Abstract
Background
17-alpha hydroxyprogesterone caproate for prevention of recurrent preterm birth is recommended for use in the United States.
Objective
To assess the clinical effectiveness of 17-alpha hydroxyprogesterone caproate to prevent recurrent preterm birth ≤ 35 weeks compared to similar births in our obstetric population prior to the implementation of 17-alpha hydroxyprogesterone caproate.
Study Design
This was a prospective cohort study of 17-alpha hydroxyprogesterone caproate in our obstetric population. The primary outcome was the recurrence of birth ≤ 35 weeks for the entire study cohort compared to a historical referent rate of 16.8% of recurrent preterm birth in our population. There were three secondary outcomes. First, did 17-alpha hydroxyprogesterone caproate modify a woman’s history of preterm birth when taking into account her prior number and sequence of preterm and term births? Second, was recurrence of preterm birth related to 17-alpha hydroxyprogesterone caproate plasma concentration? Third, was duration of pregnancy modified by 17-alpha hydroxyprogesterone caproate treatment compared to a prior preterm birth?
Results
Between January 2012 and March 2016, 430 consecutive women with prior births ≤ 35 weeks were treated with 17-alpha hydroxyprogesterone caproate. Nearly two-thirds of the women (N=267) began injections ≤ 18 weeks and 394 (92%) received a scheduled weekly injection within 10 days of reaching 35 weeks or delivery. The overall rate of recurrent preterm birth was 25% (N=106) for the entire cohort compared to the 16.8% expected rate (P = 1.0). The three secondary outcomes were also negative. First, 17-alpha hydroxyprogesterone caproate did not significantly reduce the rates of recurrence regardless of prior preterm birth number or sequence. Second, plasma concentrations of 17-alpha hydroxyprogesterone caproate were not different (P=0.17 at 24 weeks; P=0.38 at 32 weeks) between women delivered ≤ 35 weeks and those delivered later in pregnancy. Third, the mean (± standard deviation) interval in weeks of recurrent preterm birth before 17-alpha hydroxyprogesterone caproate use was 0.4 ± 5.3 weeks and the interval of recurrent preterm birth after 17-alpha hydroxyprogesterone caproate treatment was 0.1 ± 4.7 weeks (P=0.63). A side effect of weekly 17-alpha hydroxyprogesterone caproate injections was an increase in gestational diabetes. Specifically, the rate of gestational diabetes was 13.4% in 17-alpha hydroxyprogesterone caproate treated women compared to 8% in case-matched controls (P=0.001).
Conclusions
17-alpha hydroxyprogesterone caproate was ineffective for prevention of recurrent preterm birth and was associated with increased rates of gestational diabetes.
Keywords: efficacy, external validity, gestational diabetes, neonatal morbidity, prematurity, preterm birth, progesterone, progestogen, randomized trial
INTRODUCTION
Prevention of preterm birth is a major focus in obstetrics due to the burden of neonatal morbidity and mortality on mothers, infants, families, and society both medically and financially. Dollar costs due to prematurity in the United States in 2006 were estimated to exceed $26 billion.1 Moreover, the consequences of prematurity include long-term neurological complications due to immaturity related injuries to the brain.2 Consequently, development of interventions to reduce the rate of preterm birth have been emphasized in the United States for several decades. A recent example is the widespread use of progestogens to prevent preterm birth.3–9
17-alpha hydroxyprogesterone caproate (17-OHPC), a synthetic progestogen, is the first and only agent to date approved for marketing by the United States Food and Drug Administration (FDA) for prevention of recurrent preterm birth.10 This approval stems from a trial by Meis and colleagues published in 2003.3 Following FDA approval, the American College of Obstetricians and Gynecologists (ACOG) and the Society for Maternal-Fetal Medicine (SMFM) endorsed use of 17-OHPC for prevention of recurrent preterm birth in singleton gestations.11,12 Most recently (January 2017), the SMFM Publications Committee recommended 17-OHPC be used for prevention of recurrent preterm birth and that vaginal progesterone should not be considered a substitute for 17-OHPC.13 The SMFM Publications Committee also concluded that despite their recommendations, there continued to be underutilization of 17-OHPC.13 It is important to emphasize that the FDA approval of 17-OHPC was under a regulatory pathway (Subpart H of the FDA Code of Regulations) used when the decision is made on the basis of a surrogate endpoint—delivery less than 37 weeks of gestation in this case—and was deemed to require further studies.14 In fact, another placebo-controlled randomized trial of 17-OHPC is in progress in the United States and elsewhere with the FDA-preferred primary end-point of delivery less than 35 weeks gestation. Details of this ongoing trial titled, “Confirmatory Study of 17P Versus Vehicle for the Prevention of Preterm Birth in Women With a Previous Singleton Spontaneous Preterm Delivery (PROLONG),” can be found at: https://clinicaltrials.gov/ct2/show/NCT01004029.14,15 This study began in October 2009 with an originally predicted date for conclusion of October 2013 which has been moved to 2018.14,15 This trial is sponsored by the manufacturer Lumara Health, Inc.
We decided to introduce 17-OHPC into our clinical practice and organized a study for introduction. We elected not to attempt a single-center randomized trial due to the high expense of such trials as well as the fact that the prevalence of recurrent preterm birth essentially obviates a single-center trial in a practical time period. Moreover, we wanted to perform a “real-world” study given the generalizability limitations of traditional randomized trials.16,17 We now report our experience with administration of 17-OHPC to women delivered at our hospital. We were particularly interested in the effectiveness of 17-OHPC using each woman—and her specific history of preterm birth—as the benchmark to measure response to therapy. Put another way, we introduced a widely used therapy in the United States to prevent recurrent preterm birth and measured whether or not this therapy was beneficial for the women actually treated in our practice.
MATERIALS AND METHODS
Study design
Parkland Hospital serves the medically indigent women of Dallas County and has developed a neighborhood-based, administratively and medically integrated public health care system for inner-city women. All women delivering at our hospital are routinely assigned to a Parkland Hospital neighborhood clinic for antenatal and postpartum care. Upon enrollment into prenatal care, women with a history of prior preterm birth are referred to the Preterm Birth Clinic centrally located at Parkland Hospital. This is a specific high-risk prenatal clinic staffed by maternal-fetal medicine faculty and fellows from the University of Texas Southwestern Medical Center. Criteria for referral to this clinic included a singleton pregnancy and prior spontaneous preterm birth or rupture of membranes between 20 0/7 and 35 0/7 weeks gestation. All women were offered 17-OHPC therapy commencing January 1, 2012. This project was approved by the Institutional Review Board of the University of Texas Southwestern Medical Center and Parkland Hospital.
The primary outcome of interest was recurrent preterm birth in women treated with 17-OHPC. Every woman underwent a detailed review of her obstetric history by a research nurse using a pre-specified manual of operations. Review included the number of previous births, gestational age at preterm birth, reason(s) for preterm delivery, and perinatal outcome. Women with a prior medically-indicated preterm delivery—such as pregnancy hypertension or placental abruption were excluded. The study estimate of gestational age was based on the date of the last menstrual period and sonography. Data on prior obstetric history were linked to a pre-existing computerized obstetric database. This database contains maternal and infant outcomes for all women delivered at Parkland Hospital.
