Abstract
We have comprehensively demonstrated using the mouse model that intranasal immunization with recombinant chlamydial protease-like activity factor (rCPAF) leads to a significant reduction in bacterial burden, genital tract pathology and preserves fertility following intravaginal genital chlamydial challenge. In the present report, we evaluated the protective efficacy of rCPAF immunization in guinea pigs, a second animal model for genital chlamydial infection. Using a vaccination strategy similar to the mouse model, we intranasally immunized female guinea pigs with rCPAF plus CpG deoxynucleotides (CpG; as an adjuvant), and challenged intravaginally with C. trachomatis serovar D (CT-D). Immunization with rCPAF/CpG significantly reduced vaginal CT-D shedding and induced resolution of infection by day 24, compared to day 33 in CpG alone treated and challenged animals. Immunization induced robust anti-rCPAF serum IgG 2 weeks following the last immunization, and was sustained at a high level 4 weeks post challenge. Upregulation of antigen specific IFN-γ gene expression was observed in rCPAF/CpG vaccinated splenocytes. Importantly, a significant reduction in inflammation in the genital tissue in rCPAF/CpG-immunized guinea pigs compared to CpG-immunized animals was observed. Taken together, this study provides evidence of the protective efficacy of rCPAF as a vaccine candidate in a second animal model of genital chlamydial infection.
Keywords: guinea pig, Chlamydia, intravaginal challenge, protective immunity, pathology
Introduction
Chlamydia trachomatis is the leading cause of bacterial sexually transmitted diseases (STD) worldwide1. When left untreated, it leads to chronic inflammatory conditions including pelvic inflammatory disorder, ectopic pregnancies and infertility1–3. Given the asymptomatic nature of infection in a high proportion of affected individuals, and the intracellular persistence of the organism, recurring infections and chronic disease lead to significant health care costs4–6. Although the infection can be treated with antibiotics7, preventive intervention by vaccination is considered the most effective measure to control chlamydial STD.
Currently, there is no licensed vaccine against Chlamydia spp.; however, our laboratory has extensively demonstrated that immunization with recombinant chlamydial protease-like activity factor (rCPAF) is highly effective in protection against subsequent challenge in the mouse model8–16. Specifically, intranasal (i.n.) vaccination with rCPAF, with murine recombinant IL-12 or CpG deoxynucleotides as adjuvant, protects against subsequent genital chlamydial challenge and reduces the incidence of hydrosalpinx development in the upper reproductive tract10, 11. Moreover, rCPAF vaccination preserved fertility in mice repeatedly challenged with Chlamydia15. Protection by rCPAF vaccination has been found to be mediated via IFN-γ secreting antigen specific CD4+ T cells and antibodies11, 12.
Although the mouse model is widely used for Chlamydia studies17–19; the availability of other models, such as guinea pigs, pigs, minipigs, sheep, cattle, and macaques20–23, to study chlamydial pathogenesis and vaccine strategies is highly beneficial. Guinea pigs serve as a translation model between mice and human Chlamydia studies as they have a reproductive physiology and estrous cycle (15 to 17 days) similar to that of humans20, 24. Additionally, certain guinea pig strains are outbred (as used in our study) and may add to understanding the genetic basis of differences in anti-chlamydial immunity in human cohorts. To date, guinea pigs have been used to evaluate the protective efficacy of recombinant major outer membrane protein (rMOMP) against genital chlamydial infection, where reduction in genital pathology following Chlamydia challenge is associated with systematic and mucosal antibody production25–27. Furthermore, our recent report on the regulation of guinea pig-specific genes in vaccinated animals using a 96-gene transcriptome array has advanced our understanding of guinea pig immune responses to vaccination and infection28. In that report, we found that intranasal inoculation/vaccination with Chlamydia caviae elementary bodies induced robust neutralizing antibodies and regulated Th1 and Th2 related cytokine/chemokine genes, including IFN-γ28 which has been shown to play a major role in the control of chlamydial infection in mice8, 29, 30.
While C. caviae is the naturally infecting strain in guinea pigs and has been extensively used to study chlamydial infection in the guinea pig model26, 28, de Jonge et al., has developed the human C. trachomatis serovars D and E infection model in guinea pigs and reported development of upper genital tract pathology comparable to human disease31. The rCPAF vaccine extensively characterized by our group in the mouse model is derived from the human C. trachomatis L2 serovar and shares a 99% and 82% identical amino acid sequence to the human CT-D serovar and mouse C. muridarum strain, respectively32. However, this rCPAF has only 54% identity to C. caviae CPAF protein, therefore, we utilized the newly developed guinea pig CT-D infection model31 to examine the efficacy of rCPAF as a vaccine in this second animal model.
Results
Resolution of genital C. trachomatis challenge in guinea pigs immunized with rCPAF
Using a vaccination regimen similar to the one used for mice studies10, 11, 16, we evaluated the protective efficacy of rCPAF in guinea pigs. In this study, we used CpG-10109 and a vaccination dosage based on other guinea pig reports25, 33, 34 and pilot studies in our laboratory. As shown in Fig. 1a, CpG (mock) immunized guinea pigs displayed higher levels of chlamydial shedding initially which then progressively decreased with complete clearing of the infection (no detectable bacterial shedding) by day 33. In contrast, guinea pigs i.n. inoculated with 105 IFUs live CT-D (serves as positive control for immunization) exhibited significant reduction of bacterial shedding on days 3 and 6 post-challenge with complete clearance on day 9. Immunization with rCPAF+CpG resulted in a significant reduction in chlamydial shedding compared to those receiving CpG alone as early as day 3 post-challenge. The bacterial shedding profile displayed a significant reduction in CT-D and rCPAF+CpG immunized guinea pigs when compared to CpG-mock immunized animals (Figure 1b). Also as shown in figure 1c, the area under the curve showed a significant reduction in bacterial burdens in CT-D and rCPAF+CpG immunized guinea pigs compared to CpG-mock immunized guinea pigs (Figure 1c). Furthermore, 40% of the rCPAF immunized guinea pigs resolved infection on day 21 and all animals cleared infection by day 24; whereas, all CpG-mock immunized animals still exhibited chlamydial shedding on day 27 and ultimately resolved the infection by day 33 (Fig. 1d).
Figure 1.
