Abstract
Single and mixed starter cultures of lactic acid bacteria (LAB): Weissella confusa MNC20, Lactobacillus plantarum MNC21, Lactococcus lactis MNC24 and Lactobacillus fermentum MNC34 and yeasts: Issatchenkia orientalis MNC20Y and Saccharomyces cerevisiae MNC21Y were used to produce Obushera, a fermented sorghum beverage. Microbial counts, pH, sugars, organic acids, and volatile compounds in starter culture and spontaneous fermentations were monitored during 48 hrs. Maximum counts of LAB (8.4–9.4 log cfu g−1) and yeasts (7.5 ± 0.1 cfu g−1) starter cultures were attained in 6–48 hrs. Weissella confusa, Lc. lactis, and Lb. fermentum showed possible acid sensitivity while I. orientalis produced surface films. LAB starter cultures and their combinations with S. cerevisiae lowered pH from 5.83 to <4.5 (3.50–4.13) in a shorter time (12 hrs) than spontaneous fermentations (24 hrs). Lactococcus lactis and W. confusa metabolized glucose the fastest (p < .05) during the first 6 hrs. Lactobacillus fermentum, Lb. plantarum, and S. cerevisiae utilized glucose and maltose concurrently. Lactobacillus plantarum and S. cerevisiae additionally utilized fructose. S. cerevisiae metabolized sugars the fastest (p < .05) during the first 12–24 hrs. Lactobacillus plantarum and W. confusa produced the highest (p < .05) amounts of lactate (5.43 g kg−1) and diacetyl (9.5 mg kg−1), respectively. LAB also produced acetate, ethanol, acetaldehyde, acetone, and acetoin. Coculturing LAB with S. cerevisiae reduced (p < .05) lactate and diacetyl yield. Yeasts produced high amounts of acetaldehyde and methyl alcohols. Issatchenkia orientalis produced higher (p < .05) amounts of 2‐methy‐1‐propanol and 3‐methyl‐1‐butanol than S. cerevisiae. Combinations of LAB with S. cerevisiae produced a profile flavor compounds close to that of spontaneously fermented Obushera. These combinations can be adopted for controlled fermentation of Obushera and related fermented cereal products.
Keywords: Cereal fermentation, lactic acid bacteria, Obushera, sorghum, starter cultures, Yeasts
1. Introduction
Traditional fermented cereal products are important in nutrition as sources of carbohydrates, proteins, fiber, minerals, and vitamins (Nout, 2009). In different parts of Africa, the main cereals: maize, sorghum, and millet – are widely used to produce fermented nonalcoholic and alcoholic foods such as Mageu (Holzapfel & Taljaard, 2004), Togwa (Mugula, Narvhus, & Sørhaug, 2003), Gowé (Vieira‐Dalodé et al., 2007), Poto poto and Degué (Abriouel et al., 2006), and Obushera (Mukisa, Muyanja, Byaruhanga, Langsrud, & Narvhus, 2012) among others. There is great interest in industrializing traditional fermented products from different parts of the world, including Africa (Tamang & Kailasapathy, 2010).
Relying on natural fermentation is a major hindrance to large‐scale commercial processing of traditional fermented products mainly because natural fermentation is associated with variations in product quality and safety (Holzapfel, 2002). Starter cultures with desirable properties can be identified and applied to ensure reduction in processing time, consistent product quality, and safety (Holzapfel, 2002). However, selecting appropriate starter cultures requires understanding the microbial diversity of the products and the roles played by specific organisms.
Lactic acid bacteria (LAB) and yeasts are the major microbes involved in the fermentation of various traditional cereal products (Holzapfel, 2002; Nout, 2009). Culture‐ independent techniques revealed the predominance of LAB, including Lactobacillus (Lb) plantarum, Lb. fermentum, Pediococcus (P). pentosaceus, Lb. delbrueckii, Lb. casei, Lb. curvatus, Lactococcus (Lc.) lactis, Weissella (W) confusa and W. cibaria; and yeasts, including: Saccharomyces cerevisiae, Issatchenkia orientalis, Candida spp, and Pichia spp (Ampe, ben Omar, Moizan, Wacher, & Guyot, 1999; Madoroba et al., 2011; Mukisa, Porcellato, et al., 2012; ben Omar & Ampe, 2000).
Some studies evaluating starter cultures in traditional cereal fermentations focused on LAB and mostly on their ability to rapidly acidify the products (Agarry, Nkama, & Akoma, 2010; Muyanja, Narvhus, & Langsrud, 2012; Sekwati‐Monang & Gänzle, 2011). Others evaluated combinations of LAB and yeasts (Halm, Osei‐Yaw, Hayford, Kpodo, & Amoa‐Awua, 1996; Mugula et al., 2003; Omemu, Oyewole, & Bankole, 2007; Orji, Mbata, Aniche, & Ahonkhai, 2003). Products fermented with both LAB and yeasts have a flavor profile (Mugula et al., 2003) and organoleptic properties comparable to those of naturally fermented products (Halm et al., 1996; Masha, Ipsen, Petersen, & Jakobsen, 1998; Orji et al., 2003).
The flavor profile of cereal fermented products is composed of sugars, organic acids, aldehydes, ketones, alcohols, and esters (Mugula et al., 2003; Mukisa et al., 2012; Muyanja, Narvhus, & Langsrud, 2012). In sourdough fermentation, LAB are mainly responsible for acidification while yeasts produce ethanol, methyl alcohols, methyl aldehydes, and acetaldehyde among other volatiles (Gobbetti et al., 1995; Hansen & Hansen, 1996). The contribution of LAB toward production of volatile organic compounds in traditional African cereal fermented products has not been given much attention. Although the interaction between LAB and yeasts is known to enhance growth of either group of microbes (Mugula et al., 2003; Omemu et al., 2007), the effect of this interaction on flavor production has not been reported. Furthermore, differences in production of volatile organic compounds by the yeasts have also not been evaluated.
This study evaluated the individual and interactive effects of the dominant yeasts: S. cerevisiae and I. orientalis and the different LAB (Lb. plantarum, Lb. fermentum, W. confusa, Lc. lactis) on the flavor profile of traditional fermented cereals. Obushera, of the Obutoko type, a popular Ugandan fermented beverage prepared from malted sorghum (Mukisa, 2012) was used as a model for comparison. Information generated from this study will enable the identification of potential starter cultures for traditional fermented cereal products.
2. Materials and Methods
2.1. Bacteria and yeast starter cultures
Lactobacillus plantarum MNC 21, W. confusa MNC 20, Lc. lactis MNC 24, Lb. fermentum MNC 34, I. orientalis MNC 20Y and S. cerevisiae MNC 21Y (Gene bank accession numbers: JF512470, JQ754455, JF512471, JQ754464, JQ754435, and JQ754436, respectively) were used in this study. These LAB and yeasts were previously isolated from Obushera and identified by sequencing 16S rRNA and the ITS1‐5.8S ‐ITS2 region of rRNA, respectively (Mukisa, Porcellato, et al., 2012). The above strains were selected for this study since they belong to the dominant genera and species involved in the fermentation of Obushera (Mukisa et al., 2012). The LAB and yeasts were initially used as single starter cultures and later as mixed starter cultures. Mixed starter cultures included a primarily homolactic combination of Lb. plantarum + Lc. lactis. Coculturing Lc. lactis with Lb. plantarum was previously observed to enhance acid production in a cereal fermentation (Mukisa, Byaruhanga, et al., 2012). This starter culture therefore acted as a basis for two heterolactic combinations: Lb. plantarum + Lc. lactis + W. confusa and Lb. plantarum + Lc. lactis + Lb. fermentum. The yeast strain S. cerevisiae was used in two LAB/yeast mixed starter cultures: Lb. plantarum + Lc. lactis + S. cerevisiae (with homolactic LAB) and Lb. plantarum + W. confusa + S. cerevisiae (with homolactic and heterolactic LAB). I. orientalis was not used in the mixed starter cultures because it formed undesirable white films in the product.