17-alpha hydroxyprogesterone caproate (17-OHPC)
A local pharmacy provided compounded single-dose vials of 250 mg of 17-OHPC prepared in batches and delivered to the Parkland Hospital Pharmacy. Each batch was assayed for both potency and purity by an independent laboratory testing service (Eagle Analytical Services, Houston, TX). Potency testing was performed to ensure that each dose contained not less than 90% and not more than 110% of the specified 250 mg/mL 17-OHPC. Sterility testing was performed for bacteria, mold, yeast, and fungi. Our approach was similar to that reported by Chang and colleagues who evaluated the quality of 17-OHPC supplied by 15 compounding pharmacies and did not identify safety concerns when assessed as we have described.18 Each 250 mg dose was purchased retail at $24.99 including testing procedures. Sesame oil was used as the vehicle for the 17-OHPC. Injections were commenced between 16 0/7 and 20 6/7 weeks. Women received weekly injections at the Preterm Birth Clinic until 36 weeks, or delivery. This was the 17-OHPC regimen reported by Meis et al and which is in use throughout the United States.3,11–13
Assessment of clinical effectiveness
The primary outcome was the overall rate of recurrent preterm birth ≤ 35 weeks. This gestational age was chosen because it was the pre-existing criteria for referral of women to the Preterm Birth Clinic. There were three secondary questions. First, did 17-OHPC modify a woman’s history of preterm birth when taking into account her prior number and sequence of preterm and term births? Second, was recurrence of preterm birth related to 17-OHPC plasma concentration? Third, was duration of pregnancy modified by 17-OHPC treatment compared to a prior preterm birth?
Number and sequence of prior preterm births
An individual womans’ risk for recurrent preterm birth is influenced by her past number of preterm birth(s) as well as the sequence of preterm and term births.19 That is, the rate of recurrence depends upon the number of prior preterm births suffered as well as the sequence of both preterm and term infants. For example, a risk of recurrent preterm birth for a gravida 3 para 2 woman with a prior preterm birth followed by a term birth differs from a woman with a prior term birth followed by preterm birth. Our purpose was to measure the effectiveness of 17-OHPC in women with such differing past preterm and term pregnancy histories.
Relationship of 17-OHPC plasma concentration to recurrent preterm birth
Measurement of 17-OHPC concentrations in plasma became available during the trial. We opted to measure 17-OHPC concentrations to further validate use of our compounded progestogen. Specifically, we wanted to ensure that 17-OHPC was present in the plasma and concentrations were similar to those reported in the literature.20 Moreover, we wanted to examine the relationship between 17-OHPC concentration and spontaneous preterm birth. Blood was drawn coinciding with routine prenatal care blood draws at 24 and 32 weeks prior to administration of a scheduled 17-OHPC injection. The 24-week blood draw was for universal screening for gestational diabetes. Quantitative measurement of plasma concentration of 17-OHPC was performed using batch-run analyses and high-performance liquid chromatography-mass spectrometry with atmospheric pressure chemical ionization. All of these analyses were conducted by one of the co-investigators (JM). We then analyzed the relationship of 17-OHPC plasma concentrations to the rate of recurrent birth ≤ 35 weeks.
Severity of recurrent preterm birth
We sought to compare a specific womans’ weeks of gestation at a prior preterm birth not treated with 17-OHPC to the weeks gestation achieved in women who were treated.21 Put another way, we sought to determine the effects of 17-OHPC on the length of pregnancy. To do this, we compared the change in duration of pregnancy in women with recurrent preterm birth after treatment with 17-OHPC to women untreated and previously delivered preterm at our hospital.22
Screening for gestational diabetes
Universal screening for gestational diabetes has been in use at Parkland Hospital since 1998. A screening 50-gram oral glucose challenge was performed at 24 weeks in non-fasting women. Women with screening serum values ≥ 140 mg/dL were tested with a 3-hour 100-gram glucose tolerance test. Gestational diabetes was diagnosed when two or more of the following values were abnormal: fasting ≥ 105 mg/dL; 1-hour ≥ 190 mg/dL; 2-hour ≥ 165 mg/dL; 3-hour ≥ 145 mg/dL. Women with gestational diabetes were managed in coordination with a specific Gestational Diabetes Clinic held at Parkland Hospital.
Sample size calculation and statistics
The historical rate of recurrent birth ≤ 35 weeks in the Parkland Hospital general obstetric population was 16.8% when 17-OHPC was not in use.22 This rate was used to calculate the sample size necessary to assess the effectiveness of 17-OHPC. A sample size of 413 women was estimated for a 90% power to detect a one-third reduction in recurrent preterm birth (from 16.8% to 11.2%) using a one-sided, one-sample binomial test of size 0.025 (alpha = 0.025), which is equivalent to a two-sided test of 0.05. A one-sided test was chosen because the anticipated change was a lowering of the recurrent preterm birth rate. Recurrence rates according to the prior number and sequence of specific histories of preterm and term pregnancies were also based upon the Parkland Hospital general obstetric population prior to 17-OHPC implementation. Plasma concentrations of 17-OHPC in women with recurrent preterm birth were compared to concentrations in women without recurrence using the Wilcoxon rank-sum test. To assess severity of recurrent preterm birth, the change in gestational weeks of recurrent preterm births was compared using Student’s t test before and after 17-OHPC treatment.
The composition of the 17-OHPC study group was compared to the demographic characteristics of women in the historical cohort. To do this, it was necessary to match the prior preterm birth profiles of the women treated with17-OHPC to the historical cohort delivered after universal screening for gestational diabetes was instituted in 1998. A 3:1 matched control group design was used to match for prior preterm birth profile as well as maternal race and body mass index (BMI). Statistical analysis was performed using SAS 9.3 (SAS Institute Inc., Cary, NC).
RESULTS
Between 1 January 2012 and 31 March 2016, 456 consecutive women with prior births ≤ 35 weeks were treated with 17-OHPC and delivered either at Parkland Hospital (86%) or at community hospitals (14%), Figure 1. A total of 26 (6%) women were excluded from this analysis; 21 were lost to follow-up and five delivered before 20 weeks gestation. Selected demographic characteristics of the 430 remaining women treated with 17-OHPC are shown in Table 1. The women were predominantly Hispanic and 43% had BMIs of 30 kg/m2 or more. Nearly two-thirds of the women (N=267) began 17-OHPC injections ≤ 18 weeks and 394 (92%) received a scheduled weekly injection within 10 days of reaching 35 weeks or delivery. The 17-OHPC content of weekly injections was tested in 43 batches of approximately 200 doses per batch. The mean 17-OHPC content per dose was 249.25 mg.
Figure 1.