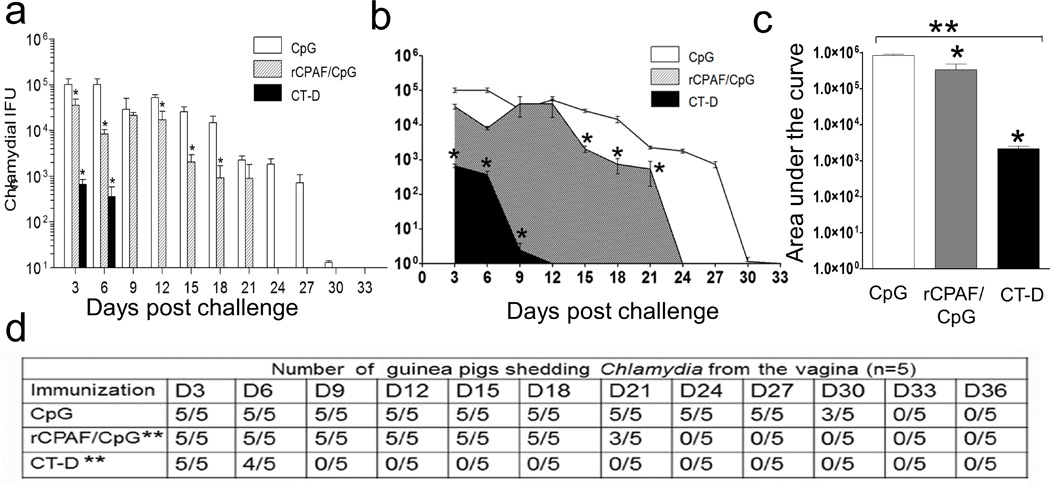
Vaccination enhanced chlamydial clearance from the guinea pigs genital tract. Groups (n =5) of guinea pigs were immunized i.n. with rCPAF/CpG or CpG alone (mock) and boosted twice at two-week intervals. Another group (n = 5) of guinea pigs received one i.n. dose of live C. trachomatis serovar D EBs (CT-D; 1×105 IFUs). One month after the final immunization, guinea pigs were challenged i.vag. with 105 IFU CT-D. Chlamydial shedding was monitored every third day post challenge until day 36 and presented as mean ± SD for each group at each time point. * Significant reductions (p < 0.05; one-way ANOVA) in bacterial shedding by (a) bar graph and (b) overall area and (c) area under the curve between the indicated group and CpG-immunized (mock) guinea pigs are shown. (d) The number of guinea pigs shedding Chlamydia after genital challenge for each immunization group is summarized. (c) ** Significant reduction (p=0.002), ANOVA with Tukey B) between groups; (d) ** Significant reduction (p=0.006 and p=0.0001, respectively, Fisher’s exact test) in number of rCPAF+CpG and CT-D immunized animals, compared to CpG immunized animals, shedding chlamydia across all time-points evaluated. Results are representative of two independent experiments.
rCPAF/CpG vaccination induced antibody production in guinea pigs
Using cyclophosphamide treatment to preferentially suppress humoral, but not cell-mediated, immunity, Rank et.al, have demonstrated that a humoral response is essential for guinea pigs to resolve primary35 and secondary36 C. caviae genital infections. To assess humoral responses induced by vaccination and challenge, we collected sera from immunized animals 2 weeks prior to challenge and 4 weeks post-challenge and measured antibody reactivity. Prior to bacterial challenge, CpG vaccinated guinea pigs produced minimal serum antibody against rCPAF and EBs (UV-inactivated CT-D) as shown in Fig. 2a. In contrast, rCPAF/CpG and CT-D vaccinated guinea pigs induced high levels of serum antibodies against rCPAF and CT-D, respectively. All animals, including CpG vaccinated guinea pigs, mounted anti- CT-D humoral responses to i.vag. CT-D challenge (Fig. 2b). Among the 3 study groups, CT-D i.n. vaccinated guinea pigs produced the highest level of anti-CT-D antibody pre and post challenge. Serum anti-CT-D antibody production also increased in CpG and rCPAF/CpG vaccinated animals after CT-D challenge (Fig. 2b).
Figure 2.
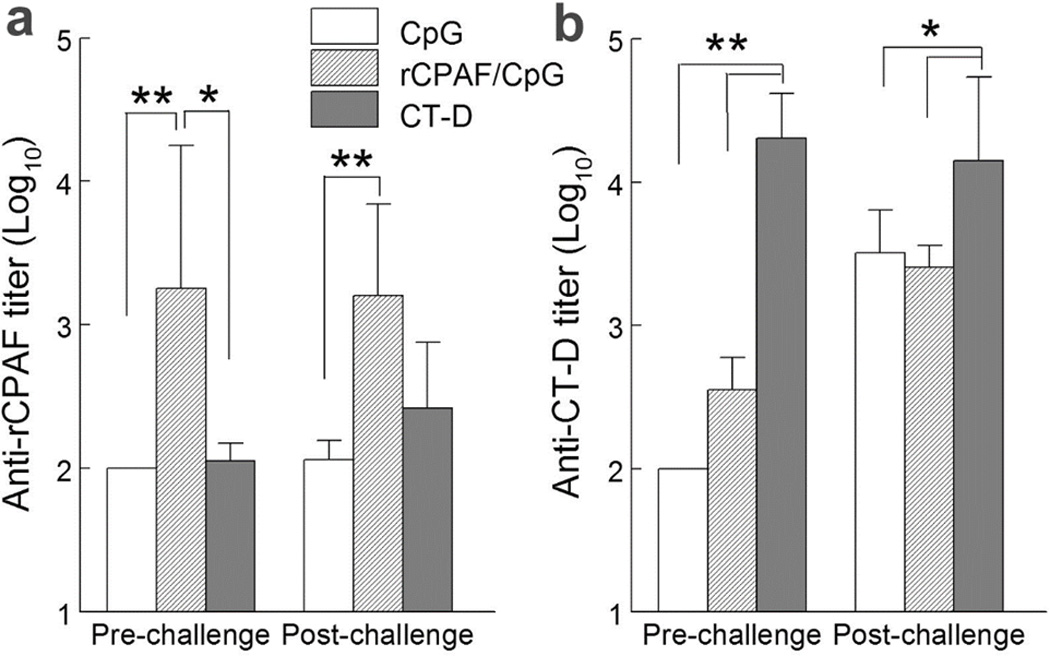
Vaccination induced antigen specific serum IgG responses in guinea pigs. Groups (n=5–6) of guinea pigs were immunized i.n. with CpG alone (mock), rCPAF/CpG or CT-D (1×105 IFUs). Guinea pigs were rested for 4 (CpG, rCPAF/CpG) or 8 (CT-D) weeks before challenging i.vag. with 1×105 IFU of CT-D. Total serum IgG reacting with rCPAF (a) and UV-inactivated CT-D (b) was determined 2 weeks prior to (pre-challenge) and 4 weeks after (post-challenge) challenge. * p < 0.05, ** p < 0.01 (One-Way ANOVA) comparison between indicated groups. Results are presented as average ± standard deviation of endpoint titer of each group. Results are representative of two independent experiments.
rCPAF/CpG vaccination induced cellular IFN-γ response in guinea pigs
We have previously demonstrated that immunization with rCPAF induced a robust cell-mediated immune response with IFN-γ production and led to protection against genital chlamydial disease in mice10, 11. To evaluate the cell-mediated response by vaccination in guinea pigs, we collected 3 spleens from each study group one day prior to challenge. Splenocytes were stimulated in vitro with antigens rCPAF and UV-inactivated CT-D (UV-CT-D) or unstimulated (with medium) for 24 hrs and the gene expression of IFN-γ was assessed by quantitative real time PCR. As shown in Fig 3, significantly higher transcript levels of IFN-γ were observed in splenocytes from the rCPAF/CpG group stimulated with rCPAF and in CT-D immunized animals stimulated with UV-CT-D compared to their respective medium stimulations. IFN-γ gene transcript levels corresponded to elevated IFN-γ secretion in supernatants of splenocytes from rCPAF/CpG or CT-D immunized guinea pigs stimulated with CPAF or CT-D compared to CpG vaccinated guinea pigs (Supplementary Figure 1). Additionally, TNF-α - a cytokine associated with its role in multifactorial Ag-specific T-cell mediated protection37–40, displayed increased/ higher transcript levels (although not statistically significant) in splenocytes from the rCPAF/CpG group stimulated with rCPAF and in CT-D immunized animals stimulated with UV-CT-D compared to their respective medium stimulations (Supplementary Figure 2). Taken together, these results indicate that both rCPAF/CpG and CT-D vaccination mounted modest increases (given the small numbers of animals/ group) in Ag-specific Th1 cytokines such as IFN-γ which contribute to the cell-mediated immune response.