Pure LAB and yeast isolates were maintained at −80°C in broth containing 15% glycerol (v/v): MRS (Merck KGaA, Darmstadt, Germany) and tryptone glucose yeast extract broth (Oxoid Ltd, Hampshire, UK), respectively. Starter cultures for inoculation were prepared by growing strains in 250 ml of respective broths at 30°C for 24 hrs. Cells were recovered by centrifugation (7,500 × g for 10 min at 4°C) and subsequently rinsed with sterile distilled water. The cell pellet was resuspended in 25 ml of Ringer's solution containing 15% glycerol and stored in aliquots at −80°C. The resulting suspension contained approximately 109 cfu ml−1 (for LAB) or 107 cfu ml−1 (for yeasts) determined by taking plate counts on de Man, Rogosa and Sharpe (MRS) agar and Rose Bengal Chloramphenicol agar (RBCA), respectively (Merck KGaA, Darmstadt, Germany).
2.2. Preparation of Obushera samples
Obushera of the Obutoko type (from malted sorghum) was prepared using flour from red sorghum (Sorghum bicolor (L.) Moench) of the Sekedo variety. One batch of sorghum malt was prepared as described earlier (Mukisa, Muyanja, Byaruhanga, Schüller et al., 2012). The formulation described by Mukisa et al. (2010) was used to prepare Obushera. Briefly, slurries were made by mixing 50 g of sorghum malt in 400 ml of sterile distilled water held in 1 L glass jars. The slurries were heated in a water bath to 90°C, held for 10 min and then cooled to 30°C prior to inoculation. The slurries were then inoculated with single or mixed starter cultures resulting in initial cell concentrations of 6.6 ± 0.2 log cfu g−1 and 4.5 ± 0.3 log cfu g−1 for individual LAB and yeasts strains, respectively (Mukisa, 2012). Natural fermentation was initiated by adding 9 g of sorghum malt (2% malt) to the slurries (Mukisa et al., 2010). After inoculation, samples were homogenized for 1 min in an Omni mixer (Omni International, Waterbury, USA). Approximately 100 ml of sample was aseptically transferred into 200 ml sterile glass jars and incubated at 30°C. Samples were taken at t = 0, 6, 12, 24, and 48 hrs for the analysis of pH, titratable acidity, sugars, organic acids, volatile organic compounds, and microbial counts. Each sampling time was allocated a separate container. Three independent fermentations were made for each starter culture combination.
2.3. Microbial counts
LAB and yeast counts were determined by plating appropriate serial dilutions of Obushera in ¼ strength Ringer's solution on MRS agar and RBCA, respectively. LAB counts were taken after incubation at 30°C for 2–3 days while yeasts were counted after incubation at 25°C for 2–5 days.
2.4. Determination of pH, sugars, organic acids, and volatile organic compounds
The pH of Obushera was measured, using a pH meter (PHM61, Radiometer, Copenhagen, Denmark) equipped with a glass electrode (Type PHC2001‐8, Radiometer Analytical SAS, Villeurbanne Cedex, France). Concentrations of sugars and organic acids were determined with high performance liquid chromatography (HPLC) as previously described (Mukisa, 2012; Narvhus, Østeraas, Mutukumira, & Abrahamsen, 1998). Volatile organic compounds were analyzed by automatic headspace gas chromatography (HS‐GC), using the method described by Narvhus et al. (1998). The compounds analyzed constitute the main flavor compounds in traditional African cereal products (Mugula et al., 2003; Mukisa, 2012; Muyanja, Narvhus, & Langsrud, 2012).
2.5. Data analysis
Results were presented as arithmetic means ± standard deviations (Mean ± SD) of three independent fermentations. Data were subjected to one‐way analysis of variance (ANOVA) to test for significant differences at p = .05. Mean comparisons were made, using the Fisher's least significant difference (LSD) test to determine which means were significantly different.
3. Results
3.1. Growth of starter cultures in Obushera
The study evaluated the ability of the individual LAB and yeast starter cultures to grow in Obushera to levels that have been reported in previous studies on naturally and starter culture fermented cereal beverages. Individual LAB and yeast starter cultures grew in Obushera increasing in numbers from 6.6 to 8.4–9.4 log cfu g−1 and 4.5 to 7.6 log cfu g−1, respectively within 6–24 hrs (Figure 1a). In contrast LAB and yeast counts in naturally fermented Obushera increased from 5.0 log cfu g−1 to 8.9 log cfu g−1 after 24 hrs and 5.2 log cfu g−1 to 7.6 log cfu g−1 in 24–48 hrs, respectively (results not shown). Lactococcus lactis and W. confusa attained maximum counts the earliest (8.4–8.8 log cfu g−1 in 6 hrs). Counts of Lc. lactis, W. confusa, and Lb. fermentum decreased significantly (p < .05) by 1.3–2.0 log cfu g−1 between 6 and 48 hrs while those of Lb. plantarum were stable at 9.0 ± 0.1 log cfu g−1 after 12 hrs. Issatchenkia orientalis produced visually disagreeable surface films, a characteristic which was also observed in spontaneously fermented Obushera kept beyond 48 hrs.
Figure 1.
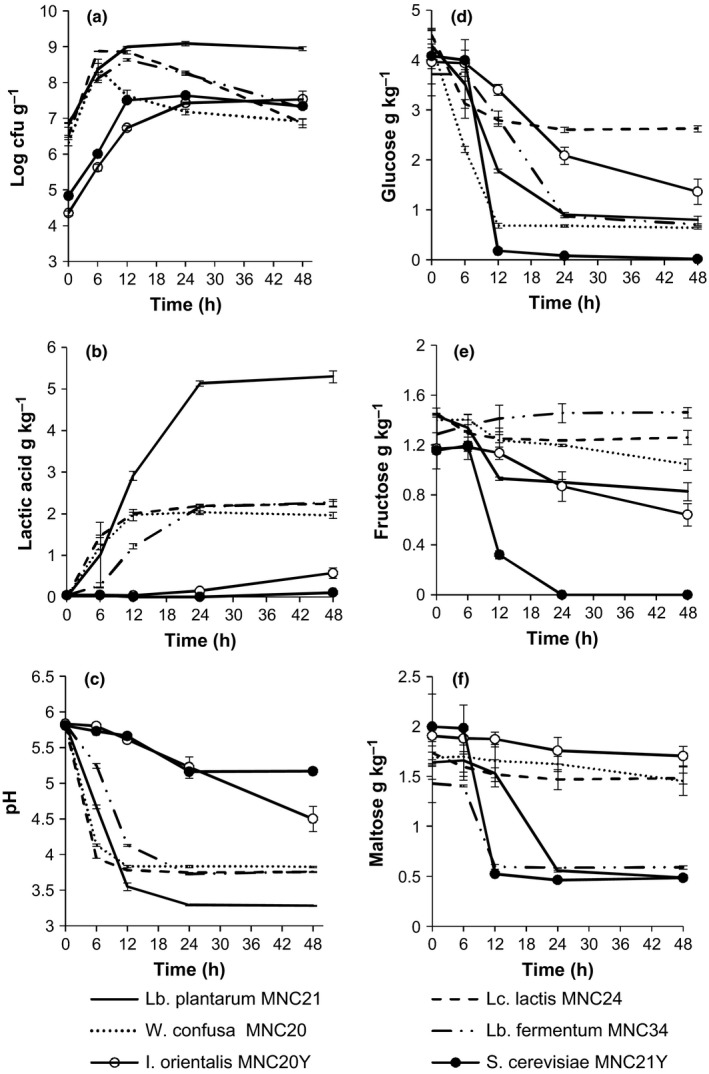
Changes in microbial counts (a), lactic acid (b), pH (c) and sugars: glucose (d), fructose (e), and maltose (f) during fermentation of Obushera inoculated with single lactic acid bacteria (LAB) and yeast starter cultures. Error bars show standard deviations of three independent fermentations
3.2. Sugar utilization by LAB and yeast starter cultures
The main sugars observed in Obushera were maltose, glucose, and fructose. Spontaneously fermented samples (inoculated with 2% malt) contained significantly higher (p < .05) starting amounts of maltose (16.5 g kg−1), glucose (5.7 g kg−1) and fructose (1.8 g kg−1) (Figure 2d, e and f) than starter culture fermented samples (1.8 g kg−1 of maltose, 4.3 g kg−1 of glucose, and 1.4 g kg−1 of fructose) (Figure 1d, e and f; Figure 2d, e and f). All single starter cultures utilized glucose while Lb. plantarum, Lb. fermentum, and S. cerevisiae in addition simultaneously catabolized maltose (Figure 1d, e and f). Lactobacillus fermentum, Lc. lactis, and I. orientalis did not utilize fructose during fermentation. Lactococcus lactis and W. confusa metabolized glucose significantly (p < .05) faster than the other starter cultures during the first 6 hrs. Saccharomyces cerevisiae had the highest rate (p < .05) of breakdown of glucose, maltose, and fructose (reduced these to a minimum in 12–24 hrs).
