Abstract
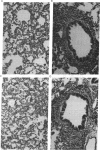
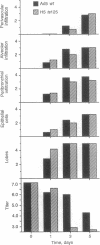
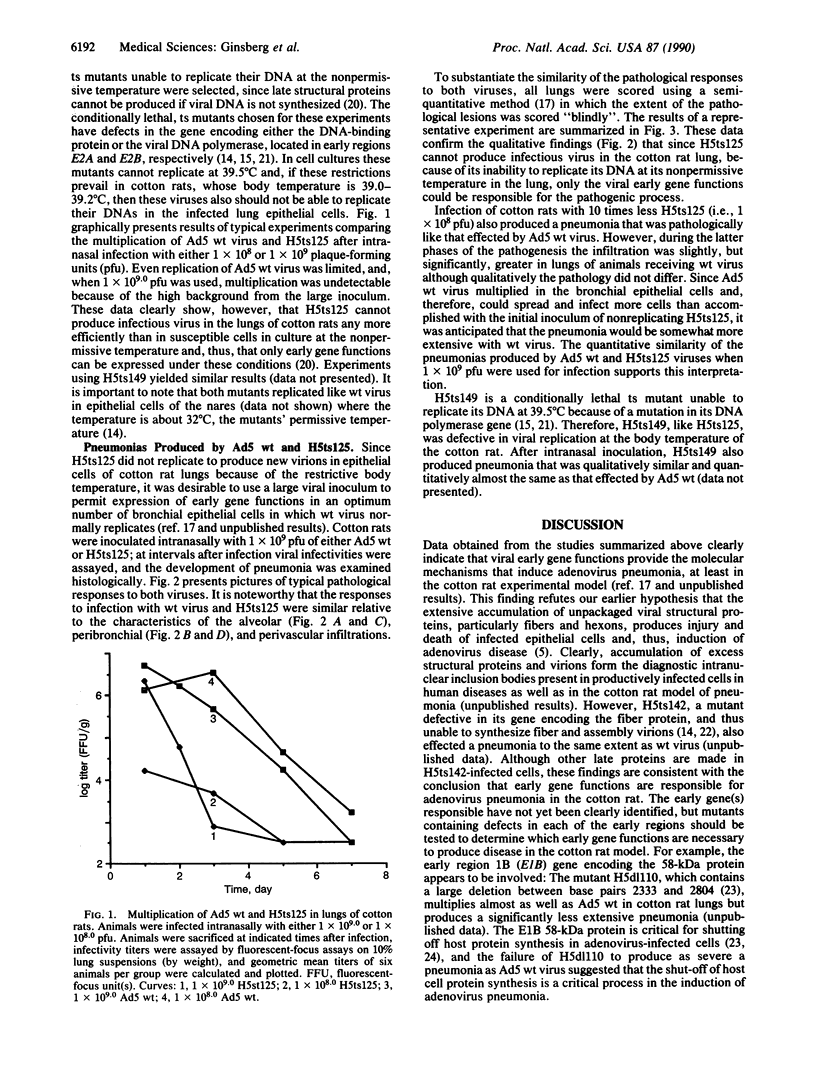
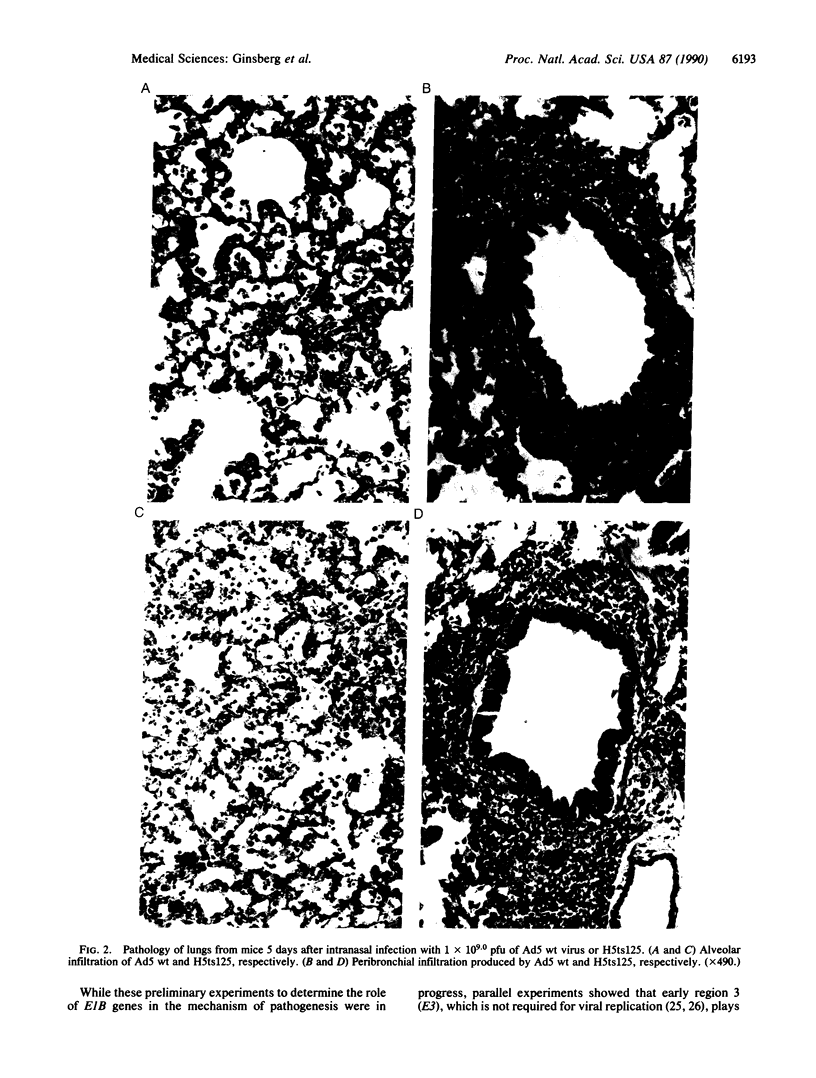
Intranasal inoculation of type 5 adenovirus into the cotton rat Sigmodon hispidus produces a pneumonia pathologically similar to that in humans, and it, therefore, provides an excellent animal model to investigate the pathogenesis of this disease. The goal of this study was to test the hypothesis that accumulation of viral structural proteins is responsible for a major portion of the cell-damage-producing disease. Since viral DNA replication is essential for synthesis of the viral structural proteins, which are products of late genes, the hypothesis was tested using mutants defective in genes required for DNA synthesis. Most experiments were done with the conditionally lethal temperature-sensitive (ts) mutant H5ts125, which contains a mutation in the early region 2A (E2A) gene encoding the DNA-binding protein. The data show that infection with 1 x 10(9.0) plaque-forming units of H5ts125 induced a pneumonia that was as extensive and qualitatively the same as that after wild-type adenovirus type 5 infection, although H5ts125 did not replicate to produce infectious virus. When cotton rats were infected with 1 x 10(8.0) plaque-forming units of wild-type adenovirus type 5 or H5ts125, the pneumonias that followed were pathologically similar; in the latter phases, however, wild-type virus produced slightly more extensive pneumonia than did H5ts125, probably because its replication permitted infection of more susceptible cells.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson M., Päbo S., Nilsson T., Peterson P. A. Impaired intracellular transport of class I MHC antigens as a possible means for adenoviruses to evade immune surveillance. Cell. 1985 Nov;43(1):215–222. doi: 10.1016/0092-8674(85)90026-1. [DOI] [PubMed] [Google Scholar]
- BOYER G. S., DENNY F. W., Jr, GINSBERG H. S. Sequential cellular changes produced by types 5 and 7 adenoviruses in HeLa cells and in human amniotic cells; cytological studies aided by fluorescein-labelled antibody. J Exp Med. 1959 Nov 1;110:827–844. doi: 10.1084/jem.110.5.