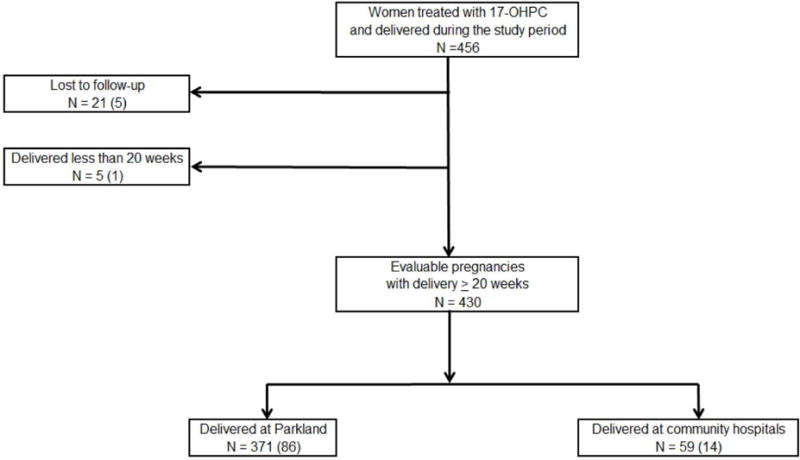
Flow diagram of women treated with 17-OHPC at Parkland Hospital from 1 January 2012 through 31 March 2016.
Table 1.
Demographic characteristics of 430 women with prior preterm births treated with 17-OHPC in a subsequent pregnancy.
| Characteristic | No. Women (%) N = 430 |
|---|---|
| Race/Ethnicity: | |
| White, non-Hispanic | 11 (3) |
| Hispanic | 342 (80) |
| Black | 73 (17) |
| Asian | 3 (1) |
| Other | 1 (0) |
| Age, years: | |
| Less than 20 | 17 (4) |
| 20 – 34 | 333 (77) |
| 35 or greater | 80 (19) |
| BMI, kg/m2: | |
| 18 – 25 | 105 (24) |
| 25 – 30 | 138 (32) |
| 30+ | 187 (43) |
| Highest level of school completed: | |
| None | 4(1) |
| ≤ 8th grade | 90 (21) |
| 9 – 12th grade | 287 (67) |
| Some college | 49 (11) |
All data shown as N (%).
As shown in Table 2, the overall rate of recurrent preterm birth was 25% (N=106) for the entire cohort treated with 17-OHPC compared to the 16.8% expected rate in the historical Parkland Hospital obstetric population (P = 1.0, one-sided test). All of the infants delivered of women treated with 17-OHPC were liveborn. We next analyzed each pregnancy according to specific obstetric history. As shown in Table 2, regardless of prior preterm birth number or sequence, 17-OHPC did not significantly reduce the rates of recurrence.
Table 2.
Prior obstetric history of 430 women with births ≤ 35 weeks and recurrence rates after 17-OHPC treatment compared to an historical cohort of 5,787 women with prior preterm birth at Parkland Hospital.
| No 17-OHPC | 17-OHPC treated | P-valueᵻ | |||
|---|---|---|---|---|---|
| Prior birth < 35 weeks | Historical cohort recurrence rate* | No. women | Recurrence | ||
| No. women | Rate | ||||
| Overall | 16.8% | 430 | 106 | 25% | 1.0 |
| Para 1 | 18% | 141 | 44 | 31% | 1.0 |
| Para 2: Both ≤ 35 weeks Only 2nd birth ≤ 35 weeks Only 1st birth ≤ 35 weeks |
43% 17% 11% |
48 52 39 |
20 11 2 |
42% 21% 5% |
0.49 0.84 0.18 |
| Para 3+: All ≤ 35 weeks Other sequences of ≤ 35 weeks |
45% 12% |
27 123 |
12 17 |
44% 14% |
0.56 0.78 |
Recurrence rate is derived from the Parkland Obstetric population for 1988–2011 prior to introduction of 17-OHPC.
P-values are one-sided
Drug concentrations were available for 116 of the 17-OHPC treated women at 24 weeks gestation and 101 at 32 weeks. The plasma concentration of 17-OHPC was 10.2 ± 5.2 ng/mL and 12 ± 5.9 ng/mL at 24 weeks and 32 weeks, respectively. When analyzed at either blood draw time-point, concentrations of 17-OHPC were not different (P=0.17 at 24 weeks; P=0.38 at 32 weeks) between women delivered ≤ 35 weeks and those delivered later in pregnancy (Figure 2). Moreover, the plasma concentrations of 17-OHPC corresponded to the concentrations reported by Caritis and colleagues.20
Figure 2.
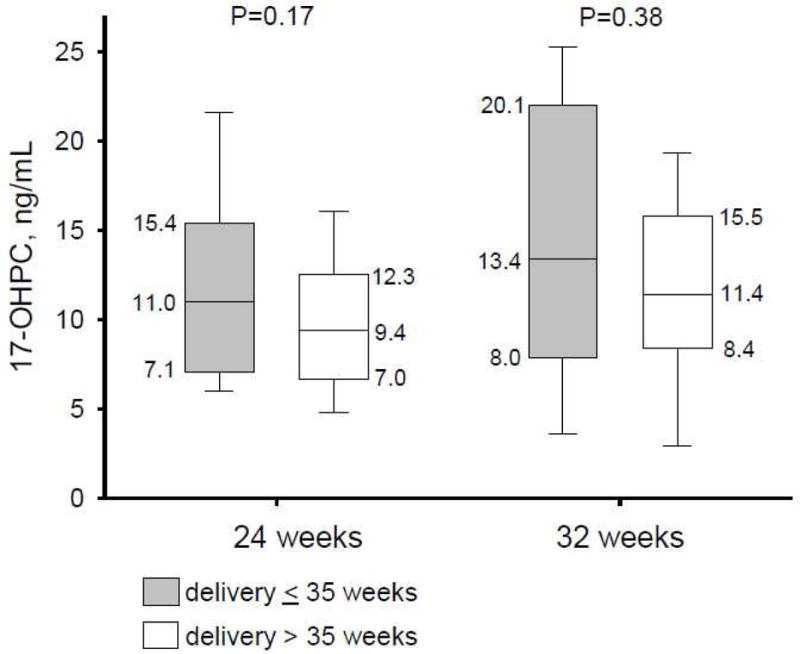
Recurrent preterm births according to 17-OHPC plasma drug concentrations measured at 24 and 32 weeks gestation. Data are shown as median [Q1,Q3] for treated women delivered ≤ 35 weeks (shaded) and > 35 weeks (not shaded) on therapy.
The change in gestational weeks of recurrent preterm births in women treated with 17-OHPC was compared to the change in weeks gestation in women previously untreated with 17-OHPC but who delivered a recurrent preterm infant (Figure 3). The mean (±SD) interval in weeks of recurrent preterm birth before 17-OHPC use was 0.4 ± 5.3 weeks and the interval of recurrent preterm birth after 17-OHPC was 0.1 ± 4.7 weeks. There was not a statistical difference (ie. improvement) in the interval weeks of recurrent preterm birth after the implementation of 17-OHPC in our practice (P=0.63).
