Yeast Aep3p, previously reported to stabilize mitochondrial ATP8 mRNA, also activates its translation. Temperature-sensitive aep3 mutants are specifically defective in translating ATP8 at the restrictive temperature. The respiratory deficiency of aep3 mutants is rescued by expression in the cytoplasm of allotopic ATP8.
Abstract
Translation of mitochondrial gene products in Saccharomyces cerevisiae depends on mRNA-specific activators that bind to the 5’ untranslated regions and promote translation on mitochondrial ribosomes. Here we find that Aep3p, previously shown to stabilize the bicistronic ATP8-ATP6 mRNA and facilitate initiation of translation from unformylated methionine, also activates specifically translation of ATP8. This is supported by several lines of evidence. Temperature-sensitive aep3 mutants are selectively blocked in incorporating [35S]methionine into Atp8p at nonpermissive but not at the permissive temperature. This phenotype is not a consequence of defective transcription or processing of the pre-mRNA. Neither is it explained by turnover of Aep3p, as evidenced by the failure of aep3 mutants to express a recoded ARG8m when this normally nuclear gene is substituted for ATP8 in mitochondrial DNA. Finally, translational of ATP8 mRNA in aep3 mutants is partially rescued by recoded allotopic ATP8 (nATP8) in a high-expression plasmid or in a CEN plasmid in the presence of recessive mutations in genes involved in stability and polyadenylation of RNA.
INTRODUCTION
Biogenesis of mitochondrial ATP synthase (F1-Fo complex) in Saccharomyces cerevisiae is assisted by a dozen nuclear gene products that intercede at all stages of the assembly pathway (Ackerman and Tzagoloff, 2005). With a few exceptions (Lefebvre-Legendre et al., 2001; Ackerman, 2002), these factors target the mitochondrial ATP6, ATP8, and ATP9 mRNAs and their products, which function in the proton-translocating activity of the ATP synthase. ATP8 and ATP6 are cotranscribed together with COX1. In some strains, the polycistronic transcript includes ENS2, which codes for a mitochondrial endonuclease (Simon and Faye, 1984).
Several nuclear genes were previously shown to affect expression of Atp6p and Atp8p by either stabilizing or activating translation of the bicistronic ATP8-ATP6 mRNAs (Camougrand et al., 1995; Pelissier et al., 1995; Ellis et al., 2004; Zeng et al., 2007). Translational activation by mRNA-specific factors is by no means unique to the ATP8-ATP6 mRNA but is a general feature of the mitochondrial genetic system in S. cerevisiae (Costanzo and Fox, 1990; Fox, 2012; Herrmann et al., 2013). Translational activators may have more than one function. Mss51p, a translational activator of the COX1 mRNA, has been shown to bind to the Cox1p product as part of a mechanism for regulating expression of this subunit of cytochrome oxidase (Perez-Martinez et al., 2003; Barrientos et al., 2004; Fontanesi et al., 2011). Translational activators have been identified for every mitochondrial gene product except Atp8p and Var1p (Fox, 2012). Even though ATP8 and ATP6 are present in the same transcript(s), their translation is probably regulated by different factors. This is supported by the finding that mutations in the ATP6 mRNA activator Atp22p prevent translation of Atp6p but not Atp8p (Zeng et al., 2007).
In the present study, we obtained temperature-sensitive (ts) aep3 mutants that are specifically defective in expression of the mitochondrial ATP8 gene at the nonpermissive temperature. Our evidence indicates that this phenotype stems from the failure of such mutants to translate the ATP8-ATP6 mRNA. In an attempt to search for additional factors that affect ATP8, we took advantage of the ability of allotopically expressed ATP8 (nATP8) to complement and restore respiratory growth of atp8 mutants (Gearing et al., 1985; Gearing and Nagley, 1986; Barros et al., 2011). (Note: nATP8 is ATP8 recoded for expression on cytoplasmic ribosomes. nATP8GPD is nATP8 downstream of the GPD promoter. nATP8GPD-CYC1 is nATP8 downstream of the GPD promoter and followed by the CYC1 terminator. nATP8ADH1 is nATP8 downstream of the ADH1 promoter followed by the ADH1 terminator.)
Using this screen, we obtained aep3 mutants that were rescued by a combination of nATP8 and mutations in pta1 or nrd1. The results of the two approaches provide strong evidence that Aep3p is required specifically for translation of ATP8. Of interest, Aep3p was first shown to stabilize the ATP8-ATP6 bicistronic messenger (Ellis et al., 2004). In a subsequent study, Aep3p was reported to be an essential cofactor for mIF2-dependent initiation of translation of mitochondrial gene products with unformylated initiator methionyl-tRNA (Lee et al., 2009). The present study further enlarges the functional repertoire of this interesting protein and highlights the role of Aep3p as a new translational regulator of ATP8-ATP6 mRNA.
RESULTS
Aep3p is required for translation of ATP8 mRNA
ATP synthase mutants, including aep3 mutants, are prone to undergo partial or complete deletions in mitochondrial DNA (Ellis et al., 2004; Zeng et al., 2007). As a result, they display a pleiotropic phenotype, with large reductions in cytochrome oxidase (COX) and the bc1 complex. This property makes it difficult to gather a detailed understanding of the underlying biochemical defect. This experimental complication has been overcome by obtaining ts alleles of the gene in question (Zeng et al., 2007). The ts mutants tend to have a more stable mitochondrial genome, thereby permitting biochemical analysis unencumbered by secondary effects stemming from loss of mtDNA.
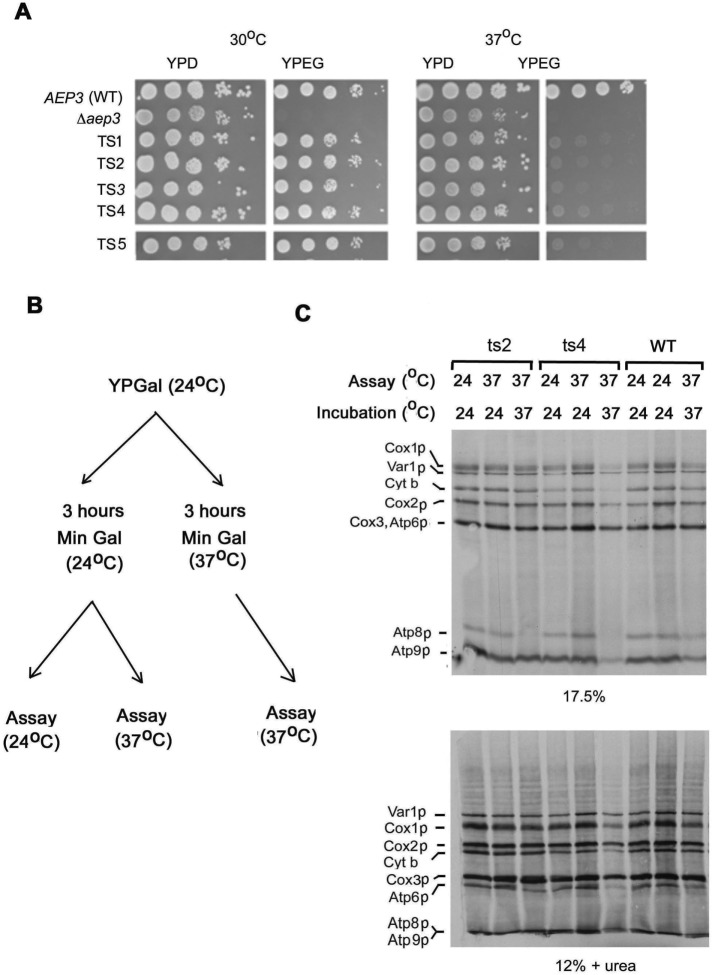
A plasmid library with mutations in aep3 was obtained by PCR mutagenesis of AEP3 under low-stringency conditions. This library was used to transform a heterozygous aep3-null mutant. Diploid transformants were sporulated and haploid progeny tested for ts growth on nonfermentable carbon sources. This protocol was used to collect seven ts mutants capable of growing on rich glycerol plus ethanol medium at 30 but not 37°C (Figure 1A).
