Abstract
Alzheimer's disease (AD) is a progressive neurodegenerative disease of the elderly. The rapid increase in its incidence has necessitated development of newer drugs. Ayurvedic herbal medications are increasingly researched due to their biosafety profile and usefulness in cognitive impairment. In this article, we critically reviewed one such Medhya Rasayana (nootropic drug) Brahmi-derived from extract of Bacopa monnieri (EBm). Studies have shown that EBm promotes free radical scavenger mechanisms and protects cells in prefrontal cortex, hippocampus, and striatum against cytotoxicity and DNA damage implicated in AD. It also reduces lipoxygenase activity reducing lipid peroxidation, increases glutathione peroxidase and chelates iron. Administration of EBm was seen to protect the cholinergic neurons and reduce anticholinesterase activity comparable to donepezil, rivastigmine, and galantamine. It also reduces hippocampal β-amyloid deposition and stress-induced hippocampal damage. The neuroprotective effect of EBm is also due to nitric oxide-mediated cerebral vasodilation. EBm improved the total memory score and maximum improvement was seen in logical memory and paired associate learning in humans and reversed phenytoin-induced memory impairment in experimental model. EBm has not shown any serious clinical, neurological, hematological complications, or vital organs damage in experimental studies. Rats showed marked reduction in fertility; however, libido was unaffected. There is no experimental evidence of genotoxicity or teratogenesis by use of EBm. Mild nausea and gastrointestinal upset are seen in humans. Brahmi promises to be a novel agent in AD; however, further human trials are recommended to verify the efficacy and rule out any side effects as evidenced by the experimental models.
Keywords: Alzheimer's disease, Bacopa monnieri, Brahmi, Nootropics, Ayurveda
Introduction
Alzheimer's disease (AD) is a chronic neurodegenerative disease of undetermined etiology, seen in the elderly albeit rarely before 60 years except when its inheritance is autosomal dominant [1, 2]. Combined presence of amyloid beta (Aβ) and tau (τ) stands out as the hallmark of progressive AD and the basis of most disease-modifying therapy [3, 4]. Initial stage of disease is characterized by the impairment of recent memory which is followed by impairment of cognitive abilities, vocabulary, and concepts [5]. Early impairment of recent memory is due to involvement of median temporal lobe and hippocampus which controls recent memory [6]. Subsequently, involvement of other areas of brain may manifest as sleep disturbances, problems in judgment, psychological changes, pyramidal and extrapyramidal motor signs [7]. According to World Alzheimer's Report 2015, global prevalence of dementia rose from 30 million (2010) to 46.8 million and global expenditure on dementia rose from US$ 604 million (2010) to US$ 818 million (2015). In India, the prevalence of dementia was 33.6 in every 1,000 people of which 54% were cases of AD [8].
The aforementioned statistics suggest a meteoric rise in the global burden of AD while the available medications remain few and constant. Herbal medications are gaining widespread acclaim globally in more than 80% of the world population, due to their higher biosafety profile over the synthetic medications. In this article, we extensively reviewed the literature of Bacopa monnieri (Brahmi), a nootropic herb that can serve as a promising agent in AD owing to its antioxidant, cholinergic, anti-beta amyloid property, and good safety profile.
Alzheimer's Disease
Genetics
Several genes play an important role in pathogenesis of AD. The most important and studied ones are presenelin-1 (PSEN-1), amyloid precursor protein (APP), presenelin-2 (PSEN-2), and apolipoprotein E (APOE). APP and PSEN-1 gene mutations result in early onset AD; APOE mutation is implemented in late-onset AD whereas PSEN-2 has a more variable onset [9].
PSEN-1 gene is present on chromosome 14q and functions to regulate intracellular Ca2+ signaling, trafficking of membrane proteins, and regulates the stabilization of β-catenin. It is observed that any mutation in PSEN-1 gene results in formation of varying lengths of Aβ peptides due to cleavage at different sites by γ-secretase. These peptides are highly fibrillogenic which in turn cause increased aggregation of Aβ plaques in brain [10].
APP gene is situated on chromosome 21q and functions to produce a protein called APP [9]. This protein is cleaved by α-, β-, and γ-secretase. In AD β- and γ-secretase cleave, the APP forms Aβ protein which has the property to get aggregated and form Aβ plaques that get deposited in the brain to cause neuronal degeneration [11].
PSEN-2 gene is located on chromosome 1q encodes PSEN-2 transmembrane protein whose isoform 2 is found in the brain. PSEN-2 mutations cause Aβ42 protein accumulation which is the hallmark of AD [12].
APOE gene is associated with production of Apo-E which helps in the removal of Aβ proteins as well as protects neurons from damage associated with it. There are 3 different forms of Apo-E protein; they are ε2, ε3, and ε4. Studies indicated that mutated oxidized form of ε4 binds readily to Aβ protein and results in deposition as amyloid plaques [9]. It has also shown decrease in cholinergic activity in brain [13]. Hence, ε4 is the isoform of Apo-E involved in pathogenesis of AD.
Pathogenesis
The pathogenesis of AD is not completely understood. However, there are 3 possible mechanisms: deposition of Aβ proteins, deposition of τ proteins in cytoplasm of neurons, and neuronal degeneration due to above 2 components [14].
APP is present in cell membrane as a transmembrane protein. It has intracellular and extracellular parts. It has cleavage sites for α-, β-, and γ-secretase enzymes. α-and β-secretase cleavage sites are present on the surface of the cell while γ-secretase has cleavage site in the intramembranous region of the protein. Normally cleavage by α-secretase is followed by cleavage by γ-secretase, which results in the formation of soluble protein. In an AD patient, β-secretase does the first cleavage which is followed by cleavage by γ-secretase resulting in the generation of Aβ protein which gets aggregated and gets deposited in various parts of the brain [14].
τ proteins provide stability to the microtubules. In AD, they undergo hyperphosphorylation to get aggregated and form neurofibrillary tangles in the cytoplasm of neurons.
Both components explained above cause degeneration of neurons in central nervous system and result in the symptoms of AD [14]. Aβ protein also gets deposited and weakens the walls of the blood vessels resulting in amyloid angiopathy [15].
Bacopa monnieri
Ayurvedic and other herbal medications have gained increased acceptance as they are found to be safer than the synthetic counterparts [16]. Ayurveda or the Indian system of Medicine viz. Sushruta Samhita, Charak Samhita, and Atharva Veda describe plants which have a prabhava (specific action) on the intellect and memory as Medhya Rasayana (Medhya – intellect or retention, Rasayana – procedure or preparation).
Traditionally, Mandukaparni, Yastimadhu, Guduchi, and Shankhapushpi have been mentioned to have a memory enhancing action. Others like Brahmi, Vacha, and Jatamamsi although inadequately emphasized have been known for their efficacy [17]. Vedic scholars of ancient India have been known to consume Medhya Rasayana that helped them memorize lengthy scriptures 3,000 years ago [18].