Figure 3.
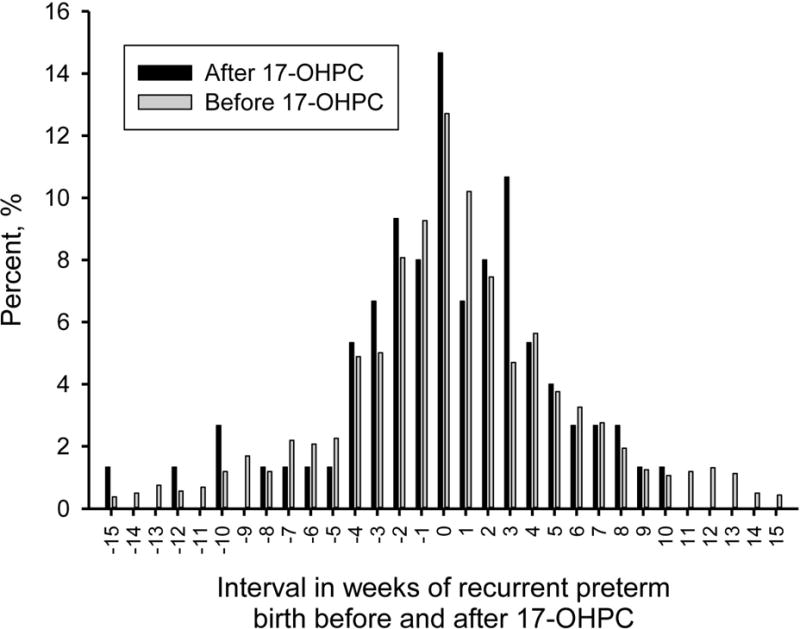
Duration of pregnancy in women delivered ≤ 35 weeks on 17-OHPC compared to similar women with recurrent preterm births between 1988 and 2011 but untreated with 17-OHPC.
A total of 56 (13.4%) women treated with 17-OHPC were diagnosed to have gestational diabetes (all but 13 women given 17-OHPC had complete evaluations for gestational diabetes). Using 3:1 matched (control: case) for prior preterm birth profile, maternal race, and BMI, a total of 104 (8%) of the matched women not given 17-OHPC were diagnosed to have gestational diabetes, P=0.001. The same 3:1 case matched control groups were applied to compare maternal demographics of women treated with 17-OHPC to those untreated in the 1998–2011 historical cohort and there were no significant differences in recurrence of preterm birth.
COMMENT
We introduced a new intervention to our obstetric service and felt a need to measure the effectiveness of 17-OHPC when given to prevent recurrent preterm birth. When prospectively compared to a historical cohort at our hospital, 17-OHPC did not improve the overall rate of recurrent preterm birth. We examined three secondary outcomes. First, the rates of recurrence were not improved by 17-OHPC when analyzed according to the specific sequence of prior preterm and term births. Second, 17-OHPC plasma concentrations were not different in women with and without recurrence. Third, 17-OHPC did not significantly increase the duration of pregnancy when those women with a recurrent preterm birth were compared to similar women not previously treated with 17-OHPC. A side effect of 17-OHPC treatment was a significantly increased rate of gestational diabetes compared to case matched historical controls, 13.4% versus 8%, for 17-OHPC treated versus untreated, respectively.
Background
The study of 17-OHPC to prevent preterm birth is not new. In 1975, Johnson and colleagues randomized 43 women with prior preterm births or spontaneous abortions to weekly 250 mg injections of 17-OHPC or placebo to test the efficacy of a progestogen in preventing premature labor.23 Prior to this study, progestogens were used primarily in the prevention of spontaneous abortion rather than prevention of preterm birth. These investigators found that 41% (9/22) of women given placebo delivered preterm compared to zero (of 14 women) given 17-OHPC, P<0.02.23 In contrast, Hauth and colleagues (1983) studied the efficacy of 17-OHPC in a heterogeneous group of women on active-military duty and found no beneficial effect.24 A total of 168 women were randomized: 80 women were allocated to 17-OHPC 1,000 mg intramuscular weekly and 88 were allocated to placebo. Premature labor occurred in 5/80 (6%) women given 17-OHPC compared to 5/88 (6%) randomized to placebo, P=0.88. Keirse analyzed seven trials of 17-OHPC published between 1964 and 1985 using meta-analysis and found that 17-OHPC was associated with a reduction in preterm birth from 28% to 16% (OR 0.5; 95%CI 0.3–0.85).25 This meta-analysis was influential in the choice of 17-OHPC for the Meis et al trial that ultimately rekindled interest in the use of progestogens to prevent preterm birth.3,25 Meis et al reported a reduction in recurrence in 463 women randomized (2:1) to receive 17-OHPC or placebo with delivery before 37 weeks in 111/306 (36%) women receiving 17-OHPC compared to 84/153 (55%) receiving placebo (P<0.001).3 As a result of this study, 17-OHPC has become established in obstetric practice in the United States for prevention of recurrent preterm birth.11–13
Some have voiced concerns about the Meis et al trial.26–29 For example, Wesley in her review of the Meis et al trial for the FDA was concerned by the unexpectedly high rate (55%) of recurrent preterm birth in the control population.26 The expected rate had been 37%. The problem was that the preterm birth rate was 36% in the 17-OHPC group, which was significant only in comparison to the unexpected 55% control group rate. One conclusion was that the benefit attributed to 17-OHPC was only seen because the rate of recurrence in the control group exceeded the expected frequency. An explanation offered for such a high rate of preterm birth in the control group was asymmetry in risk of recurrence. That is, the more prior preterm births a woman has, the greater the recurrence risk. Indeed, 41% of the control women in the Meis et al trial had two or more prior preterm births compared to 28% in the 17-OHPC group (P=0.004). Correspondence and commentary to the publication of the Meis et al report raised another possibility for the higher rate of preterm birth in the control group.29,30 Brancazio and co-authors suggested the possibility that the castor oil placebo used by Meis et al could be implicated in stimulation of preterm uterine activity.30 Specifically, Brancazio and co-authors observed that castor oil had been previously used to induce labor.30 Meis et al responded that this explanation was unlikely because castor oil was used as the vehicle in the 17-OHPC group as well the control group.30 Moreover, Meis et al contend that castor oil in the small doses used in their trial was not recognized as an effective agent for inducing labor in pregnant women.30 Recent laboratory evidence suggests otherwise with O’Sullivan and colleagues reporting that human myometrial strips exposed to castor oil resulted in enhanced oxytocin-induced contractility.31 Because of these concerns related to castor oil, we chose to use sesame oil as the vehicle for 17-OHPC in our study. We point out that 17-OHPC plasma concentrations using sesame oil as the vehicle were virtually identical to prior reported levels using castor oil.20 Romero and Stanczyk had another concern about the Meis et al trial.27 The Meis trial was completed in two phases. The first phase included 150 subjects when it had to be stopped because of reported problems with the manufacture of 17-OHPC.26 The first phase cases were not included in the final published report.27 The rate of recurrence in the control group during the first phase was 36% compared to the 55% in the control group rate published by Meis et al.3 Thus, this 36% rate was equivalent to the 17-OHPC treated groups’ expected 37% rate – meaning that 17-OHPC was ineffective in the unpublished first phase of the Meis et al trial.