Figure 2.
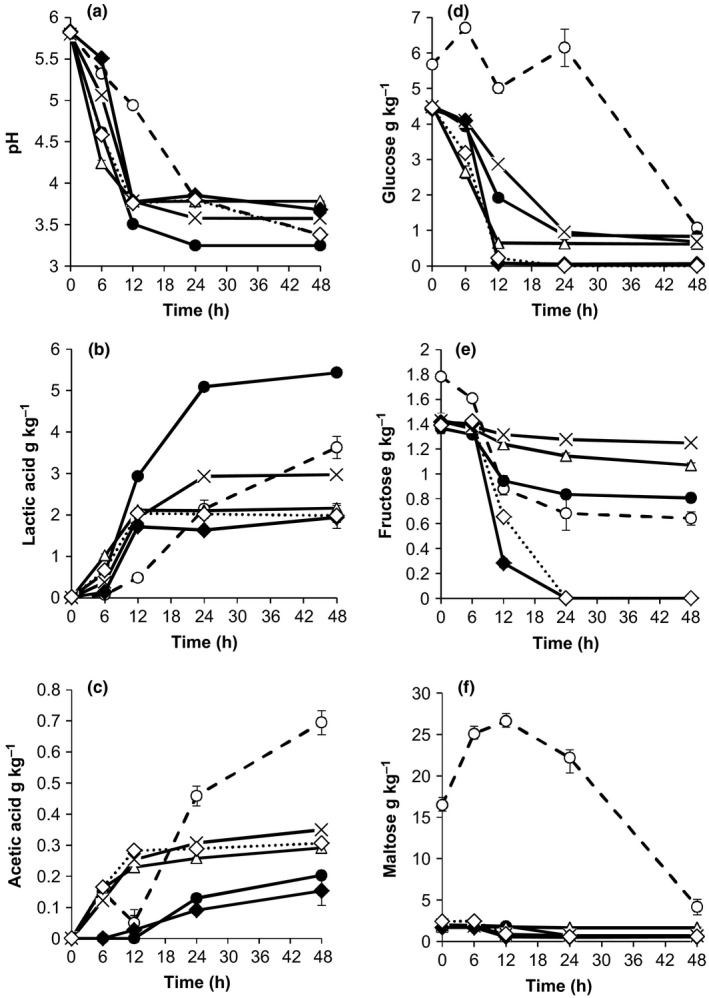
Changes in pH (a), lactate (b), acetate (c), glucose (d), fructose (e), and maltose (f) during fermentation of Obushera inoculated with sorghum malt (spontaneous fermentation) (ο) or mixed starter cultures: Lb. plantarum + Lc. lactis (•); Lb. plantarum + Lc. lactis + W. confusa (Δ); Lb. plantarum + Lc. lactis + Lb. fermentum (×); Lb. plantarum + Lc. lactis + S. cerevisiae (♦); Lb. plantarum + Lc. lactis + W. confusa + S. cerevisiae (⋄). Error bars show standard deviations of three independent fermentations
3.3. Lactate and acetate production
Lactate and acetate were the main organic acids detected in Obushera. Lactate in spontaneously fermented Obushera increased to a maximum of 3.63 g kg−1 after 48 hrs (Figure 2b). Individual and mixed LAB starter cultures produced between 1.72 and 5.30 g kg−1 lactic acid after 12–48 hrs and in effect decreased pH from 5.8 to 3.55–3.83 within 12 hrs. However, Lb. fermentum monocultures and spontaneous fermentation took 24 hrs to attain similar pH values (Figure 1c and 2a). As for the yeasts, only I. orientalis produced a significant (p < .05) amount of lactate (0.57 g kg−1) after 48 hrs and thus reduced pH to 4.5 ± 0.2 (Figure 1b and c). Lactic acid production by Lb. plantarum was significantly (p < .05) higher than for Lb. fermentum and W. confusa and Lc. lactis (Figure 2b). However, monocultures of W. confusa and Lc. lactis showed significantly faster (p < .05) lactate production during the first 6 hrs and in effect lowered the pH of Obushera from 5.83 to 3.95–4.12 (Figure 1b and c). Acetate in spontaneously fermented Obushera increased to a maximum of 0.70 g kg−1 after 48 hrs (Figure 2c). Lactobacillus fermentum and W. confusa individually, or in cocultures produced significantly (p < .05) higher amounts of acetic acid (0.25–0.35 g kg−1) than the rest of the starter cultures (≈ 0.1 g kg−1) (Figure 2). The Lb. plantarum and Lb. plantarum + Lc. lactis cultures produced the highest amounts of lactate (p < .05) (Figure 1 and 2). However, co‐culturing the Lb. plantarum + Lc. lactis starter culture with S. cerevisiae, W. confusa or Lb. fermentum significantly (p < .05) reduced lactate yields from about 5 g kg−1 to 2 g kg−1(Figure 2b).
3.4. Production of ethanol
Ethanol was the major alcohol produced in spontaneously fermented samples and ranged from 0.62 g kg−1 to 2.00 g kg−1 between 12 and 48 hrs (Table 2 and Figure 4). Only monocultures of Lb. fermentum, W. confusa, I. orientalis and S. cerevisiae produced ethanol with the later producing the highest (p < .05) amounts (1.55 g kg−1) compared to the rest (0.79–0.88 g kg−1) (Table 2). Starter cultures with S. cerevisiae showed the highest rate of ethanol production reaching peak concentrations (1.4–1.56 g kg−1) after 12 hrs (Figure 4). Ethanol production by S. cerevisiae was not affected by co‐culturing the yeast with LAB.
Table 2.
Changes in concentrations of alcohols, resulting from single starter culture and spontaneous fermentation of Obushera after 24 hrs
| Starter culture | Ethanol(g kg−1) | 2‐ME‐pro‐ol(mg kg−1) | 2‐ME‐but‐ol(mg kg−1) | 3‐ME‐but‐ol(mg kg−1) |
|---|---|---|---|---|
| Un‐fermented (T = 0 hr) | 0.01 ± 0.00a | 0.04 ± 0.00a | 0.02 ± 0.01a | 0.08 ± 0.06a |
| Spontaneous fermentation | 1.29 ± 0.04b | 1.82 ± 0.46b | 0.25 ± 0.02b | 1.17 ± 0.03b |
| Lb. plantarum MNC 21 | 0.01 ± 0.00a | 0.11 ± 0.01a | 0.07 ± 0.01a | 0.40 ± 0.02c |
| Lc. lactis MNC 24 | 0.01 ± 0.00a | 0.04 ± 0.00a | 0.03 ± 0.01a | 0.07 ± 0.01a |
| Lb. fermentum MNC 34 | 0.88 ± 0.03c | 0.06 ± 0.01a | 0.04 ± 0.01a | 0.15 ± 0.01ac |
| W. confusa MNC 20 | 0.79 ± 0.08d | 0.06 ± 0.00a | 0.03 ± 0.01a | 0.17 ± 0.05ac |
| I. orientalis MNC 20Y | 0.83 ± 0.10 cd | 5.18 ± 0.47c | 1.09 ± 0.17c | 8.47 ± 0.60d |
| S. cerevisiae MNC 20Y | 1.55 ± 0.01e | 2.78 ± 0.05d | 1.10 ± 0.09c | 5.53 ± 0.01e |
Values are means ± standard deviations of three independent fermentations. Values in the same column with similar superscripts (a – e) are not significantly different (p > .05). ND: not detected. 2‐ME‐pro‐ol (2‐methyl‐1‐propanol); 2‐ME‐but‐ol (2‐methyl‐1‐butanol); 3‐ME‐but‐ol (3‐methyl‐1‐butanol).
