ABSTRACT
Neurogenesis is associated with functional recovery after stroke. However, the underlying molecular mechanisms have not been fully investigated. Using an Ago2-based RNA immunoprecipitation to immunoprecipated Ago2-RNA complexes followed by RNA sequencing (Ago2 RIP-seq) approach, we profiled the miRNomes in neural progenitor cells (NPCs) harvested from the subventricular zone (SVZ) of the lateral ventricles of young adult rats. We identified more than 7 and 15 million reads in normal and ischemic NPC libraries, respectively. We found that stroke substantially changed Ago2-associated miRNA profiles in NPCs compared to those in non-ischemic NPCs. We also discovered a new complex repertoire of isomiRs and multiple miRNA-miRNA* pairs and numerous novel miRNAs in the non-ischemic and ischemic NPCs. Among them, pc-3p-17172 significantly regulated NPC proliferation and neuronal differentiation. Collectively, the present study reveals profiles of Ago2-associated miRNomes in non-ischemic and ischemic NPCs, which provide a molecular basis to further investigate the role of miRNAs in mediating adult neurogenesis under physiological and ischemic conditions.
KEYWORDS: Ago2, microRNA, neural progenitor cells, neurogenesis, RNA sequencing, stroke
Introduction
In the brain of adult rodent, neural progenitor cells (NPCs) are present in the subventricular zone (SVZ) of the lateral ventricle and the subgranular zone (SGZ) of the dentate gyrus, which generate new neurons throughout life of the animal.2,17,34,48 Neurogenesis impacts neurological function.6,30,54,56,60,70,80 Preclinical and clinical studies demonstrate that stroke promotes neurogenesis in the adult brain4,26,27,29,33,42,49,55,58,62,64,66,74,75,76,79,81,82 and that blockage of newly generated neuroblasts reduces spontaneous neurological recovery in experimental stroke.70 However, neurogenesis in response to stroke is insufficient to restore neurological function.4,80,54,30,78,60,70 Understanding molecular mechanisms underlying stroke-induced neurogenesis could potentially lead to the development of new therapies to amplify endogenous neurogenesis, consequently leading to the improvement of neurological function during stroke recovery.
MicroRNAs (miRNAs), a family of short noncoding RNA molecules of 20 to 25 nucleotides, are involved in physiologic function and in disease processes by decreasing gene expression.19 In the brain, growing evidence suggests that miRNAs play vital roles in cell-fate specification, neurite projection, synaptic plasticity, and neuronal function, among others.1,8,9,25 The mechanisms of miRNA action are incompletely understood. miRNAs are generated by the ribonuclease III Dicer that cleaves double-stranded RNA (dsRNA) and pre-miRNA into short double-stranded RNA. miRNAs are subsequently incorporated into an effector ribonucleoprotein complex called “miRNA-induced silencing complex (miRISC)”.24,53 The function of miRISC is that miRNAs provide sequence-specific bindings of RISC to specific mRNA targets, causing increased efficiency of mRNA destabilization and/or translational repression. miRISC is composed of RNAs and proteins. The key components of miRISC are the highly conserved proteins of the Argonaute (Ago) family (Ago1–4) in the human genome,23 which bind directly to mature miRNAs. Ago2 is the key player that possesses the activity of miRNA-guided mRNA cleavage or translational inhibition, and is a core effector of RISC.12,31,41,59,65
In combination with Ago proteins, a single miRNA can potentially target thousands of different mRNAs, and each mRNA may also be targeted by several miRNAs. It is a challenge to predict which miRNA will bind to which individual mRNA targets, because most miRNAs bind to mRNA targets through partial sequence complementarity. Computational methods are imperfect and disparate from each other, and the biochemistry underlying miRNA:mRNA interactions still needs to be fully elucidated. Recent studies have used a high-throughput approach, e.g., high-throughput sequencing of RNA isolated by crosslinking immunoprecipitation, to biochemically determine Ago:miRNA binding sites on a transcriptome wide scale. Ago proteins are the core components of RISC, and this powerful technique allows for sequencing both the miRNAs and the mRNA target regions to which they bind. These approaches have been applied in human and mouse brain and liver tissues.7,13,21,36,50
Using miRNA microarray analysis, studies have revealed the miRNA profiles in brain tissue and peripheral blood after stroke.14,28,40,61 We recently showed that the global expression of mature miRNAs was altered and that miRNAs regulate neurogenesis in ischemic SVZ NPCs.46 However, such approaches were limited in their ability to detect rare miRNAs, or tissue-specific miRNAs from tissues that are difficult to obtain. Mature miRNAs function within stable RISCs with Ago proteins to modulate target coding RNAs.10,12,18,31,41,59,65 Recent studies have asserted that the expression of miRNAs present in the total miRNA pool is not an accurate measure of the Ago RISC-associated miRNA population.16
To advance our understanding of miRNA functions in adult neurogenesis and stroke-induced neurogenesis, we profiled novel transcriptome-wide miRNAs in Ago2-RISCs in SVZ neural progenitor cells using the Ago2-based RNA immunoprecipitation (RIP) followed by RNA deep sequencing (Ago2 RIP-seq) methodology. We identified tissue-specific miRNAs and rare miRNAs related to adult neurogenesis. In addition, we discovered many miRNA variants, novel miRNAs, and a miRNA/miRNA* duplex that may modulate adult neurogenesis and stroke-induced neurogenesis.
Results
Characterization of Ago2-associated miRNomes in adult non-ischemic NPCs
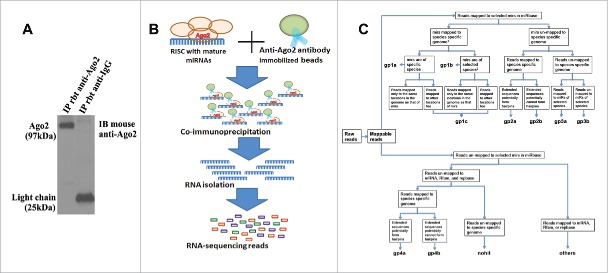
To identify Ago2-assocaited miRNAs in adult NPCs, we performed Ago2 RNA immunoprecipitation (RIP) in NPCs harvested from the SVZ of non-ischemic adult rats, in which we were able to detect the miRNAs with their guide strands that bind to Ago2.12,31 Western blot analysis demonstrated that Ago2 in NPCs was specifically immunoprecipitated (Fig. 1A). We then performed Ago2 RNA seq to analyze Ago2-associated whole genomic RNAs in NPCs (Fig. 1B).
Figure 1.
The illustration of Ago2-based RNA immunoprecipitation to immunoprecipated Ago2–RNA complexes followed by RNA sequencing (Ago2 RIP-seq) platform. (A) Western blot analysis shows the presence of Ago2 in Ago2 immunoprecipitation (IP) but not mouse IgG. (B) A scheme outlines Ago2 RIP-seq approach. A rabbit polyclonal anti-Ago2 and a mouse monoclonal anti-Ago2 antibody were used for the immunoprecipitation and Western blot analysis of Ago2, respectively. The normalization was performed by blotting the same samples with an antibody against β-actin. (C) The flow diagram outlines the various steps involved in RNA seq data analysis.
The purified cDNA library was used for cluster generation on Illumina's Cluster Station and then sequenced on Illumina GAIIx. Raw sequencing reads that range from 15 nt to 30 nt in length were used for miRNA prediction using the ACGT101-miR v4.2 program.37,73 Deep sequencing yielded a total of 7,324,417 raw reads in NPCs (Fig. S1A). For further analysis, using the criteria outlined in Fig. 1C, raw reads were mapped to the rat and other mammalian genomes, and the total mapped read count was 7,100,913 reads (96.9% of total quality reads) in NPCs (Fig. S1B). RNA sequencing showed that 95% of the read distribution was 20–24 nucleotides in length in non-ischemic NPC populations (Fig. S1C). Approximately 0.6% reads (43,946) in NPCs were mapped to Rafm or Repbase which contain repetitive DNA sequences, tRNA, rRNA or other small molecules37,73 and were not included for further analysis. Reads that mapped to protein coding mRNA sequences were considered as RNA degradation products and were also excluded.
Abundance of Ago2 binding miRNAs is inversely related to the inhibitory potential of their target gene.36 We therefore analyzed the enrichment of miRNAs in Ago2-RIP by calculating the percentage of enriched miRNA copy reads in the total library counts. Bioinformatics analysis revealed that the top 10 most abundant seed sequences in Ago2-RIP comprised nearly 60% of the total Ago2+ seed load in NPCs (Fig. 2A). To identify known miRNAs in NPCs detected by the Ago2-RIP, all mapable small RNA sequences were compared with the known rodent miRNAs in the miRBase database (version 18.0). A total number of 242 miRNAs that have the perfectly matched sequences with the known rno-miRs/premiR in miRBase were detected in non-ischemic NPCs (Table S1). The top 10 most highly enriched miRNAs included miR-26, miR-27b, miR-127, miR-99b, miR-191, miR-125b-5p, let-7f, let-7i, miR-541 and miR-151 (Fig. 2A). Using Metacore and Targetscan algorithms, we found that the signaling pathways related to genes putatively targeted by the 10 most enriched miRNAs in NPCs involved cytoskeleton remodeling, TGF, WNT, Ras and Rho protein on G1/S transition cell cycle, regulation of epithelial-to-mesenchymal transition (EMT), development genes, and cell adhesion (Fig. S1D).
Figure 2.