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOYER G. S., LEUCHTENBERGER C., GINSBERG H. S. Cytological and cytochemical studies of HeLa cells infected with adeno-viruses. J Exp Med. 1957 Mar 1;105(3):195–216. doi: 10.1084/jem.105.3.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiss L. E., Ginsberg H. S. Adenovirus type 5 early region 1b gene product is required for efficient shutoff of host protein synthesis. J Virol. 1984 Apr;50(1):202–212. doi: 10.1128/jvi.50.1.202-212.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiss L. E., Ginsberg H. S., Darnell J. E., Jr Adenovirus E1B proteins are required for accumulation of late viral mRNA and for effects on cellular mRNA translation and transport. Mol Cell Biol. 1985 Oct;5(10):2552–2558. doi: 10.1128/mcb.5.10.2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello L. J., Ginsberg H. S. Inhibition of host protein synthesis in type 5 adenovirus-infected cells. J Virol. 1967 Oct;1(5):843–850. doi: 10.1128/jvi.1.5.843-850.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello L. J., Ginsberg H. S. Relationship between deoxyribonucleic acid-like ribonucleic acid synthesis and inhibition of host protein synthesis in type 5 adenovirus-infected KB cells. J Virol. 1969 Feb;3(2):106–113. doi: 10.1128/jvi.3.2.106-113.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler B., Cerami A. Tumor necrosis, cachexia, shock, and inflammation: a common mediator. Annu Rev Biochem. 1988;57:505–518. doi: 10.1146/annurev.bi.57.070188.002445. [DOI] [PubMed] [Google Scholar]
- Bhat B. M., Brady H. A., Pursley M. H., Wold W. S. Deletion mutants that alter differential RNA processing in the E3 complex transcription unit of adenovirus. J Mol Biol. 1986 Aug 20;190(4):543–557. doi: 10.1016/0022-2836(86)90240-8. [DOI] [PubMed] [Google Scholar]
- Burgert H. G., Kvist S. An adenovirus type 2 glycoprotein blocks cell surface expression of human histocompatibility class I antigens. Cell. 1985 Jul;41(3):987–997. doi: 10.1016/s0092-8674(85)80079-9. [DOI] [PubMed] [Google Scholar]
- Challberg S. S., Ketner G. Deletion mutants of adenovirus 2: isolation and initial characterization of virus carrying mutations near the right end of the viral genome. Virology. 1981 Oct 15;114(1):196–209. doi: 10.1016/0042-6822(81)90265-8. [DOI] [PubMed] [Google Scholar]