Figure 3.
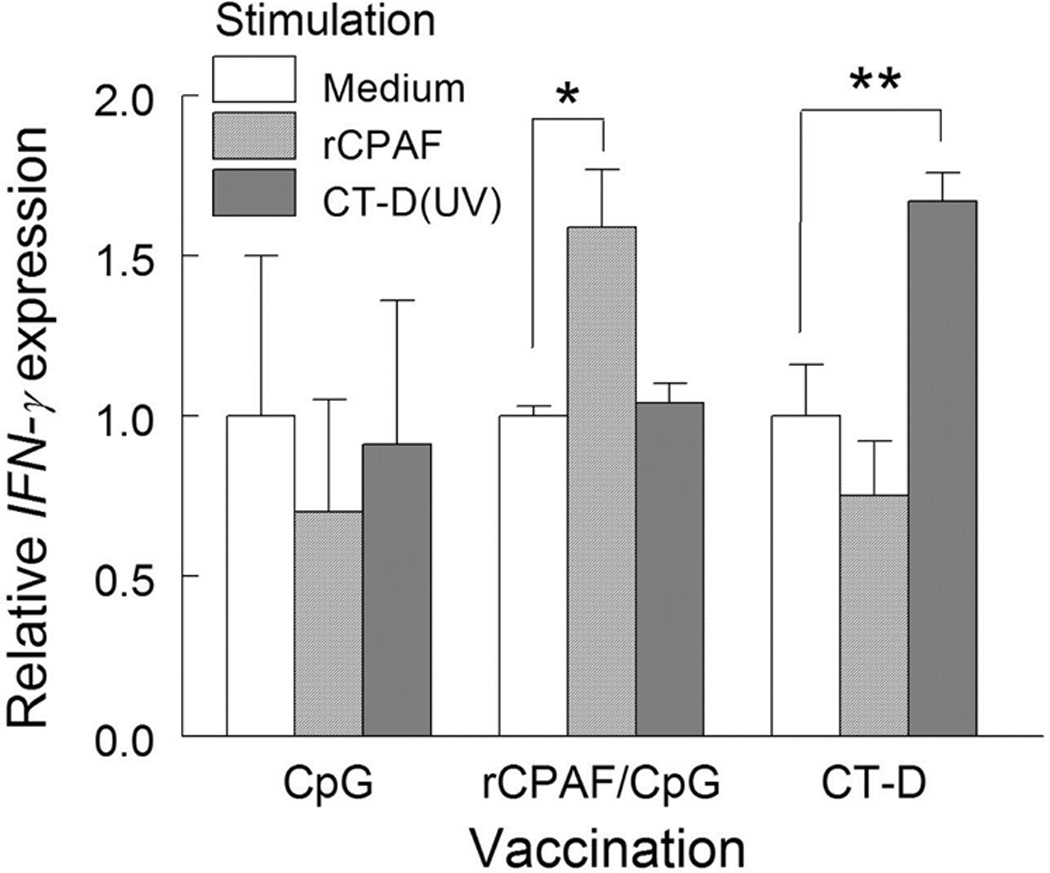
Vaccination induced antigen specific IFN-γ gene expression. Guinea pigs (n=3 per group) were euthanized one day before challenge and splenocytes were stimulated with 0.5µg of rCPAF or UV-inactivated CT-D (1×105 IFUs), or unstimulated (media alone), for 24 hrs. IFN-γ gene expression was then measured by qRT-PCR analysis and normalized to respective GAPDH expression for each sample. Subsequently, within each vaccination group, IFN-γ expression by rCPAF and CT-D (UV) stimulation was calculated and presented as a relative value to medium mock stimulation. Significant increase in IFN-γ expression between stimuli was indicated * p < 0.05, ** p < 0.01 (One-Way ANOVA). Results are representative of two independent experiments.
rCPAF/CpG vaccination reduced upper genital tract pathology
Chlamydial infections result in upper genital tract pathology and reproductive sequelae such as tubal damage and infertility10, 11. In order to evaluate the efficacy of vaccination against development of reproductive tract pathology, tissue sections were obtained from healthy and diseased guinea pigs (Figure 4a) at day 65 post challenge and the severity of pathology was scored using a comprehensive system reported previously28. Following CT-D challenge, CpG vaccinated guinea pigs developed moderate to severe congestion, hemorrhage, and edema accompanied by severe infiltration of inflammatory cells (lymphocytes and neutrophils) in uterine tissues (Fig. 4b). In contrast, the rCPAF/CpG vaccinated animals had moderate cellular inflammation and edema, and exhibited a reduction in congestion and hemorrhage. Minimal pathology was developed in CT-D vaccinated guinea pigs and was comparable to mock vaccinated healthy naive animals. The difference in pathological severity among CpG, rCPAF/CpG, and CT-D vaccinated guinea pigs (8.8, 4.8, and 2.4, respectively, sum of 4 evaluated parameters in Fig. 4b for each group) was evident and comparable to similarly vaccinated/challenged mice (5.6, 2.0, and 1.5, respectively, combination of mouse oviduct dilatation and cellular infiltration scores, in our previous report41).
Figure 4.