FIGURE 1:
Growth and mitochondrial translation activity of ts aep3 mutants. (A) The wild-type strain W303-1A (AEP3), the aep3-null mutant (Δaep3), and the null mutant transformed with plasmid bearing five independent ts aep3 mutant alleles were serially diluted and spotted on rich glucose (YPD) and two rich ethanol/glycerol (YEPG) plates, which were incubated at 30 or 37°C. (B) Three conditions used to measure the effect of temperature on mitochondrial translation in ts mutants. (C) In vivo labeling of mitochondrial gene products by the W303-1A (WT) and two aep3-null mutants transformed with two different ts aep3 alleles (ts2 and ts4). Cells were grown to early stationary phase in YPGal at 24°C, followed by 3 h of incubation at 24°C, and were then assayed at 24°C (left lane) or 37°C (middle lane). In the third assay, cells grown at 24°C were incubated for 3 h at 37°C and assayed at 37°C (right lane). Equivalent number of cells were labeled with [35S]methionine as described in Materials and Methods, and total mitochondrial proteins were separated on a 17.5% polyacrylamide gel and separately on a 12% polyacrylamide gel containing 6 M urea (to resolve Cox3p and Atp6p). Proteins were transferred to nitrocellulose and exposed to x-ray film. The mitochondrial translation products are identified on the left side of each gel.
Mitochondrial translation in the parental strain and two different ts mutants (ts2 and ts4) was assayed under different conditions (Figure 1B). Cells were grown to log phase at 24°C, transferred to minimal galactose medium, and further incubated for 3 h in minimal galactose medium at 24°C. They were then assayed at 24 or 37°C. Under both conditions, [35S]methionine incorporation into all of the mitochondrial translation products, including Atp8p, was approximately the same in the ts mutants as in wild-type cells (Figure 1C). Translation of Atp8p but not Atp6p or Atp9p, however, was markedly decreased in mutant cells grown in minimal galactose at 37°C and assayed at 37°C. The selective reduced synthesis of Atp8p in cell preincubated and assayed at the restrictive temperature could be a consequence of defective transcription/processing of the mRNA precursor, stability of the mRNA or of the translation product, or a requirement of Aep3p specifically for translation of ATP8 mRNA.
ATP8 is part of a polycistronic transcript that includes COX1 and ATP6. This primary transcript is processed by endonucleolytic cleavages that produce COX1 mRNA and 5.2- and 4.6-kb bicistronic mRNAs with ATP8 plus ATP6 (Camougrand et al., 1995; Pelissier et al., 1995; Figure 2A). It is unlikely that aep3 mutants are defective in transcription or processing of the mRNA, as they have normal amounts of COX1 mRNA and Cox1p (Ellis et al., 2004). Cox1p and Atp6p levels in the ts mutants at the nonpermissive temperature are comparable to the wild type (Figure 1C). These results suggest that the reduced 35% ATP8-ATP6 mRNAs detected in the aep3-null mutant (Ellis et al., 2004) probably stems from RNA turnover.
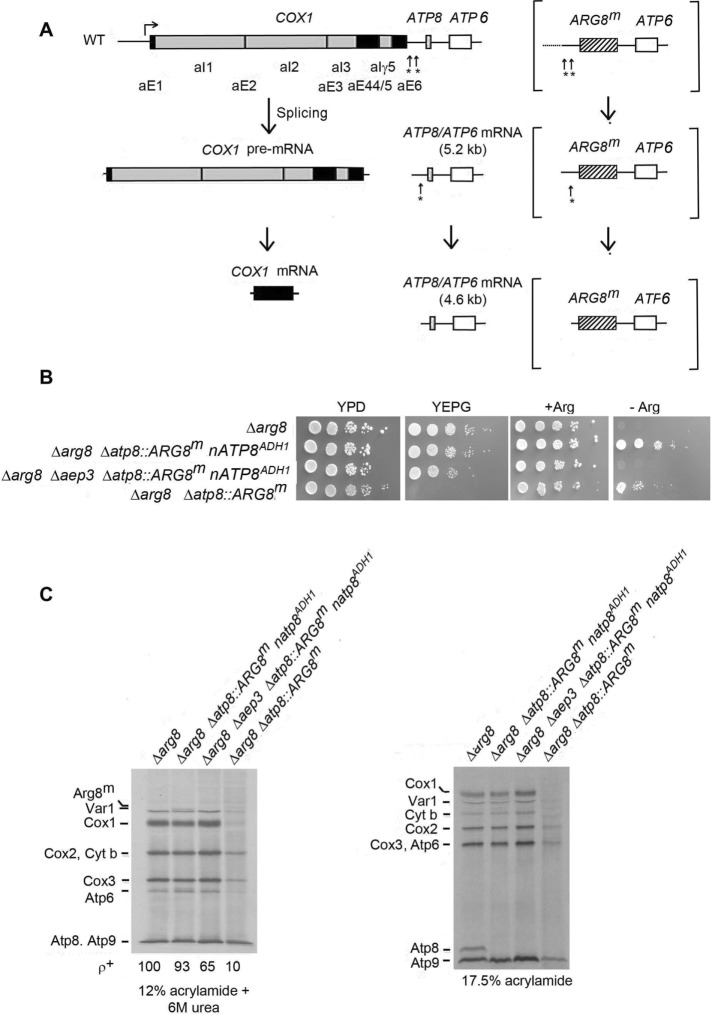
FIGURE 2:
Expression of ARG8m from the ATP8 locus. (A) Processing of the COX1-ATP8-ATP6 and COX1-ARG8-ATP6 pre-mRNAs (brackets). The introns of COX1 are in gray and the exons in black. Arrows with asterisks indicate the cleavage sites during maturation of the primary transcript. (B) Inhibition by an aep3-null mutation on growth of an atp8::ARG8m strain in the absence of arginine. The relocated nATP8ADH1 was in a plasmid with the 5′ and 3′ sequences of ADH1. Strains with the indicated genotypes were serially diluted and spotted on rich glucose (YPD), rich ethanol plus glycerol (YPEG), and minimal glucose with arginine (+ Arg) and without arginine (–Arg). The photograph was taken after 2 d of incubation at 30°C. (C) Mutants used in B were labeled in vivo in the presence of cycloheximide with [35S]methionine for 10 min. Total cellular protein were separated on 12% plus urea and 17.5% polyacrylamide gels and transferred to nitrocellulose, and radiolabeled proteins were detected as in Figure 1C.
