Abstract
Objectives: The negative publicity about menopausal hormone therapy (MHT) has led to increased use of complementary and alternative medicines (CAM) and non-pharmacological interventions (NPI) for menopausal symptom relief. We report on the prevalence and predictors of CAM/NPI among UK postmenopausal women.
Method: Postmenopausal women aged 50–74 years were invited to participate in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS). A total of 202 638 women were recruited and completed a baseline questionnaire. Of these, 136 020 were sent a postal follow-up-questionnaire between September 2006 and May 2009 which included ever-use of CAM/NPI for menopausal symptom relief. Both questionnaires included MHT use.
Results: A total of 88 430 (65.0%) women returned a completed follow-up-questionnaire; 22 206 (25.1%) reported ever-use of one or more CAM/NPI. Highest use was reported for herbal therapies (43.8%; 9725/22 206), vitamins (42.6%; 9458/22 206), lifestyle approaches (32.1%; 7137/22 206) and phytoestrogens (21.6%; 4802/22 206). Older women reported less ever-use of herbal therapies, vitamins and phytoestrogens. Lifestyle approaches, aromatherapy/reflexology/acupuncture and homeopathy were similar across age groups. Higher education, Black ethnicity, MHT or previous oral contraceptive pill use were associated with higher CAM/NPI use. Women assessed as being less hopeful about their future were less likely to use CAM/NPI.
Conclusion: One in four postmenopausal women reported ever-use of CAM therapies/NPI for menopausal symptom relief, with lower use reported by older women. Higher levels of education and previous MHT use were positive predictors of CAM/NPI use.
UKCTOCS Trial registration: ISRCTN22488978
Keywords: Menopausal symptoms, complementary and alternative medicine, CAM, non-pharmacological interventions, NPI, prevalence, UKCTOCS
Introduction
In 2002, the US Women’s Health Initiative (WHI) reported that menopausal hormone therapy (MHT) was associated with an increased risk of breast cancer, cardiovascular disease and thromboembolic events 1 . The negative publicity that ensued alarmed many women and their clinicians, resulting in a decrease in MHT use across the world 2–6 . A similar trend was observed in postmenopausal women taking part in the United Kingdom Collaborative Trial of Ovarian Cancer Screening (UKCTOCS); MHT use decreased in women recruited in 2005 (10–11%) compared with those recruited in 2001 (29%) 7 .
Complementary and alternative medicine (CAM) for menopausal symptom relief is by definition not considered part of conventional medicine 8 . The lay public regard them as being ‘fairly safe’ 9 . They can include approaches such as herbal therapies, phytoestrogens, and non-pharmacological interventions (NPI) such as exercise, acupuncture and yoga. Cognitive behavior therapy is considered part of conventional medicine and has been recommended by the National Institute of Health and Care Excellence (NICE) for management of low mood associated with the menopause 10 .
Two systematic reviews of use of CAM therapies for menopausal symptoms have been published. The first in 2013, based on nine studies with a total of 32 465 menopausal women, reported that 50.5% used CAM therapies/NPI for relief of menopausal symptoms 11 . The more recent 2014 meta-analysis restricted eligible studies to those involving more than 500 women and reported a CAM/NPI prevalence ranging from 31% to 82.5% 12 . Both reviews conclude that many surveys were of poor methodological quality, making it difficult to estimate the true prevalence of CAM/NPI use among climacteric women 11 , 12 .
Data from the UK are limited to three surveys, two of which were undertaken in 2001 before the publication of the WHI results. The remaining survey dates from 2006 involving 563 women. The overall prevalence of CAM/NPI use was not reported but the prevalence of each CAM therapy ranged from 1.2% for hypnosis to 39.6% for vitamins 13 .
Higher income, education and socioeconomic status have been shown to predict CAM/NPI use 14 , 15 with CAM/NPI consumers also using more conventional therapies than women not using CAM/NPI. Other factors associated with use of CAM/NPI therapies in the UK are White ethnicity, being physically active and a non-smoker 13 .
We report on the prevalence and predictors of CAM and NPI use for relief of menopausal symptoms in a large UK cohort of postmenopausal women participating in UKCTOCS.
Methods
Study participants
UKCTOCS is a randomized controlled trial designed to assess the impact on mortality of ovarian cancer screening. Between April 2001 and October 2005, postmenopausal women aged 50–74 were randomly invited from age-sex registers and 202 638 enrolled through 13 trial centers located in NHS Trusts in England, Wales and Northern Ireland. Further details on the study design have been reported elsewhere 16 . Postmenopausal status in these women aged over 50 was defined as >12 months amenorrhea following a natural menopause or hysterectomy or >12 months of MHT commenced for menopausal symptoms.
All women completed a baseline questionnaire at recruitment which included questions on current use of MHT, date of last period, age at first menstrual period, oral contraceptive pill (OCP) use, hysterectomy, sterilization, infertility, pregnancies lasting less or more than 6 months, personal history of cancer and family history of ovarian and breast cancer.
All women were followed up with a postal questionnaire (FUQ) 3–5 years after randomization. From September 2006, the FUQ included the question ‘Have you used any of the following to relieve menopausal symptoms?’ (yes or no) with a tick for each of the following: (1) herbal remedies, e.g. black cohosh; (2) phytoestrogens or soy products; (3) vitamins, e.g. Menopace, vitamin E; (4) homeopathic remedies; (5) aromatherapy, reflexology or acupuncture; (6) lifestyle changes, e.g. relaxation, exercise; or (7) other medical treatments, e.g. Venlafaxine, Megace.