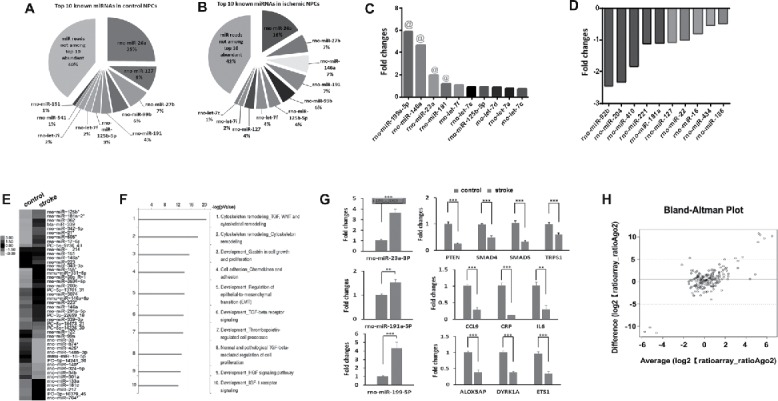
Characterization of Ago2-associated miRNomes in NPCs. Pie charts show the profiles of top 10 enriched Ago2-bound miRNAs in non-ischemic (A) and ischemic (B) NPCs. The bar-graph shows top 10 upregulated (C) and downregulated (D) miRNAs in ischemic NPCs. (E) Heat map diagram shows differential profiles of miRNAs in non-ischemic and ischemic NPCs. The color scale in (E)illustrates the relative expression level of miRNAs across all samples: red and green colors represent an expression level higher and lower, respectively, than the mean. Black represents median expression. (F) Bioinformatic analysis with Metacore and Targetscan softwares shows the signaling pathways related to highly enriched Ago2-bound miRNAs in ischemic NPCs. (G) QRT-PCR analysis validated top 3 upregulated enriched miRNAs and their target genes in NPCs after stroke. N = 3/group, **p < 0.01, ***p < 0.001. (H) Comparison of miRNA expression data between the Ago2-RIP and the miRNA microarray by means of Spearman correlation coefficient.
Characterization of Ago2-associated miRNomes in ischemic NPCs
In contrast to non-ischemic NPCs, raw reads in ischemic NPCs were substantially increased to 15,448,356 (Fig. S1E) and among them, 15,085,774 reads bound to Ago2 (Fig. S1F). Approximately 0.6% (90,514) reads in ischemic NPCs were mapped to Rafm or Repbase (Fig. S1F) and were excluded for further analysis. Similar to the non-ischemic NPCs, RNA sequencing showed that 95% of the read distribution was 20–24 nucleotides in length in ischemic NPCs (Fig. S1G). A total of 247 miRNAs perfectly matched sequences with the known rno-miRs/premiR in miRBase were detected in ischemic NPCs (Table S1). Compared to the top 10 enriched miRNAs in non-ischemic NPCs, miR-146a and miR-let-7c were specifically increased by stroke, whereas miR-541 and miR-151 were not included among the top 10 enriched miRNAs in ischemic NPCs (Fig. 2B). To examine whether stroke alters known miRNA profiles in NPCs, we compared the fold changes of miRNAs between non-ischemic and ischemic groups by setting a threshold of the average reads at 14,907. We found that the top 10 upregulated known miRNAs by stroke were rno-miR-199a-5p, rno-miR-146a, rno-miR-23a, rno-miR-191, rno-let-7f, rno-let-7e, rno-miR-125b-5p, rno-let-7d, rno-let-7a and rno-let-7c (Fig. 2C). Meanwhile, the top 10 downregulated miRNAs were rno-miR-92b, rno-miR-204, rno-miR-410, rno-miR-221, rno-miR-181a, rno-miR-127, rno-miR-22, rno-miR-16, rno-miR-434, rno-miR-186 and rno-miR-26a (Fig. 2D). Heat map also showed that stroke substantially altered Ago2-associated miRNA profiles in NPCs compared to those in non-ischemic NPCs (Fig. 2E). These data indicate that stroke dramatically alters known miRNA profiles in NPCs.
Highly abundant miRNAs in cellular miRNA pools generally play the key role in miRNAs–mediated target suppression.63,71 In order to delineate the biological processes regulated by stroke-altered miRNAs in NPCs, the gene ontology analysis of the top 10 enriched miRNAs in ischemic NPCs was conducted. We found that genes putatively targeted by stroke-upregulated miRNAs regulate multiple biological networks including thrombopoietin regulated cell processes, HGF and IGF-1 receptor signalings (Fig. 2F).
We selected the top 4 upregulated miRNAs, miR-199a-5p, miR-146a, miR-191, and miR-23, in ischemic NPCs to further verify them by means of quantitative real-time RT-PCR and Taqman probes. We previously demonstrated that stroke substantially upregulates miR-146a and downregulates its target genes of IRAK1 and TRAF6.43 We thus examined the other 3 miRNAs and found that miR-199a-5p, miR-191, and miR-23were substantially increased in ischemic NPCs, which is in line with their profiles detected by Ago2 RIP-seq (Fig. 2G). To further examine whether these upregulated miRNAs affect their putative genes, we performed bioinformatics analysis with IPA, TargetScan and miRecords algorithms to select target genes that have been experimentally validated. Quantitative RT-PCR analysis showed that mRNA levels of 4 genes targeted by miR-23a, PTEN, SMAD4, SMAD5 and TRPS1, were significantly decreased in ischemic NPCs compared to mRNA levels of these genes in non-ischemic NPCs (Fig. 2G, Table S2). In parallel, ischemic NPCs also had significantly lower levels of 3 genes targeted by miR-191a, CCL9, C-Reactive Protein (CRP), and IL6, as well as 3 genes targeted by miR-199, Arachidonate 5-Lipoxygenase-Activating Protein (ALOX5AP), Dual-Specificity Tyrosine-(Y)-Phosphorylation Regulated Kinase 1A (DYRK1A) and Avian Erythroblastosis Virus E26 (V-Ets) Oncogene Homolog-1 (ETS1) (Fig. 2G). These data suggest that the miRNAs upregulated by stroke likely regulate their target genes and consequently mediate stroke-induced neurogenesis.
Comparison of annotated miRNAs from Ago2-RIP and miRNA microarray
We previously examined miRNA expression in normal and ischemic NPCs using a miR microarray platform.46 Using a Bland-Altman plot analysis, we found that over 90% of miRNAs detected by the miRNA array assay were identified and covered by Ago2 RIP-seq analysis in non-ischemic and ischemic NPCs (Fig. 2H, Table S3). However, the previous analysis detected only 163 annotated miRNAs in NPCs, while the current analysis identified 747 known miRNAs in NPCs.
Comparison of Ago2-associated isomiRs in non-ischemic and ischemic NPCs
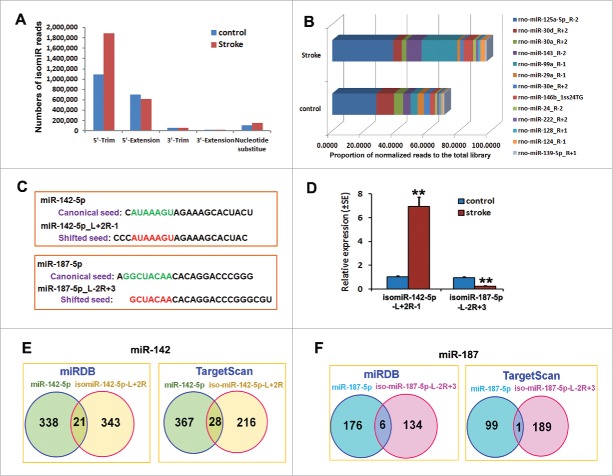
IsomiRs, variants of miRNAs, can be readily detected by deep sequencing analysis and are functional in embryonic stem cells.20 To examine whether stroke impacts isomiRs in adult NPCs, we analyzed the modifications of nucleotides with respect to the miRNA mature form in non-ischemic and ischemic NPCs (Table S4). We found that the reads of isomiRs accounted for 26% and 29% of the total 2 library reads in non-ischemic and ischemic NPCs, respectively. The modification frequently included single or more nucleotide additions or deletions to either the 5′ or the 3′ end of mature reads, base substitutions, or a combination of these changes. To further analyze their change, the isomiRs were classified into: 1) canonical miRNAs; 2) 5′-trimmed; 3) 5′- extension; 4) 3′-trimmed; 5) 3′- extension; and 6) nucleotide substitutions. Our data showed that the majority of the reads (95.6% and 97.1% in non-ischemic and ischemic NPCs, respectively) contained changes at their 5′-end, including 5′-trim and 5′-extention as well as nucleotide substitute (Fig. 3A). We also discovered that stroke markedly changed the read levels of 5′-trimmed and nucleotide substituted isomiRs compared to those in non-ischemic NPCs (Fig. 3A).
Figure 3.
Analysis of isomiRs in non-ischemic and ischemic NPCs. (A) A bar-graph shows the distribution of modified mature miRNAs in the NPCs after stroke. (B) The percentage of normalized reads in the total library of top 20 most highly expressed isomiRs in the normal and ischemic NPCs. (C) Comparison of the shifted (bold red) and canonical (bold green) seeds of isomiR-142–5p_L+2R-1 and isomiR-187-5p_L-2R+3. Detailed sequence information and average frequency are provided in Supplementary Table S4. (D) Stem-loop TaqMan PCR and qRT-PCR analysis shows the upregulation of isomiR-142-5p_L+2R-1 and downregulation of isomiR-187-5p_L-2R+3 in ischemic NPCs. N = 3/group, **p < 0.01. (E and F) Venn diagrams show the overlapped genes putatively targeted by isomiR-142-5p_L+2R-1 or isomiR-187-5p_L-2R+3 (red) as well as by their parent canonical miR-142-5p and miR-187-5p (blue). Predictions were performed using the miRDB (left box) and TargetScan (right box) softwares.