Aep3p is required for expression of ARG8m at the ATP8 locus of mtDNA
Synthesis by aep3 ts mutants of Cox1p and Atp6p but not Atp8p does not exclude the possibility that Aep3p protects newly synthesized Atp8p against proteolysis. To address this question, we tested whether Aep3p is required for expression of ARG8m when this gene is substituted for ATP8 in mtDNA. ARG8m differs from nuclear ARG8 in its sequence to accommodate for differences in the genetic code of yeast mitochondria (Steele et al., 1996). The rationale of this test is that the stability of a nonmitochondrial protein like the ARG8m product should not be affected by Aep3p. Failure of mitochondrial ARG8m to be expressed in an aep3 mutant would constitute compelling evidence that Aep3p is required for translation rather than stability of Atp8p.
In agreement with earlier studies (Rak and Tzagoloff, 2009; Barros et al., 2011), substitution of mitochondrial ATP8 with ARG8m restored growth of an arg8 mutant in medium lacking arginine (Figure 2B). This confirms the expression of a functional ARG8m when it is flanked by the 5′ and 3′ flanking sequences of ATP8. As expected, absence of ATP8 in the arginine-independent clones precluded growth on respiratory carbon sources, which was rescued by nATP8ADH1, a nuclear version of ATP8 (Gearing and Nagley, 1986; Barros et al., 2011) flanked by the ADH1 promoter and terminator in pMGL3 (Barros et al., 2011). The combination of allotopic ARG8m and nATP8ADH1 conferred both arginine prototrophy and respiratory competence on the arg8 null mutant (Figure 2B). The arginine prototrophy, however, was lost in the double arg8 and aep3–null mutant, which remained respiratory competent because of Atp8p expressed from allotropic nATP8. This indicates that translation of ARG8m and, by inference, that of ATP8 depend on Aep3p. Radiolabeled Atp8p is not seen in the respiratory-competent strain with nATP8 (Figure 2C, third lane from left), as this gene product is translated on cytoplasmic ribosomes.
Screen for mutants defective in expression of ATP8
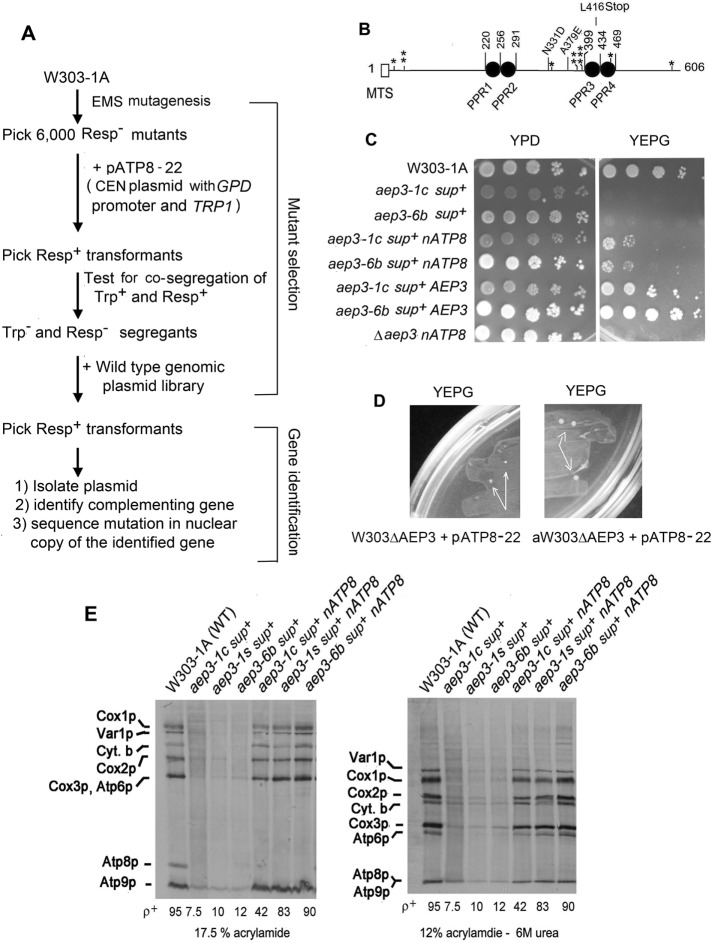
The finding that Aep3p functions in translation of ATP8 raises the question of whether there may exist additional factors necessary for ATP8 expression. The genetic screen used in this study to isolate mutants in nuclear gene products required specifically for expression of mitochondrial ATP8 was based on the ability of nATP8 to complement atp8 mutants (Gearing and Nagley, 1986; Barros et al., 2011). A library consisting of 6000 respiratory-defective mutants obtained by ethyl-methanesulfonate (EMS) mutagenesis was transformed with pATP8-22, a tryptophan-selectable CEN plasmid with the GDP promoter carrying recoded nATP8. In this screen, restoration of respiration by nATP8 is expected to identify mutations in genes that target mitochondrial ATP8. As proof of principle, the mutant library was transformed with a recoded COX2 (nCOX2) that has a W56R mutation in the first transmembrane domain of Cox2p (Supekova et al., 2010). This allotopic nCOX2 partially complemented a cox2 mutant (Supekova et al., 2010), as well as a pet111 mutant defective in translation of mitochondrial COX2 mRNA (Supplemental Figure S1A). The screen of mutants transformed with nCOX2 yielded 16 clones partially complemented for growth on rich ethanol/glycerol (yeast extract/peptone/glycerol [YEPG]). Further tests indicated that they all had mutations in PET111 (Supplemental Figure S1B).
A similar screen was used to transform the mutant library with nATP8 in plasmid pATP8-22 (Figure 3A). The screen yielded two respiratory-competent strains that were ascertained to have mutations in AEP3 (alleles aep3-1c and aep3-6b). The aep3-6b mutant had a single A379E missense mutation, and aep3-1c had the same mutation plus an N331D substitution and a premature L416Ochre termination codon in the third pentatricopeptide repeat of Aep3p (Figure 3B). The mutants with the different alleles grew at similar rates on YEPG medium in the presence of nATP8 (Figure 3C).
FIGURE 3:
Isolation of mutants with nATP8-dependent respiration. (A) Genetic screen used to isolate and identify aep3 mutants with nATP8-dependent respiration. (B) Location of the aep3-1c, aep3-6b, ts1 (single asterisk), ts2 (double asterisks), and ts3 alleles (triple asterisk) relative to the PPR motifs in Aep3p. Open bar, putative mitochondrial targeting sequence (MTS). (C) Growth property of respiratory-competent strains with nATP8 (pATP8-22) obtained by the genetic screen depicted in A and of the corresponding respiratory-deficient segregants lacking nATP8. The respiratory deficient segregants were also transformed with a plasmid containing AEP3 (bottom two rows). Mutants with the indicated phenotypes were serially diluted and spotted on rich glucose medium (YPD) and rich ethanol/glycerol medium (YPEG).(D) Respiratory-deficient aep3-null mutants with nATP8 (aW303ΔAEP3/nATP8 and W303ΔAEP3/nATP8) were plated on YEPD. The photograph was taken after 4 d of incubation at 30°C. The arrows show respiratory-competent revertant colonies. (E) The parental wild-type strain W303-1A, the two respiratory-competent aep3 point mutants, and segregants described in B were labeled in vivo and the translation products visualized as in Figure 1C. The mutant aep3-1s has the premature termination codon but not the other point mutation of aep3-1c. A plasmid (pAEP3-1S) containing this allele was integrated into the nuclear DNA of the null mutant aW303ΔAEP3/S+/ST22, which has both a suppressor and nATP8.