Botanical Characteristics
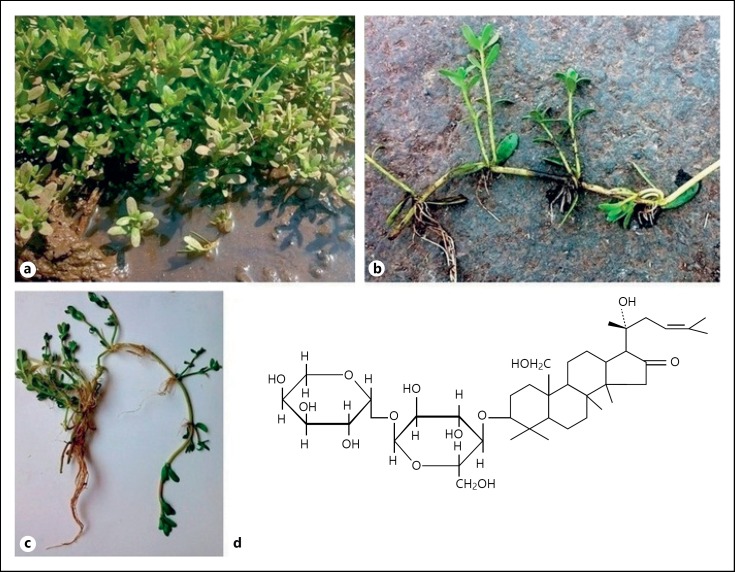
Bacopa monnieri (Hindi: Brahmi, Vietnamese: Rau Đắng, Herpestis monnieri or water hyssop), an herbal nutraceutical is recognized for its role as a Medhya Rasayana or a nootropic agent in Ayurveda [19]. It belongs to the Scrophulariaceae family growing in marshlands up to an altitude of 1,500 meters [20]. It is called “Brahmi” in India. It is seen distributed in the plains of Southeast Asia, tropical Asia, sub-tropical United States, tropical Africa, and Australia [21, 22]. The plant grows luxuriantly in wet soil near freshwater aquatic sites. It grows 6 inches to 3 feet in height and has oblong leaves and purple flowers as shown in Figure 1. The entire plant along with the roots can be used medicinally [18].
Fig. 1.
Bacopa monnieri: a Plant growing in marshlands; b, c Plant morphology; d chemical structure of bacoside.
Active Constituents
The active ingredients of extract of Bacopa monnieri (EBm) are enlisted below in Table 1. Saponins are the main active constituents of EBm responsible for most of its pharmacological actions [21].
Table 1.
Active constituents of EBm
| Chemical groups | Contents |
|---|---|
| Saponins | Bacoside A, bacoside B, bacopasaponins, D-mannitol, acid A, monnierin |
| Flavonoids | Apigenin, luteonin |
| Alkaloids | Brahmine, herpestine, hydrocotyline |
| Glycosides | Asiaticoside, thanakunicide |
| Phytochemicals | Betulinic acid, betulic acid, wogonin, oroxindin, stigmastarol, β-sitosterol |
| Sapogenin | Jujubacogenin, pseudojujubacogenin |
| Other constituents | Brahmic acid, brahamoside, brahminoside, isobrahmic acid |
Pharmacological Properties and Uses
Its main pharmacological actions are antioxidant, anti-inflammatory, anticonvulsant, cardiotonic, bronchodilator, and peptic ulcer protection [23, 24, 25]. Various indications for use of Brahmi described in Ayurvedic medicine are memory improvement, epilepsy, insomnia, and anxiolytic [26]. With regards to improvement of memory function, its effect is more on decreasing the forgetfulness rather than increasing learning [27].
Brahmi in AD
Antioxidant and Iron Chelating Properties of Brahmi
Oxidative stress is one of the most important factors in aging and age-related illnesses [28]. Various free radicals playing an important part in oxidative damage are hydroxyl (OH’), hydrogen peroxide (H2O2), peroxynitrite (ONO2−), and superoxide free radical (O2−’) [29]. Oxygen is absolutely important for survival but its excess results in the formation of reactive oxygen species (ROS) which can damage the brain [30]. Brain is particularly susceptible to free radical damage due to its high metabolic rate, unsaturated fatty acids in cell membranes, lower activity of antioxidant mechanisms like glutathione peroxidase (GPx) and catalase (CAT), and cytotoxic actions of glutamate [31].
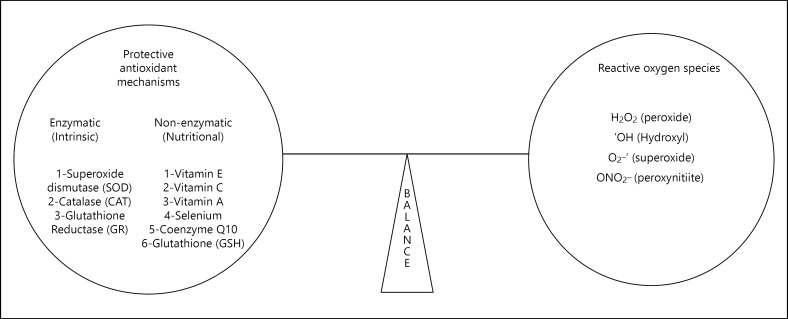
Our body has many free radical scavenger mechanisms which are enzymatic or non-enzymatic. Enzymatic ones include superoxide dismutase (SOD), CAT, glutathione reductase that act as a first line of defense against ROS while non-enzymatic ones like vitamin A, C, and E, selenium, coenzyme Q10, and glutathione (GSH) whose antioxidant actions protect neuronal tissue from free radical damage as shown in Figure 2[32]. Imbalance between protective antioxidant mechanisms and free radical species is the basis for free radical damage in elderly population resulting in aging and cognitive decline [33].
Fig. 2.
Balance between reactive oxygen species (ROS) and free radical scavenging mechanisms in the brain.
Many in vitro and animal studies have shown that EBm has an antioxidant and free radical scavenging action. Early studies showed that EBm inhibits lipid peroxidation in prefrontal cortex, hippocampus, and striatum of rats [34]. In one study conducted in rats, EBm was administered to the rats for 21 days. It showed increase in activity of enzymes SOD, CAT, and GPx in prefrontal cortex, hippocampus, and striatum. In comparison, deprenyl, a known antioxidant demonstrated increase in activity of these enzymes in prefrontal cortex and striatum but not in hippocampus [31]. In another in vitro study, human non-immortalized fibroblasts were tested for the DNA damage and free radical mediated cytotoxicity by H2O2, in which EBm was found to be protective [35].