Figure 4.
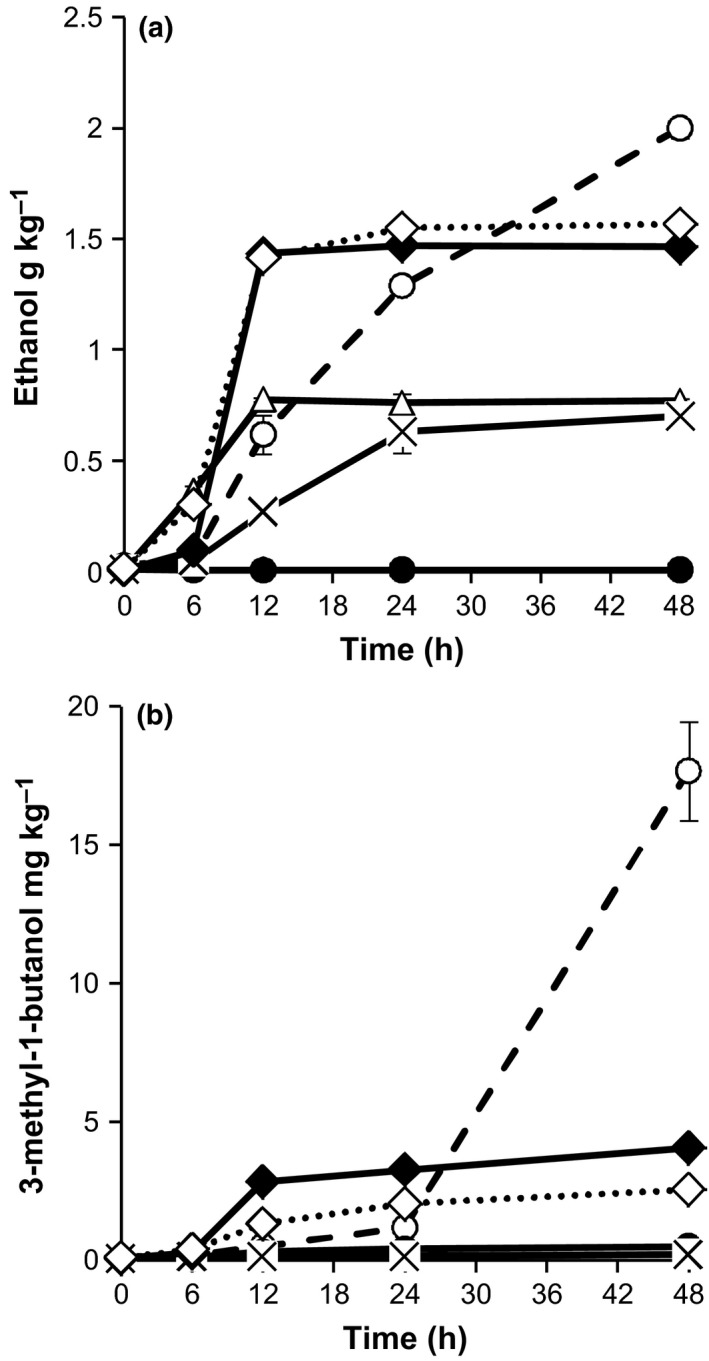
Changes in concentrations of ethanol (a) and 3‐methyl‐1‐butanol (b) during fermentation of Obushera inoculated with sorghum malt (spontaneous fermentation) (ο) or starter cultures: Lb. plantarum + Lc. lactis (•); Lb. plantarum + Lc. lactis + W. confusa (Δ); Lb. plantarum + Lc. lactis + Lb. fermentum (×); Lb. plantarum + Lc. lactis + S. cerevisiae (♦); Lb. plantarum + Lc. lactis + W. confusa + S. cerevisiae (⋄). Error bars show standard deviations of three independent fermentations
3.5. Production of acetaldehyde
Acetaldehyde reached a maximum concentration of 4.46 mg kg−1 in spontaneously fermented samples (Figure 3a). Issatchenkia orientalis and S. cerevisiae produced the highest (p < .05) amounts of acetaldehyde (8.99–10.69 mg kg−1) followed by Lb. plantarum (2.14 mg kg−1) and Lc. lactis (1.76 mg kg−1) (Table 1).
Figure 3.
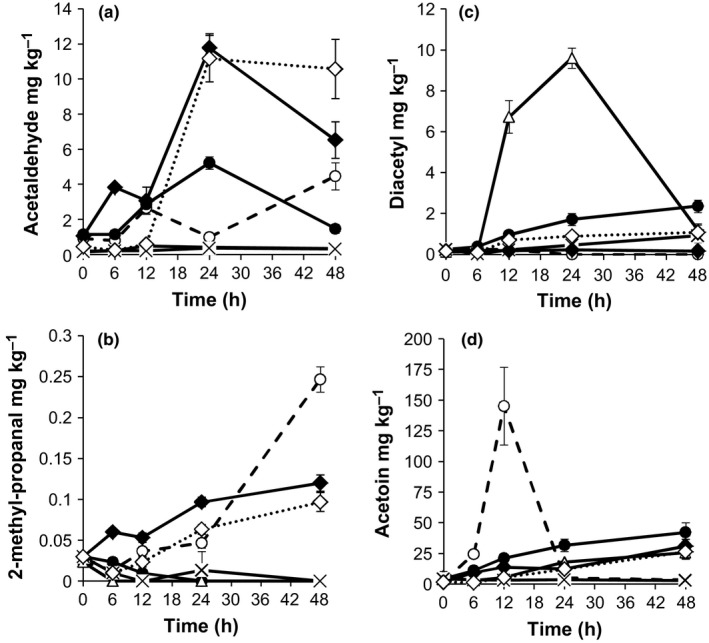
Changes in concentrations of acetaldehyde (a), 2‐methyl‐1‐propanal (b), diacetyl (c), and acetoin (d) during fermentation of Obushera inoculated with sorghum malt (spontaneous fermentation) (ο) or starter cultures: Lb. plantarum + Lc. lactis (•); Lb. plantarum + Lc. lactis + W. confusa (Δ); Lb. plantarum + Lc. lactis + Lb. fermentum (×); Lb. plantarum + Lc. lactis + S. cerevisiae (♦); Lb. plantarum + Lc. lactis + W. confusa + S. cerevisiae (⋄). Error bars show standard deviations of three independent fermentations
Table 1.
Changes in concentrations of aldehydes and ketones resulting from single starter culture and spontaneous fermentation of Obushera, a sorghum malt beverage after 24 hrs
| Starter culture | Aldehydes(mg kg−1) | Ketones(mg kg−1) | |||||
|---|---|---|---|---|---|---|---|
| Acetald | 2‐ME‐pro‐al | 2‐ME‐but‐al | 3‐ME‐but‐al | Acetone | Diacetyl | Acetoin | |
| Un‐inoculated (T = 0 h) | 0.67 ± 0.42a | 0.03 ± 0.02a | 0.04 ± 0.01a | 0.06 ± 0.03a | 0.15 ± 0.08a | 0.17 ± 0.06a | 4.11 ± 2.70a |
| Spontaneous fermentation | 0.98 ± 0.03bab | 0.05 ± 0.01a | 0.01 ± 0.00b | ND | 0.30 ± 0.05ab | ND | 4.99 ± 1.64a |
| Lb. plantarum MNC 21 | 2.14 ± 0.43b | ND | ND | 0.01 ± 0.00a | 0.15 ± 0.02a | 1.13 ± 0.15b | 18.86 ± 2.15bd |
| Lc. lactis MNC 24 | 1.76 ± 0.03b | 0.02 ± 0.00a | 0.01 ± 0.00b | 0.02 ± 0.0a | 0.13 ± 0.01a | 2.10 ± 0.21b | 30.00 ± 6.25c |
| Lb. fermentum MNC 34 | 0.30 ± 0.01a | ND | ND | ND | 0.55 ± 0.06c | ND | ND |
| W. confusa MNC 20 | 0.33 ± 0.04a | ND | ND | 0.02 ± 0.01a | 0.51 ± 0.20bc | 9.59 ± 1.86c | 14.45 ± 3.33d |
| I. orientalis MNC 20Y | 8.99 ± 2.20c | 0.04 ± 0.02a | 0.01 ± 0.00b | 0.02 ± 0.01a | 0.39 ± 0.02bc | ND | 24.89 ± 5.58bc |
| S. cerevisiae MNC 21Y | 10.69 ± 0.83c | 0.24 ± 0.00b | 0.10 ± 0.00c | 0.07 ± 0.01a | 0.62 ± 0.11c | 0.16 ± 0.03a | 3.53 ± 2.27a |
Values are means ± standard deviations of three independent fermentations. Values in the same column with similar superscripts (a – e) are not significantly different (p > .05). ND: not detected. Acetald (acetaldehyde); 2‐ME‐pro‐al (2‐methyl‐1‐propanal); 2‐ME‐but‐al (2‐methyl‐1‐butanal); 3‐ME‐but‐al (3‐methyl‐1‐butanal).