- Chee-Sheung C. C., Ginsberg H. S. Characterization of a temperature-sensitive fiber mutant of type 5 adenovirus and effect of the mutation on virion assembly. J Virol. 1982 Jun;42(3):932–950. doi: 10.1128/jvi.42.3.932-950.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensinger M. J., Ginsberg H. S. Selection and preliminary characterization of temperature-sensitive mutants of type 5 adenovirus. J Virol. 1972 Sep;10(3):328–339. doi: 10.1128/jvi.10.3.328-339.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLANAGAN J. F., GINSBERG H. S. Synthesis of virus-specific polymers in adenovirus-infected cells; effect of 5-fluorodeoxyuridine. J Exp Med. 1962 Aug 1;116:141–157. doi: 10.1084/jem.116.2.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GINSBERG H. S., DIXON M. K. Deoxyribonucleic acid (DNA) and protein alterations in HeLa cells infected with type 4 adenovirus. J Exp Med. 1959 Apr 1;109(4):407–422. doi: 10.1084/jem.109.4.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GINSBERG H. S., DIXON M. K. Nucleuc acid synthesis in types 4 and 5 adenovirus-infected HeLa cells. J Exp Med. 1961 Feb 1;113:283–299. doi: 10.1084/jem.113.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galos R. S., Williams J., Binger M. H., Flint S. J. Location of additional early gene sequences in the adenoviral chromosome. Cell. 1979 Aug;17(4):945–956. doi: 10.1016/0092-8674(79)90334-9. [DOI] [PubMed] [Google Scholar]
- Ginsberg H. S., Ensinger M. J., Kauffman R. S., Mayer A. J., Lundholm U. Cell transformation: a study of regulation with types 5 and 12 adenovirus temperature-sensitive mutants. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):419–426. doi: 10.1101/sqb.1974.039.01.054. [DOI] [PubMed] [Google Scholar]
- Ginsberg H. S., Lundholm-Beauchamp U., Horswood R. L., Pernis B., Wold W. S., Chanock R. M., Prince G. A. Role of early region 3 (E3) in pathogenesis of adenovirus disease. Proc Natl Acad Sci U S A. 1989 May;86(10):3823–3827. doi: 10.1073/pnas.86.10.3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooding L. R., Elmore L. W., Tollefson A. E., Brady H. A., Wold W. S. A 14,700 MW protein from the E3 region of adenovirus inhibits cytolysis by tumor necrosis factor. Cell. 1988 May 6;53(3):341–346. doi: 10.1016/0092-8674(88)90154-7. [DOI] [PubMed] [Google Scholar]