Dhanasekaran et al. [36] in an in vitro study demonstrated mechanisms of nootropic action of EBm derived from dried leaves powder of the plant. They found that EBm significantly reduced the lipoxygenase activity in brain. It inhibited hydrogen peroxide-induced lipid peroxidation in mouse brain homogenates and also had significant iron chelating property. Here, iron chelating property needs special emphasis as iron as well as other divalent metals interact with Aβ protein and modulate several effects that are thought to be the pathogenic effects of the protein [37]. Falangola et al. [38] studied in transgenic mouse for the presence of iron in Aβ plaques in which they histologically demonstrated that iron was present in stained brain section of all mice that were studied. In the study by Dhanasekaran et al. [36], EBm administration resulted in significant chelation of iron in an incubated substrate which demonstrates another mechanism for anti-AD activity of EBm. Similarly, in another study in diabetic rats, it was demonstrated that oral administration of EBm for 15 days showed an increase in activity of SOD, CAT, GPx, GSH, and decrease in lipid peroxidation in rat brain [39].
In a study conducted by Shinomol et al. [40] in 2011, 3-nitropropionic acid induced oxidative damage was prevented by pre-administration of EBm in both in vivo as well as in vitro conditions. The levels of oxidative markers like malondialdehyde and hydroperoxide was significantly decreased in neuronal cytoplasm of mice on EBm prophylaxis. Several other studies demonstrating anti-stress activity of EBm showed that pre-treatment with EBm and bacoside A prevented lipid peroxidation and free radical damage in experimental models of diabetes, ischemia, cigarette exposure, and aluminum toxicity [41, 42]. Russo et al. [43] exposed astrocytes to nitric oxide (NO) using a donor S-nitroso-N-acetyl-penicillamine thus generating the peroxynitrite (ONO2−) free radical. EBm administered to the substrate for 18 h had a neutralizing effect on peroxynitrite.
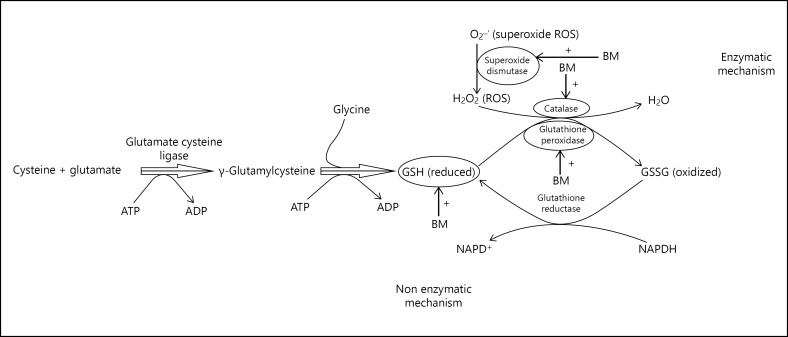
The main role of EBm as an antioxidant appears to be due to its effect on increasing concentration of GSH and enzymatic antioxidants like SOD, CAT, and GPx and as free radical scavenging agent as illustrated in Figure 3. Hence, its administration in indicated doses may act as a remedy for age-associated memory and cognitive decline in AD.
Fig. 3.
Enzymatic and non-enzymatic neuroprotective mechanism of EBm.
Brahmi on Cholinergic System
Apart from the hallmark pathological findings like Aβ plaques and neurofibrillary tangles of τ proteins, one of the very characteristic finding in AD is loss of cholinergic neurons from basal forebrain [44].
Le et al. [45] studied choline acetyltransferase (ChAT) expression in hippocampus of olfactory bulbectomized (OBX) mice compared with sham-operated control mice. OBX reduced cholinergic activity and hence also ChAT in hippocampus. Subsequent administration of EBm and tacrine to the substrate, however, reversed this effect showing amelioration OBX-induced cognition dysfunction. The above experiment demonstrated that daily administration of EBm protected the cholinergic neurons in the medial septal nucleus that projects into hippocampus. A study on rat model of AD demonstrated the inhibition of degeneration of cholinergic neurons on administration of alcoholic EBm which suggests that Brahmi could be a potential drug for treatment of AD [46].
Aforementioned studies demonstrate the cholinergic effects of EBm which are similar to the current treatments of AD like donepezil, rivastigmine, and galantamine, thus supporting the role of Brahmi as a therapeutic agent in AD.
Brahmi on Memory Impairment and Cognitive Defects
Amnesia and cognitive defects are cardinal symptoms of AD [5]. In many studies conducted on humans and animals, Brahmi has shown to improve memory performance and cognitive function. In a double-blind, randomized, placebo-controlled trial conducted in Lucknow in India, 35 subjects aged above 55 years were subjected to Wechsler Memory Scale for comparison between placebo and EBm treatment groups. Subjects were tested on various sub-tests like general information, orientation, mental control, logical memory, digit forward, digit backward, visual reproduction, and paired associated learning. Scores were given to each sub-test and total memory score was calculated by adding the score of all subtests. The test results showed that there was significant improvement in total memory score of EBm-treated patients vs. placebo-treated patients. At 8 and 12 weeks after initiation of trial, maximum increment was seen after 8th week with maximum improvement seen in logical memory and paired associate learning sub-tests [47]. This study suggests that EBm can be useful agent in treatment of age-associated memory impairment.
In another study double-blind, randomized, placebo-controlled study efficacy of Brahmi as a memory enhancer on chronic dosing in 76 adults who were given Brahmi 300 mg/day and placebo. Subjects were tested for tasks of attention, memory, and psychological state at baseline. The results observed at 6 and 12 weeks after the trial demonstrated an increase in information retaining capacity over time. This was due to decreased forgetfulness as opposed to increased procurement because Brahmi did not show any beneficial effect on learning trials [48]. In a randomized, double-blind placebo-controlled trial of 36 children with Attention Deficit Hyperactivity Disorder, improvement in logical memory was demonstrated [49]. In a randomized, double-blind, placebo-controlled trial conducted in Australia on 81 subjects aged above 55 years who were given EBm in the dose of 300 mg/day for 12 weeks, remarkable improvement was demonstrated in verbal learning, memory acquisition, and delayed recall [42].
Kumar et al. [50] investigated the effect of EBm on cold stress induced neurodegeneration in hippocampus of rats. Histologically, rat brains were divided into 4 groups: group 1 consisted of rats which were kept in ideal laboratory conditions, group 2 rats were given EBm in the dose 40 mg/kg, group 3 rats were forced to swim in the cold water (temperature: 18 ± 2°C) for 1 month which generated cold water swim stress in their body, and group 4 were given cold water swim stress for 1 month which was followed by treatment by EBm for about 1 month in the dose of 40 mg/kg. Histophotometric study of hippocampus was done in which diameter of cells, total number of cells in the square, and packing density of cells were taken into consideration. Group 3 cells showed decreased diameter of cells, number of cells per square, and packing density of cells which was indicative of stress-induced damage while group 4 cells showed increased cell diameter, number of cells per square, and cell packing density. Group 4 rats showed the above parameters comparable to that of group 1 rats. This study demonstrated that EBm has got important therapeutic effect in abolishing stress-induced hippocampal damage.