Also included in the FUQ were questions on education, alcohol use, smoking, chronic diseases (diabetes, high blood pressure, high cholesterol, heart disease, stroke, rheumatoid arthritis, osteoarthritis, osteoporosis), cancers diagnosed after trial entry, and current MHT use. Women’s broad expectations about the future and their perceived ability to attain personal goals were assessed with two statements: ‘The future seems to me to be hopeful, and I believe that things are changing for the better’ and ‘I feel that it is possible to reach the goals I would like to strive for’. Women were asked to indicate the extent to which they agreed with each statement using a 5-item scale from ‘absolutely agree’ to ‘absolutely disagree’ where higher scores indicate higher levels of hopelessness. This positively phrased measure of hopelessness has been shown to be valid and reliable in postmenopausal women in the UK 17 .
Ethical approval for the study was received from the UCL/UCLH Committees on the Ethics of Human Research, Committee A on 30th May 2006 (REC 06/Q0505/36).
Statistical analysis
Predictors of CAM/NPI use
With regard to the demographic variables, erroneous heights <120 cm and >210 cm and weights <30 and >200 kg were discarded and the data field set as missing. The body mass index (BMI) was either grouped as: underweight (< 20 kg/m2); normal weight (≥20 and <25 kg/m2); overweight (≥25 and <30 kg/m2); obese (≥30 and <40 kg/m2); and morbidly obese (≥ 40 kg/m2) or used as a continuous variable. Ethnicity was grouped as White, Black (which included Black African, Black Caribbean and Black Other), South Asian (Indian, Pakistani and Bangladeshi) and Other. Education was grouped as Higher education (college/university), Other formal qualification (nursing and teaching, ‘O’ level or equivalent, ‘A’ level or equivalent and clerical or commercial qualification) or No formal qualification (other than compulsory education). Women who had stated that they had both college/university degree and no formal education (n = 120) were grouped under college/university.
Use of CAM/NPI (yes/no and type) were examined by age group, ethnicity, education, BMI, OCP use, MHT use, hysterectomy, sterilization, number of pregnancies and cancer history.
For the women residing in England, an Index of Multiple Deprivation (IMD) score based on the women’s postcode derived at the Super Output Area level was available and used as a proxy for the socioeconomic status. For reasons of model interpretation, the IMD score was standardized to have a standard deviation of one.
Women who had used both CAM/NPI and ‘other medical treatments’ were not included in the model, but were adjusted for in the regression analysis.
Multivariate probit model of predictors of CAM/NPI use for women residing in England
To examine the associations between the six different types of CAM/NPI, tetrachoric correlations of CAM/NPI type use were calculated, which estimate the correlation between two theorized normally distributed latent variables (here the propensity for CAM/NPI type use) using observed dichotomous variables. Similarly to other correlational measures, possible values ranged from −1 (negative correlation) to +1 (positive correlation). To model the effect of the various factors on the probability of each CAM/NPI type use, it was necessary to account for the likelihood that CAM/NPI use for one type will be highly (positively) correlated with each of the other CAM/NPI types. For this reason, a multivariate probit model was fitted (with the user-written command mvprobit in Stata 18 ) which preserved this correlational structure in the six-dimensional response variable.
Age (stratified per 5-year age groups), BMI (per 5 kg/m2), age at first menstrual period (FMP, per 5 years), age at last menstrual period (LMP, per 5 years), hopelessness (HH, per unit SD), IMD score (per unit SD), number of pregnancies <6 months and number of pregnancies >6 months were modeled as continuous variables. Univariate probit models were used to check the functional form and demonstrate that linearity (in the probit scale) was a reasonable assumption.
As probit model parameters are not easily interpretable, marginal effects were estimated using Stata’s margin and lincom commands. Given that the model had 180 parameter estimates, the marginal effect (with 95% confidence interval (CI)) on each CAM/NPI type was also plotted in variable groupings to improve interpretation. Formal hypothesis testing was not considered due to the number of model estimates.
Results
Between September 2006 and May 2009, 136 020 postmenopausal women taking part in UKCTOCS were sent a follow-up questionnaire assessing use of CAM/NPI for relief of menopausal symptoms; 96 428 women returned a questionnaire (70.9%). A total of 7057 women were excluded from the analysis as the questionnaire completion date was missing. Of the remaining 89 371 participants, 941 had used ‘other medical treatment’ and were excluded. The final cohort included 88 430 (65.0%) cases. The overall median age was 64.7 years, with all women aged over 51 at completion of the survey; 97.4% (86 116/88 430) were White and 21.4% (18 902/88 430) had a higher education (Table 1).
Table 1.
Demographics of the study women and those using complementary and alternative medicine (CAM)/non-pharmacological interventions (NPIs) for menopausal symptom relief.