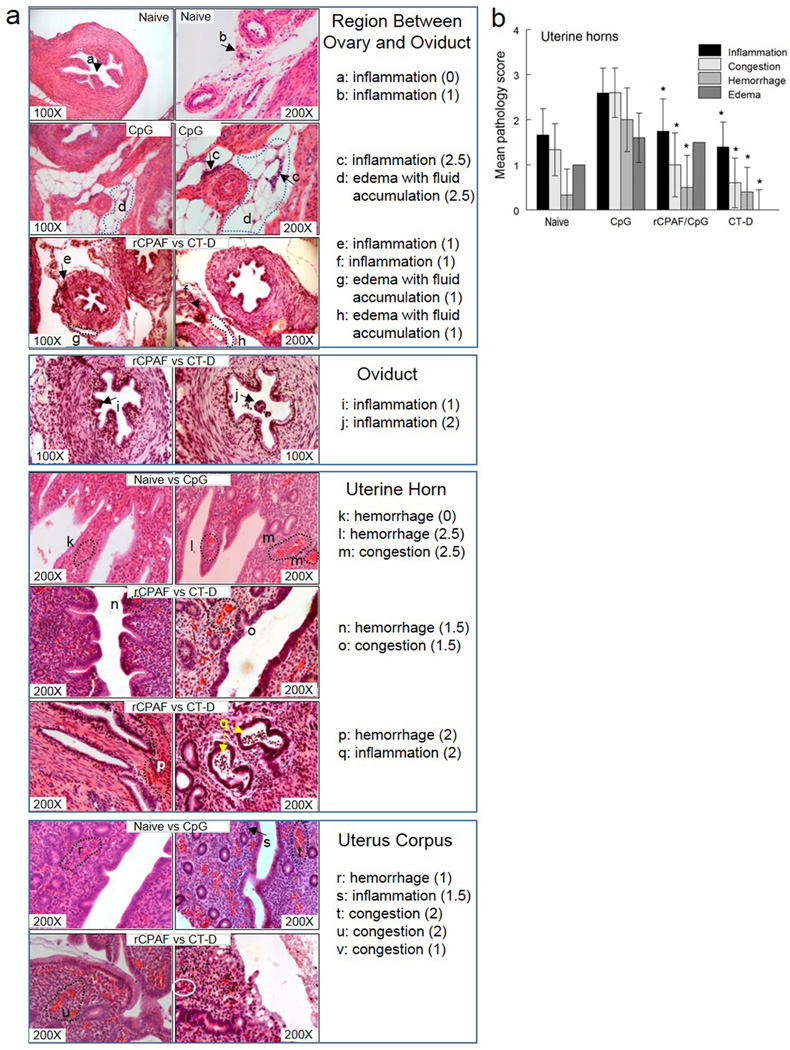
Vaccination reduced histopathological lesions in indicated genital tracts following chlamydial challenge. Histopathology was assessed in naïve (uninfected/ healthy) and Chlamydia challenged CpG-, rCPAF/CpG-, CT-D-vaccinated guinea pigs. The genital tract of each guinea pig was removed at day 65 post CT-D challenge, sectioned, H&E stained, and analyzed microscopically (original magnification of the images is 100× or 200×). Histopathological injury in the genital tract of representative healthy and diseased guinea pigs was scored (a) and graphically represented for the respective group as a whole (b), for four distinct parameters (inflammatory cell infiltration, congestion, hemorrhage and edema). Obviously, the individual micrographs shown (a) represent the types of pathology observed in different regions of the genital tract of healthy and diseased animals, whereas the graph (b) summarizes these types of observations for all sections of all regions of all animals examined. The asterisk indicates significant reductions (* p <0.05; Kruskal-Wallis test with Dunns post hoc test) between CT-D or rCPAF/CpG immunized groups in comparison to CpG group for the respective parameters.
Discussion
We have previously demonstrated that vaccination with rCPAF protects against genital chlamydial challenge by robust induction of cellular and humoral immune responses in the murine model10, 11, 15, 16. We now have demonstrated the protective efficacy of rCPAF in a second animal model, the guinea pig. Guinea pigs immunized with rCPAF/CpG exhibited lower levels of chlamydial shedding, a shortened duration of infection, and reduced upper genital tract pathology compared to CpG (mock) immunized animals. This protection was correlated with robust antibody production and cellular IFN-γ induction in an antigen specific manner. Also in line with our previous findings, we found a limited role for rCPAF specific antibodies in the CT-D immunization regimen42. Collectively, these results are consistent with the protective efficacy of rCPAF observed in the mouse model of chlamydial infection and further validate rCPAF role as a potential candidate to be used for the development of a licensed human Chlamydia vaccine.
In the current study, animals that received live i.n. C. trachomatis (CT-D) immunization exhibited rapid bacterial clearance and significant reduction in upper genital pathology following a secondary intravaginal chlamydial challenge. These results are consistent with those from the mouse model41, 43 and guinea pig vaccination studies25, 31 wherein, robust protection against genital chlamydial challenge is induced by live chlamydial EB immunization. Hydrosalpinx (fluid-filled oviduct dilatations) are a characteristic feature of pathological sequelae following chlamydial infections in their respective hosts. Hydrosalpinx was not a common feature of the guinea pig model; however, histopathology was observed in the uterine tissue sections of the previously estradiol-treated, CpG immunized and CT-D challenged animals. Estradiol, not progesterone, treatment prior to chlamydial challenge in guinea pigs has been shown to increase the intensity and duration of C. caviae infection44 and is required to establish a sustained CT-D infection31. Although genital tract pathology induced by estradiol injection has been documented in guinea pigs45, the estradiol treatment following our study regimen (two 5 mg injections 1-week apart) in the absence of chlamydial infection did not cause visible inflammation 68 days after the 2nd injection (data not shown) suggesting that hormonal treatment alone contributed minimally to upper genital pathology in our model. Furthermore, similar to other animal models20, CT-D challenge in guinea pigs resulted in milder genital tract pathology and uterine inflammation than that seen with the natural pathogen C. caviae, as demonstrated by our group and others28, 46. In this context, Chlamydiae have been shown to exhibit host tropism due to their adaptation to the restrictive influence of IFN-γ in respective hosts47.
However, despite the reduced severity of pathological outcomes following CT-D infection in the guinea pig, this model produces sufficient bacterial shedding and pathology to evaluate the effects of experimental vaccines. To this end, studies by us and others using live or UV-EBs for vaccination have demonstrated significantly accelerated chlamydial clearance within 7 days post challenge41, 48 compared to single antigens including rCPAF/CpG and MOMP which result in protection 7–10 days later49. A correlation between early protection with neutralizing antibody induced by the live-EB or UV-EB regimen may occur, whereas given that CPAF is a RB-specific protein, anti-CPAF antibodies do not neutralize chlamydial infectivity, as demonstrated using B-cell deficient mice4213. We have previously characterized the efficacy of rCPAF cloned from C. trachomatis genome49 and further evaluated the effectiveness of this vaccine candidate (with only 54% amino acid identity to C. caviae) against a CT-D challenge in this study.
Consistent with our previous findings in mouse studies, vaccination with rCPAF (derived from CT) in guinea pigs resulted in reduced chlamydial shedding and genital tract pathology, correlating with CPAF-specific immune responses. CPAF is a dominant antigen expressed in CT positive individuals50, 51. We have previously demonstrated that rCPAF immunization protects against a subsequent genital challenge in humanized HLA-DR4 transgenic mice9, and that it provides cross-serovar protection49. The vaccination regimen conferred by rCPAF plus adjuvants (CpG or IL-12) exhibited greater protection that that observed by rCPAF vaccination alone13.
Taken together, previous reports and this current study extend the importance of rCPAF as a vaccine candidate and highlight its use as a protective molecule in a second animal model against a human serovar of CT. Despite new and evolving information on cellular targets of CPAF52, 53, its role as a putative anti-Chlamydia vaccine candidate holds continued promise13, 54, 55. With rapid progression in genetic manipulation of Chlamydia56, there also is enthusiasm for the development of a safe and more effective live attenuated vaccine. Additionally, recent studies have highlighted anti-chlamydial immune responses via protective tissue resident memory T cell subsets in vaccinated mice48, and B cells enhancing Ag-specific CD4+ T cell priming upon infection56, 57.