Among the top 20 abundant isomiRs in non-ischemic and ischemic NPCs, 13 isomiRs were present in both groups, while the remaining 7 isomiRs were distinct in the non-ischemic and ischemic NPCs. The most upregulated isomiRs by stroke included rno-miR-99a_R-1, rno-miR-124_R-1, rno-miR-143_R-2, rno-miR-21_R+2, rno-miR-126_R-1, rno-miR-342-3p_R+1, rno-miR-92a_R+1, rno-miR-423*_R-1, and rno-miR-652_R+1. The downregulated isomiRs by stroke were rno-miR-30d_R+2, and rno-miR-29a_R-1 (Fig. 3B). We then compared the reads of isomiRs with their corresponding canonical miRNAs. We found that the reads of the top 20 expressed isomiRs were dominant compared with those of their canonical miRNAs, which were mostly undetectable in non-ischemic and ischemic NPCs with the exception of rno-miR-128 (Table S5). Both reads of rno-miR-128 (15315 and 21093 reads in control and ischemic NPCs) and its isomiR rno-miR-128_R+1 (27837 and 32164 reads in control and ischemic NPCs) showed comparable expression in NPCs (Table S5).
Sequence variations at the 5′-end as well as nucleotide substitutions within the seed sequence (i.e. between nucleotides 2–8 of mature sequences) have functional consequences as they may confer different targeting properties to a given miRNA.5 We therefore analyzed changes between nucleotides 2–8 of mature miRNAs, because these were expected to give rise to a modified seed sequence. On average, approximately 29.4% of the isomiRs (143 out of 485) had a modified seed sequence, due to seed-shifting caused by trimming or additions of nucleotides at the 5′-end, or due to nucleotide substitutions within the seed sequence itself (Table S6). Our RIP-seq data showed that these seed-modified isomiRs were loaded onto the Ago2 silencing complex. We selected the miR-142-5p_L+2R-1 and miR-187-5p_L-2R+3 for further analysis because the reads of both isomiRs were above 1,000 along with high fold changes in ischemic NPCs (Table S6). Fig. 3C showed the shifted seeds of these 2 isomiRs from their canonical seed sequences. Using stem-loop Taqman PCR and quantitative RT-PCR, we confirmed a significant increase of miR-142-5p_L+2R-1, but not miR-187-5p_L-2R+3 in ischemic NPCs (Fig. 3D). In silico prediction suggested that miR-142-5p L+2R-1 and miR-142-5p could putatively target distinct genes (Fig. 3E and F, Table S7). According to miRDB and TargetScan software, 94% and 87%, respectively, of genes that are potentially targeted by the isomiR-142-5p_L+2R-1 (Fig. 3E, red circles) were not targeted by the canonical miR-142-5p (Fig. 3E, blue circles). MiRDB and TargetScan software showed 96% and 99%, respectively, of genes that are potentially targeted by the isomiR-187-5p_L-2R+3 (Fig. 3F, red circles) were targeted by the canonical miR-187-5p (Fig. 3F, blue circles).
Other known mammalian miRNAs and pre-miRNAs
In addition to the mature miRNAs that mapped to rats, we found that some miRNAs from NPCs mapped to other species (Table S8). Sixty-five miRNAs were found to map to mouse genomes and 7 miRNAs were mapped to human genome matched sequences described in the rat. Two miRNAs were mapped to Sus scrofa (ssc-miR-1285_L-1R+5 and ssc-mir-1285-p5_1ss14TC), and one matched to each of the following: Pongo pygmaeus (ppy-mir-877-p3_R+1), Macaca mulatta (mml-mir-297-p5_5ss7CT8AG12GA13CT20GA), Canis familiaris (cfa-mir-574-p5), and Bos taurus (bta-miR-2887_R+10). However, the reads of miRNAs were represented by a small number of read counts in other species.
Characterization of duplex strand miRNA/miRNA* pairs in non-ischemic and ischemic NPCs
MiRNAs* play an important regulatory role in vertebrates in a tissue specific manner. We found 160 and 177 miRNAs* in non-ischemic and ischemic NPCs, respectively, (Table S9), which represented approximately 1% of total raw reads in NPCs. To find the miRNA-miRNA* pairs in NPCs, we used a criteria in which miRNA stem-loops were present if read counts reached the abundance of 0.001% in the average of both database and the ratio of miRNA* vs miRNA was above 5 times.69 We detected 5 pairs of miRNA/miRNA* in non-ischemic and ischemic NPCs including rno-miR-212/212*, rno-miR-337/337*, rno-miR-379/379*, rno-miR-411/411* and rno-miR-423/423* (Table S10). However, distribution of these pairs was distinct between non-ischemic and ischemic NPCs (Table S10), suggesting that distinct miRNA/miRNA* pairs may play an independent role in mediating neurogenesis under non-ischemic and ischemic conditions.
Novel miRNAs in non-ischemic and ischemic NPCs
To further identify unreported miRNAs in NPCs, all the mapable small RNAs were BLASTed against the rat genome sequences and mammalian pre-miRNAs in miRbase. The small RNAs that exactly map to the genome sequence and form a RNA hairpin structure but not mapped to mammalia pre-miRNAs in miRbase were considered as candidate novel miRNAs. A total of 435 and 740 new miRNAs were detected in non-ischemic and ischemic NPCs, respectively (Table S11). However, expression levels of these miRNAs were considerably low. Using a criterion by which only small RNAs with copy numbers more than 50 reads were considered, we detected 12 and 16 novel miRNAs in non-ischemic and ischemic NPCs, respectively (Table S12). The precursor length of these new miRNAs was between 55 and 100nt and the minimal folding free energy varied from −18.30 to −57.70 kcal/mol (Table S12).
The “seed” sequence of a miRNA (position 2–7 from the 5′-end) plays a critical role in the target determination of a miRNA. Using the current release of miRBase, we analyzed the seed sequences of novel miRNAs associated with annotated miRNAs and found that 9 predicted novel miRNAs displayed significant seed-paralogues to known rodent miRNAs comprising of 2 most highly expressed novel miRNAs (pc-3p-223_18254 to rno-miR-676, pc-3p-3180_242 to rno-miR-3099), another 4 novel miRNAs which could be rat species isomiRs (pc-3p-2353_379 to rno-miR-344b-1-3p, pc-5p-7206_74 to rno-miR-664-2-5p) with 1–2 neucleotide deletion at 3′- end (3′-Trim), and other 3 novel miRNAs which show seed-paralogues to mouse miRNAs (Table S12). Predicted hairpin foldings of the novel miRNAs were presented in Fig. S2. The remaining 9 putative novel miRNAs had novel, uncharacterized seed sequences in rodent and human species.
In addition, we found that among these novel miRNAs, stroke markedly upregulated and downregulated 5 and 2 miRNAs with fold change above 1.5, respectively, compared to those in non-ischemic NPCs (Table 3). We found pc-3p-17172 (mature sequence: CUGAGACUAACUCACCUGU), pc-3p-4697 (mature sequence: AACCCUGACUAAGACAUGCUCU), and pc-5p-38316 (mature sequence: AGGUCCUCAGUAAGUAUUUGUUA) in NPCs (Fig. 4A). Using a stem-loop RT-PCR system and Taqman probes, we confirmed the presence of these 3 novel miRNAs in NPCs and found that stroke significantly increased pc-3p-17172 and pc-5p-38316 levels (Fig. 4B). To examine whether the 3 novel miRNAs were specific to NPCs, we measured 3 miRNAs in primary cerebral endothelial cells isolated from non-ischemic and ischemic brains of adult rats. RT-PCR analysis showed that Ct values of all 3 miRNAs in both non-ischemic and ischemic endothelial cells were above 39 or “undetermined” (data not shown), suggesting that the levels of these novel miRNAs are undetectable. Together, our data indicate that adult NPCs contain these novel miRNAs and that stroke alters their expression.
Figure 4.
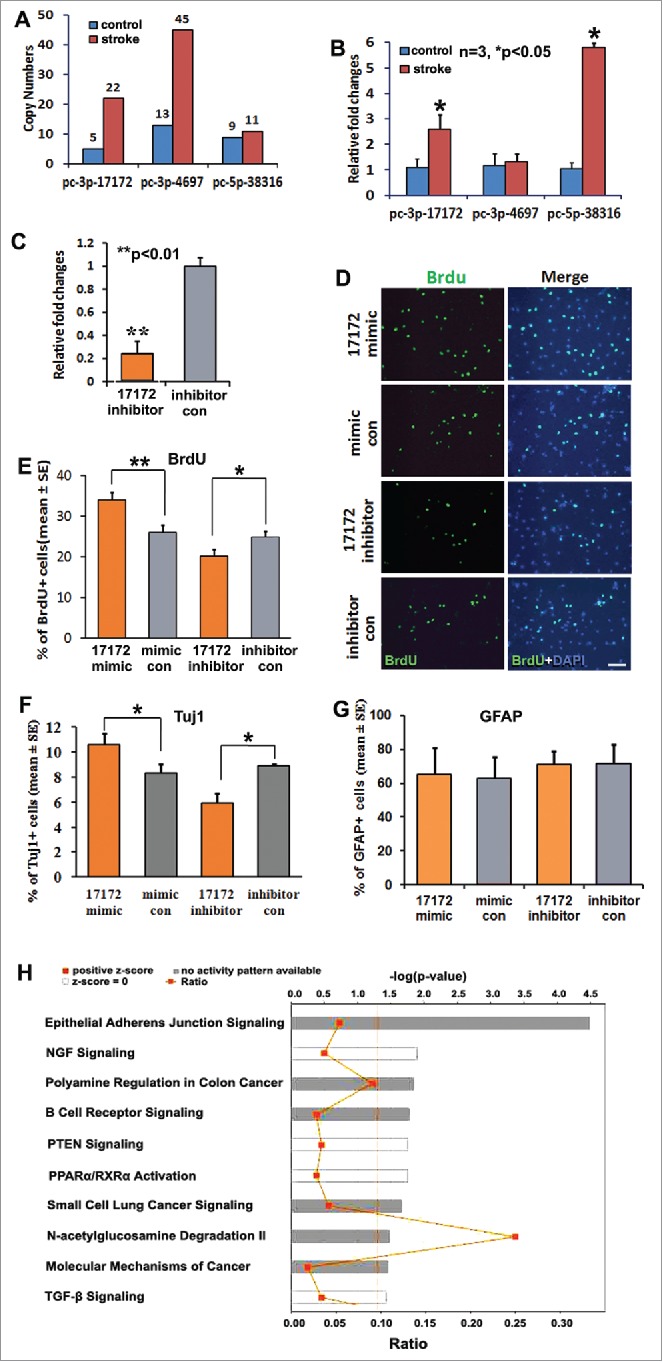
Novel miRNA pc-3p-17172 regulates neural progenitor cell proliferation and differentiation. (A) Ago2-RNA immunoprecipitation (RIP) shows the copy numbers of novel miRNAs in non-ischemic (control) and ischemic (MCAo) NPCs. (B) Quantitative RT-PCR data show the altered expression of novel miRNAs in non-ischemic (control) and ischemic (MCAo) NPCs. (C) The endogenous pc-3p-17172 expression was significantly knocked down in ischemic NPCs transfected by miScript inhibitor (pc-3p-17172 inhibitor) compared with NPCs transfected by inhibitor control (inhibitor con). Panel (D)shows the representative immunocytochemistry images of BrdU positive cells in ischemic NPCs transfected by pc-3p-17172 mimic, inhibitor or their corresponding controls. Panels E, (F)and (G)demonstrate quantitative data of BrdU (E), Tuj1 (F), GFAP (G) positive cells, respectively, in NPCs after transfection of pc-3p-17172 mimic or inhibitor. Panel (H)shows the most significantly enriched canonical signaling pathways associated with genes putatively targeted by pc-3p-17172. Data were analyzed by means of the IPA software. N = 3 for each group. * p < 0.05; ** p < 0.01 vs. the control group. Scale bar = 20µm.