In an experimental model by Saini et al. [51], an intracerebroventricular injection of the drug colchicine was given to cause oxidative stress and increased lipid peroxidation, which resulted in significant memory loss. This was demonstrated by significant reduction in retention in elevated plus maze test. However, on treatment with EBm colchicine, administered animals showed a significant increase in retention time suggestive of cognitive improvement.
Antiepileptic drug phenytoin causes cognitive impairment on regular use in many patients. Using this principle, phenytoin was given to experimental rats in a dose of 25 mg/kg for 7 days resulting in significant cognitive impairment in the rats. Administration of EBm caused significant reversal of phenytoin-induced memory impairment [52].
Benzodiazepines are known to cause dementia by their GABAergic action and by interfering with long-term potentiation. Diazepam was administered in a dose of 1.75 mg/kg to induce amnesia studied using the Morris Water Maze Test in mice. This was reversed by EBm given orally in a dose of 120 mg/kg [53].
In a study conducted by Kishore and Singh [54], it was found that Brahmi attenuated scopolamine, sodium nitrite and BN52021 (platelet activating factor antagonist) induced amnesia. The possible mechanism was by improving acetylcholine levels in mice in the above setting and was observed by improved performance on the Morris Water Maze Test. In this study, bacoside treatment decreased escape latency time which indicates that bacosides have predominant action on attenuating anterograde amnesia.
Brahmi on Cerebral Blood Flow
Vascular factors like atherosclerosis, decreased vascular density, and cerebral perfusion have been linked to AD. AD is principally associated with cerebral cortex, hippocampus, and areas of the brain that are vulnerable to vascular dysfunction [55].
In a study conducted by Kamkaew et al. [56] on in vitro vasodilator properties of Brahmi, it was found that Brahmi decreased systolic and diastolic blood pressure without significantly affecting heart rate. Its administration in isolated arteries resulted in maximum dilation of basilar artery but did not have much effect on renal and tail artery of rat which shows its brain-specific vasodilator effect. This effect was significantly blunted when NO synthesis inhibitor was co-administered which indicates that Brahmi has vasodilatory action that is mediated by NO. In the same study, they also demonstrated that K+ depolarization induced Ca2+ influx and release Ca2+ from sarcoplasmic reticulum by administration of phenylephrine and caffeine was also inhibited by co-administration of Brahmi denoting an additional mechanism of its vasodilatory property.
Another study conducted by Kamkaew et al. [57] in which they compared the cerebral blood flow (CBF) augmenting property of EBm and Ginkgo biloba. They administered EBm, G. biloba (standardized extract) and donepezil to 3 test groups and water to the control group for 8 weeks. At the end of 8 weeks, they found out that EBm enhanced cognitive function in rats and increased quiescent CBF by 25% and animals treated with G. biloba showed an increment of 29%; however, donepezil had no significant effect on CBF in chronic setting. This study showed that chronic administration of EBm and G. biloba increased the cerebral blood possibly contributing to their nootropic and neuroprotective action.
Nobili et al. [58] conducted a study comparing the CBF in AD patients administered with donepezil with those who were not, that is, controls. Patients who were not treated with donepezil had decreased CBF and those who were treated with donepezil had no significant decrease in blood flow. This suggests that donepezil slows the vascular deterioration, but it does not increase the blood flow like EBm. This can indicate therapeutic importance of EBm in future for treatment of AD.
Brahmi on Neuronal and Glial Plasticity
Neuronal or glial plasticity is the ability of the brain to adapt to stress which involves complex processes like rearrangement of synaptic connections and network of neurons [59].
Brain derived neurotrophic factor (BDNF) is a member belonging to the family neurotrophin is an important marker of neuronal plasticity and has a critical role in the transcription of gene Arc which is connected with neuronal plasticity and memory. Low levels of BDNF predispose a person to AD [60].
Glial fibrillary acidic protein (GFAP) is an important marker of glial plasticity which regulates morphology of astrocytes, interactions between neuroglia and memory forming mechanisms [61]. Expression of GFAP is also substantially decreased in amnesic conditions [62].
Konar et al. [63] demonstrated in their study that on administration of scopolamine in mice, expression of BNDF, Arc and GFAP was significantly decreased. They found out that administration of EBm alone resulted in increased expression of BDNF by 1.3 times and Arc expression by 2 times however expression of GFAP was not increased upon treatment. In scopolamine-treated mice, pre- and post-administration of EBm resulted in enhancement of plasticity markers in cerebrum the effect being more marked in expression of BDNF and Arc expression than that of GFAP. Thus EBm has a significant role in the improvement of brain plasticity by a variety of mechanisms.
Brahmi on β-Amyloid
Deposition of Aβ protein in the brain parenchyma causing neuronal degeneration is the most important mechanism of pathogenesis in AD [14]. A study done by Mathew et al. [64] on anti-amyloidogenic potentials of various herbs revealed that methanolic EBm decreased the formation of amyloid fibrils almost entirely and segregated the pre-formed amyloid fibrils up to a considerable extent. Holcomb et al. [65] in their study conducted on PSAPP mice demonstrated that administration of EBm to mice expressing APP and PSEN-1 mutation reduced amyloidogenic proteins Aβ40 and Aβ42 levels in brain by approximately 60%, which clearly signifies its potential as a therapeutic strategy in AD.
Limpeanchob et al. [66] demonstrated the neuroprotective effect of EBm in Aβ protein-induced cell death in a primary cortical culture. They found that neurons treated with Aβ protein exhibited high 2-fold rise in acetylcholine esterase (AChE) concentration (which is a component and indicator of neuronal damage), while those treated with Aβ protein and Brahmi had near normal concentration of AChE. In the same study they also found out that EBm also improved cell viability, it had reduced ROS in the cell and also had antioxidant activity of its own. This study demonstrated multiple Anti-AD mechanisms of EBm. Further research in the form of clinical trials in humans is needed to prove its usefulness in AD.
All the important human and animal studies beneficial to the understanding of EBm in AD are summarized in Table 2.
Table 2.