3.6. Production of diacetyl, acetoin, and acetone
Spontaneously fermented Obushera did not accumulate diacetyl but contained the highest amounts of acetoin after 12 hrs (Figure 3d). Among the starter cultures, W. confusa produced the highest (p < .05) amounts of diacetyl (9.59 mg kg−1) followed by Lc. lactis (2.10 mg kg−1) and Lb. plantarum (1.13 mg kg−1) (Table 1). All single starter cultures, except Lb. fermentum and S. cerevisiae, accumulated acetoin with the highest (p < .05) production in Lc. lactis (30.00 mg kg−1) followed by Lb. plantarum ,I. orientalis (18.86–24.89 mg kg−1), and W. confusa (14.45 mg kg−1) (Table 1). Spontaneously fermented Obushera also accumulated acetone (0.36–0.92 mg kg−1) within 12–48 hrs. Only W. confusa, Lb. fermentum, S. cerevisiae, and I. orientalis produced significant (p < .05) amounts (0.22–0.62 mg kg−1) of acetone (Table 1). Saccharomyces cerevisiae greatly reduced (p < .05) the production of diacetyl in mixed starter cultures containing Lb. plantarum, Lc. lactis, and/or W. confusa (Figure 3c).
3.7. Production of malty aldehydes and alcohols
Obushera initially contained the malty aldehydes 2‐methylmethyl‐1‐propanal, 2‐methylmethyl‐1‐butanal, and 3‐methylmethyl‐1‐butanal, which were depleted by all single starter cultures except S. cerevisiae which produced 2‐methyl‐1‐propanal (0.24 mg kg−1) (Table 1). Spontaneously fermented Obushera accumulated 0.05–0.25 mg kg−1 of 2‐methyl‐1‐propanal between 24 and 48 hrs (Figure 3b). Methyl‐1‐propanal was only produced by S. cerevisiae (0.06–0.12 mg kg−1) (Table 1). The methyl alcohols 2‐methyl‐1‐propanol, 2‐methyl‐1‐butanol, and 3‐methyl‐1‐butanol accumulated during spontaneous fermentation to 10.11 mg kg−1, 3.67 mg kg−1and 17.67 mg kg−1, respectively after 48 hrs following a similar trend shown for 3‐methyl‐1‐butanol (Figure 4b). Only the yeasts: I. orientalis and S. cerevisiae produced 2‐methyl‐1‐propanol (2.78–5.18 mg kg−1), 2‐methyl‐1‐butanol (1.10 mg kg−1), and 3‐methyl‐1‐butanol (5.53–8.47 mg kg−1) in significant amounts (Table 2). I. orientalis produced significantly (p < .05) higher amounts of 2‐methyl‐1‐propanol and 3‐methyl‐1‐butanol than S. cerevisiae (Table 2).
4. Discussion
4.1. Growth of starter cultures
Since not all microbial genera are of equal importance in fermentation (Holzapfel, 1997), candidate isolates for starter culture development have to be evaluated for their contribution during fermentation. Selecting appropriate starter cultures involves evaluating candidate strains for their viability and survival in the product, their ability to utilize the major carbohydrate substrates available, rate of acidification, and production of desirable flavor compounds among others (Holzapfel, 2002). It is also important to determine the effects of coculturing selected isolates on their activity such as acidification rate and production of flavor compounds.
With regard to growth of the starter cultures, individual LAB and yeast isolates used in the current study were able to grow in Obushera and attained maximum counts similar to those reported for spontaneously fermented Obushera (Muyanja, Narvhus, & Langsrud, 2004; Muyanja, Narvhus, & Langsrud, 2012). However, maximum counts for the starter cultures were attained in a much shorter time (12–24 hrs) than in spontaneous fermentation (24–48 hrs). Fast growth is desirable since it results in fast acidification and flavor development thus contributing toward shortening of processing time as well as ensuring product safety (Holzapfel, 2002). The decline in cell numbers of W. confusa, Lc. lactis, and Lb. fermentum during fermentation could imply that these strains are acid sensitive. The stability of Lb. plantarum counts can be attributed to the acid tolerance of the strain MNC 21, which has been observed in other studies (Byakika, 2015; Mukisa, 2012). Despite its viability and flavor production potential, I. orientalis was considered undesirable for production of fermented cereal beverages on the basis of surface film formation. Surface film formation by I. orientalis is commonly associated with spoilage of beverages (Stratford & James, 2003). Indeed, the development of surface films is one of the spoilage characteristics of Obushera that consumers may use to reject the product (Mukisa, 2012).
4.2. Utilization of sugars
The ability of starter cultures to metabolize sugars available in a substrate is an important aspect for starter culture selection. The major sugars in sorghum malt fermentations are maltose, glucose, and fructose. All starter cultures were able to utilize glucose thus enabling them to grow in and ferment the product. Differences were observed in the patterns and rates of utilization of maltose, glucose, and fructose by the individual LAB and yeast isolates. The ability of, and rate at which, an individual isolate metabolizes one or more sugars do influence its productivity and competiveness in a mixed starter culture (Gobbetti, 1998). Rapid utilization of sugars by S. cerevisiae makes it more competitive than LAB, when in coculture, resulting in diminished lactate production while ethanol yield was unaffected. Similar effects on lactate production were observed during sourdough fermentations when S. cerevisiae was cocultured with Lb. plantarum (Gobbetti, Corsetti, & Rossi, 1994) or Lb. sanfranciscensis (Gobbetti, 1998). In contrast, coculturing Lb. brevis with S. cerevisiae (Meignen et al., 2001) or Lb. plantarum with I. orientalis (Mugula et al., 2003) did not affect lactate production. It is possible that in the latter cases, LAB were faster than the yeasts in metabolizing the sugars while the inability of I. orientalis to utilize maltose, as seen from this study, could have made it less competitive. The rapid utilization of glucose by W. confusa and Lc. lactis in the first 8–12 hrs enables them to rapidly acidify the product to a pH below 4.5 in a period of about 6 hrs.
One key observation during the spontaneous fermentation of Obushera was that the concentration of maltose increased with maltose remaining above its taste threshold concentration of 13 g kg−1 for at least 24 hrs (Laska, Schüll, & Scheuber, 1999). This increase in sugars results from hydrolysis of starch by cereal amylases and is responsible for the desirable sweet taste of products such as Obushera. Therefore, low initial amounts of sugars in starter culture fermented samples, coupled by rapid depletion by the starter cultures could compromise the taste acceptability of some products for which sweetness is an important attribute. It may therefore be necessary to introduce a saccharification step prior to inoculation with starter culture in order to achieve desirable levels of sweetness in such products.
