Abstract
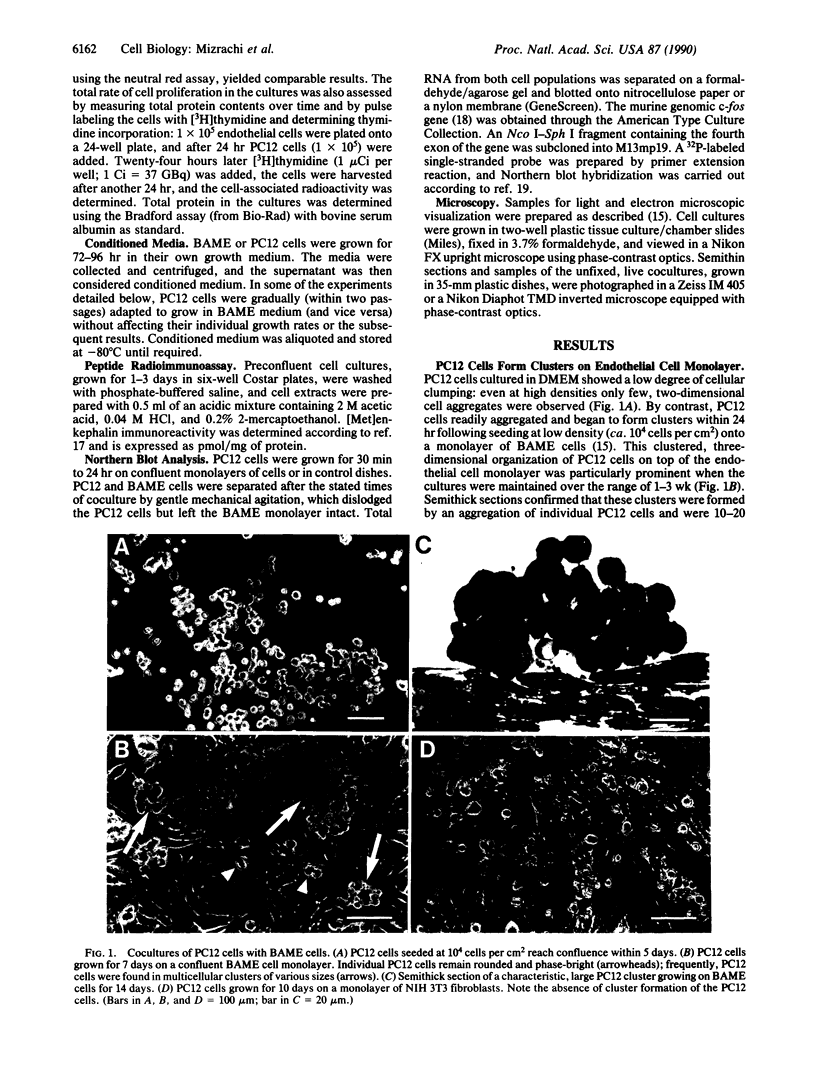
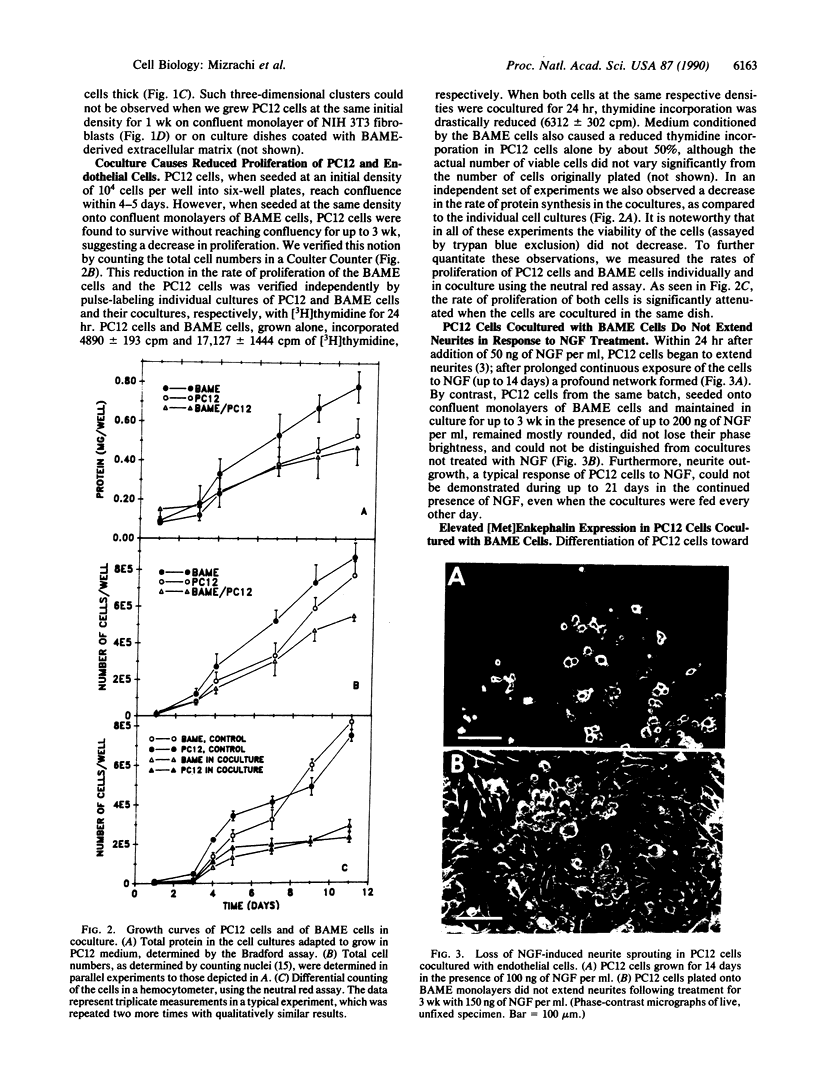
Previously we described specific in vitro interactions between PC12 cells, a cloned, catecholamine-secreting pheochromocytoma cell line derived from the rat adrenal medulla, and bovine adrenal medullary endothelial cells. We now demonstrate that these interactions induce the PC12 cells to acquire physical and biochemical characteristics reminiscent of chromaffin cells. Under coculture conditions involving direct cell-cell contact, the endothelial cells and the PC12 cells reduced their rates of proliferation; upon prolonged coculture PC12 cells clustered into nests of cells similar to the organization of chromaffin cells seen in vivo. Within 3 days in coculture with endothelial cells, but not with unrelated control cells, PC12 cells synthesized increased levels of [Met]enkephalin. In addition, PC12 cells, growing on confluent endothelial monolayers, failed to extend neurites in response to nerve growth factor. Neither medium conditioned by endothelial cells nor fixed endothelial cells could by themselves induce all of these different phenomena in the PC12 cells. These results suggest that under coculture conditions PC12 cells change their state of differentiation toward a chromaffin cell-like phenotype. The rapid, transient increase in the expression of the protooncogene c-fos suggests that the mechanism(s) inducing the change in the state of differentiation in PC12 cells in coculture with the endothelial cells may be distinct from that described for the differentiation of PC12 cells--e.g., by glucocorticoids. We propose that similar interactions between endothelial cells and chromaffin cell precursors may occur during embryonic development and that these interactions might be instrumental for the organ-specific differentiation of the adrenal medulla in vivo.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. J., Axel R. A bipotential neuroendocrine precursor whose choice of cell fate is determined by NGF and glucocorticoids. Cell. 1986 Dec 26;47(6):1079–1090. doi: 10.1016/0092-8674(86)90823-8. [DOI] [PubMed] [Google Scholar]
- Banerjee D. K., Ornberg R. L., Youdim M. B., Heldman E., Pollard H. B. Endothelial cells from bovine adrenal medulla develop capillary-like growth patterns in culture. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4702–4706. doi: 10.1073/pnas.82.14.4702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borenfreund E., Puerner J. A. Toxicity determined in vitro by morphological alterations and neutral red absorption. Toxicol Lett. 1985 Feb-Mar;24(2-3):119–124. doi: 10.1016/0378-4274(85)90046-3. [DOI] [PubMed] [Google Scholar]