Summary of some major studies done on EBm in AD
| Author and year of publication | Running title | Study design | Key findings |
|---|---|---|---|
| Kumar et al. [50], 2015 | Bacopa monnieri on cold stress induced neurodegeneration of hippocampus | Cold water swim stress-induced memory deficit in Wistar mice by depletion of norepinephrine in hippocampus resulting in reduced number of cells, diameter and packing density in the hippocampus | Cold stress in animals caused decreased in size of cells and decreased packing density of cells and cells per square on histophotometric study. This was reversed to near normal in rats Treated with EBm |
| Kamkaew et al. [56], 2013 | Bacopa monnieri increases cerebral blood flow | Cerebral blood flow in Wistar mice was tested using stereotactic laser doppler probe placed over bregma. Basilar artery showed antiChE dependent vasodilation on administration of EBm | On administration of EBm for 8 weeks EBm increased CBF by 25% and it also improved cognitive function in rats |
| Le et al. [45], 2013 | Bacopa monnieri ameliorates memory deficits in olfactory bulbectomized mice | OBX mice simulating AD were treated with EBm, tacrine, and vehicle. Memory tested using novel object recognition test, modified Y-maze test, fear conditioning test and ChAT assay | Decreases cognitive dysfunction by; enhancing synaptic plasticity related signaling and preserving cholinergic neurons. Improved object recognition, spatial memory and fear induced long-term memory deficit |
| Shinomol et al. [40], 2012 | Neuromodulatory propensity of bacopa monnieri against 3-NPA-induced oxidative stress | Pre-pubertal CFT-Swiss mice brain were subjected to oxidative damage using 3-NPA. EBm reduced the damage as assayed using DPPH radical scavenging assay, nitric oxide scavenging assay, hydroxyl radical scavenging assay, superoxide scavenging assay, deoxyribose oxidation assay and iron chelation assay | EBm prophylaxis prevented oxidative damage caused by 3-NPA indicating its antioxidant action |
| Morgan and Stevens [42], 2009 | Does bacopa monnieri improve memory performance in older persons? | Memory tests were conducted on healthy population >55 years as follows: audio-verbal and visual memory: Rey AVLT, RO-CFT and Reitan TMT. Subjective memory performance: MAC-Q | Administration of Brahmi caused significant improvement in verbal learning, memory acquisition and delayed recall |
| Dhanasekaran et al. [36], 2007 | Anti-dementia mechanisms of bacopa monnieri | C57/B16 mice brain as exposed to EBm extract. Lipoxygenase activity, free oxide radicals and iron chelation were measured. Plasmid DNA pBR322 was incubated with and without EBm at 37°C for 1 h and assayed. Both samples showed no significant differences in plasmid DNA | Administration of EBm decreased lipoxygenase activity, inhibited hydrogen peroxide-induced lipid peroxidation and chelated iron. BM does not exhibit direct genotoxicity |
| Raghav et al. [47], 2006 | RCT of bacopa monnieri extract in age-associated memory impairment | RCT involving adults >55 years treated with EBm and placebo for 12 weeks were tested for memory at 8 and 12 weeks. Memory tested using general information, orientation, mental control, logical memory, digit forward, digit backward, visual reproduction and paired associated learning. Most significant improvement in EBm was seen in logical memory and paired associated learning. | Remarkable improvement in mental control, logical memory and paired associate learning after 12 weeks EBm therapy |
AVLT, auditory verbal learning test; RO-CFT, rey-osterrieth complex figure test; TMT, trail making test; MAC-Q, memory complaint questionnaire.
Adverse Reactions
Limited studies are available demonstrating the side effects of Brahmi in human beings as well as animals. The most commonly encountered side effects are nausea, gastrointestinal upset, intestinal hypermotility which can be explained on the basis of cholinergic action of EBm [42]. Singh and Dhawan [19] in their double-blind, placebo-controlled phase 1 trial administered single dose (20–300 mg) and multiple doses (100 and 200 mg) to 31 healthy male volunteers for 4 weeks in which they found out no significant side effects or serious adverse drug reaction in clinical, hematological or biochemical parameter. In a study conducted on male rats by Allan et al. [67], it was found that at the dose of 500 mg/kg the appetite of rats decreased over time. After 90 days of treatment the rats demonstrated no change in weight of vital organs and all clinical, hematological, neurological parameters were within normal range; however, there was moderate increase in aspartate aminotransferase, albumin, globulin, urea, nitrogen, and sodium but all of them were in their normal range for controls. In the same study, they also found out that the median lethal dose for rats is 2,500 mg/kg which is a fairly high amount. Singh and Singh [68] studied effect of EBm administration in the dose of 250 mg/kg/day for 28 and 56 days in rats on fertility and male testes morphology, which demonstrated a decrease in sperm motility, viability, and sperm count. It also showed minute histological changes intraepithelial vacuolation, loosening of germinal epithelium, exfoliation of germ cells and occasionally giant cell formation. Height of germinal epithelium and diameter of seminiferous tubules was also decreased in Brahmi treated rats. Although libido remained unaffected in Brahmi treated rats, fertility was markedly reduced. EBm had no effect on dopamine and serotonin balance in rat brain, so there was no constitutional change as it is seen with other antidepressants [69].
Above studies clearly indicate that Brahmi has high therapeutic index which accounts for its safety on long term use. However most of the studies are animal studies, hence extensive human trials are absolutely necessary to prove its safety for use in human beings.
Safety
Safety profile of Brahmi has not been studied extensively; however, some in vitro studies have been performed. One such in vitro study was done by incubating plasmid DNA pBR322 (1 μg) with and without EBm (0–10 μg) at 37°C for 1 h which demonstrated no significant differences between the plasmid incubated with and without EBm, this indicated that Brahmi does not exhibit direct genotoxicity [36]. There are no studies of Brahmi regarding safety in pregnant ladies so it should not be used in pregnancy.
Drug Development
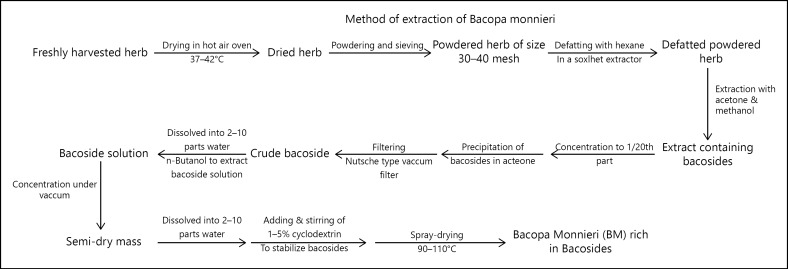
The process of extraction and drug development of EBm as patented by Kahol et al. [16] is illustrated by us in Figure 4.
Fig. 4.
Extraction of bacoside rich extract from Brahmi leaves.
Current Practice of Brahmi in AD
Brahmi is administered as various formulations. Brahmi juice (Swarasa) is extracted by mechanical pressure. Kalka containing clarified butter made from cow milk (cow ghee or Go Ghrita) is liquefied under moderate flame and the Swarasa is then added to it in prescribed quantities to form Brahmi Ghrita [70, 71]. Methanolic extract of EBm and lipid extraction of EBm showed comparable results as Brahmi Ghrita when administered in doses of 200 mg/kg to rats for 2 weeks [72].
