Abstract
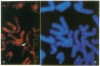
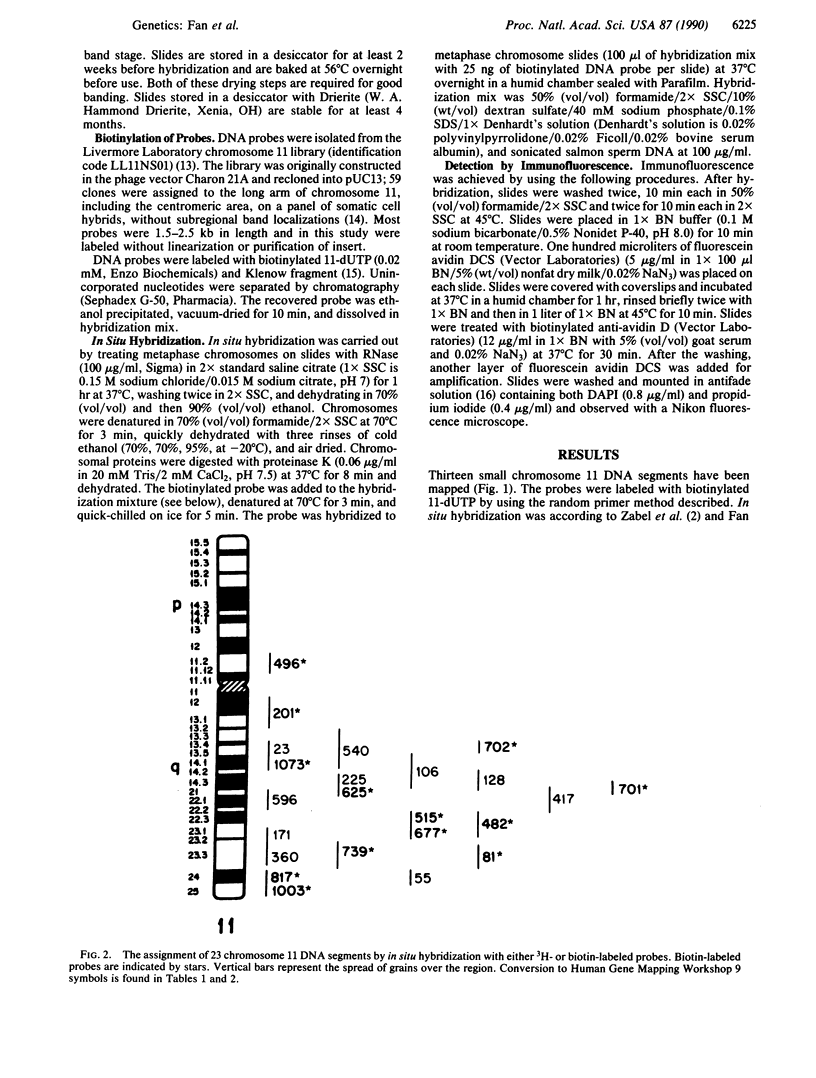
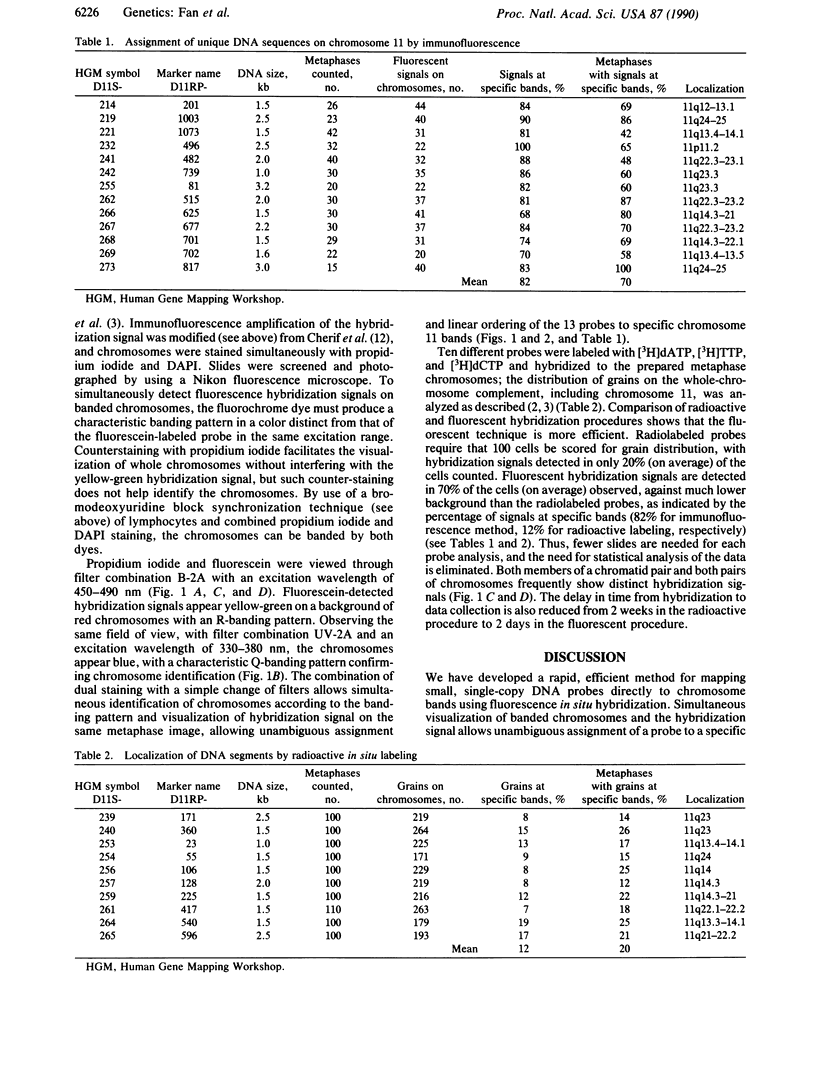
A procedure for mapping small DNA probes directly on banded human chromosomes by fluorescence in situ hybridization has been developed. This procedure allows for the simultaneous visualization of banded chromosomes and hybridization signal without overlaying two separate photographic images. This method is simple and rapid, requires only a typical fluorescence microscope, has proven successful with DNA probes as small as 1 kilobase, is applicable for larger probes, and will greatly facilitate mapping the vast number of probes being generated to study genetic disease and define the human genome. Human metaphase chromosomes were prepared from phytohemagglutinin-stimulated lymphocyte cultures synchronized with bromodeoxyuridine and thymidine. Probes were labeled with biotin-dUTP, and the hybridization signal was amplified by immunofluorescence. Chromosomes were stained with both propidium iodide and 4',6-diamidino-2-phenylindole (DAPI), producing R- and Q-banding patterns, respectively, allowing unambiguous chromosome and band identification while simultaneously visualizing the hybridization signal. Thirteen unique DNA segments have been localized to the long arm of chromosome 11 by using this technique, and localization of 10 additional probes by using radioactive in situ hybridization provides a comparison between the two procedures. These DNA segments have been mapped to all long-arm bands on chromosome 11 and in regions associated with neoplasias and inherited disorders.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cherif D., Bernard O., Berger R. Detection of single-copy genes by nonisotopic in situ hybridization on human chromosomes. Hum Genet. 1989 Mar;81(4):358–362. doi: 10.1007/BF00283691. [DOI] [PubMed] [Google Scholar]
- Davis L. M., Byers M. G., Fukushima Y., Qin S. Z., Nowak N. J., Scoggin C., Shows T. B. Four new DNA markers are assigned to the WAGR region of 11p13: isolation and regional assignment of 112 chromosome 11 anonymous DNA segments. Genomics. 1988 Oct;3(3):264–271. doi: 10.1016/0888-7543(88)90087-0. [DOI] [PubMed] [Google Scholar]