Of interest, nATP8 by itself did not rescue respiration in mutants with the aep3-1c-, aep3-6b-, or aep3-null allele. Transformation of respiratory-deficient aep3-1c or aep3-6b segregants with pATP8-22 failed to confer growth on YEPG medium (unpublished data). Similarly, transformation of the aep3 null mutant with this plasmid also failed to restore growth on YEPG medium. After 4–5 d, however, respiratory-competent revertant colonies began to appear on this medium (Figure 3D). Plasmid segregation tests on the revertants confirmed the requirement of nATP8 for respiratory competency (unpublished data). This was also true of the original respiratory-competent mutants with the aep-1c and aep3-6b alleles (Figure 3B). The nATP8 requirement correlated with the mitochondrial translational activity. Respiratory-deficient mutants with the aep3-1c, aep3-6b, and aep3-1s (a mutant construct with just the L416Ochre mutation) alleles had unstable mtDNA and very low mitochondrial translation activity compared with the respiratory-competent revertant clones (Figure 3E). No radiolabeled Atp8p was detected in any of the respiratory-deficient or competent -clones, indicating that the restoration of respiratory activity was unrelated to expression of mitochondrial ATP8. Together these results indicate that respiration in the different aep3 mutants depends on nATP8 and an extragenic suppressor.
The suppressors conferring respiratory growth on aep3 mutants in the presence of nATP8 were determined to be in nuclear DNA. A respiratory-competent aep3 revertant containing nATP8 (aW303ΔAEP3/nATP8) was depleted of mtDNA (ρ0). Respiration in this ρ0 derivative was restored by reintroduction of wild-type mtDNA from W303 (Supplemental Figure S2A). In addition, the sequences of mtDNA from the respiratory-deficient mutant W303ΔAEP3/nATP8 and the isogenic revertant W303ΔAEP3/nATP8/S+ were identical.
The nuclear suppressors were also determined to be recessive. Diploid cells (Δaep3/Δaep3 sup+/sup– nATP8) issued from a cross of the respiratory-competent revertant aW303ΔAEP3/S+/ST22 (Δaep3 sup+ nATP8) to the respiratory-deficient parental strain W303ΔAEP3 (Δaep3 sup-) were respiratory deficient (Supplemental Figure S2B). Growth on respiratory carbon sources, however, was restored by a recombinant plasmid containing AEP3 (Supplemental Figure S2B). Similarly, cross of the respiratory-competent point mutant aep3-1c (s+) or aep3-1s (s+) to W303ΔAEP3 (Δaep3 sup-) produced respiratory-deficient diploid cells. The respiratory deficiency of the different heterozygous sup+/sup- genotype is indicative of a recessive suppressor.
Suppression of aep3 mutants containing nATP8 is not affected by an fmt1 mutation
FMT1 codes for the mitochondrial methionyl-tRNA transformylase. Respiration and mitochondrial protein synthesis are not affected in fmt1 mutants, as they are able to initiate translation with unformylated initiator methionyl-tRNA (Li et al., 2000). A double fmt1 and aep3 mutant with a Y305N substitution in Aep3p, however, has been shown to lack general mitochondrial translational activity, indicating a requirement of Aep3p for translation in the absence of formylated methionyl-tRNA fMet (Lee et al., 2009). To ascertain whether respiratory competence and mitochondrial translation of the aep3-null mutant with the suppressor and nATP8 depend on Fmt1p, we crossed the respiratory-competent aep3-null mutant expressing nATP8 to an fmt1-null strain. Diploid cells issued from the cross were sporulated, and meiotic progeny with the double aep3, fmt1–null alleles and nATP8 indicated that the rescue of respiratory growth (Supplemental Figure S3A) and of mitochondrial translation (Supplemental Figure S3B) by the suppressor does not require methionyl transformylase. The extragenic suppressor in combination with nATP8, therefore, rescues translation of mitochondrial gene products in aep3 mutants even when formylation of the initiator methionyl tRNA is blocked.
Suppression by SMT1
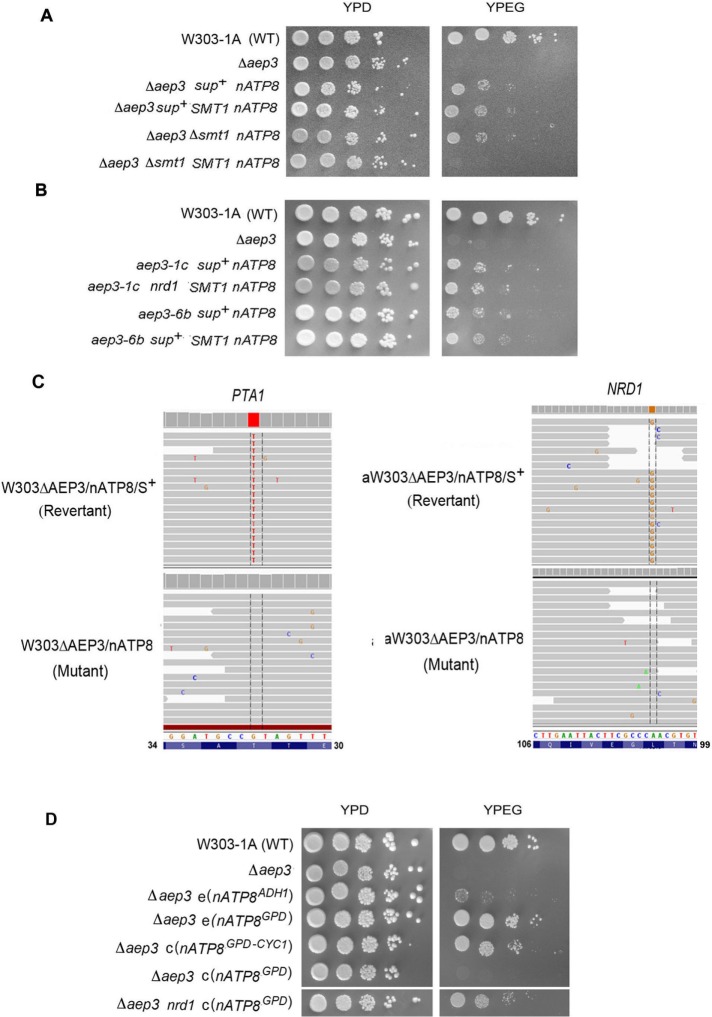
The product of the nuclear SMT1 gene was recently proposed to be a translational repressor of the mitochondrial ATP8-ATP6 mRNA (Rak et al., 2016). The translational defect of ATP6 and ATP8 in F1 mutants is suppressed by recessive mutations in SMT1 (Rak et al., 2016). Deletion of SMT1 in the presence of nATP8 partially restored respiration in the aep3-null mutant (Figure 4A). The rescue also improved mtDNA maintenance. In contrast to the smt1 deletion, overexpression of ATP22, an ATP6-specific translational activator, did not rescue the respiratory growth of the aep3-null or point mutants (unpublished data). Suppression of growth of aep3 mutants by the smt1-null mutation is recessive, as is the restoration of ATP8-ATP6 mRNA translation (Figure 4A).