|
Ever use of CAM |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
CAM (overall) |
Herbal remedies |
Homeopathic remedies |
Phytoestrogens or soy products |
Aromatherapy, reflexology or acupuncture |
Vitamins |
Lifestyle change |
||||||||||
| Number of women | % of whole cohort | n | % of total cohort | n | % | n | % | n | % | n | % | n | % | n | % | |
| Age at reporting (years) | ||||||||||||||||
| 50–54 | 2489 | 2.8 | 1051 | 42.2 | 566 | 22.7 | 119 | 4.8 | 282 | 11.3 | 160 | 6.4 | 503 | 20.2 | 328 | 13.2 |
| 55–59 | 19 063 | 21.6 | 7771 | 40.8 | 4087 | 21.4 | 918 | 4.8 | 1993 | 10.5 | 1114 | 5.8 | 3364 | 17.6 | 2456 | 12.9 |
| 60–64 | 23 965 | 27.1 | 7226 | 30.2 | 3183 | 13.3 | 898 | 3.7 | 1550 | 6.5 | 1006 | 4.2 | 3160 | 13.2 | 2194 | 9.2 |
| 65–69 | 20 422 | 23.1 | 3886 | 19.0 | 1333 | 6.5 | 534 | 2.6 | 667 | 3.3 | 597 | 2.9 | 1589 | 7.8 | 1294 | 6.3 |
| 70–74 | 16 200 | 18.3 | 1776 | 11.0 | 474 | 2.9 | 248 | 1.5 | 249 | 1.5 | 273 | 1.7 | 662 | 4.1 | 640 | 4.0 |
| 75+ | 6291 | 7.1 | 496 | 7.9 | 82 | 1.3 | 74 | 1.2 | 61 | 1.0 | 86 | 1.4 | 180 | 2.9 | 225 | 3.6 |
| All ages | 88 430 | 100.0 | 22 206 | 25.1 | 9725 | 11.0 | 2791 | 3.2 | 4802 | 5.4 | 3236 | 3.7 | 9458 | 10.7 | 7137 | 8.1 |
| Overall use of each therapy | 25.1% | 11.0% | 3.2% | 5.4% | 3.7% | 10.7% | 8.1% | |||||||||
| Overall use in those using CAM | 43.8% | 12.6% | 21.6% | 14.6% | 42.6% | 32.1% | ||||||||||
| Ethnicity | ||||||||||||||||
| White | 86 116 | 97.4 | 21 441 | 24.9 | 9470 | 11.0 | 2712 | 3.1 | 4650 | 5.4 | 3142 | 3.6 | 9112 | 10.6 | 6789 | 7.9 |
| Black | 760 | 0.9 | 294 | 38.7 | 102 | 13.4 | 28 | 3.7 | 51 | 6.7 | 34 | 4.5 | 150 | 19.7 | 133 | 17.5 |
| South Asian | 408 | 0.5 | 130 | 31.9 | 34 | 8.3 | 20 | 4.9 | 22 | 5.4 | 10 | 2.5 | 48 | 11.8 | 74 | 18.1 |
| Other | 746 | 0.8 | 255 | 34.2 | 79 | 10.6 | 22 | 2.9 | 61 | 8.2 | 35 | 4.7 | 114 | 15.3 | 114 | 15.3 |
| Missing | 400 | 0.9 | 86 | 21.5 | 40 | 10.0 | 9 | 2.3 | 18 | 4.5 | 15 | 3.8 | 34 | 8.5 | 27 | 6.8 |
| Education | ||||||||||||||||
| College/university or equivalent | 18 902 | 21.4 | 5869 | 31.0 | 2514 | 13.3 | 828 | 4.4 | 1543 | 8.2 | 976 | 5.2 | 2429 | 12.9 | 2328 | 12.3 |
| Other formal qualification | 44 233 | 50.0 | 11 990 | 27.1 | 5356 | 12.1 | 1479 | 3.3 | 2588 | 5.9 | 1785 | 4.0 | 5194 | 11.7 | 3787 | 8.6 |
| None | 23 953 | 27.1 | 4078 | 17.0 | 1753 | 7.3 | 452 | 1.9 | 633 | 2.6 | 450 | 1.9 | 1722 | 7.2 | 943 | 3.9 |
| Missing | 1342 | 1.5 | 269 | 20.0 | 102 | 7.6 | 32 | 2.4 | 38 | 2.8 | 25 | 1.9 | 113 | 8.4 | 79 | 5.9 |
| Smoking | ||||||||||||||||
| Have smoked/smoker | 37 863 | 42.8 | 9873 | 26.1 | 4510 | 11.9 | 1296 | 3.4 | 2102 | 5.6 | 1435 | 3.8 | 4255 | 11.2 | 3103 | 8.2 |
| Body mass index (kg/m2) | ||||||||||||||||
| Underweight (11–19) | 3198 | 3.6 | 830 | 26.0 | 339 | 10.6 | 112 | 3.5 | 223 | 7.0 | 112 | 3.5 | 367 | 11.5 | 271 | 8.5 |
| Normal (20–24) | 35 245 | 39.9 | 9778 | 27.7 | 4368 | 12.4 | 1204 | 3.4 | 2420 | 6.9 | 1274 | 3.6 | 4291 | 12.2 | 3281 | 9.3 |
| Overweight (25–29) | 32 142 | 36.3 | 7804 | 24.3 | 3444 | 10.7 | 987 | 3.1 | 1552 | 4.8 | 1196 | 3.7 | 3227 | 10.0 | 2451 | 7.6 |
| Obese (30–39) | 15 522 | 17.6 | 3297 | 21.2 | 1392 | 9.0 | 420 | 2.7 | 520 | 3.4 | 567 | 3.7 | 1365 | 8.8 | 972 | 6.3 |
| Morbidly obese (40+) | 1341 | 1.5 | 261 | 19.5 | 99 | 7.4 | 44 | 3.3 | 37 | 2.8 | 51 | 3.8 | 116 | 8.7 | 76 | 5.7 |
| Missing/erroneous | 982 | 1.1 | 236 | 48.0 | 83 | 16.7 | 24 | 5.0 | 50 | 9.8 | 36 | 7.5 | 92 | 18.7 | 86 | 17.6 |
| OCP (ever use) | 54 793 | 62.0 | 16 091 | 29.4 | 7561 | 13.8 | 2032 | 3.7 | 3644 | 6.7 | 2394 | 4.4 | 6875 | 12.5 | 5054 | 9.2 |
| MHT (ever use) | 14 454 | 16 | 5319 | 36.8 | 2769 | 19.2 | 619 | 4.3 | 1301 | 9.0 | 766 | 5.3 | 2204 | 15.2 | 1553 | 10.7 |
| History of hysterectomy a | 15 432 | 17.5 | 3950 | 25.6 | 1762 | 11.4 | 486 | 3.1 | 882 | 5.7 | 636 | 4.1 | 1585 | 10.3 | 1217 | 7.9 |
| Tubal ligation | 17 968 | 20.3 | 4730 | 26.3 | 2080 | 11.6 | 582 | 3.2 | 961 | 5.3 | 712 | 4.0 | 1995 | 11.1 | 1446 | 20.3 |
| Pregnancies <6 months | ||||||||||||||||