- HILLEMAN M. R., WERNER J. H. Recovery of new agent from patients with acute respiratory illness. Proc Soc Exp Biol Med. 1954 Jan;85(1):183–188. doi: 10.3181/00379727-85-20825. [DOI] [PubMed] [Google Scholar]
- Jones N., Shenk T. Isolation of deletion and substitution mutants of adenovirus type 5. Cell. 1978 Jan;13(1):181–188. doi: 10.1016/0092-8674(78)90148-4. [DOI] [PubMed] [Google Scholar]
- Kelly T. J., Jr, Lewis A. M., Jr Use of nondefective adenovirus-simian virus 40 hybrids for mapping the simian virus 40 genome. J Virol. 1973 Sep;12(3):643–652. doi: 10.1128/jvi.12.3.643-652.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence W. C., Ginsberg H. S. Intracellular uncoating of type 5 adenovirus deoxyribonucleic acid. J Virol. 1967 Oct;1(5):851–867. doi: 10.1128/jvi.1.5.851-867.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine A. J., Ginsberg H. S. Mechanism by which fiber antigen inhibits multiplication of type 5 adenovirus. J Virol. 1967 Aug;1(4):747–757. doi: 10.1128/jvi.1.4.747-757.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine A. J., Ginsberg H. S. Role of adenovirus structural proteins in the cessation of host-cell biosynthetic functions. J Virol. 1968 May;2(5):430–439. doi: 10.1128/jvi.2.5.430-439.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacini D. L., Dubovi E. J., Clyde W. A., Jr A new animal model for human respiratory tract disease due to adenovirus. J Infect Dis. 1984 Jul;150(1):92–97. doi: 10.1093/infdis/150.1.92. [DOI] [PubMed] [Google Scholar]
- ROWE W. P., HUEBNER R. J., GILMORE L. K., PARROTT R. H., WARD T. G. Isolation of a cytopathogenic agent from human adenoids undergoing spontaneous degeneration in tissue culture. Proc Soc Exp Biol Med. 1953 Dec;84(3):570–573. doi: 10.3181/00379727-84-20714. [DOI] [PubMed] [Google Scholar]
- Severinsson L., Peterson P. A. Abrogation of cell surface expression of human class I transplantation antigens by an adenovirus protein in Xenopus laevis oocytes. J Cell Biol. 1985 Aug;101(2):540–547. doi: 10.1083/jcb.101.2.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wold W. S., Deutscher S. L., Takemori N., Bhat B. M., Magie S. C. Evidence that AGUAUAUGA and CCAAGAUGA initiate translation in the same mRNA region E3 of adenovirus. Virology. 1986 Jan 15;148(1):168–180. doi: 10.1016/0042-6822(86)90412-5. [DOI] [PubMed] [Google Scholar]