FIGURE 4:
Suppressors of aep3 mutants. (A) The respiratory-competent wild-type W303-1A and aep3-null mutants with the indicated genotypes were serially diluted and spotted on rich glucose (YPD) and rich ethanol/glycerol (YPEG) media and photographed after 3 d of incubation at 30°C. Wild-type SMT1 in an integrative plasmid. (B) Growth of revertants with and without an extra copy of SMT1: aep3-1c nrd1 nATP8 and aep3-6b nrd1 nATP8 are revertants of two aep3 point mutants; aep3-1c nrd1 nATP8 and aep3-6b nrd1 nATP8 are the same revertants with an extra copy of SMT1. The strains were serially diluted and spotted on rich glucose and rich ethanol plus glycerol media as in A. (C) Whole-genome analysis of mutations in the two independent revertants W303ΔAEP3/S+/22 and aW303ΔAEP3/S+/22. Alignments, obtained with the Integrative Genomics Viewer software developed by the Broad Institute (MIT and Harvard), of reads of the revertants (top) with a consistent positional base change differing from that of the parental mutant genome (bottom). Left, alignments of reads from W303ΔAEP3/S+/22 with the G-to-T mutation in the reverse strand of PTA1. The T was present in 270 of 271 reads of the revertant. The single exception had the G of the mutant. At the same nucleotide position the mutant had a G in 260 out of 261 reads. The single exception had a C at this position. Right, results obtained with the genome of aW303ΔAEP3/S+/22 and the parental mutant. In this strain, a consistent A-to-G change was found in NRD1 (reverse strand shown). The revertant had the G in 92 and a C in two reads. (D) Growth of the following strains was measured as in A: W303-1A, the parental wild-type strain; Δaep3, the aep3-null mutant; Δaep3 e(nATP8ADH1), the aep3 mutant transformed with an episomal plasmid containing nATP8 flanked by the ADH1 promoter and terminator; Δaep3 e(nATP8GPD), the aep3 mutant transformed with an episomal plasmid containing nATP8 downstream of the GPD promoter; Δaep3 c(nATP8GPD-CYC1), the aep3 mutant transformed with an centromeric plasmid containing nATP8 flanked by the GPD promoter and CYC1 terminator; Δaep3 c(nATP8GPD), the aep3 mutant transformed with a centromeric plasmid containing nATP8 downstream of the GPD promoter; and Δaep3 nrd1 nATP8, the aep3 mutant with the nrd1 suppressor mutation transformed with a centromeric plasmid containing nATP8 downstream of the GPD promoter.
The rescue by the smt1-null mutation of respiration in the aep3 point mutants, however, was countered by an extra copy of SMT1, indicating that the smt1 was recessive in this context as well and therefore distinct from the suppressor(s) in the aep3-null mutants (Figure 4A).
Suppressors of aep3 mutations
The suppressor mutations in the aep3-null and point mutants transformed with nATP8 were identified by comparing whole-genome sequences of respiratory-competent revertants and the parental respiratory-deficient aep3 mutants containing nATP8. A total of 22,944,148 reads were obtained for the respiratory-deficient mutant W303ΔAEP3/nATP8 and 27,045,134 for the corresponding respiratory-competent revertant W303ΔAEP3/nATP8/S+. The reads of the mutant aligned 83% and of the revertant 96% with the S. cerevisiae reference genome. Only two consistent differences were found in the nuclear genome of the revertant. One was a C-to-A mutation in PTA1, resulting in a nonconservative T32K change in a subunit of the holo-CPF complex and a conservative L11V change in the product of BMH2, respectively. Similarly, 48,641,033 reads were obtained for the independent revertant aW303ΔAEP3/nATP8/S+ and 14,641,033 for the corresponding respiratory-deficient parental strain aW303ΔAEP3/nATP8. The reads of the mutant aligned 93% and of the revertant 95% with the S. cerevisiae reference genome. The following mutations were detected in this strain: a G-to-A and a C-to-A mutation in LEU3, resulting in the amino acid changes G490E and S492R, and an A-to-G mutation in NRD1, resulting in a L101R substitution. Sanger sequencing confirmed the PTA1 and NRD1 mutations. These two genes were also sequenced in the mutants with aep3-1c and aep3-6b alleles. The pta1 mutation was not found in either strain. The aep3-1c mutant, however, had A-to-G and G-to-A mutations in NRD1, resulting in Q206R and P526Q changes in the protein product, respectively. The aep3-6b mutant had only the G-to-A mutation in NRD1, leading to the P526Q substitution in the protein.
The genome sequencing data pointed to mutations in PTA1 and NRD1 as the best candidates for suppressing the respiration defect of aep3 mutants with nATP8. Common to both genes is their function in RNA metabolism and more specifically the reported role of PTA1 in cleavage and polyadenylation of nuclear RNAs (Zhao et al., 1999). This suggested that the lack of complementation of aep3 mutants by nATP8 might be related to turnover of the RNA as a result of inefficient posttranscriptional modification of mRNA lacking proper cleavage and polyadenylation signals. This is supported by the observation that nATP8 restores respiration of an aep3-null mutant when cloned in a centromeric plasmid with the strong GPD promoter (Mumberg et al., 1995) and CYC1 terminator (Figure 4D). The ability of nATP8 to rescue aep3 mutants when expressed from a high-copy plasmid with the GPD promoter indicates that the requirement of a terminator can be circumvented by overexpression of the nATP8 (Figure 4D). The inefficiency of allotopic nATP8 to bypass the Aep3p requirement is also evident in the very poor rescue by a plasmid (pATP8/ST4) that contains the weaker ADH1 promoter even though it also has the ADH1 terminator (Figure 4D).
DISCUSSION
Aep3p is a 70-kDa mitochondrial protein with four pentatricopeptide (PPR) motifs, each consisting of a degenerate 35–amino acid sequence repeated in tandem in two antiparallel α-helices leading to a helix-turn-helix domain. In series, the repeats form an RNA-binding groove (Manna, 2015). PPR proteins have been described in chloroplasts and in plant, human, and fungal mitochondria (Shikanai, 2006; Lipinski et al., 2011; Solotoff et al., 2015). Pet309p, Atp22p, and Msc6p are examples of yeast mitochondrial PPR proteins involved in the stability and/or translation of mitochondrially encoded RNAs (Krause et al., 2004; Zeng et al., 2007; Moda et al., 2016). Like other PPR proteins, Aep3p was recently shown to cosediment with mitochondrial ribosomes in a large complex termed Miorex (Kehrein et al., 2015).
Consistent with the presence in Aep3p of the PPR RNA-binding motif is its previously described function in stabilizing the ATP8-ATP6 mRNA (Ellis et al., 2004) and the requirement for mIF2-dependent initiation of translation of mitochondrial gene products with unformylated initiator methionyl-tRNA (Lee et al., 2009). Our results indicate that in addition to these activities, Aep3p is also required specifically for translation of ATP8. This is supported by three lines of evidence. At the restrictive temperature, aep3 ts mutants are impaired in expression of ATP8 but not ATP6 or other mitochondrial gene products. Second, the Cox2p deficit is not a consequence of either defective transcription or processing of the COX1-ATP8-ATP6 primary transcript. It is also unlikely that Aep3p is required for stability of Atp8p. This is evidenced by the finding that complementation of an arg8 mutant by the atp8::ARG8m allele requires the presence of wild-type AEP3. These results constitute strong evidence that Aep3p function is required for translation rather than stability of Arg8p and, by analogy, of Atp8p. Finally, although it does not exclude other possible mechanisms, rescue of aep3 mutants by allotopic nATP8 is also consistent with a role of Aep3p in translation of mitochondrial ATP8.