| 0 | 60 905 | 68.9 | 14 809 | 24.3 | 6504 | 10.7 | 1842 | 3.0 | 3109 | 5.1 | 2072 | 3.4 | 6233 | 10.2 | 4646 | 7.6 |
| 1 | 18 045 | 20.4 | 4741 | 26.3 | 2046 | 11.3 | 602 | 3.3 | 1059 | 5.9 | 728 | 4.0 | 2073 | 11.5 | 1576 | 8.7 |
| 2 | 5359 | 6.1 | 1575 | 29.4 | 694 | 13.0 | 200 | 3.7 | 377 | 7.0 | 262 | 4.9 | 667 | 12.4 | 546 | 10.2 |
| 3 | 1764 | 2.0 | 477 | 27.0 | 211 | 12.0 | 71 | 4.0 | 132 | 7.5 | 79 | 4.5 | 211 | 12.0 | 177 | 10.0 |
| 4+ | 1314 | 1.5 | 360 | 27.4 | 170 | 12.9 | 47 | 3.6 | 83 | 6.3 | 65 | 4.9 | 174 | 13.2 | 109 | 8.3 |
| Missing | 1043 | 1.2 | 244 | 23.4 | 100 | 9.6 | 29 | 2.8 | 42 | 4.0 | 30 | 2.9 | 100 | 9.6 | 83 | 8.0 |
| Parity – pregnancies >6 months | ||||||||||||||||
| 0 | 10 610 | 12.0 | 2979 | 28.1 | 1420 | 13.4 | 441 | 4.2 | 772 | 7.3 | 517 | 4.9 | 1250 | 11.8 | 936 | 8.8 |
| 1 | 10 402 | 11.8 | 2719 | 26.1 | 1230 | 11.8 | 371 | 3.6 | 621 | 6.0 | 437 | 4.2 | 1150 | 11.1 | 844 | 8.1 |
| 2 | 38 687 | 43.7 | 10 297 | 26.6 | 4598 | 11.9 | 1228 | 3.2 | 2197 | 5.7 | 1372 | 3.5 | 4427 | 11.4 | 3339 | 8.6 |
| 3 | 19 559 | 22.1 | 4419 | 22.6 | 1767 | 9.0 | 527 | 2.7 | 876 | 4.5 | 652 | 3.3 | 1887 | 9.6 | 1414 | 7.2 |
| 4+ | 8 993 | 10.2 | 1746 | 19.4 | 691 | 7.7 | 220 | 2.4 | 328 | 3.6 | 254 | 2.8 | 724 | 8.1 | 585 | 6.5 |
| Missing | 179 | 0.2 | 46 | 25.7 | 19 | 10.6 | 4 | 2.2 | 8 | 4.5 | 4 | 2.2 | 20 | 11.2 | 19 | 10.6 |
| Cancer history b | 6 878 | 7.8 | 1443 | 21.0 | 527 | 7.7 | 217 | 3.2 | 289 | 4.2 | 270 | 3.9 | 592 | 8.6 | 480 | 7.0 |
| History of breast cancer | 4 780 | 5.4 | 1008 | 21.1 | 359 | 7.5 | 152 | 3.2 | 218 | 4.6 | 188 | 3.9 | 410 | 8.6 | 337 | 7.1 |
| History of bowel cancer | 648 | 0.7 | 110 | 17.0 | 44 | 6.8 | 18 | 2.8 | 22 | 3.4 | 17 | 2.6 | 43 | 6.6 | 33 | 5.1 |
| History of gynecological (ovarian/endometrial) cancer | 376 | 0.4 | 84 | 22.3 | 39 | 10.4 | 14 | 3.7 | 12 | 3.2 | 17 | 4.5 | 31 | 8.2 | 24 | 6.4 |
OCP, oral contraceptive pill; MHT, menopausal hormone therapy
, Reported at recruitment; b, history of cancer captured at recruitment (breast, bowel, lung, other), or (breast, bowel, gynecological) at follow-up questionnaire
Ever-use of CAM/NPI
Of all the women who completed the questionnaire, 25.1% (22 206/88 430) reported ‘ever-use’ of at least one of the six types of CAM/NPI for menopausal symptom relief (Table 1). The prevalence of CAM/NPI use across responders ranged from 3.2% (2791/88 430) for homeopathy to 11.0% (9725/88 430) for herbal remedies. In those reporting CAM/NPI use, the most commonly used therapies were herbal remedies (43.8%; 9725/22 206), vitamins (42.6%; 9458/22 206) and lifestyle changes (32.1%; 7137/22 206) followed by phytoestrogens (21.6%; 4802/22 206). A smaller percentage of women used aromatherapy/reflexology/acupuncture (ARA) (14.6%; 3236/22 206) and homeopathy (12.6%; 2791/22 206) (Table 1).
Of the 4780 (5.4%) who reported a history of breast cancer, 8.3% (396/4780) had used MHT and 21.1% (1008/4780) had used one of the CAM/NPI.
Of the women who had ever-used CAM/NPI, 57.6% (12 799/22 206) indicated they had only used one type of therapy, with herbal remedies being most frequently used (3965), 25.9% (5756/22 206) had used two therapies with a combination of herbal remedies and vitamins (1254) most commonly used. Three or more therapies were used by 16.4% (3651/22 206) of the women (data not shown).
Correlations between CAM/NPI types
Correlations between the six different types of CAM/NPI were examined using tetrachoric correlations. All observed correlations were strong, with a minimum of 0.39 (herbal and lifestyle) and a maximum of 0.64 (herbal and phytoestrogens). Other correlations over 0.6 were for ARA and homeopathy (0.61) and lifestyle and ARA (0.63). These unadjusted tetrachoric correlations are shown in the lower triangle of Supplementary Table S1 (see http://dx.doi.org/10.1080/13697137.2017.1301919). The upper triangle gives the between CAM/NPI type correlations estimated following adjustment from the model and are largely similar to the unadjusted correlations, though mostly slightly lower.