- Byrd J. C., Naranjo J. R., Lindberg I. Proenkephalin gene expression in the PC12 pheochromocytoma cell line: stimulation by sodium butyrate. Endocrinology. 1987 Oct;121(4):1299–1305. doi: 10.1210/endo-121-4-1299. [DOI] [PubMed] [Google Scholar]
- Carmichael S. W., Spagnoli D. B., Frederickson R. G., Krause W. J., Culberson J. L. Opossum adrenal medulla: I. Postnatal development and normal anatomy. Am J Anat. 1987 Jul;179(3):211–219. doi: 10.1002/aja.1001790303. [DOI] [PubMed] [Google Scholar]
- Doupe A. J., Landis S. C., Patterson P. H. Environmental influences in the development of neural crest derivatives: glucocorticoids, growth factors, and chromaffin cell plasticity. J Neurosci. 1985 Aug;5(8):2119–2142. doi: 10.1523/JNEUROSCI.05-08-02119.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duband J. L., Thiery J. P. Distribution of fibronectin in the early phase of avian cephalic neural crest cell migration. Dev Biol. 1982 Oct;93(2):308–323. doi: 10.1016/0012-1606(82)90120-8. [DOI] [PubMed] [Google Scholar]
- Fujii D. K., Massoglia S. L., Savion N., Gospodarowicz D. Neurite outgrowth and protein synthesis by PC12 cells as a function of substratum and nerve growth factor. J Neurosci. 1982 Aug;2(8):1157–1175. doi: 10.1523/JNEUROSCI.02-08-01157.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita K., Lazarovici P., Guroff G. Regulation of the differentiation of PC12 pheochromocytoma cells. Environ Health Perspect. 1989 Mar;80:127–142. doi: 10.1289/ehp.8980127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentz R., Rauscher F. J., 3rd, Abate C., Curran T. Parallel association of Fos and Jun leucine zippers juxtaposes DNA binding domains. Science. 1989 Mar 31;243(4899):1695–1699. doi: 10.1126/science.2494702. [DOI] [PubMed] [Google Scholar]
- Goldstein G. W. Endothelial cell-astrocyte interactions. A cellular model of the blood-brain barrier. Ann N Y Acad Sci. 1988;529:31–39. doi: 10.1111/j.1749-6632.1988.tb51417.x. [DOI] [PubMed] [Google Scholar]
- Greenberg M. E., Greene L. A., Ziff E. B. Nerve growth factor and epidermal growth factor induce rapid transient changes in proto-oncogene transcription in PC12 cells. J Biol Chem. 1985 Nov 15;260(26):14101–14110. [PubMed] [Google Scholar]