When expressed from a plasmid lacking a proper termination sequence, rescue of aep3 point and null mutants by nATP8 depends on recessive mutations in nuclear DNA. This is true even when transcription of nATP8 is under the control of a strong promoter such as GDP (pATP8-22). NRD1 and PTA1 have been identified as nuclear suppressors that enable allotopic nATP8 on a CEN plasmid lacking a transcriptional terminator to rescue aep3 mutants. NRD1 and PTA1 are involved in mRNA termination. Pta1p is an essential 90-kDa protein required for both cleavage and poly(A) addition of mRNA precursor (Zhao et al., 1999). Nrd1p acts both on transcription termination and an RNA processing factor that has been shown to recruit RNA to the nuclear exosome, which uses its 3′ to 5′ exonuclease activity to degrade aberrant transcripts but also mature the 3′ of precursor RNAs (Heo et al., 2013). The overlapping activities of these suppressors suggest that the mechanism of suppression involves an enhancement of 3′-end maturation of the nATP8 RNA, thereby sparing the RNA from degradation. This explanation gains further support from the finding that a plasmid construct with nATP8 flanked by the GPD promoter and the CYC1 terminator rescues the aep3 mutants in the absence of additional suppressor mutations. The recessive nature of the pta1 and nrd1 suppressor mutations indicates that they probably spare the nATP8 transcript from degradation. Normal growth of the suppressor strains on rich glucose indicates that the pta1 and nrd1 mutations do not seriously compromise processing or degradation of other cellular RNAs. We have no information on whether the pta1 and nrd1 mutations would improve complementation by other relocated mitochondrial genes lacking 3′ processing and polyadenylation sequences. Conceivably, previous attempts to relocate mitochondrial genes to the nucleus (Claros et al., 1995) were unsuccessful for reasons related to incorrect 3′ processing of the primary transcripts.
Complementation of the aep3 mutants by nATP8 is also achieved by deletion of SMT1. This gene has been proposed to repress translation of the ATP8-ATP6 mRNA (Rak et al., 2016) and regulate expression of these genes by adjusting the rate of their synthesis to the availability of the F1 component of ATP synthase with which they interact during assembly of the ATP synthase complex (Rak and Tzagoloff, 2009; Rak et al., 2016). The block in translation of the ATP8-ATP6 mRNA is suppressed by mutations in SMT1. The reduction of ATP8-ATP6 mRNA in aep3 mutants (Ellis et al., 2004) would lead to a concomitant decrease of Atp6p synthesis. Mutations in smt1 may compensate for the Atp6p insufficiency by increasing its synthesis in aep3 mutants. Alternatively, the absence of Smt1p in aep3 mutants may influence some other aspect of Atp8p biogenesis from the allotopic nATP8 gene. Either of these mechanisms could explain the partial rescue by SMT1 deletion of respiration in aep3 mutants.
Mitochondrial respiration has been shown to be defective in the aep3 Y305 mutant when formylation of methionine is blocked by an fmt1-null mutation (Lee et al., 2009). Our results indicate that the respiratory deficiency and mitochondrial translation of the aep3 fmt1 double-null mutant is rescued by nATP8 in the presence of the nuclear suppressor (s+). The rescue of mitochondrial translation in a mutant lacking both Fmt1p and Aep3p (Supplemental Table S1) differs from the phenotype of the aep3 (Y305N) fmt1 double mutant, which is not only respiratory deficient, but also exhibits severely depressed mitochondrial translation (Lee et al., 2009; Supplemental Table S1). The discrepancy in the two phenotypes may be explained by the different genetic backgrounds of the strains used in the two studies, although we cannot exclude that it may also be related to the Y305N mutation itself.
In summary, our results show that in addition to its previously described activities, Aep3p is also required for translation of ATP8. The finding that mutations in pta1 and nrd1 are able to activate complementation of aep3 mutants by nATP8 suggests that they increase cellular concentration of the protein by stabilizing the mRNA through 3′-end processing and modification.
MATERIALS AND METHODS
Yeast strains and growth media
The genotypes and sources of the strains of yeast used in this study are listed in Supplemental Table S2. The compositions of YPD, YEPG, and minimal glucose medium (supplemented with the appropriate auxotrophic requirements) have been described elsewhere (Ellis et al., 2004).
Construction of aep3 ts mutants
Temperature-sensitive aep3 alleles were obtained by PCR amplification of the gene under conditions favoring misincorporation of deoxynucleotides. Primers 5′-ggaattgtgagcggataac and 5′-gggtaacgccagggttttccc used for the synthesis started from the cloning site in YEp351-AEP3 (Ellis et al., 2004). The gene was amplified in four separate reactions containing 0.25 mM MnCl2, 1.5 mM MgCl2, and 0.2 mM dITP and with the concentration of one of the four deoxynucleotides reduced from 0.2 to 0.04 mM. The four 2004–base pair products were pooled, digested with HindIII, and cloned in the centromeric plasmid YCplac22 containing TRP1 (Gietz and Sugino, 1988). The plasmid library obtained from the ligation was used to transform the heterozygous diploid strain a/αW303ΔAEP3 (Ellis et al., 2004). The pooled tryptophan prototrophic transformants were sporulated, and histidine- and tryptophan-independent meiotic progeny were checked for growth at 30 but not 37°C on YEPG. The screen yielded mutants with a clear ts phenotype. The aep3 allele in pAEP3/TS1 had five nucleotide changes that lead to amino acid changes–K20R, K154R, V336E, I460V, and I559V. The five other acids changes detected in pAEP3/TS2 were V40A, N172S, L216P, K213R, and H389R. Plasmids pAEP3/TS3, TS4, TS5, TS6, and TS7 had the single–amino acid changes F392S C347R, D310V, A480V, and E281G, respectively (Figure 3B).
Mutagenesis and plasmid isolation
The haploid strains W303-1A and W303-1B were mutagenized with EMS (Lindegren et al., 1965), and respiratory-deficient mutants were selected based on growth on rich glucose (YPD) but not on rich ethanol/glycerol (YPEG) medium. Approximately 6000 respiratory-deficient mutants were pooled and transformed with pATP8-22, a centromeric TRP1-based plasmid containing nATP8 (recoded for allotopic expression) under the control of the glyceraldehyde 3-phosphate dehydrogenase promoter (GDP; Zampol et al., 2010). This plasmid is expected to complement mutants that cannot express mitochondrial ATP8. To identify the gene responsible for the respiratory deficiency, the nATP8-dependent, respiratory-competent clones were spread for single colonies on YPD to isolate respiratory-deficient segregants auxotrophic for tryptophan. Such segregants are expected to have lost the plasmid with nATP8. To identify the mutant gene responsible for the respiratory deficiency, we transformed the respiratory-deficient segregants with a yeast genomic library consisting of partial Sau3A fragments of wild-type yeast nuclear DNA ligated to the BamHI site of the episomal plasmid YEp24 (Botstein and Davis, 1982). This plasmid library was a gift of Marian Carlson (Department of Genetics and Development, Columbia University). Respiratory-competent transforms were expected to have a recombinant plasmid of the library with the complementing gene. The screen yielded two respiratory-deficient clones that were ascertained to have mutations in aep3. The mutant alleles were named aep3-1c and aep3-6b.