Therapeutically, EBm can be administered as BrahmiGhritam (clarified butter based oral supplement) or Churna (powder) or plain tablet form. In cases of AD, Brahmi capsule are given in doses of 250–500 mg once or twice a day and Brahmi Churna or Ghritam is given in doses of 1–2 g once or twice a day.
Conclusion
Experiments on animals strongly indicate the value of Brahmi as a promising agent in AD and other forms of cognitive impairment. Due to small sample size of human trials, we recommend large multicentric clinical trials to confirm biosafety profile of Brahmi and also to explore its additional and serious side effects. Comparative studies between Brahmi and similar allopathic medicines are essential to evaluate the efficacy and safety profile of the erstwhile therapies.
Disclosure Statement
There is no funding source. Authors declare no conflict of interest. Article complies with ICMJE guidelines.
Acknowledgment
All the authors contributed to concepts, design, definition of intellectual content, literature search, clinical studies, data acquisition, manuscript preparation, manuscript editing, manuscript review, guarantor.
References
- 1.Ballard C, Gauthier S, Corbett A, Brayne C, Aarsland D, Jones E. Alzheimer's disease. Lancet. 2011;377:1019–1031. doi: 10.1016/S0140-6736(10)61349-9. [DOI] [PubMed] [Google Scholar]
- 2.Ryman DC, Acosta-Baena N, Aisen PS, Bird T, Danek A, Fox NC, et al. Symptom onset in autosomal dominant Alzheimer disease: a systematic review and meta-analysis. Neurology. 2014;83:253–260. doi: 10.1212/WNL.0000000000000596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scheltens P, Blennow K, Breteler MM, de Strooper B, Frisoni GB, Salloway S, Van der Flier WM. Alzheimer's disease. Lancet. 2016;388:505–517. doi: 10.1016/S0140-6736(15)01124-1. [DOI] [PubMed] [Google Scholar]
- 4.Akiyama H. [Development of disease-modifying therapy for Alzheimer's disease] Brain Nerve. 2016;68:463–472. doi: 10.11477/mf.1416200419. [DOI] [PubMed] [Google Scholar]
- 5.Markowitsch HJ, Staniloiu A. Amnesic disorders. Lancet. 2012;380:1429–1440. doi: 10.1016/S0140-6736(11)61304-4. [DOI] [PubMed] [Google Scholar]
- 6.Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alzheimer's Association. 2010 Alzheimer's disease facts and figures. Alzheimers Dement. 2010;6:158–194. doi: 10.1016/j.jalz.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 8.World Alzheimer Report 2015 The Global Impact of Dementia. http://www.alz.co.uk/research/worldreport2015
- 9.Selkoe DJ. Alzheimer's disease: genes, proteins, and therapy. Physiol Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- 10.Brunkan AL, Goate AM. Presenilin function and gamma-secretase activity. J Neurochem. 2005;93:769–792. doi: 10.1111/j.1471-4159.2005.03099.x. [DOI] [PubMed] [Google Scholar]
- 11.Priller C, Bauer T, Mitteregger G, Krebs B, Kretzschmar HA, Herms J. Synapse formation and function is modulated by the amyloid precursor protein. J Neurosci. 2006;26:7212–7221. doi: 10.1523/JNEUROSCI.1450-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cai Y, An SS, Kim S. Mutations in presenilin 2 and its implications in Alzheimer's disease and other dementia-associated disorders. Clin Interv Aging. 2015;10:1163–1172. doi: 10.2147/CIA.S85808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poirier J, Delisle MC, Quirion R, Aubert I, Farlow M, Lahiri D, et al. Apolipoprotein E4 allele as a predictor of cholinergic deficits and treatment outcome in Alzheimer disease. Proc Natl Acad Sci U S A. 1995;92:12260–12264. doi: 10.1073/pnas.92.26.12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Masliah E, Mallory M, Deerinck T, DeTeresa R, Lamont S, Miller A, et al. Re-evaluation of the structural organization of neuritic plaques in Alzheimer's disease. J Neuropathol Exp Neurol. 1993;52:619–632. doi: 10.1097/00005072-199311000-00009. [DOI] [PubMed] [Google Scholar]
- 15.Thal DR, Griffin WS, de Vos RA, Ghebremedhin E. Cerebral amyloid angiopathy and its relationship to Alzheimer's disease. Acta Neuropathol. 2008;115:599–609. doi: 10.1007/s00401-008-0366-2. [DOI] [PubMed] [Google Scholar]
- 16.Kahol AP, et al. Council of Scientific and Industrial Research Process for the preparation of a extract rich in Bacosides from the herb Bacopa monniera. United States Patent US006833143B1 (December 21, 2004).