Diabetogenic effects of progestogens
It has long been known that maternal hormones to include estrogen and progesterone increase and promote pancreatic beta-cell hyperplasia and increased insulin.32 As pregnancy progresses, increased levels of a variety of hormones to include cortisol, prolactin, estrogen, and progesterone lead to insulin resistance which is considered central to the glucose intolerance associated with gestational diabetes. In contrast to estradiol which is considered to be a very weak diabetogenic factor, progesterone is considered a very strong factor with peak elevation at about 32 weeks gestation. Indeed, Rebarber and colleagues studied the diagnosis of gestational diabetes in 557 women given weekly 17-OHPC injections to prevent recurrent preterm birth compared to diagnosis of gestational diabetes in 1,524 women with prior preterm births but not given 17-OHPC.33 The incidence of gestational diabetes in the 17-OHPC treated group was 12.9% compared to 4.9% in control subjects, P<0.001.33 These results are very much like our experience. We must conclude that 17-OHPC indeed has a biologic effect that may induce gestational diabetes.
Basic science observations on 17-OHPC for prevention of preterm birth
The biologic mechanisms by which progestogens prevent preterm birth are unknown. One proposed mechanism was that progestogens maintain uterine quiescence, however, the current hypothesis is that progestogens act as anti-inflammatory agents, possibly at the level of the uterine cervix.34–43 Nold and colleagues, using a murine model found that 17-OHPC had no effect on the pathways involved in uterine contractility, uterine quiescence, or cervical remodeling.44 Elovitz and Mrinalini also used a mouse model of localized uterine inflammation and found that pretreatment with 17-OHPC before intrauterine endotoxin exposure significantly decreased the preterm birth rate.45 However, such use of 17-OHPC was associated with significant maternal morbidity including behavioral changes and death45 In contrast, Furcon and colleagues found that 17-OHPC did not have local anti-inflammatory effects at the maternal-fetal interface or the cervix, and that 17-OHPC did not protect against endotoxin-induced preterm birth.43 Indeed, Furcon and co-authors reviewed the basic science on progestogen effects in their report and concluded that the laboratory evidence for 17-OHPC to influence preterm birth were weak compared to vaginal progesterone.43
Manuck and colleagues have studied the pharmacogenomics of 17-OHPC in the prevention of preterm birth.46,47 This approach to evaluating the effects of 17-OHPC was based on a human biologic fluid repository collected during the trial by Meis et al.46 Saliva was tested for 20 different progesterone receptor polymorphisms in 380 women. They found that an individuals’ response to 17-OHPC was modified by their progesterone receptor genotype. For example, 17-OHPC treatment reduced preterm births in some women with DNA variants compared to increased preterm births in women with other DNA variants. Manuck and colleagues also studied DNA extracted from stored blood buffy coats in 50 women managed at Intermountain Medical Center in Salt Lake City.47 Women who benefitted (ie “responded”) to 17-OHPC had specific overrepresented genes. Taken together, these investigations by Manuck and colleagues suggest that the benefits of 17-OHPC in prevention of recurrent preterm birth are modified by pharmacogenomics.46,47
17-OHPC pricing concerns
Other concerns about 17-OHPC for prevention of recurrent preterm birth involve the FDA approval history and subsequent price gouging claims.14,48 In 2006, a New Drug Approval application was submitted to the FDA for 17-OHPC. The initial application was denied and the FDA called for a confirmatory randomized trial with a larger sample size than the Meis et al trial.27 The rights to manufacture 17-OHPC were subsequently bought by KV Pharmaceutical. Once a confirmatory study was underway and 10% of the total sample size had been recruited from US sites, the FDA gave temporary approval to KV Pharmaceutical on 11 February 2011 to market 17-OHPC under the brand name Makena®.48 This approval was granted under the agency’s accelerated approval regulations. On 15 February 2011, KV Pharmaceutical announced the price of Makena® to be $1500 per injection. Given that pharmaceutical regulations prohibit compounding pharmacies from producing products that are commercially manufactured, KV Pharmaceutical had no competitors in pricing and was free to set the price as high as they thought the market would bear.48 There was widespread concern over pricing because the drug cost of Makena® would exceed more than $30,000 per pregnancy. This was 75–150 times more than what formerly was being charged for the same medication that previously was available only through compounding pharmacies.48 To put this pricing into context, if there are 133,000 women with prior preterm births delivered each year in the United States,49 and each woman is given a total of 20 injections of 17-OHPC (16 to 36 weeks), the income to the manufacturer using half-price, ie. $750 per 250 mg dose of 17-OHPC, totals $1,990,000,000 per year. This almost $2 billion can be compared to the $25 per 250 mg dose (including potency and purity testing) for the 17-OHPC used in our study that would total $66.5 million to treat these 133,000 women. This highlights the impact of a 30-fold increase in drug cost. Generally, the FDA has exercised enforcement discretion for most products made through traditional pharmacy compounding thus prohibiting producing compounded products that are commercially available. However, the FDA decided in the case of 17-OHPC to not take enforcement action against pharmacies that compound this drug and compounded 17-OHPC has consequently been available within the United States.
Are the results of randomized trials always validated in subsequent practice?