The aep3-1c and aep3-6b mutations were identified by PCR amplification of nuclear DNA from each mutant with primers 5′-ggggagctcctgtccaaatgccaggg and 5′-ggcctgcagggacgattccatcgaag. The 2213–base pair product was digested with SacI and PstI and cloned into YIp351 (Hill et al., 1986), yielding plasmids pAEP3-1C and pAEP3-6b. Sequencing of the plasmids revealed that aep3-1c had three nucleotide changes in aep3, resulting in amino acids changes N331D, A379E, and the nonsense mutation, L416Stop (Figure 1). The gene of aep3-6b had a single-nucleotide change, which resulted in the A379E substitution. An aep3 gene expressing the truncated product of aep3-1 without the additional mutations was reverse transcribed with the oligonucleotides 5′-ggcctgcagggacgattccatcgaag and 5′-ggcaagcttaaatcacttcttccc. The 1608–base pair product was digested with PstI plus HindIII and cloned into YIp351 to obtain pAEP3-1S.
Whole-genome sequencing
The Wizard Genomic Purification Kit (Promega) was used to extract total DNA from the parental respiratory-deficient mutant W303ΔAEP3/nATP8 and from the revertant W303ΔAEP3/nATP8/sup. The DNAs were quantified using the QUBIT DNA high-sensitivity assay, and 1 ng of the normalized DNA was tagged by the Nextera XT (Illumina) protocol. The libraries were amplified and cleaned up, and 1 μl of the undiluted libraries was analyzed on an Agilent Technology 2100 Bioanalyzer using a high-sensitivity DNA chip. The libraries were pooled and adjusted to 2 nmol in 15 μl. The pooled libraries were subjected for sequencing with the NextSeq (Illumina) equipment in the Genome Investigation and Analysis Laboratory of the Institute of Biomedical Sciences at the University of Sao Paulo. The BWA Aligner tool, version 1.1.4 (Base Space Labs Illumina), was used to align the 71,585,181 reads obtained from the four strains with the reference genomes of S. cerevisiae. The alignments were compared using the Integrative Genomics Viewer (Base Space Labs Illumina).
In vivo labeling of mitochondrial gene products in wild-type and aep3 mutants
Yeast grown to early stationary phase in YPGal were harvested, washed, and inoculated at a cell density of A600 = 2 into 2% galactose in nitrogen base without amino acids supplemented with auxotrophic requirements. After 2 h growth, or 3 h growth in the ts experiment, cells were harvested, washed twice, treated with cycloheximide, and labeled at room temperature for 30 min with [35S]methionine (1000 Ci/mmol; MP Biochemicals). Total cellular proteins were separated by SDS–PAGE on 17.5 and 12% polyacrylamide gels (Laemmli, 1970) without and with 6 M urea, respectively. Proteins were transferred to a nitrocellulose membrane and the radiolabeled mitochondrial gene products visualized by autoradiography.
Miscellaneous procedures
Standard methods were used for plasmid manipulations (Sambrook et al., 1989). The procedure of Schiestl and Gietz (1989) was used for yeast transformations. Protein concentrations were determined by the method of Lowry et al. (1951).
Supplementary Material
Acknowledgments
This research was supported by grants from the Fundação de Amparo a Pesquisa de São Paulo (2013/09482-8 and 2013/07937-8) and the Conselho Nacional Pesquisa (302935/2014-2) to M.B. and National Institutes of Health Grant HL02274 to A.T.
Abbreviations used:
- CEN plasmid
plasmid with a centromeric origin of replication
- CPF
cleavage and polyadenyation factor
- mtDNA
mitochondrial DNA
- ρ–
respiratory deficient yeast mutant with large deletion in mtDNA
- ρ0
respiratory deficient yeast mutant lacking mtDNA.
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E16-11-0775) on April 12, 2017.
REFERENCES
- Ackerman SH. Atp11p and Atp12p are chaperones for F(1)-ATPase biogenesis in mitochondria. Biochim Biophys Acta. 2002;1555:101–105. doi: 10.1016/s0005-2728(02)00262-1. [DOI] [PubMed] [Google Scholar]
- Ackerman SH, Tzagoloff A. Function, structure, and biogenesis of mitochondrial ATP synthase. Prog Nucleic Acid Res Mol Biol. 2005;80:95–133. doi: 10.1016/S0079-6603(05)80003-0. [DOI] [PubMed] [Google Scholar]
- Barrientos A, Zambrano A, Tzagoloff A. Mss51p and Cox14p jointly regulate mitochondrial translation of Cox1p in Saccharomyces cerevisiae. EMBO J. 2004;23:3274–3282. doi: 10.1038/sj.emboj.7600358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros MH, Rak M, Paulela JA, Tzagoloff A. Characterization of Gtf1p, the connector subunit of yeast mitochondrial tRNA-dependent amidotransferase. J Biol Chem. 2011;286:32937–32947. doi: 10.1074/jbc.M111.265371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botstein D, Davis RW. Principles and practice of recombinant DNA research with yeast. In: Strathern JN, Jones EW, Broach JR, editors. The Molecular Biology of the Yeast Saccharomyces Cerevisiae: Metabolism and Gene Expression. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1982. pp. 607–636. [Google Scholar]
- Camougrand N, Pelissier P, Velours G, Guerin M. NCA2, a second nuclear gene required for the control of mitochondrial synthesis of subunits 6 and 8 of ATP synthase in Saccharomyces cerevisiae. J Mol Biol. 1995;247:588–596. doi: 10.1006/jmbi.1995.0165. [DOI] [PubMed] [Google Scholar]
- Claros MG, Perea J, Shu Y, Samatey FA, Popot JL, Jacq C. Limitations to in vivo import of hydrophobic proteins into yeast mitochondria. The case of a cytoplasmically synthesized apocytochrome b. Eur J Biochem. 1995;228:762–771. [PubMed] [Google Scholar]
- Costanzo MC, Fox TD. Control of mitochondrial gene expression in Saccharomyces cerevisiae. Annu Rev Genet. 1990;24:91–113. doi: 10.1146/annurev.ge.24.120190.000515. [DOI] [PubMed] [Google Scholar]
- Ellis TP, Helfenbein KG, Tzagoloff A, Dieckmann CL. Aep3p stabilizes the mitochondrial bicistronic mRNA encoding subunits 6 and 8 of the H+-translocating ATP synthase of Saccharomyces cerevisiae. J Biol Chem. 2004;279:15728–15733. doi: 10.1074/jbc.M314162200. [DOI] [PubMed] [Google Scholar]
- Fontanesi F, Clemente P, Barrientos A. Cox25 teams up with Mss51, Ssc1, and Cox14 to regulate mitochondrial cytochrome c oxidase subunit 1 expression and assembly in Saccharomyces cerevisiae. J Biol Chem. 2011;286:555–566. doi: 10.1074/jbc.M110.188805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox TD. Mitochondrial protein synthesis, import and assembly. Genetics. 2012;192:1203–1234. doi: 10.1534/genetics.112.141267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gearing DP, McMullen GL, Nagley P. Chemical synthesis of a mitochondrial gene designed for expression in the yeast nucleus. Biochem Int. 1985;10:907–915. [PubMed] [Google Scholar]