In the context of a future Chlamydia vaccine and a possible role for CPAF, there is no consensus as to whether the desired outcome is a reduction in infectivity/transmission or a reduction in the clinically relevant chronic pathologies. Along with the reduction in bacterial shedding (around 2nd week), we have previously demonstrated superior protection against upper reproductive pathologies with the rCPAF regimen11, including protection against infertility induced following repeated chlamydial challenge15. Our findings, with regard to efficacy of rCPAF immunization, are in accordance with other reports58, 59. Additionally, the concept that a reduction in shedding does not always correlate to protection against pathology is supported by O’Meara et al60.
In summary, the guinea pig CT-D challenge model may be a useful for the evaluation of new and previously identified vaccine candidates. Vaccination with single protein antigens (such as CPAF and MOMP) may not be sufficient to generate desired protective efficacy in humans1, 4. To this end, effective vaccination strategies using multiple antigens, formulations and routes of delivery are critical61. Importantly, rCPAF with its ability to preserve fertility in mice15, and reduce infection severity in guinea pigs has the potential to serve as an ideal antigen to be formulated into multivalent vaccines or be overexpressed in an attenuated live vaccine platform to prophylactically control human Chlamydia infection.
Methods
Bacteria
Chlamydia trachomatis serovar D (from the Zhong Lab, UT Health Sciences Center, San Antonio, TX) was grown on confluent HeLa cell monolayers. Infected HeLa cells were mechanically dislodged using glass beads. The disrupted cells were then vortexed with glass beads in a falcon tube for 5 min with 30 seconds intervals on ice followed by centrifugation for 10 min at 1200 rpm 4°C. The supernatant was collected and spun at of 27000 × g for 1 hr at 4°C to obtain a bacterial pellet that was further purified on Renografin gradient as described earlier28. Purified elementary bodies were aliquoted and stored at −80°C in sucrose-phosphate-glutamate (SPG) buffer until use.
Guinea pigs
Dunkin Hartley strain guinea pigs (350g–450g) were purchased from Charles River Laboratories (Massachusetts, USA) and were housed in the AAALAC-accredited University of Texas at San Antonio Vivarium. Food and water were supplied ad libitum and all experimental studies were completed humanely and followed the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol (IS0146) was approved for conducting this study by the Institutional Animal Care and Use Committee (IACUC) of the University of Texas at San Antonio.
Immunization and challenge
Groups of guinea pigs (n=5) were immunized i.n. on day 0 with 150µg rCPAF (derived from human C. trachomatis serovar L2 with a 99% homology to serovar D32 and expressed as fusion proteins in Escherichia coli as described previously9) and 25µg of CpG nucleotides (CpG-1010925, 62), or CpG alone (mock), in 100µl of sterile phosphate buffered saline (PBS). The dose of rCPAF was based on other reported protein vaccination studies using the guinea pig infection model25, 33, 34. Booster i.n. immunizations were provided on days 14 and 28 with 100µg rCPAF and 25µg of CpG. One month following the last booster, guinea pigs were challenged i.vag. with 1×105 IFU of CT-D resuspended in 50µl SPG buffer. Another group of guinea pigs was immunized i.n. with 1×105 IFUs of live CT-D once and rested for 8 weeks before challenge. To achieve a sustained CT-D infection in guinea pigs, all animals received a subcutaneous injection of 5mg β-estradiol (Sigma) in 100µl sesame oil (Sigma) on days −10 and −3 prior to challenge as described by de Jonge et.al.31. All guinea pigs were anesthetized with 3% isoflurane before immunization and challenge procedures. Following challenge, vaginal swabs were collected at a 3-day interval for 36 days from all groups of guinea pigs and plated onto HeLa cell monolayers to determine the chlamydial burden as described previously28.
Assay of humoral immune responses
Guinea pigs were bled from the lateral saphenous leg vein 15 days after the last booster (2 weeks prior to challenge) and 4 weeks post-challenge as described63. To measure antibody reactivity, microtiter plates were coated with 1×105 IFUs of UV-inactivated CT-D or 0.5µg rCPAF and incubated at 4°C overnight. ELISA was performed using serial 2-fold diluted sera (starting with1:100) as described28 to assess antibody reactivity. Following serial dilution of serum samples, plates were incubated for 2 h, followed by incubation with goat anti-guinea pig total IgG conjugated to horseradish peroxidase (HRP) (ABD Serotec). Tetramethylbenzidine substrate was added and the absorbance quantified at 630 nm using a µQuant ELISA plate reader (BioTek Instruments, Winooski, VT). Endpoint titers of each serum for specific antigen(s) was determined by selecting the highest dilution factor at which the sample O.D. was greater than the average O.D. of 6 CpG mock immunized pre-challenge sera, plus 2.2 standard deviations (to achieve 95% confidence as suggested by Frey et.al.64), and O.D. value greater than 0.1.
Cytokine PCR and ELISA
Groups of guinea pigs (n=3) were immunized i.n. with rCPAF/CpG or live CT-D as described above and euthanized one day prior to challenge to collect spleens aseptically for antigen-specific splenocyte stimulation assay. Single splenocyte cell suspensions were prepared (1×106 cells/well in 100µl DMEM plus 10% FBS) and stimulated with 0.5µg rCPAF, UV-inactivated CT-D (1×105 IFUs) or left unstimulated with media alone in a 96 well microtiter plate for 24 hrs. Following stimulation, cells were collected to assess IFN-γ gene expression. Messenger RNA was isolated from stimulated splenocytes as per manufacturer’s instructions (RNeasy mini kit, Qiagen) and quantified using a Nanodrop spectrophotometer (Thermo Scientific NanoDropTM 1000). RNA (0.5µg) was used to generate cDNA using the Verso cDNA Synthesis (Thermo Scientific). The guinea pig IFN-γ (Forward 5’-CCATCAAGGAACAAATTATTAC and Reverse 5’-TGACCGAAATTTGAATCAG), TNF-α (Forward 5’- GGAAGAGCAGTTCTCCAG and Reverse 5’-GCTTGTCATTATCGTTTTGAG), and GAPDH (Forward 5’-CTCGTCATCAATGGAAAG, Reverse 5’- GTGGATTCCACTACATAC) gene specific primers were used to amplify gene transcripts from the synthesized cDNA. qRT-PCR was conducted under a previously optimized condition using the CFX96 Touch™ Real-Time PCR Detection System (Bio-rad)28 and IFN-γ and TNF-α mRNA levels were normalized to GAPDH mRNA and expressed as relative level to medium mock-stimulated samples using the comparative cycle threshold method65. Supernatants from each condition were collected and stored at −80°C until further use. Guinea pig IFN-γ was assessed by a quantitative competitive immunoassay (NeoScientific) according to manufacturers’ instructions. In brief, 100ul of experimental supernatants or standards were co-incubated in wells with an IFN-γ conjugate. Binding of IFN-γ HRP was visualized by production of colorimetric reaction products that were quantitatively measured by absorbance using a µQuant ELISA plate reader (BioTek Instruments, Winooski, VT).