To examine the biological function of these novel miRNAs, we selected pc-3p-17172, as it was substantially upregulated and with relatively high copy numbers in ischemic NPCs. We found that knockdown of pc-3p-17172 in non-ischemic NPCs by miRscript inhibitor (Qiagen, Fig. 4C) significantly reduced the percentage of BrdU+ NPCs (Fig. 4D, E) and Tuj1+ neuroblasts (Fig. 4F). Meanwhile, transfection of NPCs with pc-3p-17172 miScript mimics significantly increased the percentage of BrdU+ NPCs (Fig. 4D, E) and Tuj1+ neuroblasts (Fig. 4F). However, transfection of NPCs with pc-3p-17172 miScript mimics and knockdown of endogenous pc-3p-17172 by LNA did not significantly alter the percentage of GFAP+ cells (Fig. 4G). These data suggest that upregulated pc-3p-17172 by stroke promotes the proliferation and neuronal differentiation of NPCs.
Using TargetScan and miRDB algorithms, we computationally analyzed pc-3p-17172 putative target genes (Table S13). Gene ontology analysis with IPA revealed t hat pc-3p-17172 could potentially regulate multiple signaling pathway networks that are known to mediate neural stem cell functions including NGF, PTEN, TGF-β signaling, and PPAR activation (Fig. 4H).
Discussion
Using a high-throughput sequencing approach, we for the first time comprehensively identified profiles of Ago2-bound miRNAs including known and novel miRNAs in adult NPCs. In addition, we found that stroke dramatically changed the profiles of Ago2-bound miRNAs in NPCs and that genes putatively targeted by stroke-upregulated miRNAs regulate multiple biological networks that are essential for neurogenesis. These findings enhance our understanding of functional miRNAs in adult NPCs and provide a molecular basis for further study of the role of miRNAs in NPCs under non-ischemic and ischemic conditions.
Our data demonstrated highly Ago2-bound miRNAs. Among them, miR-26 was the top miRNA with the most seed enrichment in NPCs, which is in line with a previous report that miR-26 is induced in response to hypoxia and increased during smooth muscle cell differentiation35 and neurogenesis.15 The let-7 family including let-7f and 7i regulates the timing of ES cell differentiation by targeting Notch signaling.32 Multiple members of the let-7 family were highly abundant in the RISCs, suggesting a critical role of the let-7 family in regulating adult neurogenesis. Our findings that stroke induced 2-fold increases of reads in ischemia NPCs compared to non-ischemia NPCs, suggest that stroke may affect the biogenesis or pathway of mature miRNAs, which warrants further investigation. MiR-146a was one of most upregulated miRNAs in Ago2-RISCs after stroke. Despite its known role in inflammation, our recent study has revealed that miR-146a regulates neurogenesis and oligodendrogenesis.43 As proof of principle, we verified other top 3 upregulated miRNAs and their downregulated target mRNAs in ischemic NPCs. These miRNAs/mRNAs, such as miR-23/PTEN/SMAD, regulate neuronal apoptosis, myelination and neurogenesis.11,38,39 miR-191 is linked to hippocampal neuronal development and spine synapticity.22,68,44,47 Further studies are warranted to investigate the networks of miRNAs/mRNAs which mediate adult neurogenesis.
A Bland-Altman plot demonstrated 90% agreement of miRNAs between the 2 assays, microarray and Ago2 RIP-seq, for known miRNAs. The biological function of these abundant miRNAs may be related to the abundance of the corresponding target mRNAs. However, the remaining 10% of miRNAs that were not detected in the Ago2-bound miRNoms may likely have affinities to other Ago proteins whose function need to be further explored.
Our Ago2 RIP-seq study identified many new isomiRs with high seed enrichment in NPCs under normal and ischemic conditions, suggesting that isomiRs are functionally incorporated into RISCs to regulate target genes. We found that these isomiRs differed in length, sequence, expression patterns and target preference from their parental miRNAs and the expression levels of the top 20 expressed isomiRs were prominent compared with their parental canonical miRNAs, suggesting that these isomiRs may play more crucial roles in the regulation of adult neurogenesis than their parental miRNAs. Also, our data showed that 5′-end deletion and substitution isomiRs are more common in NPCs under ischemic conditions. Since isomiRs can stem from differences in RNA editing, miRNA biosynthesis, or SNPs inherent to the miRNA genes,5 our data suggest that stroke may alter biogenesis of isomiRs, especially 5′-RNA exonuclease compared to 3′-RNA exonuclease. It would be interesting to determine whether stroke affects miRNA post-transcriptional modulation. Furthermore, we verified the expression of isomiR-142 with modified seed. Bioinformatics analysis revealed the significant difference of potential predicted-targets between the isomiRs and their parent miRNAs, indicating the specific targeting properties of seed-modified isomiRs. One possible reason for the mismatch of isomiR-187 to Ago2-seq data is the use of total RNAs, for which the expression of miRNAs in total RNAs does not reflect the activity of miRNAs in Ago2 RISC.16 To our knowledge, the association between isomiR function and neurogenesis remains unknown. Further exploring the crosstalk between isomiRs and their parental miRNAs may provide novel insights into adult neurogenesis.
Our data identified several highly abundant miRNA duplexes in NPCs. The expression levels of 5 miRNAs* were more abundant than their mature miRNA siblings, suggesting that these star miRNAs may not degrade, but likely play a role in regulating stroke-induced neurogenesis. In addition, whether miRNAs* affect the passenger miRNAs and whether these miRNAs are specific for NPCs, remain to be determined.
We found several hundred new miRNAs in control and ischemic NPC libraries. However, the majority of the predicted novel miRNA had less than 10 reads, which is the reason why they were not found using other platforms. We also found that multiple predicted rno-miRNAs which displayed high expression, have been validated and renewed in the newer version of miRBase (21.0), suggesting that these predicted novel miRNAs are real. Moreover, some predicted miRNAs with high reads displayed significant homology to other mammalian species miRNAs or isomiRs, but they have not been reported in the rat, indicating that they are new miRNAs. Our data also illustrated 9 predicted miRNAs in non-ischemic and ischemic NPC libraries which were homologous to other species in the seed region. These miRNAs belong to new miRNAs in the rat. Our in vitro data demonstrated that novel miRNAs– pc-3p-17172 plays an important role in mediating stroke-induced neurogenesis.
In summary, we have implemented a technique which allowed us to quantitatively analyze miRNomes currently bound and processed in the RISC of NPCs. Our data provide new insights into the molecular mechanism underlying adult neurogenesis under non-ischemic and ischemic conditions. MiRNAs are highly promising targets for the therapy of neurological diseases.67 MiRNAs identified in the present study could potentially be used for the development of novel therapeutic approaches against brain injuries and neurodegenerative diseases.
Materials and methods
All experimental procedures were carried out in accordance with the NIH Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee (IACUC) of Henry Ford Hospital.
Animal model of middle cerebral artery occlusion (MCAo) and tissue collection
Male Wistar rats (3–4 months) were employed in this study. The right middle cerebral artery (MCA) was occluded by placement of an embolus at the origin of the right MCA. In this model, MCA occlusion (MCAo) evokes a peak increase in neurogenesis 7 d after stroke.79,81 Therefore, all rats were euthanized 7 d after MCAo. Immediately prior to sacrifice, the animals were perfused with 0.9% sterile saline solution to eliminate blood contamination. SVZ tissues, the source of NPCs, were dissected and collected from a pool of 3 rats subject to MCAo. The samples were stored at −80°C until RNA extraction.