Predictors of CAM/NPI use
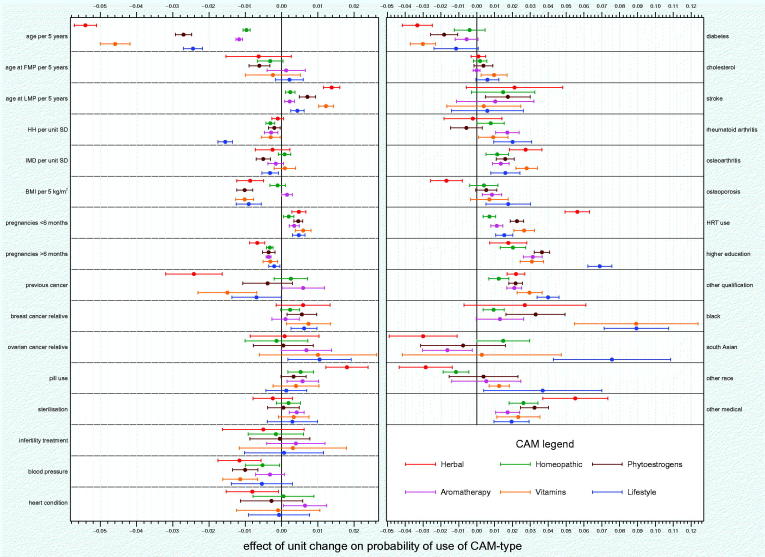
Due to the inclusion of the deprivation (IMD) variable based on women who resided in England, the modeling was restricted to 66 577 cases. There were missing covariate data in 7053 cases and excluding these meant that 59 524 cases were included in the final model. For each potential predictor, Figure 1 depicts the estimated effect on probability of ever-use of each CAM/NPI type for a unit change in that predictor, with 95% CIs. The actual values that underlie this plot are presented in Supplementary Table S2 (see http://dx.doi.org/10.1080/13697137.2017.1301919), and the original model estimates in the probit scale are presented in Supplementary Table S3 (see http://dx.doi.org/10.1080/13697137.2017.1301919).
Figure 1.
Effect on the probability of each type of CAM/NPI use for a unit change in each predictor variable. Herbal, herbal remedies; Homeopathic, homeopathic remedies; Phytoestrogens, phytoestrogens or soy products; Aromatherapy, aromatherapy, reflexology or acupuncture; Lifestyle, lifestyle changes; FMP, first menstrual period; LMP, last menstrual period; HH, hopelessness scale; IMD, index of multiple deprivation; BMI, body mass index; HRT, hormone therapy; pill, oral contraceptive pill.
There was a wide range of effect across the predictors with some having an unequivocal effect on CAM/NPI use and some being only minimally related to CAM/NPI use. In addition, it was notable that some predictors had a consistent effect across the six CAM/NPI types whilst others had a discrepant effect. In general, the predictors for herbal remedies were often quite different to those for the other CAM/NPIs, an effect which was also observed for lifestyle changes but to a lesser extent.
Age was a strong negative predictor of ever-use of CAM/NPI types, especially herbal remedies (each 5-year increase in age reduced ever-use by 5.4% (95% CI 5.1–5.7%) (Figure 1). This is also apparent in the overall set of 22 206 women (Table 1). With increasing age, there was a decline in ever-use of herbal remedies and vitamins and a less pronounced decline in ever-use of phytoestrogens (Figure 1). The uses of ARA, homeopathy and lifestyle change were similar across all the age groups (Figure 1).
Other consistently negative predictors were BMI (each additional 5 kg/m2 reduced most CAM/NPI type use by around 1%), high blood pressure and diabetes. Hopelessness (HH) had a small but clear negative effect on use of all CAM/NPI types. Women who had a low HH score (more hopeful) were more likely to use any CAM/NPI to relieve menopausal symptoms, with lifestyle changes being the most likely. Interestingly, the number of full-term pregnancies had a consistent negative effect across CAM/NPI types, whereas the number of pregnancies under 6 months had a consistent positive effect on CAM/NPI usage (around 0.5% per pregnancy).
Age at last menstrual period had a consistent positive effect on CAM/NPI usage (ranging from 0.2% for ARA to 1.4% for herbal remedies for each 5-year increase). Other clear positive effects were also found with osteoarthritis, MHT use (though much higher for herbal remedies), higher education (though much higher for lifestyle changes), other formal qualifications and being of Black ethnicity. OCP use had a positive effect on all CAM/NPI types but the effect was only unequivocal for herbal remedies (1.8%; 95% CI 1.2–2.4%).
Some predictors had a less consistent effect across the six CAM/NPI types, notably previous cancer only negatively affecting use of herbal remedies and to a lesser extent vitamins, and both osteoporosis, South Asian and ‘Other’ ethnicity appearing to have a negative effect on use of herbal remedies and a positive effect on lifestyle changes.
Discussion
In our study of over 88 000 postmenopausal women, which to our knowledge is the largest report on the subject, one in four women used at least one CAM therapy/NPI for menopausal symptom relief. Herbal therapies and vitamins were most commonly used followed by lifestyle approaches. Higher levels of education and previous MHT use were positive predictors of CAM/NPI use while women who were overweight or obese, had diabetes/hypertension or held less hopeful views about the future and their ability to achieve personal goals were less likely to use CAM/NPI.
Previous studies have reported CAM use to range from 24% to 91% 13 . The prevalence in our study (25%) was markedly lower than the 57% reported in a recent meta-analysis of three UK studies involving 3742 climacteric women 14 . However, two of these studies were undertaken prior to the publication of the WHI results in 2002.
Herbal remedies were most commonly used, as reported in other studies 19–21 , followed by vitamins and lifestyle changes. This trend persisted on subgroup analysis restricted to women who had only used one approach and is in keeping with results of a 2013 systematic review 11 . It needs to be noted, however, that some of the vitamin preparations may have contained phytoestrogens. One in five of the CAM users opted for a lifestyle change while one in four used at least two different types of CAM.
