity in C. elegans. Curr Biol. 2006;16:1641–1645. doi: 10.1016/j.cub.2006.06.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen ZW, et al. A neurosteroid analogue photolabeling reagent labels the colchicine-binding site on tubulin: A mass spectrometric analysis. Electrophoresis. 2012;33:666–674. doi: 10.1002/elps.201100434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zalucki OH, et al. Syntaxin1A-mediated resistance and hypersensitivity to isoflurane in Drosophila melanogaster. Anesthesiology. 2015;122:1060–1074. doi: 10.1097/ALN.0000000000000629. [DOI] [PubMed] [Google Scholar]
- 58.MacDonald JI, et al. Nesca, a novel neuronal adapter protein, links the molecular motor kinesin with the pre-synaptic membrane protein, syntaxin-1, in hippocampal neurons. J Neurochem. 2012;121:861–880. doi: 10.1111/j.1471-4159.2012.07729.x. [DOI] [PubMed] [Google Scholar]
- 59.Huang CF, Banker G. The translocation selectivity of the kinesins that mediate neuronal organelle transport. Traffic. 2012;13:549–564. doi: 10.1111/j.1600-0854.2011.01325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu XA, et al. New approach to capture and characterize synaptic proteome. Proc Natl Acad Sci USA. 2014;111:16154–16159. doi: 10.1073/pnas.1401483111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van Swinderen B, et al. A neomorphic syntaxin mutation blocks volatile-anesthetic action in Caenorhabditis elegans. Proc Natl Acad Sci USA. 1999;96:2479–2484. doi: 10.1073/pnas.96.5.2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nagele P, et al. Volatile anesthetics bind rat synaptic snare proteins. Anesthesiology. 2005;103:768–778. doi: 10.1097/00000542-200510000-00015. [DOI] [PubMed] [Google Scholar]
- 63.Cai Q, Pan PY, Sheng ZH. Syntabulin-kinesin-1 family member 5B-mediated axonal transport contributes to activity-dependent presynaptic assembly. J Neurosci. 2007;27:7284–7296. doi: 10.1523/JNEUROSCI.0731-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nakajima K, et al. Molecular motor KIF5A is essential for GABA(A) receptor transport, and KIF5A deletion causes epilepsy. Neuron. 2012;76:945–961. doi: 10.1016/j.neuron.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 65.Tanaka Y, et al. Targeted disruption of mouse conventional kinesin heavy chain, kif5B, results in abnormal perinuclear clustering of mitochondria. Cell. 1998;93:1147–1158. doi: 10.1016/s0092-8674(00)81459-2. [DOI] [PubMed] [Google Scholar]
- 66.Marszalek JR, Ruiz-Lozano P, Roberts E, Chien KR, Goldstein LS. Situs inversus and embryonic ciliary morphogenesis defects in mouse mutants lacking the KIF3A subunit of kinesin-II. Proc Natl Acad Sci USA. 1999;96:5043–5048. doi: 10.1073/pnas.96.9.5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hirokawa N, Tanaka Y. Kinesin superfamily proteins (KIFs): Various functions and their relevance for important phenomena in life and diseases. Exp Cell Res. 2015;334:16–25. doi: 10.1016/j.yexcr.2015.02.016. [DOI] [PubMed] [Google Scholar]
- 68.Lindhout DA, Litowski JR, Mercier P, Hodges RS, Sykes BD. NMR solution structure of a highly stable de novo heterodimeric coiled-coil. Biopolymers. 2004;75:367–375. doi: 10.1002/bip.20150. [DOI] [PubMed] [Google Scholar]
- 69.Rank KC, et al. Kar3Vik1, a member of the kinesin-14 superfamily, shows a novel kinesin microtubule binding pattern. J Cell Biol. 2012;197:957–970. doi: 10.1083/jcb.201201132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bu W, Su LK. Characterization of functional domains of human EB1 family proteins. J Biol Chem. 2003;278:49721–49731. doi: 10.1074/jbc.M306194200. [DOI] [PubMed] [Google Scholar]
- 71.Komaki S, et al. Nuclear-localized subtype of end-binding 1 protein regulates spindle organization in Arabidopsis. J Cell Sci. 2010;123:451–459. doi: 10.1242/jcs.062703. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.