- Gearing DP, Nagley P. Yeast mitochondrial ATPase subunit 8, normally a mitochondrial gene product, expressed in vitro and imported back into the organelle. EMBO J. 1986;5:3651–3655. doi: 10.1002/j.1460-2075.1986.tb04695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gietz RD, Sugino A. New yeast-Escherichia coli shuttle vectors constructed with in vitro mutagenized yeast genes lacking six-base pair restriction sites. Gene. 1988;74:527–534. doi: 10.1016/0378-1119(88)90185-0. [DOI] [PubMed] [Google Scholar]
- Heo DH, Yoo I, Kong J, Lidschreiber M, Mayer A, Choi BY, Hahn Y, Cramer P, Buratowski S, Kim M. The RNA polymerase II C-terminal domain-interacting domain of yeast Nrd1 contributes to the choice of termination pathway and couples to RNA processing by the nuclear exosome. J Biol Chem. 2013;288:36676–36690. doi: 10.1074/jbc.M113.508267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann JM, Woellhaf MW, Bonnefoy N. Control of protein synthesis in yeast mitochondria: the concept of translational activators. Biochim Biophys Acta. 2013;1833:286–294. doi: 10.1016/j.bbamcr.2012.03.007. [DOI] [PubMed] [Google Scholar]
- Hill JE, Myers AM, Koerner TJ, Tzagoloff A. Yeast/E.coli shuttle vectors with multiple unique restriction sites. Yeast. 1986;2:163–167. doi: 10.1002/yea.320020304. [DOI] [PubMed] [Google Scholar]
- Kehrein K, Möller-Hergt BV, Ott M. The MIOREX complex: lean management of mitochondrial gene expression. Oncotarget. 2015;6:16806–16807. doi: 10.18632/oncotarget.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause K, Lopes de Souza R, Roberts DG, Dieckmann CL. The mitochondrial message-specific mRNA protectors Cbp1 and Pet309 are associated in a high-molecular weight complex. Mol Biol Cell. 2004;15:2674–2683. doi: 10.1091/mbc.E04-02-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee C, Tibbetts AS, Kramer G, Appling DR. Yeast AEP3p is an accessory factor in initiation of mitochondrial translation. J Biol Chem. 2009;284:34116–34125. doi: 10.1074/jbc.M109.055350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre-Legendre L, Vaillier J, Benabdelhak H, Velours J, Slonimski PP, di Rago JP. Identification of a nuclear gene (FMC1) required for the assembly/stability of yeast mitochondrial F(1)-ATPase in heat stress conditions. J Biol Chem. 2001;276:6789–6796. doi: 10.1074/jbc.M009557200. [DOI] [PubMed] [Google Scholar]
- Li Y, Holmes WB, Appling DR, RajBhandary UL. Initiation of protein synthesis in Saccharomyces cerevisiae mitochondria without formylation of the initiator tRNA. J Bacteriol. 2000;182:2886–2892. doi: 10.1128/jb.182.10.2886-2892.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindegren G, Hwang YL, Oshima Y, Lindegren CC. Genetical mutants induced by ethyl methanesulfonate in Saccharomyces. Can J Genet Cytol. 1965;7:491–499. doi: 10.1139/g65-064. [DOI] [PubMed] [Google Scholar]
- Lipinski KA, Puchta O, Surendranath V, Kudla M, Golik P. Revisiting the yeast PPR proteins–application of an Iterative Hidden Markov Model algorithm reveals new members of the rapidly evolving family. Mol Biol Evol. 2011;28:2935–2948. doi: 10.1093/molbev/msr120. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Manna S. An overview of pentatricopeptide repeat proteins and their applications. Biochimie. 2015;113:93–99. doi: 10.1016/j.biochi.2015.04.004. [DOI] [PubMed] [Google Scholar]
- Moda BS, Ferreira-Júnior JR, Barros MH. Partial suppression of the respiratory defect of qrs1/her2 glutamyl-tRNA amidotransferase mutants by overexpression of the mitochondrial pentatricopeptide Msc6p. Curr Genet. 2016;62:607–617. doi: 10.1007/s00294-016-0566-6. [DOI] [PubMed] [Google Scholar]
- Mumberg D, Muller R, Funk M. Yeast vectors for the controlled expression of heterologous protein in different genetic backgrounds. Gene. 1995;156:119–122. doi: 10.1016/0378-1119(95)00037-7. [DOI] [PubMed] [Google Scholar]
- Pelissier P., Camougrand N, Velours G, Guerin M. NCA3, a nuclear gene involved in the mitochondrial expression of subunits 6 and 8 of the Fo–F1 ATP synthase of S. cerevisiae. Curr Genet. 1995;27:409–416. doi: 10.1007/BF00311209. [DOI] [PubMed] [Google Scholar]
- Perez-Martinez X, Broadley SA, Fox TD. Mss51p promotes mitochondrial Cox1p synthesis and interacts with newly synthesized Cox1p. EMBO J. 2003;22:5951–5961. doi: 10.1093/emboj/cdg566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rak M, Su CH, Xu JT, Azpiroz R, Singh A, Tzagoloff A. Regulation of mitochondrial translation of the ATP8-ATP6 mRNA by Smt1p. Mol Biol Cell. 2016;27:919–929. doi: 10.1091/mbc.E15-09-0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rak M, Tzagoloff A. F1-dependent translation of mitochondrially encoded Atp6p and Atp8p subunits of yeast ATP synthase. Proc Natl Acad Sci USA. 2009;106:18509–18514. doi: 10.1073/pnas.0910351106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Schiestl RH, Gietz RD. High efficiency transformation of intact yeast cells using single stranded nucleic acid as a carrier. Curr Genet. 1989;16:339–346. doi: 10.1007/BF00340712. [DOI] [PubMed] [Google Scholar]
- Shikanai T. RNA editing in plant organelles: machinery, physiological function and evolution. Cell Mol Life Sci. 2006;63:698–708. doi: 10.1007/s00018-005-5449-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon M., Faye G. Organization and processing of the mitochondrial oxi3/oli2 multigenic transcript in yeast. Mol Gen Genet. 1984;196:266–274. doi: 10.1007/BF00328059. [DOI] [PubMed] [Google Scholar]
- Solotoff V, Moseler R, Schulte U. Two pentatricopeptide repeat domain proteins are required for the synthesis of respiratory complex I. Curr Genet. 2015;61:19–29. doi: 10.1007/s00294-014-0441-2. [DOI] [PubMed] [Google Scholar]
- Steele DF, Butler CA, Fox TD. Expression of a recoded nuclear gene inserted into yeast mitochondrial DNA is limited by mRNA-specific translational activation. Proc Natl Acad Sci USA. 1996;93:5253–5257. doi: 10.1073/pnas.93.11.5253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supekova L, Supek F, Greer JE, Schultz PG. A single mutation in the first transmembrane domain of yeast COX2 enables its allotopic expression. Proc Natl Acad Sci USA. 2010;107:5047–5052. doi: 10.1073/pnas.1000735107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zampol MA, Busso C, Gomes F, Ferreira-Junior JR, Tzagoloff A, Barros MH. Over-expression of COQ10 in Saccharomyces cerevisiae inhibits mitochondrial respiration. Biochem Biophys Res Commun. 2010;402:82–87. doi: 10.1016/j.bbrc.2010.09.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X, Hourset A, Tzagoloff A. The Saccharomyces cerevisiae ATP22 gene codes for the mitochondrial ATPase subunit 6-specific translation factor. Genetics. 2007;175:55–63. doi: 10.1534/genetics.106.065821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Kessler M, Helmling S, O’Connor JP, Moore C. Pta1, a component of yeast CF II, is required for both cleavage and poly(A) addition of mRNA precursor. Mol Cell Biol. 1999;19:7733–7740. doi: 10.1128/mcb.19.11.7733. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.