- Fan Y. S., Eddy R. L., Byers M. G., Haley L. L., Henry W. M., Nowak N. J., Shows T. B. The human mineralocorticoid receptor gene (MLR) is located on chromosome 4 at q31.2. Cytogenet Cell Genet. 1989;52(1-2):83–84. doi: 10.1159/000132846. [DOI] [PubMed] [Google Scholar]
- Fan Y. S., Rowe J. M., Dal Cin P. D., Sandberg A. A. A translocation t(9;11)(p11;q23) in T-cell acute lymphoblastic leukemia (FAB-l2). Cancer Genet Cytogenet. 1988 Apr;31(2):263–269. doi: 10.1016/0165-4608(88)90226-9. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Fuscoe J. C., Clark L. M., Van Dilla M. A. Construction of fifteen human chromosome-specific DNA libraries from flow-purified chromosomes. Cytogenet Cell Genet. 1986;43(1-2):79–86. doi: 10.1159/000132301. [DOI] [PubMed] [Google Scholar]
- Gollin S. M., Perrot L. J., Gray B. A., Kletzel M. Spontaneous expression of fra(11)(q23) in a patient with Ewing's sarcoma and t(11;22)(q23;q11). Cancer Genet Cytogenet. 1986 Feb 15;20(3-4):331–339. doi: 10.1016/0165-4608(86)90092-0. [DOI] [PubMed] [Google Scholar]
- Harper M. E., Saunders G. F. Localization of single copy DNA sequences of G-banded human chromosomes by in situ hybridization. Chromosoma. 1981;83(3):431–439. doi: 10.1007/BF00327364. [DOI] [PubMed] [Google Scholar]
- Johnson G. D., Nogueira Araujo G. M. A simple method of reducing the fading of immunofluorescence during microscopy. J Immunol Methods. 1981;43(3):349–350. doi: 10.1016/0022-1759(81)90183-6. [DOI] [PubMed] [Google Scholar]
- Lawrence J. B., Villnave C. A., Singer R. H. Sensitive, high-resolution chromatin and chromosome mapping in situ: presence and orientation of two closely integrated copies of EBV in a lymphoma line. Cell. 1988 Jan 15;52(1):51–61. doi: 10.1016/0092-8674(88)90530-2. [DOI] [PubMed] [Google Scholar]
- Lichter P., Cremer T., Borden J., Manuelidis L., Ward D. C. Delineation of individual human chromosomes in metaphase and interphase cells by in situ suppression hybridization using recombinant DNA libraries. Hum Genet. 1988 Nov;80(3):224–234. doi: 10.1007/BF01790090. [DOI] [PubMed] [Google Scholar]