4.3. Lactate and acetate production
Acid production is important for both taste and safety of fermented products. Lactate imparts a sour taste which is an important sensory attribute of LAB fermented products (Mukisa et al., 2010). To ensure safety of lactic fermented products, some studies recommend attaining a rapid pH drop to ≤4.5–4.0 and a titratable acidity of about 0.7% lactic acid (Steinkraus, 1996). Fast acidification to pH below 4.0 inhibits spoilage bacteria, enteropathogens, and Bacillus sp (Holzapfel, 1997; Steinkraus, 1996). The ability to achieve rapid acidification in a shorter time than observed in the traditional process should therefore be one of the important criteria for selecting LAB starter cultures. All the LAB starter cultures used in this study were able to drop the pH of Obushera to below 4.5 in 6–12, but were not able to raise the acidity to 0.7%. Accelerated acidification by LAB starter cultures has also been reported in related studies (Mugula et al., 2003; Muyanja, Narvhus, & Langsrud, 2004; Muyanja, Narvhus, & Langsrud, 2012). Spontaneously fermented Obushera attained a pH below 4.5 in a much longer time (24 hrs) and only attained acidity of about 0.35%. Lactobacillus fermentum, Lc. lactis, and W. confusa only reached a maximum acidity of 0.2% in 12–24 hrs while Lb. plantarum was able to raise the acidity to 0.53% in 24 hrs. In this respect, Lb. plantarum would be a better candidate as a starter culture for such fermentations because of its superior acid production. This starter culture has been reported to produce up to 1.7% lactic acid from sorghum malt extracts (Byakika, 2015). High lactate production in Lb. plantarum strains is attributed to versatility in metabolism of carbohydrates coupled with tolerance to acid conditions (Van der Meulen et al., 2007). In some instances, excessive lactate production by Lb. plantarum could result in an undesirable sour taste (Hansen & Hansen, 1996). Therefore, the reduction in lactate production observed when S. cerevisiae was cocultured with the LAB‐mixed starter cultures can help prevent excessive souring during fermentation, as long as the desired level of acidity is attained. This interaction is reported to have moderated the souring effect of Lb. plantarum in sourdough bread (Hansen & Hansen, 1996).
Spontaneously fermented Obushera contained acetic acid (0.1–0.7 g kg−1) in amounts close to those observed previously (Muyanja, Narvhus, Treimo, & Langsrud, 2003). Levels exceeding 0.4–1.5 g L−1 are undesirable in wines because they impart vinegar taints (Bartowsky, Xia, Gibson, Fleet, and Henschke (2003). Excessive acetate production is also responsible for the pungent attribute which reduces the acceptability of Obushera (Mukisa et al., 2010). Acetic acid in concentrations of 0.1–0.2 g kg−1 acts as a flavor enhancer in sourdough bread (Hansen & Hansen, 1996). It is possible that the relatively low levels of acetate (0.25–0.35 g kg−1) produced by the starter cultures in this study might also contribute positively by enhancing the flavor of Obushera. Acetate production in moderate amounts could therefore be of significance in flavor of Obushera and related fermented cereal products thus necessitating the inclusion of heterofermentative starter culturers such as W. confusa and Lb. fermentum.
4.4. Ethanol production
Ethanol production in Obushera is responsible for the characteristic alcoholic flavor (Mukisa et al., 2010). Obushera of the Obutoko type is acceptable with an alcohol content of up to 10 g kg−1, but levels up to 45 g kg−1 result in a decline in acceptability (Mukisa, 2012). Concentrations of ethanol in sorghum based fermentations such as Obushera and Togwa vary with type of product and duration of fermentation ranging from 0.1 g kg−1 to 60.0 g kg−1 (Mugula et al., 2003; Mukisa, Muyanja, et al., 2012; Muyanja, Narvhus, & Langsrud, 2012). Ethanol in spontaneously fermented Obushera (1. 29–2.00 g kg−1 between 24 and 48 hrs) was within expected limits which do not negatively impact on acceptability. Saccharomyces cerevisiae produced the highest amounts of ethanol in 12 hrs (1.55 g kg−1), while ethanol production by Lb. fermentum, I. orientalis, and W. confusa was comparable (0.79–0.88 g kg−1). Ethanol is a product of carbohydrate metabolism by obligate heterofermentative LAB and yeasts, but the latter are generally recognized as high producers (Hansen & Schieberle, 2005). Although starter cultures had significantly lower yields of ethanol compared to spontaneously fermented Obushera, maximum amounts were achieved in a shorter time (12 hrs). The low yield of ethanol in samples fermented by S. cerevisiae can be attributed to substrate limitation while productivity of Lb. fermentum and W. confusa was more likely affected by increasing acidity. Lactobacillus fermentum was also less competitive in coculture with Lc. lactis and Lb. plantarum since these two utilized sugars much faster. It is therefore possible to use heterofermentative LAB, or yeast to attain either low or relatively high levels of ethanol in fermented cereal fermentations depending on the desired sensory attributes.
4.5. Production of acetaldehyde
Variations in acetaldehyde concentrations during fermentation followed a similar pattern previously observed by Muyanja, Narvhus, & Langsrud, (2012). In the current study, yeasts produced the highest concentrations of acetaldehyde (8.99–10.69 mg kg−1) during the first 24 hrs and were followed by Lb. plantarum and Lc. lactis (1.76–2.14 mg kg). Acetaldehyde is produced by LAB and yeasts, mainly as an intermediate in ethanol production (Liu & Pilone, 2000). However, yeasts and in particular S. cerevisiae, are known to produce higher amounts compared to LAB (Liu & Pilone, 2000). Coculturing Lb. fermentum or W. confusa with the Lb. plantarum + Lc. lactis starter culture prevented accumulation of acetaldehyde. The acetaldehyde was probably reduced to acetate/ethanol by action of acetaldehyde/ethanol dehydrogenase from W. confusa or Lb. fermentum (Zaunmüller, Eichert, Richter, & Unden, 2006). Acetaldehyde imparts a pleasant fruity aroma when present in low concentrations, but results in pungent odors at high concentrations (Liu & Pilone, 2000). A fruity aroma is one of the desirable sensory attributes of Obushera (Mukisa et al., 2010). The aroma threshold of acetaldehyde ranges from 0.015 mg kg−1 in water to 125 mg kg−1 in wine (Imhof, Glättli, & Bosset, 1994; Liu & Pilone, 2000). Amounts of acetaldehyde in naturally fermented Obushera fall within this range, suggesting that it is an important flavor compound. This may necessitate selection of starter cultures with the ability to accumulate acetaldehyde. In this study, the inclusion of S. cerevisiae in the LAB starter culture or elimination of W. confusa or Lb. fermentum from the Lb. plantarum + Lc. lactis ensured accumulation of acetaldehyde.
4.6. Production of diacetyl, acetoin, and acetone
Diacetyl and acetoin have been previously detected in Obushera in ranges of 0.2–2.4 mg kg−1 and 1.3–41.6 mg kg−1, respectively (Muyanja et al., 2003). However, in this study only acetoin was detected. Diacetyl and acetoin are intermediate products of reductive decarboxlaytion of excess pyruvate to 2,3‐butanediol (Bartowsky & Pretorius, 2009). The pyruvate is obtained from sugar or citrate metabolism. Diacetyl is commonly produced by citrate positive Lactococci, Lactobacilli, and yeasts (Bartowsky & Pretorius, 2009). Yeasts further rapidly convert diacetyl to acetoin and finally 2,3 butanediol (Bartowsky & Pretorius, 2009). In this study, W. confusa produced the highest amounts of diacetyl, but these were reduced in coculture with S. cerevisiae. The diacetyl produced by W. confusa in a coculture was possibly rapidly converted into 2,3 butanediol by the action of S. cerevisiae acetoin reductase (Bartowsky & Pretorius, 2009). Diacetyl and acetoin impart buttery flavors, but diacetyl is more important owing to its lower flavor threshold (0.002–2.2 mg L−1) compared to that of acetoin (150 mg L−1) (Bartowsky & Pretorius, 2009; Imhof et al., 1994). Diacetyl concentrations above 5–7 mg L−1 impart undesirable flavors in wines (Bartowsky & Pretorius, 2009). Use of W. confusa alone or in combination with Lc. lactis and Lb. plantarum could result in excess production of diacetyl and hence impart undesirable flavor. However, this can be minimized by coculturing with S. cerevisiae.