Genital tract pathology
Guinea pigs were euthanized and genital tract tissues were collected in 10% formalin, embedded in paraffin, sectioned, and stained with hematoxylin and eosin (H&E). Histopathology imaging and pathological scoring were performed using a Carl Zeiss microscope as described previously28. Pathology scores for each group was represented as mean and SD of each guinea pig in the respective group.
Animals and Statistical analyses
For experiments, female guinea pigs were age- matched and numbers/ group was selected based on previous findings11, 28. All result datasets from experiments were included for analyses and were not excluded from the study. No randomization or blinding was done. GraphPad Prism 5 (La Jolla, CA) was used to perform all statistical tests. P < 0.05 was considered statistically significant. One way ANOVA or Kruskal-Wallis test with Dunns post hoc test was used for determining the protective efficacy of rCPAF-vaccination compared to other treatment groups. Appropriate statistical tests are indicated in legends of respective figures. All data are representative of two independent experiments and each experiment was analyzed independently.
Supplementary Material
Acknowledgments
We thank Dr. Roger Rank (Arkansas Children's Hospital Research Institute) for his support in establishing the guinea pig model and insightful discussions. This work was supported by National Institutes of Health Grant (1RO3AI092621-01) and the Center for Excellence in Infection Genomics (CEIG) training grant (DOD #W911NF-11-1-0136). Partial support of this study was from the Jane and Roland Blumberg Professorship in Biology for Dr. Arulanandam.
Footnotes
Conflict of Interest: The authors have no conflicts of interest to declare in regards to this work.
References
- 1.Brunham RC, Rey-Ladino J. Immunology of Chlamydia infection: implications for a Chlamydia trachomatis vaccine. Nature reviews. Immunology. 2005;5(2):149–161. doi: 10.1038/nri1551. [DOI] [PubMed] [Google Scholar]
- 2.Hafner LM. Pathogenesis of fallopian tube damage caused by Chlamydia trachomatis infections. Contraception. 2015;92(2):108–115. doi: 10.1016/j.contraception.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 3.Menon S, Timms P, Allan JA, Alexander K, Rombauts L, Horner P, et al. Human and Pathogen Factors Associated with Chlamydia trachomatis-Related Infertility in Women. Clinical microbiology reviews. 2015;28(4):969–985. doi: 10.1128/CMR.00035-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brunham RC, Rappuoli R. Chlamydia trachomatis control requires a vaccine. Vaccine. 2013;31(15):1892–1897. doi: 10.1016/j.vaccine.2013.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mitchell C, Prabhu M. Pelvic inflammatory disease: current concepts in pathogenesis, diagnosis and treatment. Infectious disease clinics of North America. 2013;27(4):793–809. doi: 10.1016/j.idc.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schoborg RV. Chlamydia persistence - a tool to dissect Chlamydia-host interactions. Microbes and infection / Institut Pasteur. 2011;13(7):649–662. doi: 10.1016/j.micinf.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Punzio C, Neri E, Metelli P, Bianchi MS, Campa M, Fioretti P. Epidemiology and therapy of Chlamydia trachomatis genital infection in women. Journal of chemotherapy. 1992;4(3):163–166. doi: 10.1080/1120009x.1992.11739157. [DOI] [PubMed] [Google Scholar]
- 8.Murphey C, Murthy AK, Meier PA, Neal Guentzel M, Zhong G, Arulanandam BP. The protective efficacy of chlamydial protease-like activity factor vaccination is dependent upon CD4+ T cells. Cellular Immunology. 2006;242(2):110–117. doi: 10.1016/j.cellimm.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murthy AK, Cong Y, Murphey C, Guentzel MN, Forsthuber TG, Zhong G, et al. Chlamydial protease-like activity factor induces protective immunity against genital chlamydial infection in transgenic mice that express the human HLA-DR4 allele. Infection and Immunity. 2006;74(12):6722–6729. doi: 10.1128/IAI.01119-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cong Y, Jupelli M, Guentzel MN, Zhong G, Murthy AK, Arulanandam BP. Intranasal immunization with chlamydial protease-like activity factor and CpG deoxynucleotides enhances protective immunity against genital Chlamydia muridarum infection. Vaccine. 2007;25(19):3773–3780. doi: 10.1016/j.vaccine.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murthy AK, Chambers JP, Meier PA, Zhong G, Arulanandam BP. Intranasal vaccination with a secreted chlamydial protein enhances resolution of genital Chlamydia muridarum infection, protects against oviduct pathology, and is highly dependent upon endogenous gamma interferon production. Infection and immunity. 2007;75(2):666–676. doi: 10.1128/IAI.01280-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li W, Murthy AK, Guentzel MN, Seshu J, Forsthuber TG, Zhong G, et al. Antigen-specific CD4+ T cells produce sufficient IFN-gamma to mediate robust protective immunity against genital Chlamydia muridarum infection. J Immunol. 2008;180(5):3375–3382. doi: 10.4049/jimmunol.180.5.3375. [DOI] [PubMed] [Google Scholar]
- 13.Murthy AK, Guentzel MN, Zhong G, Arulanandam BP. Chlamydial protease-like activity factor-insights into immunity and vaccine development. Journal of reproductive immunology. 2009;83(1–2):179–184. doi: 10.1016/j.jri.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mascellino MT, Boccia P, Oliva A. Immunopathogenesis in Chlamydia trachomatis infected women. ISRN obstetrics and gynecology. 2011;2011:436936. doi: 10.5402/2011/436936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murthy AK, Li W, Guentzel MN, Zhong G, Arulanandam BP. Vaccination with the defined chlamydial secreted protein CPAF induces robust protection against female infertility following repeated genital chlamydial challenge. Vaccine. 2011;29(14):2519–2522. doi: 10.1016/j.vaccine.2011.01.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li W, Murthy AK, Lanka GK, Chetty SL, Yu JJ, Chambers JP, et al. A T cell epitope-based vaccine protects against chlamydial infection in HLA-DR4 transgenic mice. Vaccine. 2013;31(48):5722–5728. doi: 10.1016/j.vaccine.2013.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karunakaran KP, Yu H, Jiang X, Chan Q, Moon KM, Foster LJ, et al. Outer membrane proteins preferentially load MHC class II peptides: Implications for a Chlamydia trachomatis T cell vaccine. Vaccine. 2015;33(18):2159–2166. doi: 10.1016/j.vaccine.2015.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O'Meara CP, Andrew DW, Beagley KW. The mouse model of Chlamydia genital tract infection: A review of infection, disease, immunity and vaccine development. Current molecular medicine. 2013 doi: 10.2174/15665240113136660078. [DOI] [PubMed] [Google Scholar]