- Lichter P., Tang C. J., Call K., Hermanson G., Evans G. A., Housman D., Ward D. C. High-resolution mapping of human chromosome 11 by in situ hybridization with cosmid clones. Science. 1990 Jan 5;247(4938):64–69. doi: 10.1126/science.2294592. [DOI] [PubMed] [Google Scholar]
- Lucas J. N., Tenjin T., Straume T., Pinkel D., Moore D., 2nd, Litt M., Gray J. W. Rapid human chromosome aberration analysis using fluorescence in situ hybridization. Int J Radiat Biol. 1989 Jul;56(1):35–44. doi: 10.1080/09553008914551161. [DOI] [PubMed] [Google Scholar]
- Pinkel D., Straume T., Gray J. W. Cytogenetic analysis using quantitative, high-sensitivity, fluorescence hybridization. Proc Natl Acad Sci U S A. 1986 May;83(9):2934–2938. doi: 10.1073/pnas.83.9.2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trent J. M., Kaneko Y., Mitelman F. Report of the committee on structural chromosome changes in neoplasia. Cytogenet Cell Genet. 1989;51(1-4):533–562. doi: 10.1159/000132807. [DOI] [PubMed] [Google Scholar]
- Tucker J. D., Christensen M. L., Carrano A. V. Simultaneous identification and banding of human chromosome material in somatic cell hybrids. Cytogenet Cell Genet. 1988;48(2):103–106. doi: 10.1159/000132600. [DOI] [PubMed] [Google Scholar]
- Viegas-Pequignot E., Dutrillaux B., Magdelenat H., Coppey-Moisan M. Mapping of single-copy DNA sequences on human chromosomes by in situ hybridization with biotinylated probes: enhancement of detection sensitivity by intensified-fluorescence digital-imaging microscopy. Proc Natl Acad Sci U S A. 1989 Jan;86(2):582–586. doi: 10.1073/pnas.86.2.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabel B. U., Naylor S. L., Sakaguchi A. Y., Bell G. I., Shows T. B. High-resolution chromosomal localization of human genes for amylase, proopiomelanocortin, somatostatin, and a DNA fragment (D3S1) by in situ hybridization. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6932–6936. doi: 10.1073/pnas.80.22.6932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dekken H., Bauman J. G. A new application of in situ hybridization: detection of numerical and structural chromosome aberrations with a combination centromeric-telomeric DNA probe. Cytogenet Cell Genet. 1988;48(3):188–189. doi: 10.1159/000132622. [DOI] [PubMed] [Google Scholar]