4.7. Production of malty aldehydes and alcohols
The aldehydes 2‐methyl‐1‐propanal, 2‐methyl‐1‐butanal, and 3‐methyl‐1‐butanal are associated with malty flavors (Sheldon, Lindsay, Libbey, & Morgan, 1971) while their corresponding alcohols, at concentrations below 300 mg kg−1, impart desirable fruity notes to wine (Bartowsky & Pretorius, 2009). The higher alcohols could also be responsible for the desirable fruity notes in Obushera (Mukisa et al., 2010). The taste threshold concentrations of 2‐methyl‐1‐propanal, 2‐methyl‐1‐butanal, and 3‐methyl‐1‐butanal are 0.18, 0.13, and 0.06 mg kg−1, while those of their corresponding alcohols are 5.25, 5.50, and 4.75 mg kg−1 (Sheldon et al., 1971). These compounds have been detected in Obushera in amounts close to or above their thresholds indicating their importance in Obushera flavor (Muyanja, Narvhus, & Langsrud, 2012). Malty compounds are produced via transamination of specific amino acids to α‐keto acids followed by decarboxylation into the aldehyde and reduction to the corresponding alcohol (Hansen & Schieberle, 2005; Hazelwood, Daran, van Maris, Pronk, & Dickinson, 2008). Catabolism of valine, isoleucine, and leucine yields the aldehydes 2‐methyl‐1‐propanal, 2‐methyl‐1‐butanal, and 3‐methyl‐1‐butanal, respectively (Hazelwood et al., 2008). Their production is reported in LAB and yeasts although yeasts are often regarded as the main producers (Gobbetti, 1998; Hazelwood et al., 2008). This study indicates that yeasts are the major producers of malty compounds in sorghum malt fermentation. Thus, the inclusion of yeasts as part of the starter culture in some cereal fermented products might be inevitable. In this study, Obushera fermented by the S. cerevisiae starter culture yielded less malty compounds after 48 hrs compared to the spontaneous fermentation. This possibly resulted from a reduction in yeast metabolism following depletion of fermentable sugars, which were also initially lower in starter culture fermented samples.
5. Conclusion
This study reveals that besides producing lactic acid, LAB in cereal fermentations may also contribute to production of other flavor compounds notably acetate, ethanol, acetaldehyde, diacetyl, acetone, and acetoin. Yeasts mainly contribute toward production of ethanol, acetaldehyde, methyl aldehydes, and methyl alcohols. With regard to acidification, Lb. plantarum, Lc. lactis, W. confusa, and Lb. fermentum, or their selected combinations can be used to achieve accelerated fermentation of cereal products. However, use, or inclusion, of Lb. plantarum is encouraged because of its high acid production potential and stability to acid. Despite the acid sensitivity of Lc. lactis and Wc. confusa, their fast growth and glucose metabolism may contribute toward rapid acidification. Addition of S. cerevisiae to LAB starter cultures may be crucial for the attainment of a flavor profiles typical of traditional fermented cereal products. S. cerevisiae also prevents excessive lactate and diacetyl production, a phenomenon which could negatively or positively affect the flavor profile. The starter culture combinations: Lb. plantarum + Lc. lactis + S. cerevisiae, or Lb. plantarum + Lc. lactis +W. confusa + S. cerevisiae can be adopted for the fermentation of Obushera.
Conflict of Interest
There is no conflict of interest to be declared.
Acknowledgment
We are very grateful to Kari Olsen for the expert technical assistance with the HPLC and HSGC analyses. This research was funded by the Norwegian Universities’ Committee for Development Research and Education (NUFU, project 2007/10221) through the Norwegian University of Life Sciences and Makerere University.
Mukisa IM, Byaruhanga YB, Muyanja CMBK, Langsrud T, Narvhus JA. Production of organic flavor compounds by dominant lactic acid bacteria and yeasts from Obushera, a traditional sorghum malt fermented beverage. Food Sci Nutr. 2017;5:702–712. https://doi.org/10.1002/fsn3.450
References
- Abriouel, H. , Omar, N. B. , López, R. L. , Martínez‐Cañamero, M. , Keleke, S. , & Gálvez, A. (2006). Culture‐independent analysis of the microbial composition of the African traditional fermented foods poto poto and dégué by using three different DNA extraction methods. International Journal of Food Microbiology, 111(3), 228–233. [DOI] [PubMed] [Google Scholar]
- Agarry, O. , Nkama, I. , & Akoma, O . (2010). Production of Kunun‐zaki (A Nigerian fermented cereal beverage) using starter culture. International Research Journal of Microbiology,1(2), 018–025. [Google Scholar]
- Ampe, F. , ben Omar, N. , Moizan, C. , Wacher, C. , & Guyot, J.‐P. (1999). Polyphasic study of the spatial distribution of microorganisms in Mexican pozol, a fermented maize dough, demonstrates the need for cultivation‐independent methods to investigate traditional fermentations. Applied and Environmental Microbiology, 65(12), 5464–5473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartowsky, E. J. , & Pretorius, I. S. (2009). Microbial formation and modification of flavour and off‐flavour compounds in wine In König H., Unden G., & Fröhlich J. (Eds.), Biology of Microorganisms on grapes, in must and in wine (pp. 209–232). Berlin Heidelberg: Springer‐Verlag. [Google Scholar]
- Bartowsky, E. J. , Xia, D. , Gibson, R. L. , Fleet, G. H. , & Henschke, P. A. (2003). Spoilage of bottled red wine by acetic acid bacteria. Letters in Applied Microbiology, 36(5), 307–314. [DOI] [PubMed] [Google Scholar]
- ben Omar, N. , & Ampe, F. 2000. Microbial community dynamics during production of the Mexican fermented maize dough pozol. Applied and Environmental Microbiology, 66(9), 3664–3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byakika, S . (2015). Evaluation of sorghum malt as a growth and carrier medium for Lactobacillus plantarum MNC 21 biomass. MSc. Thesis, Makerere University, Kampala. (M.Sc), Makerere University, Kampala. [Google Scholar]
- Gobbetti, M. (1998). The sourdough microflora: Interactions of lactic acid bacteria and yeasts. Trends in Food Science & Technology, 9(7), 267–274. [Google Scholar]
- Gobbetti, M. , Corsetti, A. , & Rossi, J. (1994). The sourdough microflora. Interactions between lactic acid bacteria and yeasts: Metabolism of carbohydrates. Applied Microbiology and Biotechnology, 41(4), 456–460. doi: 10.1007/bf01982535 [DOI] [PubMed] [Google Scholar]
- Gobbetti, M. , Simonetti, M. S. , Corsetti, A. , Santinelli, F. , Rossi, J. , & Damiani, P. (1995). Volatile compound and organic acid productions by mixed wheat sour dough starters: Influence of fermentation parameters and dynamics during baking. Food Microbiology, 12, 497–507. [Google Scholar]
- Halm, M. , Osei‐Yaw, A. , Hayford, A. , Kpodo, K. , & Amoa‐Awua, W. (1996). Experiences with the use of a starter culture in the fermentation of maize for ‘kenkey'production in Ghana. World Journal of Microbiology and Biotechnology, 12(5), 531–536. [DOI] [PubMed] [Google Scholar]
- Hansen, Å. , & Hansen, B. (1996). Flavour of sourdough wheat bread crumb. Zeitschrift für Lebensmittel‐Untersuchung und Forschung, 202(3), 244–249. [Google Scholar]
- Hansen, A. , & Schieberle, P. (2005). Generation of aroma compounds during sourdough fermentation: Applied and fundamental aspects. Trends in Food Science & Technology, 16(1–3), 85–94. [Google Scholar]
- Hazelwood, L. A. , Daran, J.‐M. , van Maris, A. J. A. , Pronk, J. T. , & Dickinson, J. R. (2008). The Ehrlich Pathway for Fusel Alcohol Production: A Century of Research on Saccharomyces cerevisiae Metabolism. Applied and Environment Microbiology, 74(8), 2259–2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzapfel, W. (1997). Use of starter cultures in fermentation on a household scale. Food Control, 8(5–6), 241–258. [Google Scholar]
- Holzapfel, W. H. (2002). Appropriate starter culture technologies for small‐scale fermentation in developing countries. International Journal of Food Microbiology, 75(3), 197–212. [DOI] [PubMed] [Google Scholar]
- Holzapfel, W. , & Taljaard, J . (2004). Industrialization of mageu fermentation in South Africa (pp. 363–407). New York: Food Science and Technology‐New York‐Marcel Dekker. [Google Scholar]
- Imhof, R. , Glättli, H. , & Bosset, J. O. (1994). Volatile organic aroma compounds produced by thermophilic and mesophilic mixed strain dairy starter cultures. LWT‐Food Science and Technology, 27(5), 442–449. [Google Scholar]
- Laska, M. , Schüll, E. , & Scheuber, H.‐P. (1999). Taste preference thresholds for food‐associated sugars in Baboons (Papio hamadryas anubis). International Journal of Primatology, 20(1), 25–34. [Google Scholar]
- Liu, S.‐Q. , & Pilone, G. J. (2000). An overview of formation and roles of acetaldehyde in winemaking with emphasis on microbiological implications. International Journal of Food Science & Technology, 35(1), 49–61. [Google Scholar]
- Madoroba, E. , Steenkamp, E. T. , Theron, J. , Scheirlinck, I. , Cloete, T. E. , & Huys, G. (2011). Diversity and dynamics of bacterial populations during spontaneous sorghum fermentations used to produce ting, a South African food. Systematic and Applied Microbiology, 34(3), 227–234. [DOI] [PubMed] [Google Scholar]
- Masha, G. , Ipsen, R. , Petersen, M. , & Jakobsen, M. (1998). Microbiological, rheological and aromaticcharacteristics of fermented Uji (an East African Sour Porridge). World Journal of Microbiology and Biotechnology, 14(3), 451–456. [Google Scholar]
- Meignen, B. , Onno, B. , Gélinas, P. , Infantes, M. , Guilois, S. , & Cahagnier, B. (2001). Optimization of sourdough fermentation with Lactobacillus brevis and baker's yeast. Food Microbiology, 18(3), 239–245. [Google Scholar]
- Mugula, J. K. , Narvhus, J. A. , & Sørhaug, T. (2003). Use of starter cultures of lactic acid bacteria and yeasts in the preparation of togwa, a Tanzanian fermented food. International Journal of Food Microbiology, 83(3), 307–318. [DOI] [PubMed] [Google Scholar]
- Mukisa, I. M. (2012). Sensory characteristics, microbial diversity and starter culture development for Obushera, a traditional cereal fermented beverage from Uganda. PhD, Norwegian University of Life Sciences, Aas, Norway.