Stanley reviewed randomized controlled trials as a research method.50 A major factor in the advance of medical science over the past 50 years has been the development of the randomized controlled trial. To quote Stanley, “Nothing more clearly indicates the key role of a randomized clinical trial in modern clinical research than the placement of this research method at the top of the list of levels of evidence in evidence-based medicine.” Indeed, drugs approved for marketing by the FDA in the United States generally require two randomized trials showing efficacy of the drug in Phase III randomized clinical trials.51 Two trials are usually required because of the importance of reproducibility.52 The importance of reproducibility can be traced to Bradford-Hill who developed nine criteria—for how to separate causation from simple association. Fifty years after Bradford-Hill, Ioannidis assessed how well each of the nine criteria functioned and found reproducibility paramount in strengthening a cause-and-effect conclusion.53 Reproducibility was deemed to strengthen the cause-and-effect relationship if there were consistent findings observed by different persons in different places with different studies.53
When well conducted, randomized controlled trials have internal validity, meaning the ability to trace causal inferences to the tested intervention which in our study was 17-OHPC. Internal validity here means a carefully circumscribed experimental population that share likeliness to meet specified randomized controlled trial inclusion criteria. However, this can be a limitation when results of randomized controlled trials are applied to a more heterogeneous population deemed “real-world.” The criticism here is that the results of randomized controlled trials may not be generalizable to “real-world” populations.17,54 An example of this failure of generalizability is the recently reported trial titled, “A population-based multifaceted strategy to implement antenatal corticosteroid treatment versus standard care for the reduction of neonatal mortality due to preterm birth in low-income and middle-income countries: the ACT cluster-randomized trial” (ACT trial) where antenatal corticosteroid treatment was randomized in a study aimed at reducing neonatal mortality due to preterm birth in 349 total health facilities located in Argentina, Guatemala, India, Kenya, Pakistan, and Zambia.55 Clearly, use of antenatal corticosteroids based upon a systematic review of 21 randomized controlled trials has become a touchstone in contemporary perinatal therapy.56 Indeed, the systematic review showed a 31% relative reduction in neonatal mortality when antenatal corticosteroids were used in populations studied in the industrialized world.56 It was anticipated by the investigators of the ACT trial that administering antenatal corticosteroids to women at high-risk for preterm birth in populations where access to modern contemporary perinatal care was limited could dramatically reduce neonatal deaths.55 That is, antenatal corticosteroids administration offered the possibility of an inexpensive low-technology means of reducing neonatal mortality.55 The ACT trial took place between 2011 and March 2014 and included 48,219 women in the antenatal steroid group compared to 51,523 women in the control group.55 Among the whole population, 28-day neonatal mortality was 27.4 per 1000 livebirths for the intervention group and 23.9 per 1000 livebirths for the control group (relative risk 1.12, 1.02–1.22, P=0.0127).55 Instead of the expected reduction in neonatal mortality, there was an excess of 3.5 neonatal deaths for every 1000 women given antenatal steroids.55 This result was attributed to the lack of access to ultrasound and neonatal intensive care in the populations studied.57 A recent study of ultrasound in similar countries (Pakistan, Kenya, Zambia, and Guatemala) gives lie to the notion that ACT failed due to lack of ultrasound.58 Nonetheless, results of multiple randomized controlled trials were clearly not generalizable.
The lack of generalizability, which refers to external validity, becomes important when new therapies are applied in real-world settings. Nallamothu and co-authors writing in a report titled, “Beyond the Randomized Clinical Trial,” distinguished between efficacy (treatment which works under ideal circumstances as in a randomized controlled trial) and effectiveness (treatment that works in real-world circumstances).54 These authors concluded that observational studies are essential follow-on studies for translating findings from randomized controlled trials into routine clinical practice.54 Most recently, Sherman and colleagues writing on real-world evidence observed that there is increased interest in exploring and integrating clinical research into more diverse real-world settings by capitalizing on the exponential growth in access to data from electronic health records and other existing datasets.17 They mention studies involving historical controls such as used in our study.17 In the case of 17-OHPC, the evidence date includes only one randomized controlled trial with a FDA required second trial in progress. We note that post-market clinical research on drugs is generally considered Phase IV and that observational trials are included as a legitimate method for Phase IV studies.51
Conclusions
We wish to emphasize that our study of the effectiveness of 17-OHPC was unusual in that we not only assessed the overall effect of 17-OHPC on recurrence of preterm birth but also the effect on preterm birth recurrence in the context of variations in recurrence patterns. Specifically, the risk of recurrence intensifies in proportion to the number of prior preterm births as well as the inter-position (ie. order) of term/preterm, preterm/term births in the obstetric history. We summarize our experiences with the conclusion that we were unable to demonstrate any benefit for 17-OHPC to prevent recurrent preterm birth. We did find a side effect, specifically increased gestational diabetes when 17-OHPC was used in weekly injections. We conclude that the failure of 17-OHPC for prevention of recurrent preterm birth at our hospital and the published evidence suggests that 17-OHPC for prevention of recurrent preterm birth taken as a whole is at best problematic and has an important side effect.
CONDENSATION.
Use of 17-alpha hydroxyprogesterone caproate was not effective in prevention of recurrent preterm birth.
Short version of title: Lack of effectiveness of 17-OHPC in the prevention of recurrent preterm birth
Acknowledgments
Financial Disclosures: A portion of this study was sponsored by the National Center for Advancing Translational Sciences of the National Institutes of Health under award number UL1TR001105. Additionally, members of this research team (DBN, DDM, and KJL) were supported by the Parkland Hospital Obstetric Research Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors report no conflict of interest.
References
- 1.Institute of Medicine. Preterm Birth: Causes, Consequences, and Prevention. Washington: National Academies Press; 2007. [PubMed] [Google Scholar]
- 2.Manuck TA, Sheng X, Yoder BA, Varner MW. Correlation between initial neonatal and early childhood outcomes following preterm birth. Am J Obstet Gynecol. 2014;210:426e1–9. doi: 10.1016/j.ajog.2014.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meis PJ, Klebanoff M, Thom E, Dombrowski MP, Sibai B, Moawad AH, et al. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Engl J Med. 2003;348(24):2379–85. doi: 10.1056/NEJMoa035140. [DOI] [PubMed] [Google Scholar]
- 4.da Fonseca EB, Bittar RE, Carvalho MH, Zagaib M. Prophylactic administration of progesterone by vaginal suppository to reduce the incidence of spontaneous preterm birth in women at increased risk: a randomized placebo-controlled double-blind study. Am J Obstet Gynecol. 2003;188:419–24. doi: 10.1067/mob.2003.41. [DOI] [PubMed] [Google Scholar]
- 5.O’Brien JM, Adair CD, Lewis DF, Hall DR, Defranco EA, Fusey S, et al. Progesterone vaginal gel for the reduction of recurrent preterm birth: primary results from a randomized, double-blind, placebo-controlled trial. Ultrasound Gynecol. 2007;30:687–96. doi: 10.1002/uog.5158. [DOI] [PubMed] [Google Scholar]
- 6.Fonseca EB, Celik E, Parra M, Singh M, Nicolaides KH. Progesterone and the risk of preterm birth among women with a short cervix. Fetal Medicine Foundation Second Trimester Screening Group. N Engl J Med. 2007;357:462–9. doi: 10.1056/NEJMoa067815. [DOI] [PubMed] [Google Scholar]
- 7.Hassan SS, Romero R, Vidyadhari D, Fusey S, Baxter JK, Khandelwal M, et al. Vaginal progesterone reduces the rate of preterm birth in women with a sonographic short cervix: a multicenter, randomized, double-blind, placebo-controlled trial. PREGNANT Trial. Ultrasound Obstet Gynecol. 2011;38:18–31. doi: 10.1002/uog.9017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Romero R, Nicolaides K, Conde-Agudelo A, et al. Vaginal progesterone in women with an asymptomatic sonographic short cervix in the midtrimester decreases preterm delivery and neonatal morbidity: a systematic review and metaanalysis of individual patient data. Am J Obstet Gynecol. 2012;206:124e1–9. doi: 10.1016/j.ajog.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Romero R, Nicolaides KH, Conde-Agudelo A, et al. Vaginal progesterone decreases preterm birth ≤ 34 weeks of gestation in women with a singleton pregnancy and a short cervix: an updated meta-analysis including data from the OPPTMUM study. Ultrasound Obstet Gynecol. 2016;48:308–317. doi: 10.1002/uog.15953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.FDA approves drug to reduce risk of preterm birth in at-risk pregnancy women. 2011 Feb 4; http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm242234.htm. FDA Press release.