- Greene L. A., Tischler A. S. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruijer W., Schubert D., Verma I. M. Induction of the proto-oncogene fos by nerve growth factor. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7330–7334. doi: 10.1073/pnas.82.21.7330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Douarin N. M. Cell line segregation during peripheral nervous system ontogeny. Science. 1986 Mar 28;231(4745):1515–1522. doi: 10.1126/science.3952494. [DOI] [PubMed] [Google Scholar]
- Levi-Montalcini R. The nerve growth factor 35 years later. Science. 1987 Sep 4;237(4819):1154–1162. doi: 10.1126/science.3306916. [DOI] [PubMed] [Google Scholar]
- Mizrachi Y., Lelkes P. I., Ornberg R. L., Goping G., Pollard H. B. Specific adhesion between pheochromocytoma (PC12) cells and adrenal medullary endothelial cells in co-culture. Cell Tissue Res. 1989;256(2):365–372. doi: 10.1007/BF00218894. [DOI] [PubMed] [Google Scholar]
- Mocchetti I., Giorgi O., Schwartz J. P., Costa E. A reduction of the tone of 5-hydroxytryptamine neurons decreases utilization rates of striatal and hypothalamic enkephalins. Eur J Pharmacol. 1984 Nov 13;106(2):427–430. doi: 10.1016/0014-2999(84)90734-9. [DOI] [PubMed] [Google Scholar]
- Morin O., Normand C. Long-term maintenance of hepatocyte functional activity in co-culture: requirements for sinusoidal endothelial cells and dexamethasone. J Cell Physiol. 1986 Oct;129(1):103–110. doi: 10.1002/jcp.1041290115. [DOI] [PubMed] [Google Scholar]
- Nakao T., Ishizawa A., Ogawa R. Observations of vascularization in the spinal cord of mouse embryos, with special reference to development of boundary membranes and perivascular spaces. Anat Rec. 1988 Jun;221(2):663–677. doi: 10.1002/ar.1092210212. [DOI] [PubMed] [Google Scholar]
- Naranjo J. R., Mocchetti I., Schwartz J. P., Costa E. Permissive effect of dexamethasone on the increase of proenkephalin mRNA induced by depolarization of chromaffin cells. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1513–1517. doi: 10.1073/pnas.83.5.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newgreen D. F., Erickson C. A. The migration of neural crest cells. Int Rev Cytol. 1986;103:89–145. doi: 10.1016/s0074-7696(08)60834-7. [DOI] [PubMed] [Google Scholar]