- 19.Pan Q, Pais R, Ohandjo A, He C, He Q, Omosun Y, et al. Comparative evaluation of the protective efficacy of two formulations of a recombinant Chlamydia abortus subunit candidate vaccine in a mouse model. Vaccine. 2015;33(15):1865–1872. doi: 10.1016/j.vaccine.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Clercq E, Kalmar I, Vanrompay D. Animal models for studying female genital tract infection with Chlamydia trachomatis. Infection and immunity. 2013;81(9):3060–3067. doi: 10.1128/IAI.00357-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Entrican G, Wheelhouse N, Wattegedera SR, Longbottom D. New challenges for vaccination to prevent chlamydial abortion in sheep. Comparative immunology, microbiology and infectious diseases. 2012;35(3):271–276. doi: 10.1016/j.cimid.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 22.Boje S, Olsen AW, Erneholm K, Agerholm JS, Jungersen G, Andersen P, et al. A multi-subunit Chlamydia vaccine inducing neutralizing antibodies and strong IFN-gamma CMI responses protects against a genital infection in minipigs. Immunology and cell biology. 2015 doi: 10.1038/icb.2015.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walker E, Lee EJ, Timms P, Polkinghorne A. Chlamydia pecorum infections in sheep and cattle: A common and under-recognised infectious disease with significant impact on animal health. Veterinary journal. 2015 doi: 10.1016/j.tvjl.2015.09.022. [DOI] [PubMed] [Google Scholar]
- 24.Rank RG. Animal models for urogenital infections. Methods in enzymology. 1994;235:83–93. doi: 10.1016/0076-6879(94)35133-3. [DOI] [PubMed] [Google Scholar]
- 25.Andrew DW, Hafner LM, Beagley KW, Timms P. Partial protection against chlamydial reproductive tract infection by a recombinant major outer membrane protein/CpG/cholera toxin intranasal vaccine in the guinea pig Chlamydia caviae model. Journal of reproductive immunology. 2011;91(1–2):9–16. doi: 10.1016/j.jri.2011.06.100. [DOI] [PubMed] [Google Scholar]
- 26.Batteiger BE, Rank RG, Bavoil PM, Soderberg LS. Partial protection against genital reinfection by immunization of guinea-pigs with isolated outer-membrane proteins of the chlamydial agent of guinea-pig inclusion conjunctivitis. Journal of general microbiology. 1993;139(12):2965–2972. doi: 10.1099/00221287-139-12-2965. [DOI] [PubMed] [Google Scholar]
- 27.Volp K, Mathews S, Timms P, Hafner L. Peptide immunization of guinea pigs against Chlamydia psittaci (GPIC agent) infection induces good vaginal secretion antibody response, in vitro neutralization and partial protection against live challenge. Immunology and cell biology. 2001;79(3):245–250. doi: 10.1046/j.1440-1711.2001.01005.x. [DOI] [PubMed] [Google Scholar]
- 28.Wali S, Gupta R, Veselenak RL, Li Y, Yu JJ, Murthy AK, et al. Use of a guinea pig-specific transcriptome array for evaluation of protective immunity against genital chlamydial infection following intranasal vaccination in guinea pigs. PloS one. 2014;9(12):e114261. doi: 10.1371/journal.pone.0114261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cotter TW, Ramsey KH, Miranpuri GS, Poulsen CE, Byrne GI. Dissemination of Chlamydia trachomatis chronic genital tract infection in gamma interferon gene knockout mice. Infection and immunity. 1997;65(6):2145–2152. doi: 10.1128/iai.65.6.2145-2152.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johansson M, Schon K, Ward M, Lycke N. Genital tract infection with Chlamydia trachomatis fails to induce protective immunity in gamma interferon receptor-deficient mice despite a strong local immunoglobulin A response. Infection and immunity. 1997;65(3):1032–1044. doi: 10.1128/iai.65.3.1032-1044.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Jonge MI, Keizer SA, El Moussaoui HM, van Dorsten L, Azzawi R, van Zuilekom HI, et al. A novel guinea pig model of Chlamydia trachomatis genital tract infection. Vaccine. 2011;29(35):5994–6001. doi: 10.1016/j.vaccine.2011.06.037. [DOI] [PubMed] [Google Scholar]
- 32.Dong F, Zhong Y, Arulanandam B, Zhong G. Production of a proteolytically active protein, chlamydial protease/proteasome-like activity factor, by five different Chlamydia species. Infection and immunity. 2005;73(3):1868–1872. doi: 10.1128/IAI.73.3.1868-1872.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jacobs E, Drews M, Stuhlert A, Buttner C, Klein PJ, Kist M, et al. Immunological reaction of guinea-pigs following intranasal Mycoplasma pneumoniae infection and immunization with the 168 kDa adherence protein. Journal of general microbiology. 1988;134(2):473–479. doi: 10.1099/00221287-134-2-473. [DOI] [PubMed] [Google Scholar]
- 34.Hogarth PJ, Jahans KJ, Hecker R, Hewinson RG, Chambers MA. Evaluation of adjuvants for protein vaccines against tuberculosis in guinea pigs. Vaccine. 2003;21(9–10):977–982. doi: 10.1016/s0264-410x(02)00548-0. [DOI] [PubMed] [Google Scholar]
- 35.Rank RG, White HJ, Barron AL. Humoral immunity in the resolution of genital infection in female guinea pigs infected with the agent of guinea pig inclusion conjunctivitis. Infection and immunity. 1979;26(2):573–579. doi: 10.1128/iai.26.2.573-579.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rank RG, Barron AL. Humoral immune response in acquired immunity to chlamydial genital infection of female guinea pigs. Infection and immunity. 1983;39(1):463–465. doi: 10.1128/iai.39.1.463-465.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu H, Jiang X, Shen C, Karunakaran KP, Jiang J, Rosin NL, et al. Chlamydia muridarum T-cell antigens formulated with the adjuvant DDA/TDB induce immunity against infection that correlates with a high frequency of gamma interferon (IFN-gamma)/tumor necrosis factor alpha and IFN-gamma/interleukin-17 double-positive CD4+ T cells. Infection and immunity. 2010;78(5):2272–2282. doi: 10.1128/IAI.01374-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu H, Karunakaran KP, Jiang X, Shen C, Andersen P, Brunham RC. Chlamydia muridarum T cell antigens and adjuvants that induce protective immunity in mice. Infection and immunity. 2012;80(4):1510–1518. doi: 10.1128/IAI.06338-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaser T, Pasternak JA, Delgado-Ortega M, Hamonic G, Lai K, Erickson J, et al. Chlamydia suis and Chlamydia trachomatis induce multifunctional CD4 T cells in pigs. Vaccine. 2016 doi: 10.1016/j.vaccine.2016.11.050. [DOI] [PubMed] [Google Scholar]
- 40.O'Meara CP, Armitage CW, Kollipara A, Andrew DW, Trim L, Plenderleith MB, et al. Induction of partial immunity in both males and females is sufficient to protect females against sexual transmission of Chlamydia. Mucosal Immunology. 2016;9(4):1076–1088. doi: 10.1038/mi.2015.125. [DOI] [PubMed] [Google Scholar]