The association of age with type of CAM/NPI use for menopausal symptom relief is likely to be a cohort effect. Women of all age groups reported similar use of aromatherapy/reflexology/acupuncture, homeopathy and lifestyle changes for relief of menopausal symptoms. These approaches have been in use since the 1960s and are therefore likely to have been available when older women in our cohort were going through the menopause. In contrast, younger women reported higher ever-use of herbal remedies, vitamins and phytoestrogens, reflecting the more recent popularity of these regimes.
Consistent with the existing literature 19 , 22–24 , higher education positively influenced CAM/NPI use. This may reflect a greater propensity to seek out health information (books, magazines, internet), leading to greater knowledge on the availability of CAM for menopausal symptom relief 21 . Alternatively, as women with a higher level of education have been shown to have less severe hot flushes and night sweats 25 , it is possible that milder symptoms may have contributed to the decision to use CAM/NPI rather than MHT.
The financial cost of these therapies is known to influence women’s choices 26 . However, our data do not show a clear association between socioeconomic status, based on the deprivation (IMD) score, and use of CAM therapies/NPI for menopausal symptoms, which is at odds with previous reports 14 , 27 , 28 . Only phytoestrogens, and to some extent lifestyle changes, were less likely to be used with increasing deprivation.
A variety of other factors predicted CAM/NPI use with some having an unequivocal effect whilst others had only a minimal impact. Interestingly, predictors of herbal remedy use were often quite different to the others. In contrast to the results reported by the Study of Women’s Health across the Nation (SWAN) 14 , we found higher BMI to be negatively associated with ever use of CAM/NPI. A similarly negative correlation was also seen in those reporting hypertension and diabetes. CAM/NPI users have previously been described to smoke less 29 but we were not able to confirm this in our cohort. Although being of Black ethnicity was a strong predictor of CAM/NPI use, there were only 294 women in this group representing 1.3% of CAM/NPI users.
There is a large body of evidence suggesting that hopelessness is related to the development and outcome of mental and physical health conditions, ranging from depression to cardiovascular disease and cancer 30–37 . Women who were less hopeful about the future and their ability to attain personal goals were less likely to use any CAM/NPI and had the lowest use of lifestyle approaches to relieve menopausal symptoms. Previous studies have suggested that people who see their medical symptoms as an opportunity for personal development and learning are more likely to use CAMs 38 . Furthermore, studies in cancer patients have shown that many consider use of CAMs in addition to standard medical methods as one way of coping and actively addressing feelings of hopelessness 39 .
Use of MHT (as in the SWAN study) 27 and OCP positively predicted CAM/NPI ever use with the largest effect seen for herbal remedies. It is likely that women who are proactive in taking medicines to take control of their health are more likely to use CAM/NPI. Many women in our cohort stopped using MHT following the negative publicity of the 2002 WHI results 1 . It is possible that these women subsequently chose CAM/NPI as an alternative for menopausal symptom relief 40 .
Despite the negative publicity surrounding MHT and the initial scare about an increase in breast cancer risk, previous cancer history did not predict overall CAM/NPI use. Of the women with breast cancer history, 21% reported use of any CAM/NPI type, which is similar to the usage across the entire cohort. This is in keeping with the recommendation that women with a history of breast cancer should consider non-hormonal options 41 .
Since the initial WHI report, the re-analysis of the trial data 42 and subsequent studies have shown very beneficial risk–benefit ratios for healthy women aged 50–60 years. In 2016, the International Menopausal Society 43 and NICE 44 issued comprehensive guidance on use of MHT that reflects this revised perspective. It is likely that this may reverse the cycle of falling MHT and increasing CAM/NPI use. Currently, the North American Menopause Society only recommends cognitive behavioral therapy and clinical hypnosis as non-hormonal, non-medicinal approaches for menopausal symptom relief 45 .
Strengths and limitations
A key strength of our study is its size. The findings are likely to be more representative of CAM/NPI use in postmenopausal women in the general population, as self-selection bias in this large cohort taking part in a screening trial is likely to be less than in small studies focusing on the menopause. It is also likely that self-reporting in a clinical trial setting facilitated reporting of CAM/NPI use, as reports suggest that 55–72% of women do not wish to discuss/disclose CAM use to their health-care professionals 46 , 47 . A limitation in keeping with all patient surveys is that the reported CAM/NPI prevalence could have been influenced by a healthy volunteer effect 48 . We also had an overrepresentation of White ethnicity in our cohort (97%) compared with the 2011 Census (92.1%) 49 . While we have not reported on the duration of use and efficacy of CAM/NPI across the whole cohort, this has been reported in a subgroup of 10 000 participants who consented to a more detailed study 40 .
Conclusion
One in four postmenopausal women in the UK reported use of CAM/NPI for relief of menopausal symptoms, with herbal remedies being most commonly used. Higher CAM/NPI use was reported by women currently in their fifties and sixties. Higher education, Black ethnicity, MHT and OCP use were associated with higher CAM/NPI use while increasing BMI and diabetes/hypertension were associated with less use. Women who were less hopeful about the future and their ability to attain personal goals were less likely to use any type of CAM/NPI and had the lowest use of lifestyle approaches to relieve menopausal symptoms.
Supplementary Material
Acknowledgements
We are particularly grateful to the women throughout the UK who are participating in UKCTOCS and to the entire medical, nursing, and administrative staff who work on the trial. We thank all the staff involved in the trial for their hard work and dedication. We thank the members of the Data Monitoring and Ethics Committee and the independent Trial Steering Committee. Ethical approval: The study was approved on the 30 May 2006 by The Joint UCL/UCLH Committees on the Ethics of Human Research (Committee A), REC Reference: 06/Q0505/36.
Conflict of interest
U.M. and I.J. have a financial interest through Abcodia Ltd in the third-party exploitation of the UKCTOCS biobank. I.J. is a co-inventor of the ‘Risk of Ovarian Cancer Algorithm’, which has been licensed to Abcodia. The remaining authors declare no conflict of interest.