- 11.American College of Obstetricians and Gynecologists. Prediction and prevention of preterm birth. Practice Bulletin No. 130. Obstet Gynecol. 2012;120:964–73. doi: 10.1097/AOG.0b013e3182723b1b. [DOI] [PubMed] [Google Scholar]
- 12.Society for Maternal-Fetal Medicine Publications Committee, with the assistance of Vincenzo Berghella, MD. Progesterone and preterm birth prevention: translating clinical trials data into clinical practice. Am J Obstet Gynecol. 2012;206(5):376–86. doi: 10.1016/j.ajog.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 13.SMFM Publications Committee. SMFM Statement: The choice of progestogen for the prevention of preterm birth in women with singleton pregnancy and prior preterm birth. Am J Obstet Gynecol. 2017 doi: 10.1016/j.ajog.2017.01.022. [DOI] [PubMed] [Google Scholar]
- 14.Romero R, Yeo L, Miranda J, Hassan S, Conde-Agudelo A, Chaiworapongsa T. A blueprint for the prevention of preterm birth: vaginal progesterone in women with a short cervix. J Perinat Med. 2013 Jan;41(1):27–44. doi: 10.1515/jpm-2012-0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.https://clinicaltrials.gov/ct2/show/NCT01004029. Accessed January 6, 2017.
- 16.Booth CM, Tannock IF. Randomised controlled trials and population-based observational research: partners in the evolution of medical evidence. Br J Cancer. 2014 Feb 4;110(3):551–5. doi: 10.1038/bjc.2013.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sherman RE, Anderson SA, Dal Pan GJ, et al. Real-World Evidence—What Is It and What Can It Tell Us? N Engl J Med. 2016 Dec 8;375(23):2293–2297. doi: 10.1056/NEJMsb1609216. [DOI] [PubMed] [Google Scholar]
- 18.Chang J, Zhao Y, Zhao W, et al. Quality assessment of compounded 17-hydroxyprogesterone caproate. Am J Obstet Gynecol. 2014;210:47e1–7. doi: 10.1016/j.ajog.2013.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McManemy J, Cooke E, Amon E, Leet T. Recurrence risk for preterm delivery. Am J Obstet Gynecol. 2007;196:576e1–576.e7. doi: 10.1016/j.ajog.2007.01.039. [DOI] [PubMed] [Google Scholar]
- 20.Caritis SN, Venkataramanan R, Thom E, et al. Relationship between 17-alpha hydroxyprogesterone caproate concentration and spontaneous preterm birth. Am J Obstet Gynecol. 2014;210:128e1–6. doi: 10.1016/j.ajog.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manuck TA, Esplin MS, Biggio J, et al. Predictors of response to 17-alpha hydroxyprogesterone caproate for prevention of recurrent spontaneous preterm birth. Am J Obstet Gynecol. 2016;214(3):376e1–8. doi: 10.1016/j.ajog.2015.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bloom SL, Yost NP, McIntire DD, Leveno KL. Recurrence of preterm birth in singleton and twin pregnancies. Obstet Gynecol. 2001;98(3):379–85. doi: 10.1016/s0029-7844(01)01466-1. [DOI] [PubMed] [Google Scholar]
- 23.Johnson JW, Austin KL, Jones GS, Davis GH, King TM. Efficacy of 17alpha-hydroxyprogesterone caproate in the prevention of premature labor. N Engl J Med. 1975;293:675–80. doi: 10.1056/NEJM197510022931401. [DOI] [PubMed] [Google Scholar]
- 24.Hauth JC, Gilstrap LC, III, Brekken AL, Hauth JM. The effect of 17 alpha-hydroxyprogesterone caproate on pregnancy outcome in an active-duty military population. Am J Obstet Gynecol. 1983;146(2):187–90. doi: 10.1016/0002-9378(83)91051-7. [DOI] [PubMed] [Google Scholar]
- 25.Keirse MJ. Progestogen administration in pregnancy may prevent preterm delivery. Br J Obstet Gynecol. 1990 Feb;97(2):149–54. doi: 10.1111/j.1471-0528.1990.tb01740.x. [DOI] [PubMed] [Google Scholar]
- 26.Gestiva slides revision. (NDA 21–945).Meeting of the Advisory Committee for Reproductive Health Drugs. 2006 Aug 29; 17α-hydroxyprogesterone caproate (Gestiva). Available at: http://www.fda.gov/ohrms/dockets/ac/06/slides/2006-4227S1-02-03-FDA_Wesley_files/frame.htm. Accessed January 7, 2017.
- 27.Romero R, Stanczyk FZ. Progesterone is not the same as 17α-hydroxyprogesterone caproate: implications for obstetrical practice. Am J Obstet Gynecol. 2013;208(6):421–6. doi: 10.1016/j.ajog.2013.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O’Brien JM, Lewis DF. Prevention of preterm birth with vaginal progesterone or 17-alpha-hydroxyprogesterone caproate: a critical examination of efficacy and safety. Am J Obstet Gynecol. 2016;214(1):45–56. doi: 10.1016/j.ajog.2015.10.934. [DOI] [PubMed] [Google Scholar]
- 29.Keirse MJ. Progesterone and preterm: seventy years of “déjà vu” or “still to be seen”? Birth. 2004 Sep;31(3):230–5. doi: 10.1111/j.0730-7659.2004.00315.x. [DOI] [PubMed] [Google Scholar]
- 30.Brancazio LR, Murtha AP, Heine RP. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Engl J Med. 2003 Sep;349(11):11. 1087–8. doi: 10.1056/NEJM200309113491115. author reply 1087–8. [DOI] [PubMed] [Google Scholar]
- 31.O’Sullivan MD, Hehir MP, O’Brien, et al. 17 alpha-hydroxyprogesterone caproate vehicle, castor oil, enhances the contractile effect of oxytocin in human myometrium in pregnancy. Am J Obstet Gynecol. 2010;202:453e1–4. doi: 10.1016/j.ajog.2010.03.023. [DOI] [PubMed] [Google Scholar]
- 32.Carr DB, Gabbe S. Gestational diabetes: detection, management, and implications. Clinical Diabetes. 1998;16(1):4–11. [Google Scholar]
- 33.Rebarber A, Istwan NB, Russo-Stieglitz K, et al. Increased Incidence of Gestational Diabetes in Women Receiving Prophylactic 17α-Hydroxyprogesterone Caproate for Prevention of Recurrent Preterm Delivery. Diabetes Care. 2007 Sep;30(9):2277–80. doi: 10.2337/dc07-0564. [DOI] [PubMed] [Google Scholar]
- 34.Csapo AI, Pinto-Dantas CA. The effect of progesterone on the human uterus. Proc Natl Acad Sci USA. 1965;54:1069–76. doi: 10.1073/pnas.54.4.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saito Y, Sakamoto H, MacLusky NJ, Naftolin F. Gap junctions and myometrial steroid hormone receptors in pregnant and postpartum rats: a possible cellular basis for the progesterone withdrawal hypothesis. Am J Obstet Gynecol. 1985;151:805–12. doi: 10.1016/0002-9378(85)90525-3. [DOI] [PubMed] [Google Scholar]