- 17.Kulkarni R, Girish KJ, Kumar A. Nootropic herbs (Medhya Rasayana) in Ayurveda: an update. Pharmacogn Rev. 2012;6:147–153. doi: 10.4103/0973-7847.99949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aguiar S, Borowski T. Neuropharmacological review of the nootropic herb Bacopa monnieri. Rejuvenation Res. 2013;16:313–326. doi: 10.1089/rej.2013.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singh HK, Dhawan BN. Neuropsychopharmacological effects of the Ayurvedic nootropic Bacopa Monniera Linn. (Brahmi) Indian J Pharmacol. 1997;29:359–365. [Google Scholar]
- 20.Gupta P, Khatoon S, Tandon PK, Rai V. Effect of cadmium on growth, bacoside A, and bacopaside I of Bacopa monnieri (L.), a memory enhancing herb. ScientificWorldJournal. 2014;2014:824586. doi: 10.1155/2014/824586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Russo A, Borrelli F. Bacopa monniera, a reputed nootropic plant: an overview. Phytomedicine. 2005;12:305–317. doi: 10.1016/j.phymed.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 22.Barrett SC, Strother JL. Taxonomy and natural history of Bacopa (Scrophulariaceae) in California. Systematic Botany. 1978;3:408–419. [Google Scholar]
- 23.Basu NK, Lamsal PP. Investigations on Indian medicinal plants; Hydrocotyle asiatica Linn. Q J Pharm Pharmacol. 1947;20:135. [PubMed] [Google Scholar]
- 24.Rastogi S, Pal R, Kulshreshtha DK. Bacoside A3 – a triterpenoid saponin from Bacopa monniera. Phytochemistry. 1994;36:133–137. doi: 10.1016/s0031-9422(00)97026-2. [DOI] [PubMed] [Google Scholar]
- 25.Sairam K, Rao CV, Babu MD, Goel RK. Prophylactic and curative effects of Bacopa monniera in gastric ulcer models. Phytomedicine. 2001;8:423–430. doi: 10.1078/S0944-7113(04)70060-4. [DOI] [PubMed] [Google Scholar]
- 26.Kumar V. Potential medicinal plants for CNS disorders: an overview. Phytother Res. 2006;20:1023–1035. doi: 10.1002/ptr.1970. [DOI] [PubMed] [Google Scholar]
- 27.Pase MP, Kean J, Sarris J, Neale C, Scholey AB, Stough C. The cognitive-enhancing effects of Bacopa monnieri: a systematic review of randomized, controlled human clinical trials. J Altern Complement Med. 2012;18:647–652. doi: 10.1089/acm.2011.0367. [DOI] [PubMed] [Google Scholar]
- 28.Jenny NS. Inflammation in aging: cause, effect, or both? Discov Med. 2012;13:451–460. [PubMed] [Google Scholar]
- 29.Floyd RA, Hensley K. Oxidative stress in brain aging. Implications for therapeutics of neurodegenerative diseases. Neurobiol Aging. 2002;23:795–807. doi: 10.1016/s0197-4580(02)00019-2. [DOI] [PubMed] [Google Scholar]
- 30.Parletta N, Milte CM, Meyer BJ. Nutritional modulation of cognitive function and mental health. J Nutr Biochem. 2013;24:725–743. doi: 10.1016/j.jnutbio.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 31.Bhattacharya SK, Bhattacharya A, Kumar A, Ghosal S. Antioxidant activity of Bacopa monniera in rat frontal cortex, striatum and hippocampus. Phytother Res. 2000;14:174–179. doi: 10.1002/(sici)1099-1573(200005)14:3<174::aid-ptr624>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 32.Bourre JM. Effects of nutrients (in food) on the structure and function of the nervous system: update on dietary requirements for brain. Part 1: micronutrients. J Nutr Health Aging. 2006;10:377–385. [PubMed] [Google Scholar]
- 33.Sohal RS, Orr WC. The redox stress hypothesis of aging. Free Radic Biol Med. 2012;52:539–555. doi: 10.1016/j.freeradbiomed.2011.10.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simpson T, Pase M, Stough C. Bacopa monnieri as an antioxidant therapy to reduce oxidative stress in the aging brain. Evid Based Complement Alternat Med. 2015;2015:615384. doi: 10.1155/2015/615384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Russo A, Izzo AA, Borrelli F, Renis M, Vanella A. Free radical scavenging capacity and protective effect of Bacopa monniera L. on DNA damage. Phytother Res. 2003;17:870–875. doi: 10.1002/ptr.1061. [DOI] [PubMed] [Google Scholar]
- 36.Dhanasekaran M, Tharakan B, Holcomb LA, Hitt AR, Young KA, Manyam BV. Neuroprotective mechanisms of ayurvedic antidementia botanical Bacopa monniera. Phytother Res. 2007;21:965–969. doi: 10.1002/ptr.2195. [DOI] [PubMed] [Google Scholar]
- 37.Rottkamp CA, Raina AK, Zhu X, Gaier E, Bush AI, Atwood CS, et al. Redox-active iron mediates amyloid-beta toxicity. Free Radic Biol Med. 2001;30:447–450. doi: 10.1016/s0891-5849(00)00494-9. [DOI] [PubMed] [Google Scholar]
- 38.Falangola MF, Lee SP, Nixon RA, Duff K, Helpern JA. Histological co-localization of iron in Abeta plaques of PS/APP transgenic mice. Neurochem Res. 2005;30:201–205. doi: 10.1007/s11064-004-2442-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kapoor R, Srivastava S, Kakkar P. Bacopa monnieri modulates antioxidant responses in brain and kidney of diabetic rats. Environ Toxicol Pharmacol. 2009;27:62–69. doi: 10.1016/j.etap.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 40.Shinomol GK, Bharath MM, Muralidhara Neuromodulatory propensity of Bacopa monnieri leaf extract against 3-nitropropionic acid-induced oxidative stress: in vitro and in vivo evidences. Neurotox Res. 2012;22:102–114. doi: 10.1007/s12640-011-9303-6. [DOI] [PubMed] [Google Scholar]
- 41.Jyoti A, Sharma D. Neuroprotective role of Bacopa monniera extract against aluminium-induced oxidative stress in the hippocampus of rat brain. Neurotoxicology. 2006;27:451–457. doi: 10.1016/j.neuro.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 42.Morgan A, Stevens J. Does Bacopa monnieri improve memory performance in older persons? Results of a randomized, placebo-controlled, double-blind trial. J Altern Complement Med. 2010;16:753–759. doi: 10.1089/acm.2009.0342. [DOI] [PubMed] [Google Scholar]
- 43.Russo A, Borrelli F, Campisi A, Acquaviva R, Raciti G, Vanella A. Nitric oxide-related toxicity in cultured astrocytes: effect of Bacopa monniera. Life Sci. 2003;73:1517–1526. doi: 10.1016/s0024-3205(03)00476-4. [DOI] [PubMed] [Google Scholar]
- 44.Michaelis ML. Drugs targeting Alzheimer's disease: some things old and some things new. J Pharmacol Exp Ther. 2003;304:897–904. doi: 10.1124/jpet.102.035840. [DOI] [PubMed] [Google Scholar]
- 45.Le XT, Pham HT, Do PT, Fujiwara H, Tanaka K, Li F, et al. Bacopa monnieri ameliorates memory deficits in olfactory bulbectomized mice: possible involvement of glutamatergic and cholinergic systems. Neurochem Res. 2013;38:2201–2215. doi: 10.1007/s11064-013-1129-6. [DOI] [PubMed] [Google Scholar]
- 46.Uabundit N, Wattanathorn J, Mucimapura S, Ingkaninan K. Cognitive enhancement and neuroprotective effects of Bacopa monnieri in Alzheimer's disease model. J Ethnopharmacol. 2010;127:26–31. doi: 10.1016/j.jep.2009.09.056. [DOI] [PubMed] [Google Scholar]