- 41.Li W, Murthy AK, Guentzel MN, Chambers JP, Forsthuber TG, Seshu J, et al. Immunization with a combination of integral chlamydial antigens and a defined secreted protein induces robust immunity against genital chlamydial challenge. Infection and Immunity. 2010;78(9):3942–3949. doi: 10.1128/IAI.00346-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murthy AK, Chaganty BK, Li W, Guentzel MN, Chambers JP, Seshu J, et al. A limited role for antibody in protective immunity induced by rCPAF and CpG vaccination against primary genital Chlamydia muridarum challenge. FEMS immunology and medical microbiology. 2009;55(2):271–279. doi: 10.1111/j.1574-695X.2008.00517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tifrea DF, Pal S, Popot JL, Cocco MJ, de la Maza LM. Increased immunoaccessibility of MOMP epitopes in a vaccine formulated with amphipols may account for the very robust protection elicited against a vaginal challenge with Chlamydia muridarum. J Immunol. 2014;192(11):5201–5213. doi: 10.4049/jimmunol.1303392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rank RG, White HJ, Hough AJ, Jr, Pasley JN, Barron AL. Effect of estradiol on chlamydial genital infection of female guinea pigs. Infection and immunity. 1982;38(2):699–705. doi: 10.1128/iai.38.2.699-705.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Silva EG, Tornos C, Deavers M, Kaisman K, Gray K, Gershenson D. Induction of epithelial neoplasms in the ovaries of guinea pigs by estrogenic stimulation. Gynecologic oncology. 1998;71(2):240–246. doi: 10.1006/gyno.1998.5153. [DOI] [PubMed] [Google Scholar]
- 46.Rank RG, Sanders MM, Patton DL. Increased incidence of oviduct pathology in the guinea pig after repeat vaginal inoculation with the chlamydial agent of guinea pig inclusion conjunctivitis. Sexually transmitted diseases. 1995;22(1):48–54. doi: 10.1097/00007435-199501000-00008. [DOI] [PubMed] [Google Scholar]
- 47.Nelson DE, Virok DP, Wood H, Roshick C, Johnson RM, Whitmire WM, et al. Chlamydial IFN-gamma immune evasion is linked to host infection tropism. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(30):10658–10663. doi: 10.1073/pnas.0504198102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stary G, Olive A, Radovic-Moreno AF, Gondek D, Alvarez D, Basto PA, et al. VACCINES. A mucosal vaccine against Chlamydia trachomatis generates two waves of protective memory T cells. Science. 2015;348(6241):aaa8205. doi: 10.1126/science.aaa8205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li W, Guentzel MN, Seshu J, Zhong G, Murthy AK, Arulanandam BP. Induction of cross-serovar protection against genital chlamydial infection by a targeted multisubunit vaccination approach. Clinical and vaccine immunology : CVI. 2007;14(12):1537–1544. doi: 10.1128/CVI.00274-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sharma J, Bosnic AM, Piper JM, Zhong G. Human antibody responses to a Chlamydia -secreted protease factor. Infection and immunity. 2004;72(12):7164–7171. doi: 10.1128/IAI.72.12.7164-7171.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sharma J, Zhong Y, Dong F, Piper JM, Wang G, Zhong G. Profiling of human antibody responses to Chlamydia trachomatis urogenital tract infection using microplates arrayed with 156 chlamydial fusion proteins. Infection and immunity. 2006;74(3):1490–1499. doi: 10.1128/IAI.74.3.1490-1499.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bavoil PM, Byrne GI. Analysis of CPAF mutants: new functions, new questions (The ins and outs of a chlamydial protease) Pathogens and disease. 2014 doi: 10.1111/2049-632X.12194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.T AC, Yang Z, Ojcius D, Zhong G. A path forward for the chlamydial virulence factor CPAF. Microbes and infection / Institut Pasteur. 2013;15(14–15):1026–1032. doi: 10.1016/j.micinf.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ralli-Jain P, Tifrea D, Cheng C, Pal S, de la Maza LM. Enhancement of the protective efficacy of a Chlamydia trachomatis recombinant vaccine by combining systemic and mucosal routes for immunization. Vaccine. 2010;28(48):7659–7666. doi: 10.1016/j.vaccine.2010.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Farris CM, Morrison RP. Vaccination against Chlamydia genital infection utilizing the murine C. muridarum model. Infection and immunity. 2011;79(3):986–996. doi: 10.1128/IAI.00881-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang Y, Kahane S, Cutcliffe LT, Skilton RJ, Lambden PR, Persson K, et al. Genetic transformation of a clinical (genital tract), plasmid-free isolate of Chlamydia trachomatis: engineering the plasmid as a cloning vector. PloS one. 2013;8(3):e59195. doi: 10.1371/journal.pone.0059195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li LX, McSorley SJ. A re-evaluation of the role of B cells in protective immunity to Chlamydia infection. Immunology letters. 2015;164(2):88–93. doi: 10.1016/j.imlet.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Coler RN, Bhatia A, Maisonneuve JF, Probst P, Barth B, Ovendale P, et al. Identification and characterization of novel recombinant vaccine antigens for immunization against genital Chlamydia trachomatis. FEMS immunology and medical microbiology. 2009;55(2):258–270. doi: 10.1111/j.1574-695X.2008.00527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brown TH, David J, Acosta-Ramirez E, Moore JM, Lee S, Zhong G, et al. Comparison of immune responses and protective efficacy of intranasal prime-boost immunization regimens using adenovirus-based and CpG/HH2 adjuvanted-subunit vaccines against genital Chlamydia muridarum infection. Vaccine. 2012;30(2):350–360. doi: 10.1016/j.vaccine.2011.10.086. [DOI] [PubMed] [Google Scholar]
- 60.O'Meara CP, Armitage CW, Harvie MC, Andrew DW, Timms P, Lycke NY, et al. Immunity against a Chlamydia infection and disease may be determined by a balance of IL-17 signaling. Immunology and cell biology. 2014;92(3):287–297. doi: 10.1038/icb.2013.92. [DOI] [PubMed] [Google Scholar]
- 61.Igietseme JU, Eko FO, Black CM. Chlamydia vaccines: recent developments and the role of adjuvants in future formulations. Expert review of vaccines. 2011;10(11):1585–1596. doi: 10.1586/erv.11.139. [DOI] [PubMed] [Google Scholar]
- 62.Gu M, Hine PM, James Jackson W, Giri L, Nabors GS. Increased potency of BioThrax anthrax vaccine with the addition of the C-class CpG oligonucleotide adjuvant CPG 10109. Vaccine. 2007;25(3):526–534. doi: 10.1016/j.vaccine.2006.07.056. [DOI] [PubMed] [Google Scholar]
- 63.Vanrompay D, Lyons JM, Morre SA. Animal models for the study of Chlamydia trachomatis infections in the female genital infection. Drugs of today. 2006;42(Suppl A):55–63. [PubMed] [Google Scholar]
- 64.Frey A, Di Canzio J, Zurakowski D. A statistically defined endpoint titer determination method for immunoassays. Journal of immunological methods. 1998;221(1–2):35–41. doi: 10.1016/s0022-1759(98)00170-7. [DOI] [PubMed] [Google Scholar]
- 65.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