Neural progenitor cell cultures
SVZ cells were dissociated from adult rodents, as previously described in detail.3,45,46,52,57 The cells were plated at a density of 2 × 104 cells/ml in growth medium. Growth medium contains DMEM/F-12 medium (Life Technologies, NY, USA), 20 ng/mL of epidermal growth factor (EGF, R&D System, MN, USA), and basic fibroblast growth factor (bFGF, R&D System). DMEM/F-12 medium contains L-glutamine (2 mmol/L, Life Technologies), glucose (0.6%, Sigma Aldrich, MO, USA), putrescine (9.6 mg/Ml, Sigma Aldrich), insulin (0.025 mg/mL, Sigma Aldrich), progesterone (6.3 ng/Ml, Sigma Aldrich), apo-transferrin (0.1 mg/mL, Sigma Aldrich), and sodium selenite (5.2 ng/mL, Sigma Aldrich). The generated neurospheres (primary spheres) were passaged by mechanical dissociation and reseeded as single cells at a density of 20 cells/µl. SVZ cells from ischemic brain were extracted 7 d after MCAo. The passaged 2–5 SVZ cells were used in all experiments.
Argonaute-2 based RNA-immunoprecipitation (Ago2 RIP)
Briefly, 50 μl Dynabeads protein G slurry (Invitrogen) was immobilized with 20 μg mouse anti-mouse Ago2 monoclonal antibody (clone 2D4, Wako Pure Chemical Industries, Osaka, Japan).36,51,72 One hundred 50 µg of frozen SVZ tissues isolated from normal or ischemic adult rats were homogenized in 1.5 ml of a cell lysis solution (provided in miRNAs isolation kit, Wako) using a Polytron PT1200C homogenizer for 10 s at 4°C, and then 1.5 ml of the cell lysis solution was added into the homogenized solution. Following incubation for 15 min on ice, SVZ lysate was centrifuged at 16 000 g for 10 min at 4 °C. One milliliter of the lysate was incubated with 50 μl of the anti-mouse Ago2 Dynabead protein G for incubation for 4 hours at 4°C. After immunoprecipitation, Ago2-associated RNAs were isolated from the immunoprecipitate according to the manufacture's protocol (Wako).
To validate the sensitivity and specificity of immunoprecipitation, a rabbit polyclonal anti-Ago2 antibody (Abcam, 10µg) was used for the immunoprecipitation and a mouse monoclonal anti-Ago2 antibody (Wako, 1:1000 dilution) was utilized for Western blot analysis of Ago2. The normalization was performed by blotting the same samples with an antibody against β-actin (Abcam, 1:10,000 dilution). We confirmed that the immunoprecipitate contained mouse Ago2 protein of 100kDa in size by Western blot. Non-immune mouse IgG (Sigma) was used as a control for Ago2-immunoprecipitation.
After the elution from the beads, the RNA was prepared using a miRNeasy Mini Kit (Qiagen). To remove possible genomic DNA contamination, RNase-free DNase was used during the RNA purification steps. RNA concentration was determined using a Nanodrop ND-1000 spectrophotometer (NanoDrop Technologies Inc., Wilmington, DE) and its integrity was ascertained by migration on 2% agarose gel and analyzed by displaying 28S and 18S rRNA. RNA samples were pooled from 3 Ago2-RIP lysates in control and ischemic NPCs to obtain 600–800 ng RNA from each sample. The Ago2-associated miRNAs were analyzed using small RNA deep sequencing.
Small RNA library construction and deep sequencing
Preparation of Small RNA library construction using the Ago2-associated RNAs was conducted by using Illumina TruSeq small RNA preparation Kit following the manufacturer's instructions (LC Sciences). The general process is as follows. First, the total RNA was ligated to RNA 3′ and RNA 5′ adapters. Second, reverse transcription followed by PCR was performed to create cDNA constructs based on the small RNAs ligated with 3′ and 5′ adapters. Third, small cDNA fractions that range from 22 nt to 30 nt in length were isolated by using 6% denaturing polyacrylamide gel electrophoresis. Fourth, cDNA construct was purified, and the library was validated. The purified cDNA library was used for cluster generation on Illumina's Cluster Station and subsequently sequenced on Illumina GAIIx (Illumina, Inc., Santa Clara, CA) following the manufacturer's instruction on running the instrument. Raw sequencing reads were obtained by using related Illumina's analysis software. The sequencing data analysis was performed using the ACGT101-miR packages (version 4.2; LC Sciences, Houston, TX, USA).
Sequencing data analysis
After the raw sequence reads were extracted, adapter sequences, impurities, and sequences beyond 15 nt to 30 nt were filtered. The remaining sequences that range from 15 nt to 30 nt in length were used for miRNA prediction by using the ACGT101-miR v4.2 program (LC Sciences, Houston, TX). First, the sequences were blasted to the RFam database (RFam: rRNA, tRNA, snRNA, snoRNA, and other non-coding RNAs), repeat sequences, and mRNAs. Matched sequences were discarded. The sequences were then compared with Rattus norvegicus (rno) genome sequences downloaded from the Rattus norvegicus database (ftp://ftp.ncbi.nih.gov/genomes/Rnorvegicus). The unmatched sequences were filtered. Finally, the remaining sequences were mapped to all known mammalian miRNAs (except for Rattus norvegicus) sequences to identify the conserved miRNAs in Rattus norvegicus from the miRBase database (version 18.0, http://www.mirbase.org/). Matched sequences with no more than 3 mismatches were considered as candidate conserved miRNAs. At the same time, the unmatched sequences were reserved as candidate novel miRNAs. To identify novel miRNAs in Rattus norvegicus, predicted candidate miRNAs sequences were blasted against Rattus norvegicus genome sequences, and their flanking sequences in the genome were used to predict their secondary structures by using the mfold Web server (http://mfold.rna. albany.edu/?q=mfold/download-mfold).83
Alternative arm selection of annotated miRNA/miRNA* pairs
A potential miRNA precursor must be a non-coding sequence and must meet certain criteria. Both a candidate miRNA and its corresponding reverse sequence, namely, the candidate miRNA* sequence, must be detected in the present high-throughput sequencing. Second, the candidate miRNA and miRNA* sequences must be found on the stem, and the number of mismatched bases between them must be less than 4 (4 continuous mismatches are also not allowed). Third, within the miRNA/miRNA* duplex, the number of asymmetric bulges must be one or fewer, and the number of bases in the asymmetric bulges must fewer than two. Fourth, the miRNA and miRNA* should be located in opposite stem-arms and form a duplex with 2 nucleotide 3′ overhangs. Fifth, the potential miRNA precursor must have higher negative minimal folding energy (MFE) and minimal folding energy indexes (MFEI), with the MFEI > 0.8, to distinguish from other small RNAs.77
The ratios of the miRNA/miRNA* species percentage counts were calculated. Any annotated miRNA* sequence with at least 0.001 percentage counts (averaged over 2 libraries from control and ischemic NPCs) and at least a 5 times higher expression level compared with the relevant mature sequence was further investigated.
Discovery of novel miRNA sequences
To identify novel miRNA sequences, the output of the mapping versus rat stem–loop sequences of miRBase was screened for miRNAs with annotation only for the mature sequence, harboring 5 or more reads mapping to an identical position on the opposite arm in all 4 libraries and showing an average abundance of at least 50 read counts. Subsequently, only the putative novel star sequences lying within ±3 nt opposite of, and sharing at least 50% sequence complementarity with their respective annotated mature sequence, were accepted as novel candidates.
MiRNA target gene prediction and pathway analysis
MiRNAs with 2-fold difference and 400 genes with substantial changes in the expression were integrated into MetaCore (St. Joseph, MI) or Ingenuity Pathway Analysis (IPA, Qiagen) software to analyze the gene ontology, pathway distribution, and putative gene targets. Websites for database used in this manuscript include NCBI Entrez Nucleotide database, European rRNA database, Genomic tRNA database, RNAdb, NCBI Reference Sequence and UCSC Genome Bioinformatics Sites.
MiRNA transfection
Novel PC-3p-17172_miScript mimic or inhibitor (Qiagen) were delivered into SVZ NPCs using nucleofector electroporation. MiScript mimic or inhibitor negative control (Qiagen, cat#1027280 and 1027271) were transfected into NPCs. Briefly, miRNA oligonucleotides (200 pmol, Dharmacon) were mixed with 100 μl of nucleofector solution and were introduced into cultured NPCs using a Program A33 with NucleofectorTM machine (Lonza, NJ, USA), as previously described.44
Neurosphere assay
A neurosphere assay was employed to investigate the effect of miRNAs on SVZ NPCs as previously described.44,45,46 The SVZ NPCs were transfected with miRNA mimics or inhibitors and incubated in growth medium in the presence of EGF and bFGF for 24 hours to allow the cells to recover. To analyze cell proliferation, bromodeoxyuridine (BrdU, 30 μg/ml, Sigma Aldrich), the thymidine analog that is incorporated into the DNA of dividing cells during S-phase, was added 18 hours before the termination of incubation. The BrdU-treated cells were fixed and acid-treated, followed by immunostaining analysis with BrdU-specific antibody. BrdU positive cells were measured (see below for quantification).
To examine the SVZ cell differentiation, neurospheres were plated directly onto laminin-coated glass cover slips in DMEM/F-12 medium containing 2% fetal bovine serum (FBS), which is referred to as differentiation medium. Every 4 days, one-half of the medium was replaced with fresh medium. Incubation was terminated 10 d after plating, and immunostaining for neuronal and astrocyte markers was performed for evaluation of cell differentiation.