Source of funding
UKCTOCS was funded by the Medical Research Council (G9901012, G0801228), Cancer Research UK (C1479/A2884), and the Department of Health, with additional support from The Eve Appeal. Researchers at UCL were supported by the National Institute for Health Research (NIHR) University College London Hospitals (UCLH) Biomedical Research Centre. The funding source or the sponsor had no role in data collection, data analysis, data interpretation, or writing of the report. The researchers are independent from the funders.
References
- 1. Rossouw JE, Anderson GL, Prentice RL, et al Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA 2002;288:321–33 [DOI] [PubMed] [Google Scholar]
- 2. Hersh AL, Stefanick ML, Stafford RS.. National use of postmenopausal hormone therapy: annual trends and response to recent evidence. JAMA 2004;291:47–53 [DOI] [PubMed] [Google Scholar]
- 3. Haas JS, Kaplan CP, Gerstenberger EP, Kerlikowske K.. Changes in the use of postmenopausal hormone therapy after the publication of clinical trial results. Ann Intern Med 2004;140:184–8 [DOI] [PubMed] [Google Scholar]
- 4. Buist DS, Newton KM, Miglioretti DL, et al Hormone therapy prescribing patterns in the United States. Obstet Gynecol 2004;104:1042–50 [DOI] [PubMed] [Google Scholar]
- 5. Faber A, Bouvy ML, Loskamp L, et al Dramatic change in prescribing of hormone replacement therapy in The Netherlands after publication of the Million Women Study: a follow-up study. Br J Clin Pharmacol 2005;60:641–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gayet-Ageron A, Amamra N, Ringa V, et al Estimated numbers of postmenopausal women treated by hormone therapy in France. Maturitas 2005;52:296–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Menon U, Burnell M, Sharma A, et al Decline in use of hormone therapy among postmenopausal women in the United Kingdom. Menopause 2007;14:462–7 [DOI] [PubMed] [Google Scholar]
- 8. Complementary, Alternative, or Integrative Health : What’s In a Name? Available at: https://nccih.nih.gov/health/integrative-health. Accessed 26 January 2017
- 9. MacLennan AH, Wilson DH, Taylor AW.. The escalating cost and prevalence of alternative medicine. Prev Med 2002;35:166–73 [DOI] [PubMed] [Google Scholar]
- 10. NICE issues first guideline on menopause to stop women suffering in silence Available at: https://www.nice.org.uk/news/press-and-media/nice-issues-first-guideline-on-menopause-to-stop-women-suffering-in-silence. Accessed 26 January 2017 [Google Scholar]
- 11. Posadzki P, Lee MS, Moon TW, et al Prevalence of complementary and alternative medicine (CAM) use by menopausal women: a systematic review of surveys. Maturitas 2013;75:34–43 [DOI] [PubMed] [Google Scholar]
- 12. Peng W, Adams J, Sibbritt DW, Frawley JE.. Critical review of complementary and alternative medicine use in menopause: focus on prevalence, motivation, decision-making, and communication. Menopause 2014;21:536–48 [DOI] [PubMed] [Google Scholar]
- 13. Daley A, MacArthur C, McManus R, et al Factors associated with the use of complementary medicine and non-pharmacological interventions in symptomatic menopausal women. Climacteric 2006;9:336–46 [DOI] [PubMed] [Google Scholar]
- 14. Bair YA, Gold EB, Azari RA, et al Use of conventional and complementary health care during the transition to menopause: longitudinal results from the Study of Women’s Health Across the Nation (SWAN). Menopause 2005;12:31–9 [DOI] [PubMed] [Google Scholar]
- 15. Harris PE, Cooper KL, Relton C, Thomas KJ.. Prevalence of complementary and alternative medicine (CAM) use by the general population: a systematic review and update. Int J Clin Practice 2012;66:924–39 [DOI] [PubMed] [Google Scholar]
- 16. Menon U, Gentry-Maharaj A, Ryan A, et al Recruitment to multicentre trials-lessons from UKCTOCS: descriptive study. BMJ (Clin Res Ed) 2008;337:a2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fraser L, Burnell M, Salter LC, et al Identifying hopelessness in population research: a validation study of two brief measures of hopelessness. BMJ Open 2014;4:e005093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Multivariate probit regression using simulated maximum likelihood Available at: http://www.stata-journal.com/article.html?article=st0045. Accessed 26 January 2017 [Google Scholar]
- 19. Gollschewski S, Anderson D, Skerman H, Lyons-Wall P.. Associations between the use of complementary and alternative medications and demographic, health and lifestyle factors in mid-life Australian women. Climacteric 2005;8:271–8 [DOI] [PubMed] [Google Scholar]
- 20. Cardini F, Lesi G, Lombardo F, van der Sluijs C.. The use of complementary and alternative medicine by women experiencing menopausal symptoms in Bologna. BMC Womens Health 2010;10:7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Duffy OK, Iversen L, Hannaford PC.. The impact and management of symptoms experienced at midlife: a community-based study of women in northeast Scotland. BJOG 2012;119:554–64 [DOI] [PubMed] [Google Scholar]
- 22. Lunny CA, Fraser SN.. The use of complementary and alternative medicines among a sample of Canadian menopausal-aged women. J Midwifery Womens Health 2010;55:335–43 [DOI] [PubMed] [Google Scholar]
- 23. Bair YA, Gold EB, Greendale GA, et al Ethnic differences in use of complementary and alternative medicine at midlife: longitudinal results from SWAN participants. Am J Public Health 2002;92:1832–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Newton KM, Buist DS, Keenan NL, et al Use of alternative therapies for menopause symptoms: results of a population-based survey. Obstet Gynecol 2002;100:18–25 [DOI] [PubMed] [Google Scholar]