- 36.Mesiano S. Myometrial progesterone responsiveness and the control of human parturition. J Soc Gynecol Investig. 2004;11:193–202. doi: 10.1016/j.jsgi.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 37.Zakar T, Mesiano S. How does progesterone relax the uterus in pregnancy? N Engl J Med. 2011;364:972–3. doi: 10.1056/NEJMcibr1100071. [DOI] [PubMed] [Google Scholar]
- 38.Garfield RE, Shi L, Shi SQ. Use of progesterone and progestin analogs for inhibition of preterm birth and other uterine contractility disorders. Facts Views Vis Obgyn. 2012;4:237–44. [PMC free article] [PubMed] [Google Scholar]
- 39.Ito A, Imada K, Sato T, Kubo T, Matsushima K, Mori Y. Suppression of interleukin 8 production by progesterone in rabbit uterine cervix. Biochem J. 1994;301(Pt 1):183–6. doi: 10.1042/bj3010183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Imada K, Ito A, Sato T, Namiki M, Nagase H, Mori Y. Hormonal regulation of matrix metalloproteinase 9/gelatinase B gene expression in rabbit uterine cervical fibroblasts. Biol Reprod. 1997;56:575–80. doi: 10.1095/biolreprod56.3.575. [DOI] [PubMed] [Google Scholar]
- 41.Yellon SM, Ebner CA, Elovitz MA. Medroxyprogesterone acetate modulates remodeling, immune cell census, and nerve fibers in the cervix of a mouse model for inflammationinduced preterm birth. Reprod Sci. 2009;16:257–64. doi: 10.1177/1933719108325757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fukuyama A, Tanaka K, Kakizaki I, et al. Anti-inflammatory effect of proteoglycan and progesterone on human uterine cervical fibroblasts. Life Sci. 2012;90:484–8. doi: 10.1016/j.lfs.2011.12.024. [DOI] [PubMed] [Google Scholar]; Kim MG, Shim JY, Pak JH, et al. Progesterone modulates the expression of interleukin-6 in cultured term human uterine cervical fibroblasts. Am J Reprod Immunol. 2012;67:369–75. doi: 10.1111/j.1600-0897.2011.01094.x. [DOI] [PubMed] [Google Scholar]
- 43.Furcron A-E, Romero R, Plazyo O, et al. Vaginal progesterone, but not 17α-hydroxyprogesterone caproate, has anti-inflammatory effects at the murine maternal-fetal interface. Am J Obstet Gynecol. 2015;213:846e1–19. doi: 10.1016/j.ajog.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nold C, Maubert M, Anton L, et al. Prevention of preterm birth by progestational agents: what are the molecular mechanisms? Am J Obstet Gynecol. 2013;208:223e1–7. doi: 10.1016/j.ajog.2013.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Elovitz MA, Mrinalinia C. The use of progestational agents for preterm birth: Lessons from a mouse model. Am J Obstet Gynecol. 2006;195:1004–10. doi: 10.1016/j.ajog.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 46.Manuck TA, Lai Y, Meis PJ, et al. Progesterone receptor polymorphisms and clinical response to 17-alpha-hydroxyprogesterone caproate. Am J Obstet Gynecol. 2011;205:135e1–9. doi: 10.1016/j.ajog.2011.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Manuck TA, Watkins WS, Moore B, et al. Pharacogenomics of 17-alpha hydroxyprogesterone caproate for recurrent preterm birth prevention. Am J Obstet Gynecol. 2014;210:321e1–21. doi: 10.1016/j.ajog.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cohen AW, Copel JA, Macones GA, Menard MK, Riley L, Saade GR. Unjustified increase in cost of care resulting from U.S. Food and Drug Administration approval of Makena (17α-Hydroxyprogesterone Caproate) Obstet Gynecol. 2011;117(6):1408–12. doi: 10.1097/AOG.0b013e31821c2d75. [DOI] [PubMed] [Google Scholar]
- 49.Petrini JR, Callaghan WM, Klebanoof M, et al. Estimated Effect of 17 Alpha-Hydroxyprogesterone Caproate on Preterm Birth in the United States. Obstet Gynecol. 2005;105:267–72. doi: 10.1097/01.AOG.0000150560.24297.4f. [DOI] [PubMed] [Google Scholar]
- 50.Stanley K. Design of randomized controlled trials. Cicrulation. 2007 Mar 6;115(9):1164–9. doi: 10.1161/CIRCULATIONAHA.105.594945. [DOI] [PubMed] [Google Scholar]
- 51.https://www.nlm.nih.gov/services/ctphases.html. Accessed January 8, 2017.
- 52.Bradford Hill A. The environment and disease: association or causation? Proceedings of the Royal Society of Medicine. 1965;58:295–300. [PMC free article] [PubMed] [Google Scholar]
- 53.Ioannidis JP. Exposure-wide epidemiology: revisting Bradford Hill. Stat Med. 2016 May 20;35(11):1749–62. doi: 10.1002/sim.6825. [DOI] [PubMed] [Google Scholar]
- 54.Nallamothu BK, Hayward RA, Bates ER. Beyond the randomized clinical trial: the role of effectiveness studies in evaluating cardiovascular therapies. Circulation. 2008 Sep 16;118(12):1294–303. doi: 10.1161/CIRCULATIONAHA.107.703579. [DOI] [PubMed] [Google Scholar]
- 55.Althabe F, Belizán JM, McClure EM, et al. A population-based, multifaceted strategy to implement antenatal corticosteroid treatment versus standard care for the reduction of neonatal mortality due to preterm birth in low-income and middle-income countries: the ACT cluster-randomised trial. Lancet. 2015;385(9968):629–639. doi: 10.1016/S0140-6736(14)61651-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Roberts D, Dalziel S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2006 Jul 19;(3):CD004454. doi: 10.1002/14651858.CD004454.pub2. [DOI] [PubMed] [Google Scholar]
- 57.http://www.smfmnewsroom.org/2014/10/physicians-weigh-in-on-study-in-the-lancet-on-use-of-antenatal-corticosteroids-in-prevention-of-pre-term-birth/. Accessed January 8, 2016.
- 58.Mcclure E, Goldenberg R, Swanson D, et al. Routine antenatal ultrasound in low/middle income countries: a cluster randomized trial. Presented at the Society for Maternal-Fetal Medicine 37th Annual Meeting—The Pregnancy Meeting. 2017 Jan 23–28; Abstract No. 3. [Google Scholar]