Immunocytochemistry and quantification
Immunofluorescent staining was performed on cultured cells. The following primary antibodies were used in the present study: mouse anti-BrdU (1:1,00; Boehringer Mannheim, IN, USA), glial fibrillary acidic protein (GFAP), a marker of astrocytes, and mouse anti-β-III tubulin (TuJ-1, 1:500; Covance the Development Services Co.), a marker of neuroblasts. Cultured cells were fixed in 4% paraformaldehyde for 20 min at room temperature. Nonspecific binding sites were blocked with phosphate-buffered saline with 1% bovine serum albumin goat serum for 1 h at room temperature. The cells were then incubated with the primary antibodies listed above and with CY3-conjugated secondary antibodies. Nuclei were counterstained with 4-,6-diamidino-2-phenylindole (1:10,000, Vector Laboratories, CA, USA). BrdU staining was as previously described.45
For all measurements, we counted cells from 3 wells per group (n = 3 individual cultured cells). Six fields of the view per well were randomly imaged under a 20 × objective and measured using MCID system. All analysis was conducted with the examiner blinded to the identity of the samples being studied.
Quantification of known and novel mature miRNAs by real-time qRT-PCR
Total RNAs (10 ng) were reverse transcribed using a TaqMan® MicroRNA Reverse Transcription (RT) kit (Applied Biosystems). Each RT reaction contained 1 × stem-loop RT specific primer, 1 × reaction buffer, 0.25 mM each of dNTPs, 3.33 U/µl Multiscribe RT enzyme and 0.25 U/µl RNase inhibitor. The 15-µl reactions were incubated for 30 min at 16°C, 30 min at 42°C, and 5 min at 85°C and then held at 4°C. The PCR reaction was performed using a standard TaqMan® PCR kit protocol (Applied Biosystems). Briefly, following the RT step, 1.33 µl of the RT reaction were combined with 1 µl of a TaqMAn MicroRNA Assay (20×; forward primer, reverse primer and probe) and 17.67 µl of TaqMan® Universal PCR Master Mix, No AmpErase® UNG in a 20 µl final volume. The reactions were incubated at 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. The expression of miRNAs was normalized against the expression of U6 snRNA as an endogenous normalization control. All assays were performed in triplicate and were calculated on the basis of the ΔΔCt method. The fold change in miRNAs expression was determined according to the method of 2−ΔΔCT.
Statistical analysis
The data are presented as mean ± SE. Independent sample t-test was used for 2-group comparisons from the non-MCAo and MCAo samples. One-way analysis of variance followed by Student-Newman-Keuls test was performed for multiple sample analysis. A value of p < 0.05 was taken as significant.
Supplementary Material
Disclosure of potential confllicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by AHA Grant-in-Aid 14GRNT20460167 (XSL), National Institutes of Health Grants RO1 RDK102861A (XSL), RO1 NS075156 (ZGZ) and RO1 NS088656 (MC).
References
- 1.Akerblom M, Sachdeva R, Jakobsson J. Functional studies of microRNAs in neural stem cells: problems and perspectives. Front Neurosci 2012; 6:14; PMID:22347160; http://dx.doi.org/ 10.3389/fnins.2012.00014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alvarez-Buylla A, Herrera DG, Wichterle H. The subventricular zone: source of neuronal precursors for brain repair. Prog Brain Res 2000; 127:1-11; PMID:11142024; http://dx.doi.org/ 10.1016/S0079-6123(00)27002-7 [DOI] [PubMed] [Google Scholar]
- 3.Aranha MM, Santos DM, Xavier JM, Low WC, Steer CJ, Sola S, Rodrigues CM. Apoptosis-associated microRNAs are modulated in mouse, rat and human neural differentiation. BMC Genomics 2010; 11:514; PMID:20868483; http://dx.doi.org/ 10.1186/1471-2164-11-514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med 2002; 8(9):963-70; PMID:12161747; http://dx.doi.org/ 10.1038/nm747 [DOI] [PubMed] [Google Scholar]
- 5.Bajan S, Hutvagner G. Regulation of miRNA processing and miRNA mediated gene repression in cancer. Microrna 2014; 3(1):10-17; PMID:25069508; http://dx.doi.org/ 10.2174/2211536602666140110234046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bellenchi GC, Volpicelli F, Piscopo V, Perrone-Capano C, di Porzio U. Adult neural stem cells: an endogenous tool to repair brain injury? J Neurochem 2013; 124(2):159-67; PMID:23134340; http://dx.doi.org/ 10.1111/jnc.12084 [DOI] [PubMed] [Google Scholar]
- 7.Boudreau RL, Jiang P, Gilmore BL, Spengler RM, Tirabassi R, Nelson JA, Ross CA, Xing Y, Davidson BL. Transcriptome-wide discovery of microRNA binding sites in human brain. Neuron 2014; 81(2):294-305; PMID:24389009; http://dx.doi.org/ 10.1016/j.neuron.2013.10.062] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bushati N, Cohen SM. microRNA functions. Annu Rev Cell Dev Biol 2007; 23:175-205; PMID:17506695; http://dx.doi.org/ 10.1146/annurev.cellbio.23.090506.123406 [DOI] [PubMed] [Google Scholar]
- 9.Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer 2006; 6(11):857-66; PMID:17060945; http://dx.doi.org/ 10.1038/nrc1997 [DOI] [PubMed] [Google Scholar]
- 10.Cenik ES, Zamore PD. Argonaute proteins. Curr Biol 2011; 21(12):R446-9; PMID:21683893; http://dx.doi.org/ 10.1016/j.cub.2011.05.020 [DOI] [PubMed] [Google Scholar]
- 11.Chen Q, Xu J, Li L, Li H, Mao S, Zhang F, Zen K, Zhang CY, Zhang Q. MicroRNA-23a/b and microRNA-27a/b suppress Apaf-1 protein and alleviate hypoxia-induced neuronal apoptosis. Cell Death Dis 2014; 5:e1132; PMID:24651435; http://dx.doi.org/ 10.1038/cddis.2014.92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chendrimada TP, Gregory RI, Kumaraswamy E, Norman J, Cooch N, Nishikura K, Shiekhattar R. TRBP recruits the Dicer complex to Ago2 for microRNA processing and gene silencing. Nature 2005; 436(7051):740-44; PMID:15973356; http://dx.doi.org/ 10.1038/nature03868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chi SW, Zang JB, Mele A, Darnell RB. Argonaute HITS-CLIP decodes microRNA-mRNA interaction maps. Nature 2009; 460(7254):479-86; PMID:19536157; http://dx.doi.org/ 10.1038/nature08170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dharap A, Bowen K, Place R, Li LC, Vemuganti R. Transient focal ischemia induces extensive temporal changes in rat cerebral microRNAome. J Cereb Blood Flow Metab 2009; 29(4):675-87; PMID:19142192; http://dx.doi.org/ 10.1038/jcbfm.2008.157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dill H, Linder B, Fehr A, Fischer U. Intronic miR-26b controls neuronal differentiation by repressing its host transcript, ctdsp2. Genes Dev 2012; 26(1):25-30; PMID:22215807; http://dx.doi.org/ 10.1101/gad.177774.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flores O, Kennedy EM, Skalsky RL, Cullen BR. Differential RISC association of endogenous human microRNAs predicts their inhibitory potential. Nucleic Acids Res 2014; 42(7):4629-39; PMID:24464996; http://dx.doi.org/ 10.1093/nar/gkt1393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gage FH, Ray J, Fisher LJ. Isolation, characterization, and use of stem cells from the CNS. Annu Rev Neurosci 1995; 18:159-92; PMID:7605059; http://dx.doi.org/ 10.1146/annurev.ne.18.030195.001111 [DOI] [PubMed] [Google Scholar]
- 18.Ghildiyal M, Zamore PD. Small silencing RNAs: an expanding universe. Nat Rev Genet 2009; 10(2):94-108; PMID:19148191; http://dx.doi.org/ 10.1038/nrg2504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hammond SM, Bernstein E, Beach D, Hannon GJ. An RNA-directed nuclease mediates post-transcriptional gene silencing in Drosophila cells. Nature 2000; 404(6775):293-6; PMID:10749213; http://dx.doi.org/ 10.1038/35005107 [DOI] [PubMed] [Google Scholar]
- 20.Hinton A, Hunter SE, Afrikanova I, Jones GA, Lopez AD, Fogel GB, Hayek A, King CC. sRNA-seq analysis of human embryonic stem cells and definitive endoderm reveals differentially expressed microRNAs and novel IsomiRs with distinct targets. Stem Cells 2014; 32(9):2360-72; PMID:24805944; http://dx.doi.org/ 10.1002/stem.1739 [DOI] [PubMed] [Google Scholar]
- 21.Hsieh WJ, Wang H. Human microRNA target identification by RRSM. J Theor Biol 2011; 286(1):79-84; PMID:21736879; http://dx.doi.org/ 10.1016/j.jtbi.2011.06.022 [DOI] [PubMed] [Google Scholar]
- 22.Hu Z, Yu D, Gu QH, Yang Y, Tu K, Zhu J, Li Z. miR-191 and miR-135 are required for long-lasting spine remodelling associated with synaptic long-term depression. Nat Commun 2014; 5:3263; PMID:24535612; http://dx.doi.org/ 10.1038/ncomms4263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hutvagner G, Simard MJ. Argonaute proteins: key players in RNA silencing. Nat Rev Mol Cell Biol 2008; 9(1):22-32; PMID:18073770; http://dx.doi.org/ 10.1038/nrm2321 [DOI] [PubMed] [Google Scholar]