- 25. Hunter MS, Gentry-Maharaj A, Ryan A, et al Prevalence, frequency and problem rating of hot flushes persist in older postmenopausal women: impact of age, body mass index, hysterectomy, hormone therapy use, lifestyle and mood in a cross-sectional cohort study of 10,418 British women aged 54-65. BJOG 2012;119:40–50 [DOI] [PubMed] [Google Scholar]
- 26. Daoust JL, Mercer LC, Duncan AM.. Prevalence of natural health product use in healthy postmenopausal women. Menopause 2006;13:241–50 [DOI] [PubMed] [Google Scholar]
- 27. Bair YA, Gold EB, Zhang G, et al Use of complementary and alternative medicine during the menopause transition: longitudinal results from the Study of Women’s Health Across the Nation. Menopause 2008;15:32–43 [DOI] [PubMed] [Google Scholar]
- 28. Dailey RK, Neale AV, Northrup J, et al Herbal product use and menopause symptom relief in primary care patients: a MetroNet study. J Womens Health (Larchmt) 2003;12:633–41 [DOI] [PubMed] [Google Scholar]
- 29. Haskell SG, Bean-Mayberry B, Gordon K.. Discontinuing postmenopausal hormone therapy: an observational study of tapering versus quitting cold turkey: is there a difference in recurrence of menopausal symptoms?. Menopause 2009;16:494–9 [DOI] [PubMed] [Google Scholar]
- 30. Alloy LB, Abramson LY, Metalsky GI, Hartlage S.. The hopelessness theory of depression: attributional aspects. Br J Clin Psychol/Br Psychol Soc 1988;27:5–21 [DOI] [PubMed] [Google Scholar]
- 31. Beck AT, Steer RA, Kovacs M, Garrison B.. Hopelessness and eventual suicide: a 10-year prospective study of patients hospitalized with suicidal ideation. Am J Psychiatry 1985;142:559–63 [DOI] [PubMed] [Google Scholar]
- 32. Everson SA, Kaplan GA, Goldberg DE, Salonen JT.. Hypertension incidence is predicted by high levels of hopelessness in Finnish men. Hypertension 2000;35:561–7 [DOI] [PubMed] [Google Scholar]
- 33. Everson SA, Kaplan GA, Goldberg DE, et al Hopelessness and 4-year progression of carotid atherosclerosis. The Kuopio Ischemic Heart Disease Risk Factor Study. Arterioscler Thromb Vasc Biol 1997;17:1490–5 [DOI] [PubMed] [Google Scholar]
- 34. Whipple MO, Lewis TT, Sutton-Tyrrell K, et al Hopelessness, depressive symptoms, and carotid atherosclerosis in women: the Study of Women’s Health Across the Nation (SWAN) heart study. Stroke 2009;40:3166–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dunn SL, Corser W, Stommel M, Holmes-Rovner M.. Hopelessness and depression in the early recovery period after hospitalization for acute coronary syndrome. J Cardiopulm Rehabil 2006;26:152–9 [DOI] [PubMed] [Google Scholar]
- 36. Watson M, Homewood J, Haviland J.. Coping response and survival in breast cancer patients: a new analysis. Stress Health 2012;28:376–80 [DOI] [PubMed] [Google Scholar]
- 37. Molassiotis A, Van Den Akker OB, Milligan DW, Goldman JM.. Symptom distress, coping style and biological variables as predictors of survival after bone marrow transplantation. J Psychosom Res 1997;42:275–85 [DOI] [PubMed] [Google Scholar]
- 38. Bishop FL, Yardley L, Lewith GT.. Why do people use different forms of complementary medicine? Multivariate associations between treatment and illness beliefs and complementary medicine use. Psychol Health 2006;21:683–98 [Google Scholar]
- 39. Söllner W, Maislinger S, DeVries A, et al Use of complementary and alternative medicine by cancer patients is not associated with perceived distress or poor compliance with standard treatment but with active coping behavior: a survey. Cancer 2000;89:873–80 [DOI] [PubMed] [Google Scholar]
- 40. Gentry-Maharaj A, Karpinskyj C, Glazer C, et al Use and perceived efficacy of complementary and alternative medicines after discontinuation of hormone therapy: a nested United Kingdom Collaborative Trial of Ovarian Cancer Screening cohort study. Menopause 2015;22:384–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hamoda H, Panay N, Arya R, Savvas M.. The British Menopause Society & Women’s Health Concern 2016 recommendations on hormone replacement therapy in menopausal women. Post Reproductive Health 2016; 22:165–83 [DOI] [PubMed] [Google Scholar]
- 42. Manson JE, Chlebowski RT, Stefanick ML, et al Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA 2013;310:1353–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Baber RJ, Panay N, Fenton A. IMS Writing Group . 2016 IMS Recommendations on women’s midlife health and menopause hormone therapy. Climacteric 2016;19:109–50 [DOI] [PubMed] [Google Scholar]
- 44. National Institute for Health and Care Excellence (NICE) Menopause: diagnosis and management: NICE Guideline NG23. 2015. [PubMed] [Google Scholar]
- 45. Nonhormonal management of menopause-associated vasomotor symptoms: 2015 position statement of The North American Menopause Society. Menopause 2015;22:1155–72 [DOI] [PubMed] [Google Scholar]
- 46. Geller SE, Studee L.. Botanical and dietary supplements for menopausal symptoms: what works, what does not. J Womens Health (Larchmt) 2005;14:634–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Posadzki P, Ernst E.. Prevalence of CAM use by UK climacteric women: a systematic review of surveys. Climacteric 2013;16:3–7 [DOI] [PubMed] [Google Scholar]
- 48. Burnell M, Gentry-Maharaj A, Ryan A, et al Impact on mortality and cancer incidence rates of using random invitation from population registers for recruitment to trials. Trials 2011;12:61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Office for National Statistics Ethnicity and National Identity in England and Wales: 2011. Available at: http://www.ons.gov.uk/peoplepopulationandcommunity/culturalidentity/ethnicity/articles/ethnicityandnationalidentityinenglandandwales/2012-12-11 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.