- 47.Raghav S, Singh H, Dalal PK, Srivastava JS, Asthana OP. Randomized controlled trial of standardized Bacopa monniera extract in age-associated memory impairment. Indian J Psychiatry. 2006;48:238–242. doi: 10.4103/0019-5545.31555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stough C, Lloyd J, Clarke J, Downey LA, Hutchison CW, Rodgers T, et al. The chronic effects of an extract of Bacopa monniera (Brahmi) on cognitive function in healthy human subjects. Psychopharmacology (Berl) 2001;156:481–484. doi: 10.1007/s002130100815. [DOI] [PubMed] [Google Scholar]
- 49.Negi K, Singh Y, Kushwaha K, Rastogi C, Rathi A, Srivastava J, et al. Clinical evaluation of memory enhancing properties of Memory Plus in children with attention deficit hyperactivity disorder. Ind J Psychiatry. 2000;42 [Google Scholar]
- 50.Kumar SS, Saraswathi P, Vijayaraghavan R. Effect of bacopa monniera on cold stress induced neurodegeneration in hippocampus of wistar rats: a histomorphometric study. J Clin Diagn Res. 2015;9:AF05–AF07. doi: 10.7860/JCDR/2015/10199.5423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saini N, Singh D, Sandhir R. Neuroprotective effects of Bacopa monnieri in experimental model of dementia. Neurochem Res. 2012;37:1928–1937. doi: 10.1007/s11064-012-0811-4. [DOI] [PubMed] [Google Scholar]
- 52.Vohora D, Pal SN, Pillai KK. Protection from phenytoin-induced cognitive deficit by Bacopa monniera, a reputed Indian nootropic plant. J Ethnopharmacol. 2000;71:383–390. doi: 10.1016/s0378-8741(99)00213-5. [DOI] [PubMed] [Google Scholar]
- 53.Saraf MK, Prabhakar S, Pandhi P, Anand A. Bacopa monniera ameliorates amnesic effects of diazepam qualifying behavioral-molecular partitioning. Neuroscience. 2008;155:476–484. doi: 10.1016/j.neuroscience.2008.05.043. [DOI] [PubMed] [Google Scholar]
- 54.Kishore K, Singh M. Effect of bacosides, alcoholic extract of Bacopa monniera Linn. (brahmi), on experimental amnesia in mice. Indian J Exp Biol. 2005;43:640–645. [PubMed] [Google Scholar]
- 55.Brown WR, Thore CR. Review: cerebral microvascular pathology in ageing and neurodegeneration. Neuropathol Appl Neurobiol. 2011;37:56–74. doi: 10.1111/j.1365-2990.2010.01139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kamkaew N, Scholfield CN, Ingkaninan K, Maneesai P, Parkington HC, Tare M, et al. Bacopa monnieri and its constituents is hypotensive in anaesthetized rats and vasodilator in various artery types. J Ethnopharmacol. 2011;137:790–795. doi: 10.1016/j.jep.2011.06.045. [DOI] [PubMed] [Google Scholar]
- 57.Kamkaew N, Norman Scholfield C, Ingkaninan K, Taepavarapruk N, Chootip K. Bacopa monnieri increases cerebral blood flow in rat independent of blood pressure. Phytother Res. 2013;27:135–138. doi: 10.1002/ptr.4685. [DOI] [PubMed] [Google Scholar]
- 58.Nobili F, Vitali P, Canfora M, Girtler N, De Leo C, Mariani G, et al. Effects of long-term Donepezil therapy on rCBF of Alzheimer's patients. Clin Neurophysiol. 2002;113:1241–1248. doi: 10.1016/s1388-2457(02)00110-4. [DOI] [PubMed] [Google Scholar]
- 59.McEwen BS. Structural plasticity of the adult brain: how animal models help us understand brain changes in depression and systemic disorders related to depression. Dialogues Clin Neurosci. 2004;6:119–133. doi: 10.31887/DCNS.2004.6.2/bmcewen. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zheng F, Luo Y, Wang H. Regulation of brain-derived neurotrophic factor-mediated transcription of the immediate early gene Arc by intracellular calcium and calmodulin. J Neurosci Res. 2009;87:380–392. doi: 10.1002/jnr.21863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Drozdov O, Chorna V. Changes in the content of glial fibrillary acidic protein in the frontal cortex of rats during conditioned active avoidance training. Neurophysiology. 2003;35:98–101. [Google Scholar]
- 62.Konar A, Shah N, Singh R, Saxena N, Kaul SC, Wadhwa R, et al. Protective role of Ashwagandha leaf extract and its component withanone on scopolamine-induced changes in the brain and brain-derived cells. PLoS One. 2011;6:e27265. doi: 10.1371/journal.pone.0027265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Konar A, Gautam A, Thakur MK. Bacopa monniera (CDRI-08) upregulates the expression of neuronal and glial plasticity markers in the brain of scopolamine induced amnesic mice. Evid Based Complement Alternat Med. 2015;2015:837012. doi: 10.1155/2015/837012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mathew M, Subramanian S. Evaluation of the anti-amyloidogenic potential of nootropic herbal extracts in vitro. Int J Pharm Sci Res. 2012;3:4276–4280. [Google Scholar]
- 65.Holcomb LA, Dhanasekaran M, Hitt AR, Young KA, Riggs M, Manyam BV. Bacopa monniera extract reduces amyloid levels in PSAPP mice. J Alzheimers Dis. 2006;9:243–251. doi: 10.3233/jad-2006-9303. [DOI] [PubMed] [Google Scholar]
- 66.Limpeanchob N, Jaipan S, Rattanakaruna S, Phrompittayarat W, Ingkaninan K. Neuroprotective effect of Bacopa monnieri on beta-amyloid-induced cell death in primary cortical culture. J Ethnopharmacol. 2008;120:112–117. doi: 10.1016/j.jep.2008.07.039. [DOI] [PubMed] [Google Scholar]
- 67.Allan JJ, Damodaran A, Deshmukh N, Goudar K, Amit A. Safety evaluation of a standardized phytochemical composition extracted from Bacopa monnieri in Sprague – Dawley rats. Food Chem Toxicol. 2007;45:1928–1937. doi: 10.1016/j.fct.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 68.Singh A, Singh SK. Evaluation of antifertility potential of Brahmi in male mouse. Contraception. 2009;79:71–79. doi: 10.1016/j.contraception.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 69.Rauf K, Subhan F, Abbas M, ul Haq I, Ali G, Ayaz M. Effect of acute and sub chronic use of Bacopa monnieri on dopamine and serotonin turnover in mice whole brain. AJPP. 2012;6:2767–2774. [Google Scholar]
- 70.Yadav KD, Reddy KR, Agarwal A. Preliminary physico-chemical profile of Brahmi Ghrita. Ayu. 2013;34:294–296. doi: 10.4103/0974-8520.123130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gubbannavar JS, Chandola H, Harisha CR, Kalyani R, Shukla VJ. Analytical profile of Brahmi Ghrita: a polyherbal Ayurvedic formulation. Ayu. 2012;33:289–293. doi: 10.4103/0974-8520.105254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lohidasan S, Paradkar AR, Mahadik KR. Nootropic activity of lipid-based extract of Bacopa monniera Linn. compared with traditional preparation and extracts. J Pharm Pharmacol. 2009;61:1537–1544. doi: 10.1211/jpp/61.11.0014. [DOI] [PubMed] [Google Scholar]