- 24.Hutvagner G, Zamore PD. A microRNA in a multiple-turnover RNAi enzyme complex. Science 2002; 297(5589):2056-60; PMID:12154197; http://dx.doi.org/ 10.1126/science.1073827 [DOI] [PubMed] [Google Scholar]
- 25.Im HI, Kenny PJ. MicroRNAs in neuronal function and dysfunction. Trends Neurosci 2002; 35(5):325-34; PMID:22436491; http://dx.doi.org/ 10.1016/j.tins.2012.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iwai M, Sato K, Kamada H, Omori N, Nagano I, Shoji M, Abe K. Temporal profile of stem cell division, migration, and differentiation from subventricular zone to olfactory bulb after transient forebrain ischemia in gerbils. J Cereb Blood Flow Metab 2003; 23(3):331-41; PMID:12621308; http://dx.doi.org/ 10.1097/01.WCB.0000050060.57184.E7 [DOI] [PubMed] [Google Scholar]
- 27.Iwai M, Sato K, Omori N, Nagano I, Manabe Y, Shoji M, Abe K. Three steps of neural stem cells development in gerbil dentate gyrus after transient ischemia. J Cereb Blood Flow Metab 2002; 22(4):411-9; PMID:11919512; http://dx.doi.org/ 10.1097/00004647-200204000-00005 [DOI] [PubMed] [Google Scholar]
- 28.Jeyaseelan K, Lim KY, Armugam A. MicroRNA expression in the blood and brain of rats subjected to transient focal ischemia by middle cerebral artery occlusion. Stroke 2008; 39(3):959-66; PMID:18258830; http://dx.doi.org/ 10.1161/STROKEAHA.107.500736 [DOI] [PubMed] [Google Scholar]
- 29.Jin K, Minami M, Lan JQ, Mao XO, Batteur S, Simon RP, Greenberg DA. Neurogenesis in dentate subgranular zone and rostral subventricular zone after focal cerebral ischemia in the rat. Proc Natl Acad Sci U S A 2001; 98(8):4710-5; PMID:11296300; http://dx.doi.org/ 10.1073/pnas.081011098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jin K, Wang X, Xie L, Mao XO, Greenberg DA. Transgenic ablation of doublecortin-expressing cells suppresses adult neurogenesis and worsens stroke outcome in mice. Proc Natl Acad Sci U S A 2010; 107(17):7993-8; PMID:20385829; http://dx.doi.org/ 10.1073/pnas.1000154107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Joshua-Tor L. The argonautes. Cold Spring Harb Symp Quant Biol 2006; 71:67-72; PMID:17381282; http://dx.doi.org/ 10.1101/sqb.2006.71.048 [DOI] [PubMed] [Google Scholar]
- 32.Kawahara H, Imai T, Okano H. MicroRNAs in neural stem cells and neurogenesis. Front Neurosci 2012; 6:30; PMID:22416227; http://dx.doi.org/ 10.3389/fnins.2012.00030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kee NJ, Preston E, Wojtowicz JM. Enhanced neurogenesis after transient global ischemia in the dentate gyrus of the rat. Exp Brain Res 2001; 136(3):313-20; PMID:11243473; http://dx.doi.org/ 10.1007/s002210000591 [DOI] [PubMed] [Google Scholar]
- 34.Kirschenbaum B, Doetsch F, Lois C, Alvarez-Buylla A. Adult subventricular zone neuronal precursors continue to proliferate and migrate in the absence of the olfactory bulb. J Neurosci 1999; 19(6):2171-80; PMID:10066270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leeper NJ, Raiesdana A, Kojima Y, Chun HJ, Azuma J, Maegdefessel L, Kundu RK, Quertermous T, Tsao PS, Spin JM. MicroRNA-26a is a novel regulator of vascular smooth muscle cell function. J Cell Physiol 2011; 226(4):1035-43; PMID:20857419; http://dx.doi.org/ 10.1002/jcp.22422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leung AK, Young AG, Bhutkar A, Zheng GX, Bosson AD, Nielsen CB, Sharp PA. Genome-wide identification of Ago2 binding sites from mouse embryonic stem cells with and without mature microRNAs. Nat Struct Mol Biol 2011; 18(2):237-44; PMID:21258322; http://dx.doi.org/ 10.1038/nsmb.1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li M, Xia Y, Gu Y, Zhang K, Lang Q, Chen L, Guan J, Luo Z, Chen H, Li Y et al.. MicroRNAome of porcine pre- and postnatal development. PLoS One 2010; 5(7):e11541; PMID:20634961; http://dx.doi.org/ 10.1371/journal.pone.0011541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin ST, Fu YH. miR-23 regulation of lamin B1 is crucial for oligodendrocyte development and myelination. Dis Model Mech 2009; 2(3–4):178-88; PMID:19259393; http://dx.doi.org/ 10.1242/dmm.001065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin ST, Huang Y, Zhang L, Heng MY, Ptacek LJ, Fu YH. MicroRNA-23a promotes myelination in the central nervous system. Proc Natl Acad Sci U S A 2013; 110(43):17468-73; PMID:24101522; http://dx.doi.org/ 10.1073/pnas.1317182110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu DZ, Tian Y, Ander BP, Xu H, Stamova BS, Zhan X, Turner RJ, Jickling G, Sharp FR. Brain and blood microRNA expression profiling of ischemic stroke, intracerebral hemorrhage, and kainate seizures. J Cereb Blood Flow Metab 2010; 30(1):92-101; PMID:19724284; http://dx.doi.org/ 10.1038/jcbfm.2009.186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu J, Carmell MA, Rivas FV, Marsden CG, Thomson JM, Song JJ, Hammond SM, Joshua-Tor L, Hannon GJ. Argonaute2 is the catalytic engine of mammalian RNAi. Science 2004; 305(5689):1437-41; PMID:15284456; http://dx.doi.org/ 10.1126/science.1102513 [DOI] [PubMed] [Google Scholar]
- 42.Liu J, Solway K, Messing RO, Sharp FR. Increased neurogenesis in the dentate gyrus after transient global ischemia in gerbils. J Neurosci 1998; 18(19):7768-78; PMID:9742147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu XS, Chopp M, Pan WL, Wang XL, Fan BY, Zhang Y, Kassis H, Zhang RL, Zhang XM, Zhang ZG. MicroRNA-146a Promotes Oligodendrogenesis in Stroke. Mol Neurobiol 2016. Jan 6; PMID:26738853; http://dx.doi.org/23511639 10.1007/s12035-015-9655-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu XS, Chopp M, Wang XL, Zhang L, Hozeska-Solgot A, Tang T, Kassis H, Zhang RL, Chen C, Xu J et al.. MicroRNA-17-92 cluster mediates the proliferation and survival of neural progenitor cells after stroke. J Biol Chem 2013; 288(18):12478-88; PMID:23511639; http://dx.doi.org/ 10.1074/jbc.M112.449025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu XS, Chopp M, Zhang RL, Hozeska-Solgot A, Gregg SC, Buller B, Lu M, Zhang ZG. Angiopoietin 2 mediates the differentiation and migration of neural progenitor cells in the subventricular zone after stroke. J Biol Chem 2009; 284(34):22680-9; PMID:19553662; http://dx.doi.org/ 10.1074/jbc.M109.006551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu XS, Chopp M, Zhang RL, Tao T, Wang XL, Kassis H, Hozeska-Solgot A, Zhang L, Chen C, Zhang ZG. MicroRNA profiling in subventricular zone after stroke: MiR-124a regulates proliferation of neural progenitor cells through notch signaling pathway. PLoS One 2011; 6(8):e23461; PMID:21887253; http://dx.doi.org/ 10.1371/journal.pone.0023461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu XS, Zhang ZG, Zhang RL, Gregg S, Morris DC, Wang Y, Chopp M. Stroke induces gene profile changes associated with neurogenesis and angiogenesis in adult subventricular zone progenitor cells. J Cereb Blood Flow Metab 2007; 27(3):564-74; PMID:16835628; http://dx.doi.org/ 10.1038/sj.jcbfm.9600371 [DOI] [PubMed] [Google Scholar]
- 48.Luskin MB, Zigova T, Soteres BJ, Stewart RR. Neuronal progenitor cells derived from the anterior subventricular zone of the neonatal rat forebrain continue to proliferate in vitro and express a neuronal phenotype. Mol Cell Neurosci 1997; 8(5):351-66; PMID:9073397; http://dx.doi.org/ 10.1006/mcne.1996.0592 [DOI] [PubMed] [Google Scholar]
- 49.Macas J, Nern C, Plate KH, Momma S. Increased generation of neuronal progenitors after ischemic injury in the aged adult human forebrain. J Neurosci 2006; 26(50):13114-9; PMID:17167100; http://dx.doi.org/ 10.1523/JNEUROSCI.4667-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Malterer G, Dolken L, Haas J. The miRNA-targetome of KSHV and EBV in human B-cells. RNA Biol 2011; 8(1):30-34; PMID:21301209; http://dx.doi.org/ 10.4161/rna.8.1.13745 [DOI] [PubMed] [Google Scholar]
- 51.Mishima T, Takizawa T, Luo SS, Ishibashi O, Kawahigashi Y, Mizuguchi Y, Ishikawa T, Mori M, Kanda T, Goto T, et al.. MicroRNA (miRNA) cloning analysis reveals sex differences in miRNA expression profiles between adult mouse testis and ovary. Reproduction 2008; 136(6):811-22; PMID:18772262; http://dx.doi.org/ 10.1530/REP-08-0349 [DOI] [PubMed] [Google Scholar]
- 52.Morshead CM, Reynolds BA, Craig CG, McBurney MW, Staines WA, Morassutti D, Weiss S, van der Kooy D. Neural stem cells in the adult mammalian forebrain: a relatively quiescent subpopulation of subependymal cells. Neuron 1994; 13(5):1071-82; PMID:7946346; http://dx.doi.org/ 10.1016/0896-6273(94)90046-9 [DOI] [PubMed] [Google Scholar]