- Mukisa, I. M. , Byaruhanga, Y. B. , Muyanja, C. M. , Aijuka, M. , Schüller, R. B. , Sahlstrøm, S. , & Narvhus, J. A. (2012). Influence of cofermentation by amylolytic Lactobacillus plantarum and Lactococcus lactis strains on the fermentation process and rheology of sorghum porridge. Applied and Environment Microbiology, 78(15), 5220–5228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukisa, I. , Muyanja, C. M. , Byaruhanga, Y. , Langsrud, T. , & Narvhus, J. (2012). Changes in physico‐chemical properties and flavour compounds during fermentation of different Obushera (Sorghum and Millet) Beverages. AJFAND, 12(6), 655–6685. [Google Scholar]
- Mukisa, I. M. , Muyanja, C. M. , Byaruhanga, Y. B. , Schüller, R. B. , Langsrud, T. , & Narvhus, J. A. (2012). Gamma irradiation of sorghum flour: Effects on microbial inactivation, amylase activity, fermentability, viscosity and starch granule structure. Radiation Physics and Chemistry, 81(3), 345–351. [Google Scholar]
- Mukisa, I. , Nsiimire, D. , Byaruhanga, Y. , Muyanja, C. , Langsrud, T. , & Narvhus, J. (2010). Obushera: Descriptive sensory profiling and consumer acceptability. Journal of Sensory Studies, 25(s1), 190–214. [Google Scholar]
- Mukisa, I. M. , Porcellato, D. , Byaruhanga, Y. B. , Muyanja, C. M. , Rudi, K. , Langsrud, T. , & Narvhus, J. A. (2012). The dominant microbial community associated with fermentation of Obushera (sorghum and millet beverages) determined by culture‐dependent and culture‐independent methods. International Journal of Food Microbiology, 160(1), 1–10. [DOI] [PubMed] [Google Scholar]
- Muyanja, C. M. B. K. , Narvhus, A. , & Langsrud, T. (2004). The use of starter cultures during fermentation of bushera, a Ugandan traditional fermented sorghum beverage. Ug J Agric Sci, 8(1), 606–616. [Google Scholar]
- Muyanja, C. , Narvhus, J. , & Langsrud, T. (2012). Organic acids and volatile organic compounds produced during traditional and starter culture fermentation of Bushera, a Ugandan fermented cereal beverage. Food Biotechnology, 26(1), 1–28. [Google Scholar]
- Muyanja, C. , Narvhus, J. , Treimo, J. , & Langsrud, T. (2003). Isolation, characterisation and identification of lactic acid bacteria from bushera: A Ugandan traditional fermented beverage. International Journal of Food Microbiology, 80(3), 201–210. [DOI] [PubMed] [Google Scholar]
- Narvhus, J. A. , Østeraas, k. , Mutukumira, T. , & Abrahamsen, R. K. (1998). Production of fermented milk using a malty compound‐producing strain of Lactococcus lactis subsp. lactis biovar. diacetylactis, isolated from Zimbabwean naturally fermented milk. International journal of food microbiology, 41(1), 73–80. [DOI] [PubMed] [Google Scholar]
- Nout, M. (2009). Rich nutrition from the poorest–Cereal fermentations in Africa and Asia. Food Microbiology, 26(7), 685–692. [DOI] [PubMed] [Google Scholar]
- Omemu, A. , Oyewole, O. , & Bankole, M. (2007). Significance of yeasts in the fermentation of maize for ogi production. Food Microbiology, 24(6), 571–576. [DOI] [PubMed] [Google Scholar]
- Orji, M. , Mbata, T. , Aniche, G. , & Ahonkhai, I. (2003). The use of starter cultures to produce ‘Pito’, a Nigerian fermented alcoholic beverage. World Journal of Microbiology and Biotechnology, 19(7), 733–736. [Google Scholar]
- Sekwati‐Monang, B. , & Gänzle, M. G. (2011). Microbiological and chemical characterisation of ting, a sorghum‐based sourdough product from Botswana. International Journal of Food Microbiology, 150(2), 115–121. [DOI] [PubMed] [Google Scholar]
- Sheldon, R. M. , Lindsay, R. C. , Libbey, L. M. , & Morgan, M. E. (1971). Chemical nature of malty flavor and aroma produced by Streptococcus lactis var. maltigenes . Applied and Environmental Microbiology, 22(3), 263–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinkraus, K. (1996). Handbook of Indigenous Fermented Foods. New York, USA.: Marcel Dekker. [Google Scholar]
- Stratford, M. , & James, S. A. (2003). Non‐alcoholic beverages and yeasts In Boekhout T., & Robert V. (Eds.), Yeasts in food: Beneficial and detrimental aspects (p. 488). Cambridge, England: Woodhead Publishing Limited. [Google Scholar]
- Tamang, J. P. , & Kailasapathy, K . (2010). Fermented foods and beverages of the world. Boca Raton, FL: CRC press. [Google Scholar]
- Van der Meulen, R. , Scheirlinck, I. , Van Schoor, A. , Huys, G. , Vancanneyt, M. , Vandamme, P. , & De Vuyst, L. (2007). Population Dynamics and Metabolite Target Analysis of Lactic Acid Bacteria during Laboratory Fermentations of Wheat and Spelt Sourdoughs. Applied and Environmental Microbiology, 73(15), 4741–4750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira‐Dalodé, G. , Jespersen, L. , Hounhouigan, J. , Moller, P. , Nago, C. , & Jakobsen, M. (2007). Lactic acid bacteria and yeasts associated with gowé production from sorghum in Bénin. Journal of Applied Microbiology, 103(2), 342–349. [DOI] [PubMed] [Google Scholar]
- Zaunmüller, T. , Eichert, M. , Richter, H. , & Unden, G. (2006). Variations in the energy metabolism of biotechnologically relevant heterofermentative lactic acid bacteria during growth on sugars and organic acids. Applied Microbiology and Biotechnology, 72(3), 421–429. [DOI] [PubMed] [Google Scholar]