- Orlidge A., D'Amore P. A. Inhibition of capillary endothelial cell growth by pericytes and smooth muscle cells. J Cell Biol. 1987 Sep;105(3):1455–1462. doi: 10.1083/jcb.105.3.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozato K., Wan Y. J., Orrison B. M. Mouse major histocompatibility class I gene expression begins at midsomite stage and is inducible in earlier-stage embryos by interferon. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2427–2431. doi: 10.1073/pnas.82.8.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson P. H. Environmental determination of autonomic neurotransmitter functions. Annu Rev Neurosci. 1978;1:1–17. doi: 10.1146/annurev.ne.01.030178.000245. [DOI] [PubMed] [Google Scholar]
- Rhodin J. A., Fujita H. Capillary growth in the mesentery of normal young rats. Intravital video and electron microscope analyses. J Submicrosc Cytol Pathol. 1989 Jan;21(1):1–34. [PubMed] [Google Scholar]
- Sagen J., Pappas G. D., Perlow M. J. Fine structure of adrenal medullary grafts in the pain modulatory regions of the rat periaqueductal gray. Exp Brain Res. 1987;67(2):380–390. doi: 10.1007/BF00248558. [DOI] [PubMed] [Google Scholar]
- Schnitzer J. Astrocytes in the guinea pig, horse, and monkey retina: their occurrence coincides with the presence of blood vessels. Glia. 1988;1(1):74–89. doi: 10.1002/glia.440010109. [DOI] [PubMed] [Google Scholar]
- Sieber-Blum M. Fibronectin-regulated methionine enkephalin-like and somatostatin-like immunoreactivity in quail neural crest cell cultures. Neuropeptides. 1984 Nov;4(6):457–466. doi: 10.1016/0143-4179(84)90089-1. [DOI] [PubMed] [Google Scholar]
- Smith S. K., Giannopoulos G. Influence of pulmonary endothelial cells on fetal lung development. Pediatr Pulmonol. 1985 May-Jun;1(3 Suppl):S53–S59. [PubMed] [Google Scholar]
- Tao-Cheng J. H., Brightman M. W. Development of membrane interactions between brain endothelial cells and astrocytes in vitro. Int J Dev Neurosci. 1988;6(1):25–37. doi: 10.1016/0736-5748(88)90026-3. [DOI] [PubMed] [Google Scholar]
- Tischler A. S., Greene L. A. Phenotypic plasticity of pheochromocytoma and normal adrenal medullary cells. Adv Biochem Psychopharmacol. 1980;25:61–68. [PubMed] [Google Scholar]
- Unsicker K., Reichert-Preibsch H., Schmidt R., Pettmann B., Labourdette G., Sensenbrenner M. Astroglial and fibroblast growth factors have neurotrophic functions for cultured peripheral and central nervous system neurons. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5459–5463. doi: 10.1073/pnas.84.15.5459. [DOI] [PMC free article] [PubMed] [Google Scholar]