- 53.Mourelatos Z, Dostie J, Paushkin S, Sharma A, Charroux B, Abel L, Rappsilber J, Mann M, Dreyfuss G. miRNPs: a novel class of ribonucleoproteins containing numerous microRNAs. Genes Dev 2002; 16(6):720-8; PMID:11914277; http://dx.doi.org/ 10.1101/gad.974702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ohab JJ, Fleming S, Blesch A, Carmichael ST. A neurovascular niche for neurogenesis after stroke. J Neurosci 2006; 26(50):13007-16; PMID:17167090; http://dx.doi.org/ 10.1523/JNEUROSCI.4323-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Parent JM, Vexler ZS, Gong C, Derugin N, Ferriero DM. Rat forebrain neurogenesis and striatal neuron replacement after focal stroke. Ann Neurol 2002; 52(6):802-13; PMID:12447935; http://dx.doi.org/ 10.1002/ana.10393 [DOI] [PubMed] [Google Scholar]
- 56.Raber J, Fan Y, Matsumori Y, Liu Z, Weinstein PR, Fike JR, Liu J. Irradiation attenuates neurogenesis and exacerbates ischemia-induced deficits. Ann Neurol 2004; 55(3):381-9; PMID:14991816; http://dx.doi.org/ 10.1002/ana.10853 [DOI] [PubMed] [Google Scholar]
- 57.Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science 1992; 255(5052):1707-10; PMID:1553558; http://dx.doi.org/ 10.1126/science.1553558 [DOI] [PubMed] [Google Scholar]
- 58.Schmidt W, Reymann KG. Proliferating cells differentiate into neurons in the hippocampal CA1 region of gerbils after global cerebral ischemia. Neurosci Lett 2002; 334(3):153-6; PMID:12453618; http://dx.doi.org/ 10.1016/S0304-3940(02)01072-8 [DOI] [PubMed] [Google Scholar]
- 59.Song JJ, Smith SK, Hannon GJ, Joshua-Tor L. Crystal structure of Argonaute and its implications for RISC slicer activity. Science 2004; 305(5689):1434-7; PMID:15284453; http://dx.doi.org/ 10.1126/science.1102514 [DOI] [PubMed] [Google Scholar]
- 60.Sun F, Wang X, Mao X, Xie L, Jin K. Ablation of neurogenesis attenuates recovery of motor function after focal cerebral ischemia in middle-aged mice. PLoS One 2012; 7(10):e46326; PMID:23110048; http://dx.doi.org/ 10.1371/journal.pone.0046326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tan KS, Armugam A, Sepramaniam S, Lim KY, Setyowati KD, Wang CW, Jeyaseelan K. Expression profile of MicroRNAs in young stroke patients. PLoS One 2009; 4(11):e7689; PMID:19888324; http://dx.doi.org/ 10.1371/journal.pone.0007689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tanaka R, Yamashiro K, Mochizuki H, Cho N, Onodera M, Mizuno Y, Urabe T. Neurogenesis after transient global ischemia in the adult hippocampus visualized by improved retroviral vector. Stroke 2004; 35(6):1454-9; PMID:15073392; http://dx.doi.org/ 10.1161/01.STR.0000126480.40967.b3 [DOI] [PubMed] [Google Scholar]
- 63.Tay Y, Rinn J, Pandolfi PP. The multilayered complexity of ceRNA crosstalk and competition. Nature 2014; 505(7483):344-52; PMID:24429633; http://dx.doi.org/ 10.1038/nature12986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thored P, Arvidsson A, Cacci E, Ahlenius H, Kallur T, Darsalia V, Ekdahl CT, Kokaia Z, Lindvall O. Persistent production of neurons from adult brain stem cells during recovery after stroke. Stem Cells 2006; 24(3):739-47; PMID:16210404; http://dx.doi.org/ 10.1634/stemcells.2005-0281 [DOI] [PubMed] [Google Scholar]
- 65.Tolia NH, Joshua-Tor L. Slicer and the argonautes. Nat Chem Biol 2007; 3(1):36-43; PMID:17173028; http://dx.doi.org/ 10.1038/nchembio848 [DOI] [PubMed] [Google Scholar]
- 66.Tonchev AB, Yamashima T, Zhao L, Okano HJ, Okano H. Proliferation of neural and neuronal progenitors after global brain ischemia in young adult macaque monkeys. Mol Cell Neurosci 2003; 23(2):292-301; PMID:12812760; http://dx.doi.org/ 10.1016/S1044-7431(03)00058-7 [DOI] [PubMed] [Google Scholar]
- 67.van Rooij E, Purcell AL, Levin AA. Developing microRNA therapeutics. Circ Res 2012; 110(3):496-507; PMID:22302756; http://dx.doi.org/ 10.1161/CIRCRESAHA.111.247916 [DOI] [PubMed] [Google Scholar]
- 68.van Spronsen M, van Battum EY, Kuijpers M, Vangoor VR, Rietman ML, Pothof J, Gumy LF, van Ijcken WF, Akhmanova A, Pasterkamp RJ et al.. Developmental and activity-dependent miRNA expression profiling in primary hippocampal neuron cultures. PLoS One 2013; 8(10):e74907; PMID:24098357; http://dx.doi.org/ 10.1371/journal.pone.0074907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Voellenkle C, Rooij J, Guffanti A, Brini E, Fasanaro P, Isaia E, Croft L, David M, Capogrossi MC, Moles A et al.. Deep-sequencing of endothelial cells exposed to hypoxia reveals the complexity of known and novel microRNAs. RNA 2012; 18(3):472-84; PMID:22282338; http://dx.doi.org/ 10.1261/rna.027615.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang X, Mao X, Xie L, Sun F, Greenberg DA, Jin K. Conditional depletion of neurogenesis inhibits long-term recovery after experimental stroke in mice. PLoS One 2012; 7(6):e38932; PMID:22723908; http://dx.doi.org/ 10.1371/journal.pone.0038932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wee LM, Flores-Jasso CF, Salomon WE, Zamore PD. Argonaute divides its RNA guide into domains with distinct functions and RNA-binding properties. Cell 2012; 151(5):1055-67; PMID:23178124; http://dx.doi.org/ 10.1016/j.cell.2012.10.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wei J, Jones J, Kang J, Card A, Krimm M, Hancock P, Pei Y, Ason B, Payson E, Dubinina N et al.. RNA-induced silencing complex-bound small interfering RNA is a determinant of RNA interference-mediated gene silencing in mice. Mol Pharmacol 2011a; 79(6):953-63; PMID:21427169; http://dx.doi.org/ 10.1124/mol.110.070409 [DOI] [PubMed] [Google Scholar]
- 73.Wei Z, Liu X, Feng T, Chang Y. Novel and conserved micrornas in Dalian purple urchin (Strongylocentrotus nudus) identified by next generation sequencing. Int J Biol Sci 2011b; 7(2):180-92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yagita Y, Kitagawa K, Ohtsuki T, Takasawa K, Miyata T, Okano H, Hori M, Matsumoto M. Neurogenesis by progenitor cells in the ischemic adult rat hippocampus. Stroke 2001; 32(8):1890-6; PMID:11486122; http://dx.doi.org/ 10.1161/01.STR.32.8.1890 [DOI] [PubMed] [Google Scholar]
- 75.Yamashita T, Ninomiya M, Hernandez Acosta P, Garcia-Verdugo JM, Sunabori T, Sakaguchi M, Adachi K, Kojima T, Hirota Y, Kawase T et al.. Subventricular zone-derived neuroblasts migrate and differentiate into mature neurons in the post-stroke adult striatum. J Neurosci 2006; 26(24):6627-36; PMID:16775151; http://dx.doi.org/ 10.1523/JNEUROSCI.0149-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yoshimura S, Takagi Y, Harada J, Teramoto T, Thomas SS, Waeber C, Bakowska JC, Breakefield XO, Moskowitz MA. FGF-2 regulation of neurogenesis in adult hippocampus after brain injury. Proc Natl Acad Sci U S A 2001; 98(10):5874-9; PMID:11320217; http://dx.doi.org/ 10.1073/pnas.101034998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang BH, Pan XP, Cox SB, Cobb GP, Anderson TA. Evidence that miRNAs are different from other RNAs. Cell Mol Life Sci 2006; 63(2):246-54; PMID:16395542; http://dx.doi.org/ 10.1007/s00018-005-5467-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang L, Chopp M, Zhang RL, Wang L, Zhang J, Wang Y, Toh Y, Santra M, Lu M, Zhang ZG. Erythropoietin amplifies stroke-induced oligodendrogenesis in the rat. PLoS One 2010; 5(6):e11016; PMID:20552017; http://dx.doi.org/ 10.1371/journal.pone.0011016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang R, Zhang Z, Wang L, Wang Y, Gousev A, Zhang L, Ho KL, Morshead C, Chopp M. Activated neural stem cells contribute to stroke-induced neurogenesis and neuroblast migration toward the infarct boundary in adult rats. J Cereb Blood Flow Metab 2004; 24(4):441-8; PMID:15087713; http://dx.doi.org/ 10.1097/00004647-200404000-00009 [DOI] [PubMed] [Google Scholar]
- 80.Zhang RL, Zhang ZG, Chopp M. Neurogenesis in the adult ischemic brain: generation, migration, survival, and restorative therapy. Neuroscientist 2005; 11(5):408-16; PMID:16151043; http://dx.doi.org/ 10.1177/1073858405278865 [DOI] [PubMed] [Google Scholar]
- 81.Zhang RL, Zhang ZG, Zhang L, Chopp M. Proliferation and differentiation of progenitor cells in the cortex and the subventricular zone in the adult rat after focal cerebral ischemia. Neuroscience 2001; 105(1):33-41; PMID:11483298; http://dx.doi.org/ 10.1016/S0306-4522(01)00117-8 [DOI] [PubMed] [Google Scholar]
- 82.Zhu DY, Liu SH, Sun HS, Lu YM. Expression of inducible nitric oxide synthase after focal cerebral ischemia stimulates neurogenesis in the adult rodent dentate gyrus. J Neurosci 2003; 23(1):223-9; PMID:12514219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zuker M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res 2003; 31(13):3406-15; PMID:12824337; http://dx.doi.org/ 10.1093/nar/gkg595 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.